Abstract
Lifespan in Caenorhabditis elegans, Drosophila, and mice is regulated by conserved signaling networks, including the insulin/insulin-like growth factor 1 (IGF-1) signaling cascade and pathways depending on sirtuins, a family of NAD+-dependent deacetylases. Small molecules such as resveratrol are of great interest because they increase lifespan in many species in a sirtuin-dependent manner. However, no endogenous small molecules that regulate lifespan via sirtuins have been identified, and the mechanisms underlying sirtuin-dependent longevity are not well understood. Here, we show that in C. elegans, two endogenously produced small molecules, the dauer-inducing ascarosides ascr#2 and ascr#3, regulate lifespan and stress resistance through chemosensory pathways and the sirtuin SIR-2.1. Ascarosides extend adult lifespan and stress resistance without reducing fecundity or feeding rate, and these effects are reduced or abolished when nutrients are restricted. We found that ascaroside-mediated longevity is fully abolished by loss of SIR-2.1 and that the effect of ascr#2 requires expression of the G protein-coupled receptor DAF-37 in specific chemosensory neurons. In contrast to many other lifespan-modulating factors, ascaroside-mediated lifespan increases do not require insulin signaling via the FOXO homolog DAF-16 or the insulin/IGF-1-receptor homolog DAF-2. Our study demonstrates that C. elegans produces specific small molecules to control adult lifespan in a sirtuin-dependent manner, supporting the hypothesis that endogenous regulation of metazoan lifespan functions, in part, via sirtuins. These findings strengthen the link between chemosensory inputs and conserved mechanisms of lifespan regulation in metazoans and suggest a model for communal lifespan regulation in C. elegans.
Keywords: aging, chemosensation, β oxidation, ecology
The nematode Caenorhabditis elegans excretes a family of small molecules, the ascarosides, which regulate several different aspects of the life history of this model organism, including developmental timing (1–3), mate attraction (3–5), aggregation behavior (6, 7), and olfactory learning (8). These compounds are derived from the dideoxysugar ascarylose, which is linked to fatty acid-like side chains of varying lengths and, in some cases, features additional substituents derived from amino acid metabolism and other pathways (7). Originally, the ascarosides were identified as components of a population-density signal, the C. elegans dauer pheromone. High population density results in accumulation of the constitutively expressed ascarosides, which, in combination with additional environmental stimuli such as limited food availability or temperature stress, promote larval arrest at the dauer stage (1–3). Dauer larvae are nonfeeding and highly stress-resistant and can survive for up to 6 mo before resuming development into adults of a normal lifespan of about 2 wk (9). The signaling cascade from pheromone to dauer induction has been characterized in considerable detail. The dauer-inducing ascarosides are sensed by several different G protein-coupled receptors (GPCRs) in specific chemosensory neurons (10–12). These are coupled to several downstream signaling cascades, including the conserved insulin/insulin-like growth factor-1 (IGF-1) and transforming growth factor (TGF)-β signaling pathways (13–15). Mutations in dauer signaling genes often display long-lived lifespan phenotypes in adult C. elegans (16, 17), and homologs of dauer genes are involved in lifespan regulation of many higher organisms (13).
The strong analogies between dauer signaling and conserved aging pathways suggested that dauer pheromone components may also affect C. elegans adult lifespan. Previous studies have yielded conflicting evidence regarding the effects of dauer pheromone on adults (18–20); however, these studies tested for the lifespan-extending effects of the entire C. elegans exometabolome, which, in addition to a variety of dauer-inducing ascarosides, contains a vast assortment of other metabolites. Therefore, we investigated the effects of pure synthetic samples of dauer-inducing ascarosides on C. elegans adult lifespan.
Results
ascr#2 and ascr#3 Extend C. elegans Lifespan and Stress Resistance.
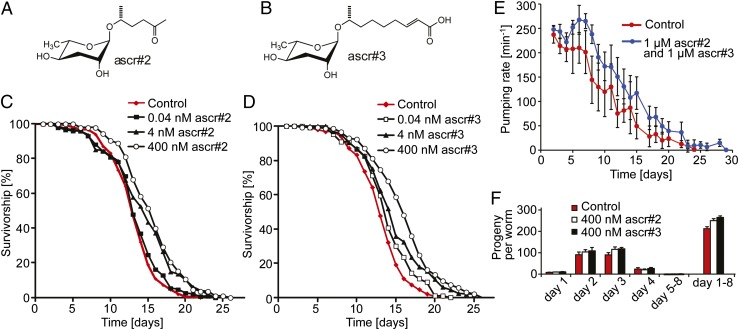
We tested the effect of ascr#2 and ascr#3 (Fig. 1 A and B), the two most consistently produced ascarosides (1, 3), on C. elegans lifespan. We found that exposure to ascarosides resulted in a concentration-dependent increase of lifespan. Mean lifespan of L4-stage worms transferred onto plates containing ascr#2 or ascr#3 was significantly increased in a concentration-dependent manner (Fig. 1 C and D and Table S1). Lifespan of worms on plates containing 400 nM ascr#2 or ascr#3, concentrations typical of high-density C. elegans cultures (7), was increased by 17% and 21%, respectively. Higher concentrations did not confer significantly higher lifespan gains, and mixtures of ascr#2 and ascr#3 extended lifespan to a similar extent as the individual compounds (Table S1 and Fig. S1).
Fig. 1.
