Summary
Cocaine addiction is characterized by long-lasting vulnerability to relapse arising because neutral environmental stimuli become associated with drug use and then act as cues that induce relapse. It is not known how cues elicit cocaine seeking, and why cocaine seeking is more difficult to regulate than seeking a natural reward. We found that cocaine-associated cues initiate cocaine seeking by inducing a rapid, transient increase in dendritic spine size and synaptic strength in the nucleus accumbens. These changes required neural activity in the prefrontal cortex. This is not the case when identical cues were associated with obtaining sucrose, which did not elicit changes in spine size or synaptic strength. The marked cue-induced synaptic changes in the accumbens were correlated with the intensity of cocaine, but not sucrose seeking, and may explain the difficulty addicts experience in managing relapse to cocaine use.
Introduction
Understanding the neurobiology of relapse to drug use will facilitate the development of pharmacotherapies to treat addiction (Kalivas and Volkow, 2011; Vocci and Ling, 2005). An important feature of the enduring vulnerability to relapse is that neutral environmental stimuli become associated with drug use and act as cues that initiate relapse (Goldstein and Volkow, 2002; See, 2002; Wilson et al., 2004). Presenting cues previously paired with cocaine use initiates craving and drug-seeking that are associated with activating the glutamatergic projection from the prefrontal cortex to the nucleus accumbens (Kalivas, 2009; Koob and Volkow, 2010; Wilson et al., 2004). Given the well-established role of the cortico-striatal projection in regulating motivated behavior (Balleine et al., 2007; Luscher and Malenka, 2011; Miller and Marshall, 2004), it is thought that cocaine-induced changes in this glutamatergic projection enable environmental stimuli associated with cocaine use to act as conditioned cues that elicit uncontrollable motivation to relapse to drug use compared with the more manageable motivation to obtain natural rewards (Garavan et al., 2000; Levy et al., 2007).
The neurobiology of relapse to cocaine use is most frequently studied in animal models by measuring long-lasting changes in brain structure and function after experimenter-injected or self-administered cocaine followed by varying periods of withdrawal. A key observation using this approach is that excitatory synapses on medium spiny neurons (MSNs) in the accumbens show evidence of long-term potentiation (LTP), including increased dendrite spine head diameter, and elevated AMPA glutamate receptor-mediated synaptic currents and AMPA receptor surface expression (Boudreau et al., 2007; Conrad et al., 2008; Kourrich and Thomas, 2009; Moussawi et al., 2009; Shen et al., 2009b; Wolf and Ferrario, 2010). This LTP-like state is suggested to mediate the enhanced motivation underlying relapse to drug use compared to natural reward (Wolf, 2010). However, it remains unknown how initiating relapse with cocaine-conditioned cues (i.e. absent the pharmacological effects of the drug) affects synaptic physiology and morphology, if synaptic changes are important for initiating relapse, or if cues initiating cocaine-seeking produce distinct synaptic changes compared with the same cues initiating seeking of a natural reward.
In order to investigate these synaptic mechanisms contributing to cocaine relapse, we used a “short-access” model of cocaine self-administration and examined the reinstatement of cocaine seeking by a light/tone cue previously associated with cocaine delivery. While this paradigm may not model compulsive drug self-administration (Koob, 2012), it allows investigation of cue-induced cocaine seeking after a period of withdrawal (Epstein et al., 2006; Shaham et al., 2003), and elicits enduring physiological and neurochemical changes in the projection from the prefrontal cortex to nucleus accumbens (Kalivas, 2009; Wolf, 2010). Using this model we show that presenting cocaine-associated cues simultaneously initiated cocaine seeking and a rapid, transient increase in dendritic spine size and synaptic strength in the nucleus accumbens. The synaptic changes were positively correlated with the intensity of reinstated cocaine seeking and required activity in the prefrontal cortex. Importantly, the increase in spine size and synaptic response did not occur when the same seeking behavior was induced by identical cues paired with a natural reward (sucrose). Our data demonstrate that associating cocaine, but not a natural reward, with environmental cues confers a capacity for these cues to transiently potentiate accumbens excitatory synaptic transmission. This distinction between cocaine and sucrose may explain the relatively uncontrollable motivation to relapse to cocaine use compared with the more manageable desire for natural rewards.
