Abstract
Background
African Americans continue to suffer disproportionately from cancer morbidity and mortality with emerging evidence suggesting potential quality of life (QOL) disparities in the survivorship period.
Objective
To assess sociodemographic, clinical, and psychosocial factors associated with physical and mental health QOL (PHQOL and MHQOL) among African American and white cancer survivors.
Methods
Patients were recruited from tumor registries. Telephone interviews were conducted with 248 African American and 244 white respondents with a history of breast, prostate, or colorectal cancers. Multivariate regression models were used to assess what factors were associated with PHQOL and MHQOL.
Results
Key racial differences in adjusted analyses included poorer MHQOL scores among African Americans compared to white survivors. Furthermore, race moderated the relationship between perceived social support and MHQOL, where higher social support levels were associated with increased MHQOL among African Americans. Other correlates of QOL impacted racial groups similarly. For example, factors associated with PHQOL scores included being unemployed, uninsured, the presence of medical comorbidities, a longer time since diagnosis and higher levels of cancer related stress appraisals. Factors associated with MHQOL scores included being unemployed, higher levels of daily stress, higher levels of stress associated with the diagnosis, higher levels of education, higher levels of perceived social support, and higher levels of spirituality.
Conclusion
Interventions aimed at increasing social support may have important implications for improving QOL outcomes among African Americans.
Implications for Practice
Measuring and understanding factors associated with QOL have important implications for patient adjustment and clinical decision-making.
Introduction
Long-observed gaps in five-year cancer survival rates for members of racial/ethnic minority groups compared to white cancer patients are beginning to narrow.1 Among African Americans the overall five-year survival rate has improved in the past four decades from a low of 27% in the 1960s to current rates of 58%.1 Despite these promising trends, African Americans continue to suffer disproportionately from cancer morbidity and mortality with emerging evidence suggesting potential quality of life (QOL) disparities in the survivorship period.2-4 The same factors that contribute to racial disparities earlier in the cancer care continuum - advanced stage of diagnosis,5 less access to timely treatments and quality of care,6 the presence of medical comorbidities,7 and poor information and decision-making support8 - are also likely to increase the risk for poor survivorship outcomes among African Americans. Beyond the experience of breast cancer patients, relatively few studies have been conducted to examine the effect of cancer diagnosis on the quality of life (QOL) of African American cancer survivors. Additionally, little is known about the demographic (e.g., age), clinical (e.g., cancer type) and psychosocial (e.g., stress and coping) factors that may influence QOL outcomes among this highly underserved subpopulation of cancer survivors.9-11 The purpose of this study was to examine physical and mental health quality of life (QOL) outcomes and the correlates of these outcomes in a sample of African American and white breast, prostate and colorectal cancer survivors. Identifying the mechanisms underlying poor QOL outcomes across diverse patient populations of cancer survivors has important implications for patient adjustment, clinical decision-making, research, and public policy.10,12
Quality of Life
Health related QOL is a multidimensional construct defined as the degree to which one's physical, emotional, social, and spiritual well-being are affected by an illness and its treatment.13 To date, studies examining the influence of race on cancer-related QOL have primarily been conducted with breast cancer patients with mixed results. In a study of African American and white women with a history of breast cancer, no differences in overall QOL were observed after controlling for socioeconomic and other clinical variables.14 Similarly, Rodrigue did not observe any differences in a heterogeneous sample of African American and white cancer survivors in psychological adjustment, mood, or health care satisfaction.15 However, other studies have provided evidence of poorer QOL among African American cancer survivors compared to other groups. For example, Paskett et al reported significantly poorer QOL in physical functioning, general health, and role limitations among African American compared to white breast cancer survivors even after taking into account education level, presence of comorbidities, age at study entry, and life events.16 Similarly, Bowen et al found that African American breast cancer survivors had lower physical health QOL compared to whites while taking into account socioeconomic factors.17 Lowered levels of physical functioning14 and more difficulty in the activities of daily living (an indicator of physical health status)18 have also been reported in African American breast cancer patients compared to their white counterparts. Alternatively, several studies have reported higher levels of emotional well being and mental health among African American breast cancer survivors relative to whites and other ethnic minority women.2,10
In the past decade, research on the QOL of African American males with a history of prostate cancer has emerged. Similar to the literature on race and QOL outcomes among African American women with breast cancer, study findings have been mixed. For example, in a study examining the effects of race on QOL of prostate cancer patients, Halbert et al found that after controlling for sociodemographic, clinical, stress and religious coping factors, African American men reported better emotional well-being compared to white men, although there were no significant racial differences observed in physical functioning.19 In contrast, after controlling for demographic, treatment type and medical comorbidities, African American men with prostate cancer reported significantly lower levels of physical and emotional functioning compared to white men.20-22 The inconsistent findings regarding race and QOL outcomes may be a result of study differences in patient sample characteristics, measurement issues, and disease factors. Additional research is warranted to better understand the relationship between race and QOL outcomes in cancer survivors.
Correlates of Quality of Life Outcomes
Research primarily focused on samples of white cancer patients suggests relationships between demographic, clinical, and psychosocial risk factors and cancer-related QOL. Demographic risk factors for low cancer-related QOL and psychosocial distress include lower socioeconomic status,10,12 being female and single marital status23, and lower levels of formal education.24 Clinical and treatment variables that affect QOL and related adjustments include younger age at diagnosis,25 more advanced stage of illness26, longer time since diagnosis27 and the presence of medical comorbidities at the time of the cancer diagnosis.28 Finally, psychosocial correlates of lower QOL and poorer adjustment include inadequate social support,29 less-adaptive coping responses,30 and lower levels of spirituality.31 Despite the well-known cancer disparities among African Americans, the relationship between the above demographic, clinical and psychosocial risk factors and QOL among African American cancer survivors has been largely unexplored. Exploration of these factors is important because race may interact with some or all of the above risk factors and influence how African Americans adjust to a cancer diagnosis. For example, cancer-related distress is a common experience of cancer patients.32-34 In most instances, elevated rates of emotional distress following a cancer diagnosis resolves over time.35 However, African American and white cancer survivors may differ in the magnitude and type of stressors associated with their cancer diagnosis. Further, there may be cultural factors associated with the availability and use of coping resources. Consequently, African Americans may be more vulnerable to the adverse psychosocial effects of cancer and experience a lower quality of life.