Ascarosides increase adult lifespan in wild-type (N2) C. elegans in a concentration-dependent manner. (A) Chemical structure of ascr#2. (B) Chemical structure of ascr#3. (C) Survivorship of worms at 20 °C exposed to 0.04 nM ascr#2: number of worms (n) = 147, average lifespan (m) = 13.4 d, P = 0.253; 4 nM ascr#2: n = 137, m = 14.5 d, P < 0.0001; 400 nM ascr#2: n = 111, m = 15.1 d, P < 0.0001; and mock-treated controls: n = 155, m = 13.2 d. (D) Survivorship of worms exposed to 0.04 nM: ascr#3, n = 139, m = 14.6 d, P < 0.0001; 4 nM ascr#3: n = 132, m = 14.9 d, P < 0.0001; 400 nM ascr#3: n = 134, m = 16.0 d, P < 0.0001; and mock-treated: n = 155, m = 13.2 d. (E) Pharyngeal pumping rate of worms exposed to 1 µM ascr#2 and ascr#3 compared with mock-treated control. (Error bars, SD.) (F) Fecundity of worms exposed to 400 nM ascr#2 or ascr#3 compared with mock-treated control. (Error bars, SD.)
Because in many animals reduced food intake [dietary restriction (DR)] results in increased lifespan, it seemed possible that ascaroside-mediated lifespan increases were the result of altered feeding behavior. Therefore, we monitored changes in pharyngeal pumping rate, a measure for C. elegans food intake, and fecundity upon ascaroside exposure (21). Pharyngeal pumping rates of ascaroside-treated worms were similar to control until day 4 of the experiments and significantly higher than control from day 5 until death (Fig. 1E), indicating that reduced food intake is not a likely cause for the observed lifespan increases. Furthermore, overall fecundity of ascaroside-treated worms was slightly higher than control (Fig. 1F).
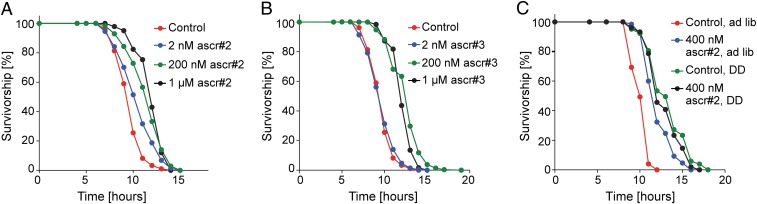
In C. elegans and other organisms, environmental conditions or genetic changes that result in increased lifespan are frequently associated with enhanced resistance to stress, including heat stress and oxidative stress (22). We found that ascarosides markedly increased survival under oxidative stress (Fig. S2A) and resistance to heat stress in a concentration-dependent manner (thermotolerance at 35 °C; Fig. 2 A and B and Table S2). We further measured pharyngeal pumping rates under heat stress and found that pumping rates of worms on ascaroside plates were significantly higher than on control plates (Fig. S2B). Consequently, we named the observed effect ascaroside-mediated increases of lifespan and stress resistance (AMILS).
Fig. 2.
Ascarosides increase thermotolerance at 35 °C in wild-type (N2) C. elegans in a concentration-dependent manner. (A) Survivorship of adult worms at 35 °C exposed to 2 nM, 200 nM, and 1 µM ascr#2; 2 nM ascr#2: n = 278, m = 10.6 h, P < 0.0001; 200 nM ascr#2: n = 313, m = 11.6 h, P < 0.0001; 1 µM ascr#2: n = 142, m = 12.1 h, P < 0.0001; and mock-treated control: n = 1062, m = 9.7 h. (B) Survivorship of adult worms at 35 °C exposed to 2 nM, 200 nM, and 1 µM ascr#2; 2 nM ascr#3: n = 278, m = 9.8 h, P = 0.004; 200 nM ascr#3: n = 289, m = 12.6 h, P < 0.0001; 1 µM ascr#3: n = 126, m = 12.6 h, P < 0.0001; and mock-treated control: n = 1062, m = 9.7 h. (C) Survivorship of adult worms at 35 °C exposed to 400 nM ascr#2 compared with mock-treated controls, using two different nutritional conditions [adlib (with bacteria) and DD (without bacteria)]; adlib ascr#2: n = 65, m = 12.2 h, P < 0.0001; adlib control: n = 75, m = 10.2 h; DD ascr#2: n = 103, m = 12.9 h, P = 0.145; DD control: n = 103, m = 13.3 h.
AMILS Depends on Nutritional Conditions.
Next, we asked whether nutritional conditions influence AMILS. For thermotolerance assays under dietary deprivation (DD) conditions, we used a protocol in which young adult worms are transferred to plates without bacteria before exposure to heat stress (23). Mean heat stress survival time under DD conditions was higher than for worms with bacteria, in accordance with previous studies demonstrating increased stress resistance under these conditions (23). Notably, addition of ascarosides did not further increase thermotolerance of DD worms (Fig. 2C). Additional lifespan assays using DR conditions (24) showed a similar pattern (Fig. S3). As expected, DR control worms lived longer than non-DR controls; however, ascarosides increased lifespan of DR worms less than of non-DR worms (Fig. S3). These results show that the effect of ascarosides on C. elegans thermotolerance and lifespan strongly depends on nutritional conditions.
AMILS Requires the Histone Deacetylase SIR-2.1.
To investigate the mechanism of AMILS, we probed conserved genetic pathways known to regulate lifespan in C. elegans and other metazoans. The observation that AMILS is reduced or abolished under starvation conditions led us to investigate a possible role of the sirtuin SIR-2.1, a NAD+-dependent histone deacetylase (25). DR, DD, and other forms of caloric restriction (CR) (26) have been shown to increase lifespan and stress resistance in many organisms, and in several cases, including yeast and mice, this effect has been found to depend on sirtuins (13, 27–32). In C. elegans, SIR-2.1 has been shown to be required for lifespan increases by resveratrol and other compounds (25, 33), although it is unclear to what extent SIR-2.1 is required for DR-dependent lifespan increases. For example, SIR-2.1 is not required for lifespan extension associated with DD of nonfertile worms (23), whereas lifespan increases associated with milder forms of DR appear to require this gene (13). The role of sirtuins in C. elegans and Drosophila lifespan continues to be discussed extensively (13, 34, 35).
