Abstract
Previous studies have demonstrated that local application of hypertonic KCl or NaCl to the cerebral cortex induces tolerance to a subsequent episode of ischemia. The objective of the present study was to determine whether application of these salts increases the levels of mRNAs encoding inhibitors of inflammation. Hypertonic KCl or NaCl was applied for 2 hours to the frontal cortex of Sprague-Dawley rats. After recovery periods up to 24 hours, levels of selected mRNAs were measured in samples from frontal and parietal cortex using Northern blots. Application of hypertonic KCl caused a rapid and widespread increase in the levels of mRNA coding for tumor necrosis factor (TNF), tristetraprolin (TTP), suppressor of cytokine signaling-3 (SOCS3), and brain-derived neurotrophic factor (BDNF), and a 24-hour delayed induction of ciliary neurotrophic factor (CNTF) mRNA. Application of hypertonic NaCl caused alterations in mRNA levels that were restricted to the frontal cortex. In this region, application of NaCl rapidly increased levels of mRNA encoding TNF, TTP, and SOCS3, but not BDNF, and caused a delayed induction of CNTF mRNA. These results suggest that upregulation of inhibitors of inflammation may contribute to the induction of tolerance to ischemia following preconditioning with hypertonic salt solutions.
Keywords: Inflammation, Hypertonic salt solution, Ischemic tolerance, Preconditioning, Cortical spreading depression, SOCS3, Tristetraprolin, TNF
1. Introduction
Preconditioning the brain with a variety of sublethal stimuli induces profound tolerance to a subsequent episode of ischemia (Dirnagl et al. 2003; Kirino 2002). One of the preconditioning stimuli that has been employed is cortical spreading depression (CSD)(Kawahara et al. 1995; Kobayashi et al. 1995; Matsushima et al. 1996). In experimental models of preconditioning, CSD is commonly evoked by applying a high concentration of KCl to the cerebral cortex for a period of 1-2 hours. Application of KCl not only triggers multiple episodes of CSD, but also produces a small cortical lesion at the application site (Kobayashi et al. 1995). Thus, the induction of tolerance to ischemia following application of KCl may be a consequence of CSD, the cortical lesion, or both. Recently, cortical application of hypertonic NaCl, like KCl, was shown to cause a small cortical lesion and induce tolerance to ischemia (Muramatsu et al. 2004). Importantly, application of NaCl, unlike KCl, failed to evoke CSD. Thus, the presence of a cortical lesion by itself appears to be sufficient to induce tolerance to ischemia. The molecular mechanisms by which application of hypertonic salt solutions trigger neuroprotective pathways, however, remain poorly understood.
Application of KCl to the cerebral cortex has previously been shown to increase the expression of proinflammatory cytokines, including tumor necrosis factor (TNF) and interleukin-1ß (IL-1ß) (Jander et al. 2001). Expression of these cytokines has been linked to ischemic tolerance in other models of cerebral preconditioning (Tasaki et al. 1997; Wang et al. 2000). Indeed, direct administration of TNF or IL-1ß has been shown to induce tolerance to ischemia (Nawashiro et al. 1997; Ohtsuki et al. 1996). These results suggest that proinflammatory cytokines trigger neuroprotective mechanisms in experimental models of preconditioning. Proinflammatory cytokine-signaling normally activates counter-regulatory mechanisms that limit the degree, duration, and spatial dissemination of inflammation. The counter-regulatory mechanisms include upregulation of anti-inflammatory cytokines, decoy receptors, and intracellular feedback inhibitors (Kariko et al. 2004). Recent studies have identified a number of intracellular feedback inhibitors that suppress the inflammatory response to harmful stimuli (Table 1). The presence of these inhibitors following a preconditioning stimulus would be expected to attenuate inflammation during a subsequent episode of ischemia and, thus, diminish the extent of ischemic injury. However, the induction of inhibitors of inflammation has not been previously investigated in models of cerebral preconditioning. Thus, the primary objective of the present study was to determine whether preconditioning with hypertonic salts triggered expression of selected inhibitors of inflammation. A secondary objective was to compare the induction of the inhibitors after preconditioning with KCl and NaCl to determine whether CSD is required for their induction. A final objective was to compare the effects of KCl and NaCl on levels of mRNA encoding ciliary neurotrophic factor (CNTF), which has recently been associated with the induction of inhibitors of inflammation (Kelly et al. 2004).
Table 1.
Intracellular Feedback Inhibitors of Inflammation
| Inhibitor | Function | Reference |
|---|---|---|
| Tristetraprolin (TTP) | Promotes degradation of transcripts encoding proinflammatory cytokines |
(Carballo et al. 1998) |
| Suppressor of Cytokine Signaling-3 (SOCS3) |
Blocks activation of Janus kinases/signal transducers and activators of transcription (JAK/STAT) |
(Cacalano et al. 2001) |
| IL-1 Receptor- associated Kinase M (IRAK-M) |
Inhibits function of IL-1 receptor-associated kinase (IRAK), inhibits IL-1 and TLR signal transduction |
(Kobayashi et al. 2002) |
| Toll-interacting Protein (TOLLIP) |
Binds to and sequesters IRAK, inhibits IL-1 and TLR signal transduction |
(Zhang and Ghosh 2002) |
2. Results
2.1. Physiologic Variables
Physiologic variables were in the normal range prior to application of KCl or NaCl (Table 2). In animals undergoing application of KCl, the numbers of episodes of CSD detected were 20 ± 3 (mean ± SD), 16 ± 2, 16 ± 6, and 18 ± 4 for the 0 hour, 2 hour, 4 hour, and 24 hour groups, respectively. CSD was not detected in animals undergoing application of NaCl.
Table 2.