In summary, research examining racial differences in QOL outcomes in the survivorship period has resulted in mixed results. Further, little is known about the survivorship experiences of African Americans with cancer diagnoses other than breast cancer or the factors that may be associated with poorer outcomes. To date, much of the research on QOL outcomes among diverse populations of cancer survivors has been atheoretical. However, previous research suggests that constructs such as clinical, psychological, social, and spiritual well-being are important contributors to QOL13. More recent work by Ashing–Giwa36,37 and others16,17 have described the importance of constructs related to sociodemographic and health care factors on QOL, especially in racial minority and other underserved groups. We use the above definition of quality of life and constructs to guide the variables used in our research models.
Research Questions
This paper addresses a significant gap in the literature by examining the mental and physical health QOL outcomes among African American and white cancer survivors with a history of breast, prostate, and colorectal cancer. Based on previous research, we posit that QOL among cancer survivors is associated with the following clinical and non-clinical factors: demographic characteristics of patients, clinical factors, cancer-related stress, and psychosocial factors. Specific research questions to be addressed were: 1) Do African American and white cancer patients differ on physical and mental health QOL outcomes? and 2) Are observed racial differences in QOL outcomes accounted for by sociodemographic, clinical, and psychosocial (i.e., stress and coping resources) factors.
Methods
Sample Design and Data Collection
Data for this paper come from a larger survey study aimed at examining the treatment decision-making and cancer related outcomes of cancer patients.8 The larger study sample included 248 African American and 244 White patients diagnosed with breast, prostate, or colorectal cancer and diagnosed within the past three years. The following sampling approaches were used to obtain a sample of urban and non-urban African American cancer patients in Illinois. First, all 79 Illinois hospitals with a cancer registry and located in the 15 Illinois counties with at least 10 reported African American cancer cases (any site) per year were included in the initial recruitment plan. Additionally, three other hospitals located in bordering states but known to serve Illinois residents were included. Finally, to increase the overall number of non-urban African Americans in the sample, additional non-overlapping cases were identified from the Illinois State Cancer Registry (ISCR). After each African American patient was recruited, a similar white comparison patient was identified in terms of cancer site, gender, age, and time since diagnosis and recruited from the same hospital pool as the African American (see Table 1). This method was used as a recruitment strategy for both the hospitals and ISCR. A total of 753 patients meeting eligibility criteria (African American or white, breast, prostate or colorectal cancer diagnosis, and within three years of an initial diagnosis) were identified through 33 hospital registries and the Illinois State Cancer Registry and forwarded to the Survey Research Laboratory (SRL) at the University of Illinois, who conducted telephone interviews. Upon telephone contact by SRL, 149 cases were found to be ineligible (more than 36 months since diagnosis, other cancer site, deceased, non-English speaking). Of the qualifying cases (N=604), 492 (81.5%) completed the interview (44 were not found, 51 refused to participate and 17 started the interview, but did not complete the majority of the questions and were therefore excluded from the analysis).
Table 1. Comparisons between African American and white Cancer Patients on Hospital and Clinical Characteristicsa.
| African American | White | Comparison Test | |
|---|---|---|---|
|
|
|
|
|
| N = 248 | N = 244 | χ2 | |
| Geographic area of diagnosing hospital | |||
| Urban | 50.0 | 39.8 | 8.22b |
| Suburban | 29.4 | 29.1 | |
| Small city/rural | 20.6 | 31.2 | |
| Bed Size | |||
| 200 or less | 15.7 | 13.5 | 4.31 |
| 201–400 | 38.3 | 47.5 | |
| 401–600 | 40.3 | 34.3 | |
| More than 600 | 5.7 | 4.5 | |
| Hospital has cancer left/program | |||
| No | 35.6 | 41.8 | 1.97 |
| Yes | 64.4 | 58.2 | |
| Teaching hospital | |||
| No | 28.2 | 35.7 | 3.12 |
| Yes | 71.8 | 64.3 | |
| Type of Cancer | 4.77 | ||
| Colorectal (Females) | 10.5 | 13.1 | |
| Colorectal (Males) | 8.1 | 13.1 | |
| Breast | 53.0 | 46.3 | |
| Prostate | 28.3 | 27.5 | |
| Age at diagnosis (years) | |||
| 26–49 | 22.6 | 16.0 | 9.10b |
| 50–64 | 44.8 | 39.3 | |
| 65–74 | 23.4 | 29.1 | |
| 75+ | 9.3 | 15.6 | |
| Time since diagnosis | |||
| 12 months or less | 16.5 | 19.7 | 1.41 |
| 13–18 months | 31.9 | 27.9 | |
| 19–24 months | 33.5 | 34.8 | |
| 25-36 months | 18.2 | 17.6 | |
Variables above were the study's recruitment matching characteristics and were used to identify white cancer patients from the same hospitals where African American cancer patients were identified.
p< .05
There was no attempt to achieve race concordance between patients and interviewers: however, SRL interviewers are carefully trained and supervised and are experienced in surveying diverse populations. On average interviews lasted between 60 to 90 minutes. Respondents received a $30 gift card for participation. Prior to study implementation, the survey and study protocol were reviewed and approved by The Institutional Review Boards at the University of Chicago and the University of Illinois at Chicago.
Study Measures
Quality of Life
The physical and mental health component summary scales of the Medical Outcomes Study 36-item Short Form Health Survey (SF36) were used to measure QOL outcomes.38 Four subscales make up the physical health QOL summary measure including physical functioning, role limitations due to physical problems, bodily pain, and general health and four subscales make up the mental health QOL summary measure including energy/vitality, social functioning, role limitations due to emotional problems, and mental health. In the current sample, the internal consistency as measured by Cronbach's alpha was 0.84 for the physical health summary scale and 0.81 for the mental health summary scale.
Sociodemographic Factors
Socioeconomic factors included education (<high school, high school/GED, some college, and ≥college degree), income (≤$30,000, $30,000-$50,000, and >$50,000), employment status [employed, unemployed (this category included disabled), or retired], and health insurance status [none, public (Medicare/Medicaid), and private (including military and other)]. Demographic variables included race (African American and white) and gender.
Clinical Factors
Clinical and treatment-related factors measured were: age at diagnosis in years (26-49, 50-64, 65-74, and ≥75), time since diagnosis in months (≤ 12, 13-18, 19-24, and 25-36), cancer site (colorectal, breast, or prostate), currently in treatment (yes/no), and other medical comorbidities (0, 1 or 2). We grouped cancer stage at diagnosis into three categories (early = 1 and 2, late = 3 and 4, and unknown stage of diagnosis). We included a category for unknown due to more than 50% of participants reporting stage as unknown.