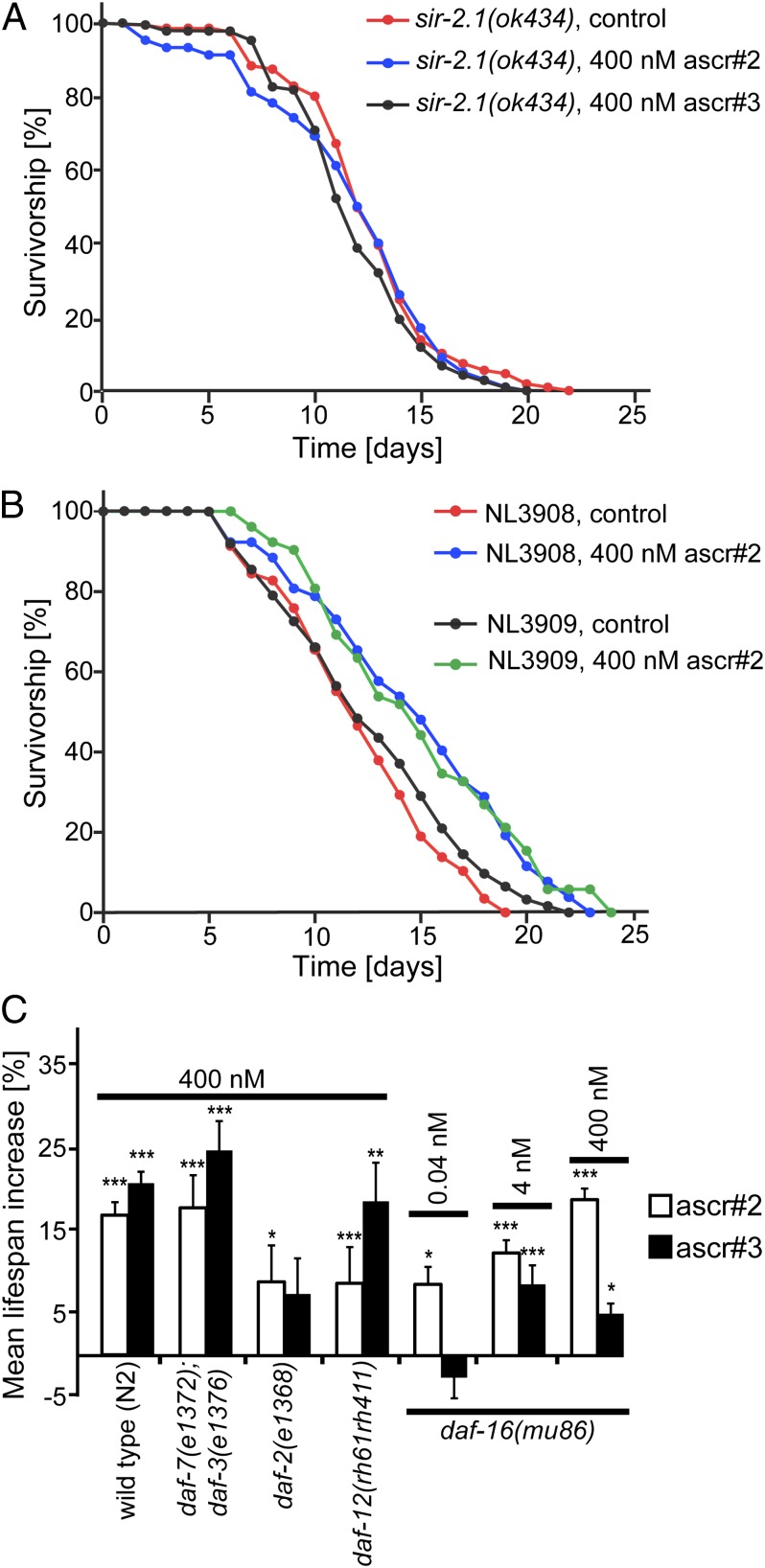
We found that neither ascr#2 nor ascr#3 extend lifespan or thermotolerance in the null allele sir-2.1(ok434) (lifespan: Fig. 3A and Table S1; thermotolerance: Fig. S4 A and B and Table S2). We then investigated the effect of ascr#2 on an outcrossed SIR-2.1–overexpressing strain, NL3909, relative to its control, NL3908 (34, 36, 37). ascr#2 increased lifespan of NL3909 worms to a similar extent as for NL3908 control worms (Fig. 3B and Table S1). These results indicate that SIR-2.1 is required for AMILS, but overexpression of SIR-2.1 does not significantly affect the amount of lifespan extension.
Fig. 3.
AMILS requires SIR-2.1 but not classical dauer pathway genes. (A) Survivorship of sir-2.1(ok434) mutant worms exposed to 400 nM ascr#2 or ascr#3; ascr#2: n = 100, m = 11.8 d; ascr#3: n = 119, m = 11.9 d; mock-treated control: n = 109, m = 12.5 d. (B) Survivorship of the sir-2.1 overexpressor NL3909 and its control NL3908 treated with ascr#2 400 nM; NL3909 ascr#2: n = 178, m = 13.7 d, P = 0.002; NL3909 mock-treated control: n = 174, m = 12.4 d; NL3908 ascr#2: n = 111, m = 15.2 d, P < 0.0001; NL30908 mock-treated control: n = 125, m = 12.0 d. (C) Mean lifespan extension for mutant strains of genes involved in dauer induction. (Error bars, SEM for highlighted experiments.) For full data, see Table S1. ***P < 0.0001; **P < 0.001; *P < 0.05.
AMILS Is Largely Independent of Classical Dauer Signaling Pathways.
