Abstract
Amphibian tadpoles display extensive anti-predator phenotypic plasticity, reducing locomotory activity and, with chronic predator exposure, developing relatively smaller trunks and larger tails. In many vertebrates, predator exposure alters activity of the neuroendocrine stress axis. We investigated predator-induced effects on stress hormone production and the mechanistic link to anti-predator defences in Rana sylvatica tadpoles. Whole-body corticosterone (CORT) content was positively correlated with predator biomass in natural ponds. Exposure to caged predators in mesocosms caused a reduction in CORT by 4 hours, but increased CORT after 4 days. Tadpoles chronically exposed to exogenous CORT developed larger tails relative to their trunks, matching morphological changes induced by predator chemical cue; this predator effect was blocked by the corticosteroid biosynthesis inhibitor metyrapone. Tadpole tail explants treated in vitro with CORT increased tissue weight, suggesting that CORT acts directly on the tail. Short-term treatment of tadpoles with CORT increased predation mortality, likely due to increased locomotory activity. However, long-term CORT treatment enhanced survivorship, likely due to induced morphology. Our findings support the hypothesis that tadpole physiological and behavioural/morphological responses to predation are causally interrelated. Tadpoles initially suppress CORT and behaviour to avoid capture, but increase CORT with longer exposure, inducing adaptive phenotypic changes.
Keywords: anti-predator response, stress, tadpole, corticosterone, phenotypic plasticity
1. Introduction
Environmental factors impacting the fitness of organisms generally vary in time and space, and this variation often creates mismatches between the phenotype and the environment. Moreover, in many cases this variation occurs on time scales that are not conducive to constitutive evolutionary responses. Consequently, most organisms exhibit some capacity to refashion the phenotype during the life cycle to reduce the mismatch between the phenotype and environment. Interest in such phenotypic plasticity has increased markedly in ecology and evolution recently, and many studies have shown that this plasticity can be adaptive, incur costs and have large consequences at the population and community levels [1–7]. However, few studies have explored the modulation of such responses from perception of the environmental condition, through physiological mechanisms, to fitness consequences. This is a critical gap in our knowledge, as a more mechanistic understanding of such responses will enable a better understanding of their evolution and the constraints on this evolution. Moreover, understanding the mechanistic underpinnings of phenotypic plasticity can uncover trade-offs not apparent from investigations that simply demonstrate a relationship between an environmental agent and a phenotypic response.
For example, the mismatch between phenotype and environment is often manifest at the organismal level by physiological stress responses that can shape an organism's phenotype, making the physiological stress response a candidate proximate mechanism underlying phenotypic plasticity [8]. In vertebrates, many stressors lead to activation of the neuroendocrine stress axis, increasing circulating levels of corticosteroids (e.g. corticosterone, CORT), which direct energy expenditure away from less critical activities and boost survival responses in the face of an acute stressor [9,10]. However, when a stressor is chronic, many of these actions become damaging, and, therefore, organisms must balance responding adaptively to survive a stressor in the short-term and contending with the long-term consequences of a chronic stress response. It is of considerable interest then to understand how organisms modulate phenotypic responses to stress over time, and to what extent these responses are adaptive.
A ubiquitous environmental challenge for most organisms is the presence of predation risk [11–14], and this risk has sometimes been shown to activate [15–18] or suppress [19] the neuroendocrine stress axis. Predator presence or density can fluctuate widely in many systems, and studies of many taxa have demonstrated physiological, behavioural and morphological phenotypic plasticity to the presence of predators. For example, larval amphibians have been used as a model system for examining phenotypic plasticity induced by predator presence and the consequences to their interactions with other species [20–24]. Tadpoles are able to detect the presence of predators using visual and chemical cues [20,25,26], and in response modify their behaviour and morphology [27–29] in ways that increase fitness under high predation risk [30]. Chronic exposure to predator chemical cue results in a relatively smaller trunk and larger tail [31,32] that can confer enhanced burst locomotion for escape, and may deflect predator strikes from the more vulnerable trunk [31,33]. However, the specific interactions among predator presence, stress physiology, and effects on fitness correlates are poorly understood in this and many other systems.
In this study, we tested the hypothesis that chemical cues of predation risk modulate the amphibian tadpole neuroendocrine stress axis, and that expression of morphological anti-predator defences is mediated by CORT. We used field studies to show that naturally occurring variation in predator densities in ponds is positively correlated with whole-body CORT content in Rana sylvatica tadpoles. In mesocosm and laboratory experiments, we found that exposure of tadpoles to predator chemical cue caused a biphasic response in CORT content, with a decrease in the short term (hours; as shown previously; [19]), but an increase with chronic exposure (days). We provide compelling evidence based on in vivo and in vitro experiments that the elevated CORT is the proximate mechanism for expression of the anti-predator body morphology. Furthermore, we show that the effects of CORT on fitness (survival) depend on the timing and the duration of hormone exposure: short-term treatment decreased survivorship, likely due to increased locomotory activity and thus exposure to predators, whereas long-term treatment increased survivorship, likely due to the morphological response. Our findings provide important insight into the connections between environment, physiological response, and resulting phenotypic plasticity and fitness consequences in a model ecological system.
2. Material and methods
(a). Animals
For the following experiments, we collected R. sylvatica egg masses or tadpoles from natural ponds on the University of Michigan's E.S. George Reserve (ESGR) in Livingston Co., Michigan.
(b). Relationship between baseline tadpole whole-body corticosterone content and predator density in natural ponds
We collected R. sylvatica tadpoles at Gosner stages 27–30 [34] from 10 natural ponds on the ESGR that historically contain consistent R. sylvatica populations and represent a range of pond sizes and environmental characteristics (four were closed-canopy ponds and six were open-canopy; [35]). Tadpoles were flash frozen in test tubes immersed in an ethanol-dry ice bath within 2 min of capture. Two to three tadpoles (approx. 100–200 mg body weight, BW) were pooled to generate a sample for CORT analysis (described below), and ten replicate samples were collected from each pond. Within a week, we also sampled the ponds as part of a long-term survey of the tadpole and tadpole predator community [35]. Biomass per square metre of potential predators of tadpoles in these ponds (adult newts, adult and larval dytiscids, larval hydrophylids, adult belostomatids and larval aeshnids) was used as a measure of predation pressure; these predators are largely present in ponds well before amphibian egg masses are deposited in the spring. Potential competitors (tadpoles of all species) and snails (potential source of trematode parasites) were also sampled and length–weight regressions calculated to estimate biomass per square metre (see [35] for details). We conducted regression analyses using whole-body CORT content as the dependent variable and predator, competitor or snail biomass as the predictor; each data point was a pond. We used the software package SPSS v. 16.0 (SPSS Inc., Chicago, IL, USA) here and for subsequent experiments for statistical analysis, unless otherwise noted. All data for this and the following studies are archived in the Dryad repository (doi:10.5061/dryad.1kf76).
