Abstract
An individual's environmental history may have delayed effects on its physiology and life history at later stages in life because of irreversible plastic responses of early ontogenesis to environmental conditions. We chose a marine fish, the common sole, as a model species to study these effects, because it inhabits shallow marine areas highly exposed to environmental changes. We tested whether temperature and trophic conditions experienced during the larval stage had delayed effects on life-history traits and resistance to hypoxia at the juvenile stage. We thus examined the combined effect of global warming and hypoxia in coastal waters, which are potential stressors to many estuarine and coastal marine fishes. Elevated temperature and better trophic conditions had a positive effect on larval growth and developmental rates; warmer larval temperature had a delayed positive effect on body mass and resistance to hypoxia at the juvenile stage. The latter suggests a lower oxygen demand of individuals that had experienced elevated temperatures during larval stages. We hypothesize that an irreversible plastic response to temperature occurred during early ontogeny that allowed adaptive regulation of metabolic rates and/or oxygen demand with long-lasting effects. These results could deeply affect predictions about impacts of global warming and eutrophication on marine organisms.
Keywords: fish, environmental programming, climate change, hypoxia, nutrition
1. Introduction
Global climate warming is presently causing a monotonic decrease in dissolved oxygen concentration in numerous coastal and estuarine ecosystems around the world, resulting in the increased frequency, intensity and length of hypoxia episodes in shallow areas [1–4]. A primary consequence of sea water warming for marine fishes is the progressive widening of the gap between the availability of dissolved oxygen in the water and their metabolic oxygen demand [5]. In shallow coastal and estuarine ecosystems, this situation is made even more stressful by concomitant eutrophication-related oxygen depletion. As temperature reaches the upper limit of a fish species' tolerance range, the cardio-respiratory system cannot meet the organism's increasing oxygen demands and its capacity to perform aerobic activities is diminished [6]. In such instances, key physiological performance traits are affected, with potential consequences for locomotion, behaviour, growth, reproduction and, ultimately, the fate of the species [7].
In marine fishes, early ontogenesis is associated with a sequence of life-history transitions characterized by critical changes in morphology, physiology and behaviour generally coupled with shifts in habitat or diet. Apart from exerting selection pressures, environmental conditions also influence ontogeny itself [8], through phenotypically plastic responses [9,10]. Plastic responses provide species with a first important means of coping with changing environmental conditions without altering their genetic constitution, and they may have long-term consequences for population dynamics and evolution [11]. However, these delayed physiological and life-history effects resulting from irreversible plastic responses of early ontogenesis to environmental conditions remain poorly understood. One explanation for this situation could be that, with the exception of short-lived species [12], experimental approaches are generally unable to examine the long-term, fitness determining, delayed consequences of environment effects on ontogenesis [13]. Only long-term experiments can adequately address questions about the cohort effects that may occur in wild fish populations. Cohort effects occur when common environmental conditions experienced by individuals of a given year-class induce differences in their future physiological performance and life history that make them distinguishable from those of other individuals.
Most marine fish species have a pelagic larval stage that takes place offshore, where temperature is generally recognized as the most influential environmental factor in determining ontogenic trajectories. As larvae age and develop, they classically drift to inshore shallow nurseries where eutrophication-related hypoxia represents a new environmental constraint. Eutrophication also influences trophic conditions as it stimulates nutrient-based primary production, and thus secondary production available as food for fish larvae and juveniles with a direct effect on lipid storage, particularly in estuarine areas [14].
The combination of global warming and eutrophication may, therefore, affect marine fishes both through temperature-related effects at the larval stage and hypoxia-related effects at the juvenile stage, with potential consequences for population structure and dynamics [15].
Here, we report on an original approach for examining how environmental conditions experienced during early ontogeny affect physiological performance traits at later ontogenetic stages. The present study was designed to mainly consider the adaptive role of phenotypically plastic responses rather than that of genetic (evolutionary) ones. We examined how temperature and nutritional conditions experienced during larval development, in addition to juvenile dietary regime, affect hypoxia tolerance at the juvenile stage within a population of common sole Solea solea. Our results make an important contribution to understanding and predicting the consequences of the combined trends in climate and eutrophication for marine fish populations.
2. Material and methods
(a). Specimen collection and rearing treatments
(i). Phase 1: larval rearing and treatments (from hatching until day 30 post-hatching)
Forty thousand North Sea sole (S. solea) eggs, resulting from crossing 11 females with 25 males, were purchased from a commercial hatchery (SOLEA BV, Ijmuiden, The Netherlands) and put in two 60 l incubators at 14°C (water temperature) until hatching. To achieve the crosses that produced these eggs, breeders had been put in several tanks with roughly three females and six males each and allowed to mate freely with each other. This procedure insured that the progeny included a representative sample of genetic variability within the wild population despite potential differential contribution of breeders to the eggs collected within a tank. One day post-hatching (dph) larvae were distributed among 12 cylindrical fibreglass 67 l tanks (5300 larvae per tank). Throughout the experiment, water salinity and oxygenation were maintained at 35‰ and more than 6 mg l−1, respectively. Water temperature was progressively increased from 14°C to 16°C and then to 20°C between 1 and 5 dph for acclimation purposes.
From 10 dph, larvae were exposed to four treatments, three replicate tanks each, according to a factorial design between two water temperature levels, 16°C and 20°C, and two feeding regimes, standard one-day-old (A1) Artemia (named A) or A1 Artemia enriched in polyunsaturated fatty acids (named AE). Details on larvae rearing parameters and equipment are given in [16]. In each treatment, larval metamorphosis was assessed at 18, 22 and 26 dph by counting the number of metamorphosed individuals in a random sample of ca 15 individuals. In each treatment, post-metamorphic standard length was measured on approximately 20 individuals sampled randomly at 26 dph. In each tank, larval survival probability was assessed at 30 dph, as the ratio of the number of individuals alive to the initial number.
(ii). Phase 2: juvenile rearing treatments
Following phase 1, replicate tanks were pooled in one 300 l raceway tank per treatment (approx. 1200 juveniles per tank). Fish were then fed with a commercial diet and maintained at 16°C for seven months.