Physiologic Variables
| Salt/ Recovery Time |
Body Weight (g) |
Arterial pH |
Arterial pCO2 (mm Hg) |
Arterial pO2 (mm Hg) |
MABP (mm Hg) |
Rectal Temp (°C) |
|---|---|---|---|---|---|---|
| KCl | ||||||
| 0 hours | 262 ± 14 | 7.39 ± 0.03 | 42 ± 5 | 130 ± 35 | 90 ± 11 | 37.3 ± 0.3 |
| 2 hours | 344 ± 43 | 7.42 ± 0.03 | 41 ± 4 | 138 ± 34 | 63 ± 2 | 37.4 ± 0.6 |
| 4 hours | 343 ± 71 | 7.45 ± 0.12 | 43 ± 9 | 118 ± 29 | 82 ± 8 | 37.7 ± 0.2 |
| 24 hours | 270 ± 28 | 7.40 ± 0.02 | 38 ± 5 | 147 ± 30 | 86 ± 1 | 37.2 ± 0.3 |
| NaCl | ||||||
| 0 hours | 201 ± 78 | 7.41 ± 0.05 | 39 ± 7 | 114 ± 41 | 80 ± 8 | 37.4 ± 0.0 |
| 2 hours | 302 ± 36 | 7.45 ± 0.03 | 36 ± 2 | 130 ± 30 | 88 ± 14 | 37.5 ± 0.4 |
| 4 hours | 341 ± 53 | 7.45 ± 0.05 | 39 ± 2 | 104 ± 45 | 78 ± 7 | 37.5 ± 0.2 |
| 24 hours | 313 ± 27 | 7.45 ± 0.03 | 36 ± 2 | 139 ± 45 | 70 ± 6 | 37.2 ± 0.1 |
Values are means ± SD, n=4 animals per group.
2.2. Application of KCl: Northern Blots
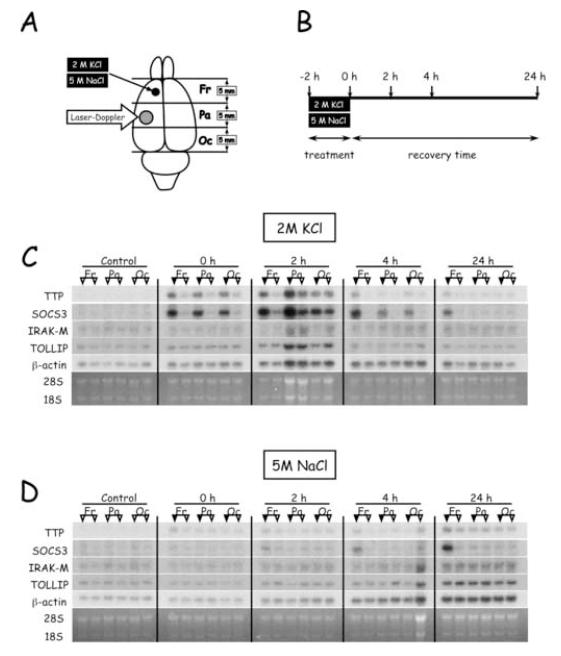
Application of 2 M KCl to the frontal cortex for 2 hours caused a rapid and widespread increase in cortical levels of mRNAs encoding TTP and SOCS3 (Fig. 1). The induction of TTP and SOCS3 mRNA levels was most pronounced in the frontal cortex, which included the KCl application site. However, the levels of these transcripts were also increased in the parietal and occipital cortex, most prominently at 0 and 2 hours of recovery (Fig. 1C). By contrast, mRNAs encoding IRAK-M and TOLLIP were not induced in any region of the ipsilateral cortex at the times tested.
Figure 1.
Effect of hypertonic salts on regional levels of mRNAs encoding inhibitors of inflammation. (A) Location of salt application, laser-Doppler flowmetry, and brain sectioning into frontal (Fr), parietal (Pa), and occipital (Oc) samples for RNA extraction. (B) Timecourse of experimental protocol and animal sacrifice for tissue sampling. (C) Northern blots of representative animals sacrificed at various times after KCl application. For each animal, samples from the hemisphere ipsilateral to the application site are indicated by the filled triangles; samples from the contralateral hemisphere are indicated by the open triangles. Control is an unoperated animal. (D) Northern blots of representative animals sacrificed at various times after application of NaCl.
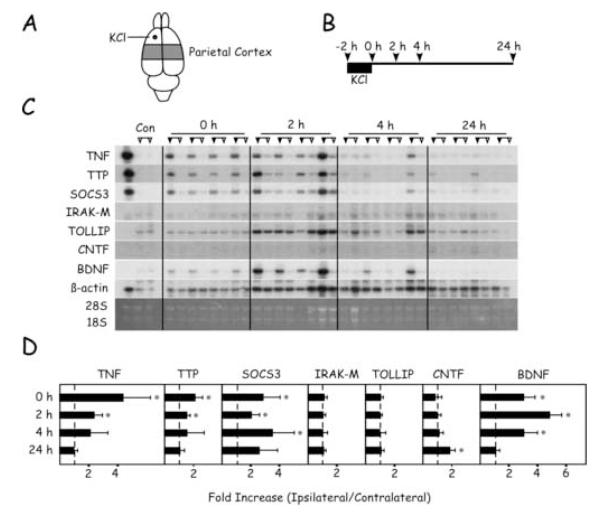
Samples from frontal and parietal cortex were analyzed quantitatively for levels of mRNA encoding inflammation-related proteins and neurotrophic factors (Figs. 2 and 3). Application of KCl triggered a rapid increase in the levels of TNF, TTP, SOCS3, and BDNF mRNAs in both regions of the ipsilateral hemisphere. In the frontal cortex, ipsilateral levels of TTP and SOCS3 mRNAs were significantly higher than those in the contralateral hemisphere at all times tested, while TNF and BDNF mRNAs remained elevated for 4 hours (Fig. 2). IRAK-M and TOLLIP mRNA levels were similar in each hemisphere. Interestingly, levels of CNTF mRNA were not different between hemispheres until 24 hours recovery. In the parietal cortex, the changes in mRNA levels were similar to those described for the frontal cortex, but were attenuated in duration (Fig. 3). Thus, by 24 hours recovery there were no significant differences in transcipt levels between hemispheres in the parietal cortex, with one exception. At 24 hours, CNTF mRNA was detectable in the ipsilateral but not in the contralateral hemispheres.
Figure 2.
Timecourse of changes in mRNA levels in the frontal cortex after application of KCl. (A) Region of frontal cortex sampled and location of KCl application. (B) Timecourse of experimental protocol and animal sacrifice for tissue sampling. (C) Northern blots of samples from each of the four animals sacrificed at the different time points. For each animal, samples from the hemisphere ipsilateral to the application site are indicated by the filled triangles; samples from the contralateral hemisphere are indicated by the open triangles. The control lanes are samples from two unoperated control animals. In the far left-hand lane, a positive control sample was generated from activated splenocytes. (D) Quantitation of Northern blots. For each transcript, the ratio of ipsilateral/contralateral level was calculated in each animal. Values are expressed in means ± SD. Asterisks denote significant differences between hemispheres, p < 0.05.