Because ascarosides induce formation of dauer larvae in C. elegans, we asked whether AMILS depends on conserved aging pathways involved in dauer formation, including insulin/IGF-1, TGF-β, and nuclear hormone receptor (DAF-12) signaling (38). By testing the effect of ascarosides on the insulin/IGF-1 receptor mutant strain daf-2(e1368) (13), we found that both ascr#2 and ascr#3 further increased lifespan and thermotolerance of this long-lived mutant (Fig. 3C, Fig. S5, and Table S1). DAF-2 negatively regulates the FOXO-like transcription factor DAF-16, which is required for many lifespan- or stress resistance-enhancing mutations described for C. elegans (13). Ascarosides significantly increased lifespan and thermotolerance of the short-lived null allele daf-16(mu86) even at low concentrations (Fig. 3C, Fig. S5, and Table S1), although the observed lifespan increases were somewhat smaller than for wild type. We confirmed lifespan increase in a second daf-16 allele, a daf-16(mgDf50) mutant in sir-2.1–overexpressing background (Fig. S6 and Table S1). AMILS, therefore, is largely independent of insulin signaling via DAF-16.
AMILS is also maintained in the null allele of another key component of dauer signaling, the nuclear hormone receptor DAF-12 (Fig. 3C and Table S1) (39). To determine a possible role of TGF-β signaling, we tested the effect of ascarosides on lifespan and thermotolerance of a daf-7;daf-3 double mutant strain. daf-7 and daf-3 encode the TGF-β ligand and a co-SMAD protein that functions as a transcriptional regulator downstream of daf-7, respectively (40). daf-7(e1372);daf-3(e1376) double mutant worms responded to ascarosides similarly to wild type (Fig. 3C, Fig. S5, and Table S1). We conclude that AMILS functions largely independently from the main dauer signaling pathways, requiring SIR-2.1 but not insulin/IGF-1 or TGF-β signaling.
Sensory Neurons and a GPCR Mediate ascr#2-Dependent Lifespan Extension.
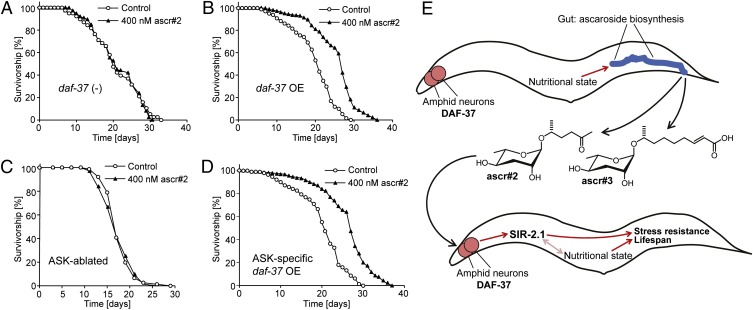
Next, we asked whether AMILS is mediated by chemosensory neurons and receptors that have been shown to participate in ascaroside sensing (4, 6, 10–12). For these investigations, we focused on ascr#2 for which we had recently described a highly specific GPCR, DAF-37 (12). DAF-37 expression in the ASI chemosensory neurons is required for dauer formation in response to ascr#2, whereas the behavioral phenotypes of ascr#2 depend on DAF-37 expression in the ASK neurons. ascr#2 directly binds to DAF-37, and it was further shown that daf-37 null worms are defective in their dauer and behavioral responses only to ascr#2 but not to other ascarosides (12). We found that ascr#2 does not increase lifespan of daf-37 null worms (Fig. 4A), which could be rescued by daf-37 cDNA under its own promoter (Fig. 4B). In addition, ascr#2-dependent lifespan increase was fully abolished in worms lacking the ASK neurons (Fig. 4C) because of cell-specific expression of mammalian caspase in the developing neurons. ascr#2-induced lifespan increase was also rescued by daf-37 cDNA driven by the ASK-selective srbc-64 promoter (Fig. 4D). In contrast, ascr#2 treatment of worms expressing daf-37 cDNA under the ASI-specific gpa-4 promoter resulted in variable results, whereby one experiment showed no change in lifespan compared with control and two subsequent experiments showed a small but significant lifespan decrease (Fig. S7 and Table S1). These results indicate that daf-37 expression in the ASK neurons is sufficient for ascr#2-dependent lifespan increase, in contrast to the dauer-inducing effects of ascr#2, which require expression in ASI (12).
Fig. 4.
ASK neurons and the GPCR DAF-37 are required for lifespan extension via ascr#2. (A) Survivorship of daf-37 (−) mutant worms exposed to 400 nM ascr#2 (n = 42, m = 20.9 d), compared with mock-treated controls (n = 38, m = 21.3 d). (B) Survivorship of daf-37;mIs41[daf-37p::cMyc::daf-37] (daf-37 OE) worms exposed to 400 nM ascr#2 (n = 56, m = 25.5 d, P < 0.0001), compared with mock-treated controls (n = 77, m =19.8 d). (C) Survivorship of worms with genetically ablated ASK neurons exposed to 400 nM ascr#2 (n = 79, m = 18.8 d), compared with mock-treated controls (n = 74, m = 19.0 d). (D) Survivorship of daf-37; unc-119; mEx188[srbc-64p::daf-37,unc-119(+)] (ASK neuron-specific daf-37 OE in daf-37 null background) worms exposed to 400 nM ascr#2 (n = 60, m = 26.2 d, P < 0.0001), compared with mock-treated controls (n = 85, m = 20.0 d). (E) Model for communal lifespan regulation via pheromone signaling. C. elegans constitutively produce ascr#2 and ascr#3, resulting in population density-dependent ascaroside concentrations. Chemosensation of the ascarosides integrated with environmental and nutritional cues results in activation of longevity and/or stress-resistance genes in a SIR-2.1-dependent manner.