After this intermediate growing phase, 160 individuals were randomly drawn from each tank, tagged subcutaneously (passive integrated transponder; PIT-tag) and randomly distributed among eight 67 l tanks, i.e. two tanks per larval treatment. For two months, these fish were maintained at 16°C and fed with commercial diets containing either 11 or 20 per cent lipids: one tank per larvae group. The experimental design was therefore a three-way full factorial design between two larval rearing temperatures, two larval feeding regimes and two juvenile dietary lipid contents.
In each treatment, initial (just after the intermediate growing phase) and final juvenile body mass were measured on all individuals.
(b). Hypoxia challenge tests
Individuals (268 dph) were pooled in a single tank (1 m3) and left undisturbed and unfed for 48 h. Water oxygenation was then decreased from 100 to 10% within 1 h, followed by a slower descent at 2 per cent per hour to 4 per cent. Ambient oxygenation was controlled by bubbling nitrogen at the intake of a submersible pump placed in the tank. As fish lost their equilibrium, they were removed, identified (PIT-tag reading) and quickly placed in fully aerated conditions. The corresponding time and oxygenation level were recorded. The hypoxia challenge test (HCT) ended when the last fish was removed to aerated conditions.
Based on the frequency distribution of tolerance time during HCT, fish situated below the 20th percentile (tolerance time: 3 h from the beginning of the challenge) or above the 80th percentile (tolerance time: 5 h) were identified as hypoxia sensitive and hypoxia tolerant, respectively.
(c). Blood biochemical analyses
One month later, the response of blood parameters to hypoxia was compared between hypoxia-sensitive and hypoxia-resistant animals. Only soles that were fed on plain Artemia (A) during the larval stage were considered, as preliminary analyses had shown that Artemia enrichment (AE) had no effect on resistance to hypoxia (see §3). Ten hypoxia-sensitive and 10 hypoxia-resistant animals were selected from each relevant experimental treatment. Among these, five resistant and five sensitive individuals were submitted to a second HCT as above. As water oxygenation reached 11 per cent, five resistant and five sensitive individuals were removed from the tank, weighed and a blood sample was taken from their caudal vein. The remaining five resistant and five sensitive individuals were maintained in normoxia, and samples were taken in the same way.
Blood samples were used to measure three biochemical parameters. Haematocrit was measured using heparinized micro-capillaries (Becton Dickinson & Co., NJ, USA, reference no. 361021). Commercial kits were used for measuring glucose (glucose RTU reference no. 61269, from bioMérieux, Marcy l'Etoile, France) and lactate (lactate PAP reference no. 61192, from bioMérieux) levels in blood plasma.
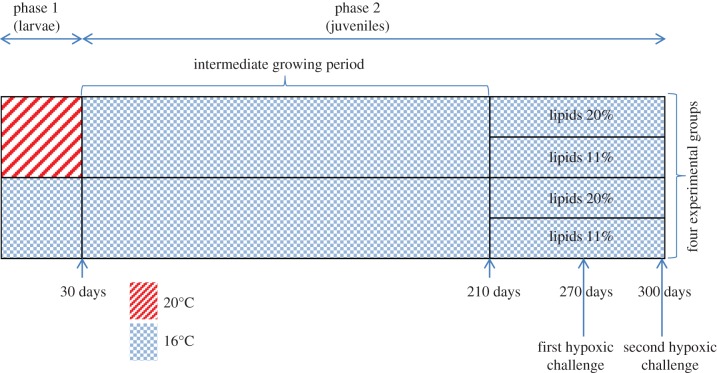
All experiments were conducted in strict compliance with the Guide for the Care and Use of Laboratory Animals [17]. A diagram illustrating the main steps of the experimental design is given in figure 1.
Figure 1.
Main steps of the experimental design for sole that were fed the (A)-regime during the larval period. Sole that were fed the (AE)-regime during the larval period underwent the same treatments in the same experimental design until the first hypoxic challenge. See the text for more details. (Online version in colour.)
(d). Statistical analyses
(i). Larval and juvenile life-history traits
Larval and juvenile life-history traits were analysed using generalized linear (mixed) effect models (see the electronic supplementary material for details). Larval traits (survival probability, proportion of metamorphosed individuals and post-metamorphic standard length) were modelled according to a two-way full factorial design between larval rearing temperature T (16°C/20°C) as a continuous effect and larval feeding regime F (A/AE) as a factor. Juvenile traits (body mass during phase 2 and final body mass) were analysed following a three-way full factorial design based on T, F and juvenile dietary lipid content L (11%/20%) as a continuous effect. Models of the proportion of metamorphosed individuals and juvenile body mass during phase 2 also included time t (days) as a continuous effect, with the two slopes representing the increase in the proportion of metamorphosed individuals with age and growth rate in juvenile body mass, respectively, and interactions with the factors examined in the experiment. Larval survival was considered as Bernoulli data, the proportion of metamorphosed individuals as binomial data, and post-metamorphic standard length and juvenile body mass as Gaussian data. Analyses were performed using the ‘stats’, ‘nlme’ [18], ‘lme4’ [19] and ‘car’ [20] packages in the statistical environment R.
(ii). Resistance to hypoxia at the juvenile stage
HCTs generate data similar to those of survival studies: data are expressed as waiting times until loss of equilibrium for HCTs and until death for survival studies. Therefore, time of resistance to hypoxia during HCT was analysed using survival analysis techniques [21]. We used a Cox proportional hazards model to analyse hypoxia resistance following a three-way full factorial design based on T, F and L, with body mass as an additional continuous covariate. The latter was intended to control for any potential-independent effect of body mass on hypoxia resistance. Cox models allow the hazard rate to be modelled, here the rate of occurrence of equilibrium loss, as being affected proportionally (multiplicatively) by the explanatory variables. In practice, a logarithmic transformation of the hazard rate allows a linear predictor to be obtained (see the electronic supplementary material for details). Analyses were performed using the ‘survival’ [22] and ‘car’ packages in the statistical environment R.
(iii). Blood biochemical correlates of resistance to hypoxia
Haematocrit, lactate level and glycaemia were analysed using linear models. The three transformed blood biochemical parameters (see the electronic supplementary material for details) were modelled according to a four-way full factorial design between the hypoxia sensitivity group S (resistant/sensitive) as a factor, oxygenation level at sampling O2 (100%/11%) as a factor, T, and L. Model terms excluding oxygenation level O2 allowed for the effects of S, T and L to be tested under normoxia, while those involving O2 allowed evaluation of their effects on the response to hypoxia. Analyses were performed using the ‘stats’ and ‘car’ packages in the statistical environment R.