Psychosocial Factors
Stress variables included life disruption associated with their illness (1 = not at all disruptive to 10 = extremely disruptive), stress associated with diagnosis (1 = not at all stressful to 10 = extremely stressful), and daily stress due to cancer and other factors (1 = not at all stressful to 10 = extremely stressful). These items were used in a previous study of information seeking among cancer patients.37
Coping resources measured included marital status, perceived support, and spirituality. Marital status was measured as married/with partner or not currently married/widowed. Perceived social support was measured using three items from the Multidimensional Scale of Perceived Social Support39: emotional help and support from family, presence of a special person to help in time of need, and whether or not the family helped with decisions (1 = strongly disagree to 4 = strongly agree). A composite measure of social support was generated by summing these three variables. Responses ranged from 3 to 12, with higher scores indicating higher levels of perceived social support. The internal consistency as measured by Cronbach's alpha was 0.75. A measure of social network size used for coping was created by summing the types of people from whom patients sought help including: other cancer patients, medical providers, clergy, a spouse or partner, family members, and friends (1 = yes, 0 = no, range 0-6). The internal consistency as measured by Cronbach's alpha was 0.71. The Functional Assessment of Chronic Illness Therapy-Spiritual Factors Scale (FACIT-SP)40 was used to measure spirituality (alpha = 0.87 for the current sample).
Data Analysis
African American and white cancer participants were compared on all study variables using X2 tests for categorical variables and two-sample Student's t-tests for continuous variables and scales. Multivariate regression models were used to assess what factors were associated with physical and mental health QOL. As discussed in the methods section, African Americans and a comparison sample of white cancer patients were selected based on 1) geographic area of diagnosis hospital, 2) bed size, 3) hospital has cancer center/program, 4) teaching hospital, 5) type of cancer, 6) age at diagnosis, and 7) time since diagnosis. Despite sampling procedures intended to minimize group differences on these hospital and clinical characteristics, a greater percentage of African American participants were diagnosed in an urban hospital and tended to be younger at diagnosis compared to white participants (Table 1). Therefore, in multivariate regression analyses we adjusted for geographic area of diagnosing hospital and age at diagnosis. Regression models also included cancer type and time since diagnosis since these clinical factors are known to be associated with QOL. Finally, multivariate regressions with interactions terms (e.g. race × spirituality) assessed whether race impacted the relationship between psychosocial factors (stress and coping resources) and the QOL outcomes. Data were analyzed using Stata/SE version 11.
Results
Sample Characteristics
Table 2 describes participants' characteristics, the physical and mental health QOL outcomes, and comparisons by race. African American participants differed from whites on a range of demographic variables including education, income, employment, insurance coverage and marital status. The only medical characteristic that differed between the groups were younger age of diagnosis and the greater likelihood of medical comorbidities among African Americans compared to white patients (Table 2). Measures of stress did not differ based on race. However, compared to white participants, African Americans had lower mean scores for social support but higher mean spirituality scores. Additionally, unadjusted mean scores for both physical and mental health QOL outcomes were lower for African American compared to white participants.
Table 2.
Comparisons Between African American and white Cancer Patients in Sociodemographic Factors, Clinical Factors, Psychosocial Factors and QOL Outcomes.
| African American | White | Comparison Test | |
|---|---|---|---|
|
|
|
|
|
| N = 248 % | N = 244 % | χ2 | |
| Sociodemographic Factors | |||
| Education | |||
| Less than H. S. | 21.5 | 9.6 | 19.29d |
| H. S./G.E.D. | 22.7 | 32.5 | |
| Some college | 29.6 | 23.8 | |
| College degree or above | 26.3 | 34.2 | |
| Income | |||
| Less than $30,000 | 47.7 | 26.1 | 23.35d |
| $30,000–$50,000 | 25.7 | 35.4 | |
| $50,000+ | 26.6 | 38.5 | |
| Employment status | |||
| Employed full/part time | 39.2 | 48.8 | 32.75d |
| Unemployed (includes disabled) | 21.6 | 4.2 | |
| Retired | 39.2 | 47.1 | |
| Insurance | |||
| None | 15.4 | 6.6 | 18.81d |
| Public (Medicare/Medicaid) | 22.7 | 37.9 | |
| Private, military, other | 61.9 | 55.6 | |
| Marital status | |||
| Married or with partner | 48.4 | 71.5 | 27.12d |
| Not married/widowed | 51.6 | 28.5 | |
| Clinical Factors | |||
| Stage 1 and 2 | 34.7 | 34.8 | 0.49 |
| Stage 3 and 4 | 7.7 | 10.7 | |
| Stage Unknown | 57.7 | 54.5 | |
| Currently receiving treatment | 22.7 | 17.3 | 2.22 |
| Comorbidities | 10.04c | ||
| None | 34.3 | 48.4 | |
| One | 33.1 | 27.9 | |
| Two | 32.7 | 23.8 | |
| Psychosocial Factors | Mean(SD) | Mean(SD) | T-value |
| Disruptiveness of treatment | 4.4 (3.2) | 4.7 (3.0) | 1.11 |
| Daily stress levels | 3.8 (2.4) | 3.8 (2.2) | 0.06 |
| Stress associated with diagnosis | 6.8 (3.1) | 6.6 (2.9) | -0.76 |
| Social support (scale) | 10.0 (1.8) | 10.3 (1.8) | 2.02 b |
| Support network for coping (scale) | 4.0 (1.8) | 3.8(1.6) | -1.50 |
| FACIT-SP a(scale) | 39.4 (7.7) | 37.1(8.4) | -3.20 c |
| SF-36 Outcomes | |||
| Physical Health QOL Summary Scale | 44.5 (10.9) | 47.5 (11.2) | 3.03 c |
| Mental Health QOL Summary Scale | 51.6 (10.0) | 53.7 (8.8) | 2.48 b |
Functional Assessment of Chronic Illness Therapy-Spiritual Factors Scale
p< .05
p< .01
p< .001
Physical Health QOL
Multivariate regressions examined the relationship between independent variables and physical health QOL. As shown in Table 3, Model 1 explained 35% of the variance in physical health QOL [F(26, 430) = 10.62, p<.001, adjusted R2= 0.354]. Factors that were significantly associated with poorer physical health QOL included being unemployed, uninsured, having one or two comorbidities, having greater life disruptions due to treatment and having higher levels of daily stress. In contrast, a longer time since diagnosis and having greater levels of stress due to the diagnosis were associated with better physical health QOL. After controlling for study covariates, race was not an independent predictor of physical health QOL. Furthermore, differences in physical health QOL by gender and cancer type were not significant.