(c). Effect of non-lethal predator presence on tadpole whole-body corticosterone content
We established 20 outdoor experimental mesocosms consisting of 1000 l tanks filled with well water, 200 g of leaves (primarily Quercus) for substrate, a 4 l aliquot of pond water for zooplankton and periphyton colonization and 15 g of rabbit chow as an initial food source [36]. We collected six R. sylvatica egg masses to hatch in a stock pool, from which 40 free-swimming tadpoles were haphazardly chosen and placed into each experimental mesocosm. Each mesocosm was randomly assigned to one of two treatments: no predator (control) or non-lethal predator presence, each replicated 10 times. Predator treatment tanks received three cages each containing one late-instar dragonfly larva (Anax sp.); the no predator treatments received empty cages. Predators were fed approximately 0.3 g of live R. sylvatica tadpoles three times per week to produce predator chemical cue [26,37]; empty predator cages in control tanks were similarly manipulated to simulate disturbance caused by feeding. On day 25 of the experiment, four tadpoles were collected from each of the experimental tanks, pooled into two samples, and immediately flash frozen for later analysis of baseline whole-body CORT content by extraction and radioimmunoassay (RIA; described below).
To determine whether tadpole environment influenced whole-body CORT content after metamorphosis (a possible carry-over effect), we sampled newly metamorphosed frogs. The remaining tadpoles with tail stubs at or less than 50 per cent of their snout–vent length were collected from tanks and placed into plastic containers maintained in the laboratory to finish metamorphosis. Once metamorphosis was complete (tail stub completely resorbed, 2–3 days following removal from the tank), two metamorphic frogs originating from each tank were pooled and immediately flash frozen for baseline CORT analysis (n = 10 per treatment). To investigate whether the metamorphic frog stress response was influenced by prior exposure to predator chemical cue as a tadpole, an additional two metamorphic frogs from each tank were subjected to a confinement stressor (placed into a 16.5 × 15 cm plastic zip-top bag) for 90 min [38], after which they were pooled by tank and flash frozen for subsequent CORT analysis (n = 10 per treatment).
For statistical analyses, tank was used as the experimental unit, and independent-samples t-tests were used to determine effects of predator chemical cue on tadpole baseline whole-body CORT content and BW at metamorphosis. To determine if exposure to predator chemical cue influenced baseline or stressor-induced whole-body CORT content of juvenile frogs, we used a one-way ANOVA with CORT as the dependent variable, and larval treatment and stress measure as fixed factors.
(d). Time course of the corticosterone response to predator chemical cue
We filled 20 outdoor wading pools (200 l capacity) with well water, and covered them with shade cloth to prevent colonization by other organisms. Ten pools received a caged late-instar dragonfly larva (non-lethal predator treatment), and ten received an empty cage (control treatment). Fifteen partial R. sylvatica egg masses were collected from a single pond and allowed to hatch in a stock pool. We haphazardly chose five Gosner stage 27 tadpoles for each of 100, 1.5 l plastic containers. We added 3–4 g rabbit chow (Purina) to each container to provide sufficient food for the 8-day experimental period, topped the containers with screen mesh to allow for water exchange and placed five containers into each of the wading pools immediately after feeding the caged dragonfly larvae three live tadpoles to produce predator chemical cue. The dragonfly larvae feeding/cage manipulation was continued every day thereafter.
Tadpoles were housed in individual containers to eliminate sampling bias and reduce disturbance (i.e. dip-netting would have induced a scatter response in the tadpoles, and may have resulted in a non-random sample with respect to behaviour). At 4 h, 1, 2, 4 and 8 days following initiation of the experiment one container of tadpoles was removed from each tank for analysis; technical problems resulted in the loss of the 2 day sample. Tadpoles were immediately flash frozen as described above and pooled (two animals per pool) for CORT analysis (n = 20 per treatment). We used ANOVA to test for differences between the two treatment groups across the sampling time points.
(e). Effect of chronic exposure to predator chemical cue, corticosterone or the corticosteroid biosynthesis blocker metyrapone on tadpole body morphology
Ten R. sylvatica egg masses collected from a single pond were hatched in outdoor wading pools. When tadpoles reached Gosner stage 26, 12 animals were haphazardly chosen and transferred to 10 l plastic tanks (37 × 23.5 × 13 cm) filled with 8 l of aged well water. The tanks were maintained in the laboratory at a temperature of 22–23°C on a 14 L : 10 D cycle; tadpoles were fed rabbit chow ad libitum. Treatments were added to the tank water and consisted of predator chemical cue (Pred; see below), predator chemical cue + the corticosteroid biosynthesis blocker metyrapone (MTP; Aldrich Chemical Co.; Pred + MTP), CORT (Sigma Chemical Co., St Louis, MO, USA) or control; each treatment was replicated 16 times and tanks were randomly assigned to treatments.
To produce water conditioned with predator chemical cue, late-instar dragonfly larvae were housed individually in 1 l containers filled with 0.5 l water and allowed to feed on 2–3 live R. sylvatica tadpoles for a 2–3 h period. Water was decanted and strained for use as predator chemical cue, and prepared fresh before each experiment by pooling water from 12–18 dragonfly containers. A 50 ml aliquot of the conditioned water was then added to each of the treatment tanks. The CORT was dissolved in 100 per cent ethanol and added to tanks to produce a final concentration of 125 nM; this concentration of CORT elevates whole-body CORT content by approximately 35 per cent, which is within the physiological range [39]. The MTP was also dissolved in 100 per cent ethanol and added to tanks for a final concentration of 110 μM that can reduce whole-body CORT content by two thirds in tadpoles [40]. Control tanks received a 50 ml aliquot of unconditioned water (vehicle control) plus 100 per cent ethanol added to a final concentration of 0.0005 per cent (to match the final concentration of ethanol in the hormone treatments). Treatments were maintained for 14 days, with water changes and fresh predator chemical cue, CORT or MTP added every other day.
At the termination of the experiment, four tadpoles from each of the 16 tanks per treatment were haphazardly chosen for morphological analysis and preserved in formalin (64 tadpoles total per treatment; the sample was the tank mean for n = 16 per treatment). Six tadpoles from each of the 16 tanks per treatment were flash frozen for hormone analysis (described below; 96 tadpoles total per treatment; the sample was the tank mean for n = 16 per treatment). We observed very low mortality: only two of 768 tadpoles died during the experiment, one from the Pred + MTP treatment and one from the Pred treatment. These tadpoles were not replaced.
Formalin-preserved tadpoles were photographed using a digital camera (FujiFilm FinePix S1 Pro, Nikon AF Micro-Nikkor 60 mm 1 : 2.8 D lens) mounted on a camera stand for morphological measurements. Trunk length, trunk width, tail length, and tail depth were measured using Image J v. 1.36b (National Institutes of Health, Bethesda, MD, USA) using the landmarks described by Relyea [20]. Morphological measurements were corrected for BW and the residuals used in statistical analysis. Measurements from tadpoles housed in the same tank were averaged, and the resulting replicate mean was used as the experimental unit in ANOVA to assess differences among treatments.
(f). Effect of one week corticosterone treatment in vitro on tadpole tail explant weight
We prepared tail explants from tadpoles of Xenopus laevis and cultured them in the presence or absence of CORT as described by Bonett et al. [41]. This experiment was conducted outside of the season when R. sylvatica tadpoles were available, so we used a laboratory colony of X. laevis. Briefly, tadpole tails were cultured for one week in six-well plates containing amphibian strength Dulbeco's Modified Eagle's Medium (DMEM; Gibco BRL; diluted 1 : 1.5). The cultures were maintained at 25°C in a sterile, humidified atmosphere of 5 per cent CO2 and 95 per cent O2 with gentle rotation (50 r.p.m.). The CORT was dissolved in 100 per cent EtOH and added to DMEM to a final concentration of 100 or 500 nM. The final concentration of EtOH in all wells, including the controls was 0.001 per cent. The medium with fresh hormone was replenished every 12 h. At the end of the experiment we blotted the tails on paper towels, weighed them and then dried them in a drying oven for one week before recording the final dry weight.