(iv). Significance tests and effect estimates
Significance of the effects at 5 per cent was tested by likelihood ratio tests (LRTs; larval and juvenile traits, and resistance to hypoxia) or F tests (blood biochemical parameters) between nested models respecting marginality of the effects (type II tests) [20]. The effects of active covariates were then estimated from the reduced models obtained by backward elimination on the basis of the significance at 5 per cent of LRTs or F tests, according to the parameter, between nested models.
For all analyses in this paper, larval rearing temperature T and juvenile dietary lipid content L were centred on 16°C and 11%, respectively, and plain A1 Artemia (F = A) was defined as the reference larval feeding regime. Consequently, the model intercepts represent mean values for individuals that were reared with all of these three conditions. As T and L had only two levels each, it makes no difference whether they were modelled as continuous effects or discrete factors. The reason for choosing the first option was to express their effects proportionally to their values.
3. Results
(a). Effect of experimental treatments on larval and juvenile life-history traits
Graphs illustrating larval and juvenile life-history traits are given in the electronic supplementary material, figure S1.
Larvae metamorphosed at a significantly younger age at warmer temperature (age at which 50% of individuals are metamorphosed, A50: 22.9±1.10 dph versus 18.9±1.36 dph at 16°C and 20°C, respectively; positive T effect; table 1; electronic supplementary material, figure S1a) or with an enriched feeding regime (A50: 21.8±2.65 dph versus 20.1±2.90 dph for A and AE feeding regimes, respectively; positive F effect; table 1; electronic supplementary material, figure S1a). Post-metamorphic standard length was significantly larger at warmer temperatures (11.1±1.05 mm versus 12.4±2.25 mm; positive T effect; table 1; electronic supplementary material, figure S1b), which indicates faster average growth rate throughout the larval stage. Larval survival (0.47±0.14) was unaffected by the experimental treatments (see table 1 and electronic supplementary material, figure S1c).
Table 1.
Analysis of the effect of experimental treatments on larval and juvenile traits by generalized linear (mixed effect) modelling. (Results shown concern reduced models issued from backward elimination. For each trait and covariate, the following elements are provided: the effect estimate α together with its standard error (s.e.) (α) in the reduced model; the χ2 statistic (with 1 d.f. for all tests) and the resulting p-value for the type II test from the complete model. Larval survival probability: generalized linear mixed effect model for Bernoulli data with logit link, and replicate tanks as a random effect; probability of metamorphosis: generalized linear model for binomially distributed data with logit link; post-metamorphic standard length: generalized least-squares model with residual variance differing across T × F levels to account for heteroscedasticity. Juvenile body mass growth and final juvenile body mass: generalized least-squares models with residual variance differing across T × F × L and t × T × F × L combinations to account for heteroscedasticity. For juvenile body mass growth, terms excluding time t measure effects on initial body mass just after the intermediate growing phase, since t was set at 0 on this date, while terms including t evaluate effects on body mass growth rate. α0, intercept; T, larval rearing temperature (continuous); F, larval feeding regime (factor: A/AE); L, juvenile dietary lipid content (continuous); t, time (dph); n.a., not applicable.)
| trait | covariate | α | s.e. (α) | 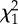 |
p-value |
|---|---|---|---|---|---|
| larval survival | α0 | −0.141 | 0.180 | n.a. | n.a. |
| proportion of metamorphosed individuals | α0 | −24.702 | 3.828 | n.a. | n.a. |
| t | 1.034 | 0.160 | 129.763 | <0.001 | |
| T | 1.095 | 0.208 | 54.333 | <0.001 | |
| F | 1.572 | 0.580 | 8.540 | 0.003 | |
| final larval standard length | α0 | 11.114 | 0.184 | n.a. | n.a. |
| T | 0.274 | 0.099 | 6.939 | 0.008 | |
| juvenile body mass growth | |||||
| initial body mass | α0 | 9.993 | 0.082 | n.a. | n.a. |
| T | 0.132 | 0.027 | 209.742 | <0.001 | |
| F | −1.012 | 0.116 | 3.148 | 0.076 | |
| T × F | 0.445 | 0.038 | 125.733 | <0.001 | |
| growth rate | t | 0.069 | 0.003 | 540.040 | <0.001 |
| t × F | −0.009 | 0.003 | 8.225 | 0.004 | |
| t × L | −0.003 | 0 | 59.410 | <0.001 | |
| final juvenile body mass | α0 | 14.859 | 0.252 | n.a. | n.a. |
| T | 0.109 | 0.081 | 56.591 | <0.001 | |
| F | −1.795 | 0.267 | 15.549 | <0.001 | |
| L | −0.193 | 0.024 | 59.410 | <0.001 | |
| T × F | 0.532 | 0.103 | 23.732 | <0.001 | |
Initial juvenile body mass (i.e. just after the intermediate growing phase) was significantly higher for individuals that had experienced warmer larval rearing temperature (9.5±1.15 g versus 10.9±1.02 g; positive T effect; table 1; electronic supplementary material, figure S1d), and this effect was stronger as larval feeding regime was enriched (positive T × F effect; table 1; electronic supplementary material, figure S1d). Subsequent juvenile growth rate, estimated by terms involving time t, was significantly slower for individuals that had an enriched larval feeding regime (negative t × F effect; table 1; electronic supplementary material, figure S1d) or higher juvenile dietary lipid content (negative t × L effect; table 1; electronic supplementary material, figure S1d). Consequently, final juvenile body mass was significantly higher in individuals that had experienced warmer larval rearing temperature (13.2±2.83 g versus 14.5±2.85 g; positive T effect; table 1; electronic supplementary material, figure S1d), a non-enriched larval feeding regime (14.2±3.00 g versus 13.5±2.79 g dph; negative F effect; table 1; electronic supplementary material, figure S1d) or a low juvenile dietary lipid content (14.7±3.12 g versus 13.0±2.43 g at 11 and 20% dietary lipid content, respectively; negative L effect; table 1; electronic supplementary material, figure S1d).
