Abstract
Background
Hepatitis C virus (HCV) antiviral therapy entails a long treatment course, as well as significant side effects that can lead to medication non-adherence and premature termination of treatment. Few large studies have comprehensively examined patient perspectives on the treatment experience, particularly the social and personal effects.
Objective
We sought to understand how a diverse group of patients’ lives were affected during HCV treatment, and to obtain suggestions about how to better support patients during treatment.
Methods
On average, 13 months after therapy we interviewed by telephone a consecutive sample of 200 patients treated for hepatitis C with ribavirin and pegylated interferon in a comprehensive, integrated health plan in the years 2008–2010. Mixed (quantitative and qualitative) survey methods were used.
Results
The response rate was 68.9 %. Mean age at treatment was 51 years; 63.0 % were men; and Black, Hispanic, Asian, and White non-Hispanic racial/ethnic groups were similarly represented. Patients whose treatment was managed by nurses or clinical pharmacists (vs. physicians) were more likely to report their providers as being part of their support system (83.5 % vs. 58.9 %; p < 0.001). Most patients reported flu-like symptoms (93.5 %) and psychiatric problems (84.5 %), and 43.0 % reported side effects lasted up to 6 months after treatment. Black patients reported discontinuing treatment prematurely due to side effects more often than non-Blacks (29.4 % vs.12.1 %; p < 0.001). Physical side effects (69.5 % of patients), psychiatric issues (43.5 %), and employment (27.4 %) were ranked among the three most difficult challenges. Patients desired help in anticipating and arranging work modifications during treatment. Most patients rated peer support, nutritional guidance, and weekly provider contact by telephone as potentially helpful resources for future patients undergoing HCV treatment.
Conclusions
Patient perspectives can help formulate and refine HCV treatment support programs. Effective support programs for diverse populations are crucial as the complexities and costs of HCV treatment increase. The call for greater support from peers, providers, and employers demands new systems such as patient-centered care teams.
1 Background
Chronic hepatitis C virus (HCV) infection affects over 150 million individuals worldwide, and is the leading cause of chronic liver disease and liver transplantation in the USA [1, 2]. Without treatment, up to a third of patients with chronic hepatitis C will develop cirrhosis and complications of end-stage liver disease including hepatocellular carcinoma [3]. In the USA, the majority of chronic liver disease and related deaths are attributable to hepatitis C [4, 5]. Furthermore, hepatitis C-related mortality is on the rise [6] as the majority of affected individuals (baby boomers, born 1945–1964) age. Hepatitis C antiviral treatment is initiated to reduce such morbidity and mortality.
Until 2011, the standard treatment for all chronic hepatitis C infection has been dual therapy with injectable pegylated interferon and oral ribavirin (PegIFN/RBV) for either 24 or 48 weeks depending on the HCV genotype [7]. The goal of therapy is sustained virologic response (SVR), defined as the absence of circulating HCV RNA at 24 weeks after treatment completion. Patients infected with different HCV genotypes have distinct response rates to treatment [8, 9]. Race and race-related host genetic markers such as IL28B are also established predictors of treatment outcome [10–14]. The recently approved direct-acting antiviral agents (DAAs; telaprevir and boceprevir) have shown substantially improved SVR rates, as well as potentially shorter treatment courses, for patients with HCV genotype 1 (the most common genotype worldwide). The regimens involve triple therapy, with either of the DAAs being added to PegIFN/RBV therapy.
Adherence to HCV therapy is often complicated by medication side effects [15]. Dual therapy with PegIFN/RBV most frequently produces flu-like symptoms, anemia, and depression. For some, supplemental therapy is required, including injection medications such as erythropoietin or other hematopoietic growth factors, or oral medications such as antidepressants. However, still over 10 % of patients terminate treatment prematurely due to side effects [8]. Since the new DAAs each bring their own unique side effects, they may add to or worsen the already substantial challenges of dual therapy.
While the medical side effects of hepatitis C antiviral therapy are well known, less is known about the broader impact of treatment on patients’ lives. Patient quality of life during HCV treatment has been shown to affect medication adherence [16], reinforcing the need to think broadly about treatment management. Few large studies describe how treatment affects patients’ lives generally, such as in their relationships or employment. One in-depth US study employed focus group methods with 33 treated patients to elucidate common themes, including the need for broad types of support [17]. Several qualitative and mixed-methods studies with sample sizes ranging from single case reports to about 100 previously treated patients also have explored the medical, social, and employment challenges of HCV treatment in the USA and other nations [18–23].
A comprehensive, patient-centered understanding of the treatment experience can inform the development of novel support tools to improve not only medication adherence and subsequent treatment outcome but also quality of life during and after therapy. To this end, we conducted the PATH-C (Patient Perspectives after Treatment for Hepatitis C) Study to learn, through telephone interviews, about the experiences of 200 patients who received hepatitis C treatment with PegIFN/RBV in an integrated healthcare setting. We hypothesized that patients would reveal issues related to treatment that were not generally recognized, that satisfaction would vary by type of provider, and that they would suggest novel types of support for patients undergoing treatment.
2 Methods
2.1 Study Population
We studied patients who had undergone HCV treatment within the Northern California Kaiser Permanente Medical Care Program (KPNC). This comprehensive, integrated healthcare delivery system serves over 3.2 million members in the San Francisco and Sacramento Greater Metropolitan areas. The membership includes over 25 % of the area’s total insured population, and is representative except for persons with extremes in income [24, 25]. In 2010, members included over 18,000 patients with hepatitis C: 57 % White non-Hispanic, 16 % Black, 15 % Hispanic, 10 % Asian, and 2 % Native American.
The Viral Hepatitis Registry (VHR) maintains comprehensive, electronic administrative and clinical data for all KPNC patients with hepatitis C dating from 1995 to the present. Using VHR databases, we identified patients who were enrolled in the health plan for at least 6 months prior to treatment and had at least 5 weeks of PegIFN/RBV treatment in the years 2008–2010. We excluded patients with a preferred language other than English (5 %); a diagnosis of psychosis within 12 months prior to recruitment (<1 %); terminal illness, HIV, or hepatitis B virus (HBV) co-infection (2 %); or a history of liver cancer or liver transplant (<1 %). Patients were not required to be health plan members at the time of recruitment. The study protocol was approved by the Institutional Review Board of the Kaiser Foundation Research Institute.
2.2 Recruitment and Interview Process
Based on race/ethnicity from administrative and clinical records, health plan members treated for hepatitis C in the years 2008–2010 were 62 % White non-Hispanic, 9 % Black, 12 % Asian, 14 % Hispanic, and 3 % Native American. Our goal was to recruit 200 patients with approximately equal representation of the four largest racial/ethnic groups. We recruited by mail all eligible Asians and Blacks (consecutive sample) because of the limited number of potential participants; and we contacted a random sample of eligible non-Hispanic Whites and Hispanics. To reach the target, we contacted 63 Asians, 64 Blacks, 84 Hispanics, and 69 non-Hispanic Whites. If no response was received within 2 weeks, telephone contact was attempted up to three times. Patients were offered a US$20 gift card for participation.