(g). Effect of corticosterone or metyrapone on tadpole survival in lethal predation trials
We measured tadpole survival in the presence of a lethal (uncaged) predator following either short (3 h) or long-term (8 days) exposure to CORT or MTP. For the short-term experiment, we placed 80 haphazardly chosen Gosner stage 28 tadpoles into three 75 l aquaria maintained in the laboratory at a temperature of 23–24°C. One aquarium received ethanol vehicle (0.0005%), another received CORT (125 nM) and a third received MTP (110 μM). Tadpoles were maintained in the aquaria for 3 h before lethal predation trial. We set up lethal predation trial tanks (not containing hormone) by filling 60, 10 l tanks with aged well water, then adding one 4 × 50 cm strip of vinyl screen and one late-instar dragonfly larva to each tank. At the end of the 3 h treatment period, we transferred four tadpoles to each 10 l tank, generating 20 tanks per treatment with four tadpoles in each tank. We then counted the number of live tadpoles in each tank every 30 min for 5 h.
For the long-term exposure experiment, extended-time housing requirements necessitated the use of smaller replicate tanks for conditioning. Gosner stage 26 tadpoles were haphazardly assigned to 10 l tanks filled with 8 l of aged well water, and maintained in the laboratory at a temperature of 23–24°C and a 14 L : 10 D cycle; tadpoles were fed rabbit chow ad libitum. Experimental treatments again consisted of ethanol vehicle, CORT (125 nM) or MTP (110 μM), each with sixteen replicate tanks per treatment and eight tadpoles per tank. Treatments were continued for 8 days, with water changes and fresh CORT or MTP added every other day. At the end of the treatment period we transferred four tadpoles from each treatment tank to 10 l tanks containing dragonfly larvae and conducted lethal predation trials as described. Survivorship analyses were conducted using the software package JMP (SAS Institute Inc, Cary, NC, USA).
(h). Whole-body corticosterone analysis
We conducted steroid hormone extraction on whole tadpoles as described by Denver [42] and analysed whole-body CORT content by RIA as described by Licht and colleagues [43]. The anti-CORT serum was purchased from MP Biomedicals (Irvine, CA, USA). This antiserum is highly specific for CORT: cross-reaction with the immediate precursor in the biosynthetic pathway 11-deoxycorticosterone was only 6.1 per cent; cross-reaction with 18 other steroids or cholesterol was greater than 0.3% (MP Biomedicals). Samples from a single study were assayed in either a single RIA, or multiple RIAs conducted on the same day. Potency estimates from the RIA were corrected for recoveries, and inter- and intra-assay coefficients of variation calculated using a quality control standard averaged 13 and 10%, respectively. We observed some variation in baseline (control) CORT content in tadpoles across different experiments, which likely reflects variation in the developmental stage of animals used in each study, or perhaps exposure to different environmental factors that we could not control for in the experimental mesocosms; the important comparison here is the relative difference in CORT content among treatments within an experiment.
3. Results
(a). Relationship between baseline tadpole whole-body corticosterone content and predator density in natural ponds
Whole-body CORT content was positively related to predator (slope = 44.1, t = 2.777, p = 0.032; figure 1) and snail (potential source of tadpole parasites; slope = 2.7, t = 3.195, p = 0.019; data not shown) biomass in natural ponds; there was no significant effect of tadpole competitor biomass (slope =−0.052, t =−0.194, p = 0.379; data not shown; full model: F3,9 = 6.035, R2 = 0.751, p = 0.030). None of the explanatory variables in the regression model were correlated.
Figure 1.
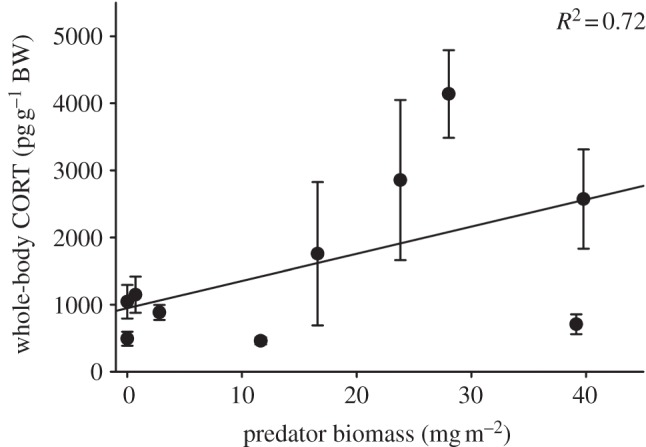
Whole-body corticosterone (CORT) content in field-collected tadpoles was positively correlated with predator biomass per square metre sampled in the same ponds (slope = 44.1, p = 0.032). Points represent pond means+2 s.e. (n = 10 ponds, with 10 samples collected in each pond).
(b). Effect of non-lethal predator presence on tadpole whole-body corticosterone content
Tadpoles reared in mesocosms containing caged predators had significantly greater whole-body CORT content compared with no predator controls (t9 = 2.387, p = 0.041; figure 2). Juvenile frogs exposed to predators as tadpoles also had significantly greater whole-body CORT content compared with no-predator controls (approx. twofold; F1,38 = 1.462, p = 0.049). Confinement stress of juvenile frogs caused a significant increase in whole-body CORT content in controls, but not in animals that were exposed to predators as larvae (treatment x stress interaction; F1,38 = 4.882, p = 0.034; figure 2). Body weight at metamorphosis was not different among treatments.
Figure 2.
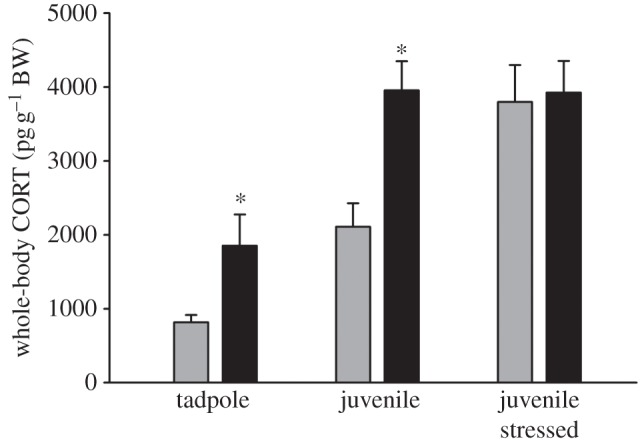
Tadpole whole-body CORT content was increased when animals were raised in mesocosms containing caged larval aeshnid predators fed conspecific tadpoles compared with controls raised in the absence of predators (n = 10 mesocosms per treatment, p = 0.041). Metamorphic frogs emerging from the mesocosms with predators retained elevated baseline CORT content but did not mount a further stress response to confinement compared with control frogs (treatment × stress interaction, p = 0.034). Black bars represent predators and grey bars represent no predators. Bars represent the mean±s.e.m.