Discussion
We demonstrate here that sensing of excreted small molecules regulates C. elegans lifespan and stress resistance via pathways that require the sirtuin SIR-2.1. Ascarosides increase adult lifespan at physiological concentrations typical of high-density C. elegans cultures, without reducing fecundity or feeding rate. In contrast to many other metazoan examples for life extension, AMILS does not require insulin signaling via DAF-16. Furthermore, AMILS does not depend on DAF-12, which is required for germ-line–dependent modulation of C. elegans lifespan (41). These observations starkly differentiate AMILS from dauer formation.
Previous studies suggested a complex role for sir-2.1 in C. elegans lifespan regulation (13, 20). There is increasing evidence that sirtuin overexpression increases lifespan across model systems (37, 42), although, in some cases, this effect may not be as robust as initially reported (34, 35, 43). In addition, genetic studies of sir-2.1-deletion strains suggest that sir-2.1 influences lifespan in a daf-16–independent manner as part of the CR pathway (44–46). Recent work connecting sirtuins and longevity in mouse affirmed a conserved role for sirtuins in lifespan regulation (47). With regard to sirtuin dependence, AMILS resembles the lifespan-increasing effects of exogenous small molecules such as resveratrol. Comparatively high concentrations of resveratrol (10–100 µM) increase lifespan in C. elegans and Drosophila, and this effect has been shown to require SIR-2.1 in C. elegans and its homolog Sir2 in Drosophila (25). Similar to resveratrol-mediated lifespan increases, AMILS does not carry any apparent cost of reproduction, is reduced or abolished when nutrients are restricted, and does not require daf-16. It has been suggested that resveratrol increases metazoan lifespan via pathways related to CR (25); however, the mechanisms of resveratrol-mediated lifespan increases are not well understood (13), and it has been suggested that the relatively weak lifespan-increasing effects of resveratrol in C. elegans could merely reflect activation of detoxification mechanisms (35). Given that AMILS requires much lower (compared with resveratrol) concentrations of compounds that are endogenously produced and perceived by highly specific membrane receptors, it is unlikely that detoxification plays a role for the lifespan-mediating effects of ascarosides.
In dauer formation, the ascarosides act through several amphid neurons (4, 48), and six GPCRs have been associated with ascaroside-mediated dauer induction (10–12). We recently showed that DAF-37 is required specifically for sensing of ascr#2 and that DAF-37 expression in the ASK neurons is required for the behavioral phenotypes of ascr#2, whereas DAF-37 expression in the ASI neurons is required for ascr#2-mediated dauer induction (12). Here, we show that DAF-37 is also required for ascr#2-mediated lifespan extension and that DAF-37 expression in the ASK neurons is sufficient for this phenotype, further distinguishing AMILS from dauer. Therefore, AMILS provides a direct example for a lifespan-regulatory mechanism based on chemosensation of an endogenously produced small-molecule signal.
Genetic and small-molecule screens have previously suggested a connection between GPCR signaling and lifespan in Drosophila (49, 50). In these studies, GPCR mutation or inhibition of downstream signaling components extended fly lifespan, but it is unclear whether chemosensation played a role. Notably, GPCRs have also been implicated in many age-related diseases (26). In Fig. 4E, we present a simplified model for AMILS in C. elegans. As products of peroxisomal β-oxidation and other primary metabolic pathways (7), ascarosides integrate input from the nutritional state of the producing animal. The primary site of ascaroside biosynthesis appears to be the worm gut (51), and it seems likely that ascarosides are also excreted via the gut. In the perceiving worm, GPCRs in the amphid chemosensory neurons trigger downstream signaling cascades promoting longevity and stress resistance via SIR-2.1, additionally integrating information about the perceiving animal’s nutritional state. In essence, AMILS may represent a model for communal mechanism for lifespan regulation (Fig. 4E).
Our study highlights the significance of sirtuin-dependent signaling pathways for endogenous lifespan regulation, and ascarosides may serve as useful tools to investigate the mechanisms of sirtuin-dependent longevity, which remain poorly understood (27). The ascarosides are derived from highly conserved peroxisomal fatty acid β-oxidation, and their biosynthesis is regulated, in part, by nutritional state (7), suggesting a link between nutritional state, fat metabolism, and sirtuin-dependent lifespan regulation. Furthermore, analysis of the adaptive significance of sirtuin-dependent lifespan regulation via ascarosides in C. elegans may lead to a better understanding of the evolutionary forces that determine lifespan and may inspire a search for endogenous small molecules that promote sirtuin-dependent longevity in other metazoans.
Materials and Methods
Worm Strains.
The following C. elegans strains were obtained from the Caenorhabditis Genetics Center: wild type (N2), daf-2(e1368), daf-16(mu86), sir-2.1(ok434), daf-12(rh61rh411). The strain daf-7(e1372);daf-3(e1376) was the kind gift of Paul Sternberg (Caltech, Pasadena, CA), and the low-copy SIR-2.1-overexpressing strain NL3909 pkIs1642(unc-119[+] sir-2.1[+]), and its control NL3908 pkIs1641(unc-119[+]), as well as a daf-16(mgDf50);NL3909, were kind gifts of Sylvia Lee (Cornell University, Washington, DC). For genetic ablation of the ASK neuron, we used the transgenic strain PS6025 qrIs2[sra-9::mCasp1], which expresses mammalian caspase in the ASK neuron under the sra-9 promoter [this strain was a kind gift of Tokumitsu Wakabayashi (Iwate University, Iwate, Japan)]. The following daf-37 overexpressor and mutant strains were created as described earlier: daf-37(DR2585) (daf-37 null mutant), daf-37; mIs41[daf-37p::daf-37,rol-6(su1006)] (daf-37 overexpressor in daf-37 mutant background), daf-37; unc-119; mEx188[srbc-64p::daf-37,unc-119(+)] (ASK-specific daf-37 overexpressor in daf-37 mutant background), and daf-37;unc-119;mEx187[gpa-4p::daf-37,unc-119(+)] (ASI-specific daf-37 overexpressor in daf-37 mutant background) (12). Nematode stocks were maintained on nematode growth medium (NGM) (52) plates with added bacteria (Escherichia coli strain OP50) at 20 °C (www.wormbook.org), unless indicated otherwise.