(b). Effect of experimental treatments on resistance to hypoxia at the juvenile stage
Thermal conditions during the larval stage significantly affected fish tolerance to hypoxia at the juvenile stage (negative T effect; table 2). Fish reared at 16°C were significantly less tolerant to hypoxia than those reared at 20°C (figure 2a), with a diminution of 7.5 per cent of the instantaneous rate of equilibrium loss for each additional degree (table 2). By contrast, feeding conditions during the larval stage did not affect fish resistance to hypoxia (figure 2b; F effect; table 2). Dietary lipid content during the juvenile stage also significantly affected hypoxia tolerance, those individuals fed with a 11 per cent lipid diet resisting better than those fed with a 20 per cent lipid diet with an estimated increase of 6 per cent of the instantaneous rate of equilibrium loss for each additional percentage of lipid (figure 2c; positive L effect; table 2). None of the interactions affected hypoxia tolerance, nor did body mass within the size range tested.
Table 2.
Analysis of the effect of experimental treatments on resistance to hypoxia at the juvenile stage by a Cox proportional hazards model. (The reduced model resulting from backward elimination is presented. For each covariate, the following elements are provided: its effect estimate α together with its s.e. (α) and the corresponding multiplicative effect exp (α) on instantaneous hazard rate in the reduced model; the χ2 statistic (with 1 d.f. for all tests) and the resulting p-value for the type II test from the complete model. T, larval rearing temperature (continuous); L, juvenile dietary lipid content (continuous).)
| covariate | α | s.e. (α) | exp (α) | 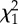 |
p-value |
|---|---|---|---|---|---|
| T | −0.077 | 0.021 | 0.925 | 11.732 | <0.001 |
| L | 0.059 | 0.010 | 1.060 | 30.455 | <0.001 |
Figure 2.
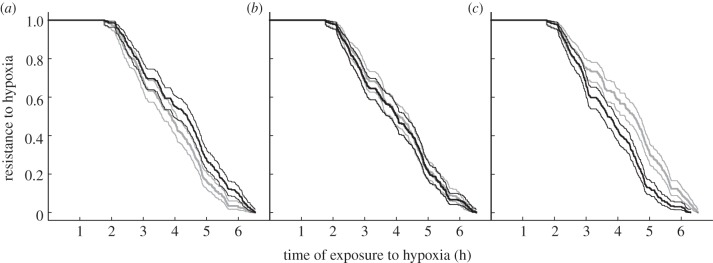
Resistance of juvenile sole to hypoxia according to experimental treatment. Thick lines represent the percentage of individuals that resisted hypoxia up to the time point considered (also called the Kaplan–Meier estimator in survival analysis) and thin lines represent the associated 95% confidence intervals. (a) effect of larval rearing temperature (16°C (grey) or 20°C (black)); (b) effect of larval feeding regime (plain or enriched Artemia, A (grey) or AE (black)); (c) effect of juvenile dietary lipid content (11% (grey) or 20% (black)).
(c). Blood biochemical correlates of resistance to hypoxia
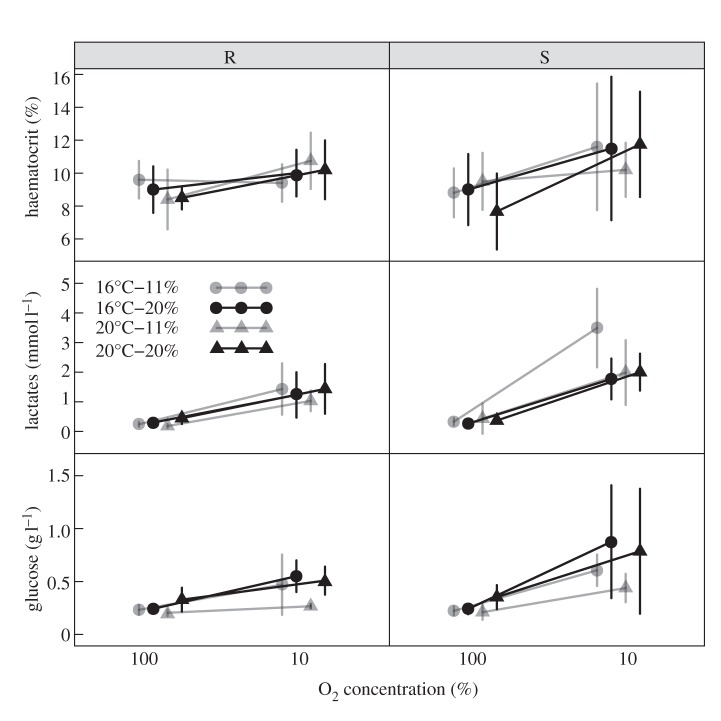
Haematocrit increased significantly with exposure to hypoxia (figure 3; positive O2 effect; table 3) but neither its level under normoxia (figure 3; S, T and L effects; table 3) nor its response to hypoxia (figure 2; O2 × S, O2 × T, O2 × L effects; table 3) depended on the hypoxia tolerance group or the experimental treatment considered.
Figure 3.
Response of blood biochemical parameters to water oxygenation level (100 versus 11%) in resistant (R) and sensitive (S) individuals according to experimental treatment. Circles and triangles represent individuals that had experienced 16°C and 20°C, respectively, during larval rearing. Grey symbols/lines and black symbols/lines represent individuals that were fed with 11% and 20% lipid diets, respectively, during juvenile rearing. Means±standard deviations are given (n = 5). Successive treatment curves are slightly shifted along the x-axis to ease visualization.
Table 3.
Analysis of the blood biochemical correlates (haematocrit, lactate level and glycaemia) of sensitivity to hypoxia (R and S individuals) at two oxygen concentration levels (100% and 11%) by linear modelling. (Results shown concern reduced models issued from backward elimination. For each blood biochemical parameter and covariate, the following elements are given: its effect coefficient α together with its s.e. (α) in the reduced model; the associated F statistic with its degrees of freedom (at numerator and denominator as indices) and the resulting p-value for the type II test from the complete model. Terms excluding O2 test effects on the constitutive levels of the blood biochemical parameters under normoxia, while terms involving O2 test effects on the response of blood biochemical parameters to hypoxia. α0, intercept; S, sensitivity group (factor: sensitive/resistant); T, larval rearing temperature (continuous); L, juvenile dietary lipid content (continuous); O2, water concentration level (factor: 100%/11%); n.a., not applicable.)