(c). Time course of the corticosterone response to predator chemical cue
We observed a biphasic pattern in the tadpole stress response to predator chemical cue; there was an initial decrease in whole-body CORT content in predator-exposed tadpoles at 4 h (univariate contrasts: p = 0.065), which returned to baseline (equivalent to control treatment) by 24 h (p = 0.162). In contrast, long-term exposure to predator chemical cue led to significant increases in whole-body CORT at 4 and 8 days, compared with controls (p = 0.008 and p = 0.001, respectively; figure 3). In the full model, sample time (F = 8.382, p < 0.001) and treatment (F = 6.795, p = 0.011) both had significant effects on CORT content in tadpoles, and the time x treatment interaction was also significant (F = 9.415, p < 0.001).
Figure 3.
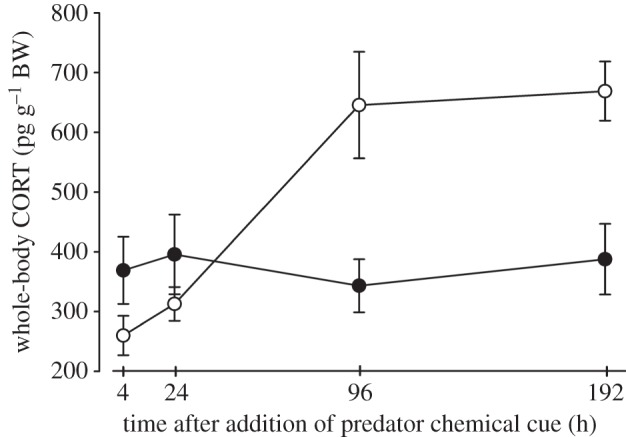
Exposure of tadpoles to the non-lethal presence of predators causes a biphasic response in whole-body CORT. Rana sylvatica tadpoles were exposed to caged aeshnid predators fed conspecific tadpoles in mesocosms and sampled at the indicated times for measurement of whole-body CORT by extraction and radioimmunoassay. The tadpoles initially reduced whole-body CORT content at 4 h post-exposure, but then showed elevated CORT by 4 days post-exposure (time × treatment interaction: p < 0.001 ANOVA). Each point represents the mean±s.e.m. (n = 10 per treatment and time point). Filled circles represent control and open circles represent predator cue.
(d). Effect of chronic exposure to predator chemical cue, corticosterone or the corticosteroid biosynthesis blocker metyrapone on tadpole body morphology
Tadpole BW differed among treatments (F3,60 = 34.55, p < 0.0001; figure 4). The predator chemical cue and CORT-exposed tadpoles did not significantly differ from one another (p = 0.07), and Pred + MTP and control treatments also grouped together (p = 0.471), but the CORT and Pred cluster significantly different from the Pred + MTP and control cluster (p < 0.0001). Controlling for these differences in BW, experimental treatments caused significant changes in tadpole tail depth (F3,60 = 7.310, p = 0.003; figure 4a) and trunk length (F3,60 = 9.406, p < 0.0001; figure 4b). Tadpoles exposed to predator chemical cue for 14 days increased tail depth (p = 0.0003) and decreased trunk length (p = 0.0001) relative to controls. Tadpoles exposed to exogenous CORT for 14 days had greater tail depth (p = 0.019) and showed a trend towards decreased trunk length (p = 0.101) relative to controls. The predator chemical cue and CORT-exposed tadpoles were not significantly different from one another in either measure. By contrast, blockade of corticosteroid synthesis by MTP reversed the effects of predator chemical cue on tail depth (p = 0.033) and body length (p = 0.0004).
Figure 4.
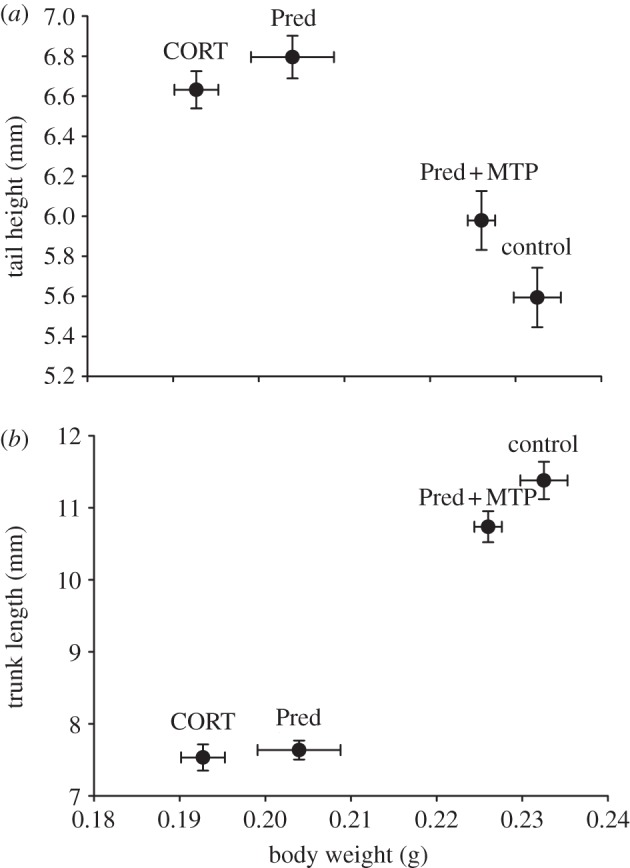
Exposure of tadpoles to predator chemical cue or CORT generate similar anti-predator morphology, and the effect of the predator chemical cue can be blocked by co-treatment with the corticosteroid synthesis inhibitor metyrapone (MTP). Tadpoles were treated with predator chemical cue (Pred), CORT (125 nM) or Pred plus MTP (110 μM). At the end of the treatment period tail height, trunk length and body weight were measured. Tail height (a) and trunk length (b) shown in the graphs were corrected for body weight, and both measures were significantly affected by the treatments. Tadpoles treated with Pred or CORT both developed deeper tails (Pred: p = 0.0003; CORT: p = 0.019) and shorter trunks (Pred: p = 0.0001; CORT non-significant trend: p = 0.101) compared with controls, and were not significantly different from each other in either measure (tail depth: p = 0.998; trunk length: p = 0.171). Tadpoles treated with predator chemical cue plus MTP had shallower tails (p = 0.033) and longer trunks (p = 0.0004) than tadpoles exposed to predator chemical cue alone. Error bars represent standard error of the mean (n = 16 per treatment).
(e). Effect of one week corticosterone treatment in vitro on tadpole tail explant weight
Treatment of tadpole tail explant cultures with two doses of CORT for one week caused significant increases in both wet and dry final tail weight (see the electronic supplementary material, table S1).
(f). Effect of corticosterone or metyrapone on tadpole survival in lethal predation trials
Exposure to exogenous CORT altered tadpole survival probability, the direction of which was opposite depending on whether hormone exposure was short (3 h) or long (8 days). After short-term exposure to CORT, tadpoles were more likely to be captured by predators than the control or MTP-treated animals (Wilcoxon X2 = 8.299, p = 0.016; figure 5a; control and MTP treatments were not significantly different). Following long-term exposure to CORT there was a trend towards tadpoles being less likely to be captured than in the control or MTP treatments (Wilcoxon X2 = 4.751, p = 0.093; figure 5b; control and MTP treatments were not significantly different).