Plate Preparation.
Ascarosides ascr#2 and ascr#3 were synthesized as described previously (3). For preparation of ascaroside-containing NGM plates, ascr#2 and ascr#3 were dissolved in 100% ethanol producing 10.6 mM (ascr#2) and 7.6 mM (ascr#3) stock solutions, which were further diluted with water to yield stock solutions of micromolar and nanomolar concentrations, as needed. Controls for mock treatment had corresponding amounts of ethanol added. Plates were allowed to dry for 24 h. For the heat stress and lifespan assays, 6-cm diameter Petri dish plates were prepared using noble agar-based NGM prepared according to www.wormbook.org. Minimal growth medium NGM without peptone (“-pep”, for DR studies) was prepared as described for NGM plates but without adding peptone. Two hundred microliters of ascaroside solutions or mock solutions were spread on the surface of NGM plates. After drying overnight, 60 μL of bacteria (E. coli OP50 pellet), freshly grown at 37 °C in LB media, was spread onto the plates, and plates were incubated for 24 h at 23.5 °C. For thermotolerance assays, plates were exposed to UV radiation for 1 h to kill the bacteria before putting worms on the plates.
Lifespan Assays.
For lifespan assays, worm strains were thawed from frozen stock and grown for at least two generations on well-fed conditions. Six-centimeter NGM plates containing ascr#2, ascr#3, 1:1 mixtures of ascr#2 and ascr#3, and mock-treated control plates were used. Late L4-stage worms were picked from synchronized NGM plates and transferred (25–30 worms per plate) to experimental plates at 20 °C. Table S1 contains full lifespan data. Survival was monitored by scoring for touch-provoked movement every other day, in some cases every day. Live worms were transferred every other day to remove progeny until day 10. Day 0 corresponds to the time of transfer of the worms at the L4 stage. All experiments, except the experiment using 0.04 nM either ascr#2 and ascr#3 (Fig. 1), were carried out at least twice at two different times, started at least 1 wk apart. For testing the effect of ascarosides on C. elegans adult lifespan without peptone supplementation, NGM without peptone plates were used. For some lifespan experiments (Table S1), 2′-deoxy-5-fluorouridine (FUdR) was used. The SPSS version 19 (IBM) statistical analysis package was used for all lifespan and thermotolerance statistics. P values were calculated using the log-rank (Mantel–Cox) method.
Lifespan Assays Using FUdR.
FUdR (Sigma) was added to a final concentration of 4 µg/mL after media were autoclaved. Ascaroside and control solutions were added as described above. Late L4-stage worms were picked from synchronized NGM plates and transferred (30 worms per plate) to control plates or plates containing ascarosides that also contained FUdR.
Thermotolerance Assays.
Six-centimeter NGM plates containing ascr#2, ascr#3, 1:1 mixtures of ascr#2 and ascr#3, and mock-treated control plates were used. Experimental plates were prepared using noble agar-based NGM (52). E. coli OP50 was UV-killed before placing worms on plates. Late L4-stage worms were picked from synchronized NGM plates and transferred (15–25 worms per plate) to experimental plates. After completion of development to adults at 20 °C (14–20 h, depending on the strain), plates were incubated at 35 °C. Survival was scored hourly, beginning after 5–8 h of heat exposure, by checking for touch-provoked movement until all worms had died. At least four plates (usually five) for each ascaroside concentration were used, in addition to six control plates, and all experiments were carried out at least twice at two different times. Thermotolerance assays reported in Table S2 were performed as above, except that experimental plates with live E. coli OP50 were used instead of UV-killed bacteria. The SPSS version 19 (IBM) statistical analysis package was used for all thermotolerance statistics. P values were calculated using the log-rank (Mantel–Cox) method.
Thermotolerance Assays with DD.
Late stage-L4 worms were picked from synchronized NGM plates and placed on experimental plates. After completion of development to adults at 20 °C (16 h), worms were transferred to “adlib” or “DD” plates; adlib plates: NGM plates with bacteria containing 400 nM ascr#2 or control; DD plates: NGM plates without bacteria containing 400 nM ascr#2 or control, additionally treated with 200 µL of 5 mg/mL streptomycin sulfate and penicillin solution (23). Immediately following worm transfer, adlib or DD plates were exposed to 35 °C. Survival was monitored as described above. The SPSS version 19 (IBM) statistical analysis package was used for all thermotolerance statistics. P values were calculated using the log-rank (Mantel–Cox) method.
Oxidative Stress (H2O2) Resistance Assay.
Synchronized L4-stage worms were placed on NGM plates (mock-treated control or containing various concentrations ascr#2 and ascr#3) with bacteria (OP50) at 20 °C. After reaching young adult stage (12–16 h), worms were transferred to NGM plates (mock-treated control or containing various concentrations ascr#2 or ascr#3) with bacteria and containing 0.6 mM H2O2. Survival was scored after 6 h at 20 °C.
Brood Size Measurement.
To determine the average brood size per worm, living eggs and L1 progeny were counted on the plates used for the lifespan experiments described above. Worms were transferred 8–10 times over the course of the experiments. The number of progeny per worm was determined by dividing the total number of eggs and L1 worms counted per plate by the number of live worms transferred. Five to six plates per condition (ascaroside-treated and control) with 20–30 worms per plate were counted.
