Abstract
Tissue fibrosis represents one of the largest groups of diseases for which there are very few effective therapies. In the heart, myocardial infarction (MI) resulting in the loss of cardiac myocytes can culminate in adverse cardiac remodeling leading to eventual heart failure. Adverse cardiac remodeling includes myocyte hypertrophy, fibrosis, and electrical remodeling. We have previously demonstrated the beneficial effects of several potent soluble epoxide hydrolase inhibitors (sEHIs) in different models of cardiac hypertrophy and failure. Here, we directly determine the molecular mechanisms underlying the beneficial effects of sEHIs in cardiac remodeling post-MI. Treatment with a potent sEHI, 1-trifluoromethoxyphenyl-3-(1-propionylpiperidine-4-yl)urea (TPPU), which was started 1 wk post-MI in a murine model, results in a significant improvement in cardiac function. Importantly, treatment with TPPU results in a decrease in cardiac fibrosis as quantified using histological and immunostaining techniques. Moreover, single-cell–based assays demonstrate that treatment with TPPU results in a significant decrease not only in the percentages but also the proliferative capacity of different populations of cardiac fibroblasts as well as a reduction in the migration of fibroblasts into the heart from the bone marrow. Our study provides evidence for a possible unique therapeutic strategy to reduce cardiac fibrosis and improve cardiac function post-MI.
Keywords: epoxyeicosatrienoic acids, dihydroxyeicosatrienoic acids, thymocyte differentiation antigen, Cluster of Differentiation 90, fibroblast specific protein 1
Tissue fibrosis represents one of the largest groups of diseases for which there are very few effective therapies. In the heart, adverse cardiac remodeling consists of cardiac myocyte hypertrophy, fibrosis, and electrical perturbations and represents the prevailing response of the heart to extrinsic and intrinsic stimuli including myocardial infarction (MI) as well as pressure or volume overload. Cardiac fibrosis is characterized by the accumulation of extracellular matrix in the myocardium and is associated with increased stiffness that contributes significantly to diastolic dysfunction (1, 2). Even though important progress has been made in the treatment of systolic dysfunction, at the present time, there is no effective therapy for diastolic dysfunction (3, 4).
Tissue injury results in acute and robust inflammatory responses, involving the synthesis and release of chemokines and cytokines and the recruitment of leukocytes and fibroblasts. One of the important inflammatory responses involves the inflammatory lipid mediators with the activation of phospholipase A2 and the release of arachidonic acid (AA). Eicosanoids are oxylipids that are potent modulators of immune responses and are derived from AA or similar fatty acids. AA is metabolized through three enzymatic pathways, namely, cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 (CYP450) pathways. Whereas the COX and LOX pathways have been studied in detail, underpinned by the translation of inhibitors of these enzymatic pathways exemplified by aspirin and zileuton in the treatment of inflammatory diseases, the translational manipulation of the CYP450 pathway remains unexplored mechanistically and underused clinically. We hypothesize that the CYP450s may represent the last robust frontier in the inflammatory pathway that we may be able to manipulate to reduce inflammatory responses in ischemic cardiomyopathy and pressure overload hypertrophy.
AA when metabolized through the CYP450 epoxygenase pathway generates epoxyeicosatrienoic acids (EETs). EETs function as autocrine and paracrine effectors in the cardiovascular system (5, 6) and are shown to have cardioprotective properties. However, EETs are further metabolized by soluble epoxide hydrolase (sEH) to form the corresponding dihydroxyeicosatrienoic acids (DHETs) (7).
We have previously revealed the beneficial effects of several sEH inhibitors (sEHIs) in the prevention of hypertrophy and electrical remodeling in cardiac myocytes from animal models of myocardial infarction and pressure-overload hypertrophy (8–14). In the present study, we directly demonstrate that treatment with sEHIs can prevent cardiac fibrosis by inhibiting cardiac fibroblast proliferation. Single-cell–based phenotyping was used to quantify the percentages and the proliferative response in different populations of cardiac fibroblasts (CFs) in two models of cardiac injury, namely ischemic cardiomyopathy and pressure overload hypertrophy. Our study demonstrates the molecular mechanisms of selective and potent inhibitors of inflammation that reduce fibrosis and adverse cardiac remodeling. Treatment with sEHIs results in the improvement of cardiac function by preventing the development of cardiac fibrosis.
Results
1-Trifluoromethoxyphenyl-3-(1-Propionylpiperidine-4-yl)Urea (TPPU) Prevents the Development of Cardiac Hypertrophy in a Murine MI Model.
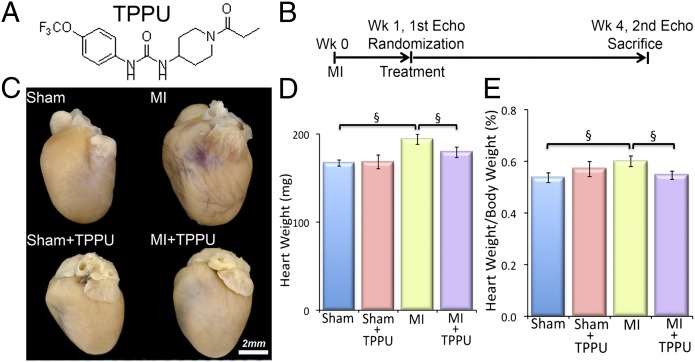
After determining the potency, pharmacokinetics, and physiochemical properties of 11 different sEHIs, TPPU (containing a piperidine ring; Fig. 1A) was found to have high inhibitory potency, drug-like physicochemical properties, pharmacokinetics with high area under the curve values, a relatively longer half-life, and lower plasma protein binding properties than many previous compounds (15).
