Abstract
Proteins of lens fiber cells are prone to accumulate extensive post-translational modifications because of very little protein turnover. Lens proteins are degraded via the lens proteolytic systems into peptides, which are subsequently hydrolyzed by downstream aminopeptidases. Inefficient degradation can lead to accumulation of protein fragments and subsequent aggregation. Previously we showed that αA-66-80 peptide and its truncated products accumulate in aging and cataract human lenses. These peptides interact with crystallins, causing crystallin aggregation and precipitation. N- and C-terminal-blocked peptides that have the cleavage sites to generate the αA-66-80 fragment were used to test lens extracts for sequence-specific proteases in lens extracts. An internally quenched fluorogenic peptide substrate containing the sequence-specific site for a lens protease to generate αA-66-80 peptide was designed, synthesized and used to characterize protease(s) that are capable of generating this peptide in bovine and human lenses. We show that proteases with the potential to generate αA-66-80 peptide are present in bovine and human lenses. We also show that the αA-66-80 peptides are resistant to hydrolysis by aminopeptidases present in the lenses and they can suppress the degradation of other peptides. Failure of complete hydrolysis of these peptides in vivo can lead to their accumulation in the lens and subsequent lens protein aggregation, which may ultimately lead to the formation of cataract.
Keywords: αA-66-80 peptide, crystallin, cataract, lens, protease, peptidase, hydrolysis
1. Introduction
The eye lens is composed of fiber cells that are endowed with highly stable, long-lived proteins known as crystallins. The primary function of crystallins is to maintain lens transparency (Bloemendal et al., 2004). A member of the small heat shock protein family, α-crystallin is composed of αA and αB subunits which are known to act as molecular chaperones and suppress misfolding and aggregation of denatured lens proteins (Horwitz, 1992). With aging, α-crystallin begins to lose its protective action, aggregates of crystallin begin to form and the lens starts accumulating water-insoluble modified crystallins that scatter light and lead to cataract (Sharma and Santhoshkumar, 2009).
Since protein turnover is negligible in differentiated lens fiber cells, lens proteins, including α-crystallin, undergo age-related extensive post-translational modifications that include but are not limited to deamidation, truncation, glycation, oxidation and phosphorylation, which are believed to induce functional changes (Chaves et al., 2008; Hanson et al., 2000; Kumar et al., 2007; Lund et al., 1996; Miesbauer et al., 1994; Sharma and Santhoshkumar, 2009; Truscott, 2005; Wilmarth et al., 2006). Many crystallin subunits, including αA- and αB-crystallin (Lampi et al., 1998; Srivastava et al., 2004) and βB1-crystallin (Alcala et al., 1988; Shih et al., 1998), are known to be extensively fragmented, even in young human lenses, generating crystallin-derived fragments and increasing the propensity for aggregation, cross-linking and insolubilization of lens proteins with age (David and Shearer, 1989; Hanson et al., 2000; Truscott, 2005). Degradation and removal of modified crystallins is necessary for maintaining the clarity of the lens. The proteolytic system that plays a crucial role in maintaining the health and clarity of the lens is believed to help in the degradation of modified lens proteins (David and Shearer, 1989; Shih et al., 2001; Wride et al., 2006). Several proteolytic enzymes have been shown to play a role in aging of the lens and cataract formation (Mathur et al., 2000; Sharma and Kester, 1996; Swanson et al., 1985; Wride et al., 2006). Despite the presence of the proteolytic systems that help maintain lens clarity, the truncated proteins and peptides accumulate in aged lenses, perhaps because of excessive truncated proteins and peptides production or due to the failure of the degradation system to break down the protein fragments (Hosler et al., 2003; Ruotolo et al., 2003; Viteri et al., 2004; Zeng et al., 2006).
In the aged human lens, a number of crystallin-derived peptides are present in the nuclear region, with most being in the innermost region (Sharma and Santhoshkumar, 2009; Su et al., 2010). In our earlier studies we showed that the crystallin peptides derived from aged lenses increase the molecular mass, polydispersity and hydrodynamic radius of αA- and αB-crystallins. Some of these peptides act as antichaperones by binding to α-crystallin subunits, whose function is to prevent aggregation of β- and γ-crystallins and non-crystallin protein substrates, a process believed to be essential for lens transparency (Santhoshkumar et al., 2008). Of the peptides identified from the nuclear region, 66SDRDKFVIFLDVKHF80 (αA-66-80) and its truncated forms viz, 66–75, 67–80 and 67–75 are the most prominent members, which also contain residues contributing to the chaperone site of αA-crystallin (Sharma et al., 2000). We have shown that this peptide can inhibit the chaperone activity of α-crystallin and cause aggregation and precipitation of lens crystallins. In presence of this peptide, α-crystallin aggregates and functions as a nucleus for protein aggregation, attracting additional α-, β- and γ-crystallins. In human lens this peptide starts to accumulate as early as 18 years of age and by 70 years of age, the peptide concentration is increased at least by 7-fold (Santhoshkumar et al., 2011). How this peptide is generated in aging lenses remains unknown. Nor is it clear why only a few peptides from crystallins, including αA-66-80 and its truncated forms, are present in the cortical and nuclear regions of the lens and why the rest of the peptides generated during proteolysis of α-crystallins are completely degraded into amino acids.
In this study, we report the role of lens enzymes in the generation of αA-66-80 peptide and its truncated forms. We describe an internally quenched fluorescence substrate we designed to discover specific proteases present in lenses. Our results indicate that more than one protease may be responsible for the generation of αA-66-80 peptides from α-crystallin. We also demonstrate that αA-66-80 peptides are resistant to hydrolysis by peptidases found in lenses, which explains their presence in aged lenses and their role in cataract formation.
2. Materials and Methods
Fresh young bovine lenses were purchased from Pel-Freez Arkansas LLC (Rogers, AR), and human lenses were obtained from the Lions Eye Tissue Bank of Missouri (Columbia, MO). Lenses were stored at −70 °C until use. The fluorogenic peptide MCA-SEVRSDRD[K-Dnp]RR-NH2 used in this study was custom synthesized at Pi Proteomics, LLC (Huntsville, AL). The peptide substrates, Succinyl-ISEVRSDRDK-Biotin (αA-61-70) and Succinyl-DVKHFSPEDK-Biotin (αA-76-85), were obtained from United Biosystems, Inc (Rockville, MD). Arg-MCA was obtained from Sigma-Aldrich (St. Louis, MO). αA-66-80 peptide was from GenScript Corporation (Piscataway, NJ). The purity level of all the peptides used in the study was > 95%. The peptides were dissolved in either sterile water or 200 μL phosphate buffer (50 mM, pH 7.2) containing 150 mM NaCl (PB).
2.1. Hydrolysis of synthetic peptides by lens proteases
Bovine and human (60 years) lenses were thawed, decapsulated and homogenized separately at 4°C in PB. The homogenates were centrifuged for 30 min at 30,000 × g. The supernatant was filtered through 0.45μM syringe filter, dialyzed overnight using 10 kDa membrane against PB at 4°C and used for the study after estimating the protein content using Bio-Rad Protein assay reagent. Two synthetic peptides, Succinyl-ISEVRSDRDK-Biotin (αA-61-70) and Succinyl-DVKHFSPEDK-Biotin (αA-76-85), 100 μg each, the cleavage of which would indicate the mechanism of αA-66-80 generation in vivo, were separately incubated with bovine and human lens extract containing 5 mg protein for 4, 8 and 16 h at 37 °C. A similar peptide-protease mixture without incubation and peptides and lens extract by themselves in assay buffer were used as controls. After incubation, the hydrolyzed peptides were separated from the protease mixture using 10 kDa centrifuge filters. The filtrate thus obtained was purified by RP-HPLC using C18 reverse-phase analytical column (Grace Vydac, Hesperia, CA) over a linear gradient (0 to 70%) of acetonitrile containing 0.1% trifluoroacetic acid. The HPLC was run for 35 min, at a flow rate of 1 mL/min. The elution was monitored at 220 nm using a PDA detector. The amount of peptide hydrolyzed was calculated from the difference in the peptide peak area. The 10 kDa filtrates from the control and 8 h incubation mixtures were also analyzed by LC-MS/MS (Proxeon Easy nLC-II HPLC system attached to an Orbitrap mass spectrometer) to identify the fragments generated during protease assy.
