Abstract
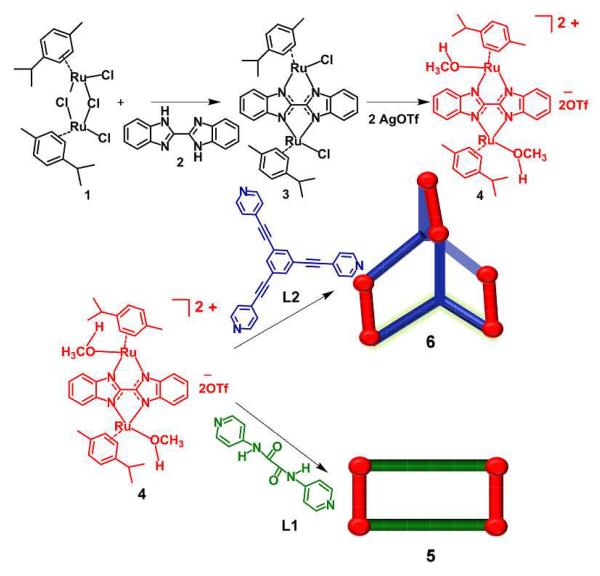
Two new supramolecular coordination complexes (SCCs), were obtained from the self-assembly of a new bis-benzimidazole bridged Ru acceptor, 4, with dipyridyl and tripyridyl donors, respectively. As part of a growing library of anticancer-active Ru-based SCCs, metalla-prism 6 selectively showed high cytotoxicities relative to cisplatin for a series of cancer cell lines, with IC50 values as low as 8.41 μM for MCF7 cells, as determined from MTS assays.
Mainly, molecular clips are a specific class of dinuclear building blocks which could be comprised of two metal centers bridged by organic spacers, wherein two substitutionally labile ligands are oriented to give parallel coordination vectors. When such species are used in coordination-driven self-assembly reactions they represent a 0° ditopic building block, where the angle indicates the relative orientation of the two labile sites. As such, molecular clips are suitable for constructing a variety of 2D and 3D SCCs that contain a 90°-spacer-90° motif, such as squares, rectangles and prisms.1 These designs have historically favoured Pt and Re-based metal acceptors, owing to their predictable coordination geometries and well-established chemistry.2 However, the desire for functional SCCs has motivated the use of alternative acceptors based on Ir, Rh and Ru, due to the novel physiochemical properties that such metals can impart to their resulting structures.3 Recently, a variety of arene-Ru molecular clips bridged by O,O-chelating ligands have been reported for the self-assembly of metalla-rectangles and prisms.4 In this context, ruthenium is not simply a structural element; the metal fulfils a functional role as the impetus for antitumor activity.5
One of the strengths of coordination-driven self-assembly is the modularity and tunability of the building blocks, which can often be modified without significant synthetic redesign. Along these lines, we explore here Ru complexes wherein the O,O-bridging moieties are replaced by an N,N-chelating species, prompted by observations of cisplatin-levels of cytotoxicity in analogous small molecule Ru complexes.6 Specifically, we demonstrate the use of a bis-benzimidazole bridging ligand in the formation of Ru-based SCCs with inherent biological activities.7
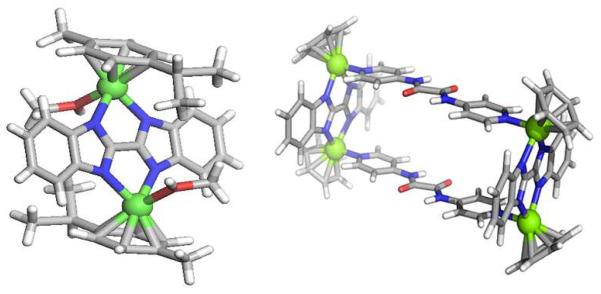
