Abstract
Background
By supplementing an index composed of HIV biomarkers and age (Restricted Index) with measures of organ injury, the Veterans Aging Cohort Study (VACS) Index more completely reflects risk of mortality. We compare the accuracy of the VACS and Restricted Indices 1) among subjects outside the Veterans Healthcare System (VA), 2) over 1–5 years of prior exposure to antiretroviral therapy (ART), and 3) within important patient subgroups.
Methods
We used data from 13 cohorts in the North American AIDS Cohort Collaboration (NA-ACCORD, n=10, 835) limiting analyses to HIV-infected subjects with at least 12 months exposure to ART. Variables included demographic, laboratory (CD4 count, HIV-1 RNA, hemoglobin, platelets, aspartate and alanine transaminase, creatinine and hepatitis C status), and survival. We used C statistic and net reclassification improvement (NRI) to test discrimination varying prior ART exposure from 1–5 years. We then combined VA (n=5,066) and NA-ACCORD data, fit a parametric survival model, and compared predicted to observed mortality by cohort, gender, age, race, and HIV-1 RNA level.
Results
Mean follow-up was 3.3 years (655 deaths). Compared with the Restricted Index, the VACS Index showed greater discrimination (C statistic: 0.77 vs. 0.74; NRI 12%; p<0.0001). NRI was highest among those with HIV-1 RNA<500 copies/ml (25%) and age ≥50 years (20%). Predictions were similar to observed mortality among all subgroups.
Conclusion
VACS Index scores discriminate risk and translate into accurate mortality estimates over 1–5 years of exposures to ART and for diverse patient subgroups from North American
Keywords: HIV, Aging, Prognosis
With the advent of effective antiretroviral therapy (ART), the spectrum of disease experienced by those with HIV infection has changed. Viral suppression is common1 and incident AIDS defining events are rare.2 Yet, those with HIV infection continue to experience excess mortality 3;4 which is incompletely described by age, CD4 count, and HIV-1 RNA alone.5
Despite ART, chronic HIV infection appears to exacerbate generic pathophysiologic processes associated with aging which increase physiologic vulnerability relative to demographically similar uninfected individuals.6–8 Consistent with current treatment guidelines9, HIV providers routinely monitor general indicators of organ system injury including hemoglobin, platelets, aspartate and alanine transaminase (AST and ALT), creatinine, and viral hepatitis C infection (HCV) but have no index with which to integrate these data into an overall estimate of disease burden or mortality risk. Such a comprehensive measure would be useful as a means of more effectively motivating behavior change in the clinical setting10, improved risk stratification in the analysis of observational data11 and more effective randomized trials12. For example, indices such as the Framingham Risk Index has enhanced research and care in cardiovascular disease13 and several geriatric risk indices are enhancing research and care for those aging without HIV infection.14
While the cumulative evidence supporting the accuracy and generalizability of the VACS Index exceeds that for any prior HIV risk index, the VACS Index builds upon important prior research.15–22 Most prior indices emphasized AIDS defining conditions, CD4 cell count, and HIV-1 RNA. Some recognized the importance of age and anemia16;20. However much has changed since these indices were developed and validated. Specifically, the increasing role of multi-organ system injury (reflected by FIB 4, eGFR, and hemoglobin) and of hepatitis C infection (HCV), and the decreasing role of AIDS Defining Illnesses, CD4 count, and HIV-1 RNA. By including FIB-4, HCV, eGFR, hemoglobin and age, and placing less weighting upon CD4 count and HIV-1 RNA, the VACS Index better reflects more of the major common pathways of physiologic injury among those on antiretroviral therapy. As a result, the Veterans Aging Cohort Study Index (VACS Index) discriminates risk of mortality more effectively than an index restricted to CD4 count, HIV-1 RNA and age (Restricted Index).23 24
Importantly, the discrimination of the VACS Index rivals that of indices in clinical use including the Framingham Index13 and those recommended for use among geriatric patients.14 Nevertheless, prognostic indices developed in one sample (those within the Veterans Affairs Healthcare System (VA)) may not generalize to a new sample or important subgroups.25 Further, indices effective at one particular point in clinical care (ART initiation) may not generalize beyond treatment initation.25 We use data from the North American AIDS Cohort Collaboration (NA-ACCORD) to test the generalizability of the VACS Index outside the VA and at differing intervals of exposure to ART. We then combine data from NA-ACCORD and VA to translate index scores to an estimated absolute risk of mortality and compare predicted to observed mortality by cohort and subgroups defined by sex, age, race, and HIV-1 RNA titer.
METHODS
Study Population
NA-ACCORD has been described in detail.26–28 It is a multi-site collaboration of interval and clinical cohort studies in the United States and Canada, and represents the North American region of the International epidemiologic Databases to Evaluate AIDS (IeDEA). VACS has also been described in detail.29 Although VACS is a participating cohort within NA-ACCORD, we separated VACS patients from the NA-ACCORD for this analysis to demonstrate the generalizability of our findings outside the Veterans Healthcare Administration.
We used data from 13 NA-ACCORD cohorts that routinely collect and contribute the laboratory data required for construction of the VACS Index. All included cohorts monitor deaths at local sites and regionally using death registries. United States cohorts also check for deaths using national registries (Social Security Administration or National Death Index). Among these cohorts, eligible subjects were HIV-infected individuals on ART for at least 1 year from 2000–2007 (n=15,938). Of these, 10,835 (68%) had complete data after 12 months of ART (90 days before to 180 days after) and constituted our sample with full data (complete cases). Using the same eligibility criterion, 5,066 VACS subjects were available for analyses. Both VACS and NA-ACCORD studies are approved by affiliated institutional review boards.