Figure 5.
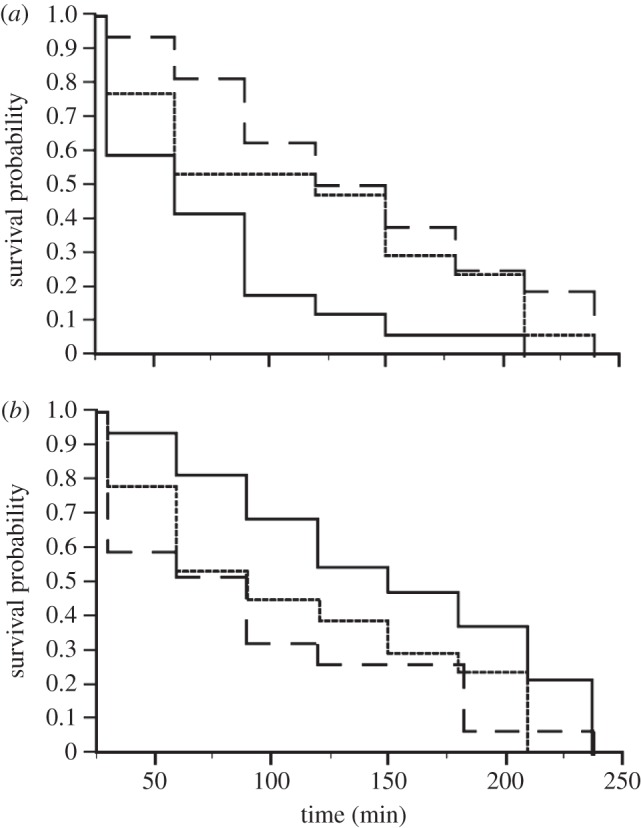
Tadpole survivorship patterns indicate possible temporal trade-offs during the development of the stress response. Survival probability for the first tadpole consumed by a larval dragonfly predator differed following a (a) 3 h or (b) 8 days pre-treatment with exogenous CORT (solid line), the CORT synthesis blocker MTP (dashed line), or ethanol control (dotted line). (a) After a 3 h pre-treatment, CORT-treated tadpoles were consumed at a significantly higher rate than MTP-treated or controls (p = 0.016; n = 20 per treatment). (b) After 8 days of treatment, however, CORT-treated tadpoles trended towards surviving at a higher rate than MTP-treated or control tadpoles (p = 0.093; n = 16 per treatment).
4. Discussion
Amphibian tadpoles display extensive phenotypic plasticity in anti-predator defences [19,29,37,44] that have been shown to enhance fitness by increasing the probability of survival to metamorphosis [30]. While the induction of these defences and their evolutionary and ecological significance have been studied extensively (see [31] for review), the proximate mechanisms that underlie these changes are largely unknown. Moreover, because these responses are used with different lags to predator exposure, it is critical that we understand how these responses are mechanistically integrated and what trade-offs are implicated. Here, we show that tadpole anti-predator defences are controlled, in part, by a bimodal physiological stress response to predation. Tadpoles suppress behaviour and their neuroendocrine stress axis in the short-term: behavioural inhibition enhances survivorship by reducing exposure to predators, and this response is facilitated by suppression of the stress axis [19]. However, tadpoles increase stress hormonal activity over a longer time frame, which induces adaptive changes in tail and body morphology that enhance survivorship by facilitating escape behaviour, or providing a decoy (large tail) to deflect lethal predator attacks from the more vulnerable body [30,31].
Rana sylvatica is an excellent system for investigating the mechanistic underpinnings of phenotypic plasticity to predators, because populations of this species inhabit a range of pond types and associated predation risks [35] and, therefore, tadpoles exhibit a high degree of both behavioural and morphological plasticity [6,21]. Using a series of field surveys, and mesocosm and laboratory experiments, we found that exposure to predator chemical cue altered the activity of the tadpole's neuroendocrine stress axis as a function of exposure time. We confirmed that initial exposure of tadpoles to predator chemical cue causes suppression of whole-body CORT content; Fraker et al. [19] showed that this response is rapid (lower mean CORT by the earliest time measured (1 h), statistically significant by 2 h), dependent on the dose of predator chemical cue, and occurred in two amphibian species, R. sylvatica and R. clamitans. The rapid behavioural and physiological response to predation is induced by a chemical cue released from tadpole skin by active secretion (an alarm pheromone—biochemical purification of the predator chemical cue from tadpole skin showed that it composed of two distinct components that must be combined for biological activity; mass spectrometry identified a series of small peptides as candidate components of the alarm pheromone [19]). Importantly, corticosteroids generally stimulate locomotion and foraging by amphibian tadpoles [45,46] and, therefore, the predator-induced suppression of the tadpole stress axis is consistent with the reduction in activity usually reported in amphibian tadpoles exposed to predators [19,37]. Furthermore, the behavioural inhibition can be reversed by treatment with CORT [19]. The behavioural response with the concomitant physiological response is clearly adaptive in reducing exposure to predators [47]. It is noteworthy that the suppression of the tadpole neuroendocrine stress axis differs from the response reported for many other vertebrate taxa exposed to predators or predator odours, where corticosteroid production tends to show rapid increases (i.e. in a classical fight or flight response; [48,49]). The optimal short-term response, however, may depend upon predator hunting mode [50]. In contrast, we found that long-term exposure of tadpoles to predators or predator chemical cue in both the field and mesocosm led to activation of the stress axis, producing chronically elevated whole-body CORT content. Activation of the stress axis of prey by exposure to predators or predator chemical cues has also been demonstrated in other vertebrate taxa (mammals [12], birds [14,18] reptiles [17], and fish [51]).
A large literature has documented that longer-term exposure to predators induces a range of morphological responses in tadpoles [47]. Because chronic predator exposure increased whole-body CORT, and prior work from our laboratory showed increased tail muscle depth in R. pipiens tadpoles following treatment with CORT [39,41], we hypothesized that the elevated CORT was causal for the induced anti-predator morphology. Here, we provide compelling evidence that the increased tail size in tadpoles chronically exposed to predator chemical cue is caused by direct actions of the stress hormone CORT on the tadpole tail. Tadpoles exposed to exogenous CORT over a two-week period developed tail and body morphology similar to tadpoles exposed chronically to predator chemical cue [21]. Importantly, the corticosteroid synthesis inhibitor MTP blocked the predator chemical cue-induced morphology. We replicated the effect of CORT on tadpole tail size in tissue culture, thus showing that the action of the hormone is direct on the tail. Hossie et al. [52] reported that exposure to a predator increased body size of R. pipiens tadpoles, and that this could be blocked by treatment with MTP.
We also show that both short- and long-term responses to stress hormone action positively affect fitness. Countering the short-term suppression of the stress axis in predator presence by treatment with CORT decreased survivorship of tadpoles reared with lethal predators; this was likely due to the increased locomotory activity of tadpoles caused by CORT, which increased the likelihood of encountering a sit-and-wait predator such as the larval aeshnid. By contrast, longer-term treatment with CORT tended to increase survivorship, presumably due to the development of the anti-predator morphological responses. Relyea [53] has demonstrated that these morphological responses in Hyla versicolor tadpoles can develop in 4–8 days after exposure to cues of predator presence, which is within the time frame of our long-term exposure experiment.