Measurement of Pharyngeal Pumping Rates.
Pumping of worms was counted over 1-min intervals by eye. Ten worms per condition and time point in the lifespan assays were scored, beginning at day 1 of the experiment and continued daily until all worms had died. In the thermotolerance assays, pharyngeal pumping rates of at least five worms were scored every 30 min starting 8 h after placing the worms in the incubator.
Statistical Analysis.
The SPSS version 19 (IBM) statistical analysis package was used for all lifespan and thermotolerance statistics. P values were calculated using the log-rank (Mantel–Cox) method.
Supplementary Material
Acknowledgments
We thank Sylvia Lee (Cornell University), Art Edison (University of Florida), Jagan Srinivasan (Caltech), and Paul Sternberg (Caltech) for helpful comments on the manuscript. This work was supported by National Institutes of Health Grants T32GM008500 (to Y.I.) and AG033839 and GM088290 (to F.C.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1214467110/-/DCSupplemental.
References
- 1.Butcher RA, Fujita M, Schroeder FC, Clardy J. Small-molecule pheromones that control dauer development in Caenorhabditis elegans. Nat Chem Biol. 2007;3(7):420–422. doi: 10.1038/nchembio.2007.3. [DOI] [PubMed] [Google Scholar]
- 2.Jeong PY, et al. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature. 2005;433(7025):541–545. doi: 10.1038/nature03201. [DOI] [PubMed] [Google Scholar]
- 3.Pungaliya C, et al. A shortcut to identifying small molecule signals that regulate behavior and development in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2009;106(19):7708–7713. doi: 10.1073/pnas.0811918106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srinivasan J, et al. A blend of small molecules regulates both mating and development in Caenorhabditis elegans. Nature. 2008;454(7208):1115–1118. doi: 10.1038/nature07168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Izrayelit Y, et al. Targeted metabolomics reveals a male pheromone and sex-specific ascaroside biosynthesis in Caenorhabditis elegans. ACS Chem Biol. 2012;7(8):1321–1325. doi: 10.1021/cb300169c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srinivasan J, et al. A modular library of small molecule signals regulates social behaviors in Caenorhabditis elegans. PLoS Biol. 2012;10(1):e1001237. doi: 10.1371/journal.pbio.1001237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Reuss SH, et al. Comparative metabolomics reveals biogenesis of ascarosides, a modular library of small-molecule signals in C. elegans. J Am Chem Soc. 2012;134(3):1817–1824. doi: 10.1021/ja210202y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamada K, et al. Olfactory plasticity is regulated by pheromonal signaling in Caenorhabditis elegans. Science. 2010;329(5999):1647–1650. doi: 10.1126/science.1192020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golden JW, Riddle DL. The Caenorhabditis elegans dauer larva: Developmental effects of pheromone, food, and temperature. Dev Biol. 1984;102(2):368–378. doi: 10.1016/0012-1606(84)90201-x. [DOI] [PubMed] [Google Scholar]
- 10.Kim K, et al. Two chemoreceptors mediate developmental effects of dauer pheromone in C. elegans. Science. 2009;326(5955):994–998. doi: 10.1126/science.1176331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGrath PT, et al. Parallel evolution of domesticated Caenorhabditis species targets pheromone receptor genes. Nature. 2011;477(7364):321–325. doi: 10.1038/nature10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park D, et al. Interaction of structure-specific and promiscuous G-protein-coupled receptors mediates small-molecule signaling in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2012;109(25):9917–9922. doi: 10.1073/pnas.1202216109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kenyon CJ. The genetics of ageing. Nature. 2010;464(7288):504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 14.Riddle DL. Genetic pathway for dauer larva formation in nematode, Caenorhabditis elegans. Genetics. 1977;86(2):S51–S52. [Google Scholar]
- 15.Shaw WM, Luo S, Landis J, Ashraf J, Murphy CT. The C. elegans TGF-beta Dauer pathway regulates longevity via insulin signaling. Curr Biol. 2007;17(19):1635–1645. doi: 10.1016/j.cub.2007.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larsen PL, Albert PS, Riddle DL. Genes that regulate both development and longevity in Caenorhabditis elegans. Genetics. 1995;139(4):1567–1583. doi: 10.1093/genetics/139.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299(5611):1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- 18.Alcedo J, Kenyon C. Regulation of C. elegans longevity by specific gustatory and olfactory neurons. Neuron. 2004;41(1):45–55. doi: 10.1016/s0896-6273(03)00816-x. [DOI] [PubMed] [Google Scholar]
- 19.Kawano T, et al. Lifespan extending activity of substances secreted by the nematode Caenorhabditis elegans that include the dauer-inducing pheromone. Biosci Biotechnol Biochem. 2005;69(12):2479–2481. doi: 10.1271/bbb.69.2479. [DOI] [PubMed] [Google Scholar]
- 20.Kenyon C. The plasticity of aging: Insights from long-lived mutants. Cell. 2005;120(4):449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Huang C, Xiong C, Kornfeld K. Measurements of age-related changes of physiological processes that predict lifespan of Caenorhabditis elegans. Proc Natl Acad Sci USA. 2004;101(21):8084–8089. doi: 10.1073/pnas.0400848101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lithgow GJ, White TM, Melov S, Johnson TE. Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proc Natl Acad Sci USA. 1995;92(16):7540–7544. doi: 10.1073/pnas.92.16.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee GD, et al. Dietary deprivation extends lifespan in Caenorhabditis elegans. Aging Cell. 2006;5(6):515–524. doi: 10.1111/j.1474-9726.2006.00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosono R, Nishimoto S, Kuno S. Alterations of life span in the nematode Caenorhabditis elegans under monoxenic culture conditions. Exp Gerontol. 1989;24(3):251–264. doi: 10.1016/0531-5565(89)90016-8. [DOI] [PubMed] [Google Scholar]