| parameter | covariate | α | s.e. (α) | F | p-value |
|---|---|---|---|---|---|
| haematocrit | |||||
| under normoxia | α0 | 2.159 | 0.036 | n.a. | n.a. |
| response to hypoxia | O2 | 0.181 | 0.049 | F1,52 = 12.110 | 0.001 |
| lactates | |||||
| under normoxia | α0 | −1.360 | 0.169 | n.a. | n.a. |
| S | 0.353 | 0.200 | F1,63 = 10.826 | 0.002 | |
| T | −0.070 | 0.041 | F1,63 = 0.019 | 0.892 | |
| L | 0.0001 | 0.022 | F1,63 = 0.322 | 0.572 | |
| S × L | −0.051 | 0.025 | F1,63 = 4.017 | 0.050 | |
| T × L | 0.016 | 0.006 | F1,63 = 6.054 | 0.017 | |
| response to hypoxia | O2 | 1.453 | 0.162 | F1,63 = 217.026 | <0.001 |
| O2 × S | 0.510 | 0.228 | F1,63 = 4.614 | 0.036 | |
| glycaemia | |||||
| under normoxia | α0 | −2.592 | 0.174 | n.a. | n.a. |
| S | 0.033 | 0.169 | F1,62 = 6.157 | 0.016 | |
| T | −0.024 | 0.052 | F1,62 = 0.550 | 0.461 | |
| L | 0.034 | 0.018 | F1,62 = 26.780 | <0.001 | |
| T × L | 0.018 | 0.007 | F1,62 = 7.304 | 0.009 | |
| response to hypoxia | O2 | 1.408 | 0.206 | F1,62 = 120.686 | <0.001 |
| O2 × S | 0.533 | 0.235 | F1,62 = 4.732 | 0.033 | |
| O2 × T | −0.163 | 0.059 | F1,62 = 6.965 | 0.011 | |
Lactate under normoxia was significantly higher in sensitive individuals (figure 3; positive S effect; table 3), but this difference lessened as juvenile dietary lipid content increased (figure 3; negative S × L effect; table 3). Lactate under normoxia was lower for individuals that experienced warmer larval rearing temperature and low juvenile dietary lipid content (figure 3; negative T effect and positive L effect; table 3), but these effects were not fully additive (figure 3; positive T × L effect; table 3). Lactate level increased significantly with hypoxia (figure 3; positive O2 effect; table 3) and this was more pronouncedly in sensitive individuals (fourfold) than in tolerant ones (twofold; figure 3; positive O2 × S effect; table 3).
Glycaemia under normoxia was significantly higher in sensitive individuals (figure 3; positive S effect; table 3). It was significantly lower in individuals that had experienced warmer larval temperature and low juvenile dietary lipid content (figure 3; negative T effect and positive L effect; table 3), but the effects acted non-additively (figure 3; positive T × L effect; table 3). The increase in glycaemia under hypoxia (figure 3; positive O2 effect; table 3) was significantly stronger for sensitive sole (2.6-fold) than for tolerant ones (1.8-fold; figure 3; positive O2 × S effect; table 3). In addition, this response was significantly higher in animals that had experienced warmer larval rearing temperature (figure 3; positive O2 × T effect; table 3).
4. Discussion
Realistic scenarios of global change require, from a conservation perspective, integrated analyses based on multiple stressors acting simultaneously on organisms [23]. From a mechanistic perspective, however, responses to multiple stressors are poorly understood although crucial for defining the capacity of fishes to adapt to climate change. One puzzling issue relates to the fact that early environmental stresses could potentially affect the ability of fishes to face environmental constraints at later developmental stages. This study specifically addressed this question by considering the combined effect of two major abiotic factors affected by global warming and eutrophication, temperature and water oxygenation, acting at two different life stages, larvae and juveniles. We found that elevated temperature and better trophic conditions had a positive effect on larval growth and developmental (earlier metamorphosis) rates; warmer temperature experienced during larval stage had a positive delayed effect on body mass and resistance to hypoxia at juvenile stage. We hypothesize that an irreversible plastic response to temperature occurred during early ontogeny that allowed adaptive regulation of metabolic rates and/or oxygen demand with long-lasting effects. Also, lower levels of lactate and glycaemia in blood were associated with better hypoxia tolerance both within and between experimental treatments, suggesting, respectively, a genetic and an environmental source of variation in individuals' hypoxia resistance.
(a). Ecological relevance of the model species and tested temperatures
Common sole (S. solea) is a coastal benthic species that is exposed to global change (warming and eutrophication). Geographical distribution of this species ranges from the Mediterranean Sea to the Baltic Sea (see the electronic supplementary material for details on the main life events of this fish species). Common sole is a batch-spawner. Spawning occurs in coastal waters from winter to spring, in temperature ranges from 14°C to 22°C depending on the geographical location and season [24,25]. The temperatures tested in the present experiment, 16°C and 20°C, therefore fall within the likely seasonal thermal amplitude in coastal temperate sea water, from winter to spring.
(b). Immediate effects of environmental conditions on larval life-history traits
In common sole, the duration of metamorphosis (period during which larvae switch from a pelagic to a benthic life mode) is approximately 10 days depending on temperature. Settlement occurs about 25 days after hatching [24]. In the present experiment, metamorphosis occurred earlier at warmer temperature and in enriched trophic conditions, and lasted less than 6 days irrespective of treatment. Body lengths at the end of the larval period (post-metamorphic standard length) were greater at warmer temperature, thus revealing faster average growth rate throughout the larval stage, but very similar to those reported in the field or in laboratory conditions [24,26]. Unfortunately, size at metamorphosis per se could not be assessed because of the lack of length-at-age data during the metamorphosis period.
It has been proposed that, in wild populations of flatfishes, year-class strength is determined during larval and early post-settlement stages [25] and that the availability of suitable food is a strong determinant for survival. Our finding indicates that, contrary to other marine fish species, food lipid content did not directly influence larval sole survival.
(c). Delayed effects of larval environmental history on juvenile life-history traits and tolerance to hypoxia
At the end of phase 2, sole were 10 months old, which corresponds to 0-group fish present on nursery grounds at the end of the summer. Fish lengths found in the present experiment were comparable to field reports [25], i.e. approximately 10 cm and were lower than those observed in farmed individuals of the same age [27]. Therefore, juvenile growth occurred normally in our 10 month experiment, without any negative impact of the rearing protocol that would have impaired the validity of our observations and findings.