Figure 3.
Timecourse of changes in mRNA levels in the parietal cortex after application of KCl. (A) Region of parietal cortex sampled and location of KCl application. (B) Timecourse of experimental protocol and animal sacrifice for tissue sampling. (C) Northern blots of samples from each of the four animals sacrificed at the different time points. For each animal, samples from the hemisphere ipsilateral to the application site are indicated by the filled triangles; samples from the contralateral hemisphere are indicated by the open triangles. The control lanes are samples from an unoperated control animal. In the far left-hand lane, a positive control sample was generated from activated splenocytes. (D) Quantitation of Northern blots. For each transcript, the ratio of ipsilateral/contralateral level was calculated in each animal. Values are expressed in means ± SD. Asterisks denote significant differences between hemispheres, p < 0.05.
2.3 Application of NaCl: Northern Blots
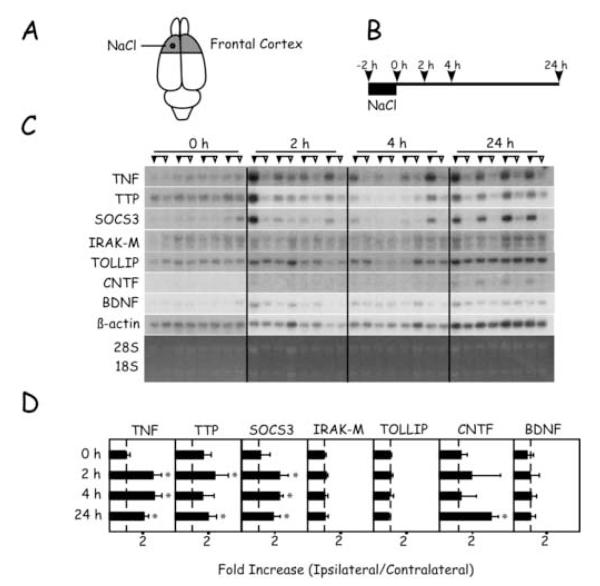
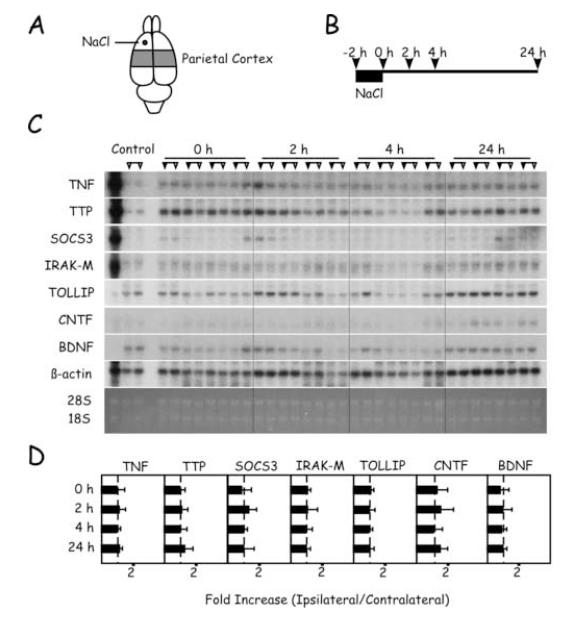
Application of 5 M NaCl to the frontal cortex for 2 hours caused alterations in mRNA levels that were restricted to the frontal cortex (Figs. 1D and 4). Thus, by 2 hours recovery, levels of TNF, TTP, and SOCS3 mRNAs were significantly increased in the ipsilateral hemisphere. These changes persisted for 24 hours, with the exception of TTP mRNA at 4 hours. Similar to KCl application, CNTF mRNA level was increased in a delayed fashion after application of NaCl. Thus, at 24 hours, CNTF mRNA was detectable in the ipsilateral but not in the contralateral hemispheres. No changes were detected in levels of IRAK-M or TOLLIP mRNAs. Interestingly, application of NaCl, unlike KCl, failed to alter levels of BDNF mRNA. In the parietal cortex, no significant changes in mRNA levels between the hemispheres were detectable (Fig. 5).
Figure 4.
Timecourse of changes in mRNA levels in the frontal cortex after application of NaCl. (A) Region of frontal cortex sampled and location of NaCl application. (B) Timecourse of experimental protocol and animal sacrifice for tissue sampling. (C) Northern blots of samples from each of the four animals sacrificed at the different time points. For each animal, samples from the hemisphere ipsilateral to the application site are indicated by the filled triangles; samples from the contralateral hemisphere are indicated by the open triangles. (D) Quantitation of Northern blots. For each transcript, the ratio of ipsilateral/contralateral level was calculated in each animal. Values are expressed in means ± SD. Asterisks denote significant differences between hemispheres, p < 0.05.
Figure 5.
Timecourse of changes in mRNA levels in the parietal cortex after application of NaCl. (A) Region of parietal cortex sampled and location of NaCl application. (B) Timecourse of experimental protocol and animal sacrifice for tissue sampling. (C) Northern blots of samples from each of the four animals sacrificed at the different time points. For each animal, samples from the hemisphere ipsilateral to the application site are indicated by the filled triangles; samples from the contralateral hemisphere are indicated by the open triangles. The control lanes are samples from an unoperated control animal. In the far left-hand lane, a positive control sample was generated from activated splenocytes. (D) Quantitation of Northern blots. For each transcript, the ratio of ipsilateral/contralateral level was calculated in each animal. Values are expressed in means ± SD.
3. Discussion
The present results are the first to show that cerebral preconditioning is associated with upregulation of transcripts encoding inhibitors of inflammation. Importantly, these results suggest that suppression of inflammation is one mechanism which may contribute to the induction of tolerance to ischemia following preconditioning. Cerebral ischemia triggers a robust inflammatory response that is believed to exacerbate ischemic brain damage (Barone and Feuerstein 1999; del Zoppo et al. 2000). Suppression of the inflammatory response to ischemia should, therefore, limit the overall extent of tissue injury. The present results demonstrate that among the feedback inhibitors studied, transcripts encoding TTP and SOCS3 were rapidly elevated following preconditioning with hypertonic salts. By contrast, no alterations were detected in the levels of IRAK-M and TOLLIP mRNAs. Thus, TTP and SOCS3 may play major roles in suppressing the inflammatory response to ischemia in models of cerebral preconditioning.