- 25.Wood JG, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430(7000):686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 26.Alemany R, et al. G protein-coupled receptor systems and their lipid environment in health disorders during aging. Biochim Biophys Acta. 2007;1768(4):964–975. doi: 10.1016/j.bbamem.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 27.Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460(7255):587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mair W, Dillin A. Aging and survival: The genetics of life span extension by dietary restriction. Annu Rev Biochem. 2008;77:727–754. doi: 10.1146/annurev.biochem.77.061206.171059. [DOI] [PubMed] [Google Scholar]
- 29.Bamps S, Wirtz J, Savory FR, Lake D, Hope IA. The Caenorhabditis elegans sirtuin gene, sir-2.1, is widely expressed and induced upon caloric restriction. Mech Ageing Dev. 2009;130(11-12):762–770. doi: 10.1016/j.mad.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Greer EL, Brunet A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell. 2009;8(2):113–127. doi: 10.1111/j.1474-9726.2009.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jadiya P, et al. Sir-2.1 modulates ‘calorie-restriction-mediated’ prevention of neurodegeneration in Caenorhabditis elegans: Implications for Parkinson’s disease. Biochem Biophys Res Commun. 2011;413(2):306–310. doi: 10.1016/j.bbrc.2011.08.092. [DOI] [PubMed] [Google Scholar]
- 32.Raynes R, Leckey BD, Jr, Nguyen K, Westerheide SD. Heat shock and caloric restriction have a synergistic effect on the heat shock response in a sir2.1-dependent manner in Caenorhabditis elegans. J Biol Chem. 2012;287(34):29045–29053. doi: 10.1074/jbc.M112.353714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller DL, Roth MB. Hydrogen sulfide increases thermotolerance and lifespan in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2007;104(51):20618–20622. doi: 10.1073/pnas.0710191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burnett C, et al. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature. 2011;477(7365):482–485. doi: 10.1038/nature10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bass TM, Weinkove D, Houthoofd K, Gems D, Partridge L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech Ageing Dev. 2007;128(10):546–552. doi: 10.1016/j.mad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Berdichevsky A, Viswanathan M, Horvitz HR, Guarente L. C. elegans SIR-2.1 interacts with 14-3-3 proteins to activate DAF-16 and extend life span. Cell. 2006;125(6):1165–1177. doi: 10.1016/j.cell.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 37.Rizki G, et al. The evolutionarily conserved longevity determinants HCF-1 and SIR-2.1/SIRT1 collaborate to regulate DAF-16/FOXO. PLoS Genet. 2011;7(9):e1002235. doi: 10.1371/journal.pgen.1002235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fielenbach N, Antebi A. C. elegans dauer formation and the molecular basis of plasticity. Genes Dev. 2008;22(16):2149–2165. doi: 10.1101/gad.1701508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gerisch B, et al. A bile acid-like steroid modulates Caenorhabditis elegans lifespan through nuclear receptor signaling. Proc Natl Acad Sci USA. 2007;104(12):5014–5019. doi: 10.1073/pnas.0700847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patterson GI, Koweek A, Wong A, Liu Y, Ruvkun G. The DAF-3 Smad protein antagonizes TGF-beta-related receptor signaling in the Caenorhabditis elegans dauer pathway. Genes Dev. 1997;11(20):2679–2690. doi: 10.1101/gad.11.20.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamawaki TM, et al. The somatic reproductive tissues of C. elegans promote longevity through steroid hormone signaling. PLoS Biol. 2010;8(8):e1000468. doi: 10.1371/journal.pbio.1000468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Banerjee KK, et al. dSir2 in the adult fat body, but not in muscles, regulates life span in a diet-dependent manner. Cell Rep. 2012;2(6):1485–1491. doi: 10.1016/j.celrep.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 43.Viswanathan M, Guarente L. Regulation of Caenorhabditis elegans lifespan by sir-2.1 transgenes. Nature. 2011;477(7365):E1–E2. doi: 10.1038/nature10440. [DOI] [PubMed] [Google Scholar]
- 44.Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410(6825):227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, et al. C. elegans 14-3-3 proteins regulate life span and interact with SIR-2.1 and DAF-16/FOXO. Mech Ageing Dev. 2006;127(9):741–747. doi: 10.1016/j.mad.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, Tissenbaum HA. Overlapping and distinct functions for a Caenorhabditis elegans SIR2 and DAF-16/FOXO. Mech Ageing Dev. 2006;127(1):48–56. doi: 10.1016/j.mad.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 47.Kanfi Y, et al. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483(7388):218–221. doi: 10.1038/nature10815. [DOI] [PubMed] [Google Scholar]
- 48.Macosko EZ, et al. A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature. 2009;458(7242):1171–1175. doi: 10.1038/nature07886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin YJ, Seroude L, Benzer S. Extended life-span and stress resistance in the Drosophila mutant methuselah. Science. 1998;282(5390):943–946. doi: 10.1126/science.282.5390.943. [DOI] [PubMed] [Google Scholar]
- 50.Spindler SR, Li R, Dhahbi JM, Yamakawa A, Sauer F. Novel protein kinase signaling systems regulating lifespan identified by small molecule library screening using Drosophila. PLoS ONE. 2012;7(2):e29782. doi: 10.1371/journal.pone.0029782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Butcher RA, et al. Biosynthesis of the Caenorhabditis elegans dauer pheromone. Proc Natl Acad Sci USA. 2009;106(6):1875–1879. doi: 10.1073/pnas.0810338106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shivers RP, Youngman MJ, Kim DH. Transcriptional responses to pathogens in Caenorhabditis elegans. Curr Opin Microbiol. 2008;11(3):251–256. doi: 10.1016/j.mib.2008.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.