Abstract
The rapid adoption of genetically engineered (GE) plants that express insecticidal Cry proteins derived from Bacillus thuringiensis (Bt) has raised concerns about their potential impact on non-target organisms. This includes the possibility that non-target herbivores develop into pests. Although studies have now reported increased populations of non-target herbivores in Bt cotton, the underlying mechanisms are not fully understood. We propose that lack of herbivore-induced secondary metabolites in Bt cotton represents a mechanism that benefits non-target herbivores. We show that, because of effective suppression of Bt-sensitive lepidopteran herbivores, Bt cotton contains reduced levels of induced terpenoids. We also show that changes in the overall level of these defensive secondary metabolites are associated with improved performance of a Bt-insensitive herbivore, the cotton aphid, under glasshouse conditions. These effects, however, were not as clearly evident under field conditions as aphid populations were not correlated with the amount of terpenoids measured in the plants. Nevertheless, increased aphid numbers were visible in Bt cotton compared with non-Bt cotton on some sampling dates. Identification of this mechanism increases our understanding of how insect-resistant crops impact herbivore communities and helps underpin the sustainable use of GE varieties.
Keywords: Gossypium hirsutum, genetically engineered crops, gossypol, non-target effects, plant-mediated competition, plant secondary chemistry
1. Introduction
Varieties of genetically engineered (GE) cotton, Gossypium hirsutum (L.) (Malvales: Malvaceae), that produce Cry proteins derived from Bacillus thuringiensis (Berliner) (Bt) are highly resistant against major lepidopteran pests [1] and their widespread planting has led to area-wide population suppression of key pest species [2,3]. The Cry toxins currently deployed in Bt cotton varieties are not known to cause direct toxic effects to herbivorous pests other than those in the order Lepidoptera [1]. Thus, management of non-lepidopteran pests remains a requirement for the sustainable deployment of Bt-transgenic cotton [1,4]. Some studies have reported increased populations of sap-feeding herbivores, such as aphids and leafhoppers, as well as plant-feeding hemipterans such as plant bugs (Miridae) and stink bugs (Pentatomidae), in Bt cotton [1]. In the case of plant-feeding hemipterans, increased damage to Bt cotton has been linked to the reduced use of broad-spectrum insecticides to control pest Lepidoptera [5–7]. However, increased abundance of non-lepidopteran herbivores in Bt cotton was also reported from some studies that excluded chemical insecticide treatments on both Bt and non-Bt cotton [8,9], suggesting that reduced insecticide use alone is an insufficient explanation for some emergent pests in cotton.
Cotton produces a range of closely related terpenoids (e.g. gossypol) that act as anti-feedants and possess insecticidal properties [10–12]. The levels of these defensive compounds are systemically increased by the plant damage caused by tissue feeders, such as lepidopteran larvae [13–16]. The intensity of the damage affects the levels of induced terpenoids [14]. This can have cascading effects on the performance of tissue feeders [15,17] and sap-feeding herbivores, such as aphids [18], which by themselves do not appear to induce this plant resistance [19,20].
We demonstrate here in a glasshouse and field study that the reduced damage caused by larvae of Heliothis virescens (Fabricius) (Lepidoptera: Noctuidae) on Bt cotton leads to a reduced induction of cotton terpenoids, which enhances the crop's susceptibility to a non-target pest, namely the cotton aphid Aphis gossypii (Glover) (Hemiptera: Aphididae). This indirect, plant-mediated effect of Bt cotton on non-target species has not been previously considered and may constitute an additional mechanism that benefits non-target herbivores.
2. Material and methods
(a). Glasshouse experiments
(i). Plants
Two varieties of GE cotton plants, which were provided by Monsanto Company (St. Louis, USA), were used for the experiments. One variety (‘Bt cotton’), Deltapine DPL143B2 RF (event: MON15985 × MON88913), expresses two Bt toxins (Cry1Ac and Cry2Ab, Bollgard II) and carries an herbicide-tolerance trait (targeting glyphosate). The second variety (‘non-Bt cotton’), Deltapine DPL147 RF (event: MON88913), has a similar genetic background but only contains the herbicide-tolerance trait. For insect rearing, the closely related non-GE variety Deltapine DPL491 was used. Plants were grown in 3 l plastic pots containing heat-sterilized humus-rich soil. At planting, 15 mg of the slow-release fertilizer Osmocote (16% N, 11% P2O5, 11% K2O; Scotts UK Professional, Bramford, UK) was added to each plant. Subsequently, the plants were fertilized weekly using 10N : 10P : 8K at 20 ml l−1 and were watered daily with water containing no fertilizer. Plants were enclosed in gauze cages (height, 71 cm; diameter, 35 cm; mesh-width, 0.264 mm) to protect them from glasshouse pests. Bt and non-Bt cotton plants were used when they had four fully expanded true leaves.