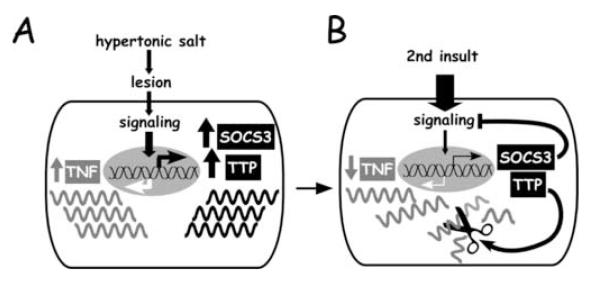
The cellular functions of TTP and SOCS3 have been investigated previously. TTP, also known as TIS11, was first described as one of several immediate early genes induced in rat brain following ischemia (Gubits et al. 1993). TTP is a proline-rich protein that promotes degradation of mRNAs encoding inflammatory mediators such as interleukin (IL)-2 (Ogilvie et al. 2005), IL-3 (Lai and Blackshear 2001), granulocyte-macrophage colony-stimulating factor (Carballo et al. 2000), cyclooxygenase-2 (Sawaoka et al. 2003), and, most importantly, TNF (Carballo et al. 1998) (see Fig. 6). TTP is not detectable in unstressed brain and resting cells, but is rapidly expressed in response to exposure to lipopolysaccharide (LPS) or TNF (Cao et al. 2004; Zhu et al. 2001). The functional importance of TTP was demonstrated in a null mouse mutant, which spontaneously develops a severe inflammatory syndrome, primarily from TNF over-production due to increased stability of TNF mRNA (Taylor et al. 1996). In a canine model of cardiac ischemia-reperfusion, TTP was identified as a potential mediator of protection following ischemic preconditioning (Zubakov et al. 2003). SOCS is a family of regulatory proteins that inhibit signaling through a wide range of cytokine and Toll-like receptors (TLRs), thereby inhibiting inflammation (Alexander and Hilton 2004). Similar to TTP, SOCSs are also products of immediate early genes and function as classical feedback inhibitors (Cacalano et al. 2001). SOCSs primarily suppress signal transduction of cytokines acting through the JAK/STAT (Janus kinases/signal transducers and activators of transcription) pathways. Family member SOCS3, for example, inhibits inflammation by negatively regulating the action of cytokines such as IL-1, IL-6, TNF, and interferon-γ (Kubo et al. 2003) (see Fig. 6). SOCSs are normally expressed at low levels, but are induced rapidly in response to treatment with a wide range of bioactive molecules, including most interleukins and interferons, TNF, erythropoietin, LPS, and other TLR ligands (Baetz et al. 2004; Bode et al. 1999; Starr et al. 1997). Interference with SOCS3 synthesis has been shown to exacerbate ischemic damage in rat brain (Rao et al. 2002), suggesting a neuroprotective role for SOCS3. In summary, SOCS3 and TTP are prime candidates contributing to the induction of tolerance to ischemia following preconditioning with hypertonic salts.
Figure 6.
A model for suppression of inflammation following preconditioning with hypertonic salts. (A) Application of KCl or NaCl cause lesion which leads to the expression of TNF and its feedback inhibitors, SOCS3 and TTP. (B) Functions of feedback inihibors. Once expressed, SOCS3 functions to suppress TLR/cytokine signaling, attenuating the expression of TNF and other proinflammatory cytokines. Simultaneously, TTP functions to hasten the degradation of TNF mRNA, further attenuating the expression of TNF and other proinflammatory cytokines.
Tolerance to cerebral ischemia is induced by application of either KCl or NaCl (Kobayashi et al. 1995; Muramatsu et al. 2004). In the present study, however, these salts produced remarkably distinct effects on mRNA levels. Application of KCl produced robust increases in TNF, TTP, SOCS3, and BDNF, which were widespread and long-lasting. By comparison, application of NaCl caused increases in mRNA levels that were restricted to the frontal cortex. The widely distributed changes following application of KCl are presumably due to CSD, which spreads across most of cortex in the ipsilateral hemisphere. By contrast, the focal changes observed following application of NaCl are consistent with inability of NaCl to evoke CSD (Muramatsu et al. 2004). Thus, the alterations in mRNA levels measured in the parietal cortex after application of KCl are most likely consequences of CSD. Conversely, the alterations measured in the frontal cortex after application of NaCl are likely not due to CSD, but rather to local effects of hypertonic NaCl. Application of hypertonic NaCl (or KCl) is known to produce a small cortical lesion at the application site (Muramatsu et al. 2004). Thus, the developing cortical lesion may be responsible for elevated levels of transcripts encoding TNF, TTP, and SOCS3 in the frontal cortex following application of NaCl and may contribute to those changes observed in the frontal cortex following application of KCl. Interestingly, there were marked increases in BDNF mRNA levels following application of KCl, but not NaCl. Thus, in the experimental model used in the present study, the induction of BDNF is strictly dependent on CSD. Finally, both KCl and NaCl triggered a 24-hour delayed increase in CNTF mRNA in the frontal cortex, but only KCl increased the level of this transcript in the parietal cortex. In summary, the comparison between the effects of KCl and NaCl provide evidence for two important conclusions. First, both salts increased the levels of transcripts encoding TTP and SOCS3, two feedback inhibitors of inflammation and, thus, potential contributors to neuroprotection. Second, the differences in expression following application of the two salts indicate which of the changes can be attributed to CSD and which cannot.
As noted above, application of either KCl or NaCl caused a delayed increase in CNTF mRNA. Previous studies have shown that a mechanical lesion in rat brain increased the expression of CNTF mRNA and protein, which was localized in reactive astrocytes (Ip et al. 1993). Administration of CNTF was reported to be neuroprotective in several experimental models of cerebral ischemia (Hermann et al. 2001; Kumon et al. 1996; Wen et al. 1995). Thus, upregulation of CNTF following preconditioning with hypertonic salts may contribute to the induction of tolerance to ischemia. Importantly, recent studies have demonstrated that administration of CNTF increases the expression of SOCS3 and TTP (Bjorbaek et al. 1999; Kelly et al. 2004). Thus, one of the mechanisms by which CNTF protects the brain against ischemia may depend on CNTF-mediated upregulation of these inhibitors of inflammation. In the present study, increased levels of TTP and SOC3 mRNAs, paradoxically, occurred prior to the increase in CNTF mRNA. Activation of signaling pathways associated with TLRs and cytokine receptors are likely responsible for the rapid induction of TTP and SOCS3 mRNAs following application of KCl and NaCl (Kariko et al. 2004). However, the delayed expression of CNTF may serve to reinforce and prolong the expression of TTP and SOCS3, thus extending the duration of suppressed inflammatory signaling.