Because participants resided within in a large geographic range, telephone survey methods were used. Interviews (30–90 minutes in length) were conducted by a single investigator (RM) from April 2010 to May 2011. Informed consent was obtained verbally prior to each interview. Ms. Murphy is an experienced hepatitis researcher, having conducted almost 500 telephone and direct interviews with viral hepatitis patients for previous studies.
2.3 Survey Instrument
The mixed-methods 40-item instrument included a combination of prompts, Likert scales, and multiple choice and open-ended questions (see Online Resource). The survey opened with basic demographic questions. Medication adherence and adjustments, the need for supplemental medication, treatment length, and response were assessed with structured questions. Semi-structured questions allowed free responses about treatment preparation by category (e.g., lifestyle changes, work modifications). Semi-structured questions probed specific mental and physical side effects, and relationships with family, friends, healthcare providers, and employers. These detailed queries were intended not only to capture quantitative information but also to help patients revisit and recall broad aspects of the treatment experience before open-ended questions were presented.
Patients were asked to name the provider they saw most regularly for their treatment and related care (we subsequently identified the provider as a KPNC medical doctor [MD], registered nurse, or clinical pharmacist for analytic purposes). A Likert scale question evaluated provider interactions. The survey closed with general reflections on the treatment experience and patient suggestions to improve the treatment process. Open-ended questions included listing the most challenging aspects of treatment and queried the advice patients would offer to someone starting treatment. Finally, a Likert scale was used to rate the helpfulness of potential resources for future patients undergoing treatment.
Selected questions were adapted from previous surveys [4]. The survey was reviewed by three colleagues who had undergone HCV treatment, and minor revisions were made based on their suggestions.
2.4 Data Analysis
All telephone survey responses were recorded on a paper form by a single investigator (RM) and subsequently entered into a relational database (double entry). Selected clinical, pharmacy, and demographic data were extracted from the VHR. Most data presented in this article are from patient report; information obtained from the medical records is noted accordingly. Self-reported race/ethnicity from the interview was used in all analyses.
For comparisons of continuous variables, T-, Mann-Whitney U-, Kruskal-Wallis, or conventional ANOVA testing was used. Chi-squared tests were used for categorical variables, employing exact methods as needed. Comparisons were conducted between the four substantially represented racial/ethnic groups (White non-Hispanic, Hispanic, Asian, Black), and for some responses, between specific groups and all others (e.g., Blacks versus non-Blacks). The Native American participants (n = 5) were included in the overall frequencies, and in the ‘non’ comparison groups. STATA version 11 (Stata Corp, College Station, TX, USA) and SPSS version 18.1 (SPSS Inc., Chicago, IL, USA) were used.
Qualitative analytic methods were used for the open-ended questions. Two investigators (RM and CH) individually reviewed all text responses in the database and assigned each to a category. These categories were not assigned a priori, but were created subsequent to the review of responses. After initial categorization, RM and CH established a consensus on broader themes and the final assignment of more encompassing categories. For example, one person’s response to ‘What were the most challenging aspects of your HCV treatment?’ was “really tired all the time,” “nausea,” and “sad and depressed.” Initially, these were coded as fatigue, nausea, and depression. After further review, both fatigue and nausea were grouped into ‘physical side effects’ (counting once) and depression categorized as ‘mental side effects.’ The most frequent themes were considered as binary variables for analysis.
3 Results
3.1 Characteristics of the Participants
A total of 290 patients were recruited by mail to meet the goal of 200 telephone interviews (69.0 % participation rate). The refusal rate was just 15.5 %; most non-participation was due to unsuccessful follow-up contact. Due to differences in follow-up intensity required to accrue the target size per racial/ethnic group, the response rates differed: Asians 68.3 %, Blacks 81.3 %, Hispanics 66.7 %, and non-Hispanic Whites 60.9 %. Patient characteristics such as gender, age, provider type, time since treatment, or treatment response did not vary between participants and non-participants. Similar numbers (43–51) of non-Hispanic White, Black, Asian, and Hispanic patients participated, as did five who identified themselves as Native American.
Table 1 details the demographic and selected clinical characteristics of the study population overall and among the four targeted racial/ethnic groups. More than half (58.0 %) of patients were working full-time at the start of treatment. Of those, 41.5 % were doing manual or physical labor. A small portion of patients (8.5 %) reported taking a leave of absence or modifying their work hours prior to treatment initiation. Educational level varied by race/ethnicity; notably, almost 60 % of Hispanic patients reported educational levels of high school or less. Annual household income did not vary significantly between groups.
Table 1.