2.2. Design of an internally quenched fluorogenic substrate and detection of protease activity
An internally quenched peptide substrate with sequence similarity of αA-crystallin 62–70, MCA-SEVRSDRD[Lys(Dnp)]RR-NH2 (MCA-62-70), having the susceptible peptide bond between R-S and the cleavage of which will release the N-terminal end of αA-66-80 peptide, was custom synthesized to investigate the presence of specific protease activity in bovine and human lens homogenates. The peptide substrate also contained two Arg residues at the C-terminal region to increase the solubility. To determine protease activity, 50 μL (1 mg protein) of the lens homogenates or partially purified lens extracts were added to 1.4 nmoles of the MCA-62-70 substrate in 200 μL of PB (final volume) and incubated on a 96-well plate at 37 °C. Assay mixtures containing substrate as such or protein fractions without added substrate served as controls. Fluorescence was measured with a plate reader (FLx 800; BioTek, Winooski, VT), having filters for excitation at 340 ± 30 nm and emission at 395 ± 25 nm. The readings were recorded every minute for up to 30 min and the ΔFU/min was noted.
2.3. Cleavage pattern of MCA-62-70 substrate by lens protease(s)
To determine the hydrolysis pattern of the substrate by the lens enzyme, 5 mg of the partially purified lens protease extract was incubated with 50 μg of MCA-62-70 substrate in PB (pH 7.2) in a total volume of 500 μL at 37 °C for 5 h. Since the recovery of the products after 10 kDa centrifuge filtration was low, the hydrolytic products of the peptide were separated from the rest of the proteins by isopropanol extraction that causes larger proteins to precipitate while peptides are maintained in solution (Marney et al., 2008). For isopropanol precipitation an equal volume of ice-cold isopropanol was added to the reaction mixture, and the mixture was maintained at 4 °C for 15 min. The precipitated proteins were separated by centrifuging at 30,000 × g for 30 min. The supernatant was concentrated by vacuum evaporation and further purified by HPLC as described above and the elution of the fluorescent peptides was monitored using a fluorescence detector (Shimadzu), with excitation and emission set at 320 and 405 nm, respectively. The resultant peptide peaks were collected and analyzed by MALDI TOF MS (AB Sciex Voyager DEPro mass spectrometer). The spectra were processed with “Data Explorer” (v 4.0.0.0) software and the masses were calculated using “MS Isotope” calculator at http://prospector.ucsf.edu to identify the fragments.
2.4. Determination of Km value
Partially purified protease fraction, 1 mg, was added to different concentrations of the MCA-62-70 substrate (ranging from 1 to 14 μM) and incubated at 37 °C for 1 h. The fluorescence intensity of the resulting solution was measured as described earlier. Kinetic parameters were determined by estimating Michaelis-Menten parameters (Km, Vmax) by nonlinear curve fitting using the scientific software GraphPad Prism 6.0 (San Diego, CA).
2.5. Distribution of protease activity in bovine and human lenses
To determine the relative distribution of protease activity in bovine lens, decapsulated lenses were stirred with 50 mM sodium phosphate and 150 mm NaCl (pH 7.2) 4 °C until the outer cortical fibers (about a third of the wet weight) were dissolved. Soluble proteins from the disrupted lens fiber cells were separated and homogenized. With the remaining portion of the lens, stirring with fresh buffer was continued until the inner cortical fibers (about a third of the original weight) were dissolved. Following this, the remainder of the lens, often referred as lens nucleus, was homogenized in buffer. Each of the three homogenates were centrifuged at 30,000 × g for 30 min and the supernatants were designated as outer cortical, inner cortical and nuclear extracts. Cortical and nuclear fractions of young (20 ± 2 years), middle aged (40 ± 2 years) and old (60 ± 2 years) human lenses were also prepared by carefully separating the cortical and nuclear regions of the frozen lenses. One mg of each of the extracts was separately incubated with 25 μg of MCA-62-70 substrate for 2 h at 37 °C. Fluorescence was measured before and after incubation in a Jasco FP750 spectrofluorometer at excitation 326 nm and emission 398 nm.
2.6. Partial purification of the protease and peptidase from bovine lens extract
Bovine lens extract was prepared as described earlier (section 2.1). The extract was then run on a pre-equilibrated 16 × 600 mm Superdex 200 column (GE Life Sciences) using PB as mobile phase, and fractions of 1 mL were collected. The elution was monitored using a 280 nm absorption detector. The protease activity was monitored by MCA-62-70 substrate as described under section 2.2. The peak of MCA-62-70 active fractions were pooled, concentrated using 10 kDa cutoff centrifuge filters (Amicon, Millipore) and dialyzed against 25 mM Tris buffer, pH 7.5, (TB). The preparation was then run on a strong anion exchange column (Q Sepharose fast flow, 16 × 200 mm, GE Healthcare) which had been pre-equilibrated with TB. Elution was performed with a linear gradient of salt, up to 1 M NaCl in TB over five bed volumes. The elution was monitored using a 280 nm absorption detector. Fractions of 3 mL were collected at a flow rate of 1 mL/min and screened for protease activity using MCA-62-70 substrate, and the pooled concentrated protease was used for substrate hydrolysis study. In a separate elution of bovine lens extract through Sephadex G 200 column, using similar conditions as described above, the fractions were screened for peptidase activity using Arg-MCA substrate (Sharma and Ortwerth, 1986b) and the pooled concentrated peptidase active fractions were used for the hydrolysis of αA-66-80 substrate.
2.7. Effect of inhibitors on hydrolysis of MCA-62-70 substrate by lens protease
The bovine lens protease-rich fraction (1 mg) was pre-incubated with different protease inhibitors’ belonging to serine, cysteine and metalloproteinase classes at 37 °C for 30 min. Residual enzyme activity was measured using MCA-62-70 substrate as described above.
2.8. Hydrolysis of αA-66-80 peptide by bovine lens peptidase fraction
The peptidase-rich fraction of bovine lens extract (200 μg protein) was incubated with αA-66-80 peptide (12.5 nmoles) for 0, 2, 4, 6, 8 and 24 h at 37 °C in the presence and absence of bovine α-crystallin (12.5 nmoles) in a total volume of 0.25 ml. Bradykinin (12.5 nmoles), a known substrate that gets hydrolyzed rapidly by lens prolyl oligopeptidase and aminopeptidases (Chaerkady and Sharma, 2004), was used as a positive control. αA-66-80 peptide without peptidase served as the negative control. A mixture of αA-66-80 peptide and bradykinin was also incubated with peptidases. The resulting samples were analyzed by HPLC using a C8 reverse-phase analytical column (Grace Vydac, Hesperia, CA). The peptides were separated using a linear gradient of acetonitrile (0% to 10%), containing 0.1% trifluoroacetic acid and run for 5 min, followed by 10% to 60% run over 35 min at a flow rate of 1 mL/min.