Several growth-related traits were influenced by individuals' environmental history. For instance, juveniles that had experienced elevated temperature during their larval period exhibited higher initial and final body masses. Therefore, the observed advantage in terms of juvenile body mass was mainly owing to a long-lasting body size consequence of faster larval growth and not to enhanced juvenile growth potential.
Hypoxia tolerance is a complex performance trait that integrates the functioning of a series of convective and diffusive processes known as the oxygen cascade [3]. This cascade allows oxygen to move down the pressure gradient from ambient water to the mitochondria. Impairments at any step in this cascade are liable to affect the ability of an organism to meet its oxygen requirement when faced with reduced oxygen availability. Moreover, metabolic rate and efficiency also contribute to fixing individual oxygen demand and, therefore, individual susceptibility to limiting oxygenation conditions.
Although often alleged, the link between initial thermal and dietary conditions and metabolic performance at later developmental stages is poorly documented. The present investigation sheds a new light on this aspect by suggesting that metabolic rates at the juvenile stage are lower for individuals that have experienced warm larval temperature. This result is the opposite of the immediate positive effect of temperature on metabolic rates [28] and suggests that increased temperature during early ontogeny may trigger an irreversible plastic response that compensates for the temperature-induced elevation in metabolic rates.
The acute effect of water temperature on fish metabolism has been documented in numerous studies and is generally considered as the strongest environmental determinant of fish performance [29]. What is less documented, however, is the effect of temperature on the phenotype of developing fishes. Fish plastic responses to early thermal conditions have been reported to have long-lasting repercussions upon sex determination [30], muscles structure [31] and swimming performance [32]. The present investigation increases current knowledge by documenting the fact that hypoxia tolerance is a phenotypic trait under thermal control. This result profoundly changes our perception of contemporary environmental trends upon fish adaptation ability [33] and the possible repercussions at population and ecosystem levels [34]. Hypoxia tolerance is a determinant of Darwinian fitness for many coastal fish species, and the demonstration that early thermal and dietary history affects this performance clearly deserves further study. This would be particularly important for improving the predictive ability of current models of the impact of climate change and eutrophication of coastal areas on fish stock production, dynamics and evolution.
(d). Other sources of variation of sensitivity to hypoxia: trophic conditions and genetic variability
Nutritional conditions, particularly dietary lipid content, had a short-term impact on sole tolerance to hypoxia. Juvenile sole exhibited a lower tolerance to hypoxia when fed with a lipid-rich diet. Relationships among diet lipid content and fish metabolic rate and aerobic performance have been clearly established in juvenile fishes [35,36]. High dietary lipid contents are known to adversely affect growth and nutrient utilization of flatfish, and particularly sole, which is considered to have low lipid tolerance [37]. The relative difficulty that sole have at handling high dietary lipid contents was confirmed in our study by their slower growth, and it is reasonable to consider that this difficulty has a metabolic cost. High dietary lipid levels, when not correctly metabolized by the organism, increase lipid peroxidation and affect mitochondria functioning through the production of reactive oxygen species. They are likely to impair tolerance to hypoxia [38].
One interesting result of this study is that individuals which had experienced the same environmental history could exhibit varying levels of sensitivity to hypoxia (as shown by our classification of ‘tolerant’ versus ‘sensitive’ individuals). This diversity probably has a genetic basis and possibly relates to individuals' capacity to extract oxygen by increasing the water flow over their gills and/or a higher capacity to reduce their oxygen consumption. Recently, Flight et al. [39] revealed a highly significant effect of sex on survivorship under hypoxic conditions, with females surviving longer than males. Reasons for this difference were not fully uncovered although not related to size. Variation in individuals' reproductive state could be potentially responsible for the observed difference between sexes, but this explanation cannot apply to our results as the fish had not started sexual maturation within the time of the experiment.
(e). Physiological aspects of resistance to hypoxia
As expected, haematological variables (i.e. haematocrit, lactate and glucose) rose during the hypoxia challenge. The increase in haematocrit is classically reported during acute hypoxia. It is aimed at increasing oxygen transport capacities [40]. This adaptive response was not influenced by any of the environmental conditions tested (temperature at early stages or trophic conditions) nor by the degree of resistance to hypoxia of individuals.
Conversely, plasma lactate and glucose levels were influenced by fish environmental history. During hypoxia, plasma levels in these two metabolites rose to a lesser extent in animals that had experienced 20°C than in those that had only experienced 16°C during their larval period. Inversely, the levels of these two metabolites under normoxia and hypoxia conditions increased with dietary lipid content and/or an individuals' sensitivity to hypoxia. The high plasma level of glucose could be owing to lipid-induced insulin resistance, since high levels of dietary fat impair glucose homeostasis in fishes [41]. The impairment of glucose homeostasis could be a sign of the metabolic cost of high dietary lipid levels on sole metabolism, particularly during hypoxia. The better resistance to hypoxia of fishes that experienced warmer temperatures during their larval period was unexpected since these animals also exhibited larger body masses at the juvenile stage. Fast-growing fishes tend to have a higher standard metabolic rate and therefore higher oxygen requirements. They should, therefore, be less tolerant to hypoxia than slow-growing individuals [38]. We suggest that thermal conditions during early developmental stages may have affected cellular actors involved in hypoxia-dependent paths [42], such as those activated by the hypoxia-inducible factor (HIF) or associated with the activation of the unfolded protein response (UPR), to an extent that they distorted the relationship between body size and hypoxia tolerance. A set of multiple transcription factors are regulated by HIF and UPR, and it would not be surprising if their potential activation during early developmental stages could have led to metabolic programming of some genes (involved in metabolism and/or mitochondria biogenesis), with long-term effects on hypoxia tolerance during the juvenile stage.
5. Conclusion
This study clearly demonstrates that environmental conditions experienced during early developmental stages are important in controlling environmental adaptation performance at later life stages. In particular, sole that had experienced elevated temperatures during their early-life exhibited higher body masses and tolerance to hypoxia, probably through long-term programming of metabolic pathways. Such a cohort effect on growth performance and hypoxia tolerance could have major implications for population dynamics. Our study also indicates that developmental plasticity in animals may allow adaptation to changing environment conditions to have delayed effects, and that this may attenuate some of the more severe predictions about organisms' responses to global warming and eutrophication.
Acknowledgements
All experiments were conducted in strict compliance with the Guide for the Care and Use of Laboratory Animals.
We are grateful to the Singer-Polignac Foundation for the grant it provided. We also thank Helen McCombie for English language editing.