Fig. 1.
TPPU prevents the development of cardiac hypertrophy in a murine myocardial infarction (MI) model. (A) Structure of the sEHI, 1-trifluoromethoxyphenyl-3-(1-propionylpiperidine-4-yl) urea (TPPU) used in our studies. (B) Schematic representation of the experimental protocol. (C) Examples of whole hearts from sham-operated, TPPU-treated–sham-operated, MI, and TPPU-treated MI mice. Mice were killed after 3 wk of follow-up. (Scale bar, 2 mm.) (D) Summary data for heart weight (in milligrams), and (E) heart weight/body weight ratio (percentage) from MI mice compared with untreated MI mice. Error bars represent SE, n = 12 per group, and §P < 0.05 by Student t test.
MI was generated in 8- to 10-wk-old male C57BL/6J mice (Charles River) using previously described techniques (16). One week after the surgery, mice were randomized to receive either drinking water containing TPPU (Fig. 1A, 15 mg/L) or vehicle alone for 3 wk (Fig. 1B) (17). Sham-operated mice were also randomized to receive either TPPU or vehicle alone at week one for 3 wk. The investigators were blinded to the treatment groups.
Whole hearts from the MI mice after 3 wk of follow up exhibited cardiac dilatation, which was prevented in the MI animals treated with TPPU (Fig. 1C). As expected, whole hearts from the sham-operated and sham-operated-TPPU–treated groups showed no significant hypertrophy or dilatation. Summary data in Fig. 1 D and E illustrate the significant increase in the heart weight and the ratio of heart weight/body weight in the MI group compared with sham-operated hearts. Treatment with TPPU resulted in a significant decrease in the heart weight and the heart weight/body weight ratio in the MI animals. There were no significant changes in the sham-operated mice treated with TPPU.
Treatment with TPPU Results in a Significant Improvement in Cardiac Function as Assessed by Echocardiography.
The chamber size and systolic function were assessed in the four groups of animals using echocardiography. Two-dimensional and motion-mode (M-mode) echocardiography showed evidence of cardiac chamber dilatation in the MI mice that was prevented in TPPU-treated animals (Fig. 2A and Table S1). However, there were no significant differences between the two sham-operated groups. Fig. 2B and Table S1 summarize the percentages of the fractional shortening (FS) before and 3 wk after treatment with TPPU. Indeed, treatment with TPPU in the MI mice resulted in a significant improvement in the FS compared with the MI alone. In contrast, there were no significant differences in FS between TPPU-treated sham-operated mice compared with sham alone. Taken together, these data suggest that the treatment with TPPU prevented adverse cardiac remodeling and improved cardiac function in the MI model.
Fig. 2.
Noninvasive echocardiographic assessment of the effect of TPPU on cardiac function and immunohistochemistry. (A) Examples of 2D and M-mode echocardiography in sham-operated, MI, and MI treated with TPPU after 3 wk of treatment showing evidence of chamber dilatation in MI mice. TPPU prevented the development of chamber dilatation in MI mice. (B) Summary data for percentage of fractional shortening (FS). (C) Cardiac sections stained with Sirius Red to demonstrate the amount of collagen deposition. (D) Quantification of the percentage of infarct zone. (E) Confocal images of wheat germ agglutinin (WGA) stains showing a significant decrease in collagen deposition in the MI model treated with TPPU compared with MI alone. (Scale bar, 50 mm.) Error bars represent SE, n = 12 per group. §P < 0.05 by Student t test and *P < 0.05 by ANOVA.
Beneficial Effect of TPPU Treatment on Cardiac Fibrosis in the Infarct Zone.
Here, we specifically sought to determine the effect of a sEHI on cardiac fibrosis within the infarct zone as well as the remote zone. To this end, cardiac sections (100 µm) from corresponding areas in the four groups of animals were stained using Picrosirius Red to quantify the amount of collagen (18, 19). Histological analysis demonstrated that treatment with TPPU resulted in a marked decrease in the infarct size and prevented the development of cardiac dilatation post-MI (Fig. 2C). Direct quantification of the infarcted area from the four groups showed a significant reduction in the amount of collagen in the treated MI hearts compared with the MI alone (Fig. 2D). There were no significant differences between the two sham-operated groups. Examination of collagen in the fibrotic areas using wheat germ agglutinin showed a marked decrease in the amount of collagen deposition in the treated MI hearts compared with the MI alone (Fig. 2E). The data suggest that treatment with TPPU post-MI prevents adverse cardiac remodeling at least in part by reducing infarct size and cardiac fibrosis.
Effects of TPPU on Cardiac Fibrosis in the Remote Zone in the MI Model.