3. Results
3.1. Hydrolysis of αA-61-70 and αA-76-85 peptides by lens extracts
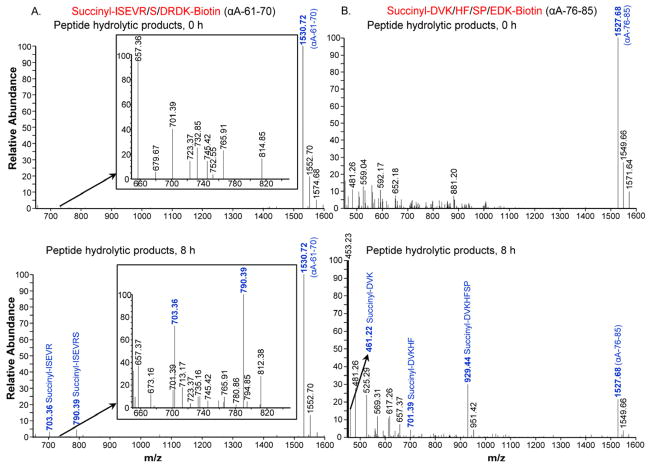
To determine whether lens extract contains proteases capable of cleaving peptide bonds corresponding to αA-65-66 and αA-80-81, N- and C-terminal-blocked αA61-70 and αA76-85 peptides were used with bovine lens extracts and the products were analyzed by LC-MS. The LC-MS profile of the hydrolyzed synthetic peptide αA-61-70 showed that the peptide was cleaved at two sites, with nearly equal intensity, to generate fragments corresponding to αA-61-65 (m/z 703.36) and αA-61-66 (m/z 790.39) (Fig. 1A). The results were reproducible when repeated with the enzyme fraction isolated from human lenses (data not shown). Peaks corresponding to αA-76-78 (m/z 461.22), αA-76-80 (m/z 701.39) and αA-76-82 (m/z 929.44) were identified in LC-MS analysis when lens extract was used to hydrolyze αA-76-85 peptide substrate (Fig. 1B). αA-76-82 fragment was the most abundant followed by αA-76-78 and αA-76-80. HPLC analysis of the incubation mixtures showed that αA-76-85 peptide was 6 times more susceptible to hydrolysis than αA-61-70 peptide (Table 1). Ther esults suggest that the αA-66-80 and its truncated forms identified earlier in human lenses accumulate because of enzymatic hydrolysis of αA-crystallin.
Fig. 1. LC-MS profile of peptide substrates hydrolyzed by bovine lens protease-rich fraction.
(A) αA-61-70 (Succinyl-ISEVRSDRDK-Biotin), (B) αA-76-85 (Succinyl-DVKHFSPEDK-Biotin), was incubated with protease-rich bovine lens fraction for 8 h at 37 °C. Similar peptide-protease mixture without incubation was used as control. The hydrolyzed peptides were separated from bulk of the proteins by 10 kDa cutoff centrifuge filters and analyzed by LC-MS. αA-61-70 peptide was cleaved at two sites to generate the fragments corresponding to αA-61-65 (m/z 703.36) and αA-61-66 (m/z 790.39) with nearly equal intensity. Peaks corresponding to αA-76-78 (m/z 461.22), αA-76-80 (m/z 701.39) and αA-76-82 (m/z 929.44) were identified when αA-76-85 peptide was analyzed. Insets in A are expanded portions of the profiles. Additional peptide signals seen in the spectrum are due the presence of crystallin fragments present in the enzyme preparation and were not analyzed further.
Table 1.
Hydrolysis of αA-61-70 and αA-76-85 peptides by bovine lens protease extracts.
| Peptide | Incubation time (h) | % Peptide hydrolyzed |
|---|---|---|
|
| ||
| αA-61-70 | 0 | 0 |
| 4 | 10.0 | |
| 8 | 26.7 | |
| 16 | 26.8 | |
|
| ||
| αA-76-85 | 0 | 0 |
| 4 | 97.8 | |
| 8 | 100 | |
Synthetic peptides were incubated with bovine lens extract at 37°C for different time intervals. The hydrolyzed peptides obtained after 10 kDa centrifuge filtration were purified using RP-HPLC using C18 analytical column. The amount of substrate hydrolyzed was calculated from peptide peak area. αA-76-85 peptide was completely hydrolyzed in 4-8 h, whereas αA-61-70 peptide was only partially hydrolyzed even after prolonged incubation. Lens extract incubated without synthetic peptides and analyzed at the same time points did not have any peak matching the synthetic peptides used in this study or the products generated by the action of protease.
3.2. Hydrolysis of MCA-62-70 substrate
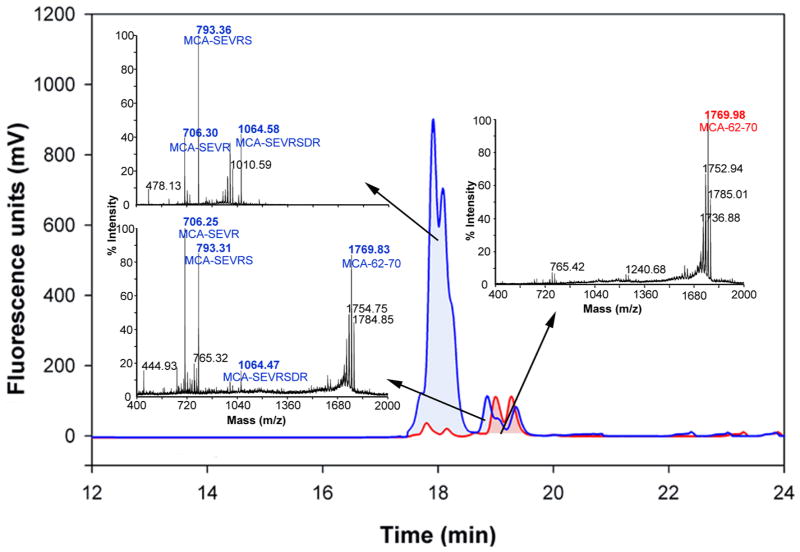
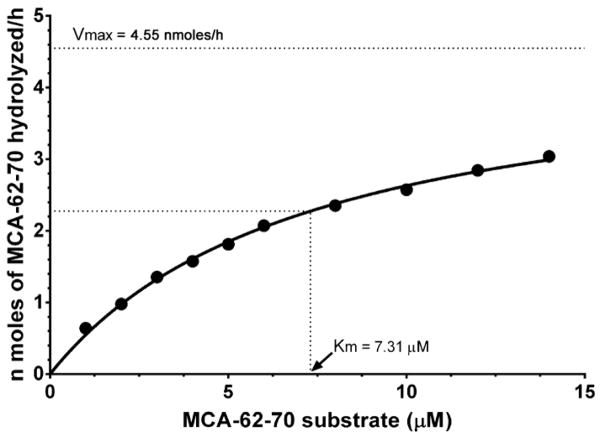
When intact MCA-62-70 substrate was excited at 320 nm, nearly complete suppression of MCA (donor) fluorescence occurred due to its close proximity to Dnp (acceptor) group. Proteolysis at any backbone peptide bonds that separate the donor and acceptor will result in removal of the Dnp quenching effect and sharp rise in MCA fluorescence. A time-dependent increase in the fluorescence was observed when the MCA-62-70 substrate was incubated with lens extracts suggesting the hydrolysis of the peptide. To establish the preferred sites of cleavage(s) in MCA-62-70 substrate, mass spectral studies of the product was performed. When the products were separated from the lens enzyme fraction by HPLC and analyzed by LC-MS, fragments corresponding to MCA-62-65 (MCA-SEVR), MCA-62-66 (MCA-SEVRS) and MCA-62-68 (MCA-SEVRSDR) were observed, with MCA-62-66 the predominant fragment (Fig. 2). Similar results were obtained with hydrolysis of substrate with partially purified human and bovine lens enzyme fractions (data not shown). Figure 3 shows the hydrolysis of MCA-62-70 at different substrate concentrations by lens protease. The kinetic parameters Km and Vmax for MCA-62-70 are 7.3 μM and 4.6 nmoles hour−1, respectively.