VACS and Restricted Index Scores
The development and internal validity of the VACS Index has been described (Table 1).30 It includes age and routinely monitored laboratory tests: CD4 count, HIV-1 RNA, hemoglobin, platelets, AST, ALT, creatinine, and HCV status. Composite markers of liver and renal injury (FIB-4 and eGFR) are computed. FIB-4, composed of AST, ALT, platelets, and age, has been validated as an indicator of liver fibrosis [FIB-4=(years of age × AST)/(platelets in 100/L × sqrt of ALT.31 eGFR composed of serum creatinine age, gender, and race, was included as a validated indicator of impaired renal function [eGFR=186.3 × (creatinine)-1.154 × (age)-0.203 × (0.742 for women) × (1.21 if Black)].32 HCV status was defined as positive if the patient ever had a positive antibody test or detectable virus prior to the anchoring point of our analysis (12 months of ART). Points are added to calculate score. The Restricted Index was developed solely for the purposes of comparing the accuracy and generalizability of an index restricted to CD4 count, HIV-1 RNA, and age with that of a more completely specified index. All predictors are categorized according to previously established cut points.5;31–34
Table 1.
Components of the Restricted and VACS Indices, showing point values assigned and distribution of selected characteristics in 10,835 HIV positive patients (NA-ACCORD subjects only).
| Points Assigned | Distribution | ||||
|---|---|---|---|---|---|
| Component | Level | Restricted Index |
VACS Index |
N | % |
| Age (years) | <50 | 0 | 0 | 8428 | (78) |
| 50 to 64 | 23 | 12 | 2245 | (20) | |
| ≥ 65 | 44 | 27 | 162 | (2) | |
| CD4 (cells/mm3) | ≥ 500 | 0 | 0 | 3789 | (35) |
| 350 to 499 | 10 | 6 | 2443 | (23) | |
| 200 to 349 | 10 | 6 | 2660 | (25) | |
| 100 to 199 | 19 | 10 | 1293 | (12) | |
| 50 to 99 | 40 | 28 | 346 | (3) | |
| < 50 | 46 | 29 | 304 | (3) | |
|
HIV-1 RNA (copies/ml) |
< 500 | 0 | 0 | 8324 | (77) |
| 500 to 1×105 | 11 | 7 | 2161 | (20) | |
| ≥ 1×105 | 25 | 14 | 332 | (3) | |
| Hemoglobin (g/dL) | ≥ 14 | 0 | 5897 | (54) | |
| 12 to 13.9 | 10 | 3720 | (34) | ||
| 10 to 11.9 | 22 | 1062 | (10) | ||
| < 10 | 38 | 156 | (1) | ||
| FIB-4 | < 1.45 | 0 | 8103 | (75) | |
| 1.45 to 3.25 | 6 | 2213 | (20) | ||
| > 3.25 | 25 | 519 | (5) | ||
| eGFR (mL/min) | ≥ 60 | 0 | 10149 | (94) | |
| 45 to 59.9 | 6 | 430 | (4) | ||
| 30 to 44.9 | 8 | 141 | (1) | ||
| < 30 | 26 | 115 | (1) | ||
|
Hepatitis C Co- Infection |
5 | 2605 | (24) | ||
| Sex | Male | 7853 | (72) | ||
| Race | White | 4723 | (44) | ||
| Black | 3557 | (33) | |||
| Hispanic | 1734 | (16) | |||
| Other | 821 | (7) | |||
| HIV Transmission by IDU | 1721 | (16) | |||
|
Year of ART initiation, median (IQR) |
2000 | (1998–2003) | |||
| Person Years of Observation | 35598 | ||||
| Deaths | |||||
| Total | 655 | NA | |||
| In First 5 Years | 571 | NA | |||
Statistical Analysis
Statistical analyses were conducted by S Modur, JP Tate, and S Gange. Using the point system described above, VACS and Restricted Index scores were assigned to each subject at 1, 2, 3, 4, and 5 years of ART exposure. We evaluated the discrimination of the indices at these anchoring points. Observation time ended at death or was censored at the date of last follow-up, December 30, 2007 (administrative censoring) or five years from each anchoring point, whichever came first. For Year 1, labs were obtained −90 to +180 days of the anchor date; and +/− 180 days for years 2 to 5. These 5 anchors were then used to assess and compare discrimination of the indices using Cox proportional hazards models and Harrell’s C-statistics (C statistic) among NA-ACCORD subjects only. We also measured C statistics among NA-ACCORD subjects after stratifying by sex (men, women), age (<50 years, ≥50 years), race (White, Black, and Hispanic), and HIV-1 RNA (<500 copies/ml, ≥500 copies/ml). Proportion of NA-ACCORD subjects reclassified by VACS Index compared with Restricted Index was calculated using the method by Cook, et al. (Appendix).35;36
Of 15,938 eligible NA-ACCORD subjects, 32% were missing at least one required laboratory value and were excluded from the complete case analyses. Those with complete data differed from those with missing data on gender, race, injection drug use (IDU), hemoglobin, platelets, AST, ALT, and FIB-4. We applied multiple imputation methods to the entire NA-ACCORD sample; results were similar compared to the complete case analyses (Appendix).
To translate scores to predicted mortality with maximal precision, we combined NA-ACCORD and VA subjects and fit a parametric (Gamma) regression model predicting all cause mortality using VACS Index score as the only predictor. This model provided an equation for calculating predicted mortality over 1–5 years for each value of the VACS Index score (Figure 1). Five year mortality predictions were compared graphically with observed mortality among NA-ACCORD and VA subjects separately and among designated subgroups. For each five-point interval of score (collapsed if necessary to maintain at least 5 deaths and 10 survivors in each interval), a Kaplan-Meier (KM) mortality estimate and 95% confidence interval were calculated.
Figure 1.