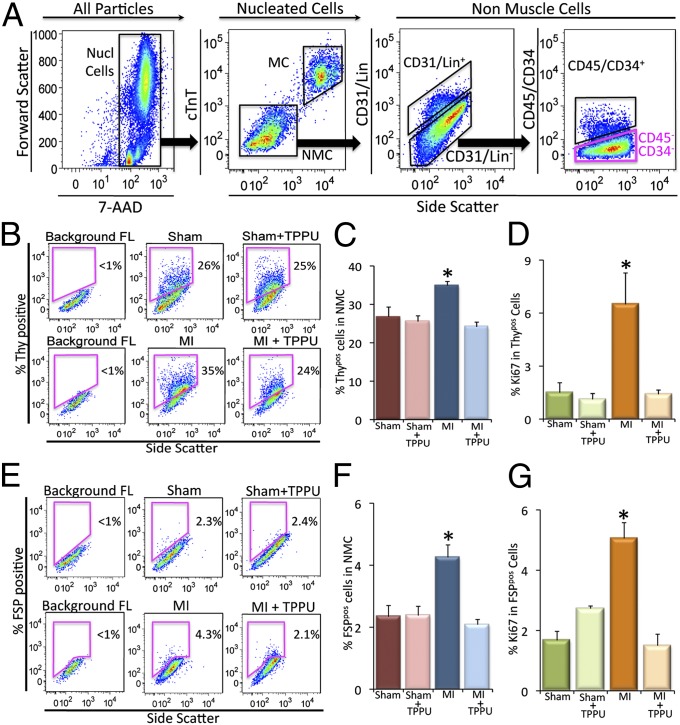
Because the heart contains a mixture of heterogenous populations of cells, to directly quantify the percentages of CFs, single-cell–based assays using flow cytometric analyses were performed as we have previously described (20, 21). The nucleated cells from the fresh myocardial preparation were enumerated based on the incorporation of 7-aminoactinomycin D (7-AAD) (Fig. 3A). A cardiac-specific Troponin T (cTnT) antibody labeled the myocytes (MCs), which show a higher fluorescence level and gate them separately from the nonmuscle cells (NMCs) to increase the cell specificity of this analysis. Controls were stained with isotype-matched IgG antibodies to set gates that identified positive cells.
Fig. 3.
Flow cytometric analysis of cardiac fibroblasts from the remote zone in the in vivo MI model. (A) Selection of nucleated cells (Nucl cells) from the mixed population based on the incorporation of 7-AAD and the separation of the myocytes (MCs) from the nonmuscle cells (NMCs) using cardiac troponinT (cTnT)-specific antibody. All of the analyses were conducted on the Lin−/CD31−/CD45−/CD34− cells (pink box). X and Y axes represent arbitrary units. (B) Flow cytometric analysis of Thy1.2+/Lin−/CD31−/CD45−/CD34− (Thypos) cells from sham-operated, TPPU-treated–sham-operated, MI and TPPU-treated MI mice. (C) Summary data from B (n = 3 per group). (D) Summary data showing the proliferative Thypos cells using Ki67 proliferative marker. (E) Flow cytometric analysis of FSP+/Lin−/CD31−/CD45−/CD34− (FSPpos) cells from sham-operated, TPPU-treated–sham-operated, MI and TPPU-treated MI mice. (F) Summary data from E (n = 3 per group). (G) Summary data showing the proliferative FSPpos cells using Ki67 proliferative marker. Representative results are shown. Error bars represent SE and *P < 0.05.
Two populations of CFs were identified in the remote zone away from the infarct zone. CFs were defined by Thy1.2 (22) and fibroblast-specific protein 1 (FSP-1) expression (23–25) and the lack of other lineage markers (Lin). Thy1 [thymocyte differentiation antigen or Cluster of Differentiation 90 (CD90)] is a small glycoprotein localized at the surface of several cell types including CF (22). Further characterization of Thy1.2+ cells using fluorescence-activated cell sorting (FACS) and PCR revealed the expression of collagen Ia and IIIa. The Thy1.2+ cells lacked the expression of platelet endothelial cell adhesion molecule (PECAM) and Von Willebrand factor (vWF) for endothelial cells and Nkx2.5 (Fig. S1). FSP-1, also known as S100A4 is a member of the S100 superfamily of EF-hand calcium-binding proteins, has been shown to be specific for CFs (23–25). In addition, a population of CD34+CD45+ fibroblasts has previously been shown to be derived from bone marrow and contributes to cardiac fibrosis in angiotensin II (AngII)-induced cardiac hypertrophy (18). This population was also analyzed separately in our study.
For flow cytometric analysis, Thypos CFs were identified in our study as Thy1.2+/Lin−/CD31−/CD34−/CD45− cells (22) (Fig. 3A). Flow cytometric analyses demonstrated that there was a significant increase in Thypos cells in the remote area in MI mice compared with the two groups of sham animals (Fig. 3 B and C). Moreover, treatment with TPPU in the MI animals resulted in a significant decrease in Thypos cells compared with MI alone.
Next, Ki67, a nuclear antigen that is expressed in actively cycling cells (26, 27), was used to directly test the hypothesis that there is a significant increase in the proliferative capacity among CFs isolated from the MI model. There was a significant increase in the percentage of Ki67 in the Thypos population in MI mice compared with sham animals. Treatment with TPPU in the MI animals resulted in a significant decrease in Ki67 positivity in Thypos cells (Fig. 3D).
Using FSP-1 as a second marker for CFs, we also documented the same beneficial effects of TPPU on the percentages and proliferative capacity of FSP-1. Specifically, there was a significant increase in FSPpos cells (FSP-1+/Lin−/CD31−/CD34−/CD45− cells) in the remote area in MI mice compared with sham animals. Treatment with TPPU in MI animals resulted in a significant decrease in the percentages and Ki67 positivity of FSPpos cells compared with MI alone (Fig. 3 E–G). Finally, treatment with TPPU in MI animals significantly decreased the percentages of CD34+CD45+ fibroblasts compared with MI alone (Fig. S2). Taken together, our data suggest that treatment with sEHI prevents the proliferation of the resident CFs as well as the migration of CFs into the heart post-MI, leading to a significant decrease in cardiac fibrosis both in the infarct zone and the remote area from the infarct.
Mechanistic Insights into the Effect of TPPU on the Activation of CFs.