Importantly, inhibition of TLR- and cytokine-mediated signaling may be a common mechanism by which other preconditioning stimuli, cytokines and trophic factors exert their neuroprotective effects. Several lines of evidence support this suggestion. First, TLR-ligands (LPS), trophic factors (NGF, bFGF, G-CSF, CNTF, leukemia inhibitory factor, erythropoietin), inflammatory cytokines (TNF, IL-1β, IL-6), and anti-inflammatory cytokines (IL-10, TGF-β) have well documented neuroprotective effects against ischemic injury (Digicaylioglu and Lipton 2001; Gibson et al. 2005; Kumon et al. 1996; Loddick et al. 1998; Nawashiro et al. 1997; Nozaki et al. 1993; Ohtsuki et al. 1996; Pechan et al. 1995; Spera et al. 1998; Suzuki et al. 2005; Tasaki et al. 1997). Second, all of these bioactive molecules have also been shown to induce feedback inhibitors of inflammation, including SOCS3 and/or TTP (Arenander et al. 1989; Auernhammer et al. 1999; Bode et al. 1999; Carballo et al. 1998; Fox et al. 2003; Ito et al. 1999; Kelly et al. 2004; Kreider and Rovera 1992; Nakajima and Wall 1991; Peng et al. 1995; Starr et al. 1997; Terstegen et al. 2000). Third, TLRs and receptors for the cytokines and trophic factors listed above have been demonstrated on cells of CNS (Bsibsi et al. 2002; Keswani et al. 2004; Szelenyi 2001) and, thus, are capable of mediating the induction of feedback inhibitors of inflammation in response to the corresponding stimuli. Thus, suppression of inflammation is a mechanism that may be common to a number of known neuroprotective agents.
In summary, the present results demonstrate that preconditioning with hypertonic salts increases the expression of TTP and SOCS3, two important feedback inhibitors of inflammation. It should be cautioned, however, that the present results represent only a first step in determining the timecourse and cellular location of expression. Nevertheless, the results suggest that endogenous suppression of inflammation may contribute to the induction of tolerance to ischemia following application of hypertonic salts and other preconditioning stimuli.
4. Experimental procedures
4.1 Application of Hypertonic Salt Solutions
Application of hypertonic KCl or NaCl to the cerebral cortex was performed using previously described procedures (Otori et al. 2003). In brief, male Sprague-Dawley rats, weighing 250-400 g, were anesthetized with halothane, intubated, and ventilated with a mixture of 1% halothane/70% nitrous oxide/29% oxygen. The tail artery was cannulated for measurement of arterial pressure and blood gases. Core temperature was regulated at 37.5°C using a rectal thermistor and heating blanket. The head of the animal was placed in a stereotaxic frame, and a 2-mm burr-hole was made over the left frontal cortex (3 mm rostral to bregma, 2 mm lateral to the midline), leaving the dura intact. KCl was applied to the frontal cortex using a 1-mm2 filter paper soaked in 2 M KCl, refreshed every 20 min for 2 hours. NaCl was applied in a similar fashion using filter paper soaked in 5 M NaCl. The occurrence of CSD was monitored using laser Doppler flowmetry through a fenestration made by thinning the bone over the ipsilateral cortex (2 mm caudal to bregma, 4 mm lateral to the midline) (Figure 1A) as described previously (Kariko et al. 1998; Rangel et al. 2001). At the conclusion of the 2 hours of KCl or NaCl application, the filter paper was removed and the burr-hole irrigated with physiologic saline. For rats destined to recover, the laser-Doppler probe, rectal probe, and arterial catheter were removed and the scalp wounds sutured. The animals were then extubated and returned to their cages. Groups of 4 animals were sacrificed with an overdose of halothane at the end of the 2-hour period of KCl application (0 hour of recovery), or at 2, 4, or 24 hours of recovery (Figure 1B).
4.2. Brain sampling and Northern blotting
At the time of sacrifice, the brain was rapidly removed and samples were dissected for Northern blot analysis. The brain was placed in a rodent brain matrix slicer and sectioned in the coronal plane at 0, 5, 10, and 15 mm behind the frontal pole (Fig. 1A). Each of the sections was divided at the midline, and paired samples of neocortex (100-180 mg) from the left and right hemispheres were isolated. Fresh samples were homogenized in the presence of 300-600 μl Trizol (Invitrogen, Carlsbad, CA) using a glass pestle and Eppendorf tube, and total RNA was extracted according to the manufacturer’s instructions. RNA pellets were reconstituted in nuclease-free water, and following three freeze-thaw cycles, the RNA concentration was determined spectrophotometrically. RNA samples were stored in siliconized tubes at −20°C. For Northern analyses, 2 μg RNA was denatured and separated in a 1.4% agarose, 0.22 M formaldehyde gel submerged into MESA buffer (Sigma, St. Louis, MO) supplemented with formaldehyde (0.22 M). RNA was transferred to NYTRAN SuperCharge filters (Schleicher and Schuell, Keene, NH) and UV cross-linked. The filters were prehybridized at 68°C for 1 hour in MiracleHyb (Stratagene, La Jolla, CA). To probe the Northern blots, 50 ng of DNA was labeled using Redivue [α-32P] dCTP (Amersham, Arlington Heights, IL) with a random prime labeling kit (Boehringer Mannheim, Indianapolis, IN). The filters were hybridized at 68°C for 20 hours with MiracleHyb containing the labeled and denatured probe. The filters were washed and exposed to Kodak MS film using an MS intensifier screen at −70°C for 2-72 hours. Autoradiograms of the blots were digitized using a Vista-S6E scanner equipped with a transparency adapter and analyzed using image analysis software (Molecular Analyst, Bio-Rad, Hercules, CA). Densitometric values of mRNA were normalized to those of the housekeeping gene β-actin.