Fig. 2. C18 RP-HPLC elution profile of protease-rich fraction of human (20 years) lens extract and MCA-62-70 and LC-MS results.
MCA-62-70 substrate (MCA-SEVRSDRD[Lys(Dnp)]RR, 50 μg) was incubated with protease-rich human (20 years) lens extract (5 mg) for 5 h at 37 °C. The hydrolyzed peptides were separated from bulk of the proteins by 1:1 isopropanol extraction. The extracted peptides were eluted through C18 RP-HPLC column (
 ). The elution was monitored using a fluorescence detector with excitation 320 nm and emission 405 nm. A similar sample without incubation served as a control (
). The elution was monitored using a fluorescence detector with excitation 320 nm and emission 405 nm. A similar sample without incubation served as a control (
 ). The collected fractions of peaks were analyzed by LC-MS analysis. Hydrolytic cleavages were observed at 62-65 (MCA-SEVR), 62-66 (MCA-SEVRS) and 62-68 (MCA-SEVRSDR) regions, with 62-66 being the predominant.
). The collected fractions of peaks were analyzed by LC-MS analysis. Hydrolytic cleavages were observed at 62-65 (MCA-SEVR), 62-66 (MCA-SEVRS) and 62-68 (MCA-SEVRSDR) regions, with 62-66 being the predominant.
Fig. 3. Kinetic properties of MCA-62-70 substrate.
The Km value was determined by incubating different concentrations of substrate (1 to 14 μM) with partially purified protease fraction for 1 h at 37 °C.
3.3. Fractionation of protease and peptidases from bovine lens extract
The elution profile of a crude bovine lens extract on a Superdex 200 column is shown in Figure 4. When all fractions were assayed with internally quenched MCA-62-70 substrate, the protease activity was found to elute between βH and βL-crystallin peaks. The pooled active protease fraction was further purified using strong anion-exchange column, Q-Sepaharose (GE healthcare). The protease activity bound to this column was eluted using buffer containing 0.5 M NaCl (data not shown) and this fraction, showing 8-fold purification of the activity was used to investigate inhibitor susceptibility of the protease. The peptidase activity assayed with Arg-MCA eluted as a broad peak from the Superdex 200 column and overlapped the MCA-62-70 activity (Figure 4). However, as the endopeptidase and aminopeptidase activities hydrolyze different peptide bonds, we were able to use the fractions that contained both activities in different assays. Further, aminopeptidase will not hydrolyze the protease substrates used in this study since both N- and C-terminals were blocked. When our protease preparation was treated with bestatin, a known aminopeptidase inhibitor, complete inhibition of Arg-MCA hydrolyzing activity (aminopeptidase activity) was observed but bestatin had very little effect on the hydrolysis of MCA-62-70 substrate (Table 2). This suggests that that MCA-62-70 hydrolyzing activity we observed is exclusively due to protease(s) under investigation.
Fig. 4. Elution profile of bovine lens extract (BLE) in Superdex 200 column.
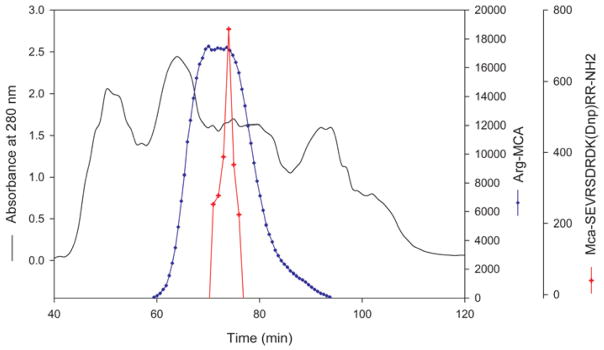
Water-soluble bovine lens extract was passed through pre-equilibrated Superdex 200 column (16 × 600 mm). The elution was monitored using a 280 nm absorption detector. Fractions of 1 mL were collected. The protease activity in the fractions was monitored using MCA-62-70 as substrate (
 ). The peptidase activity (
). The peptidase activity (
 ) was monitored by Arg-MCA substrate. The MCA-62-70 hydrolyzable protease eluted between βH and βL-crystallin peaks.
) was monitored by Arg-MCA substrate. The MCA-62-70 hydrolyzable protease eluted between βH and βL-crystallin peaks.
Table 2.
Hydrolysis of MCA-62-70 substrate by lens protease in the presence of inhibitors.
| Mechanistic type | Inhibitor | Concentration | Relative activity (%) |
|---|---|---|---|
| 1. Serine | DFP | 1mM | 78 |
| 3, 4-DCI | 500μM | 15 | |
|
| |||
| 2. Cysteine | E-64 | 1mM | 100 |
| N-methylmaleimide | 0.2mM | 12 | |
| H2O2 | 0.5mM | 48 | |
| DTNB | 0.2mM | 1 | |
|
| |||
| 3. Metallo-proteinase | 1,10 Phenanthroline | 1mM | 1 |
| 500μM | 8 | ||
| 100μM | 59 | ||
|
| |||
| EDTA | 5mM | 46 | |
| 10mM | 15 | ||
|
| |||
| EGTA | 1mM | 51 | |
|
| |||
| 4. Others | Bestatin | 0.5mM | 88 |
| Iodoacetamide | 1mM | 97 | |
| DTT | 2.5mM | 4 | |
| TCEP | 1mM | 49 | |
| CaCl2 | 2mM | 94 | |
| ZnCl2 | 1mM | 72 | |
| MgCl2 | 1mM | 111 | |
Bovine lens protease-rich fraction (1 mg) was pre-incubated with different reagents at 37 °C for 30 min, and the residual proteolytic activity was measured using MCA-62-70 (5μg) substrate as described under methods.
3.4. Effect of inhibitors on MCA-62-70 hydrolytic activity
Different inhibitors capable of inhibiting serine, cysteine and metalloproteinases were pre-incubated with the bovine lens protease-rich fraction for 30 min at 37 °C, before adding MCA-62-70 substrate. The measurement of the rate of enzymatic hydrolysis of substrate revealed that 1, 10 phenanthroline (1 mM), a metalloproteinase inhibitor, and DTNB (0.2 mM), a cysteine proteinase inhibitor, almost completely inhibited the hydrolysis of internally quenched substrate by the lens protease (Table 2). N-Methylmaleimide, another cysteine proteinase inhibitor, suppressed the hydrolysis of the substrate by 88%. However, cysteine protease inhibitor E-64 did not have an effect on proteolytic activity at 1 mM concentration. EDTA and EGTA, the metalloproteinase inhibitors used in the study, reduced the protease activity only by 50%. Serine protease inhibitor 3, 4-DCI inhibited proteolytic activity up to 85% at 500 μM concentration. However, diisopropylfluorphosphate(DFP) inhibited the hydrolysis of substrate partially, about 22% at 1 mM concentration.