Results
Rats were trained to self-administer cocaine by pressing one of two levers to receive an intravenous cocaine injection. Rats self-administered cocaine for 2 hours a day over 10 days to achieve stable daily cocaine use, and lever pressing was then extinguished over another 14 days of 2 hour sessions (Figure S1). A light/tone compound stimulus was paired with cocaine infusions during the self-administration sessions and lever pressing during extinction training yielded neither cocaine nor the light/tone stimulus. A parallel yoked-saline control group was included consisting of rats administered an intravenous infusion of saline when a paired rat self-administered cocaine. Once rats achieved a stable extinguished baseline of lever pressing (Figure S1), the light/tone cue was presented with each press of the lever that previously provided cocaine (active lever), but no cocaine was delivered. Returning the conditioned light/tone cue resulted in a marked reinstatement of lever pressing (Figures 1A and 1B), which was used to model cue-induced relapse (Epstein et al., 2006).
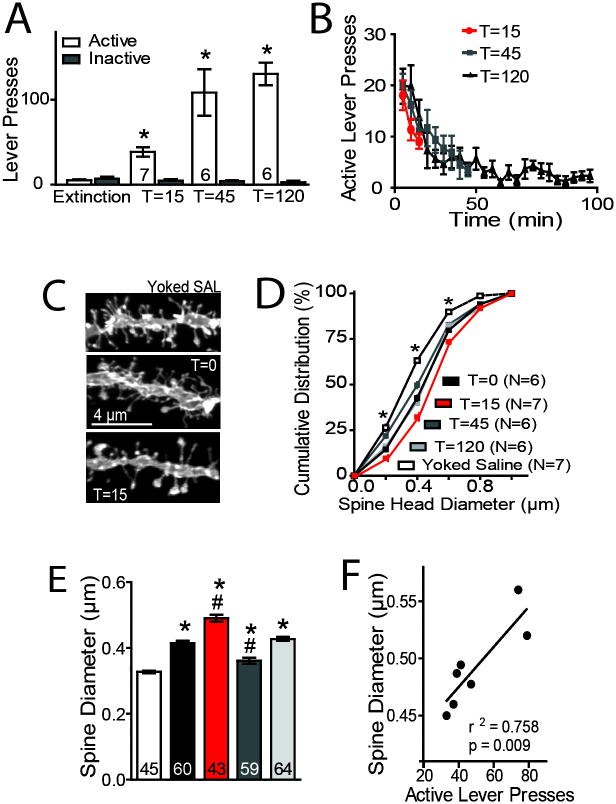
Figure 1. Cue-induced cocaine-seeking rapidly enlarges spine head diameter in NAcore MSNs.
(A) Cue-induced reinstatement of cocaine-seeking increased active lever pressing over the 15, 45, or 120 min prior to euthanizing rats for morphological or A/N measurements (F(7,92)=39.93, P< 0.0001). (B) Time course of active lever pressing during the cue reinstatement session. (C) Sample dendrites from NAcore MSNs in yoked saline (dh= 0.329 μm) or cocaine-trained rats at T=0 (0.422 μm), T=15 (0.528 μm) min after initiating cue-induced reinstatement. (D) Cumulative distribution of spine head diameter reveals changes in dh between treatment groups (group: F(5,1698)= 8769, P< 0.0001; dh F(4,1698)= 115.2, P< 0.0001; interaction: F(20,1698)= 21, P< 0.0001). (E) Cocaine self-administration increased dh (F(6,326)= 43.98, P< 0.0001). Spine dh was elevated at T=15, decreased below pre-reinstatement levels at T=45, and returned to pre-reinstatement levels at T=120. (F) The increase in dh at 15 min was significantly correlated with active lever pressing. N in panel D is the number of rats, and N shown as the number in bars corresponds to either number of animals (panel A) or the number of neurons quantified (panel E). Five to 12 neurons were measured from each rat. Data are shown as mean ± SEM.
*p< 0.05, compared to yoked saline or extinction lever presses, #p< 0.05, compared to T=0 cocaine.
As described above, cocaine self-administration causes stable LTP-like synaptic potentiation in the core subcompartment of the nucleus accumbens (NAcore) that endures for months after discontinuing cocaine use and is proposed to contribute to cocaine relapse. To test if synaptic alterations initiated in the NAcore by presentation of cocaine-conditioned cues contribute to relapse, animals were examined just prior to beginning cue-induced reinstatement (time= 0), or at 15, 45 or 120 min after beginning the reinstatement trial for two measures of synaptic plasticity. To quantify spine density and dh, 3-dimensional confocal images were made of neurons in the NAcore that were diolistically labeled with the lipophilic dye DiI (Figure 1C) (Shen et al., 2011). Synaptic strength was also estimated by calculating the ratio of AMPA to NMDA currents (A/N) (Malenka and Bear, 2004) using whole cell patch recordings from MSNs in NAcore tissue slices (Moussawi et al., 2011; Shen et al., 2011).