CFs have been shown to play critical roles in cardiac remodeling (1, 23). In response to injury, CFs differentiate into myofibroblasts that express contractile proteins including α-smooth muscle actin and exhibit increased proliferative, migratory, and secretory properties. CFs respond to proinflammatory cytokines, vasoactive peptides (e.g., AngII and endothelin-1), and hormones (e.g., norepinephrine). These factors play critical roles in cardiac fibrosis (1, 23–25, 28). Chemokines such as monocyte chemoattractant protein-1 (MCP-1) also play important roles in cardiac fibrosis (2, 29).
Here, we demonstrate that treatment with TPPU resulted in a significant decrease in inflammatory cytokines and chemokines including interleukin-12 (IL-12), tumor necrosis factor-α (TNF-α), and MCP-1 (Fig. S3). The results are consistent with our previous findings in an acute treatment in the MI model (12). Moreover, sEHIs have been shown to block AngII-induced cardiac hypertrophy (10) and AngII is a well-characterized profibrotic molecule whose downstream mediators include mitogen-activated protein kinases (MAPKs) (1, 23). An unbiased approach of metabolic profiling of oxylipids was also performed to document the target engagement by TPPU, demonstrating significant increases in EETs/DHETs ratios in the sEHI-treated groups compared with no-treatment groups (Fig. S4).
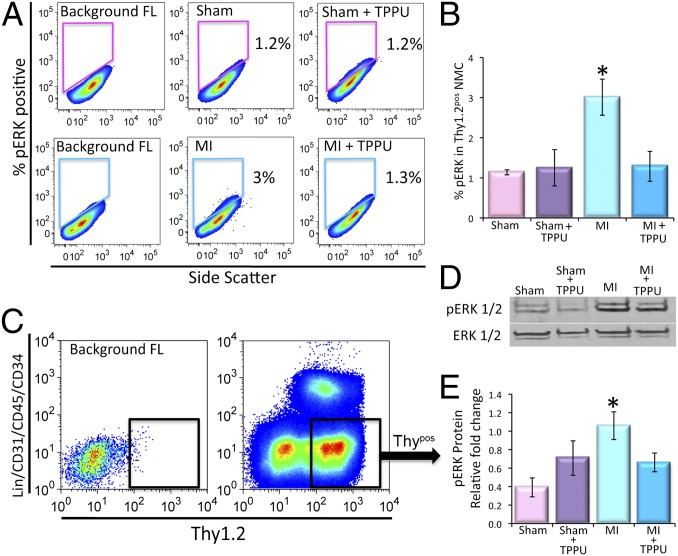
To investigate the mechanisms underlying the observed beneficial effects of TPPU on cardiac fibrosis, the activation of the major downstream signaling molecules in the MAPK pathway, extracellular signal-regulated kinases 1 and 2 (ERK1 and 2), was examined in the Thypos subpopulation of the CFs. Our analysis showed a significant increase in the levels of phosphorylated ERK1/2 (pERK1/2) in the MI mice compared with the sham-operated animals (3 ± 0.4% and 1.1 ± 0.06%, respectively). Indeed, there was a significant decrease in phosphorylated ERK1/2 by treatment with TPPU in the MI model (1.3 ± 0.3%, Fig. 4 A and B). Moreover, in vitro treatment of cultured mouse cardiac fibroblasts with AngII plus TPPU showed a significant decrease in the levels of pERK1/2 compared with AngII treated Thypos CFs (Fig. S5).
Fig. 4.
Activation of CFs in cardiac fibrosis through the pERK1/2 pathway. (A) Flow cytometric analysis of pERK1/2+ nonmuscle cells from sham-operated, TPPU-treated–sham-operated, MI and TPPU-treated MI mice. (B) Summary data from A (n = 3 per group). (C) Florescent activated cell sorting (FACS) of Thy1.2+/Lin−/CD31−/CD45−/CD34− cells (Thypos). Representative results are shown. (D) Representative lanes of Western blot assay and (E) summary data for pERK1/2 and ERK1/2 (loading control) performed from FACS sorted Thypos cells from sham-operated, TPPU-treated–sham-operated, MI and TPPU-treated MI mice (n = 3). Error bars represent SE and *P < 0.05.
The Thypos subpopulation of the CFs was then sorted using FACS from the four groups of animals (Fig. 4C). Consistently, Western blot analyses from the sorted cells showed an increase in pERK1/2 levels in MI mice compared with sham animals. Treatment with TPPU for 3 wk resulted in a significant decrease in the pERK1/2 toward the control level (Fig. 4 D and E, n = 3, P < 0.05). Our data suggest that sEHIs are beneficial in preventing adverse ventricular remodeling at least in part, by decreasing the production of proinflammatory cytokines and chemokines leading to a reduction in the proliferation and activation of resident CFs and/or recruitment of fibroblast progenitors.
Treatment with TPPU in the MI Model Prevents Cardiac Myocyte Hypertrophy as Assessed by Cell Volume and Hypertrophic Markers.
To further elucidate how TPPU antagonizes the initiation of hypertrophic response, we examined the effect of TPPU on the size of the cardiac myocytes isolated from the remote zone using myocyte volume measurements by a Coulter multisizer assay (21). Our data show that the myocyte volume significantly increased in the MI mice, which was restored to the sham-operated control levels by the TPPU treatment (Fig. S6 A and B).
Beta-myosin heavy chain (β-MyHC) is a well known myosin fetal isoform that is reexpressed in the adult heart during pathological states in minor subpopulations of myocytes (21). We used the validated NOQ7.5.4D antibody to assess the effect of TPPU on the reexpression of β-MyHC in single myocytes. Our results demonstrated that MI resulted in significant up-regulation of percentages of myocytes expressing β-MyHC (Fig. S6 C and D). Treatment with TPPU in the MI animals resulted in a significant reduction in the percentages of myocytes expressing β-MyHC to similar levels as the control groups.