Observed (Kaplan-Meier estimates) five-year mortality according to index score in 10,835 HIV infected patients after one year of antiretroviral therapy. The I bars denote 95% confidence intervals. Bubble size is proportional to the number of subjects at each data point. (NA-ACCORD Subjects Only)
RESULTS
Characteristics of the Population
The NA-ACCORD sample (n=10,835) included 2,982 women, 2,407 people ≥50 years, and 3,557 Black individuals. After one year of ART, 77% of the entire sample had HIV-1 RNA < 500 copies/ml, 34% had hemoglobin values between 12–13.9 g/dL, 10% had hemoglobin values between 10–11.9 g/dL, 25% had FIB 4 consistent with fibrosis (>3.25), 6% had stage III renal insufficiency (eGFR <60mL/min), and 24% had HCV co-infection (Table 1). The overall mortality was 1.6 per 100 person years. Median scores were 16 with a 1%-99% range of 0–80 for the VACS Index and 10 with a range of 0–71 for the Restricted Index.
Prognostic Accuracy in NA-ACCORD
Among NA-ACCORD subjects overall, the VACS Index was more discriminating of all cause mortality than the Restricted Index (Table 2, C statistic: 0.77 vs. 0.74) and among men and women; Whites, Blacks and Hispanics; those < and ≥50 years of age; and those with HIV-1 RNA< and ≥ 500 copies/mL (p<0.0001 in all cases). Discrimination of the Restricted Index declined with increased prior ART exposure (C statistics: 0.74 at 2-year anchor; 0.72 at 5-year anchor), whereas discrimination of the VACS Index remained strong (C statistics: 0.79 at 2 years; 0.81 at 5 years). When compared with the Restricted Index, the VACS Index resulted in the reclassification of 53% of patients: 22% to a higher risk group and 31% to a lower risk group. The net gain in reclassification proportions at 5 years were 9% for survivors and 3% for those who died for a Net Reclassification Improvement (NRI) of 12% (p<0.0001). NRI was 25% among those with undetectable HIV-1 RNA and 20% among those 50 years and over. When 5-year Kaplan Meier observed mortality estimates were graphed, the VACS Index demonstrated a finer gradation of risk for more patients and a wider range of observed mortality compared with the Restricted Index (Figure 1a–b).
Table 2.
Discrimination of the VACS and Restricted Indices for 5-year, all-cause mortality (NA-ACCORD subjects only)
| Restricted Index | VACS Index | p-value | ||
|---|---|---|---|---|
| Time on ART | 1 Year | 0.74 | 0.77 | <0.0001 |
| 2 Years | 0.74 | 0.79 | <0.0001 | |
| 3 Years | 0.72 | 0.77 | <0.0001 | |
| 4 Years | 0.72 | 0.79 | <0.0001 | |
| 5 Years | 0.72 | 0.81 | <0.0001 | |
| Sex | Male | 0.76 | 0.77 | <0.001 |
| Female | 0.72 | 0.76 | <0.001 | |
| Race | White | 0.76 | 0.79 | <0.001 |
| Black | 0.71 | 0.74 | <0.001 | |
| Hispanic | 0.70 | 0.77 | <0.001 | |
| Age, years | <50 | 0.75 | 0.78 | <0.001 |
| >= 50 | 0.63 | 0.70 | <0.0001 | |
| HIV-1 RNA, | <500 | 0.67 | 0.74 | <0.0001 |
| copies/ml | >=500 | 0.7 | 0.71 | <0.0001 |
When components of the VACS Index were evaluated separately and in combination in NA-ACCORD and VACS cohorts (Table 3) we found that age alone offered modest risk discrimination (C stat: 0.56, 0.59 respectively). HIV biomarkers were more discriminating in NA-ACCORD than VACS data (c stat: 0.72, 0.67). Organ system biomarkers were less discriminating in NA-ACCORD than VACS data (c stat: 0.70, 0.75). When age, HIV biomarkers and organ system biomarkers were combined, discrimination was improved in both cohorts (c stat: 0.78 for both).
Table 3.
Incremental Discrimination of VACS Index Components in NA-ACCORD and VACS Cohorts (C statistic and 95% CI).
| Components of VACS Index | NA-ACCORD | VACS | ||
|---|---|---|---|---|
| Age | 0.56 | (0.54,0.58) | 0.59 | (0.57,0.61) |
| HIV Biomarkers (CD4, HIV1 RNA) | 0.72 | (0.70,0.75) | 0.67 | (0.64,0.69) |
| Organ System Biomarkers (Hgb, FIB4, eGFR, HCV) | 0.70 | (0.68,0.72) | 0.75 | (0.73,0.76) |
| Age + HIV Biomarkers (Restricted Index) | 0.74 | (0.72,0.77) | 0.72 | (0.70,0.74) |
| Age + Organ System Biomarkers | 0.71 | (0.69,0.74) | 0.75 | (0.73,0.77) |
| Complete VACS Index | 0.78 | (0.75,0.79) | 0.78 | (0.77,0.80) |
Calibration of Model Predictions Using Combined NA-ACCORD and VA Data
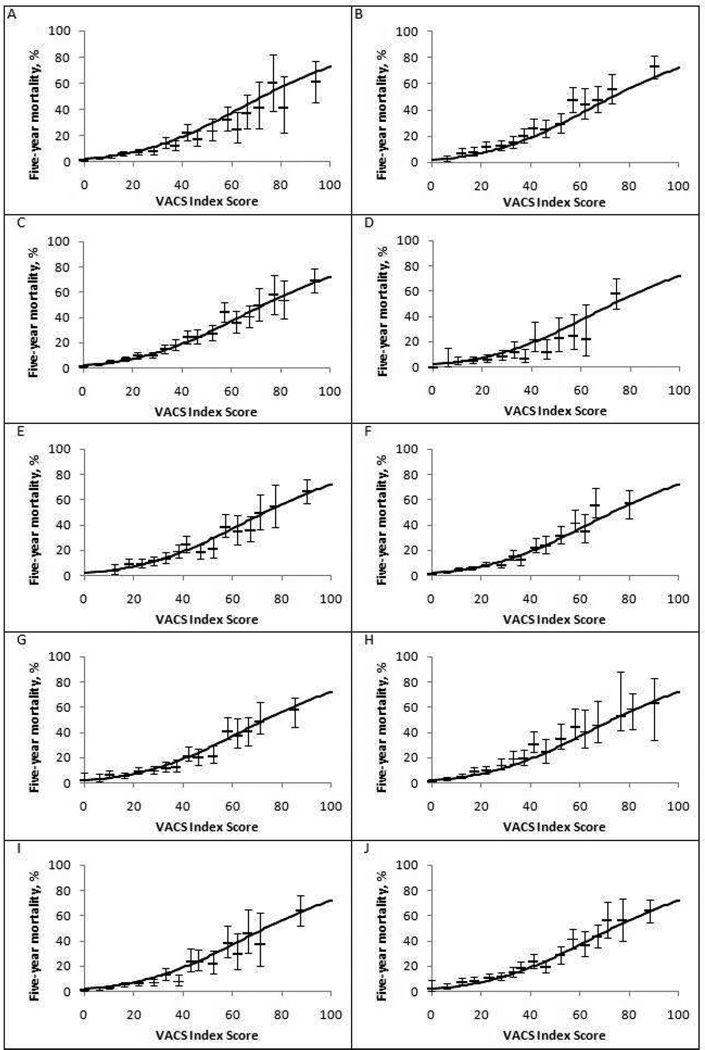
When a parametric survival model was calculated (Figure 2), predicted mortality was similar to observed mortality at 5 years for both NA-ACCORD and VA subjects (Figure 3 a and b) and when stratified by important subgroups (Figure 3c–j).