Conditioned cues reinstated robust active lever pressing compared to inactive lever pressing or to active lever pressing during extinction (Figure 1A). The increase in lever pressing was maximal during the first 10 min of the reinstatement session and progressively decreased thereafter for the remainder of the session (Figure 1B). At T=0, dh was increased in rats extinguished from cocaine self-administration (0.415 ± 0.007 μm) compared to yoked saline controls (0.327 ± 0.004 μm) (Figures 1C,D,E). Cue-induced reinstatement further increased dh at 15 min after the cue was presented (0.491 ± 0.009 μm). By 45 min after initiating reinstatement, dh decreased below the resting (T=0) cocaine levels (0.361 ± 0.009 μm), and had returned to pre-reinstatement levels by 120 min after initiating the reinstatement session (0.427 ± 0.011 μm). Importantly, the amount of reinstated active lever pressing at 15 min was positively correlated with the increase in dh (Fig 1F). No difference in spine density was found between groups (Figure S2A).
To test whether cue-induced morphological plasticity was specific for reinstating lever pressing for cocaine and not for a natural reward, rats were trained to self-administer sucrose pellets and pellet delivery was paired with the same light/tone stimulus used for cocaine training. While the sucrose-trained rats showed robust cue-induced reinstatement of lever pressing (Figure 2A), no change in mean dh (Fig 2B) or spine density (Fig S2B) was measured at 15 or 45 min following the cue (Figs. 2C). To further test that the cue-induced increase in dh depended on a contingent association between the cocaine-paired lever and the light/tone cue, rats were exposed to the chamber either without presenting cues or when cues were presented independent of lever pressing, and these animals also showed no increase in dh, spine density, or lever pressing at 15 min after beginning the session (Figures S3A-C). Taken together, the morphological measurements show that withdrawal from daily cocaine self-administration causes a resting enlargement of dh, and that cue-induced reinstatement of cocaine, not sucrose, seeking is accompanied by a further rapid, transient enlargement of the spines that is significantly correlated with reinstated behavior.
Figure 2. Sucrose trained rats to not show increased spine head diameter during cue-induced reinstatement.
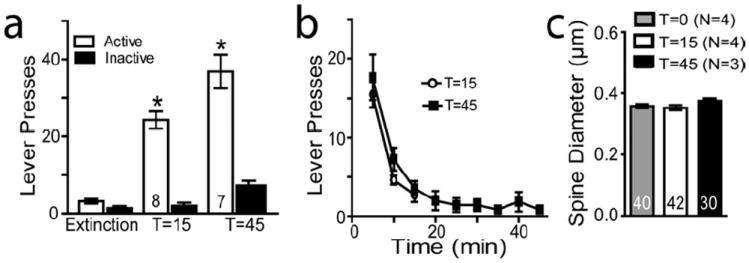
(A,B) Sucrose-trained rats show significant cue-induced reinstatement (F(3,31)= 86.981, P< 0.001). (C) Sucrose reinstatement was not accompanied by a change in dh. N is the number in bars and corresponds to either number of animals (panel A) or the number of neurons quantified (panel C). Five to 12 neurons were measured from each rat. Data are shown as mean ± SEM.
*p< 0.05, compared to yoked saline or extinction lever presses.
Whole cell patch recordings revealed parallel evidence for rapid synaptic potentiation during cue-induced reinstatement (Figures 3A and 3B). Withdrawal from investigator- or self-administered cocaine increased A/N compared to yoked saline rats. The A/N was further increased 15 minutes after initiating cue-induced reinstatement and returned to pre-reinstatement levels after 120 minutes. In contrast with the decrease in dh (Figure 1F), the A/N remained elevated at T=45. Similar to dh, the increase A/N at 15 min was significantly correlated with the number of reinstated active lever presses (Figure 3C), and cue-induced reinstatement of sucrose did not show a change in A/N at 15 or 45 min after initiating the reinstatement session (Figure 3D).