We also analyzed the induction of another hypertrophic marker, atrial natriuretic factor (ANF) in the MI and MI-treated animals using whole-heart lysates by Western blot analysis. MI showed an up-regulation of ANF, which was reduced with the treatment of TPPU (Fig. S6 E and F).
TPPU Reduces Cardiac Fibrosis in a Murine Thoracic Aortic Constriction Model.
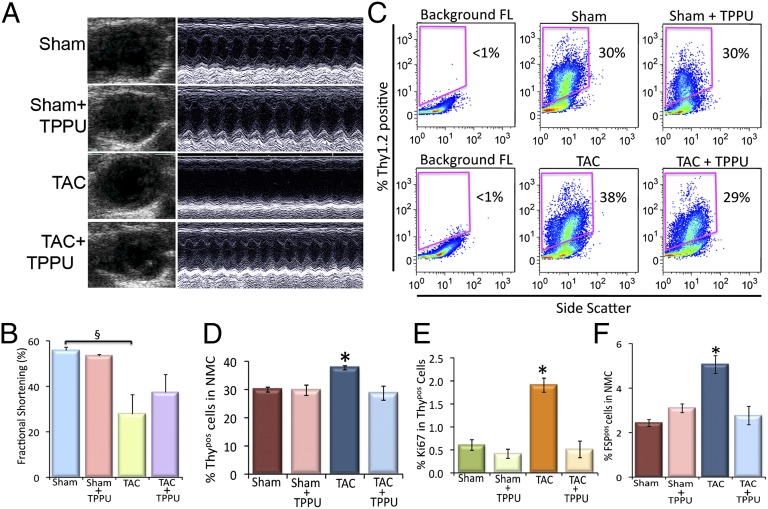
To directly demonstrate that the observed beneficial effects of TPPU in the prevention of cardiac fibrosis were model independent, a thoracic aortic constriction (TAC) model was used, resulting in a chronic pressure overload-induced hypertrophy. One week after the surgery, the TAC mice were randomized to receive TPPU for 3 wk. The effect of TPPU on cardiac function was assessed by 2D and M-mode echocardiography. There was an increase in chamber dilation associated with a significant decrease in FS in the TAC mice that was prevented in the TPPU-treated group (Fig. 5 A and B). There were no significant differences in the two sham-operated groups. Cardiac sections (100 µm) from four groups of animals stained using Picrosirius Red demonstrated that treatment with sEHI resulted in a decrease in chamber fibrosis as assessed using Picrosirius Red (Fig. S7).
Fig. 5.
Beneficial effect of TPPU on cardiac function in TAC animals. (A) Examples of 2D and M-mode echocardiography in mouse models with sham operation, TAC, and TAC treated with TPPU after 3 wk of treatment showing evidence of cardiac failure with chamber dilatation in TAC mice. (B) Summary data for percentage of fractional shortening (FS). (C) Flow cytometric analysis of Thy1.2+/Lin−/CD31−/CD45−/CD34− (Thypos) cells from sham-operated, TPPU-treated–sham-operated, TAC and TPPU-treated TAC mice. (D) Summary data from C (n = 3 per group). (E) Summary data showing the proliferative Thypos cells using Ki67 proliferative marker. (F) Summary data of FSP+/Lin−/CD31−/CD45−/CD34− (FSPpos) cells from sham-operated, TPPU-treated–sham-operated, TAC and TPPU-treated TAC mice (n = 3 per group). Representative results are shown. Error bars represent SE and *P < 0.05.
The analysis of Thypos and FSPpos CFs isolated from the left ventricular free wall and the septum showed a significant increase in Thypos and FSPpos CFs in the TAC mice compared with the sham-operated mice. Treatment with TPPU in TAC animals resulted in a significant decrease in Thypos and FSPpos cells in the TAC mice (Fig. 5 C, D, and F). The proliferating Thypos cells as assessed by the presence of Ki67 showed a similar trend in the TAC-treated and the MI-treated mice (Fig. 5E), suggesting a model independent action of TPPU in the prevention of cardiac fibrosis.
Discussion
In the present study, we provide mechanistic insights into the beneficial effects of sEHIs on adverse cardiac remodeling in two animal models with distinct pathophysiology, namely, ischemic cardiomyopathy and pressure-overload hypertrophy. We demonstrate the beneficial effect of sEHI on the reduction in the percentages and the proliferative capacity of the different populations of CFs resulting in a significant decrease in cardiac fibrosis and adverse cardiac remodeling. Treatment with sEHI significantly decreases the systemic levels of inflammatory cytokines and chemokines. Moreover, treatment with sEHI results in a significant decline in the phosphorylation of one of the key signaling molecules, ERK1/2, in the MAPK pathway in CF population. Our data provide evidence that an increase in the biological activities of EETs prevents adverse cardiac remodeling by reducing the percentages, proliferative capacity, and activation of the different populations of CFs. Moreover, treatment with sEHI leads to a significant decrease in chemokines, resulting in a decrease in the migration and recruitment of a population of fibroblasts derived from circulating bone marrow cells. Taken together, treatment with sEHI not only prevents the development of pathologic cardiac myocyte hypertrophy, sEHI may also result in an improvement in diastolic dysfunction by decreasing CF proliferation and cardiac fibrosis.
Effects of sEHI on the Subpopulations of Cardiac Fibroblasts.