(ii). Insects
A colony of A. gossypii was obtained from Syngenta (Stein, Switzerland) and reared on four- to eight-week-old cotton plants (Deltapine DPL491). The H. virescens larvae used in the experiments were regularly obtained from Syngenta.
(iii). Experiments
The glasshouse experiments were conducted at 25°C ± 5°C, relative humidity 70 ± 10% and 16 L : 8 D long-day conditions.
Plants were either infested with a single H. virescens larva (3rd instar) that was caged on the youngest fully developed leaf for 7 days in a gauze bag, or left uninfested (control). Heliothis virescens larval weight was recorded before placing the larva on the plant and after 7 days to calculate the weight gain. In total, 27 plants were initially tested per treatment. Plants damaged accidentally during the experiment or found to be infected by any glasshouse pest were removed, resulting in n = 15–21 per treatment.
After the 7 days, 20 A. gossypii (mixed stages) were transferred to the youngest fully developed leaf of each plant (not identical to the leaf on which the larva was released). The aphids were enclosed in a clip-cage that was removed after 24 h. After 14 days, the aphid numbers were recorded, and all aphids were transferred into vials with 70 per cent ethanol. Subsequently, hind-tibia lengths were measured for 10 randomly chosen adults (fewer if 10 were not available) from each plant.
After termination of the experiment, leaf damage caused by H. virescens larvae was recorded. For this, the damaged leaf from each plant was collected and photographed and the damaged leaf area was measured with ImageJ v. 1.42 software.
A separate set of Bt and non-Bt cotton was treated as described earlier, with one group from each plant type being infested with H. virescens while the second remained uninfested (10 plants per treatment). After 7 days, the youngest fully developed leaf and the oldest true leaf from each plant were collected, shock-frozen in liquid nitrogen, stored at −80°C, and later freeze-dried for high-performance liquid chromatography (HPLC) analyses (see §2c).
To test whether the Bt and non-Bt cotton plants respond similarly to a known resistance inducer, plants were treated with jasmonic acid (JA) [11]. A 100 µM JA solution was prepared by dissolving JA in 100 per cent ethanol. The JA–ethanol solution was then diluted in water 4 : 1000 (JA–ethanol : water). Cotton plants (fourth-full-leaf stage) were watered with 25 ml of the JA solution. The water was applied by pouring it on the stem of the plant, thereby allowing uptake of JA by above- and below-ground tissue. The control plants were treated with an ethanol : water (4 : 1000) solution. Each treatment included 10 plants. After 7 days, the youngest fully developed leaf and the oldest true leaf from each plant were collected, shock-frozen in liquid nitrogen, stored at −80°C and later freeze-dried for HPLC analyses (see §2c).
(b). Field experiment
The field experiment was conducted at the USDA-ARS Coastal Plains Experiment Station, Tifton, GA (USA). Bt cotton (Deltapine DPL143B2 RF) and non-Bt cotton (Deltapine DPL147 RF) were planted on 30 April 2009, with three plots (27 × 49 m) of each variety distributed in a randomized complete block design over an area of ca 3.5 ha. The rest of the field was planted with plots of soybean (Stoneville 78-G7, Maturity Group 7), peanut (Georgia green) and cotton. Plots were separated by 6 m of bare, tilled soil. Aldicarb (Temik) was applied to furrows at 5.6 kg ha−1 at planting to protect the plants from early season pests. Otherwise, the crop was grown and managed according to agronomic practices recommended by the Georgia Cooperative Extension Service except that no insecticides were applied after planting. Glyphosate (Roundup Ultra Max) was applied on 29 May, and the plant growth regulator Mepiquat chloride (Compact) was applied on 25 June. Plants were fertilized with liquid nitrogen in mid-June. The plots were irrigated as required. Data from two plots (one Bt and one non-Bt) were not considered for the analyses because they contained substantial numbers of fire ants, which probably affected herbivore abundance.
When plants were four to five weeks old, 30 randomly selected plants in each of the four plots were tagged with coloured flags and ribbons to be able to resample the same plants. Ten of these 30 plants in each plot were artificially infested with Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae), a species that infests cotton fields early in the season compared with other Bt-sensitive herbivores and is well known to induce the production of cotton terpenoids [13,16]. Each week, the same 10 plants in each plot were infested with three 2nd instar S. exigua larvae. The larvae were caged onto a randomly selected leaf from the middle third of the plant. Each week plants were infested on a new leaf.