Study population
| Totala (n = 200) |
Asian (n = 43) |
Black (n = 51) |
Hispanic (n = 51) |
Whiteb (n = 50) |
P valuec | |
|---|---|---|---|---|---|---|
| Characteristics at treatment start | ||||||
| Age, years [mean (SD)]d | 51.5 (9.0) | 47.8 (12.4) | 54.3 (6.370) | 49.3 (8.914) | 52.3 (6.899) | 0.002e |
| Sex | ||||||
| Men | 126 (63.0) | 22 (51.2) | 29 (56.9) | 44 (86.3) | 29 (58.0) | 0.001 |
| Marital Status | ||||||
| Married/domestic partner | 123 (61.5) | 28 (65.1) | 28 (54.9) | 34 (66.7) | 30 (60.0) | |
| Single | 38 (19.0) | 12 (27.9) | 10 (19.6) | 6 (11.8) | 9 (18.0) | 0.063 |
| Divorced/separated | 35 (17.5) | 3 (7.0) | 10 (19.6) | 11 (21.6) | 11 (22.0) | |
| Widowed | 4 (2.0) | 0 | 3 (5.9) | 0 | 0 | |
| Employment status | ||||||
| Working (full/part time) | 135 (67.5) | 28 (65.1) | 37 (72.5) | 36 (70.6) | 30 (60.0) | 0.469 |
| Leave of absence | 14 (7.0) | 3 (7.0) | 2 (3.9) | 6 (11.8) | 3 (6.0) | |
| Not working | 22 (11.0) | 7 (16.3) | 3 (5.9) | 4 (7.8) | 8 (16.0) | |
| Retired/disabled | 29 (14.5) | 5 (11.6) | 9 (17.6) | 5 (9.8) | 9 (18.0) | |
| Annual household income | ||||||
| <US$25,000 | 28 (14.0) | 6 (14.0) | 5 (9.8) | 11 (21.6) | 5 (10.0) | 0.239 |
| US$25,000–49,000 | 54 (27.0) | 13 (30.2) | 15 (29.4) | 14 (27.5) | 11 (22.0) | |
| US$50,000–99,000 | 83 (41.5) | 14 (32.6) | 26 (51.0) | 18 (35.3) | 24 (48.0) | |
| >US$99,000 | 32 (16.0) | 10 (23.3) | 4 (7.8) | 6 (11.8) | 10 (20.0) | |
| Refused to answer | 3 (1.5) | 0 | 1 (2.0) | 2 (3.9) | 0 | |
| Education | ||||||
| High school or less | 71 (35.5) | 12 (27.9) | 15 (29.4) | 30 (58.9) | 13 (26.0) | 0.005 |
| Some college/technical | 97 (48.5) | 18 (41.9) | 29 (56.9) | 18 (35.3) | 28 (56.0) | |
| College graduate | 32 (16.0) | 13 (30.2) | 7 (13.7) | 3 (5.9) | 9 (18.0) | |
| Co-morbiditiesd | ||||||
| Diabetes | 35 (17.5) | 9 (20.9) | 13 (25.5) | 8 (15.7) | 5 (10.0) | 0.208 |
| Depression | 60 (30.0) | 6 (14.0) | 11 (21.6) | 17 (33.3) | 24 (48.0) | 0.002 |
| Cirrhosis | 24 (12.0) | 6 (14.0) | 5 (9.8) | 9 (17.6) | 4 (8.0) | 0.456 |
| Treatment-related results | ||||||
| Treatment length | ||||||
| Full course (48 weeks) | 77 (38.5) | 20 (46.5) | 9 (17.6) | 25 (49.0) | 21 (42.0) | <0.001f |
| Full course (24 weeks) | 45 (22.5) | 12 (27.9) | 9 (17.6) | 7 (13.7) | 17 (34.0) | |
| Stopped, non-response | 41 (20.5) | 4 (9.3) | 18 (35.3) | 9 (17.6) | 7 (14.0) | |
| Stopped, side effects | 37 (18.5) | 7 (16.3) | 15 (29.4) | 10 (19.6) | 5 (10.0) | |
| Provider type | ||||||
| Physician | 73 (36.5) | 9 (20.9) | 24 (47.1) | 20 (39.2) | 19 (38.0) | 0.117 |
| RN/CP | 127 (63.5) | 34 (79.1) | 27 (52.9) | 31 (60.8) | 31 (62.0) | |
| Response to therapy | ||||||
| SVR | 66 (33.0) | 21 (48.8) | 11 (21.6) | 15 (29.4) | 18 (36.0) | 0.056 |
| Pending test results | 51 (25.5) | 9 (20.9) | 11 (21.6) | 16 (31.4) | 15 (30.0) | |
| Not successful, no SVR | 83 (41.5) | 13 (30.2) | 29 (56.9) | 20 (39.2) | 17 (34.0) | |
| Interval, treatment to interview,d months [median (range)] | 10 (1–39) | 10 (1–39) | 13 (2–34) | 10 (1–38) | 8 (1–26) | 0.500g |
All values are expressed as number (%) unless otherwise indicated
Information is from patient report unless otherwise stated
RN/CP registered nurse or clinical pharmacist management, SVR sustained virologic response to therapy (viral clearance)
Total includes the five Native American participants
Non-Hispanic White patients
All p values are for comparisons among the four racial/ethnic groups shown and reflect the chi-squared heterogeneity test unless otherwise indicated
Information from medical records
ANOVA
P value for chi-squared analysis of those with full course versus all others
Kruskal-Wallis test
The median length of KPNC health plan enrollment prior to treatment was 89 months (range 3–122) and 66.5 % of participants were health plan members for at least 5 years before treatment; this did not vary significantly by racial/ethnic group. Overall, 15.5 % reported having received a HCV treatment course prior to the one being considered in the interview, with no variation between racial/ethnic groups (p = 0.974). Treatment length and response status varied as expected based on the established larger predominance of HCV genotype 1 and poorer response to therapy in Blacks. While 76.5 % of Black participants had genotype 1 (based on the medical record), 60–70.5 % of the other three groups did.
Over half of the patients (63.5 %) named a nurse or clinical pharmacist (RN/CP) as the primary person who managed their treatment, while 36.5 % named a physician (MD) as their treatment manager. The type of provider varied by health plan medical service area; within each area, either a physician or RN/CP approach is used. Participants identified a total of 18 different registered nurses (including one nurse practitioner) and two CPs (all RN/CP were supervised by gastroenterology or infectious disease specialists), and 20 MDs (17 gastroenterology or infectious disease specialists, three internists supervised by gastroenterologists) as their primary treatment providers. Due to demographic differences in the populations served by the 15 medical service areas, Blacks were the least likely and Asians the most likely to have RN/CP management of treatment.
3.2 Preparation for Treatment
While no standard KPNC pre-treatment curriculum was in place during the study period, several medical facilities offered general or treatment-specific hepatitis C classes; 61.5 % of patients reported attending such a class for preparation. Patients also reported treatment preparation with internet resources (47.0 %), decreasing alcohol and tobacco use (32.0 %), support groups/group counseling (23.0 %), diet and exercise (23.0 %), and stress reduction and alternative therapy (7.0 %).
Overall, 28.0 % of patients reported receiving preparatory support or advice from someone who had previously undergone HCV treatment. White non-Hispanics and Hispanics were more likely to report this than were Asians and Blacks (43.6 % and 35.3 % vs. 18.6 % and 11.8 %, respectively; p < 0.001 for all comparisons). Of the 31 patients with a previous HCV treatment course, 11 noted their previous treatment helped prepare them. Several patients contrasted the usefulness of peer support with the preparatory information from healthcare providers:
“The doctor can only inform you about side effects, but my friend described the experience in detail. I knew what to expect.”
“They say it's tough but they don't know how hard it is unless they've walked in those shoes.”
“A friend who had been on treatment warned me to save money because the end of treatment and end of disability [benefits] will happen at the same time.”
“My friend went through treatment and coached me through mine. This was very helpful.”
3.3 Medication Use
Few (15.5 %) patients reported that PegIFN or RBV dose reductions were required. Only 9.5 % and 23.5 % reported ever missing a weekly injection of PegIFN or an RBV dose, respectively. Supplemental erythropoietin therapy was reported by 20.5 % and granulocyte colony stimulating factor use by 10.5 %. Many (41.5 %) noted using antidepressants during treatment, and 29.5 % required prescription medication for sleep.