The p-cymene complex [(p-cymene)RuCl2]2 (1) reacts with bis-benzimidazole (2) and sodium acetate in 1:1:2 molar ratio to furnish the dimeric species 3, which subsequently converts into molecular clip 4 upon treatment with silver triflate in methanol (Scheme1). The pure product, fully characterized by 1H and 13C NMR and HR-ESI-MS spectrometry, is isolated as a yellowish brown solid upon addition of diethyl ether. The 1H NMR spectrum exhibited two multiplets at δ = 8.00 and 7.50 ppm, corresponding to bis-benzimidazole protons. The p-cymene protons resulted in two doublets at δ = 6.43 and 6.30 ppm. HR- ESI-MS analysis of molecular clip 4 showed a peak at 852.0 for [4 –O3SCF3−]+ with an isotopic distribution consistent with its theoretical pattern. Single crystals of 4 suitable for X-ray structural studies were grown by vapour diffusion of diethyl ether into a methanol solution, confirming the molecular clip nature of the compound with two labile sites, occupied by MeOH (Figure 1). Neither NMR nor ESI-MS experiments indicated MeOH coordination, suggesting that these sites are readily exchanged and labile in solution, a requirement for efficient SCC formation. A similar reaction of [(p-cymene)RuCl2]2 with bis-benzimidazole was attempted by Carmona et al.7g afforded the mixture of mononuclear complex [(p-cymene)Ru(H2Bbzim)Cl]Cl along with dinuclear derivative [{(p-cymene)RuC1}2μ-Bbzim) and failed to obtain a discrete product as described in this report.
Scheme 1.
Synthesis of acceptor 4, metalla-rectangle 5 and metalla-prism 6.
Figure 1.
X-ray crystal structure of molecular clip 4 (left) and DFT-optimized computational structure of a model of rectangle 5 (right) (iPr and Me groups were omitted for DFT calculation). Atom (color): Ru (green), O (red), N (blue), C (grey), H (white).
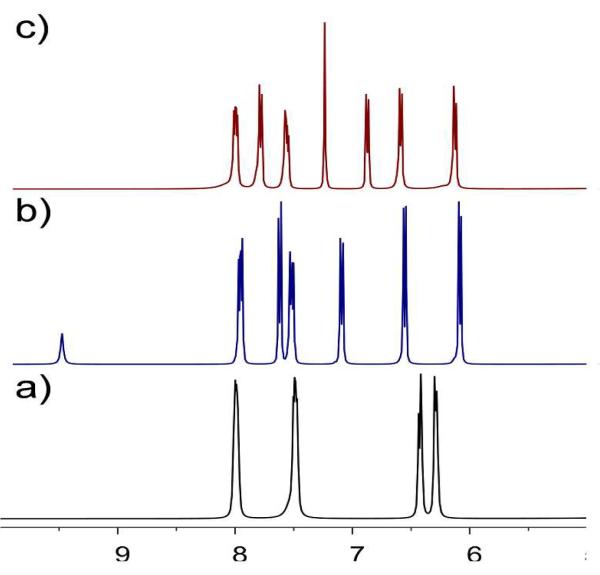
Treatment of molecular clip 4 with N, N-di(pyridine-4yl)oxalamide (L1) in 1:1 molar ratio afforded a new metalla-rectangle 5. A similar treatment with 1, 3, 5-tris (4-pyridylethynyl) benzene (L2)in 3:2 molar ratio resulted in a self-assembled metalla-prism 6, both with quantitative yields. The 1H NMR spectra of 5 and 6 show two doublets (δ = 7.68 and 7.14 ppm for 5; δ = 7.85 and 6.93 ppm for 6) for pyridyl protons and two multiplets (δ = 8.03 and 7.58 ppm for 5; δ = 8.08 and 7.64 ppm for 6) for the bis-benzimidazole protons. Additionally, two singlets at δ = 9.57 ppm (amidic NH proton for donor L1) and δ = 7.29 ppm (benzyl protons of donor L2) were observed for 5 and 6, respectively. The p-cymene protons were observed as two doublets (δ = 6.59 and 6.11 ppm for 5; δ = 6.64 and 6.17 ppm for 6), significantly shifted from those in the spectrum of molecular clip 4 (Figure 2).