Figure 3. Synaptic potentiation initiated by cue-induced cocaine seeking.
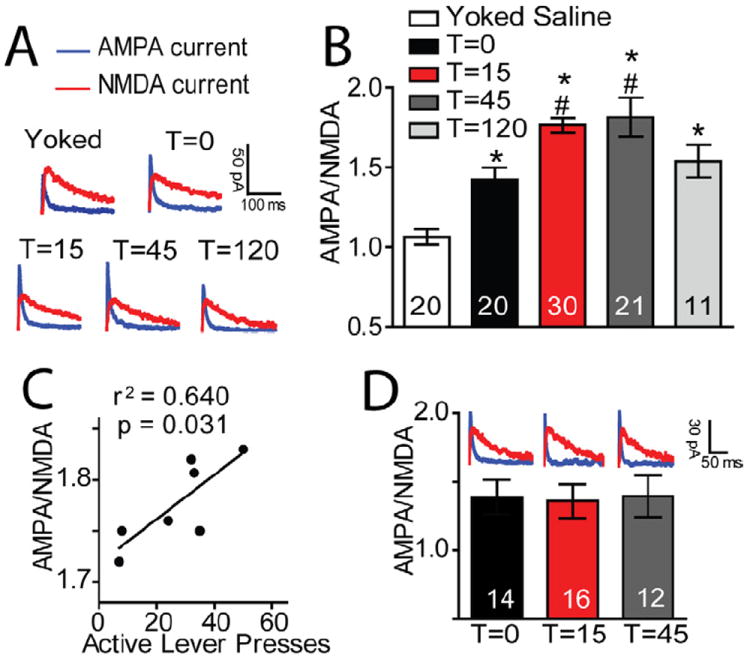
(A) Sample AMPA and NMDA current traces from each group. (B) AMPA to NMDA ratios (A/N) were significantly elevated in animals withdrawn with extinction training from cocaine self-administration (1.423 ± 0.075) compared to yoked saline animals (1.064 ± 0.050). In addition, the initiation of cue-induced reinstatement further elevated A/N at T=15 (1.780 ± 0.060). Ratios remained elevated at T=45 (1.815 ± 0.122), and returned to pre-reinstatement levels by T=120 (1.538 ± 0.103) (F(4,101)= 14.45, P< 0.001). (C) The increase in A/N at 15 min was significantly correlated with the number of active lever presses. (D) Cue-induced reinstatement of sucrose seeking did not alter A/N. Two to five neurons were recorded from each animal. Data are shown as mean ± SEM.
*p< 0.05 compared to yoked saline animals at T=0 (white bar); #p< 0.01 compared to T=0 (black bar).
The NAcore receives glutamatergic input from various sources, and increased release of glutamate from the prelimbic cortex (PL) into the NAcore is required for reinstating drug-seeking (LaLumiere and Kalivas, 2008; McFarland et al., 2003). We found that neural activity in the PL is also critical for the cue-induced synaptic changes in the NAcore. Inhibiting the PL by microinjecting GABA agonists (baclofen plus muscimol) prior to the reinstatement session prevented cue-induced increases in dh and A/N in NAcore MSNs, as well as blocked reinstated active lever pressing (Figure 4; Figure S4 for histology).
Figure 4. Inactivation of the PL prevents cue-induced reinstatement and the increase in dh and A/N in NAcore.