3.5. MCA-62-70 Hydrolytic activity in different regions of bovine lenses
We determined relative enzymatic activity in different regions of the bovine lens by incubating MCA-62-70 substrate with 1 mg protein extracts prepared form outer cortex, inner cortex and nuclear regions. The outer cortical region of the lens had relatively higher enzymatic activity (0.58 ± 0.04 nmoles/hr/mg protein) compared to the inner cortex (0.10 ± 0.01 nmoles/hr/mg protein). The nuclear region of bovine lens showed the least enzymatic activity (0.04 ± 0.003 nmoles/hr/mg protein), which suggests that the newly formed lens fiber cells have higher enzymatic activity than the older fiber cells present in the nuclear region. Because of the higher specific activity of the protease in the outer cortical region we conclude that outermost lens fibers contained the bulk of the total MCA-62-70 hydrolytic activity in a given lens.
3.6. Hydrolysis of MCA-62-70 by human lenses of different age
We determined relative enzymatic activity in water-soluble proteins in different age groups of human lenses. Young (20 ± 2 years), middle-aged (40 ± 2 years) and old (60 ± 2 years) human lens extracts were incubated with MCA-62-70 substrate. The total protease activity in young lenses hydrolyzed 8.84 ± 0.96 nmoles/h of MCA-62-70 substrate, which was greater than the protease activity in middle-aged (7.26 ± 0.83 n moles/h) and older human lenses (5.49 ± 0.29 nmoles/h), indicating an age-related decrease in protease activity in the soluble fraction of the lens (The water-insoluble fraction was not tested for protease activity). As in bovine lenses, the human lens cortical region of all age groups had nearly 90% of the total lens enzymatic activity and the nuclear region accounted for the remainder of activity (Table 3).
Table 3.
Comparison of the rate of hydrolysis of MCA-62-70 substrate in different age groups of human and young bovine lens extracts.
| Type of lens | Cortex | Nucleus | ||
|---|---|---|---|---|
|
| ||||
| Specific activity (nmoles/mg protein/h) | Total activity in cortex (nmoles/h) | Specific activity (nmoles/h) protein/h) | Total activity in nucleus (nmoles/mg | |
| Human young (20 years) | 0.28 ± 0.04** | 7.86 ± 0.89 | 0.043 ± 0.003** | 0.98 ± 0.07 |
| Human middle aged (40 years) | 0.37 ± 0.04*** | 6.50 ± 0.78 | 0.032 ± 0.001*** | 0.76 ± 0.05 |
| Human old aged (60 years) | 0.29 ± 0.02** | 4.92 ± 0.27 | 0.037 ± 0.001** | 0.57 ± 0.02 |
| Bovine young | 0.68 ± 0.07*** | 95.87 ± 3.26 | 0.035 ± 0.001*** | 7.53 ± 0.31 |
Water-soluble cortical and nuclear extracts (1 mg protein) of young, middle-aged and old human lenses were incubated with MCA-62-70 substrate (25 μg) at 37 °C for 2 h. Difference in fluorescence intensity was measured before and after incubation in a spectrofluorometer after excitation at 326 nm and emission at 398 nm. The amount of MCA-62-70 hydrolyzed was calculated by extrapolation of standard graph prepared by tryptic digestion of different concentrations of MCA-62-70 substrate. The values presented are average ± SE of four independent experiments. The data was analyzed by one-way ANOVA followed by Tukey’s multiple comparison test where **p<0.001 and ***p<0.0001, compared within lens groups of cortex and nucleus. Differences between lens groups of different ages were not significant.
3.7. Hydrolysis of αA-66-80 peptide by bovine lens peptidases
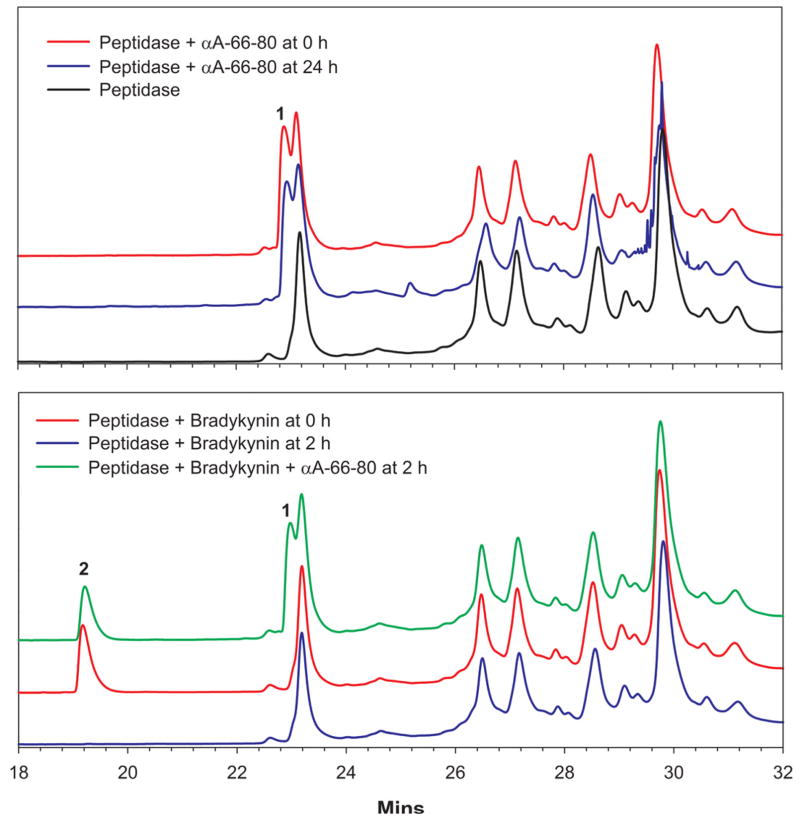
Earlier we reported that αA-66-80 peptide accumulates in the lens. Aminopeptidases are believed to act in concert to completely degrade the products of proteolysis into amino acids. To investigate whether αA-66-80 accumulates in lens because of the lack of peptidase activity, we tested the susceptibility of bradykinin as well as αA-66-80 peptide to hydrolysis by peptidases present in lenses. We used the peptidase fraction from bovine lens, since both human and bovine lenses contain similar peptidases (Sharma et al., 1996). When αA-66-80 peptide was incubated with peptidase-rich bovine lens extract, even after incubation for 24 h at 37 °C, we did not find significant degradation of the peptide (Fig. 5, upper panel). However, under similar conditions, bradykinin was completely hydrolyzed by peptidases within 2 h of incubation at 37 °C. Furthermore, the addition of 12.5 nmoles of α-crystallin to the peptidase decreased to 30 minutes the time required for complete hydrolysis of bradykinin but had no effect on hydrolysis of αA-66-80 peptide (data not shown). Interestingly, when bradykinin and αA-66-80 peptides were simultaneously incubated with bovine lens peptidases, bradykinin was hydrolyzed, but at a 40% slower rate than when bradykinin was incubated alone (Fig. 5, lower panel, peak 2).
Fig. 5. Degradation of αA-66-80 peptide by peptidase-rich bovine lens extract.
RP-HPLC using C8 column was used to analyze the hydrolysis of αA-66-80 peptide. Top panel – Incubation of αA-66-80 peptide with peptidase-rich fraction from bovine lens at 37 °C for 24 h did not hydrolyze the peptide (blue chromatogram, peak 1). Bottom panel – Incubation of bradykinin for 2 h at 37 °C with peptidase rich fraction from bovine lens resulted in complete hydrolysis of substrate (compare red and blue chromatogram, peak 2). The addition of αA-66-80 peptide to the incubation mixture containing bradykinin and peptidase prevented the complete hydrolysis of bradykinin (green chromatogram, peak 2). Peaks 1 and 2 represent αA-66-80 peptide and bradykinin, respectively. Other peaks in the chromatogram are from the proteins present in the enzyme fraction used in the assay.