Figure 2.
Partial 1H NMR spectra of 4(a), 5(b) and 6(c) in CD3NO2.
The formations of rectangle 5 and prism 6 were further supported by HR-ESI-MS analysis. Two charge states were observed at m/z = 1094.1 [5-2O3SCF3−]2+ and 679.8 [5-3O3SCF3−]3+ for 5 and m/z = 1106.2 [6-3O3SCF3−]3+ and 604.1 [6-5O3SCF3−]5+ for 6 (See SI). These peaks were isotopically resolved and showed good agreement with their theoretical distributions. A theoretical structure indicative the 2D rectangular nature of 5 is shown in Figure 1, as determined from a density functional theory (DFT) geometry optimization with the iPr and Me groups of the p-cymene omitted.
The absorption and emission spectra of molecular clip 4, rectangle 5, and prism 6 in methanol are shown in Figure 3. High energy bands were observed at λabs = 323 nm for 4; λabs = 316 and 289 nm (shoulder) for 5 and λabs = 310 nm for 6. Low energy metal-ligand charge transfer bands are also seen at λabs = 449 nm for all three species. Upon excitation at 290 nm (Figure 3, right), emission bands are observed at λem= 358 nm for 4; λem= 380 nm for 5 and λem= 365 nm for 6. The relatively intense fluorescence of 6 is ascribed to the presence of ethynyl groups which result in extended π conjugation.1a
Figure 3.
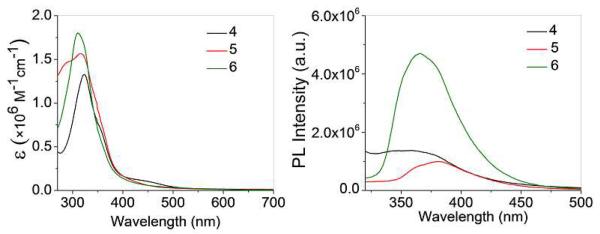
UV-vis. (left) and Fluorescence (right) spectra of 4, 5 and 6 in Methanol.
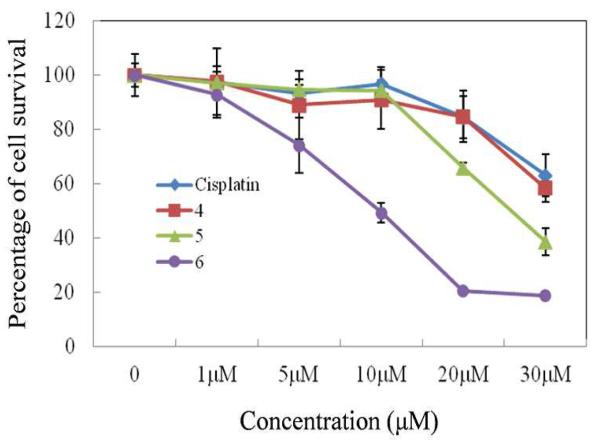
Due to the anticancer activity of existing O,O-bridged Ru SCCs and N,N-bridged Ru small molecules, the anti-proliferative activity of 4 and its self-assembled supramolecular derivatives 5 and 6 was investigated against various cancer cell lines such as Colo320 (colorectal cancer), A549 (lung cancer), MCF-7 (breast cancer) and H1299 (lung cancer). All cancer cells were exposed for 24 h to increasing concentrations of the compounds, and their activities were determined using a 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) cell proliferation assay with the chemotherapeutic drug cisplatin used as a control. Based on the results of the MTS assays, only one cell line, Colo320, was found to be sensitive to cisplatin while A549, MCF-7, and H1299, were resistant to it. The effects of 4–6 on these cell lines are summarized in Table 1. All four cell lines were resistant to 4. Rectangle 5 showed activity only with the Colo320 cell line. Interestingly, the prism 6 was found to inhibit the proliferation of all four cell lines and their growths were effectively inhibited at a very low concentration (Table 1 and Figure 4).