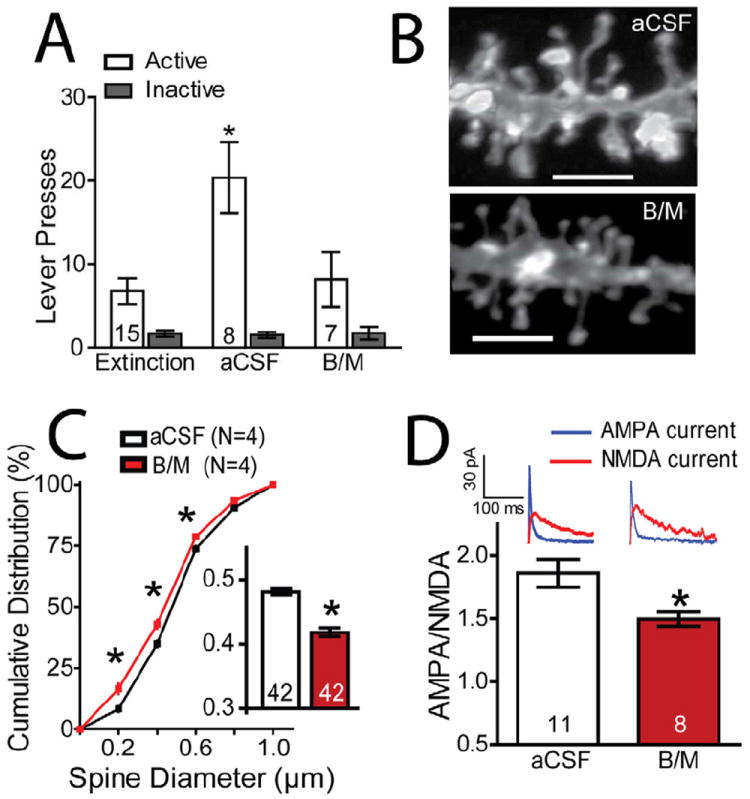
(A) B/M infusions into PL inhibited cue-induced reinstatement (T=15; F(5,59)= 11.971, P< 0.001; N is shown in bars). (B) Sample dendrites of animals receiving either aCSF or B/M into PL prior to initiating cue-induced reinstatement and sacrificed 15 min later. (C,D) B/M into PL inhibited the mean increase in dh (t(82)= 7.504, P< 0.001; see inset) and shifted the cumulative distribution to the left. (E) B/M into PL inhibited the increase in A/N (t(17)= 2.554, P= 0.021). Data are shown as mean ± SEM.
*p< 0.05, comparing aCSF to B/M
Discussion
We show here that the reinstatement of cocaine seeking by conditioned cues, but not the reinstatement of seeking a natural reward, was accompanied by rapid, transient synaptic potentiation in NAcore MSNs. The rapid potentiation contrasts with previous reports showing that cocaine use reduces the ability of prefrontal input to induce classical forms of synaptic plasticity, such as LTP and LTD (Martin et al., 2006; Moussawi et al., 2009). Thus, while cocaine use diminishes the capacity of stimuli not associated with drug use to induce synaptic plasticity, LTP-like plasticity is readily induced by stimuli paired with cocaine use. The importance in relapse of synaptic plasticity selectively coded by cocaine-associated cues was supported by a significant correlation between the intensity of cocaine seeking and both morphological and electrophysiological measures of synaptic potentiation.
Changes in spine density and/or head diameter (dh) are a structural substrate for synaptic plasticity, with larger dh being associated with LTP, and reduced dh with LTD (Carlisle and Kennedy, 2005; De Roo et al., 2008; Yang and Zhou, 2009). Consistent with previous reports (Kourrich et al., 2007; Moussawi et al., 2011; Shen et al., 2009a), withdrawal from investigator- or self-administered cocaine increased dh and A/N compared to yoked saline rats. The dh and A/N was further increased 15 minutes after initiating cue-induced reinstatement and returned to pre-reinstatement levels after 120 minutes. Although there was a decrease in dh at T=45, the A/N remained elevated. The slower normalization of the A/N is consistent with previous in vitro studies indicating that although both dh and A/N are reliable markers of synaptic plasticity, they are regulated in part by distinct signaling pathways (Fukazawa et al., 2003; Henley et al., 2011). For example, inhibiting protein phosphatase 1 prevents electrophysiological measures of LTP without affecting enlargement of dendritic spines (Zhou et al., 2004). Conversely, inhibiting actin polymerization reduces spine size in cultured neurons (Gu et al., 2010), but inhibits only enduring LTP (> 1 hour), leaving intact short-term synaptic potentiation that is akin to what we show here being initiated by cocaine-conditioned cues (Fukazawa et al., 2003; Krucker et al., 2000; Ramachandran and Frey, 2009).