Between 1 June and 20 July, the aphid population was counted twice each week on the youngest fully developed leaf and on a random leaf from the middle third of each of the 120 tagged plants, i.e. 40 plants that were artificially infested with S. exigua and 80 plants that were exposed only to natural infestation by herbivores. Once per week, starting on 10 June, the damage to the foliage of every plant was recorded in a non-destructive way by photographing every damaged leaf and measuring the area of removed tissue using ImageJ v. 1.42 software.
For chemical analyses, the youngest fully developed leaf of all tagged plants was collected on 20 July 2009. Samples were placed immediately on ice in the field and were subsequently transferred to −80°C and later freeze-dried for terpenoid quantification (see §2c).
(c). Terpenoid quantification
Samples were prepared and extracted according to the procedure of Benson et al. [21]. An 8–10 mg sample of freeze-dried and ground leaf tissue per plant was extracted with 1 ml of a mixture of acetonitirile (Multisolvent HPLC grade, Scharlau, Sentmenat, Spain), water (purified by a Gradient A10, Millipore, Billerica, USA) and 85 per cent phosphoric acid (Fluka, Buchs, Switzerland) (80 : 20 : 0.1) for 3 min in an ultra-sonicator at room temperature. After 3 min of centrifugation (8000g), the extract was directly transferred into glass vials for separation and detection on an liquid chromatography system (1090 Series II, Hewlett-Packard, Palo Alto, USA). All substances to be analysed in the plant extracts were baseline separated at 40°C on a Varian Polaris Amide C-18 column (150 × 2.0 mm, 3 µm) equipped with a precolumn (C18, 4 × 3.0 mm, Supelco Security Guard System) and were detected with a single wavelength absorbance detector at 272 nm. The following elution gradient was applied: 0 min: 60 per cent B (40% A); 2 min: 60 per cent B; 20 min: 70 per cent B; 21 min: 100 per cent B; 25 min: 100 per cent B; 26 min: 60 per cent B; 35 min: 60 per cent B. Eluent A consisted of MilliQ-water, and eluent B consisted of acetonitrile. Eluent A was acidified with 0.1 per cent trifluoroacetic acid (greater than or equal to 99%, Riedel de Haën, Seelze Germany) to pH = 2.5 (based on [22]). The flow rate of the mobile phase was 0.125 ml min–1, and a 10 µl sample was injected.
Gossypol (≥ 95%, Sigma, St. Louis) was identified by comparing the retention time in the extract with the retention time of standard solutions. The order of elution of hemigossypolone and the heliocides 1–4 was ascertained from previously published chromatograms [12,21] and analytically confirmed by mass spectrometry. Terpenoid concentration was expressed in terms of gossypol equivalents [16].
(d). Statistical analyses
All analyses were conducted using R v. 2.13.1.
(i). Glasshouse experiments
Plant damage caused by H. virescens was analysed using the Welch t-test for non-homogeneous variances. Aphid abundance was compared among treatments using one-way analysis of variance (ANOVA). Means were subsequently separated using the Tukey Honestly Significant Difference (HSD) test. Data were log-transformed to meet the ANOVA assumptions when necessary. Aphid tibia length was analysed using Kruskal–Wallis ANOVA.
(ii). Field experiment
Data obtained from plants of the same plot were pooled to adjust for dependency. Separate analyses were conducted for naturally infested plants and plants that were artificially infested with S. exigua. Aphid data from the field and damage levels of plants in the plots were analysed by repeated measures ANOVA. Differences of aphid abundance on specific sampling dates were separated using Fisher's Least Significant Difference (LSD) test. Correlations between aphid counts from a specific date and plant damage, as well as total terpenoid concentration of plants, were evaluated using Kendall's τ rank correlation. For the correlation the data were not pooled by plot.
(iii). Terpenoid analyses
For analyses of cotton terpenoids in glasshouse grown plants, one-way ANOVA was used. Means were subsequently separated using the Tukey HSD test. Separate analyses were conducted for young and old cotton leaves. Data were log-transformed, if required. Terpenoid concentrations in leaf samples from the field were compared between Bt and non-Bt plots using Student's t-test.
3. Results
(a). Glasshouse experiments
When exposed to a single H. virescens larva for 7 days, Bt cotton plants remained nearly undamaged (average leaf area consumed ± s.e.: 0.12 ± 0.02 cm2; n = 12) compared with non-Bt cotton plants (31.79 ± 5.66 cm2; n = 13; t-test: t =−5.37, p < 0.0001). No larvae (n = 18) were found alive after 7 days on Bt cotton, but all larvae on the non-Bt cotton survived and gained considerable weight (average weight gain ± s.e. per larva: 136.4 ± 34.4 mg; n = 15).