Table 3. Correlates of Physical and Mental Health Quality of Life Outcomes.
| Adjusted coefficients (Standard Errors) | ||||
|---|---|---|---|---|
| Physical health QOL | Mental health QOL | |||
|
|
|
|||
| Model 1: Adjusted a | Model 2: Interactions a | Model 3: Adjusted a | Model 4: Interactions a | |
| Sociodemographic Factors | ||||
| Race | ||||
| African American | -1.12(0.97) | 11.46 (6.34) | -1.90 (0.83) c | -21.24 (5.38) e |
| White | Ref | Ref | Ref | Ref |
| Gender | ||||
| Male | 3.44 (1.90) | 3.47 (1.91) | -1.99 (1.63) | -1.87 (1.62) |
| Female | Ref | Ref | Ref | Ref |
| Education b | 0.07 (0.45) | 0.06 (0.45) | 0.90 (0.38) c | 0.89 (0.38) c |
| Income b | 1.35 (0.67) | 1.45 (0.68) c | 1.03 (0.58) | 1.07 (0.57) |
| Employment Status | ||||
| Employed | Ref | Ref | Ref | Ref |
| Unemployed | -5.47 (1.39) e | -5.54 (1.39) e | -3.83 (1.19) d | -3.76 (1.18) d |
| Retired | -1.11 (1.21) | -1.14 (1.23) | -0.92 (1.03) | -0.51 (1.04) |
| Health Insurance Status | ||||
| No Insurance | -3.19 (1.52) c | -3.18 (1.52) c | 0.65 (1.30) | 0.44 (1.29) |
| Public Insurance | -1.37 (1.22) | -1.20 (1.24) | 1.39 (1.05) | 0.93 (1.05) |
| Private | Ref | Ref | Ref | Ref |
| Clinical Factors | ||||
| Age at diagnosis b | -0.64 (0.71) | -0.70 (0.71) | -0.14 (0.60) | -0.25 (0.60) |
| Time since diagnosis b | 1.61 (0.46) e | 1.62 (0.46) e | -0.65 (0.39) | -0.70 (0.39) |
| Cancer Type | ||||
| Colorectal | Ref | Ref | Ref | Ref |
| Breast | 0.59 (1.45) | 0.37 (1.47) | -0.39 (1.24) | -0.13 (1.24) |
| Prostate | -1.19 (1.59) | -1.50 (1.59) | 0.85 (1.36) | 1.04 (1.35) |
| Cancer Stage | ||||
| Unknown | 0.23 (0.98) | -0.45 (0.99) | -0.21 (0.84) | -0.18 (0.84) |
| Stage 1 and 2 | Ref | Ref | Ref | Ref |
| Stage 3 and 4 | -2.18 (1.59) | -2.13 (1.60) | 1.40 (1.36) | 1.66 (1.35) |
| Currently receiving treatment | -0.90 (1.10) | -0.89 (1.10) | 0.51 (0.94) | 0.58 (0.93) |
| Number of comorbidities | ||||
| None | Ref | Ref | Ref | Ref |
| One | -2.19 (1.05) c | -2.05 (1.06) | 0.96 (0.90) | 0.70 (0.90) |
| Two | -9.20 (1.15) e | -9.00 (1.15) e | 1.20 (0.98) | 0.95 (0.98) |
| Psychosocial Factors | ||||
| Greater disruptiveness of treatment | -0.47 (0.15) d | -0.30 (0.23) | -0.09 (0.13) | -0.16 (0.20) |
| Greater daily stress | -0.44 (0.20) c | -0.34 (0.29) | -1.00 (0.17) e | -1.35 (0.25) e |
| Greater stress associated with diagnosis | 0.43 (0.16) d | 0.77 (0.25) c | -0.41 (0.14) d | -0.45 (0.21) c |
| Married | 0.46 (1.01) | 0.63 (1.47) | 0.07 (0.86) | -0.41 (1.25) |
| Greater perceived social support | -0.14 (0.25) | -0.24 (0.37) | 0.67 (0.22) d | -0.03 (0.32) |
| Greater resources used for coping | -0.17 (0.31) | -0.58 (0.49) | -0.54 (0.26) c | -0.51 (0.42) |
| Greater Spirituality | 0.12 (0.06) | 0.26 (0.08) d | 0.44 (0.05) e | 0.41 (0.07) e |
| Interactions: Stress and race | ||||
| Disruptiveness of treatment × AA | -0.26(0.40) | 0.09 (0.26) | ||
| Daily stress levels × AA | -0.21 (0.40) | 0.63 (0.34) | ||
| Stress associated with diagnosis × AA | -0.56 (0.32) | 0.12 (0.27) | ||
| Interactions: Coping resources and race | ||||
| Married × AA | -0.17 (1.87) | 0.70 (1.58) | ||
| More Social support × AA | 0.08 (0.52) | 1.35 (0.44) d | ||
| Greater resources used for coping × AA | 0.62 (0.62) | -0.23 (0.53) | ||
| Higher spiritual levels × AA | -0.26 (0.12) c | 0.06 (0.10) | ||
| Adjusted R-squared | 0.35 | 0.36 | 0.36 | 0.37 |
Abbreviations: AA, African American
Variable for location of diagnosing hospital is not included in table (not a significant predictor)
Variables operationalized as follows: Education (<High School (HS), HS/GED, some college,≥ college degree); Income (<$30,000, $30-50,000, ≥$50,000); Age at diagnosis (26-49,50-64,65-74, ≥75); Time since diagnosis in months (≤12, 13–18, 19–24, 25-36)
P< .05
P< .01
P< .001
In Model 2 (Table 3), the regression included interaction terms accounting for the influence of race on the relationship between psychosocial factors (stress and coping resources) and physical health QOL. The model explained 36% of the variance in physical health QOL [F(33,423) =8.68, p< .001, adjusted R2=0.357]. Despite the significant interaction between spirituality and race, inclusion of interaction terms for stress and coping resources did not improve the overall model fit [F (7,423) =1.30, p=>.05, adj R2 change =0.013], therefore Model 1 is the final model. We further examined a reduced model (not shown) including only the spirituality by race interaction. The interaction term was not significant and the model fit also did not improve significantly [F(1,429) =1.17, p >.05, adj R2 change =0.0003].