Fibrotic scar tissue accumulation leads to adverse remodeling and a significant loss of cardiac function (2). In the pathologic myocardium, CFs are considered to be the most important contributors of the collagen matrix deposition (23, 24). In the past, fibroblasts were defined based on morphological characteristics or collagen synthesis (24, 30). However, CFs represent a heterogeneous population that are derived from various distinct tissue niches including resident fibroblasts, endothelial cells, bone marrow sources (23–25, 31), circulating progenitors, as well as progenitors residing within the vascular walls (32, 33). In addition, a truly definitive cell-specific marker has yet to be defined. The origin of proliferating CFs in pathological conditions is not completely defined. It also becomes clear that a particular marker only labels a subset of CFs (23, 24, 31).
To overcome some of these challenges, we have used single-cell phenotyping to evaluate and quantify the percentages and the proliferative capacity of the different subpopulations of CFs. The CFs were identified by a combination of markers. We demonstrate that treatment with sEHI prevents the proliferation and migration of different populations of fibroblasts (Fig. 3 and Fig. S2) post-MI, leading to a significant decrease in cardiac fibrosis. However, additional markers may need to be explored to define subpopulations of CFs and their responses to treatment with sEHI.
Mechanistic Insights into the Signaling Pathways Involved in Cardiac Fibrosis.
CFs maintain the homeostasis of the extracellular matrix by modulating the expression of proteins such as collagens (I, III, IV, V, and VI) and matrix metalloproteinases (MMPs). CFs also secrete growth factors and cytokines that exert autocrine and paracrine effects on cellular processses such as proliferation and apoptosis. However, under pathological conditions, in response to the increase in proinflammatory cytokines (e.g., TNF-α, IL-1, IL-6, and TGFβ), CFs exhibit up-regulated proliferation, migration, differentiation, secretion of collagens, cytokines, and activation MMPs. All these factors contribute to the development of perivascular and interstitial fibrosis, which ultimately leads to diastolic and systolic ventricular dysfunction (1, 23–25, 28).
In addition, studies have provided compelling evidence to support the roles of chemokines in cardiac fibrosis (2, 29). One of the best-studied chemokines is MCP-1 (also known as CCL2). Effects of MCP-1 in cardiac fibrosis are highly complex including (i) recruitment and activation of mononuclear cell subsets and fibroblast progenitors, and (ii) possible direct effects on resident fibroblasts (2, 29).
Our previous and current data support the notion that sEHIs are potent anti-inflammatory agents that significantly decrease the systemic levels of cytokines and chemokines (Fig. S3) (8–14, 34, 35). Of considerable relevance are our findings that there is a significant decrease in several inflammatory cytokines and chemokines including TNF-α, IL-12, and MCP-1 levels by treatment with sEHIs. IL-6 has been shown to be involved in the increased proliferation of fibroblasts via the MAPK pathway (36). TNF-α and MCP-1 also contribute to cardiac fibrosis via the extracellular signal regulated kinase (ERK) signaling cascade (2, 37). Our flow cytometric and immunoblot data provide evidence that sEHI results in a significant decrease in the activation of ERK1/2 in CFs in the MI mice (Fig. 4). Taken together, our data suggest that treatment with sEHI decreases CF proliferation at least in part, via the inhibitory effects on the MAPK pathway.
Future Directions.
CFs are pleiomorphic and pleiotropic cells that can respond to multiple profibrotic factors that are highly complex in nature, many of which exert synergistic effects with one another with cross-talk at multiple levels. AngII is a well-characterized profibrotic molecule. Prominent downstream mediators from AngII include TGFβ and MAPKs (1, 23). AngII up-regulates the three inactive isoforms of TGFβ (TGFβ1, TGFβ2, and TGFβ3), which are complexed with latent TGFβ binding proteins (LTBPs). Upon activation, the proteolytic cleavage of LTBP dissociates active TGF by molecules such as thrombospondin 1 (tsp-1), plasmin proteases, and integrin αvβ6 (38, 39).
AngII induces another profibrotic factor, connective tissue growth factor (CTGF), which promotes cardiac fibrosis through the protein kinase-C pathway. CTGF has also been shown to promote fibroblast proliferation (38).
Cardiac fibrosis also triggers endothelial–mesenchymal transition (EndMT), which contributes to about 30% of the activated fibroblasts. TGFβ1 has been shown to induce endothelial cells to undergo EndMT to acquire a more fibroblast-like phenotype and enter the interstitium where they contribute to cardiac fibrosis (25). Another downstream mediator of TGFβ and AngII in CFs is endothelin 1 (ET-1), which acts as a growth factor to induce cardiac fibroblast proliferation and increase collagen synthesis via the activation of cell surface ETA and ETB receptors (1, 39). ET-1 can also produce a profibrotic effect by reducing the collagenase activity of MMPs via the ETA receptors (1). It has also been shown that ET-1 can induce CTGF expression (39).
Here, we have demonstrated that treatment with TPPU significantly decreases the activation of the MAPK pathway in CFs. However, CFs contribute to cardiac fibrosis through several synergistic factors including AngII, ERK, TGFβ, ET-1, and CTFG. Hence, other signaling cascades have to be investigated to test whether these factors are also down-regulated by sEHIs.
Materials and Methods
A detailed description of the methods is provided in SI Methods.
MI Model in Mice.
The MI model in mice was created using procedure as previously described (16). Echocardiograms were performed 1 wk after surgery after which mice were randomized to receive TPPU (15 mg/L) (17) in the drinking water or water alone for a period of 3 wk (Fig. 1B), at which time repeat echocardiograms were performed.