Thus, we demonstrate adaptive aspects of temporal dynamics of stress hormone production and action in tadpoles. However, understanding this mechanistic link between the neuroendocrine stress response and predator-induced phenotypic plasticity in tadpoles exposes trade-offs not apparent from experiments simply documenting the relationship between environmental agents and the phenotypic response. For example, although activity reduction is a nearly universal response of animals to sit-and-wait predators [54], a reduction in stress hormone production compatible with reduced activity is not viable in the long term as the animal enters a state of negative energy balance, and CORT is involved with mobilizing energy for survival [9,10]. Earlier, we showed that low CORT is permissive for reduced locomotory activity and foraging [19]. The rise in CORT production following several days of predator exposure (and associated behavioural quiescence) may stimulate foraging while inducing morphology that reduces predation susceptibility. It is not clear if this transition results in a window of vulnerability; i.e. increasing locomotory activity before morphological development is possible. It is also possible that the stimulatory effects of CORT on locomotion become refractory over time, resulting in maintenance of behaviour quiescence over the long term. However, our results indicate that elevated CORT is required for development of the anti-predator morphology, so the short-term suppression of CORT would be expected to delay this development.
Corticosteroids have complex effects on growth and metamorphosis of larval amphibians. For example, elevated CORT (e.g. owing to an endocrine stress response caused by pond drying) accelerates metamorphosis by synergizing with the metamorphic hormone, thyroid hormone [8]. Tadpoles must reach a threshold stage of development (mid-prometamorphosis) before their neuroendocrine system is sufficiently mature to respond to the environmental signal by accelerating development [8]. By contrast, elevated corticosteroids reduce tadpole growth at all developmental stages [8,55]. Exposure to predators slows tadpole growth ([31]; and see §3), which may be due in part to the growth inhibitory actions of the elevated CORT. Therefore, while the development of anti-predator morphology in response to CORT can promote survival, it can incur costs such as reduced growth rate.
It is well documented that chronic stress responses in juvenile and adult vertebrates can have important costs and lasting effects on phenotype and fitness. In our mesocosm study, predator-exposed tadpoles metamorphosed into juveniles that retained a high baseline CORT content, and did not mount a stress response to a novel stressor. We do not know if the elevated CORT would have persisted as the animals matured, but earlier work from our laboratory [54] showed that exposure of early prometamorphic X. laevis tadpoles to elevated CORT led to long term, stable changes in physiology that included elevated baseline plasma CORT concentration and a blunted stress response. These changes in the neuroendocrine stress axis may have been mediated, at least in part, by an alteration in negative feedback by CORT on the hypothalamus and limbic system, because the glucocorticoid receptor was decreased in these brain areas in frogs exposed to CORT as tadpoles [55].
Taken together, our findings suggest that there are a series of important and likely costly trade-offs involved with both the short- and long-term responses to predators mediated through the stress axis. Our results support that corticosteroids mediate the cost/benefit trade-off in the development of anti-predator morphology and growth rate, and hint at the time frame whereby these responses switch in tadpoles from immediate responses to predators to strategies more compatible with chronic exposure. Thus, the optimal timing of the transition from the short-term to chronic physiological responses, accounting for the costs associated with the different responses, is an important but conceptually underdeveloped area.
Acknowledgements
All experiments were conducted in accordance with the guidelines of the University Committee on the Use and Care of Animals at the University of Michigan.
This work was conducted on the University of Michigan's E.S. George Reserve. We thank Chris Davis, Mike Benard, Amanda Zellmer, Sarah Seiter, Mike Fraker, John Marino, Leah Penn Boris, Brian Duchemin and Fang Hu for assistance in the field and laboratory. This research was supported in part by a University of Michigan Department of Ecology and Evolutionary Biology Block grant to J.M.M., NSF grant no. IOS 0909703 to E.E.W. and J.M.M., NSF grants nos. IBN 0235401 and IOS 0641587 to R.J.D., and NSF LTREB grant no. DEB 0454519 to E.E.W., D. Skelly, R. Relyea and K. Yurewicz.
References
- 1.Werner EE, Peacor SD. 2003. A review of trait-mediated indirect interactions in ecological communities. Ecology 84, 1083–1100 10.1890/0012-9658(2003)084[1083:AROTII]2.0.CO;2 (doi:10.1890/0012-9658(2003)084[1083:AROTII]2.0.CO;2) [DOI] [Google Scholar]
- 2.Auld JR, Agrawal AA, Relyea RA. 2010. Re-evaluating the costs and limits of adaptive phenotypic plasticity. Proc. R. Soc. B 277, 503–511 10.1098/rspb.2009.1355 (doi:10.1098/rspb.2009.1355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeWitt TJ, Sih A, Wilson DS. 1998. Costs and limits of phenotypic plasticity. Trends Ecol. Evol. 13, 77–81 10.1016/S0169-5347(97)01274-3 (doi:10.1016/S0169-5347(97)01274-3) [DOI] [PubMed] [Google Scholar]
- 4.Miner BG, Sultan SE, Morgan SG, Padilla DK, Relyea RA. 2005. Ecological consequences of phenotypic plasticity. Trends Ecol. Evol. 20, 685–692 10.1016/j.tree.2005.08.002 (doi:10.1016/j.tree.2005.08.002) [DOI] [PubMed] [Google Scholar]
- 5.Pigliucci M. 2005. Evolution of phenotypic plasticity: where are we going now? Trends Ecol. Evol. 20, 481–486 10.1016/j.tree.2005.06.001 (doi:10.1016/j.tree.2005.06.001) [DOI] [PubMed] [Google Scholar]
- 6.Relyea RA. 2002. Costs of phenotypic plasticity. Am. Nat. 159, 272–282 10.1086/338540 (doi:10.1086/338540) [DOI] [PubMed] [Google Scholar]