Mental Health QOL
Model 3 (Table 3) explained 36% of the variance in mental health QOL [F (26,430) =10.85, p<.001, adjusted R2=0.360]. Factors that were significantly associated with poorer mental health QOL included being African American, being unemployed, having higher levels of daily stress, having higher levels of stress associated with the diagnosis, and using more resources for coping. In contrast, higher levels of education, more perceived social support, and higher spirituality scores were associated with better mental health QOL. Finally, similar to the physical health QOL outcome, differences in mental health QOL were not significantly influenced by cancer type and gender.
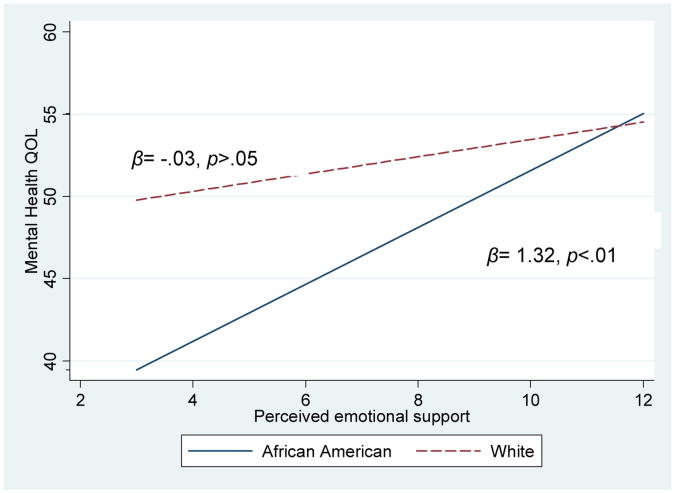
In Model 4 (Table 3) results of the regression model with interaction terms are presented. The model explained 37% of the variance in mental health QOL [F (33,423) =9.23, p < .001, adjusted R2 =0.373]. Model 4 is the final model as including the interaction terms improved the model fit [F (7,423) =2.38, p< .05, adj R2 change=0.014]. There was one significant interaction. Race moderated the effect of perceived social support on mental health QOL. The difference in mental health QOL between African Americans and white survivors is represented by the value of the coefficient for the interaction (β = 1.35, p<.01). As perceived social support increased for African Americans their mental health QOL scores also increased (β=1.32, p<.001); however, this relationship was non-significant for whites (β = -0.03, p>.05). (See the Figure)
Figure.
The Effect of Race on the Relationship Between Levels of Perceived Social Support and Mental Health QOL.
Discussion
The overall objective of this study was to evaluate differences in physical and mental health QOL between African American and white cancer survivors and to determine the influence of sociodemographic, clinical and psychosocial factors on QOL outcomes. The majority of our study participants were well adjusted, with cancer survivors who were on average three years beyond diagnosis and treatment, reporting mental and physical health QOL scores similar to those of older adults (65-74 years) in the general U.S. population.38 These findings are consistent with recent reports suggesting that cancer-related distress resolves over time.35 However, differences in adjustment were observed based on race. First, we found that in bivariate analyses, African Americans had lower physical and mental health QOL scores. However, after adjusting for sociodemographic, clinical and psychosocial factors only racial differences in mental health QOL scores remained. Second, we found that correlates of QOL impacted racial groups similarly, except for perceived social support. Higher social support levels were associated with increased mental health QOL among African Americans, although not for white counterparts. A summary of key findings are described below and implications for treatment services discussed.
Factors Associated with Physical Health QOL
The attenuated relationship between poorer physical health QOL scores and race after adjustment for other patient characteristics are consistent with previous research highlighting the role of sociodemographic factors in outcomes of cancer patients.37 In this study, being uninsured and unemployed predicted physical health QOL, independent of race. These findings suggest that differences in cancer-related physical health outcomes are due to disparities in insurance status and treatment access. In the current study, African American cancer patients had lower education levels, were less likely to be married, and were more likely to be unemployed and lack health insurance compared with whites. These sociodemographic risk factors may increase the vulnerability of African Americans to poorer outcomes across a myriad of health disorders including physical health QOL following a cancer diagnosis.
Beyond race and sociodemographic characteristics, clinical and psychosocial factors also play a role in physical health QOL outcomes. In terms of clinical factors, we found similarities to other study findings.26,28 Not surprisingly, having a higher number of medical comorbidities was negatively associated with physical health QOL, while a longer-time since diagnosis was positively associated with physical health QOL. Unlike other studies,23,41 we did not find gender differences in physical health QOL. Research findings examining the relationship between cancer type and QOL are mixed. For example, one study found similarities in symptoms and adjustment across several different cancer types when diagnosed at early stages,42 while others have reported differences in physical health QOL based on cancer type. In a large study comparing the QOL outcomes between breast and colon cancer patients, breast cancer patients reported, on average, worse physical health-related scores.43 However, after adjustment for age, education and gender, most of the differences observed between breast and colon cancer patients disappeared. Cancer type was not associated with physical health QOL in our sample of cancer patients. It may be that other factors such as level of disease burden and the availability of appropriate clinical and psychosocial care are stronger predictors of QOL than actual cancer tumor type. However, additional research will be needed to determine which factors influence QOL outcomes in patients with differing tumor types. Several psychosocial factors measured in our study were associated with physical health QOL outcomes. For example, cancer-related stress appraisals were independent predictors of physical health QOL. Counter intuitively, higher stress at diagnosis predicted better physical health QOL in patients on average three years after being diagnosed. This finding may be influenced by unmeasured factors such as increased motivation among patients experiencing stress to engage in health promoting activities such as physical exercise. African American and white cancer patients were similar in their appraisal of the treatment experience, including the stress associated with their diagnosis. This appraisal of treatment is a critical determinant of successful coping. Specifically, appraisals of threat or harm associated with an event initiate the use of coping mechanisms.44 In a sample of patients receiving radiation therapy, appraisals mediated the effects of negative mood states, self-care burden, and reduced symptom distress.45 Another study of African American breast cancer survivors26 found that appraisals mediated the relationship between current concerns and QOL. Additional research is needed to better understand the relationship between stress appraisals and QOL outcomes by race.