3.4 Specific Side Effects
Patients were asked about specific side effects in semi-structured questions. As anticipated, the most commonly reported physical side effects of treatment were flu-like symptoms (93.5 %), skin-related reactions (80.5 %), and gastrointestinal (GI) problems (79.5 %). Also common were fatigue (91.0 %), hair loss (50.0 %), and rash (48.0 %). Injection site reactions were noted by 24.0 %. Most patients (84.5 %) reported psychiatric symptoms including anxiety (66.5 %), depression (58.0 %), and impaired concentration or ‘brain fog’ (53.0 %).
Overall, 18.5 % of patients reported stopping therapy due to side effects. Blacks were more likely to report this than non-Blacks (29.4 % vs. 15.4 %; p < 0.001), even among HCV genotype 1 patients. Interestingly, several patients hesitated to tell their providers about the severity of their side effects, fearing their treatment might be discontinued as a result. One patient described hiding painful mouth sores from the nurse and physician, while another chose not to report severe suicidal ideation. Increasing numbers of psychiatry visits were not disclosed to the treating nurse in another case.
When asked whether any problems occurred after treatment ended, 42.5 % reported at least one specific medical side effect that persisted for up to 6 months. Persistent physical side effects were mentioned by 26.5 %. Slow resolution of dermatologic problems such as rash and alopecia were reported by 7.0 %. Continued mental side effects were described by 16.0 %, with persistent loss of memory noted by 10 patients (5.0 %). Many such patients expressed disappointment in the lack of medical aftercare or preparation for continued side effects:
“I felt disregarded at the end of treatment.”
"Feels like the world left me behind during treatment and I can't seem to plug back in."
"Not sure if I'll ever get my short-term memory back."
"Treatment ended abruptly, with no aftercare. I was not prepared for the recovery time."
“I’m still experiencing a lot of fatigue. My body is still out of whack.”
“I had difficulty recovering from a malnourished and sedentary lifestyle.”
3.5 Work, Family, and Friends
Of the 135 patients working during treatment, just 28.1 % reported co-workers or supervisors as supportive. This proportion did not vary between those doing physical/manual labor and others. Many (37.8 %) hid their treatment situation from their employer and co-workers, ranging from 28.6 % of Asians to 47.2 % of Hispanics, with no significant difference between those in physical/manual labor jobs and others. Serious financial consequences of treatment were reported by 34.8 % (e.g., job loss, decreased work hours, difficulty paying for medications, or loss of insurance).
Some patients shared how their irritability affected them in the work place. Others experienced being ostracized, and several expressed regret at telling co-workers or supervisors about their HCV treatment. Several shared disappointment that their treatment provider did not suggest or facilitate work modifications such as a medical leave of absence.
“Because of my shabby work and anger during treatment, I lost my big customers. They would no longer do business with me.”
"My boss thought I had cancer, and I let him think that."
“Co-workers made me feel like I was dirty and different. I was harassed at work and written up for poor performance.”
“I wish someone told me I should have gone on disability [benefits].”
“I took a leave of absence. I would never have made it through treatment if I had to work.”
Over half (53.0 %) of patients overall cited difficulty attending social functions, and 24.5 % hid their condition and/or treatment from their friends. Whites reported support from friends more often than did Blacks or Hispanics (52.0 % vs. 29.4 % or 31.4 %, respectively; p = 0.04, both comparisons). Numerous patients described that they purposefully isolated themselves from friends and activities, sometimes to avoid having to explain or discuss their situation:
“I stayed home and became withdrawn. I felt alienated from people.”
“I didn’t feel like going out; I felt cemented to the bed.”
“I avoided social contacts because of my personality change.”
“I felt that if I told my friends, they would avoid me.”
Most (79.5 %) reported family members as helpful during treatment. Among the 122 participants who lived with their spouse or partner at the start of treatment, most (80.3 %) listed their spouse as a source of support during treatment. Just 14.8 % noted lack of support from their spouse as a treatment challenge, while 48.4 % reported being unable to meet daily obligations to their spouse/partner. These proportions varied neither by patient gender nor by race/ethnicity.
“My husband was very supportive; he took time off work to care for me and the kids. He did the injections and talked me through it.”
“My wife was my only support. Otherwise I felt like I was going through treatment alone.”
“My wife was supportive initially, but financially, mentally … everything took its toll. We split up for 2 months.”
“The mental and emotional side effects were extremely difficult. My behavior and rage tore my family apart. I had to move out of the house.”
3.6 Ranking of Treatment Challenges
Patients were asked to list the three most challenging aspects of their HCV treatment. Table 2 displays the ranked challenges overall and among the four racial/ethnic groups. Not surprisingly, physical side effects were most commonly cited overall and among the groups. Mental side effects were the second most commonly cited challenge overall; however, among Blacks the second ranked challenge was performing self-injection. Ranking of challenges did not vary significantly by type of treatment provider (not shown).
Table 2.
Most difficult HCV treatment challenges (%)
| Totala (n = 200) |
Asian (n = 43) |
Black (n = 51) |
Hispanic (n = 51) |
Whiteb (n = 50) |
P valuec | |
|---|---|---|---|---|---|---|
| Physical side effects | 69.5 | 74.4 | 45.1 | 76.5 | 84.0 | <0.001 |
| Mental side effects | 43.5 | 41.9 | 33.3 | 41.2 | 56.0 | 0.140 |
| Performing self-injection | 26.0 | 32.6 | 37.3 | 21.6 | 14.0 | 0.036 |
| Workd | 27.4 | 14.3 | 16.2 | 33.3 | 40.0 | 0.048 |
| Family/social | 17.0 | 16.3 | 17.7 | 13.7 | 16.0 | 0.960 |
| Anxiety regarding treatment outcome | 13.0 | 7.0 | 19.6 | 9.8 | 16.0 | 0.251 |
The table displays the percentage of patients (the total and within each group) citing that challenge as one of the three most difficult in an open-ended question
Total includes the five Native American participants
Non-Hispanic White patients
P values are from the chi-squared heterogeneity test for the four racial/ethnic groups shown
Percentage of patients who were working prior to starting treatment
HCV hepatitis C virus
Compared with Whites, both Asians and Blacks were more likely to rank injection issues among the top three challenges, (odds ratio [OR] 2.97, 95 % CI 1.09–8.24, and OR 3.65, 95 % CI 1.37–9.72, respectively). Virtually identical results were found when adjusting for provider type (which was not associated with ranking injection issues; p = 0.30). Among White patients only, there was a suggestion that patients with RN/CP management were less likely than those with MD management to rank injection issues (OR 0.19, 95 % CI 0.03–1.12; p = 0.07).
"Once you start this stuff, it dictates your life, mood, feelings. The medications told me what my life was going to be like.”
“Nothing could have made treatment easier. It was all bad. I just felt like dying.”