References
- 1.Diaz RJ. 2001. Overview of hypoxia around the world. J. Environ. Qual. 30, 275–281 10.2134/jeq2001.302275x (doi:10.2134/jeq2001.302275x) [DOI] [PubMed] [Google Scholar]
- 2.Wu RSS. 2002. Hypoxia: from molecular responses to ecosystem responses. Mar. Pollut. Bull. 45, 35–45 10.1016/S0025-326X(02)00061-9 (doi:10.1016/S0025-326X(02)00061-9) [DOI] [PubMed] [Google Scholar]
- 3.Chabot D, Claireaux G. 2008. Environmental hypoxia as a metabolic constraint on fish: the case of Atlantic cod, Gadus morhua. Mar. Pollut. Bull. 57, 287–294 10.1016/j.marpolbul.2008.04.001 (doi:10.1016/j.marpolbul.2008.04.001) [DOI] [PubMed] [Google Scholar]
- 4.Rabalais NN, Turner RE, Díaz RJ, Justić D. 2009. Global change and eutrophication of coastal waters. ICES J. Mar. Sci. 66, 1528–1537 10.1093/icesjms/fsp047 (doi:10.1093/icesjms/fsp047) [DOI] [Google Scholar]
- 5.Wang T, Overgaard J. 2007. The heartbreak of adapting to global warming. Science 315, 49–50 10.1126/science.1137359 (doi:10.1126/science.1137359) [DOI] [PubMed] [Google Scholar]
- 6.Pörtner HO, Mark FC, Bock C. 2004. Oxygen limited thermal tolerance in fish? answers obtained by nuclear magnetic resonance techniques. Resp. Physiol. Neurobiol. 141, 243–260 10.1016/j.resp.2004.03.011 (doi:10.1016/j.resp.2004.03.011) [DOI] [PubMed] [Google Scholar]
- 7.Pörtner HO, Knust R. 2007. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315, 95–97 10.1126/science.1135471 (doi:10.1126/science.1135471) [DOI] [PubMed] [Google Scholar]
- 8.Hamdoun A, Epel D. 2007. Embryo stability and vulnerability in an always changing world. Proc. Natl Acad. Sci. USA 104, 1745–1750 10.1073/pnas.0610108104 (doi:10.1073/pnas.0610108104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kingsolver JG, Massie KR, Ragland GJ, Smith MH. 2007. Rapid population divergence in thermal reaction norms for an invading species: breaking the temperature–size rule. J. Evol. Biol. 20, 892–900 10.1111/j.1420-9101.2007.01318.x (doi:10.1111/j.1420-9101.2007.01318.x) [DOI] [PubMed] [Google Scholar]
- 10.Frank SA. 2011. Natural selection. II. Developmental variability and evolutionary rate. J. Evol. Biol. 24, 2310–2320 10.1111/j.1420-9101.2011.02373.x (doi:10.1111/j.1420-9101.2011.02373.x) [DOI] [PubMed] [Google Scholar]
- 11.Beckerman A, Benton TG, Ranta E, Kaitala V, Lundberg P. 2002. Population dynamic consequences of delayed life-history effects. Trends Ecol. Evol. 17, 263–269 10.1016/S0169-5347(02)02469-2 (doi:10.1016/S0169-5347(02)02469-2) [DOI] [Google Scholar]
- 12.Kong RYC, et al. 2008. Development of a marine fish model for studying in vivo molecular responses in ecotoxicology. Aquat. Toxicol. 86, 131–141 10.1016/j.aquatox.2007.10.011 (doi:10.1016/j.aquatox.2007.10.011) [DOI] [PubMed] [Google Scholar]
- 13.Metcalfe NB, Monaghan P. 2001. Compensation for a bad start: grow now, pay later?. Trends Ecol. Evol. 16, 254–260 10.1016/S0169-5347(01)02124-3 (doi:10.1016/S0169-5347(01)02124-3) [DOI] [PubMed] [Google Scholar]
- 14.Breitburg D, et al. 2009. Nutrient enrichment and fisheries exploitation: interactive effects on estuarine living resources and their management. Hydrobiologia 629, 31–47 10.1007/s10750-009-9762-4 (doi:10.1007/s10750-009-9762-4) [DOI] [Google Scholar]
- 15.van der Veer HW, Berghahn R, Miller JM, Rijnsdorp AD. 2000. Recruitment in flatfish, with special emphasis on North Atlantic species: progress made by the flatfish symposia. ICES J. Mar. Sci. 57, 202–215 10.1006/jmsc.1999.0523 (doi:10.1006/jmsc.1999.0523) [DOI] [Google Scholar]
- 16.Zambonino-Infante JL, Cahu C. 1994. Development and response to a diet change of some digestive enzymes in sea bass (Dicentrarchus labrax) larvae. Fish Physiol. Biochem. 12, 399–408 10.1007/bf00004304 (doi:10.1007/bf00004304) [DOI] [PubMed] [Google Scholar]
- 17.National-Research-Council 2010. Guide for the care and use of the laboratory animals. Washington, DC: National Academic Press [Google Scholar]
- 18.Pinheiro J, Bates D, DebRoy S, Sarkar D. & The R Development Core Team 2011. nlme: Linear and nonlinear mixed effects models. R package version 3.1–103. See http://cran.r-project.org/web/packages/nlme/index.html.
- 19.Bates D, Maechler M, Bolker B. 2011. lme4: Linear mixed-effects models using S4 classes. R package version 0.999375-42 See http://CRAN.R-project.org/package=lme4.