Table 1.
Cytotoxicity of the complexes in human cancer cells.
| Compound | IC50 μM[a] |
|||
|---|---|---|---|---|
| Colo320 | A549 | H1299 | MCF7 | |
| 4 | >100 | >100 | >100 | >100 |
| 5 | 13.94 ± 3.49 | >100 | >100 | >100 |
| 6 | 78.86 ± 12.11* | 15.42 ± 4.73 | 15.65 ± 4.55 | 8.41 ± 2.19 |
| Cisplatin | 38.6 ± 6.26 | >100 | >100 | >100 |
drug concentration necessary for 50% inhibition of cell viability,
mean±SD
Figure 4.
Viability of H1299 lung cancer cells under treatment with 4– 6 and cisplatin.
H1299 cells were treated with 10 μM of both 6 and cisplatin for 12 h, after which they were stained with TUNEL and examined by FACS analysis. Cisplatin and metalla-prism 6 caused apoptosis in 25% and 18% of H1299 cell population, respectively, suggesting that the inhibitory effect of the metalla-prism 6 was not obtained from the induction of apoptosis..
This result indicated that metalla-prism 6 was much more effective than cisplatin in the inhibition of cancer cell growth and could be a candidate for the development of chemotherapeutic drug against cisplatin-resistant cancer cells. As such, a careful examination of the anti-proliferative activity of the prism 6 is warranted to guide future Ru-based drug design.
In conclusion, we have reported the synthesis and characterization of a novel N, N-bridged Ru acceptor and its subsequent coordination-driven self-assembly chemistry in the formation of both 2D and 3D species. The antitumor action of this suite of compounds was evaluated, revealing that bis-benzimidazole bridged SCCs have potential to act as potent anticancer agents, particularly in cell lines which are resistant to Pt-based molecules. Further studies are under progress to investigate the biological mechanism of these derivatives.
Supplementary Material
Acknowledgment
This work was supported by the World Class University (WCU) program (R33-2008-000-10003) and Priority Research Centers program (2009-0093818) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology. P. J. S. thanks the NIH (GM-57052) for financial support.
Footnotes
Supporting Information Available: Synthetic details, spectral data and crystallographic data for the molecular clip 4. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).(a) Vajpayee V, Song YH, Cook TR, Kim H, Lee Y, Stang PJ, Chi K-W. J. Am. Chem. Soc. 2011;133:19646–19649. doi: 10.1021/ja208495u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yan H, Süss-Fink G, Neels A, Stoeckli-Evans H. J. Chem. Soc. Dalton Trans. 1997:4345–4350. [Google Scholar]; (c) Vajpayee V, Jung YJ, Kang SC, Kim H, Kim IS, Wang M, Cook TR, Stang PJ, Chi K-W. Dalton Trans. 2012;41:3046–3052. doi: 10.1039/c2dt11811d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Kaim W, Schwederski B, Dogan A, Fiedler J, Kuehl CJ, Stang PJ. Inorg. Chem. 2002;41:4025–4028. doi: 10.1021/ic020122n. [DOI] [PubMed] [Google Scholar]; (e) Liao R-T, Yang W-C, Thanasekaran P, Tsai C-C, Sathiyendiran M, Liu Y-H, Rajendran T, Lin H-M, Tseng T-W, Lu K-L. Chem. Commun. 2008:3175–3177. doi: 10.1039/b802777c. [DOI] [PubMed] [Google Scholar]