3.7 The Type of Treatment Provider
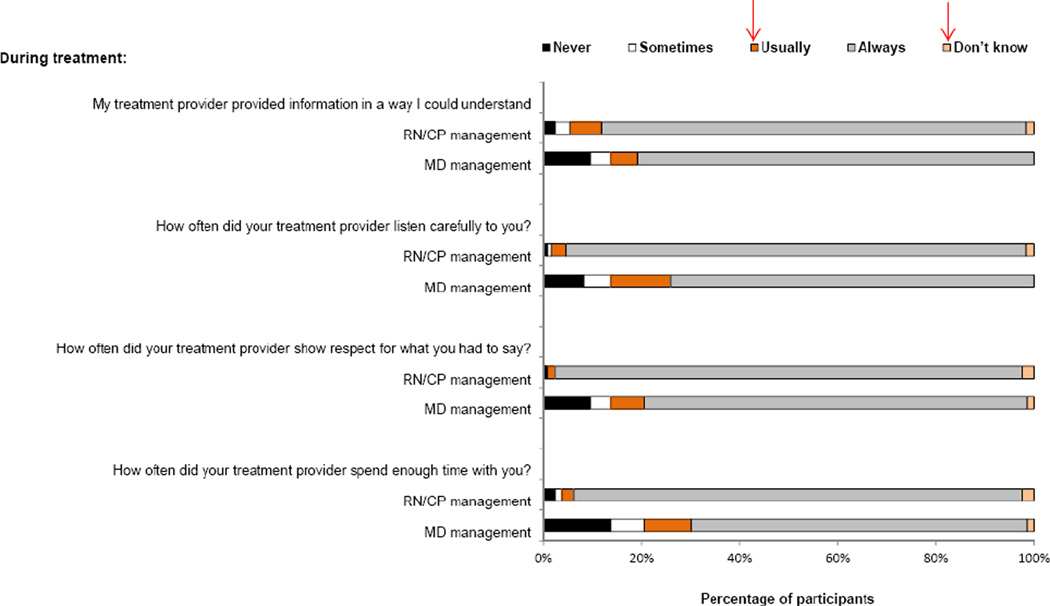
We examined differences in perceived support and quality of interactions by type of treatment provider. When asked to identify ‘what actually did help you during treatment?’ patients with RN/CP management were more likely to mention their treatment providers than were those with MD management (83.5 % vs. 58.9 %; p < 0.001). This discrepancy was consistent among racial/ethnic groups, and regardless of treatment response status (not shown). In response to a Likert scale question (never, sometimes, usually, always, don’t know), patients with RN/CP providers more often reported that their treatment provider always: listened carefully to them (93.7 % [RN/CP management] vs. 74.0 % [MD management]; p = 0.001); showed respect for what they had to say (95.3 % vs. 78.1 %; p = 0.01); spent enough time with them (91.3 % vs. 68.5 %; p = 0.001); and provided information in an understandable way (86.6 % vs. 80.8 %, p = 0.17). Fig. 1 shows complete responses to these four queries.
“The nurse practitioner was great. He coached me; he called me at night and at work.”
“I thought I was going to die; I called the nurse at midnight and she answered the phone!”
Fig. 1.
Ranking of interactions with treatment providers. MD physician management, RN/CP registered nurse or clinical pharmacist management
3.8 Improving the Treatment Experience
We asked patients to rank how helpful different types of support might be for future patients undergoing treatment. Table 3 displays the patient rankings of specific options presented by the interviewer. The most highly ranked options pointed to more frequent provider contact by telephone, and to peer support availability, either with others simultaneously undergoing treatment or with someone treated previously. Less favorable options involved in-person and internet-based interactions. Responses did not vary substantially by racial/ethnic group or by the type of treatment provider.
“I would have appreciated support from someone who had experienced treatment.”
“I wanted to talk to others about my HCV treatment but felt unable to because of the stigma and embarrassment. I would be interested in being a peer support person for others.”
Table 3.
Ranking of potential resources for future patients (%)
| Very helpful |
Somewhat helpful |
Not helpful |
|
|---|---|---|---|
| Weekly phone check-ins with a treatment provider | 81.0 | 11.5 | 5.5 |
| Peer support from someone treated in the past | 75.0 | 13.5 | 7.5 |
| In-person support group with other patients undergoing treatment | 71.5 | 15.0 | 6.5 |
| Phone contact with other patients undergoing treatment | 66.0 | 19.0 | 11.0 |
| A nutritionist/dietician available during treatment | 65.5 | 8.0 | 7.0 |
| Individual, professional mental health counseling/therapy offered every 1–2 weeks during treatment | 63.0 | 14.0 | 10.0 |
| E-mail or internet access to a treatment provider | 62.5 | 12.5 | 4.5 |
| Counseling/support for you with your family before or during treatment | 62.0 | 9.5 | 14.0 |
| Internet chat group with other patients undergoing treatment | 53.5 | 17.5 | 12.0 |
| Weekly in-person meetings with a treatment provider | 43.0 | 27.0 | 26.5 |
The table displays the percentage of participants ranking by scale the potential resources for patients undergoing HCV treatment in the future
The Likert scale included: very helpful, somewhat helpful, not very helpful, not at all helpful, don’t know
Not very and not at all helpful responses are combined for presentation
The few don’t know responses are not shown, thus percentages may not total 100
HCV hepatitis C virus
We asked ‘What would you advise someone about to begin HCV treatment?’ and up to three answers were collected. The five most common suggestions were: obtain an adequate support network (28.5 %); ‘just do it’ (24.0 %); prepare for treatment (23.5 %); switch to a healthy lifestyle (20.5 %); and modify work hours or take a leave of absence (16.5 %). The types of advice offered did not vary substantially by racial/ethnic group. Many patients commented generally about needing to understand and anticipate the potential enormity and breadth of the side effects of treatment:
“Life may change completely for you.”
“This is huge. Make sure you have a support group and your family really understands what they're in for as well. You really need to get your head wrapped around treatment before you use it.”
“Mentally know that you'll be going to war. Get mentally right.”
"Remember, when you think you are crazy, it's just the medication"
“If you can't take intense mental and emotional anguish, don't do it.”
“Having a positive attitude is extremely important."
Among the 90 patients who did not yet know their treatment response status or reported they did not have SVR, 70.3 % said they would try HCV treatment again.
4 Discussion
The objective of this study was to broadly characterize patient perspectives on hepatitis C treatment. While the treatment experience varied between patients, several common themes consistently emerged, including the severity and breadth of medication side effects, the desire for increased support, and the difficulties balancing work and treatment. Our findings confirm and expand upon findings from smaller, more homogeneous study populations.
Our 200 participants recalled a diverse spectrum of challenges as being the most difficult, reinforcing the need to understand, manage, and support each patient individually. While physical and mental side effects were the most commonly ranked challenges overall, Black patients were distinct in that performing self-injection was the second most commonly ranked challenge (over 37 % of Black patients). A prior report suggested that nurses can help to minimize patients’ injection-related issues,[26] although we found a suggestion of this only among White patients.