A link between reinstated cocaine seeking and the rapid LTP-like plasticity was also indicated by inactivating the PL and showing necessary involvement of this region of the PFC in cue-induced increases in dh and A/N. It is likely that the glutamatergic projection from the PL to the NAcore is contributing to the effects of inactivation since double-dissociation pharmacological inactivation and more selective optogenetic inhibition show that this pathway is necessary for reinstated cocaine seeking (McFarland and Kalivas, 2001; Stefanik et al., 2012). This mechanism is also consistent with in vivo recordings showing increased activation of NAcore neurons in response to cocaine-conditioned cues after a period of extinction training (Hollander and Carelli, 2007), and with neuron culture studies indicating that glutamate induces LTP-like synaptic changes (Shepherd and Huganir, 2007). In addition, the lack of rapid LTP-like plasticity accompanying reinstated sucrose seeking supports a role for PL glutamatergic input since cocaine reinstatement requires a marked rise in the release of synaptic glutamate from the PL into the NAcore, but reinstated sucrose seeking does not induce measurable glutamate release (McFarland et al., 2003). However, it is possible that PL projections to other brain regions innervating the NAcore known to regulate reinstated behavior may also play a role, such as dopamine projections from the ventral tegmental area or glutamatergic input from the basolateral amygdala (Koob and Volkow, 2010). A role for dopaminergic afferents is supported by the fact that in co-cultured prefrontal and accumbens neurons, D1 receptor stimulation facilitates trafficking of AMPA receptors to the surface and co-stimulation of NMDA receptors promotes D1 synaptic insertion (Sun et al., 2008). In this regard, it will be of interest in future studies to determine if the changes identified here are selective for D1 or D2 receptor expressing MSNs.
Cocaine addiction is defined in part by the unmanageable motivation to take cocaine, and differs markedly from relative control over engaging natural rewards. The lack of change in dh and A/N after cue-induced sucrose seeking indicates that associating cues with cocaine delivery is conferring neuroadaptations that are not occurring when the identical cues are associated with sucrose delivery. This supports the possibility that the rapid, transient synaptic potentiation may be a biomarker for a cocaine seeking neuropathology, and poses the possibility that countermanding the synaptic potentiation may selectively disrupt the vulnerability to relapse to cocaine use without affecting the motivation to seek natural rewards.
Experimental Procedures
Animal housing and surgery
Male Sprague Dawley rats (250 g; Charles River Laboratories) were individually housed with a 12:12 hr dark/light cycle. All experimentation occurred in the dark cycle. Rats received food ad libitum until the day prior to behavioral training, after which food restriction (20 g of rat chow per day) was implemented and maintained throughout the experiment. Rats were allowed 1 week to acclimate to the vivarium before inducing anesthesia and implanting indwelling jugular catheters, and in some experiments microinjection guide cannula were also implanted in the PL (surgical details in Supplement). All procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Assessment and Accreditation of Laboratory Animal Care.
Cocaine self-administration procedures
Seven days after surgery, rats began daily 2-hr cocaine self-administration sessions, in which one response on the active lever yielded one intravenous cocaine infusion (0.2 mg/infusion, followed by a 20-sec timeout period), paired with a white cue light above the active lever and a discrete tone cue. An inactive lever was also available throughout each session. Following 10 consecutive sessions of self-administration (≥ 10 infusions/day), rats were placed into daily extinction training sessions (no cocaine delivery or cues) for at least 14 sessions, or until extinction criteria were met (≤ 25 active lever responses for a minimum of 2 sessions). Reinstatement was elicited by cues (tone + light delivery following an active lever press).
Microinfusion procedures and histology
Rats were stereotaxically implanted immediately after catheterization with bilateral guide cannulae aimed above PL (see Supplement for surgical details). Obturators were placed into the guide cannulae, and were removed during bilateral injection of 0.3 μl baclofen/muscimol cocktail (0.3/0.03 nmol, GABAB/GABAA receptor agonists, respectively) over 1 min (McFarland and Kalivas, 2001). Rats were placed in the operant chamber 10 min following removal of injection cannulae and replacement of the obturators. Rats were sacrificed at various times for either dendritic spine or electrophysiological quantification. When appropriate, coronal slices (100 μm thick) of PL were mounted and stained via cresyl violet to verify guide cannulae placement (Figure S4).
Quantification of dendritic spines
All dendritic spine quantification procedures have been described previously (Shen et al., 2009b). Briefly, a confocal microscope was used to image DiI-labeled sections, and DiI was excited using the Helium/Neon 543 nm laser line. Images of DiI-labeled dendrites (see Figure 1C) were acquired via optical sectioning using a 63x oil immersion objective (Plan-Apochromat, Zeiss; NA = 1.4, WD = 90 μm) with pixel size 0.07 μm at XY plane and 0.1 μm intervals along the z-axis. Images were deconvoluted prior to analysis, and a 3-D perspective was rendered by the Surpass module of Imaris software package (Bitplane; Saint Paul, MN). Only spines on dendrites beginning at >75 μm and ending at ≤ 200 μm distal to the soma and after the first branch point were quantified from cells localized to the NA core (see Table S1). The length of quantified dendrites was 45-55 μm. Five-12 neurons were analyzed from each animal, and the minimum end segment diameter (spine head) was set at ≥0.143 μm.