- 7.van Kleunen M, Fischer M. 2007. Progress in the detection of costs of phenotypic plasticity in plants. New Phytol. 176, 727–730 10.1111/j.1469-8137.2007.02296.x (doi:10.1111/j.1469-8137.2007.02296.x) [DOI] [PubMed] [Google Scholar]
- 8.Denver RJ. 2009. Stress hormones mediate environment-genotype interactions during amphibian development. Gen. Comp. Endocrinol. 164, 20–31 10.1016/j.ygcen.2009.04.016 (doi:10.1016/j.ygcen.2009.04.016) [DOI] [PubMed] [Google Scholar]
- 9.Sapolsky RM, Romero LM, Munck AU. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55–89 10.1210/er.21.1.55 (doi:10.1210/er.21.1.55) [DOI] [PubMed] [Google Scholar]
- 10.Wingfield JC, Romero LM. 2001. Andrenocortical responses to stress and their modulation in free-living vertebrates. In Handbook of physiology; section 7: the endocrine system; volume iv: coping with the environment: neural and endocrine mechanisms (eds McEwen BS, Goodman HM.), pp. 211–234 Oxford, UK: Oxford University Press [Google Scholar]
- 11.Adamec R, Head D, Blundell J, Burton P, Berton O. 2006. Lasting anxiogenic effects of feline predator stress in mice: Sex differences in vulnerability to stress and predicting severity of anxiogenic response from the stress experience. Physiol. Behav. 88, 12–29 10.1016/j.physbeh.2006.03.005 (doi:10.1016/j.physbeh.2006.03.005) [DOI] [PubMed] [Google Scholar]
- 12.Blanchard RJ, Nikulina JN, Sakai RR, McKittrick C, McEwen B, Blanchard DC. 1998. Behavioral and endocrine change following chronic predatory stress. Physiol. Behav. 63, 561–569 10.1016/S0031-9384(97)00508-8 (doi:10.1016/S0031-9384(97)00508-8) [DOI] [PubMed] [Google Scholar]
- 13.Canoine V, Hayden TJ, Rowe K, Goymann W. 2002. The stress response of European stonechats depends on the type of stressor. Behaviour 139, 1303–1311 10.1163/156853902321104172 (doi:10.1163/156853902321104172) [DOI] [Google Scholar]
- 14.Scheuerlein A, Van't Hof TJ, Gwinner E. 2001. Predators as stressors? Physiological and reproductive consequences of predation risk in tropical stonechats (Saxicola torquata axillaris). Proc. R. Soc. Lond. B 268, 1575–1582 10.1098/rspb.2001.1691 (doi:10.1098/rspb.2001.1691) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barcellos LJG, Ritter F, Kreutz LC, Quevedo RM, Silva LBda, Bedin AC, Finco J, Cericato L. 2007. Whole-body cortisol increases after direct and visual contact with a predator in zebrafish, Danio rerio. Aquaculture 272, 774–778 10.1016/j.aquaculture.2007.09.002 (doi:10.1016/j.aquaculture.2007.09.002) [DOI] [Google Scholar]
- 16.Roseboom PH, Nanda SA, Bakshi VP, Trentani A, Newman SM, Kalin NH. 2007. Predator threat induces behavioral inhibition, pituitary-adrenal activation and changes in amygdala CRF-binding protein gene expression. Psychoneuroendocrinology 32, 44–55 10.1016/j.psyneuen.2006.10.002 (doi:10.1016/j.psyneuen.2006.10.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berger S, Wikelski M, Romero LM, Kalko EK, Roedl T. 2007. Behavioral and physiological adjustments to new predators in an endemic island species, the Galapagos marine iguana. Horm. Behav. 52, 653–663 10.1016/j.yhbeh.2007.08.004 (doi:10.1016/j.yhbeh.2007.08.004) [DOI] [PubMed] [Google Scholar]
- 18.Clinchy M, Zanette L, Charlier TD, Newman AE, Schmidt KL, Boonstra R, Soma KK. 2011. Multiple measures elucidate glucocorticoid responses to environmental variation in predation threat. Oecologia 166, 607–614 10.1007/s00442-011-1915-2 (doi:10.1007/s00442-011-1915-2) [DOI] [PubMed] [Google Scholar]
- 19.Fraker ME, Hu F, Cuddapah V, McCollum SA, Relyea RA, Hempel J, Denver RJ. 2009. Characterization of an alarm pheromone secreted by amphibian tadpoles that induces behavioral inhibition and suppression of the neuroendocrine stress axis. Horm. Behav. 55, 520–529 10.1016/j.yhbeh.2009.01.007 (doi:10.1016/j.yhbeh.2009.01.007) [DOI] [PubMed] [Google Scholar]
- 20.Relyea RA. 2000. Trait-mediated indirect effects in larval anurans: reversing competition with the threat of predation. Ecology 81, 2278–2289 10.1890/0012-9658(2000)081[2278:TMIEIL]2.0.CO;2 (doi:10.1890/0012-9658(2000)081[2278:TMIEIL]2.0.CO;2) [DOI] [Google Scholar]
- 21.Relyea RA. 2004. Fine-tuned phenotypes: tadpole plasticity under 16 combinations of predators and competitors. Ecology 85, 172–179 10.1890/03-0169 (doi:10.1890/03-0169) [DOI] [Google Scholar]
- 22.Skelly DK. 1994. Activity level and the susceptibility of anuran larvae to predation. Anim. Behav. 47, 465–468 10.1006/anbe.1994.1063 (doi:10.1006/anbe.1994.1063) [DOI] [Google Scholar]
- 23.Van Buskirk J, Schmidt BR. 2000. Predator-induced phenotypic plasticity in larval newts: trade-offs, selection, and variation in nature. Ecology 81, 3009–3028 10.1890/0012-9658(2000)081[3009:PIPPIL]2.0.CO;2 (doi:10.1890/0012-9658(2000)081[3009:PIPPIL]2.0.CO;2) [DOI] [Google Scholar]
- 24.Van Buskirk J, Yurewicz KL. 1998. Effects of predators on prey growth rate: relative contributions of thinning and reduced activity. Oikos 82, 20–28 10.2307/3546913 (doi:10.2307/3546913) [DOI] [Google Scholar]
- 25.Gallie JA, Mumme RL, Wissinger SA. 2001. Experience has no effect on the development of chemosensory recognition of predators by tadpoles of the American toad, Bufo americanus. Herpetologica 57, 376–383 [Google Scholar]
- 26.McCollum SA, Leimberger JD. 1997. Predator-induced morphological changes in an amphibian: predation by dragonflies affects tadpole shape and color. Oecologia 109, 615–621 10.1007/s004420050124 (doi:10.1007/s004420050124) [DOI] [PubMed] [Google Scholar]
- 27.Relyea RA, Auld JR. 2005. Predator- and competitor-induced plasticity: how changes in foraging morphology affect phenotypic trade-offs. Ecology 86, 1723–1729 10.1890/04-1920 (doi:10.1890/04-1920) [DOI] [Google Scholar]
- 28.Van Buskirk J, McCollum SA. 2000. Functional mechanisms of an inducible defence in tadpoles: morphology and behaviour influence mortality risk from predation. J. Evol. Biol. 13, 336–347 10.1046/j.1420-9101.2000.00173.x (doi:10.1046/j.1420-9101.2000.00173.x) [DOI] [Google Scholar]
- 29.Relyea RA. 2001. Morphological and behavioral plasticity of larval anurans in response to different predators. Ecology 82, 523–540 10.1890/0012-9658(2001)082[0523:MABPOL]2.0.CO;2 (doi:10.1890/0012-9658(2001)082[0523:MABPOL]2.0.CO;2) [DOI] [Google Scholar]