4.3. Plasmids for probes
Plasmids containing rat-specific SOCS3, CNTF and β-actin cDNAs (accession: AI059528, CB326787 and AA900159, respectively) were purchased from Open Biosystems (Huntsville, AL). A clone with rat TTP-specific cDNA (accession: AA858882) was obtained from ATCC (Manassas, VA). Plasmids with rat-specific TNF, IRAK-M, TOLLIP and BDNF were generated by TOPO TA cloning (Invitrogen) using the corresponding RT-PCR products generated from rat brain RNA. For TNF specific PCR product, a 5′ primer (5′-CAGAACTCCAGGCGGTGTC-3′) and 3′ primer (5′-AGTAGACCTGCCCGGACTC-3′) corresponding to nt 73-91 and nt 688-670 of the coding sequence of rat TNF (accession: NM_012675) were used. To obtain IRAK-M specific PCR product, 5′ primer (5′-TCCAACCCAAACTAACCGAT-3′) and 3′ primer (5′ -AAGAATGGCCTGGAACTTC-3′) correspond to nt 1085-1104 and nt 1885-1867 of the coding sequence of rat IRAK-M (Accession: XM_235183) were used. For TOLLIP, 5′ primer (5′-CATGGCGACCACCGTCA-3′) and 3′ primer (5′-TAATCATGCCCTCCTTGTCGT-3′) corresponding to nt 193-209 and nt 672-652 of the coding sequence of rat TOLLIP cDNA were used (Accession: XM_341961). The probe for BDNF was generated as described previously (Rangel et al. 2001). The specificity of all probes was confirmed by sequencing (DNA Sequencing Facility, University of Pennsylvania). All probes were excised and gel-purified inserts.
4.4. Statistical Analysis
Differences in mRNA levels between hemispheres were tested for statistical significance using paired “t”-tests. Differences in mRNA levels between groups of animals were tested for statistical significance using unpaired “t”-tests.
Acknowledgments
This work was supported by NIH (grants NS29331 and NS43126).
REFERENCES
- Alexander WS, Hilton DJ. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annual Review of Immunology. 2004;22:503–529. doi: 10.1146/annurev.immunol.22.091003.090312. [DOI] [PubMed] [Google Scholar]
- Arenander AT, Lim RW, Varnum BC, Cole R, de Vellis J, Herschman HR. TIS gene expression in cultured rat astrocytes: multiple pathways of induction by mitogens. J Neurosci Res. 1989;23:257–265. doi: 10.1002/jnr.490230303. [DOI] [PubMed] [Google Scholar]
- Auernhammer CJ, Bousquet C, Melmed S. Autoregulation of pituitary corticotroph SOCS-3 expression: Characterization of the murine SOCS-3 promoter. PNAS. 1999;96:6964–6969. doi: 10.1073/pnas.96.12.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baetz A, Frey M, Heeg K, Dalpke AH. Suppressor of cytokine signaling (SOCS) proteins indirectly regulate Toll-like receptor signaling in innate immune cells. J. Biol. Chem. 2004;279:54708–54715. doi: 10.1074/jbc.M410992200. [DOI] [PubMed] [Google Scholar]
- Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: new opportunities for novel therapeutics. J Cereb Blood Flow Metab. 1999;19:819–834. doi: 10.1097/00004647-199908000-00001. [DOI] [PubMed] [Google Scholar]
- Bjorbaek C, Elmquist JK, El-Haschimi K, Kelly J, Ahima RS, Hileman S, Flier JS. Activation of SOCS-3 Messenger Ribonucleic Acid in the Hypothalamus by Ciliary Neurotrophic Factor. Endocrinology. 1999;140:2035–2043. doi: 10.1210/endo.140.5.6736. [DOI] [PubMed] [Google Scholar]
- Bode JG, Nimmesgern A, Schmitz J, Schaper F, Schmitt M, Frisch W, Haussinger D, Heinrich PC, Graeve L. LPS and TNF[alpha] induce SOCS3 mRNA and inhibit IL-6-induced activation of STAT3 in macrophages. FEBS Letters. 1999;463:365–370. doi: 10.1016/s0014-5793(99)01662-2. [DOI] [PubMed] [Google Scholar]
- Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002;61:1013–1021. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- Cacalano NA, Sanden D, Johnston JA. Tyrosine-phosphorylated SOCS-3 inhibits STAT activation but binds to p120 RasGAP and activates Ras. Nat Cell Biol. 2001;3:460–465. doi: 10.1038/35074525. [DOI] [PubMed] [Google Scholar]
- Cao H, Tuttle JS, Blackshear PJ. Immunological characterization of tristetraprolin as a low abundance, inducible, stable cytosolic protein. J. Biol. Chem. 2004;279:21489–21499. doi: 10.1074/jbc.M400900200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballo E, Lai WS, Blackshear PJ. Feedback inhibition of macrophage tumor necrosis factor-alpha production by tristetraprolin. Science. 1998;281:1001–1005. doi: 10.1126/science.281.5379.1001. [DOI] [PubMed] [Google Scholar]
- Carballo E, Lai WS, Blackshear PJ. Evidence that tristetraprolin is a physiological regulator of granulocyte-macrophage colony-stimulating factor messenger RNA deadenylation and stability. Blood. 2000;95:1891–1899. [PubMed] [Google Scholar]
- del Zoppo G, Ginis I, Hallenbeck JM, Iadecola C, Wang X, Feuerstein GZ. Inflammation and stroke: putative role for cytokines, adhesion molecules and iNOS in brain response to ischemia. Brain Pathol. 2000;10:95–112. doi: 10.1111/j.1750-3639.2000.tb00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digicaylioglu M, Lipton SA. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature. 2001;412:641–647. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26:248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- Fox SW, Haque SJ, Lovibond AC, Chambers TJ. The possible role of TGF-{beta}-induced suppressors of cytokine signaling expression in osteoclast/macrophage lineage commitment in vitro. J Immunol. 2003;170:3679–3687. doi: 10.4049/jimmunol.170.7.3679. [DOI] [PubMed] [Google Scholar]