Metabolomic Profiling of Oxylipins.
Oxylipin profiling was performed using a modification of a previously published method (12, 40).
Measurement of Plasma Cytokine Levels.
Plasma cytokine levels were analyzed using a Cytometric Bead Array kit (BD Biosciences) (12).
Flow Cytometric Analysis of Mouse Fibroblasts.
Isolated cells were fixed and treated with anti-cTnT, anti-CD31, anti-CD45, anti-CD34, anti-Thy1.2, anti-FSP-1, anti-Ki67, anti–β-MyHC, anti-pERK1/2, and anti-ERK1/2 antibodies.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (NIH) HL85727 and HL85844 and Veterans Administration Merit Review Grant I01 BX000576 (to N.C.); Howard Hughes Medical Institute Med-into-Grad Training Program to University of California Davis (UCD) (P.S.); American Heart Association (AHA) Western States Affiliate Predoctoral Fellowship Award (to P.S.); P.S. is supported by NIH T32 Training Grant in Basic and Translational Cardiovascular Science (T32 HL86350). Fellow-to-Faculty Award from Sarnoff Cardiovascular Research Foundation (to J.E.L.); an AHA Western States Affiliate Beginning Grant-in-Aid (to J.E.L.); and Harold Amos Medical Faculty Development Award from Robert Wood Johnson Foundation (to J.E.L.). Partial supports were provided by the National Institute of Environmental Health Sciences (NIEHS) Grant R37 ES02710, the NIEHS Superfund Basic Research Program (P42 ES04699), the NIEHS Center for Children’s Environmental Health and Disease Prevention (P01 ES11269), and a Technology Translational Grant from UCD Health System. B.D.H. is a George and Judy Marcus Senior Fellow of the American Asthma Society.
Footnotes
Conflict of interest statement: B.D.H. and N.C. have filed patents with the University of California for sEH inhibitors and cardiac hypertrophy therapy.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1221972110/-/DCSupplemental.
References
- 1.Porter KE, Turner NA. Cardiac fibroblasts: At the heart of myocardial remodeling. Pharmacol Ther. 2009;123(2):255–278. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Dobaczewski M, Frangogiannis NG. Chemokines and cardiac fibrosis. Front Biosci (Schol Ed) 2009;1:391–405. doi: 10.2741/s33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kass DA, Bronzwaer JG, Paulus WJ. What mechanisms underlie diastolic dysfunction in heart failure? Circ Res. 2004;94(12):1533–1542. doi: 10.1161/01.RES.0000129254.25507.d6. [DOI] [PubMed] [Google Scholar]
- 4.Ouzounian M, Lee DS, Liu PP. Diastolic heart failure: Mechanisms and controversies. Nat Clin Pract Cardiovasc Med. 2008;5(7):375–386. doi: 10.1038/ncpcardio1245. [DOI] [PubMed] [Google Scholar]
- 5.Roman RJ, Maier KG, Sun CW, Harder DR, Alonso-Galicia M. Renal and cardiovascular actions of 20-hydroxyeicosatetraenoic acid and epoxyeicosatrienoic acids. Clin Exp Pharmacol Physiol. 2000;27(11):855–865. doi: 10.1046/j.1440-1681.2000.03349.x. [DOI] [PubMed] [Google Scholar]
- 6.Schmelzer KR, et al. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci USA. 2005;102(28):9772–9777. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu Z, et al. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ Res. 2000;87(11):992–998. doi: 10.1161/01.res.87.11.992. [DOI] [PubMed] [Google Scholar]
- 8.Xu D, et al. Prevention and reversal of cardiac hypertrophy by soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci USA. 2006;103(49):18733–18738. doi: 10.1073/pnas.0609158103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiamvimonvat N, Ho CM, Tsai HJ, Hammock BD. The soluble epoxide hydrolase as a pharmaceutical target for hypertension. J Cardiovasc Pharmacol. 2007;50(3):225–237. doi: 10.1097/FJC.0b013e3181506445. [DOI] [PubMed] [Google Scholar]
- 10.Ai D, et al. Soluble epoxide hydrolase plays an essential role in angiotensin II-induced cardiac hypertrophy. Proc Natl Acad Sci USA. 2009;106(2):564–569. doi: 10.1073/pnas.0811022106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris TR, Li N, Chiamvimonvat N, Hammock BD. The potential of soluble epoxide hydrolase inhibition in the treatment of cardiac hypertrophy. Congest Heart Fail. 2008;14(4):219–224. doi: 10.1111/j.1751-7133.2008.08430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li N, et al. Beneficial effects of soluble epoxide hydrolase inhibitors in myocardial infarction model: Insight gained using metabolomic approaches. J Mol Cell Cardiol. 2009;47(6):835–845. doi: 10.1016/j.yjmcc.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li N, et al. Use of metabolomic profiling in the study of arachidonic acid metabolism in cardiovascular disease. Congest Heart Fail. 2011;17(1):42–46. doi: 10.1111/j.1751-7133.2010.00209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu H, et al. Soluble epoxide hydrolase inhibitors and heart failure. Cardiovasc Ther. 2011;29(2):99–111. doi: 10.1111/j.1755-5922.2010.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ulu A, et al. Pharmacokinetics and in vivo potency of soluble epoxide hydrolase inhibitors in cynomolgus monkeys. Br J Pharmacol. 2012;165(5):1401–1412. doi: 10.1111/j.1476-5381.2011.01641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tarnavski O, et al. Mouse cardiac surgery: Comprehensive techniques for the generation of mouse models of human diseases and their application for genomic studies. Physiol Genomics. 2004;16(3):349–360. doi: 10.1152/physiolgenomics.00041.2003. [DOI] [PubMed] [Google Scholar]