- 20.Fox J, Weisberg S. 2011. An R companion to applied regression, 2nd edn Los Angeles, CA: SAGE Publications [Google Scholar]
- 21.Therneau TM, Grambsch PM. 2000. Modeling survival data: extending the Cox model. New York, NY: Springer [Google Scholar]
- 22.Therneau TM. 1999. A package for survival analysis. Technical report series No 53, Department of Health Science Research, Mayo Clinic, Rochester, MN, USA
- 23.Pörtner HO. 2008. Ecosystem effects of ocean acidification in times of ocean warming: a physiologist's view. Mar. Ecol. Prog. Ser. 373, 203–217 10.3354/meps07768 (doi:10.3354/meps07768) [DOI] [Google Scholar]
- 24.Amara R, Galois R. 2004. Nutritional condition of metamorphosing sole: spatial and temporal analyses. J. Fish. Biol. 64, 72–88 10.1111/j.1095-8649.2004.00284.x (doi:10.1111/j.1095-8649.2004.00284.x) [DOI] [Google Scholar]
- 25.Amara R. 2004. 0-group flatfish growth conditions on a nursery ground (Bay of Canche, Eastern English Channel). Hydrobiologia 518, 23–32 10.1023/B:HYDR.0000025053.62966.cd (doi:10.1023/B:HYDR.0000025053.62966.cd) [DOI] [Google Scholar]
- 26.Heath P, Moore C. 1997. Rearing Dover sole larvae on Tisbe and Artemia diets. Aquacult. Int. 5, 29–39 10.1007/bf02764785 (doi:10.1007/bf02764785) [DOI] [Google Scholar]
- 27.Imsland AK, Foss A, Conceição LEC, Dinis MT, Delbare D, Schram E, Kamstra A, Rema P, White P. 2003. A review of the culture potential of Solea solea and S. senegalensis. Rev. Fish Biol. Fisher. 13, 379–408 10.1007/s11160-004-1632-6 (doi:10.1007/s11160-004-1632-6) [DOI] [Google Scholar]
- 28.Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL. 2001. Effects of size and temperature on metabolic rate. Science 293, 2248–2251 10.1126/science.1061967 (doi:10.1126/science.1061967) [DOI] [PubMed] [Google Scholar]
- 29.Clarke A, Johnston NM. 1999. Scaling of metabolic rate with body mass and temperature in teleost fish. J. Anim. Ecol. 68, 893–905 10.1046/j.1365-2656.1999.00337.x (doi:10.1046/j.1365-2656.1999.00337.x) [DOI] [PubMed] [Google Scholar]
- 30.Koumoundouros G, Pavlidis M, Anezaki L, Kokkari C, Sterioti A, Divanach P, Kentouri M. 2002. Temperature sex determination in the European sea bass, Dicentrarchus labrax (L., 1758) (Teleostei, Perciformes, Moronidae): critical sensitive ontogenetic phase. J. Exp. Zool. 292, 573–579 10.1002/jez.10095 (doi:10.1002/jez.10095). [DOI] [PubMed] [Google Scholar]
- 31.Johnston IA. 2006. Environment and plasticity of myogenesis in teleost fish. J. Exp. Biol. 209, 2249–2264 10.1242/jeb.02153 (doi:10.1242/jeb.02153) [DOI] [PubMed] [Google Scholar]
- 32.Koumoundouros G, Ashton C, Sfakianakis DG, Divanach P, Kentouri M, Anthwal N, Stickland NC. 2009. Thermally induced phenotypic plasticity of swimming performance in European sea bass Dicentrarchus labrax juveniles. J. Fish Biol. 74, 1309–1322 10.1111/j.1095-8649.2009.02206.x (doi:10.1111/j.1095-8649.2009.02206.x) [DOI] [PubMed] [Google Scholar]
- 33.Dillon ME, Wang G, Huey RB. 2010. Global metabolic impacts of recent climate warming. Nature 467, 704–706 10.1038/nature09407 (doi:10.1038/nature09407) [DOI] [PubMed] [Google Scholar]
- 34.O'Connor MI, Piehler MF, Leech DM, Anton A, Bruno JF. 2009. Warming and resource availability shift food web structure and metabolism. PLoS Biol. 7, e1000178. 10.1371/journal.pbio.1000178 (doi:10.1371/journal.pbio.1000178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKenzie DJ. 2001. Effects of dietary fatty acids on the respiratory and cardiovascular physiology of fish. Comp. Biochem. Physiol. A 128, 605–619 10.1016/S1095-6433(00)00338-X (doi:10.1016/S1095-6433(00)00338-X) [DOI] [PubMed] [Google Scholar]
- 36.Chatelier A, McKenzie DJ, Prinet A, Galois R, Robin J, Zambonino J, Claireaux G. 2006. Associations between tissue fatty acid composition and physiological traits of performance and metabolism in the seabass (Dicentrarchus labrax). J. Exp. Biol. 209, 3429–3439 10.1242/jeb.02347 (doi:10.1242/jeb.02347) [DOI] [PubMed] [Google Scholar]
- 37.Borges P, Oliveira B, Casal S, Dias J, Conceição L, Valente LMP. 2009. Dietary lipid level affects growth performance and nutrient utilisation of Senegalese sole (Solea senegalensis) juveniles. Br. J. Nutr. 102, 1007–1014 10.1017/S0007114509345262 (doi:10.1017/S0007114509345262) [DOI] [PubMed] [Google Scholar]
- 38.Pörtner HO. 2002. Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp. Biochem. Physiol. A 132, 739–761 10.1016/S1095-6433(02)00045-4 (doi:10.1016/S1095-6433(02)00045-4) [DOI] [PubMed] [Google Scholar]
- 39.Flight PA, Nacci D, Champlin D, Whitehead A, Rand DM. 2011. The effects of mitochondrial genotype on hypoxic survival and gene expression in a hybrid population of the killifish, Fundulus heteroclitus. Mol. Ecol. 20, 4503–4520 10.1111/j.1365-294X.2011.05290.x (doi:10.1111/j.1365-294X.2011.05290.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White AJ, Schreer JF, Cooke SJ. 2008. Behavioral and physiological responses of the congeneric largemouth (Micropterus salmoides) and smallmouth bass (M. dolomieu) to various exercise and air exposure durations. Fish Res. 89, 9–16 10.1016/j.fishres.2007.08.008 (doi:10.1016/j.fishres.2007.08.008) [DOI] [Google Scholar]
- 41.Figueiredo-Silva AC, Panserat S, Kaushik S, Geurden I, Polakof S. 2012. High levels of dietary fat impair glucose homeostasis in rainbow trout. J. Exp. Biol. 215, 169–178 10.1242/jeb.063933 (doi:10.1242/jeb.063933) [DOI] [PubMed] [Google Scholar]
- 42.Tagliavacca L, Caretti A, Bianciardi P, Samaja M. 2012. In vivo up-regulation of the unfolded protein response after hypoxia. BBA Gen. Subjects 1820, 900–906 10.1016/j.bbagen.2012.02.016 (doi:10.1016/j.bbagen.2012.02.016) [DOI] [PubMed] [Google Scholar]