The constitutive expression of foliar terpenoids did not differ between undamaged Bt and non-Bt cotton plants in their oldest and youngest fully developed leaves (p > 0.05) (figure 1a). This was true for the four terpenoid classes that we analysed: gossypol, hemigossypolone, heliocides 1 + 4 and heliocides 2 + 3 (see table 1 and electronic supplementary material, table S1). Herbivory by H. virescens induced terpenoid synthesis by the non-Bt plants (figure 1a): terpenoid levels were significantly increased in young leaves of herbivore-challenged non-Bt plants compared with Bt cotton and undamaged plants (F3,36 = 27.98, p < 0.0001). This increase was significant for three of the four major classes of cotton terpenoids that were analysed (table 1). Terpenoid levels in the oldest true leaves were unaffected by H. virescens feeding and did not differ among treatments (F3,35 = 0.41, p = 0.75; figure 1a; electronic supplementary material, table S1).
Figure 1.
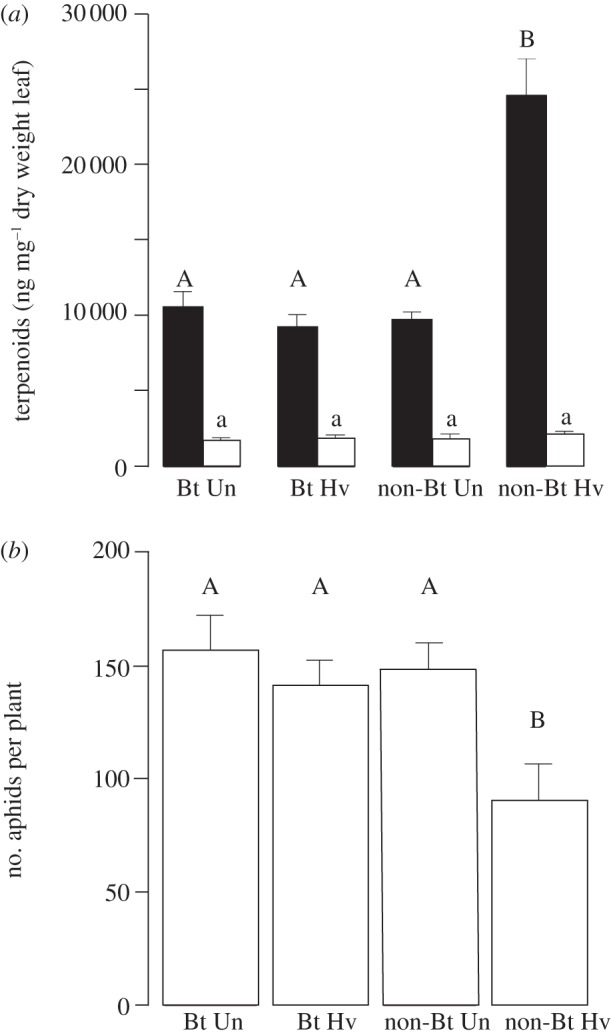
Terpenoid levels (ng mg–1 dw) and aphid numbers on Bt and non-Bt cotton as affected by prior infestation with Heliothis virescens in a glasshouse experiment. (a) Total terpenoid concentration (mean + s.e.) in the youngest and oldest true leaf of Bt and non-Bt cotton plants that were uninfested (Un) or infested (Hv) with H. virescens. (b) Numbers of Aphis gossypii per Bt and non-Bt cotton plant (mean ± s.e.) that was uninfested or infested with H. virescens. Different letters above bars indicate significant differences among treatments (p ≤ 0.05; ANOVA followed by Tukey HSD test), (a) uppercase letters, young leaves; lowercase letters, old leaves.
Table 1.