Of note, almost 43 % of patients reported specific physical and/or mental side effects that lasted several months beyond the end of treatment. The persistent loss of memory reported by 5 % of our patients contrasts with conclusions from the HALT-C (Hepatitis C Antiviral Long-term Treatment against Cirrhosis) clinical trial that HCV treatment did not worsen cognitive function[27]. The need for aftercare has been mentioned previously: in a study of 106 patients who had undergone HCV treatment with nurse managers, ‘more support post-treatment’ was noted as an unmet need by 15 % [28]. Clearly, attention to side effects should not cease abruptly with the end of treatment.
The most frequent point of advice mentioned for future patients was to obtain support, consistent with the major theme found in a US focus group study of 33 patients [17]. In a study of 44 patients who had decided against treatment, 39 % noted a lack of a supportive environment as an important factor in that decision [19]. Higher levels of tangible support were predictive of higher quality-of-life scores during HCV treatment in an interview study of 115 patients in Taiwan [29].
When asked to rank potentially helpful resources for future patients, almost all our study participants ranked peer support and/or support groups highly. Interestingly, after the interview, four patients volunteered (without prompting) to serve as peer supporters; two of them had not responded to therapy and planned to try again. Others have considered peer support in the context of HCV treatment. Previously, patients revealed that social support was the most important factor in the preparation for, and initiation and adherence to HCV therapy [17]. In-person and online hepatitis C support groups, and other peer support approaches have been described [30–32]. Patients report using peers to prepare for treatment, to give and receive support during treatment, and to continue treatment despite side effects. Given that persons with hepatitis C are reported to experience limited social support, relationship difficulties with family, and social isolation and stigmatization due to their disease [33, 34], peer support may offer an antidote to such isolation.
Many of our patients mentioned the need for support related to the workplace. And our findings suggest that providers can help patients by advising and facilitating a leave of absence, temporary disability benefits, or modified work hours during treatment. Over one-third of working patients suffered severe financial problems, such as job loss, during treatment. Such work challenges continued beyond treatment completion for some patients. In a study of 65 previously treated patients in Victoria, Australia, almost half ranked ‘limits my ability to work’ as a major challenge to staying on treatment [19]. In the context of a clinical trial, patients reported up to 40 % impairment while working due to their health during treatment [35]. Also, hepatitis C patients in general are less likely to be in the labor force and to have higher rates of absenteeism and work impairment than uninfected persons [36, 37].
Most of our patients reported family members as supportive. Family members have been shown to play a positive role in symptom management among patients undergoing chemotherapy [38]. A study of five patients and their partners explored the positive aspects and the challenges of spousal roles in the HCV treatment experience [23]. Patients have suggested that family members attend support groups and educational sessions to better prepare them for the HCV treatment experience [17]. Similarly, almost two-thirds of our patients rated counseling and support groups with family members as potentially very helpful resources for future patients. Further work is needed to determine how best to educate and engage family members or other informal caregivers to benefit patients undergoing HCV treatment.
We found more satisfaction with RN/CP care versus MD treatment providers in our study. Others have reported patient frustration with both communication among physicians and communication between the hepatitis C patient and the physician [17, 39]. Furthermore, a desire for access to multidisciplinary services was common among our patients (Table 3). Communication quality is certainly impacted by the time constraints of providers. To address such limitations, some US healthcare systems have increasingly relied on nurse practitioners and physician assistants to care for patients with hepatitis C [40]. The important role of nurses in patients’ quality of life during HCV treatment has been mentioned in previous studies [20, 23], and support from nurses has been rated highly [22].The role of pharmacists in improving the HCV treatment experience has also been explored, particularly in increasing adherence [41, 42]. Mental health providers have also proven helpful to HCV treatment adherence [43], and a pilot study suggests that weekly telephone meetings with a mental health professional are effective [44]. Other studies and guidelines suggest that integrated care models can help to optimize HCV treatment [45–47], and such systems are in place de facto in countries with interdisciplinary, integrated national health services.
In a study of patients with nurse management of HCV treatment, dietary advice was mentioned as an unmet need, [28] consistent with our patients’ suggestion to provide nutritionist/dietician support during treatment. Given that GI side effects are frequently associated with HCV therapy, professional nutritional advice could help increase medication tolerance, especially with new DAAs that require administration thrice daily with food. Unmitigated GI side effects can decrease medication adherence, and for DAAs this can result in the emergence of drug resistance [7], a potentially serious complication. Even with the development of interferon-free regimens, RBV and DAAs will independently contribute to side effects and adherence challenges [48, 49].
One limitation of our study is the potential for recall bias. Although a substantial portion (64.5 %) of participants were interviewed within a year of ending treatment, the range of time since treatment was broad (up to 39 months). Fortunately, this interval did not vary substantially between the groups compared in our analyses. Also, the interview was conducted only in English. Almost 30 % of otherwise eligible Asian patients and 10 % of eligible Hispanic patients were excluded from recruitment due to language preference. Thus, our findings may not have captured the entire breadth of patient experiences, particularly among non-English speakers. However, our conclusions do clearly support and expand upon existing findings that HCV treatment poses a broad spectrum of significant challenges. Furthermore, given the racial/ethnic diversity of our cohort, and representativeness of the health plan membership, our results may be reflective of many insured populations within in the USA.
While these findings are currently informing interventions to improve the quality of care and patient satisfaction within the Kaiser Permanente Medical Care Program, such programs remain to be broadly implemented. Nurses and clinical pharmacists are increasingly deployed as HCV treatment providers, and monthly support groups for patients on HCV treatment have begun. HCV treatment nurses from throughout the health plan region meet monthly to discuss cases and recent HCV treatment literature. These programs are being guided by an interdisciplinary hepatitis C leadership team that includes physicians, nurses, pharmacists, and researchers. Integrated care teams are successfully managing HIV treatment in our healthcare system [50], providing a useful model as we continue to improve systems for HCV therapy. As we progress, our approaches to engage support from peers, family, the healthcare team, and employers must be rigorously evaluated in terms of treatment outcome, medication adherence, and patient satisfaction.
5 Conclusions
This patient-centered investigation provides a panoramic snapshot of the HCV treatment experience with PegIFN/RBV. The call for greater support from peers, providers, and employers demands that new systems of care be considered for hepatitis C treatment. Multidisciplinary, patient-centered care teams can provide the appropriate infrastructure. Robust support systems will be crucial as HCV treatment becomes increasingly complicated with new antiviral agents.
Supplementary Material
Key Points for Decision Makers.