Figure 2.
Predicted mortality by VACS Index score based on 15,901 HIV infected patients with one year of antiretroviral therapy (ART). (NA-ACCORD and VACS Subjects)
Figure 3.
Kaplan-Meier estimates of five-year mortality according to VACS Index score after one year of antiretroviral therapy, by subgroup. (NA-ACCORD and VACS Subjects)
A. NA-ACCORD (N = 10835), B. VACS (N=5066) C. Men (N = 12785), D. Women (N = 3116), E. Age < 50 years (N = 11191), F. Age >50 years (N = 4710), G. Black (N= 5878), H. White (N = 6079), I. HIV RNA <500 copies/ml (N=8715), J. HIV RNA > 500 copies/ml (N= 7186). The I bars denote the 95% confidence intervals. Solid line is fitted curve for the overall study sample (N = 15901).
DISCUSSION
The VACS Index provided a more discriminating prediction of all cause mortality among HIV-infected subjects from North America on ART than the Restricted Index. This was true overall, with increasing exposure to ART, and among important subgroups, most notably among persons with low HIV-1 RNA and those ≥50 years of age—two rapidly growing populations in treatment. Based on established criteria,13;25 the VACS Index has demonstrated excellent generalizability and is likely to accurately predict mortality among HIV-infected patients on ART in North America. Importantly, after demonstrating that this translation is accurate in demographically and clinically diverse subgroups, we provide a table and nomogram (Table 1 and Figure 2) and a website (http://vacs.med.yale.edu) to facilitate calculating VACS Index scores and translating them to predicted mortality rates. Potential applications for the VACS Index include patient management and clinical research.
C statistics are a commonly employed metric for evaluating the discrimination of prognostic indices.37 In uncensored data, the C statistic is the likelihood that, if any two subjects were drawn from the sample, the subject with the higher score would die before the subject with the lower score. Although these categories are somewhat arbitrary, C statistics between 0.50–0.59 are considered poor; 0.60–0.69, fair; 0.70–0.79, good; 0.80–0.89 very good; and above 0.89, excellent.14 While Restricted Index C statistics ranged from 0.63–0.76 (“fair” to “good”), VACS Index C statistics ranged from 0.70 to 0.81 (“good” to “very good”). Discrimination was particularly better among those with undetectable HIV-1 RNA (C statistics: 0.67 vs. 0.74) and those over 50 years of age (C statistics: 0.63 vs. 0.70). C statistics for the VACS Index for all cause mortality meet or surpass those reported for prognostic indices used in clinical practice including the Framingham Index for predicting Cardiovascular Disease and validated indices predicting all cause mortality among aging uninfected individuals.13;38;39
A newer metric, developed and popularized by the methodologists working on the Framingham Risk score, is the net reclassification improvement (NRI)35;36. This is calculated by separating those who died and those who lived and asking in each group whether the VACS Index resulted in a change in risk classification compared with the Restricted Index. Among those who died, a higher risk classification is considered an improvement and a lower risk classification is considered an error. Among those who lived, a lower risk classification is considered an improvement and a higher classification is an error. The NRI is the sum of the differences. The net gain in reclassification proportions at 5 years was 9% for survivors and 3% for those who died for an overall statistic of 12% (p<0.0001). Further, the NRI was even higher among those with undetectable HIV-1 RNA (25%) and those 50 years and over (20%). These NRIs suggest a highly clinically significant improvement in discrimination36;40 and are greater than improvements seen by the addition of D-dimer to the VACS Index.24
For maximal clinical and research utility, providers and investigators need a means of translating VACS Index scores to mortality risk. We combined NA-ACCORD and VACS data to provide as precise a translation as possible. We then considered the accuracy of this translation by cohort and among important subgroups. Because such translations depend upon the overall mortality rate in the cohort,13;25;38 we conducted this work among cohorts with uniform access to regional and/or national death registries. In these analyses, the predicted mortality based upon VACS Index score was similar to observed mortality among veteran (VACS) and nonveteran (NA-ACCORD) subjects; and among: men and women; Black and non-Black patients; those <and ≥50 years old; and those with HIV-1 RNA < and ≥ 500 copies/ml.
To understand how the VACS Index reclassifies risk, consider an HIV-infected 45-year-old man who, after 12 months of ART, has a CD4 count of 500 cells/mm3 and an undetectable HIV-1 RNA but is HCV co-infected with a FIB-4 >3.25. He, like one in four NA-ACCORD subjects, was assigned 0 points by the Restricted Index with a 2% predicted 5-year mortality. Using the VACS Index, he was assigned 5 points for HCV co-infection and 25 points for his FIB-4 value for a score of 30 and an predicted 5-year mortality of 12%. Fifty-three percent of NA-ACCORD subjects assigned a score of 0 by the Restricted Index were assigned a higher score by the VACS Index.