Slice preparation and whole cell recordings
Rats were anesthetized with ketamine, decapitated and coronal accumbens brain slices were collected into a vial containing artificial cerebrospinal fluid (aCSF). All recordings were collected at 32°C in the dorsomedial NAcore, where the prefrontal inputs are most dense (Gorelova and Yang, 1997). Inhibitory synaptic transmission blocked with picrotoxin (50 μM), and AMPA and NMDA currents recorded in whole cell patch-clamp configuration. Glass microelectrodes (1-2 MΩ) were filled with cesium-based internal solution. To evoke postsynaptic currents, a bipolar stimulating electrode was placed ~300 μm dorsomedial of the recorded cell to maximize chances of stimulating PL afferents. The stimulation intensity chosen evoked a ~50% of maximal AMPA current. Recordings were collected every 20 sec and begun >10 min after the cell membrane was ruptured, to allow diffusion of the internal solution into the cell. AMPA currents were first measured at -80 mV to ensure stability of response. Then the membrane potential was gradually increased until +40 mV. Recording of currents was resumed 5 min after reaching +40 mV to allow stabilization of cell parameters. Currents composed of both AMPA and NMDA components were then obtained. Then D-AP5 was bath-applied (50 μM) to block NMDA currents and recording of AMPA currents at +40 mV was started after 2 min. NMDA currents were obtained by subtracting the AMPA currents from the total current at +40 mV.
Statistics
All spine density and dh data were statistically analyzed after averaging the values for all the neurons in each animal. The number of determinations in each group was established using an analysis of statistical power based on previous morphological data from our laboratory (Shen et al., 2009b). A/N data was analyzed using one-way analysis of variance (ANOVA). Behavioral data were analyzed using repeated-measures ANOVA, and t tests were used to compare dh and A/N in animals receiving aCSF or B/M. Additionally, linear regression was used to determine the association between magnitude of reinstated lever pressing and dh or A/N. Post-hoc comparisons were conducted using Bonferroni-corrected t tests.
Supplementary Material
Highlights.
-
▪
Cue-induced relapse to cocaine requires rapid neuronal plasticity.
-
▪
Relapse-induced plasticity correlates with the intensity of cocaine-seeking.
-
▪
Cue-induced synaptic plasticity requires neuronal activity in the prefrontal cortex.
-
▪
Cue-induced seeking of a natural reward does not induce neuronal plasticity.
Acknowledgments
We thank Dr. Rachel Smith, Dr. Joshua Beckmann, Megan Hensley, Brenton Mahaffey, Rebecca Szer, and Phong Do for technical assistance. This work was supported by DA007288, DA033690 (CDG) and DA03906, DA012513 and DA015369 (PWK) grants from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci. 2007;27:10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle HJ, Kennedy MB. Spine architecture and synaptic plasticity. Trends Neurosci. 2005;28:182–187. doi: 10.1016/j.tins.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Roo M, Klauser P, Garcia PM, Poglia L, Muller D. Spine dynamics and synapse remodeling during LTP and memory processes. Prog Brain Res. 2008;169:199–207. doi: 10.1016/S0079-6123(07)00011-8. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukazawa Y, Saitoh Y, Ozawa F, Ohta Y, Mizuno K, Inokuchi K. Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo. Neuron. 2003;38:447–460. doi: 10.1016/s0896-6273(03)00206-x. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelova N, Yang CR. The course of neural projection from the prefrontal cortex to the nucleus accumbens in the rat. Neuroscience. 1997;76:689–706. doi: 10.1016/s0306-4522(96)00380-6. [DOI] [PubMed] [Google Scholar]
- Gu J, Lee CW, Fan Y, Komlos D, Tang X, Sun C, Yu K, Hartzell HC, Chen G, Bamburg JR, Zheng JQ. ADF/cofilin-mediated actin dynamics regulate AMPA receptor trafficking during synaptic plasticity. Nature Neuroscience. 2010;13:1208–1215. doi: 10.1038/nn.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley JM, Barker EA, Glebov OO. Routes, destinations and delays: recent advances in AMPA receptor trafficking. Trends Neurosci. 2011;34:258–268. doi: 10.1016/j.tins.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander JA, Carelli RM. Cocaine-associated stimuli increase cocaine seeking and activate accumbens core neurons after abstinence. J Neurosci. 2007;27:3535–3539. doi: 10.1523/JNEUROSCI.3667-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Molecular psychiatry. 