- (2).(a) Resendiz MJE, Noveron JC, Disteldorf H, Fischer S, Stang PJ. Org. Lett. 2004;6:651–653. doi: 10.1021/ol035587b. [DOI] [PubMed] [Google Scholar]; (b) Kuehl C, Huang SD, Stang PJ. J. Am. Chem. Soc. 2001;123:9634–9641. doi: 10.1021/ja0114355. [DOI] [PubMed] [Google Scholar]; (c) Kuehl C, Mayne CL, Arif AM, Stang PJ. Org. Lett. 2000;2:3727–3729. doi: 10.1021/ol006638x. [DOI] [PubMed] [Google Scholar]; (d) Dinolfo PH, Williams ME, Stern CL, Hupp JT. J. Am. Chem. Soc. 2004;126:12989–13001. doi: 10.1021/ja0473182. [DOI] [PubMed] [Google Scholar]; (e) Benkstein KD, Hupp JT, Stern CL. Angew. Chem. Int. Ed. 2000;39:2891–2893. doi: 10.1002/1521-3773(20000818)39:16<2891::aid-anie2891>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]; (f) Dinolfo PH, Hupp JT. Chem. Mater. 2001;13:3113–3125. [Google Scholar]
- (3).(a) Zhang W-Z, Han Y-F, Lin Y-J, Jin G-X. Dalton Trans. 2009:8426–8431. doi: 10.1039/b909357e. [DOI] [PubMed] [Google Scholar]; (b) Han Y-F, Fei Y, Jin G-X. Dalton Trans. 2010;39:3976–3984. doi: 10.1039/b925098k. [DOI] [PubMed] [Google Scholar]; (c) Vajpayee V, Song YH, Lee MH, Kim H, Wang M, Stang PJ, Chi K-W. Chem. Eur. J. 2011;17:7837–7844. doi: 10.1002/chem.201100242. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Furrer MA, Furrer J, Therrien B. Organometallics. 2012;31:3149–3154. [Google Scholar]; (e) Mishra A, Vajpayee V, Kim H, Lee MH, Jung H, Wang M, Stang PJ, Chi K–W. Dalton Trans. 2012;41:1195–1201. doi: 10.1039/c1dt11612f. [DOI] [PubMed] [Google Scholar]
- (4).(a) Wang M, Vajpayee V, Shanmugaraju S, Zheng YR, Zhao Z, Kim H, Mukherjee PS, Chi K–W, Stang PJ. Inorg. Chem. 2011;50:1506–1512. doi: 10.1021/ic1020719. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Therrien B. Eur. J. Inorg. Chem. 2009:2445–2453. [Google Scholar]; (c) Barry NPE, Furrer J, Freudenreich J, Süss-Fink G, Therrien B. Eur. J. Inorg. Chem. 2010:725–728. [Google Scholar]; (d) Vieille-Petit L, Therrien B, Süss-Fink G, Ward TRJ. Organomet. Chem. 2003;684:117–123. [Google Scholar]; (e) Ang WH, Grote Z, Scopelliti R, Juillerat-Jeanneret L, Severin K, Dyson PJJ. Organomet. Chem. 2009;694:968–972. [Google Scholar]; (f) Govender P, Renfrew AK, Clavel CM, Dyson PJ, Therrien, Smith BGS. Dalton Trans. 2011;40:1158–1167. doi: 10.1039/c0dt00761g. [DOI] [PubMed] [Google Scholar]; (g) Vajpayee V, Lee S, Kim S-H, Kang SC, Cook TR, Kim H, Kim DW, Verma S, Lah MS, Kim IS, Wang M, Stang PJ, Chi K-W. Dalton Trans. 2012 doi: 10.1039/c2dt31014g. DOI: 10.1039/c2dt31014g. [DOI] [PubMed] [Google Scholar]