Having an accurate, generalizable, responsive, and feasible method for estimating individual risk can improve effectiveness and efficiency of chronic disease management in major ways41–43. First, it can inform decision making when an intervention puts the patient at some immediate risk for longer term gain44;45. This is true whenever patients are asked to undergo a risk of immediate harm from treatment in the hope of averting long term disease incidence or progression-- commonly the case in cancer screening and primary and secondary prophylaxis for cardiovascular disease and stroke. It is also true when considering aggressive treatment protocols (toxic chemotherapy, organ transplant, or major surgery) for cancer or heart disease. Second, it can motivate patients to modify health behaviors such as adherence to medication, smoking, diet, exercise, and alcohol use by quantifying the impact these changes have on risk and by charting progress after modifying risk46–50. Third, it can identify patients in need of intensive management either with respect to site of care (outpatient, inpatient, intensive care unit, skilled nursing facility, nursing home) or care management (case management, frequency of follow up).
Of note, none of these applications require that index identify all modifiable sources of risk, only that it accurately, generalizably, responsively, and feasibly estimate risk of mortality—including risk associated with the modifiable factors of interest.25;39 Because many sources of modifiable risk have a similar common pathway to physiologic injury, it is not efficient or feasible for a single index to include all modifiable sources of risk. Instead, separate analyses can map changes in risk score associated with changes in modifiable factors of interest. We are currently undertaking a series of analyses demonstrating this for adherence to ART, alcohol use, smoking, and substance use, but these are beyond the scope of this paper.
The VACS Index also predicts cardiovascular mortality51, hospitalization and medical intensive care unit admission52 and is correlated with functional performance53 and fragility fractures54. It offers an improved means of balancing patient enrollment by severity of illness in randomized trials and of controlling for disease severity in observational analyses, and it may eventually prove a useful intermediate outcome for interventional and observational research. Because the discrimination of the VACS Index for mortality is maintained over extended prior exposure to ART it also offers a means of charting a patient’s progress over time.
Although the utility of the VACS Index for clinical management can only be established through a randomized trial comparing management with and without the Index, evidence to date suggests that it offers useful insight. We have previously shown that hemoglobin, FIB-4, and eGFR, as well as CD4 count and HIV-1 RNA, change substantially in response to ART initiation, not always in the same direction,33 and that the VACS Index is more responsive to ART initiation and differing levels of ART adherence than the Restricted Index.33 Third, we have shown that the VACS Index is more correlated with biomarkers of inflammation (IL-6), microbial translocation (D-dimer), and hyper coagulability (sCD14) than the Restricted Index.5 Taken together, these data suggest that the VACS Index provides a more comprehensive means of tracking disease burden, including the effects of chronic inflammation, over time.
Further, to facilitate use of the VACS Index in the clinical setting, we have developed a web site calculator accessible via smart phone or computer with an automatic conversion of the VACS Index score to a risk estimate (HTTP://VACS.MED.YALE.EDU). The site includes regularly updated links to supporting evidence for the index. As we develop information regarding how behavior change alters risk we will include this information as an additional link for the calculator. We also provide SAS programming for any who wish to include the calculation as part of their electronic medical record system or for analyses of grouped clinical data (WWW.VACOHORT.ORG).
Our analysis has substantial strengths. We demonstrated the generalizability of the VACS Index in a large, independent sample on ART, over differing periods of ART exposure and among important subgroups of patients.25 By combining NA-ACCORD and VA samples we were powered to precisely translate VACS Index scores to predicted mortality and to consider whether predicted mortality matched observed deaths overall and among important subgroups. Further, the VACS Index is based on laboratory tests currently ordered in the course of routine management and therefore offers enhanced clinical insight requiring only that providers calculate and interpret the score. We have simplified this process by providing a web site and smart phone calculator (http://vacs.med.yale.edu). Eventually the Index could be calculated by the clinical laboratory (or the electronic medical record) every time component tests are ordered, as is current practice for eGFR.
A limitation of any large observational study is missing data. While subjects with missing values in NA-ACCORD tended to have more liver disease and less anemia, the imputed analyses yielded results similar to complete cases (Appendix), suggesting that missing data did not compromise our findings. Further, the VACS Index may be improved in the future. Our choice of risk factors was based on prior work,16;20;21;23;55 a desire to base the index on consistently measured metrics such as clinical laboratory tests, and the need to validate findings. As the population with HIV ages, higher age thresholds will likely become relevant. Of note, we have evaluated whether: BMI, lipid profiles, smoking status, hypertension;56 inflammatory biomarkers (D-dimer, IL-6, and soluble CD14)24, and functional status57 improve the discrimination of the VACS Index. While many of these predict mortality in unadjusted analyses, VACS Index scores co vary with these factors. When added to the VACS Index, only D-dimer and/or sCD14 resulted in risk reclassification that exceeded 1%. Additional factors (such as D-dimer) may be added to the VACS Index in the future if they can be consistently measured and they improve discrimination sufficiently to justify added cost and complexity.13;36
In summary, we have demonstrated that a novel index composed of routine clinical data can predict mortality among HIV-infected individuals on ART with good to very good discrimination and consistent calibration across important subgroups. Measures of general organ system function included in the VACS Index substantially enhance discrimination. While it would be a reasonable precaution to verify the calibration of the VACS Index among younger patients and subjects outside North America, predicted mortality from the VACS Index is likely generalizable to HIV-infected individuals over 30 years of age in care in North America. Among these individuals, the VACS index is ready for clinical and research application.
Supplementary Material
Acknowledgements
We would like to thank all individuals involved with the NA-ACCORD collaboration, including staff, investigators, and patients, for their valuable contributions to this work.