- Gibson CL, Bath PMW, Murphy SP. G-CSF reduces infarct volume and improves functional outcome after transient focal cerebral ischemia in mice. 2005;25:431–439. doi: 10.1038/sj.jcbfm.9600033. [DOI] [PubMed] [Google Scholar]
- Gubits RM, Burke RE, Casey-McIntosh G, Bandele A, Munell F. Immediate early gene induction after neonatal hypoxia-ischemia. Brain Res Mol Brain Res. 1993;18:228–238. doi: 10.1016/0169-328x(93)90194-t. [DOI] [PubMed] [Google Scholar]
- Hermann DM, Kilic E, Kugler S, Isenmann S, Bahr M. Adenovirus-Mediated GDNF and CNTF Pretreatment Protects against Striatal Injury Following Transient Middle Cerebral Artery Occlusion in Mice. Neurobiology of Disease. 2001;8:655–666. doi: 10.1006/nbdi.2001.0399. [DOI] [PubMed] [Google Scholar]
- Ip NY, Wiegand SJ, Morse J, Rudge JS. Injury-induced regulation of ciliary neurotrophic factor mRNA in the adult rat brain. Eur J Neurosci. 1993;5:25–33. doi: 10.1111/j.1460-9568.1993.tb00201.x. [DOI] [PubMed] [Google Scholar]
- Ito S, Ansari P, Sakatsume M, Dickensheets H, Vazquez N, Donnelly RP, Larner AC, Finbloom DS. Interleukin-10 inhibits expression of both interferon alpha- and interferon gamma-induced genes by suppressing tyrosine phosphorylation of STAT1. Blood. 1999;93:1456–1463. [PubMed] [Google Scholar]
- Jander S, Schroeter M, Peters O, Witte OW, Stoll G. Cortical spreading depression induces proinflammatory cytokine gene expression in the rat brain. J Cereb Blood Flow Metab. 2001;21:218–225. doi: 10.1097/00004647-200103000-00005. [DOI] [PubMed] [Google Scholar]
- Kariko K, Harris VA, Rangel Y, Duvall ME, Welsh FA. Effect of cortical spreading depression on the levels of mRNA coding for putative neuroprotective proteins in rat brain. J Cereb Blood Flow Metab. 1998;18:1308–1315. doi: 10.1097/00004647-199812000-00005. [DOI] [PubMed] [Google Scholar]
- Kariko K, Weissman D, Welsh FA. Inhibition of toll-like receptor and cytokine signaling--a unifying theme in ischemic tolerance. J Cereb Blood Flow Metab. 2004;24:1288–1304. doi: 10.1097/01.WCB.0000145666.68576.71. [DOI] [PubMed] [Google Scholar]
- Kawahara N, Ruetzler CA, Katzo I. Protective effect of spreading depression against neuronal damage following cardiac arrest cerebral ischemia. Neurol Res. 1995;17:9–16. doi: 10.1080/01616412.1995.11740281. [DOI] [PubMed] [Google Scholar]
- Kelly JF, Elias CF, Lee CE, Ahima RS, Seeley RJ, Bjorbaek C, Oka T, Saper CB, Flier JS, Elmquist JK. Ciliary neurotrophic factor and leptin induce distinct patterns of immediate early gene expression in the brain. Diabetes. 2004;53:911–920. doi: 10.2337/diabetes.53.4.911. [DOI] [PubMed] [Google Scholar]
- Keswani SC, Buldanlioglu U, Fischer A, Reed N, Polley M, Liang H, Zhou C, Jack C, Leitz GJ, Hoke A. A novel endogenous erythropoietin mediated pathway prevents axonal degeneration. Ann Neurol. 2004;56:815–826. doi: 10.1002/ana.20285. [DOI] [PubMed] [Google Scholar]
- Kirino T. Ischemic tolerance. J Cereb Blood Flow Metab. 2002;22:1283–1296. doi: 10.1097/01.WCB.0000040942.89393.88. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Harris VA, Welsh FA. Spreading depression induces tolerance of cortical neurons to ischemia in rat brain. J Cereb Blood Flow Metab. 1995;15:721–727. doi: 10.1038/jcbfm.1995.92. [DOI] [PubMed] [Google Scholar]
- Kreider BL, Rovera G. The immediate early gene response to a differentiative stimulus is disrupted by the v-abl and v-ras oncogenes. Oncogene. 1992;7:135–140. [PubMed] [Google Scholar]
- Kubo M, Hanada T, Yoshimura A. Suppressors of cytokine signaling and immunity. Nat Immunol. 2003;4:1169–1176. doi: 10.1038/ni1012. [DOI] [PubMed] [Google Scholar]
- Kumon Y, Sakaki S, Watanabe H, Nakano K, Ohta S, Matsuda S, Yoshimura H, Sakanaka M. Ciliary neurotrophic factor attenuates spatial cognition impairment, cortical infarction and thalamic degeneration in spontaneously hypertensive rats with focal cerebral ischemia. Neurosci Lett. 1996;206:141–144. doi: 10.1016/s0304-3940(96)12450-2. [DOI] [PubMed] [Google Scholar]
- Lai WS, Blackshear PJ. Interactions of CCCH zinc finger proteins with mRNA. Tristetraprolin-mediated AU-rich element-dependent mRNA degradation can occur in the absence of a poly(A) tail. J. Biol. Chem. 2001;276:23144–23154. doi: 10.1074/jbc.M100680200. [DOI] [PubMed] [Google Scholar]
- Loddick SA, Turnbull AV, Rothwell NJ. Cerebral interleukin-6 is neuroprotective during permanent focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1998;18:176–179. doi: 10.1097/00004647-199802000-00008. [DOI] [PubMed] [Google Scholar]
- Matsushima K, Hogan MJ, Hakim AM. Cortical spreading depression protects against subsequent focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1996;16:221–226. doi: 10.1097/00004647-199603000-00006. [DOI] [PubMed] [Google Scholar]
- Muramatsu H, Kariko K, Welsh FA. Induction of tolerance to focal ischemia in rat brain: dissociation between cortical lesioning and spreading depression. J Cereb Blood Flow Metab. 2004;24:1167–1171. doi: 10.1097/01.WCB.0000134714.38679.2C. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Wall R. Interleukin-6 signals activating junB and TIS11 gene transcription in a B-cell hybridoma. Mol Cell Biol. 1991;11:1409–1418. doi: 10.1128/mcb.11.3.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawashiro H, Tasaki K, Ruetzler CA, Hallenbeck JM. TNF-alpha pretreatment induces protective effects against focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 1997;17:483–490. doi: 10.1097/00004647-199705000-00001. [DOI] [PubMed] [Google Scholar]