4. Discussion
There is increasing evidence that peptide-induced aggregation of proteins can lead to many disease conditions, including neurodegenerative diseases, prion diseases and Type 2 diabetes mellitus (Du et al., 2006; Hoppener and Lips, 2006; Koo et al., 1999; Temussi et al., 2003). The αA-66-80 peptide and its truncated forms are implicated in lens protein aggregation and cataract (Santhoshkumar et al., 2011). These peptides carry a portion of the αA-crystallin chaperone site and possess sequence similarity to that of a region responsible for fibril formation in β-amyloid (Santhoshkumar and Sharma, 2004). In vivo breakdown of crystallin leading to the generation of peptides can occur due to different reasons. It may result from the action of lens proteolytic enzymes (David and Shearer, 1989; Shih et al., 2001; Wride et al., 2006) or from non-enzymatic mechanisms (Voorter et al., 1988). Occasionally, crystallin fragmentation is part of the normal lens maturation process (Miesbauer et al., 1994). It is likely that the fragments of αA-66-80 peptides viz., αA-66-75 and αA-67-75 are generated in vivo either from αA-66-80 peptide by the action of aminopeptidases and carboxypeptidases present in the lens (Nakajima et al., 2009; Sharma and Kester, 1996) or by the action of protease on αA-crystallin. Our study suggests that the generation of αA-66-80 and its truncated forms in the lens may be due to proteolytic hydrolysis of αA-crystallin. In MALDI imaging mass spectrometric studies of different aged human lenses, multiple truncated products of α-crystallin were found to be altered in intensity and distribution with lens cell age and tissue age (Grey and Schey, 2009). Among the identified truncated products were αA-1-65 and αA-1-80. A protease responsible for the generation of αA-1-65 fragment can also act on αA-1-80 fragment, leading to the release of αA-66-80 peptide in lenses. At this time it is unclear whether αA-66-80 is generated in vivo by the action of a single protease on native or modified αA-crystallin since our attempts to purify a protease from lens extract and to demonstrate the release of αA-66-80 peptide from isolated WT-αA-crystallin have not yet been successful.
The αA-66-80 peptide and its truncated forms are mostly found in the nuclear region of aged and cataract lenses (Santhoshkumar et al., 2011; Su et al., 2010). We determined relative enzymatic activity in different regions of the lens to identify any correlation between protease activity and the level of αA-66-80. We found the lowest level of enzymatic activity in the nuclear region, compared to a moderate level in the inner cortex and the highest level in the outer cortical region (Table 3). When we estimated the relative protease activity in different age groups of human lenses, we found that aged (60 ± 2 years) lenses had 38% less activity as compared to young (20± 2 years) human lenses. Again, the cortical region of all age groups had nearly 90% of the total lens enzymatic activity. Relatively less proteolytic activity in the nuclear region of lens may be due to the age-related inactivation of the enzymes.
Lens is known to contain leucine aminopeptidase (Henson and Frohne, 1976; Kim and Lipscomb, 1994; Spector, 1963; Taylor et al., 1981) and other peptidases, including aminopeptidase III (Sharma and Ortwerth, 1986a, b), dipeptidyl peptidases II and III (Swanson et al., 1981), prolyloligopeptidases (Sharma and Ortwerth, 1994; Swanson et al., 1984), acylpeptide hydrolase (Sharma and Ortwerth, 1993), carboxypeptidase (Nakajima et al., 2009) and exopeptidases A, B, D, E and S (Lafferty et al., 1984). Studies have also shown that the activities of these peptidases are significantly reduced in the nuclear region of the lenses, where older fiber cells reside. The peptidases are believed to help in the complete degradation of the products of proteolysis into amino acids. In spite of the presence of these peptidases, as we age large numbers of peptides, including αA-66-80, accumulate in lenses. Interestingly, several peptides that accumulate in aged and cataract lenses are different from the ones that are found in young lenses. It is possible that the peptides that accumulate in aged and cataract lenses are either resistant to the action of peptidases or they are not completely hydrolyzed due to very low activity of the peptidases, at least in the nuclear region, whereas the peptides from young lenses are being degraded into amino acids in due course of time. To test our hypothesis that the peptides that accumulate in the lens are resistant to hydrolysis by lens peptidases, we incubated αA-66-80 peptide, which is primarily found in aged and cataract lens (Santhoshkumar et al., 2011; Sharma et al., 2000), with the peptidase-rich fraction from bovine lens extract and found that the peptide is resistant to complete hydrolysis. When 12.5 nmoles of bovine α-crystallin was incubated with αA-66-80 peptide and bovine peptidases, there was nearly a 20% reduction in intact αA-66-80 peptide after 24 h of incubation at 37 °C, suggesting that αA-66-80 peptide is relatively resistant to hydrolysis. We found that the addition of 12.5 nmoles of α-crystallin to the incubation mixture increases the ability of peptidase-rich bovine lens fraction to hydrolyze Arg-MCA substrate by nearly 4 fold (data not shown) but α-crystallin did not help in the hydrolysis of αA-66-80. The increase in the Arg-MCA hydrolysis was most likely due to stabilization of peptidase(s) by α-crystallin. The addition of a similar concentration of α-crystallin to the mixture of bradykinin and bovine peptidase decreased by half the time required for hydrolysis of bradykinin. The reduction in the amount of αA-66-80 peptide hydrolyzed may be due to the binding of αA-66-80 peptide to bovine α-crystallin. In another experiment, when αA-66-80 was incubated along with bradykinin and bovine peptidases, bradykinin was partially hydrolyzed whereas αA-66-80 was not hydrolyzed, indicating that αA-66-80 peptide may also be interfering with the hydrolysis of bradykinin. It is unlikely that beta-crystallins present in our enzyme preparation interfere in the hydrolysis of bradykinin because the same fraction without added 66–80 peptide completely hydrolyzed bradykinin. Similar mechanisms may prevent the complete hydrolysis of peptides accumulating in aging lenses, which in turn can interact with crystallins and cause aggregation and precipitation.
When inhibitors capable of differentiating proteases belonging to different mechanistic classes were pre-incubated with an enzyme-rich lens fraction and tested with MCA-62-70 substrate, almost complete inhibition of substrate hydrolysis was induced by the metalloproteinase inhibitor 1,10-phenanthroline and by the cysteine proteinase inhibitor DTNB, (Table 2). N-Methylmaleimide, a cysteine proteinase inhibitor, also showed good inhibition of hydrolysis (~88%) of the substrate. The partial inhibition of the protease activity by other inhibitors tested (Table 2) indicate that more than one protease may be involved in the hydrolysis of synthetic peptide substrate. However, it is unlikely that the major protease responsible for the hydrolysis of MCA-62-70 is calpain because both E-64 and Ca2+ showed negligible effect on the hydrolytic activity. Further, significant resistance to DFP, bestatin and iodoactamide suggest that the MCA-62-70 hydrolytic activity is other than the know peptidases present in lens such as leucineaminopeptidase, aminopeptidase III, prolyloligopeptidase or acylpeptidehydrolase.
These studies provide the first evidence that the antichaperone αA-66-80 peptide is most likely generated in the aged human lens by enzymatic hydrolysis of αA-crystallin and that the peptide is resistant to hydrolysis by peptidases present in the lens. To our knowledge this is the first report of the synthesis of an internally quenched fluorescence peptide to investigate a sequence-specific lens protease. The half-life of most peptides generated in vivo is only a few seconds (Reits et al., 2004), which prevents the accumulation of potentially hazardous aggregation-prone peptides. Failure of complete degradation of proteins can lead to protein accumulation and subsequent aggregation. Many such peptides accumulate over the age in lens, particularly in nuclear region, and may be resistant to hydrolysis by peptidases due to the binding of the peptides to other proteins. Further, the aggregation-prone β-sheet peptides such as αA-66-80 may also prevent hydrolysis of other, easily hydrolysable peptides by interaction with those peptides. The peptides are known to bind to lens crystallins (Santhoshkumar et al., 2011) to form high molecular weight aggregates similar to those found in the nuclear region of human lenses, which may ultimately lead to the cataract.