Funding Statement: This work was financially supported by National Institutes of Health: NIAAA (U10-AA13566), NHLBI (R01-HL095136; R01-HL090342; RCI-HL100347), and NIA (R01-AG029154; K23 AG024896). Ms. Janet Tate was supported by the Training Program in Environmental Epidemiology funded under grant no.T32 ES07069. This work was supported by grants from the National Institutes of Health: U01-AI069918, U01-AA013566, UO1-AI-35042, 5-MO1-RR-00052 (GCRC), UO1-AI-35043, UO1-AI-35039, UO1-AI-35040, UO1-AI-35041, U01-AI38855: ALLRT; U01-AI38858; U01-AI68634; U01-AI68636; AI-69432; AI-69434, UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590, UO1-HD-32632, UL1-RR024131, P30-AI27757; K23-AI-61-0320, P30-AI27767, P30-AI50410; RR025747, P30-AI54999, R01-DA04334; R01-DA12568, R01-MH54907, R24-AI067039, N02-CP55504; Z01-CP010176, AHQ290-01-0012, K01-AI071754, K24-00432; R01-DA11602, K01-AI071725, R01 AG026250: Kelly Gebo. This work was also supported by the Centers for Disease Control (CDC200-2006-18797), the Canadian Institutes for Health Research (CIHR: TGF-96118; HCP-97105; CBR-86906; CBR-94036; KRS-86251; 169621) and the Canadian Trials Network (project number 242)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: No conflicts of interest.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention (CDC) or those of the Veterans Affairs.
Reference List
- 1.McKinnell JA, Willig JH, Westfall AO, Nevin C, Allison JJ, Raper JL, Mugavero MJ, Saag MS. Antiretroviral prescribing patterns in treatment-naive patients in the United States. AIDS Patient Care STDS. 2010;24:79–85. doi: 10.1089/apc.2009.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D'Arminio MA, Sabin CA, Phillips A, Sterne J, May M, Justice A, Dabis F, Grabar S, Ledergerber B, Gill J, Reiss P, Egger M. The changing incidence of AIDS events in patients receiving highly active antiretroviral therapy. Arch Intern Med. 2005;165:416–423. doi: 10.1001/archinte.165.4.416. [DOI] [PubMed] [Google Scholar]
- 3.Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372:293–299. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Losina E, Schackman BR, Sadownik SN, Gebo KA, Walensky RP, Chiosi JJ, Weinstein MC, Hicks PL, Aaronson WH, Moore RD, Paltiel AD, Freedberg KA. Racial and sex disparities in life expectancy losses among HIV-infected persons in the united states: impact of risk behavior, late initiation, and early discontinuation of antiretroviral therapy. Clin Infect Dis. 2009;49:1570–1578. doi: 10.1086/644772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Justice AC, Freiberg MS, Tracy R, Tate J, Goetz M, Butt AA, Rodriguez-Barradas M, Gibert C, Oursler KA, Bryant KJ the VACS Project Team. Does an Index Composed of Clinical Data Reflect Effects of Inflammation, Coagulation, and Mnoocyte Activation on Mortality Among those Aging with HIV? Clinical Infectious Diseases. 2012 doi: 10.1093/cid/cir989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Justice AC. HIV and aging: time for a new paradigm. Curr HIV /AIDS Rep. 2010;7:69–76. doi: 10.1007/s11904-010-0041-9. [DOI] [PubMed] [Google Scholar]
- 7.Deeks SG. Immune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapy. Top HIV Med. 2009;17:118–123. [PubMed] [Google Scholar]
- 8.Phillips AN, Neaton J, Lundgren JD. The role of HIV in serious diseases other than AIDS. AIDS. 2008;22:2409–2418. doi: 10.1097/QAD.0b013e3283174636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Department of Health and Human Services. :1–166. 1-10-2011. 6-16-2011. [Google Scholar]
- 10.DiMatteo MR, Haskard KB, Williams SL. Health beliefs, disease severity, and patient adherence: a meta-analysis. Med Care. 2007;45:521–528. doi: 10.1097/MLR.0b013e318032937e. [DOI] [PubMed] [Google Scholar]
- 11.Feinstein AR. Clinical biostatistics, XIV: the purposes of prognostic stratification. Clin Parmacol Therapy. 1972;13:285–297. doi: 10.1002/cpt1972132285. [DOI] [PubMed] [Google Scholar]
- 12.Feinstein AR, Landis RJ. The role of prognostic stratification in preventing the bias permitted by random allocation of treatment. J Chron Dis. 1979;29:277–284. doi: 10.1016/0021-9681(76)90080-1. [DOI] [PubMed] [Google Scholar]
- 13.Vasan RS. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation. 2006;113:2335–2362. doi: 10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]
- 14.Yourman LC, Lee SJ, Schonberg MA, Widera EW, Smith AK. Prognostic indices for older adults: a systematic review. JAMA. 2012;307:182–192. doi: 10.1001/jama.2011.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turner BJ, Kelly JV, Ball JK. A severity classification system for AIDS hospitalizations. Med Care. 1989;27:423–437. doi: 10.1097/00005650-198904000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Justice AC, Feinstein AR, Wells CK. A new prognostic staging system for the acquired immunodeficiency syndrome. N Engl J Med. 1989;320:1388–1393. doi: 10.1056/NEJM198905253202106. [DOI] [PubMed] [Google Scholar]
- 17.Egger M, May M, Chene G, Phillips AN, Ledergerber B, Dabis F, Costagliola D, D'Arminio MA, de Wolf F, Reiss P, Lundgren JD, Justice AC, Staszewski S, Leport C, Hogg RS, Sabin CA, Gill MJ, Salzberger B, Sterne JA. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 18.May M, Royston P, Egger M, Justice AC, Sterne JA. Development and validation of a prognostic model for survival time data: application to prognosis of HIV positive patients treated with antiretroviral therapy. Stat Med. 2004;23:2375–2398. doi: 10.1002/sim.1825. [DOI] [PubMed] [Google Scholar]