Patients undergoing hepatitis C treatment expressed a need for both peer support and improved provider support during treatment
Patients treated by nurses or clinical pharmacists felt more supported than those treated directly by physicians
The impact of antiviral treatment on employment was significant, and patients desired help with arranging for modified work hours or leaves of absence
Physical and mental side effects continued for months after treatment for many patients, revealing a need to continue treatment-related care beyond the end of treatment
Patient perspectives can be invaluable to the design and refinement of innovative support strategies
Acknowledgments
This study was supported by The Permanente Medical Group. Dr. Ho was supported by NIH T32 DK060414. We appreciate helpful suggestions from Drs. Carol Somkin, Douglas Corley, and Theodore Levin. We thank the many KPNC HCV treatment providers for their input in designing the interview. Special thanks to Dr. Philip Madvig for his interest in this work and subsequent efforts to bring the results into clinical practice. Most importantly, we thank the patients who generously participated in the PATH-C Study and shared their experiences so openly.
Footnotes
Conflicts of Interest The authors have no conflicts of interest that are directly relevant to the content of this article.
Author Contributions MM and RM were responsible for study and survey design. RM conducted all patient recruitment and interviews. VS retrieved and analyzed all medical record data and identified eligible patients. CH and RM performed qualitative and quantitative survey data analysis. MM led the project overall and was primarily responsible for manuscript preparation, with contributions from CH and RM. MM is the guarantor for the overall content of this article.
References
- 1.World Health Organization. Prevention and control of viral hepatitis infection: framework for global action. Geneva: World Health Organization; 2012. [Google Scholar]
- 2.Institute of Medicine. Hepatitis and liver cancer: a national strategy for prevention and control of hepatitis B and C. Washington DC: The National Academies Press; 2010. [PubMed] [Google Scholar]
- 3.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C: The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 4.Bell BP, Manos MM, Zaman A, et al. The epidemiology of newly diagnosed chronic liver disease in gastroenterology practices in the United States: results from population-based surveillance. Am J Gastroenterol. 2008;103:2727–2736. doi: 10.1111/j.1572-0241.2008.02071.x. quiz 2737. [DOI] [PubMed] [Google Scholar]
- 5.Manos MM, Leyden WA, Murphy RC, et al. Limitations of conventionally derived chronic liver disease mortality rates: results of a comprehensive assessment. Hepatology. 2008;47:1150–1157. doi: 10.1002/hep.22181. [DOI] [PubMed] [Google Scholar]
- 6.Wise M, Bialek S, Finelli L, et al. Changing trends in hepatitis C-related mortality in the United States, 1995–2004. Hepatology. 2008;47:1128–1135. doi: 10.1002/hep.22165. [DOI] [PubMed] [Google Scholar]
- 7.Ghany MG, Nelson DR, Strader DB, et al. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54:1433–1444. doi: 10.1002/hep.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 9.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 10.Jeffers LJ, Cassidy W, Howell CD, et al. Peginterferon alfa-2a (40 kd) and ribavirin for black American patients with chronic HCV genotype 1. Hepatology. 2004;39:1702–1708. doi: 10.1002/hep.20212. [DOI] [PubMed] [Google Scholar]
- 11.Hepburn MJ, Hepburn LM, Cantu NS, et al. Differences in treatment outcome for hepatitis C among ethnic groups. Am J Med. 2004;117:163–168. doi: 10.1016/j.amjmed.2004.02.043. [DOI] [PubMed] [Google Scholar]
- 12.Muir AJ, Bornstein JD, Killenberg PG. Peginterferon alfa-2b and ribavirin for the treatment of chronic hepatitis C in blacks and non-Hispanic whites. N Engl J Med. 2004;350:2265–2271. doi: 10.1056/NEJMoa032502. [DOI] [PubMed] [Google Scholar]
- 13.Thomas DL, Astemborski J, Rai RM, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA. 2000;284:450–456. doi: 10.1001/jama.284.4.450. [DOI] [PubMed] [Google Scholar]
- 14.Balagopal A, Thomas DL, Thio CL. IL28B and the control of hepatitis C virus infection. Gastroenterology. 2010;139:1865–1876. doi: 10.1053/j.gastro.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McHutchison JG, Manns M, Patel K, et al. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology. 2002;123:1061–1069. doi: 10.1053/gast.2002.35950. [DOI] [PubMed] [Google Scholar]
- 16.Rodis JL, Kibbe P. Evaluation of medication adherence and quality of life in patients with hepatitis C virus receiving combination therapy. Gastroenterol Nurs. 2010;33:368–373. doi: 10.1097/SGA.0b013e3181f443cb. [DOI] [PubMed] [Google Scholar]
- 17.Fraenkel L, McGraw S, Wongcharatrawee S, et al. Patients' experiences related to anti-viral treatment for hepatitis C. Patient Educ Couns. 2006;62:148–155. doi: 10.1016/j.pec.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 18.Taylor-Young P, Hildebrandt E. The multidimensional burden of hepatitis C and its treatment: a case study. Gastroenterol Nurs. 2009;32:180–187. doi: 10.1097/SGA.0b013e3181a80655. [DOI] [PubMed] [Google Scholar]
- 19.McNally S, Temple-Smith M, Sievert W, et al. Now, later or never? Challenges associated with hepatitis C treatment. Aust N Z J Public Health. 2006;30:422–427. doi: 10.1111/j.1467-842x.2006.tb00457.x. [DOI] [PubMed] [Google Scholar]
- 20.Kinder M. The lived experience of treatment for hepatitis C. Gastroenterol Nurs. 2009;32:401–408. doi: 10.1097/SGA.0b013e3181c1497f. [DOI] [PubMed] [Google Scholar]
- 21.Hopwood M, Treloar C. The experience of interferon-based treatments for hepatitis C infection. Qual Health Res. 2005;15:635–646. doi: 10.1177/1049732304273932. [DOI] [PubMed] [Google Scholar]
- 22.Grogan A, Timmins F. Patients' perceptions of information and support received from the nurse specialist during HCV treatment. J Clin Nurs. 2010;19:2869–2878. doi: 10.1111/j.1365-2702.2010.03239.x. [DOI] [PubMed] [Google Scholar]
- 23.Sgorbini M, O'Brien L, Jackson D. Living with hepatitis C and treatment: the personal experiences of patients. J Clin Nurs. 2009;18:2282–2291. doi: 10.1111/j.1365-2702.2009.02806.x. [DOI] [PubMed] [Google Scholar]