- 17.Hwang SH, Tsai HJ, Liu JY, Morisseau C, Hammock BD. Orally bioavailable potent soluble epoxide hydrolase inhibitors. J Med Chem. 2007;50(16):3825–3840. doi: 10.1021/jm070270t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haudek SB, et al. Monocytic fibroblast precursors mediate fibrosis in angiotensin-II-induced cardiac hypertrophy. J Mol Cell Cardiol. 2010;49(3):499–507. doi: 10.1016/j.yjmcc.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matkovich SJ, et al. MicroRNA-133a protects against myocardial fibrosis and modulates electrical repolarization without affecting hypertrophy in pressure-overloaded adult hearts. Circ Res. 2010;106(1):166–175. doi: 10.1161/CIRCRESAHA.109.202176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anversa P, et al. Myocardial aging—a stem cell problem. Basic Res Cardiol. 2005;100(6):482–493. doi: 10.1007/s00395-005-0554-3. [DOI] [PubMed] [Google Scholar]
- 21.López JE, et al. β-myosin heavy chain is induced by pressure overload in a minor subpopulation of smaller mouse cardiac myocytes. Circ Res. 2011;109(6):629–638. doi: 10.1161/CIRCRESAHA.111.243410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hudon-David F, Bouzeghrane F, Couture P, Thibault G. Thy-1 expression by cardiac fibroblasts: Lack of association with myofibroblast contractile markers. J Mol Cell Cardiol. 2007;42(5):991–1000. doi: 10.1016/j.yjmcc.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Krenning G, Zeisberg EM, Kalluri R. The origin of fibroblasts and mechanism of cardiac fibrosis. J Cell Physiol. 2010;225(3):631–637. doi: 10.1002/jcp.22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeisberg EM, Kalluri R. Origins of cardiac fibroblasts. Circ Res. 2010;107(11):1304–1312. doi: 10.1161/CIRCRESAHA.110.231910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeisberg EM, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13(8):952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 26.Epting CL, et al. Stem cell antigen-1 regulates the tempo of muscle repair through effects on proliferation of alpha7 integrin-expressing myoblasts. Exp Cell Res. 2008;314(5):1125–1135. doi: 10.1016/j.yexcr.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landberg G, Tan EM, Roos G. Flow cytometric multiparameter analysis of proliferating cell nuclear antigen/cyclin and Ki-67 antigen: A new view of the cell cycle. Exp Cell Res. 1990;187(1):111–118. doi: 10.1016/0014-4827(90)90124-s. [DOI] [PubMed] [Google Scholar]
- 28.Leask A. Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res. 2010;106(11):1675–1680. doi: 10.1161/CIRCRESAHA.110.217737. [DOI] [PubMed] [Google Scholar]
- 29.Dobaczewski M, Frangogiannis NG. Chemokines in myocardial infarction: Translating basic research into clinical medicine. Future Cardiol. 2008;4(4):347–351. doi: 10.2217/14796678.4.4.347. [DOI] [PubMed] [Google Scholar]
- 30.Kompa AR, et al. Soluble epoxide hydrolase inhibition exerts beneficial anti-remodeling actions post-myocardial infarction. Int J Cardiol. 2012 doi: 10.1016/j.ijcard.2011.12.062. 10.1016/j.ijcard.2011.12.062. [DOI] [PubMed] [Google Scholar]
- 31.Souders CA, Bowers SL, Baudino TA. Cardiac fibroblast: The renaissance cell. Circ Res. 2009;105(12):1164–1176. doi: 10.1161/CIRCRESAHA.109.209809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res. 2005;65(1):40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 33.Sartore S, et al. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: From innocent bystander to active participant. Circ Res. 2001;89(12):1111–1121. doi: 10.1161/hh2401.100844. [DOI] [PubMed] [Google Scholar]
- 34.Liu JY, et al. Metabolic profiling of murine plasma reveals an unexpected biomarker in rofecoxib-mediated cardiovascular events. Proc Natl Acad Sci USA. 2010;107(39):17017–17022. doi: 10.1073/pnas.1011278107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu JY, et al. Inhibition of soluble epoxide hydrolase enhances the anti-inflammatory effects of aspirin and 5-lipoxygenase activation protein inhibitor in a murine model. Biochem Pharmacol. 2010;79(6):880–887. doi: 10.1016/j.bcp.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leicht M, Briest W, Zimmer HG. Regulation of norepinephrine-induced proliferation in cardiac fibroblasts by interleukin-6 and p42/p44 mitogen activated protein kinase. Mol Cell Biochem. 2003;243(1-2):65–72. doi: 10.1023/a:1021655023870. [DOI] [PubMed] [Google Scholar]
- 37.Westermann D, et al. Tumor necrosis factor-alpha antagonism protects from myocardial inflammation and fibrosis in experimental diabetic cardiomyopathy. Basic Res Cardiol. 2007;102(6):500–507. doi: 10.1007/s00395-007-0673-0. [DOI] [PubMed] [Google Scholar]
- 38.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18(7):816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 39.Leask A. TGFbeta, cardiac fibroblasts, and the fibrotic response. Cardiovasc Res. 2007;74(2):207–212. doi: 10.1016/j.cardiores.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 40.Morisseau C, et al. Structural refinement of inhibitors of urea-based soluble epoxide hydrolases. Biochem Pharmacol. 2002;63(9):1599–1608. doi: 10.1016/s0006-2952(02)00952-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.