2011;16:974–986. doi: 10.1038/mp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Animal models of psychiatric disorders. In: Vinken PJ, Bruyn GW, editors. Handbook of clinical neurology. Vol. 106. 2012. pp. 137–166. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourrich S, Thomas MJ. Similar neurons, opposite adaptations: psychostimulant experience differentially alters firing properties in accumbens core versus shell. J Neurosci. 2009;29:12275–12283. doi: 10.1523/JNEUROSCI.3028-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krucker T, Siggins GR, Halpain S. Dynamic actin filaments are required for stable long-term potentiation (LTP) in area CA1 of the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:6856–6861. doi: 10.1073/pnas.100139797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28:3170–3177. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Shabat-Simon M, Shalev U, Barnea-Ygael N, Cooper A, Zangen A. Repeated electrical stimulation of reward-related brain regions affects cocaine but not “natural” reinforcement. J Neurosci. 2007;27:14179–14189. doi: 10.1523/JNEUROSCI.4477-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Martin M, Chen BT, Hopf FW, Bowers MS, Bonci A. Cocaine self-administration selectively abolishes LTD in the core of the nucleus accumbens. Nat Neurosci. 2006;9:868–869. doi: 10.1038/nn1713. [DOI] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Altered prelimbic cortex output during cue-elicited drug seeking. J Neurosci. 2004;24:6889–6897. doi: 10.1523/JNEUROSCI.1685-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12:182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Zhou W, Shen H, Reichel CM, See RE, Carr DB, Kalivas PW. Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proc Natl Acad Sci U S A. 2011;108:385–390. doi: 10.1073/pnas.1011265108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran B, Frey JU. Interfering with the actin network and its effect on long-term potentiation and synaptic tagging in hippocampal CA1 neurons in slices in vitro. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:12167–12173. doi: 10.1523/JNEUROSCI.2045-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav. 2002;71:517–529. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shen H, Moussawi K, Zhou W, Toda S, Kalivas PW. Heroin relapse requires long-term potentiation-like plasticity mediated by NMDA2b-containing receptors. Proc Natl Acad Sci U S A. 2011;108:19407–19412. doi: 10.1073/pnas.1112052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HW, Toda S, Moussawi K, Bouknight A, Zahm DS, Kalivas PW. Altered dendritic spine plasticity in cocaine-withdrawn rats. J Neurosci. 2009a;29:2876–2884. doi: 10.1523/JNEUROSCI.5638-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HW, Toda S, Moussawi K, Bouknight A, Zahm DS, Kalivas PW. Altered dendritic spine plasticity in cocaine-withdrawn rats. J Neurosci. 2009b;29:2876–2884. doi: 10.1523/JNEUROSCI.5638-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annual review of cell and developmental biology. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- Stefanik MT, Moussawi K, Kupchik YM, Smith KC, Miller RL, Huff ML, Deisseroth K, Kalivas PW, Lalumiere RT. Optogenetic inhibition of cocaine seeking in rats. Addict Biol. 2012 doi: 10.1111/j.1369-1600.2012.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Milovanovic M, Zhao Y, Wolf ME. Acute and chronic dopamine receptor stimulation modulates AMPA receptor trafficking in nucleus accumbens neurons cocultured with prefrontal cortex neurons. J Neurosci. 2008;28:4216–4230. doi: 10.1523/JNEUROSCI.0258-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocci F, Ling W. Medications development: successes and challenges. Pharmacol Ther. 2005;108:94–108. doi: 10.1016/j.pharmthera.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nat Neurosci. 2004;7:211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. The Bermuda Triangle of cocaine-induced neuroadaptations. Trends Neurosci. 2010;33:391–398. doi: 10.1016/j.tins.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci Biobehav Rev. 2010;35:185–211. doi: 10.1016/j.neubiorev.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Zhou Q. Spine modifications associated with long-term potentiation. Neuroscientist. 2009;15:464–476. doi: 10.1177/1073858409340800. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Homma KJ, Poo MM. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.