Factors Associated with Mental Health QOL
Mental health QOL scores were lower for African Americans compared to whites. Unlike the physical health QOL outcome, race remained an independent predictor of mental health QOL even after controlling for other factors. Previous reports have described both poorer22 and better 2,19 mental health outcomes among African American breast and prostate cancer patients relative to white survivors. The continued significance of race in mental health outcomes after controlling for background differences suggests the influence of culture or other life experiences between African Americans and whites. Other factors may have contributed to the association between race and poorer mental health QOL. For example, social support plays an important role in emotional adjustment to illness and is thought to ameliorate psychological distress in medical patients by providing a buffer against the harmful effects of stress.46 In our study the perceived availability of support differed between racial groups, with African Americans reporting lower levels of social support, although, as levels of social support increased for African Americans, mental health QOL scores improved. This relationship did not hold among whites. Differences in levels of social support between African Americans and white patients is significant, given the association between decreased social support and increased death rate among breast cancer patients.12 These findings support the need for the assessment and provision of additional psychosocial supportive services to under-resourced African Americans with cancer. Our findings suggest that interventions to identify and increase social support levels among African American may improve their mental health QOL. In addition, understanding racial differences in patients' help-seeking and use of specific types of coping resources is an area in need of further research to help inform culturally targeted interventions for increasing adjustment to cancer and its aftermath.
Medical factors were not associated with mental health QOL, although several psychosocial factors were associated with mental health QOL outcomes as they were for physical health QOL. For example, cancer-related stress appraisals were independent predictors of mental health QOL. Higher levels of stress associated with patients' cancer diagnoses were associated with poorer mental health QOL. Higher levels of spirituality were also associated with improved mental health QOL outcomes. African Americans were more likely to report relying on their spiritual beliefs to cope with their illnesses, suggesting that higher rates of spirituality among African Americans may be important to improving their physical and emotional health following a cancer diagnosis and treatment. A review of the literature showed that strong religious beliefs were associated with lower levels of pain, anxiety, hostility, and social isolation, as well as higher levels of life satisfaction in cancer patients.47 Strong religious faith has also been linked to higher levels of QOL.48 Religion and spirituality may serve for some as a means for adjusting to a cancer diagnosis,47 much remains to be understood about the relationship between spirituality and health.49 Although the evidence is inconclusive, possible mechanisms linking psychosocial factors such as stress and coping resources to improved QOL outcomes include the body's physiological response to the stress produced by the diagnosis and the disease50 as well as behavioral pathways such as engagement in health risk behaviors.
Strengths and Limitations
This study contributes to the literature on QOL of cancer survivors by providing additional information about the QOL of African Americans. The study sample had several important strengths. First, the African American sample included cancer patients drawn from all hospitals in Illinois that reported treating 10 or greater African American patients per year. Thus, our study findings may be more generalizable than those from studies that have used samples of convenience based on one or a few hospitals. Second, each African American patient was matched as closely as possible with a white patient on demographic (i.e., gender), disease (i.e., type of cancer) and characteristics of the diagnosing hospital (i.e., bed size). Our sample of cancer survivors was also heterogeneous in terms of gender and cancer type, thus expanding our knowledge base about African Americans with cancers other than breast cancer. Finally, we measured psychosocial variables that may help to account for differences observed in QOL outcomes based on race.
Despite the strengths of the study, limitations should also be noted. Study findings are limited by the cross-sectional study design that did not allow us to examine QOL over time. Many Illinois counties were excluded from the study because they did not have any African American cancer patients, possibly under-representing white patients residing in more affluent suburban counties or in some rural areas. Comparison by race might have produced different results if we had selected a sample more representative of all white cancer patients in Illinois. Given the focus on African American patients and the importance of where patients are treated, we chose to ensure comparability on hospitals at diagnosis over greater generalizability of the white sample. The cancer registry provided health data including date of diagnosis, cancer type, time since diagnosis and the name and location of the diagnosing hospital. However, all other information was based on self-report including the presence of medical comorbidities and stage of diagnosis, which may have introduced recall bias into our findings. Furthermore, only 44% of participants had knowledge about their stage at diagnosis. Future studies should use chart reviews or other means to obtain accurate medical information on these important variables. The stress variables used in this study have been used in previous studies of primarily White cancer patients. Findings about the relationship between stress and QOL were counter-intuitive suggesting the need for additional research in this area. Although the stress measures were previously used in a large study of cancer survivors, a potential limitation of our measures of stress is that they were based on responses to three single items. It could be that the measures of stress used in this study were not sensitive measures in this population and other standardized measures of stress would be more appropriate. Finally, the focus of this study was on understanding the QOL of patients with breast, prostate and colorectal cancer. These cancer types were selected due to availability of early detection screening tests and thus the increased likelihood of longer-term cancer survivors with these diseases. Additional research is needed to measure QOL and predicting factors among African Americans with different types of cancers.
Conclusions
Findings have implications for better understanding of factors influencing QOL among African American and white cancer patients and may inform the development of psychosocial supportive services needed to address the general and culturally specific needs of this underserved population of cancer survivors.
Results of our findings make a significant contribution to the extant literature on race and QOL outcomes among cancer survivors and have important implications for research, treatment, and public policy. We explored the impact of a range of domains with known or theoretically plausible associations with physical and mental health outcomes for cancer survivors in general and for African American cancer survivors in particular. Identification of general and culturally-specific predictors of QOL in cancer patients has implications for developing targeted interventions that may affect outcomes and survival. Although additional research will be needed to confirm these results and to further clarify the most salient QOL issues for African Americans, our findings expand the knowledge of the cancer survivorship experience for African Americans.
Acknowledgments
This research was supported by grants from the National Cancer Institute: R01CA775-01A1 and R25CA057699. We thank participants for their time.
Footnotes
The first author was at the University of Chicago, Department of Psychiatry when this project was conducted.
Conflict of Interest Disclosures: All authors have completed and submitted the LWW Copyright Transfer and Disclosure Form and no Conflicts of Interest were reported.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dr. Alicia K. Matthews, College of Nursing, University of Illinois at Chicago, Chicago, Illinois.
Dr. Silvia Tejeda, Institute for Health Research and Policy, University of Illinois at Chicago, Chicago, Illinois.
Dr. Timothy P. Johnson, Institute for Health Research and Policy, University of Illinois at Chicago, Chicago, Illinois; Survey Research Laboratory, University of Illinois at Chicago, Chicago, Illinois.
Dr. Michael L. Berbaum, Institute for Health Research and Policy, University of Illinois at Chicago, Chicago, Illinois.
Dr. Clara Manfredi, Institute for Health Research and Policy, University of Illinois at Chicago, Chicago, Illinois.