- Nozaki K, Finklestein SP, Beal MF. Basic fibroblast growth factor protects against hypoxia-ischemia and NMDA neurotoxicity in neonatal rats. J Cereb Blood Flow Metab. 1993;13:221–228. doi: 10.1038/jcbfm.1993.27. [DOI] [PubMed] [Google Scholar]
- Ogilvie RL, Abelson M, Hau HH, Vlasova I, Blackshear PJ, Bohjanen PR. Tristetraprolin down-regulates IL-2 gene expression through AU-rich element-mediated mRNA decay. J Immunol. 2005;174:953–961. doi: 10.4049/jimmunol.174.2.953. [DOI] [PubMed] [Google Scholar]
- Ohtsuki T, Ruetzler CA, Tasaki K, Hallenbeck JM. Interleukin-1 mediates induction of tolerance to global ischemia in gerbil hippocampal CA1 neurons. J Cereb Blood Flow Metab. 1996;16:1137–1142. doi: 10.1097/00004647-199611000-00007. [DOI] [PubMed] [Google Scholar]
- Otori T, Greenberg JH, Welsh FA. Cortical spreading depression causes a long-lasting decrease in cerebral blood flow and induces tolerance to permanent focal ischemia in rat brain. J Cereb Blood Flow Metab. 2003;23:43–50. doi: 10.1097/01.WCB.0000035180.38851.38. [DOI] [PubMed] [Google Scholar]
- Pechan PA, Yoshida T, Panahian N, Moskowitz MA, Breakefield XO. Genetically modified fibroblasts producing NGF protect hippocampal neurons after ischemia in the rat. Neuroreport. 1995;6:669–672. doi: 10.1097/00001756-199503000-00021. [DOI] [PubMed] [Google Scholar]
- Peng X, Greene LA, Kaplan DR, Stephens RM. Deletion of a conserved juxtamembrane sequence in Trk abolishes NGF-promoted neuritogenesis. Neuron. 1995;15:395–406. doi: 10.1016/0896-6273(95)90043-8. [DOI] [PubMed] [Google Scholar]
- Rangel YM, Kariko K, Harris VA, Duvall ME, Welsh FA. Dose-dependent induction of mRNAs encoding brain-derived neurotrophic factor and heat-shock protein-72 after cortical spreading depression in the rat. Brain Res Mol Brain Res. 2001;88:103–112. doi: 10.1016/s0169-328x(01)00037-7. [DOI] [PubMed] [Google Scholar]
- Rao VLR, Bowen KK, Dhodda VK, Song G, Franklin JL, Gavva NR, Dempsey RJ. Gene expression analysis of spontaneously hypertensive rat cerebral cortex following transient focal cerebral ischemia. J Neurochem. 2002;83:1072–1086. doi: 10.1046/j.1471-4159.2002.01208.x. [DOI] [PubMed] [Google Scholar]
- Sawaoka H, Dixon DA, Oates JA, Boutaud O. Tristetraprolin binds to the 3′-untranslated region of cyclooxygenase-2 mRNA. A polyadenylation variant in a cancer cell line lacks the binding site. J Biol Chem. 2003;278:13928–13935. doi: 10.1074/jbc.M300016200. [DOI] [PubMed] [Google Scholar]
- Spera PA, Ellison JA, Feuerstein GZ, Barone FC. IL-10 reduces rat brain injury following focal stroke. Neurosci Lett. 1998;251:189–192. doi: 10.1016/s0304-3940(98)00537-0. [DOI] [PubMed] [Google Scholar]
- Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, Gonda TJ, Alexander WS, Metcalf D, Nicola NA, Hilton DJ. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Yamashita T, Tanaka K, Hattori H, Sawamoto K, Okano H, Suzuki N. Activation of cytokine signaling through leukemia inhibitory factor receptor (LIFR)/gp130 attenuates ischemic brain injury in rats. J Cereb Blood Flow Metab. 2005;25:685–693. doi: 10.1038/sj.jcbfm.9600061. [DOI] [PubMed] [Google Scholar]
- Szelenyi J. Cytokines and the central nervous system. Brain Research Bulletin. 2001;54:329–338. doi: 10.1016/s0361-9230(01)00428-2. [DOI] [PubMed] [Google Scholar]
- Tasaki K, Ruetzler CA, Ohtsuki T, Martin D, Nawashiro H, Hallenbeck JM. Lipopolysaccharide pre-treatment induces resistance against subsequent focal cerebral ischemic damage in spontaneously hypertensive rats. Brain Res. 1997;748:267–270. doi: 10.1016/s0006-8993(96)01383-2. [DOI] [PubMed] [Google Scholar]
- Taylor GA, Carballo E, Lee DM, Lai WS, Thompson MJ, Patel DD, Schenkman DI, Gilkeson GS, Broxmeyer HE, Haynes BF, Blackshear PJ. A pathogenetic role for TNF alpha in the syndrome of cachexia, arthritis, and autoimmunity resulting from tristetraprolin (TTP) deficiency. Immunity. 1996;4:445–454. doi: 10.1016/s1074-7613(00)80411-2. [DOI] [PubMed] [Google Scholar]
- Terstegen L, Gatsios P, Bode JG, Schaper F, Heinrich PC, Graeve L. The Inhibition of interleukin-6-dependent STAT activation by mitogen-activated protein kinases depends on Tyrosine 759 in the cytoplasmic tail of glycoprotein 130. J. Biol. Chem. 2000;275:18810–18817. doi: 10.1074/jbc.M904148199. [DOI] [PubMed] [Google Scholar]
- Wang X, Li X, Erhardt JA, Barone FC, Feuerstein GZ. Detection of tumor necrosis factor-alpha mRNA induction in ischemic brain tolerance by means of real-time polymerase chain reaction. J Cereb Blood Flow Metab. 2000;20:15–20. doi: 10.1097/00004647-200001000-00004. [DOI] [PubMed] [Google Scholar]
- Wen TC, Matsuda S, Yoshimura H, Kawabe T, Sakanaka M. Ciliary neurotrophic factor prevents ischemia-induced learning disability and neuronal loss in gerbils. Neurosci Lett. 1995;191:55–58. doi: 10.1016/0304-3940(95)11574-8. [DOI] [PubMed] [Google Scholar]
- Zhu W, Brauchle MA, Di Padova F, Gram H, New L, Ono K, Downey JS, Han J. Gene suppression by tristetraprolin and release by the p38 pathway. Am J Physiol Lung Cell Mol Physiol. 2001;281:L499–508. doi: 10.1152/ajplung.2001.281.2.L499. [DOI] [PubMed] [Google Scholar]
- Zubakov D, Hoheisel JD, Kluxen FW, Brandle M, Ehring T, Hentsch B, Frohme M. Late ischemic preconditioning of the myocardium alters the expression of genes involved in inflammatory response. FEBS Lett. 2003;547:51–55. doi: 10.1016/s0014-5793(03)00667-7. [DOI] [PubMed] [Google Scholar]