- 30.Van Buskirk J, McCollum SA, Werner EE. 1997. Natural selection for environmentally induced phenotypes in tadpoles. Evolution 51, 1983–1992 10.2307/2411018 (doi:10.2307/2411018) [DOI] [PubMed] [Google Scholar]
- 31.Benard MF. 2004. Predator-induced phenotypic plasticity in organisms with complex life histories. Annu. Rev. Ecol. Evol. Syst. 35, 651–673 10.1146/annurev.ecolsys.35.021004.112426 (doi:10.1146/annurev.ecolsys.35.021004.112426) [DOI] [Google Scholar]
- 32.Relyea RA. 2007. Getting out alive: how predators affect the decision to metamorphose. Oecologia 152, 389–400 10.1007/s00442-007-0675-5 (doi:10.1007/s00442-007-0675-5) [DOI] [PubMed] [Google Scholar]
- 33.Johnson JB, Burt DB, DeWitt TJ. 2008. Form, function, and fitness: pathways to survival. Evolution 62, 1243–1251 10.1111/j.1558-5646.2008.00343.x (doi:10.1111/j.1558-5646.2008.00343.x) [DOI] [PubMed] [Google Scholar]
- 34.Gosner KL. 1960. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16, 183–190 [Google Scholar]
- 35.Werner EE, Skelly DK, Relyea RA, Yurewicz KL. 2007. Amphibian species richness across environmental gradients. Oikos 116, 1697–1712 10.1111/j.0030-1299.2007.15935.x (doi:10.1111/j.0030-1299.2007.15935.x) [DOI] [Google Scholar]
- 36.Relyea RA. 2001. The lasting effects of adaptive plasticity: predator-induced tadpoles become long-legged frogs. Ecology 82, 1947–1955 10.1890/0012-9658(2001)082[1947:TLEOAP]2.0.CO;2 (doi:10.1890/0012-9658(2001)082[1947:TLEOAP]2.0.CO;2) [DOI] [Google Scholar]
- 37.Fraker ME. 2008. The dynamics of predation risk assessment: responses of anuran larvae to chemical cues of predators. J. Anim. Ecol. 77, 638–645 10.1111/j.1365-2656.2008.01386.x (doi:10.1111/j.1365-2656.2008.01386.x) [DOI] [PubMed] [Google Scholar]
- 38.Yao M, Westphal NJ, Denver RJ. 2004. Distribution and acute stressor-induced activation of corticotrophin-releasing hormone neurones in the central nervous system of Xenopus laevis. J. Neuroendocrinol. 16, 880–893 10.1111/j.1365-2826.2004.01246.x (doi:10.1111/j.1365-2826.2004.01246.x) [DOI] [PubMed] [Google Scholar]
- 39.Glennemeier KA, Denver RJ. 2002. Small changes in whole-body corticosterone content affect larval Rana pipiens fitness components. Gen. Comp. Endocrinol. 127, 16–25 10.1016/S0016-6480(02)00015-1 (doi:10.1016/S0016-6480(02)00015-1) [DOI] [PubMed] [Google Scholar]
- 40.Glennemeier KA, Denver RJ. 2002. Role for corticoids in mediating the response of Rana pipiens tadpoles to intraspecific competition. J. Exp. Zool. 292, 32–40 10.1002/jez.1140 (doi:10.1002/jez.1140) [DOI] [PubMed] [Google Scholar]
- 41.Bonett RM, Hoopfer ED, Denver RJ. 2010. Molecular mechanisms of corticosteroid synergy with thyroid hormone during tadpole metamorphosis. Gen. Comp. Endocrinol. 168, 209–219 10.1016/j.ygcen.2010.03.014 (doi:10.1016/j.ygcen.2010.03.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Denver RJ. 1998. Hormonal correlates of environmentally induced metamorphosis in the Western spadefoot toad, Scaphiopus hammondii. Gen. Comp. Endocrinol. 110, 326–336 10.1006/gcen.1998.7082 (doi:10.1006/gcen.1998.7082) [DOI] [PubMed] [Google Scholar]
- 43.Licht P, McCreery BR, Barnes R, Pang R. 1983. Seasonal and stress related changes in plasma gonadotropins, sex steroids, and corticosterone in the bullfrog, Rana catesbeiana. Gen. Comp. Endocrinol. 50, 124–145 10.1016/0016-6480(83)90249-6 (doi:10.1016/0016-6480(83)90249-6) [DOI] [PubMed] [Google Scholar]
- 44.Benard MF, Fordyce JA. 2003. Are induced defenses costly? Consequences of predator-induced defenses in western toads, Bufo boreas. Ecology 84, 68–78 10.1890/0012-9658(2003)084[0068:AIDCCO]2.0.CO;2 (doi:10.1890/0012-9658(2003)084[0068:AIDCCO]2.0.CO;2) [DOI] [Google Scholar]
- 45.Crespi EJ, Denver RJ. 2004. Ontogeny of corticotropin-releasing factor effects on locomotion and foraging in the Western spadefoot toad (Spea hammondii). Horm. Behav. 46, 399–410 10.1016/j.yhbeh.2004.03.011 (doi:10.1016/j.yhbeh.2004.03.011) [DOI] [PubMed] [Google Scholar]
- 46.Crespi EJ, Denver RJ. 2005. Roles of stress hormones in food intake regulation in anuran amphibians throughout the life cycle. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 141, 381–390 10.1016/j.cbpb.2004.12.007 (doi:10.1016/j.cbpb.2004.12.007) [DOI] [PubMed] [Google Scholar]
- 47.Cooper C, et al. 2003. The Barker hypothesis: early life influences on bone and cardiovascular diseases. J. Bone Miner. Res. 18, 1356–1356 [Google Scholar]
- 48.Apfelbach R, Blanchard CD, Blanchard RJ, Hayes RA, McGregor IS. 2005. The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci. Biobehav. Rev. 29, 1123–1144 10.1016/j.neubiorev.2005.05.005 (doi:10.1016/j.neubiorev.2005.05.005) [DOI] [PubMed] [Google Scholar]
- 49.Schreck CB. 2010. Stress and fish reproduction: the roles of allostasis and hormesis. Gen. Comp. Endocrinol. 165, 549–556 10.1016/j.ygcen.2009.07.004 (doi:10.1016/j.ygcen.2009.07.004) [DOI] [PubMed] [Google Scholar]
- 50.Schmitz OJ. 2008. Effects of hunting model on grassland ecosystem function. Science 319, 952–954 10.1126/science.1152355 (doi:10.1126/science.1152355) [DOI] [PubMed] [Google Scholar]
- 51.Bell AM, Backstrom T, Huntingford FA, Pottinger TG, Winberg S. 2007. Variable neuroendocrine responses to ecologically-relevant challenges in sticklebacks. Physiol. Behav. 91, 15–25 10.1016/j.physbeh.2007.01.012 (doi:10.1016/j.physbeh.2007.01.012) [DOI] [PubMed] [Google Scholar]
- 52.Hossie TJ, Ferland-Raymond B, Burness G, Murray DL. 2010. Morphological and behavioural responses of frog tadpoles to perceived predation risk: a possible role for corticosterone mediation? Ecoscience 17, 100–108 10.2980/17-1-3312 (doi:10.2980/17-1-3312) [DOI] [Google Scholar]
- 53.Relyea RA. 2003. Predators come and predators go: the reversibility of predator-induced traits. Ecology 84, 1840–1848 10.1890/0012-9658(2003)084[1840:PCAPGT]2.0.CO;2 (doi:10.1890/0012-9658(2003)084[1840:PCAPGT]2.0.CO;2) [DOI] [Google Scholar]
- 54.Lima SL, Dill LM. 1990. Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68, 619–640 10.1139/z90-092 (doi:10.1139/z90-092) [DOI] [Google Scholar]
- 55.Hu F, Crespi EJ, Denver RJ. 2008. Programming neuroendocrine stress axis activity by exposure to glucocorticoids during postembryonic development of the frog, Xenopus laevis. Endocrinology 149, 5470–5481 10.1210/en.2008-0767 (doi:10.1210/en.2008-0767) [DOI] [PubMed] [Google Scholar]