- 19.Mocroft AJ, Johnson MA, Sabin CA, Lipman M, Elford J, Emery V, Morcinek J, Youle M, Janossy G, Lee CA, Phillips AN. Staging system for clinical AIDS patients. Lancet. 1995;346:12–17. doi: 10.1016/s0140-6736(95)92649-6. [DOI] [PubMed] [Google Scholar]
- 20.Mocroft A, Kirk O, Barton SE, Dietrich M, Proenca R, Colebunders R, Pradier C, dArminio Monteforte A, Ledergerber B, Lundgren JD. Anaemia is an independent predictive marker for clinical prognosis in HIV-infected patients from across Europe. EuroSIDA study group. AIDS. 1999;13:943–950. doi: 10.1097/00002030-199905280-00010. [DOI] [PubMed] [Google Scholar]
- 21.Harris RJ, Sterne JA, Abgrall S, Dabis F, Reiss P, Saag M, Phillips AN, Chene G, Gill JM, Justice AC, Rockstroh J, Sabin CA, Mocroft A, Bucher HC, Hogg RS, Monforte AD, May M, Egger M. Prognostic importance of anaemia in HIV type-1-infected patients starting antiretroviral therapy: collaborative analysis of prospective cohort studies. Antivir Ther. 2008;13:959–967. [PMC free article] [PubMed] [Google Scholar]
- 22.Lundgren JD, Mocroft A, Gatell JM, Ledergerber B, D'Arminio MA, Hermans P, Goebel FD, Blaxhult A, Kirk O, Phillips AN. A clinically prognostic scoring system for patients receiving highly active antiretroviral therapy: results from the EuroSIDA study. J Infect Dis. 2002;185:178–187. doi: 10.1086/338267. [DOI] [PubMed] [Google Scholar]
- 23.Justice AC, McGinnis KA, Skanderson M, Chang CC, Gibert CL, Goetz MB, Rimland D, Rodriguez-Barradas MC, Oursler KK, Brown ST, Braithwaite RS, May M, Covinsky KE, Roberts MS, Fultz SL, Bryant KJ. Towards a combined prognostic index for survival in HIV infection: the role of 'non-HIV' biomarkers. HIV Med. 2009 doi: 10.1111/j.1468-1293.2009.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Justice AC, Freiberg MS, Tracy R, Kuller L, Tate JP, Goetz MB, Fiellin DA, Vanasse GJ, Butt AA, Rodriguez-Barradas MC, Gibert C, Oursler KA, Deeks SG, Bryant K. Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV? Clin Infect Dis. 2012;54:984–994. doi: 10.1093/cid/cir989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Justice AC, Covinsky KE, Berlin JA. Assessing the generalizability of prognostic information. Ann Intern Med. 1999;130:515–524. doi: 10.7326/0003-4819-130-6-199903160-00016. [DOI] [PubMed] [Google Scholar]
- 26.Gange SJ, Kitahata MM, Saag MS, Bangsberg DR, Bosch RJ, Brooks JT, Calzavara L, Deeks SG, Eron JJ, Gebo KA, John GM, Haas DW, Hogg RS, Horberg MA, Jacobson LP, Justice AC, Kirk GD, Klein MB, Martin JN, McKaig RG, Rodriguez B, Rourke SB, Sterling TR, Freeman AM, Moore RD. Cohort Profile: The North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) Int J Epidemiol. 2007;.:294–301. doi: 10.1093/ije/dyl286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lau B, Gange SJ, Moore RD. Interval and clinical cohort studies: epidemiological issues. AIDS Res Hum Retroviruses. 2007;23:769–776. doi: 10.1089/aid.2006.0171. [DOI] [PubMed] [Google Scholar]
- 28.Lau B, Gange SJ, Moore RD. Risk of non-AIDS-related mortality may exceed risk of AIDS-related mortality among individuals enrolling into care with CD4+ counts greater than 200 cells/mm3. J Acquir Immune Defic Syndr. 2007;44:179–187. doi: 10.1097/01.qai.0000247229.68246.c5. [DOI] [PubMed] [Google Scholar]
- 29.Fultz SL, Skanderson M, Mole LA, Gandhi N, Bryant K, Crystal S, Justice AC. Development and verification of a "virtual" cohort using the National VA Health Information System. Med Care. 2006;44:S25–S30. doi: 10.1097/01.mlr.0000223670.00890.74. [DOI] [PubMed] [Google Scholar]
- 30.Tate JP, Justice AC, Hughes MD, Bonnet F, Reiss P, Mocroft A, Nattermann J, Lampe FC, Bucher HC, Sterling TR, Crane HM, Kitahata MM, May M Sterne JAC. The VACS Index: An internationally generalizable risk index for mortality after one year of antiretroviral therapy. AIDS. 2012 doi: 10.1097/QAD.0b013e32835b8c7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, Sulkowski S, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 32.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 33.Tate JP, Justice AC for the VACS Project Team. Change in a prognostic index for survival in HIV infection after one year on cART by level of adherence. 48th Annual Meeting of the Infectious Disease Society of America.2010. [Google Scholar]
- 34.Tate JP, Hughes MD, Justice AC for the VACS Project Team. Do risk factors for mortality change with time on antiretroviral therapy?. 48th Annual Meeting of the Infectious Disease Society of America.2010. [Google Scholar]
- 35.Cook NR, Ridker PM. Advances in measuring the effect of individual predictors of cardiovascular risk: the role of reclassification measures. Ann Intern Med. 2009;150:795–802. doi: 10.7326/0003-4819-150-11-200906020-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cook NR. Assessing the Incremental Role of Novel and Emerging Risk Factors. Curr Cardiovasc Risk Rep. 2010;4:112–119. doi: 10.1007/s12170-010-0084-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;20(115):928–935. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]