- (5).(a) Vajpayee V, Yang YJ, Kang SC, Kim H, Kim IS, Wang M, Stang PJ, Chi K-W. Chem. Commun. 2011;47:5184–5186. doi: 10.1039/c1cc10167f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Barry NPE, Edafe F, Therrien B. Dalton Trans. 2011;40:7172–7180. doi: 10.1039/c1dt10489f. [DOI] [PubMed] [Google Scholar]; (c) Barry NPE, Zava O, Furrer J, Dyson PJ, Therrien B. Dalton Trans. 2010;39:5272–5277. doi: 10.1039/c001521k. [DOI] [PubMed] [Google Scholar]; (d) Therrien B, Süss-Fink G, Govindaswamy P, Renfrew AK, Dyson PJ. Angew. Chem., Int. Ed. 2008;47:3773–3776. doi: 10.1002/anie.200800186. [DOI] [PubMed] [Google Scholar]; (e) Zava O, Mattsson J, Therrien B, Dyson PJ. Chem.–Eur. J. 2010;16:1428–1431. doi: 10.1002/chem.200903216. [DOI] [PubMed] [Google Scholar]; (f) Barry NPE, Abd Karim NH, Vilar R, Therrien B. Dalton Trans. 2009:10717–10719. doi: 10.1039/b913642h. [DOI] [PubMed] [Google Scholar]; (g) Vajpayee V, Song YH, Yang YJ, Kang SC, Cook TR, Kim DW, Lah MS, Kim IS, Wang M, Stang PJ, Chi K–W. Organometallics. 2011;30:6482–6489. doi: 10.1021/om200908c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Mishra A, Jung H, Park JW, Kim HK, Kim H, Stang PJ, Chi K-W. Organometallics. 2012;31:3519–3526. doi: 10.1021/om2012826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).(a) Morris RE, Aird RE, Mudroch S, Chen HM, Cummings J, Hughes ND, Parsons S, Parkin A, Boyd G, Jodrel DI, Sadler PJ. J. Med. Chem. 2001;44:3616–3621. doi: 10.1021/jm010051m. [DOI] [PubMed] [Google Scholar]; (b) Wang F, Bella J, Parkinson JA, Sadler PJ. J. Biol. Inorg. Chem. 2005;10:147–155. doi: 10.1007/s00775-004-0621-5. [DOI] [PubMed] [Google Scholar]; (c) Betanzos-Lara S, Salassa L, Habtemariam A, Novakova O, Pizarro AM, Clarkson GJ, Liskova B, Brabec V, Sadler PJ. Organometallics. 2012;31:3466–3479. [Google Scholar]
- (7).(a) Haga M, Bond AM. Inorg. Chem. 1991;30:475–480. [Google Scholar]; (b) Mann J, Baron A, Opoku-Boahen Y, Johansson E, Parkinson G, Kelland LR, Neidle S. J. Med. Chem. 2001;44:138–144. doi: 10.1021/jm000297b. [DOI] [PubMed] [Google Scholar]; (c) Mo H-J, Niu Y-L, Zhang M, Qiao Z-P, Ye B-H. Dalton Trans. 2011;40:8218–8225. doi: 10.1039/c0dt01446j. [DOI] [PubMed] [Google Scholar]; (d) Saha D, Das S, Maity D, Dutta S, Baitalik S. Inorg. Chem. 2011;50:46–61. doi: 10.1021/ic100905u. [DOI] [PubMed] [Google Scholar]; (e) Rau S, Büttner T, Temme C, Ruben M, Görls H, Walther D. Inorg. Chem. 2000;39:1621–1624. doi: 10.1021/ic991225h. [DOI] [PubMed] [Google Scholar]; (f) Rau S, Ruben M, Büttner T, Temme C, Dautz S, Görls H, Rudolph M, Walther D, Brodkorb A, Duati M, O'Connor C, Vos JG. J. Chem. Soc. Dalton Trans. 2000:3649–3657. [Google Scholar]; (g) Carmona D, Ferrer J, Mendoza A, Lahoz FJ, Oro LA, Viguri F, Reyes J. Organometallics. 1995;14:2066–2080. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.