- 24.Gordon N. Internal report: Kaiser Permanente Division of Research. Oakland (CA): 2012. Similarity of the Adult Kaiser Permanente Membership in Northern California to the insured and general population in Northern California: statistics from the 2007 California Health Interview Survey. [Google Scholar]
- 25.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez M, Moreno L, Dosal A, et al. Interferon and the fear of needles: a case report. Gastroenterol Nurs. 2011;34:384–388. doi: 10.1097/SGA.0b013e31822c3a3a. [DOI] [PubMed] [Google Scholar]
- 27.Fontana RJ, Bieliauskas LA, Lindsay KL, et al. Cognitive function does not worsen during pegylated interferon and ribavirin retreatment of chronic hepatitis C. Hepatology. 2007;45:1154–1163. doi: 10.1002/hep.21633. [DOI] [PubMed] [Google Scholar]
- 28.Grogan A, Timmins F. Side effects of treatment in patients with hepatitis C: implications for nurse specialist practice. Australian Journal of Advanced Nursing. 2009;27:70–77. [Google Scholar]
- 29.Chang SC, Ko WS, Wu SS, et al. Factors associated with quality of life in chronic hepatitis C patients who received interferon plus ribavirin therapy. J Formos Med Assoc. 2008;107:454–462. doi: 10.1016/S0929-6646(08)60153-9. [DOI] [PubMed] [Google Scholar]
- 30.Cormier M. The role of hepatitis C support groups. Gastroenterol Nurs. 2005;28(3 Suppl):S4–S9. doi: 10.1097/00001610-200505001-00002. [DOI] [PubMed] [Google Scholar]
- 31.Jessop AB, Cohen C, Burke MM, et al. Hepatitis support groups: meeting the information and support needs of hepatitis patients. Gastroenterol Nurs. 2004;27:163–169. doi: 10.1097/00001610-200407000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Galindo L, Maginnis T, Wallace G, et al. Education by peers is the key to success. Int J Drug Policy. 2007;18:411–416. doi: 10.1016/j.drugpo.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Blasiole JA, Shinkunas L, Labrecque DR, et al. Mental and physical symptoms associated with lower social support for patients with hepatitis C. World J Gastroenterol. 2006;12:4665–4672. doi: 10.3748/wjg.v12.i27.4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zickmund S, Ho EY, Masuda M, et al. "They treated me like a leper": stigmatization and the quality of life of patients with hepatitis C. J Gen Intern Med. 2003;18:835–844. doi: 10.1046/j.1525-1497.2003.20826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perrillo R, Rothstein KD, Rubin R, et al. Comparison of quality of life, work productivity and medical resource utilization of peginterferon alpha 2a vs the combination of interferon alpha 2b plus ribavirin as initial treatment in patients with chronic hepatitis C. J Viral Hepat. 2004;11:157–165. doi: 10.1046/j.1365-2893.2003.00482.x. [DOI] [PubMed] [Google Scholar]
- 36.Su J, Brook RA, Kleinman NL, et al. The impact of hepatitis C virus infection on work absence, productivity, and healthcare benefit costs. Hepatology. 2010;52:436–442. doi: 10.1002/hep.23726. [DOI] [PubMed] [Google Scholar]
- 37.DiBonaventura M, Wagner JS, Yuan Y, et al. The impact of hepatitis C on labor force participation, absenteeism, presenteeism and non-work activities. J Med Econ. 2011;14:253–261. doi: 10.3111/13696998.2011.566294. [DOI] [PubMed] [Google Scholar]
- 38.Silveira MJ, Given CW, Cease KB, et al. Cancer carepartners: improving patients' symptom management by engaging informal caregivers. BMC Palliat Care. 2011;10:21. doi: 10.1186/1472-684X-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zickmund S, Hillis SL, Barnett MJ, et al. Hepatitis C virus-infected patients report communication problems with physicians. Hepatology. 2004;39:999–1007. doi: 10.1002/hep.20132. [DOI] [PubMed] [Google Scholar]
- 40.Gujral H, Viscomi C, Collantes R. The role of physician extenders in managing patients with chronic hepatitis C. Cleve Clin J Med. 2004;71(Suppl. 3):S33–S37. doi: 10.3949/ccjm.71.suppl_3.s33. [DOI] [PubMed] [Google Scholar]
- 41.Smith JP, Dong MH, Kaunitz JD. Evaluation of a pharmacist-managed hepatitis C care clinic. Am J Health Syst Pharm. 2007;64:632–636. doi: 10.2146/ajhp060153. [DOI] [PubMed] [Google Scholar]
- 42.Smith JP. Treatment options for patients with hepatitis C: role of pharmacists in optimizing treatment response and managing adverse events. Pharmacotherapy. 2008;28:1151–1161. doi: 10.1592/phco.28.9.1151. [DOI] [PubMed] [Google Scholar]
- 43.Knott A, Dieperink E, Willenbring ML, et al. Integrated psychiatric/medical care in a chronic hepatitis C clinic: effect on antiviral treatment evaluation and outcomes. Am J Gastroenterol. 2006;101:2254–2262. doi: 10.1111/j.1572-0241.2006.00731.x. [DOI] [PubMed] [Google Scholar]
- 44.Silberbogen AK, Ulloa E, Mori DL, et al. A telehealth intervention for veterans on antiviral treatment for the hepatitis C virus. Psychol Serv. 2012;9:163–173. doi: 10.1037/a0026821. [DOI] [PubMed] [Google Scholar]
- 45.Ho SB, Groessl E, Dollarhide A, et al. Management of chronic hepatitis C in veterans: the potential of integrated care models. Am J Gastroenterol. 2008;103:1810–1823. doi: 10.1111/j.1572-0241.2008.01877.x. [DOI] [PubMed] [Google Scholar]
- 46.Evon DM, Simpson K, Kixmiller S, et al. A randomized controlled trial of an integrated care intervention to increase eligibility for chronic hepatitis C treatment. Am J Gastroenterol. 2011;106:1777–1786. doi: 10.1038/ajg.2011.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yee HS, Chang MF, Pocha C, et al. Update on the management and treatment of hepatitis C virus infection: recommendations from the Department of Veterans Affairs Hepatitis C Resource Center Program and the National Hepatitis C Program Office. Am J Gastroenterol. 2012;107:669–689. doi: 10.1038/ajg.2012.48. quiz 690. [DOI] [PubMed] [Google Scholar]
- 48.Sharma P, Lok AS. Interferon-free treatment regimens for hepatitis C: are we there yet? Gastroenterology. 2011;141:1963–1967. doi: 10.1053/j.gastro.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 49.Poordad F, Chee GM. Interferon free hepatitis C treatment regimens: the beginning of another era. Curr Gastroenterol Rep. 2012;14:74–77. doi: 10.1007/s11894-011-0229-1. [DOI] [PubMed] [Google Scholar]
- 50.Horberg MA, Hurley LB, Towner WJ, et al. Determination of optimized multidisciplinary care team for maximal antiretroviral therapy adherence. J Acquir Immune Defic Syndr. 2012;60:183–190. doi: 10.1097/QAI.0b013e31824bd605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.