- 38.D'Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–187. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 39.Yourman LC, Lee SJ, Schonberg MA, Widera EW, Smith AK. Prognostic indices for older adults: a systematic review. JAMA. 2012;307:182–192. doi: 10.1001/jama.2011.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cook NR. Comments on 'Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond' by M. J. Pencina et al., Statistics in Medicine (DOI: 10.1002/sim.2929). Stat Med. 2008;27:191–195. doi: 10.1002/sim.2987. [DOI] [PubMed] [Google Scholar]
- 41.Kent DM, Rothwell PM, Ioannidis JP, Altman DG, Hayward RA. Assessing and reporting heterogeneity in treatment effects in clinical trials: a proposal. Trials. 2010;11:85. doi: 10.1186/1745-6215-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hayward RA, Kent DM, Vijan S, Hofer TP. Multivariable risk prediction can greatly enhance the statistical power of clinical trial subgroup analysis. BMC Med Res Methodol. 2006;6:18. doi: 10.1186/1471-2288-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eddy DM, Adler J, Patterson B, Lucas D, Smith KA, Morris M. Individualized guidelines: the potential for increasing quality and reducing costs. Ann Intern Med. 2011;154:627–634. doi: 10.7326/0003-4819-154-9-201105030-00008. [DOI] [PubMed] [Google Scholar]
- 44.Braithwaite RS. Can life expectancy and QALYs be improved by a framework for deciding whether to apply clinical guidelines to patients with severe comorbid disease? Med Decis Making. 2011;31:582–595. doi: 10.1177/0272989X10386117. [DOI] [PubMed] [Google Scholar]
- 45.Braithwaite RS, Concato J, Chang CC, Roberts MS, Justice AC. A framework for tailoring clinical guidelines to comorbidity at the point of care. Arch Intern Med. 2007;167:2361–2365. doi: 10.1001/archinte.167.21.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chan CW, Witherspoon JM. Health risk appraisal modifies cigarette smoking behavior among college students. J Gen Intern Med. 1988;3:555–559. doi: 10.1007/BF02596099. [DOI] [PubMed] [Google Scholar]
- 47.Dapp U, Anders JA, von Renteln-Kruse W, Minder CE, Meier-Baumgartner HP, Swift CG, Gillmann G, Egger M, Beck JC, Stuck AE. A randomized trial of effects of health risk appraisal combined with group sessions or home visits on preventive behaviors in older adults. J Gerontol A Biol Sci Med Sci. 2011;66:591–598. doi: 10.1093/gerona/glr021. [DOI] [PubMed] [Google Scholar]
- 48.Kreuter MW, Strecher VJ. Do tailored behavior change messages enhance the effectiveness of health risk appraisal? Results from a randomized trial. Health Educ Res. 1996;11:97–105. doi: 10.1093/her/11.1.97. [DOI] [PubMed] [Google Scholar]
- 49.Maron DJ, Forbes BL, Groves JR, Dietrich MS, Sells P, DiGenio AG. Health-risk appraisal with or without disease management for worksite cardiovascular risk reduction. J Cardiovasc Nurs. 2008;23:513–518. doi: 10.1097/01.JCN.0000338933.81587.b4. [DOI] [PubMed] [Google Scholar]
- 50.Stuck AE, Kharicha K, Dapp U, Anders J, von Renteln-Kruse W, Meier-Baumgartner HP, Iliffe S, Harari D, Bachmann MD, Egger M, Gillmann G, Beck JC, Swift CG. The PRO-AGE study: an international randomised controlled study of health risk appraisal for older persons based in general practice. BMC Med Res Methodol. 2007;7:2. doi: 10.1186/1471-2288-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Justice AC, Tate JP, Freiberg MS, Rodriguez-Barradas MC, Tracy R. Reply to Chow et al. Clin Infect Dis. 2012 [Google Scholar]
- 52.Akgun KM, Pisani MA, Fried TR, Butt AA, Gibert CL, McGinnis K, Huang L, Rimland D, Justice AC, Rodriguez-Barradas MC, Crothers K. Risk Factors for Medical Intensive Care Unit Admission in HIV Infected Veterans. American Throacic Society. 2010 [Google Scholar]
- 53.Erlandson KM, Allshouse AA, Jankowski C. Prospective comparison of three functional assessments with the Veteran's Aging Cohort Study index in virologically suppressed HIV-infected adults. 2nd International Workshop on HIV and Aging. abstract #8. 10-27-0011. [Google Scholar]
- 54.Womack JA, Goulet JL, Gibert C, Brandt C, Chang CC, Gulanski B, Fraenkel L, Mattocks K, Rimland D, Rodriguez-Barradas MC, Tate J, Yin MT, Justice AC. Increased risk of fragility fractures among HIV infected compared to uninfected male veterans. PLoS One. 2011;6:e17217. doi: 10.1371/journal.pone.0017217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.May MT, Sterne JA, Costagliola D, Sabin CA, Phillips AN, Justice AC, Dabis F, Gill J, Lundgren J, Hogg RS, de WF, Fatkenheuer G, Staszewski S, D'Arminio MA, Egger M. HIV treatment response and prognosis in Europe and North America in the first decade of highly active antiretroviral therapy: a collaborative analysis. Lancet. 2006;368:451–458. doi: 10.1016/S0140-6736(06)69152-6. [DOI] [PubMed] [Google Scholar]
- 56.Tate J, Freiberg M, Justice AC. Do Risk Factors for Cardiovascular Disease Improve VACS Index Prediction of All Cause Mortality? International Cohorts Meeting. 2012 [Google Scholar]
- 57.Oursler K, Tate J, Gill T, Crothers K, Brown T, Crystal S, Womack J, Leaf D, Sorkin J, Justice A Veterans Aging Cohort Study Project Team. Comorbidity is Predictive of Muscle Strength in HIV-infected Veterans: Results from the VACS Index. 19th Conference on Retroviruses and Opportunistic Infections; 3-8-0012.2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.