References
- 1.American Cancer Society. Cancer Facts & Figures 2008. Atlanta: 2008. [Google Scholar]
- 2.Janz N, Mujahid M, Hawley S, et al. Racial/ethnic differences in quality of life after diagnosis of breast cancer. Journal of Cancer Survivorship. 2009;3(4):212–222. doi: 10.1007/s11764-009-0097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powe BD, Hamilton J, Hancock N, et al. Quality of life of African American cancer survivors. Cancer. 2007;109(S2):435–445. doi: 10.1002/cncr.22358. [DOI] [PubMed] [Google Scholar]
- 4.Garofalo J, Hamann H, Ashworth K, et al. Stress and quality of life in African American cancer survivors. Ethn Dis. 2006;16(3):732–738. [PubMed] [Google Scholar]
- 5.Jemal A, Siegel R, Xu J, et al. Cancer Statistics 2010. CA Cancer J Clin. 2010 Septemper;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 6.Smith-Bindman R, Miglioretti DL, Lurie N, et al. Does Utilization of Screening Mammography Explain Racial and Ethnic Differences in Breast Cancer? Annals of Internal Medicine. 2006;144(8):541–W589. doi: 10.7326/0003-4819-144-8-200604180-00004. [DOI] [PubMed] [Google Scholar]
- 7.Braithwaite D, Tammemagi CM, Moore DH, et al. Hypertension is an independent predictor of survival disparity between African-American and white breast cancer patients. International Journal of Cancer. 2009;124(5):1213–1219. doi: 10.1002/ijc.24054. [DOI] [PubMed] [Google Scholar]
- 8.Manfredi C, Kaiser K, Matthews AK, et al. Are Racial Differences in Patient–Physician Cancer Communication and Information Explained by Background, Predisposing, and Enabling Factors? Journal of Health Communication: International Perspectives. 2010;15(3):272–292. doi: 10.1080/10810731003686598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aziz NM, Rowland JH. Cancer survivorship research among ethnic minority and medically underserved groups. Oncology nursing forum. 2002 Jun;29(5):789–801. doi: 10.1188/02.ONF.789-801. [DOI] [PubMed] [Google Scholar]
- 10.Giedzinska A, Meyerowitz B, Ganz P, et al. Health-related quality of life in a multiethnic sample of breast cancer survivors. Annals of Behavioral Medicine. 2004 Aug;28(1):39–51. doi: 10.1207/s15324796abm2801_6. [DOI] [PubMed] [Google Scholar]
- 11.Meyerowitz BE, Richardson J, Hudson S, et al. Ethnicity and cancer outcomes: behavioral and psychosocial considerations. Psychological bulletin. 1998 Jan;123(1):47–70. doi: 10.1037/0033-2909.123.1.47. [DOI] [PubMed] [Google Scholar]
- 12.Soler-Vila H, Kasl SV, Jones BA. Prognostic significance of psychosocial factors in African-American and white breast cancer patients: a population-based study. Cancer. 2003 Sep 15;98(6):1299–1308. doi: 10.1002/cncr.11670. [DOI] [PubMed] [Google Scholar]
- 13.Ferrell BR, Dow KH, Grant M. Measurement of the quality of life in cancer survivors. Quality of Life Research. 1995 Dec;4(6):523–531. doi: 10.1007/BF00634747. [DOI] [PubMed] [Google Scholar]
- 14.Ashing-Giwa K, Ganz PA, Petersen L. Quality of life of African-American and white long term breast carcinoma survivors. Cancer. 1999 Jan 15;85(2):418–426. doi: 10.1002/(sici)1097-0142(19990115)85:2<418::aid-cncr20>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 15.Rodrigue JR. An examination of race differences in patients' psychological adjustment to cancer. Journal of Clinical Psychology in Medical Settings. 1997;4(3):271–280. [Google Scholar]
- 16.Paskett ED, Alfano CM, Davidson MA, et al. Breast cancer survivors' health-related quality of life: racial differences and comparisons with noncancer controls. Cancer. 2008 Dec 1;113(11):3222–3230. doi: 10.1002/cncr.23891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowen DJ, Alfano CM, McGregor BA, et al. Possible socioeconomic and ethnic disparities in quality of life in a cohort of breast cancer survivors. Breast Cancer Res Treat. 2007 Nov;106(1):85–95. doi: 10.1007/s10549-006-9479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bourjolly JN, Kerson TS, Nuamah IF. A comparison of social functioning among black and white women with breast cancer. Social Work in Health Care. 1999;28(3):1–20. doi: 10.1300/J010v28n03_01. [DOI] [PubMed] [Google Scholar]
- 19.Halbert CH, Coyne J, Weathers B, et al. Racial Differences in Quality of Life Following Prostate Cancer Diagnosis. Urology. 2010;76(3):559–564. doi: 10.1016/j.urology.2009.09.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eton DT, Lepore SJ, Helgeson VS. Early quality of life in patients with localized prostate carcinoma: an examination of treatment-related, demographic, and psychosocial factors. Cancer. 2001 Sep 15;92(6):1451–1459. doi: 10.1002/1097-0142(20010915)92:6<1451::aid-cncr1469>3.0.co;2-r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lubeck DP, Kim H, Grossfeld G, et al. Health related quality of life differences between black and white men with prostate cancer: data from the cancer of the prostate strategic urologic research endeavor. J Urol. 2001 Dec;166(6):2281–2285. [PubMed] [Google Scholar]
- 22.Jayadevappa R, Johnson JC, Chhatre S, et al. Ethnic variation in return to baseline values of patient-reported outcomes in older prostate cancer patients. Cancer. 2007;109(11):2229–2238. doi: 10.1002/cncr.22675. [DOI] [PubMed] [Google Scholar]
- 23.Parker PA, Baile WF, de Moor C, et al. Psychosocial and demographic predictors of quality of life in a large sample of cancer patients. Psychooncology. 2003 Mar;12(2):183–193. doi: 10.1002/pon.635. [DOI] [PubMed] [Google Scholar]
- 24.Dean C. Psychiatric morbidity following mastectomy: Preoperative predictors and type of illness. Journal of Psychosomatic Research. 1988;31:385–392. doi: 10.1016/0022-3999(87)90059-6. [DOI] [PubMed] [Google Scholar]