Highlights.
Reasons for the generation and accumulation of αA-66-80 peptide in the lens.
An internally quenched fluorogenic substrate was used to identify the proteases.
One or more proteases are responsible for generation of these peptides.
αA-66-80 peptide is resistant to hydrolysis by lens peptidases.
Accumulation of αA-66-80 may promote protein aggregation and cataract development.
Acknowledgments
Supported by Grant EY19878 from National Institutes of Health grants and an unrestricted grant-in-aid from Research to Prevent Blindness to the Department of Ophthalmology. We thank Sharon Morey for help with the preparation of manuscript and Proteomics Center, University of Missouri-Columbia for performing mass spectrometry analysis.
Abbreviations
- EDTA
ethylene diamine tetraacetic acid
- EGTA
ethylene glycol tetraacetic acid
- LC-MS
liquid chromatography-mass spectrometry
- MALDI TOF MS
matrix-assisted laser desorption ionization time-of-flight mass spectrometry
- PB
phosphate buffer
- PDA
photodiode array
- RP-HPLC
reverse-phase high-performance liquid chromatography
- TB
tris buffer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alcala J, Katar M, Rudner G, Maisel H. Human beta crystallins: regional and age related changes. Curr Eye Res. 1988;7:353–359. doi: 10.3109/02713688809031784. [DOI] [PubMed] [Google Scholar]
- Bloemendal H, de Jong W, Jaenicke R, Lubsen NH, Slingsby C, Tardieu A. Ageing and vision: structure, stability and function of lens crystallins. Prog Biophys Mol Biol. 2004;86:407–485. doi: 10.1016/j.pbiomolbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Chaerkady R, Sharma KK. Characterization of a bradykinin-hydrolyzing protease from the bovine lens. Invest Ophthalmol Vis Sci. 2004;45:1214–1223. doi: 10.1167/iovs.03-0769. [DOI] [PubMed] [Google Scholar]
- Chaves JM, Srivastava K, Gupta R, Srivastava OP. Structural and functional roles of deamidation and/or truncation of N- or C-termini in human alpha A-crystallin. Biochemistry. 2008;47:10069–10083. doi: 10.1021/bi8001902. [DOI] [PubMed] [Google Scholar]
- David LL, Shearer TR. Role of proteolysis in lenses: a review. Lens Eye Toxic Res. 1989;6:725–747. [PubMed] [Google Scholar]
- Du HN, Li HT, Zhang F, Lin XJ, Shi JH, Shi YH, Ji LN, Hu J, Lin DH, Hu HY. Acceleration of alpha-synuclein aggregation by homologous peptides. FEBS Lett. 2006;580:3657–3664. doi: 10.1016/j.febslet.2006.05.050. [DOI] [PubMed] [Google Scholar]
- Grey AC, Schey KL. Age-related changes in the spatial distribution of human lens alpha-crystallin products by MALDI imaging mass spectrometry. Invest Ophthalmol Vis Sci. 2009;50:4319–4329. doi: 10.1167/iovs.09-3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson SR, Hasan A, Smith DL, Smith JB. The major in vivo modifications of the human water-insoluble lens crystallins are disulfide bonds, deamidation, methionine oxidation and backbone cleavage. Exp Eye Res. 2000;71:195–207. doi: 10.1006/exer.2000.0868. [DOI] [PubMed] [Google Scholar]
- Henson H, Frohne M. Crystalline leucine aminopeptidase from lens (alpha-aminoacyl-peptide hydrolase; EC 3.4.11.1) Methods Enzymol. 1976;45:504–520. doi: 10.1016/s0076-6879(76)45045-0. [DOI] [PubMed] [Google Scholar]
- Hoppener JW, Lips CJ. Role of islet amyloid in type 2 diabetes mellitus. Int J Biochem Cell Biol. 2006;38:726–736. doi: 10.1016/j.biocel.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci USA. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosler MR, Wang-Su ST, Wagner BJ. Targeted disruption of specific steps of the ubiquitin-proteasome pathway by oxidation in lens epithelial cells. Int J Biochem Cell Biol. 2003;35:685–697. doi: 10.1016/s1357-2725(02)00397-7. [DOI] [PubMed] [Google Scholar]
- Kim H, Lipscomb WN. Structure and mechanism of bovine lens leucine aminopeptidase. Adv Enzymol Relat Areas Mol Biol. 1994;68:153–213. doi: 10.1002/9780470123140.ch4. [DOI] [PubMed] [Google Scholar]
- Koo EH, Lansbury PT, Jr, Kelly JW. Amyloid diseases: abnormal protein aggregation in neurodegeneration. Proc Natl Acad Sci USA. 1999;96:9989–9990. doi: 10.1073/pnas.96.18.9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar PA, Kumar MS, Reddy GB. Effect of glycation on alpha-crystallin structure and chaperone-like function. Biochem J. 2007;408:251–258. doi: 10.1042/BJ20070989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty MA, Raducha M, Harris H. Soluble exopeptidases of bovine and human lens: characterization by electrophoresis. Curr Eye Res. 1984;3:1017–1031. doi: 10.3109/02713688409011748. [DOI] [PubMed] [Google Scholar]
- Lampi KJ, Ma Z, Hanson SR, Azuma M, Shih M, Shearer TR, Smith DL, Smith JB, David LL. Age-related changes in human lens crystallins identified by two-dimensional electrophoresis and mass spectrometry. Exp Eye Res. 1998;67:31–43. doi: 10.1006/exer.1998.0481. [DOI] [PubMed] [Google Scholar]
- Lund AL, Smith JB, Smith DL. Modifications of the water-insoluble human lens alpha-crystallins. Exp Eye Res. 1996;63:661–672. doi: 10.1006/exer.1996.0160. [DOI] [PubMed] [Google Scholar]
- Marney LC, Laha TJ, Baird GS, Rainey PM, Hoofnagle AN. Isopropanol protein precipitation for the analysis of plasma free metanephrines by liquid chromatography-tandem mass spectrometry. Clin Chem. 2008;54:1729–1732. doi: 10.1373/clinchem.2008.104083. [DOI] [PubMed] [Google Scholar]
- Mathur P, Gupta SK, Wegener AR, Breipohl W, Ahrend MH, Sharma YD, Gupta YK, Vajpayee RB. Comparison of various calpain inhibitors in reduction of light scattering, protein precipitation and nuclear cataract in vitro. Curr Eye Res. 2000;21:926–933. doi: 10.1076/ceyr.21.6.926.6990. [DOI] [PubMed] [Google Scholar]
- Miesbauer LR, Zhou X, Yang Z, Sun Y, Smith DL, Smith JB. Post-translational modifications of water-soluble human lens crystallins from young adults. J Biol Chem. 1994;269:12494–12502. [PubMed] [Google Scholar]
- Nakajima E, David LL, Riviere MA, Azuma M, Shearer TR. Human and monkey lenses cultured with calcium ionophore form alphaB-crystallin lacking the C-terminal lysine, a prominent feature of some human cataracts. Invest Ophthalmol Vis Sci. 2009;50:5828–5836. doi: 10.1167/iovs.09-4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reits E, Neijssen J, Herberts C, Benckhuijsen W, Janssen L, Drijfhout JW, Neefjes J. A major role for TPPII in trimming proteasomal degradation products for MHC class I antigen presentation. Immunity. 2004;20:495–506. doi: 10.1016/s1074-7613(04)00074-3. [DOI] [PubMed] [Google Scholar]