- 25.Costanzo ES, Lutgendorf SK, Mattes ML, et al. Adjusting to life after treatment: distress and quality of life following treatment for breast cancer. British Journal of Cancer. 2007;97(12):1625–1631. doi: 10.1038/sj.bjc.6604091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Northouse LL, Caffey M, Deichelbohrer L, et al. The quality of life of African American women with breast cancer. Research in Nursing & Health. 1999 Dec;22(6):449–460. doi: 10.1002/1098-240x(199912)22:6<449::aid-nur3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 27.Okamura H, Watanabe T, Narabayashi M, et al. Psychological distress following first recurrence of disease in patients with breast cancer: prevalence and risk factors. Breast Cancer Res Treat. 2000 May;61(2):131–137. doi: 10.1023/a:1006491417791. [DOI] [PubMed] [Google Scholar]
- 28.Kornblith AB, Anderson J, Cella DF, et al. Hodgkin disease survivors at increased risk for problems in psychosocial adaptation. The Cancer and Leukemia Group B. Cancer. 1992 Oct 15;70(8):2214–2224. doi: 10.1002/1097-0142(19921015)70:8<2214::aid-cncr2820700833>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 29.Mehnert A, Koch U. Psychological comorbidity and health-related quality of life and its association with awareness, utilization, and need for psychosocial support in a cancer register-based sample of long-term breast cancer survivors. J Psychosom Res. 2008 Apr;64(4):383–391. doi: 10.1016/j.jpsychores.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Peled R, Carmil D, Siboni-Samocha O, et al. Breast cancer, psychological distress and life events among young women. BMC Cancer. 2008;8:245. doi: 10.1186/1471-2407-8-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edmondson D, Park CL, Blank TO, et al. Deconstructing spiritual well-being: existential well-being and HRQOL in cancer survivors. Psycho-Oncology. 2008;17(2):161–169. doi: 10.1002/pon.1197. [DOI] [PubMed] [Google Scholar]
- 32.Hewitt M, Herdman R, Holland J. Meeting psychosocial needs of women with breast cancer. Washington, DC: Institute of Medicine and National Research Council of the National Academies, National Academy of Sciences; 2004. [PubMed] [Google Scholar]
- 33.Bultz BD, Carlson LE. Emotional distress: the sixth vital sign in cancer care. Journal of Clinical Oncology. 2005 Sep 10;23(26):6440–6441. doi: 10.1200/JCO.2005.02.3259. [DOI] [PubMed] [Google Scholar]
- 34.Ell K, Sanchez K, Vourlekis B, et al. Depression, correlates of depression, and receipt of depression care among low-income women with breast or gynecologic cancer. Journal of Clinical Oncology. 2005 May 1;23(13):3052–3060. doi: 10.1200/JCO.2005.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Badger TA, Braden CJ, Mishel MH, et al. Depression burden, psychological adjustment, and quality of life in women with breast cancer: patterns over time. Res Nurs Health. 2004 Feb;27(1):19–28. doi: 10.1002/nur.20002. [DOI] [PubMed] [Google Scholar]
- 36.Ashing-Giwa KT. The contextual model of HRQoL: a paradigm for expanding the HRQoL framework. Qual Life Res. 2005 Mar;14(2):297–307. doi: 10.1007/s11136-004-0729-7. [DOI] [PubMed] [Google Scholar]
- 37.Ashing-Giwa KT, Lim JW. Examining the impact of socioeconomic status and socioecologic stress on physical and mental health quality of life among breast cancer survivors. Oncol Nurs Forum. 2009 Jan;36(1):79–88. doi: 10.1188/09.ONF.79-88. [DOI] [PubMed] [Google Scholar]
- 38.Ware JE, Kosinski M, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. Lincoln: Quality Metric Incorporated; 2000. [Google Scholar]
- 39.Zimet GD, Dahlem NW, Zimet SG, et al. The Multidimensional Scale of Perceived Social Support. Journal of Personality Assessment. 1988;52:30–41. [Google Scholar]
- 40.Peterman AH, Fitchett G, Brady MJ, et al. Measuring spiritual well-being in people with cancer: the functional assessment of chronic illness therapy--Spiritual Well-being Scale (FACIT-Sp) Annals of Behavioral Medicine. 2002 Winter;24(1):49–58. doi: 10.1207/S15324796ABM2401_06. [DOI] [PubMed] [Google Scholar]
- 41.Baider L, Perez T, De-Nour AK. Gender and adjustment to chronic disease. A study of couples with colon cancer. Gen Hosp Psychiatry. 1989 Jan;11(1):1–8. doi: 10.1016/0163-8343(89)90018-2. [DOI] [PubMed] [Google Scholar]
- 42.Portenoy RK, Thaler HT, Kornblith AB, et al. Symptom Prevalence, Characteristics and Distress in a Cancer Population. Quality of Life Research. 1994;3(3):183–189. doi: 10.1007/BF00435383. [DOI] [PubMed] [Google Scholar]
- 43.Apolone G, Filiberti A, Cifani S, et al. Evaluation of the EORTC QLQ-C30 questionnaire: A comparison with SF-36 Health Survey in a cohort of Italian long-survival cancer patients. Annals of Oncology. 1998 May 1;9(5):549–557. doi: 10.1023/a:1008264412398. [DOI] [PubMed] [Google Scholar]
- 44.Lazarus R, Folkman S. Stress Appraisal and Coping. New York: Springer; 1984. [Google Scholar]
- 45.Oberst MT, Hughes SH, Chang AS, et al. Self-care burden, stress appraisal, and mood among persons receiving radiotherapy. Cancer nursing. 1991 Apr;14(2):71–78. [PubMed] [Google Scholar]
- 46.Taylor SE, Lichtman RR, Wood JV. Attributions, beliefs about control, and adjustment to breast cancer. J Pers Soc Psychol. 1984 Mar;46(3):489–502. doi: 10.1037//0022-3514.46.3.489. [DOI] [PubMed] [Google Scholar]
- 47.Jenkins RA, Pargament KI. Religion and spirituality as resources for coping with cancer. Journal of Psychosocial Oncology. 1995;13(1-2):51–74. [Google Scholar]
- 48.Brady MJ, Peterman AH, Fitchett G, et al. A case for including spirituality in quality of life measurement in oncology. Psychooncology. 1999 Sep-Oct;8(5):417–428. doi: 10.1002/(sici)1099-1611(199909/10)8:5<417::aid-pon398>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 49.Daugherty CK, Fitchett G, Murphy PE, et al. Trusting God and medicine: spirituality in advanced cancer patients volunteering for clinical trials of experimental agents. Psychooncology. 2005 Feb;14(2):135–146. doi: 10.1002/pon.829. [DOI] [PubMed] [Google Scholar]
- 50.Flint MS, Baum A, Chambers WH, et al. Induction of DNA damage, alteration of DNA repair and transcriptional activation by stress hormones. Psychoneuroendocrinology. 2007;32(5):470–479. doi: 10.1016/j.psyneuen.2007.02.013. [DOI] [PubMed] [Google Scholar]