- Ruotolo R, Grassi F, Percudani R, Rivetti C, Martorana D, Maraini G, Ottonello S. Gene expression profiling in human age-related nuclear cataract. Mol Vis. 2003;9:538–548. [PubMed] [Google Scholar]
- Santhoshkumar P, Raju M, Sharma KK. alphaA-crystallin peptide SDRDKFVIFLDVKHF accumulating in aging lens impairs the function of alpha-crystallin and induces lens protein aggregation. PLoS One. 2011;6:e19291. doi: 10.1371/journal.pone.0019291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhoshkumar P, Sharma KK. Inhibition of amyloid fibrillogenesis and toxicity by a peptide chaperone. Mol Cell Biochem. 2004;267:147–155. doi: 10.1023/b:mcbi.0000049373.15558.b8. [DOI] [PubMed] [Google Scholar]
- Santhoshkumar P, Udupa P, Murugesan R, Sharma KK. Significance of interactions of low molecular weight crystallin fragments in lens aging and cataract formation. J Biol Chem. 2008;283:8477–8485. doi: 10.1074/jbc.M705876200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma KK, Elser NJ, Kester K. Comparison of leucine aminopeptidase and aminopeptidase III activities in lens. Curr Eye Res. 1996;15:774–781. doi: 10.3109/02713689609003462. [DOI] [PubMed] [Google Scholar]
- Sharma KK, Kester K. Peptide hydrolysis in lens: role of leucine aminopeptidase, aminopeptidase III, prolyloligopeptidase and acylpeptidehydrolase. Curr Eye Res. 1996;15:363–369. doi: 10.3109/02713689608995826. [DOI] [PubMed] [Google Scholar]
- Sharma KK, Kumar RS, Kumar GS, Quinn PT. Synthesis and characterization of a peptide identified as a functional element in alphaA-crystallin. J Biol Chem. 2000;275:3767–3771. doi: 10.1074/jbc.275.6.3767. [DOI] [PubMed] [Google Scholar]
- Sharma KK, Ortwerth BJ. Aminopeptidase III activity in normal and cataractous lenses. Curr Eye Res. 1986a;5:373–380. doi: 10.3109/02713688609025176. [DOI] [PubMed] [Google Scholar]
- Sharma KK, Ortwerth BJ. Isolation and characterization of a new aminopeptidase from bovine lens. J Biol Chem. 1986b;261:4295–4301. [PubMed] [Google Scholar]
- Sharma KK, Ortwerth BJ. Bovine lens acylpeptide hydrolase. Purification and characterization of a tetrameric enzyme resistant to urea denaturation and proteolytic inactivation. Eur J Biochem. 1993;216:631–637. doi: 10.1111/j.1432-1033.1993.tb18183.x. [DOI] [PubMed] [Google Scholar]
- Sharma KK, Ortwerth BJ. Purification and characterization of prolyl oligopeptidase from bovine lens. Exp Eye Res. 1994;59:107–115. doi: 10.1006/exer.1994.1086. [DOI] [PubMed] [Google Scholar]
- Sharma KK, Santhoshkumar P. Lens aging: effects of crystallins. Biochim Biophys Acta. 2009;1790:1095–1108. doi: 10.1016/j.bbagen.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih M, David LL, Lampi KJ, Ma H, Fukiage C, Azuma M, Shearer TR. Proteolysis by m-calpain enhances in vitro light scattering by crystallins from human and bovine lenses. Curr Eye Res. 2001;22:458–469. doi: 10.1076/ceyr.22.6.458.5483. [DOI] [PubMed] [Google Scholar]
- Shih M, Lampi KJ, Shearer TR, David LL. Cleavage of beta crystallins during maturation of bovine lens. Mol Vis. 1998;4:4. [PubMed] [Google Scholar]
- Spector A. Lens aminopeptidase. I Purification and properties. J Biol Chem. 1963;238:1353–1357. [PubMed] [Google Scholar]
- Srivastava OP, Kirk MC, Srivastava K. Characterization of covalent multimers of crystallins in aging human lenses. J Biol Chem. 2004;279:10901–10909. doi: 10.1074/jbc.M308884200. [DOI] [PubMed] [Google Scholar]
- Su SP, McArthur JD, Andrew Aquilina J. Localization of low molecular weight crystallin peptides in the aging human lens using a MALDI mass spectrometry imaging approach. Exp Eye Res. 2010;91:97–103. doi: 10.1016/j.exer.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Swanson AA, Davis RM, Albers-Jackson B, McDonald JK. Lens exopeptidases. Exp Eye Res. 1981;32:163–173. doi: 10.1016/0014-4835(81)90005-1. [DOI] [PubMed] [Google Scholar]
- Swanson AA, Davis RM, McDonald JK. The identification of prolyl endopeptidase in bovine lenses: a preliminary report. Curr Eye Res. 1984;3:659–661. doi: 10.3109/02713688409003068. [DOI] [PubMed] [Google Scholar]
- Swanson AA, Davis RM, Meinhardt NC. Proteases in human lenses and their possible significance. Curr Eye Res. 1985;4:43–48. doi: 10.3109/02713688508999965. [DOI] [PubMed] [Google Scholar]
- Taylor A, Tisdell FE, Carpenter FH. Leucine aminopeptidase (bovine lens): synthesis and kinetic properties of ortho-, meta-, and para-substituted leucyl-anilides. Arch Biochem Biophys. 1981;210:90–97. doi: 10.1016/0003-9861(81)90167-3. [DOI] [PubMed] [Google Scholar]
- Temussi PA, Masino L, Pastore A. From Alzheimer to Huntington: why is a structural understanding so difficult? Embo J. 2003;22:355–361. doi: 10.1093/emboj/cdg044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truscott RJ. Age-related nuclear cataract-oxidation is the key. Exp Eye Res. 2005;80:709–725. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Viteri G, Carrard G, Birlouez-Aragon I, Silva E, Friguet B. Age-dependent protein modifications and declining proteasome activity in the human lens. Arch Biochem Biophys. 2004;427:197–203. doi: 10.1016/j.abb.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Voorter CE, de Haard-Hoekman WA, van den Oetelaar PJ, Bloemendal H, de Jong WW. Spontaneous peptide bond cleavage in aging alpha-crystallin through a succinimide intermediate. J Biol Chem. 1988;263:19020–19023. [PubMed] [Google Scholar]
- Wilmarth PA, Tanner S, Dasari S, Nagalla SR, Riviere MA, Bafna V, Pevzner PA, David LL. Age-related changes in human crystallins determined from comparative analysis of post-translational modifications in young and aged lens: does deamidation contribute to crystallin insolubility? J Proteome Res. 2006;5:2554–2566. doi: 10.1021/pr050473a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wride MA, Geatrell J, Guggenheim JA. Proteases in eye development and disease. Birth Defects Res C Embryo Today. 2006;78:90–105. doi: 10.1002/bdrc.20063. [DOI] [PubMed] [Google Scholar]
- Zeng J, Dunlop RA, Rodgers KJ, Davies MJ. Evidence for inactivation of cysteine proteases by reactive carbonyls via glycation of active site thiols. Biochem J. 2006;398:197–206. doi: 10.1042/BJ20060019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su SP, McArthur JD, Andrew Aquilina J. Localization of low molecular weight crystallin peptides in the aging human lens using a MALDI mass spectrometry imaging approach. Exp Eye Res. 2010;91:97–103. doi: 10.1016/j.exer.2010.04.010. [DOI] [PubMed] [Google Scholar]