Terpenoid levels (ng mg–1 dw) in Bt and non-Bt cotton as affected by prior infestation with H. virescens in a glasshouse experiment. (Terpenoid concentration (mean ± s.e.; n = 10) in the youngest fully developed leaf of Bt and non-Bt cotton plants that were uninfested or infested with H. virescens (3rd instar) for 7 days. HGQ, hemigossypolone; G, gossypol; H1–H4, heliocide 1–4. Means within one line followed by different letters are significantly different (p ≤ 0.05; Tukey HSD test).)
| terpenoids | ANOVA |
concentration (ng mg–1 dw±s.e.) |
|||||
|---|---|---|---|---|---|---|---|
| d.f. | F | p-value | Bt uninfested | non-Bt uninfested | Bt H. virescens | non-Bt H. virescens | |
| HGQ | 3,36 | 23.7 | <0.001 | 6939±521.5 b | 6764±429.1 b | 5921±661.2 b | 15831±1461.7 a |
| G | 3,36 | 32.3 | <0.001 | 2688±541.3 b | 2014±131.2 b | 2473±161.3 b | 7388±921.0 a |
| H1/H4 | 3,36 | 5.3 | <0.001 | 335±69.9 b | 284±26.6 b | 278±23.3 b | 471±48.8 a |
| H2/H3 | 3,36 | 3.7 | 0.02 | 573±43.2 ab | 642±87.4 ab | 548±63.5 b | 874±75.8 a |
The application of JA confirmed that terpenoid production is equally inducible in Bt and non-Bt cotton plants (F3,36 = 6.22, p < 0.001; figure 2). This increase was significant for the four major classes of cotton terpenoids that were analysed (table 2). Terpenoid levels in the oldest true leaf were not inducible and did not differ among treatments (F3,36 = 0.28, P = 0.84; figure 2; electronic supplementary material, table S2).
Figure 2.
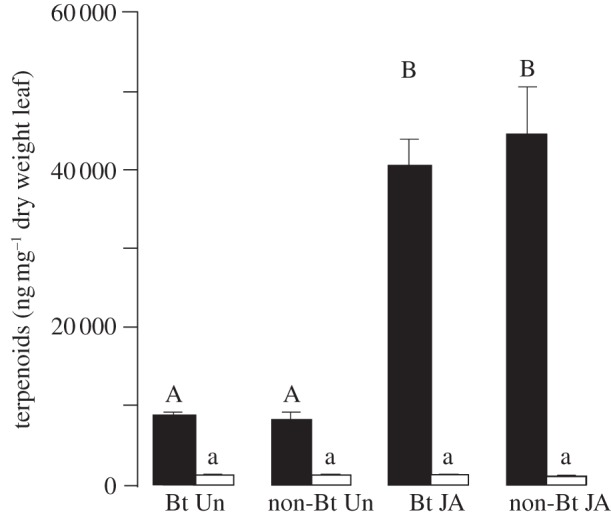
Terpenoid levels (ng mg–1 dw) in Bt and non-Bt cotton plants as affected by treatment with JA. Total terpenoid concentration (mean+s.e.) in the youngest and oldest true leaf of Bt and non-Bt cotton plants that were untreated (Un) or treated with JA. Different letters above bars indicate significant differences among treatments (p ≤ 0.05; ANOVA followed by Tukey HSD test); uppercase letters, young leaves; lowercase letters, old leaves.
Table 2.
Terpenoid levels (ng mg–1 dw) in Bt and non-Bt cotton plants as affected by treatment with JA in a glasshouse experiment. (Terpenoid concentration (mean±s.e., n = 10) in the youngest fully developed leaf of Bt and non-Bt cotton plants that were untreated or treated with JA. HGQ, hemigossypolone; G, gossypol; H1–H4, heliocide 1–4. Means within one line followed by different letters are significantly different (p ≤ 0.05; Tukey HSD test).)
| terpenoids | ANOVA |
concentration (ng mg–1 dw±s.e.) |
|||||
|---|---|---|---|---|---|---|---|
| d.f. | F | p-value | Bt untreated | non-Bt untreated | Bt JA | non-Bt JA | |
| HGQ | 3,36 | 23.6 | <0.001 | 4901±359.6 b | 4655±572.5 b | 19610±2041.9 a | 19974±2845.9 a |
| G | 3,36 | 32.5 | <0.001 | 2288±292.9 b | 2434±448.9 b | 17482±1557.1 a | 21622±3125.9 a |
| H1/H4 | 3,36 | 11.2 | <0.001 | 521±62.2 b | 399±42.7 b | 1029±119.5 a | 995±131.8 a |
| H2/H3 | 3,36 | 16.5 | <0.001 | 884±113.3 b | 642±91.1 b | 2094±226.11 a | 1892±226.8 a |
To investigate how the Bt-mediated differences in induction of terpenoids by H. virescens affect Bt-insensitive herbivores, we measured the population growth of A. gossypii on Bt and non-Bt cotton in the glasshouse. Uninfested Bt and non-Bt cotton plants were equally suitable as aphid hosts (p = 0.99; figure 1b). When cotton plants had previously been exposed to H. virescens larvae, however, aphids were significantly more abundant on the Bt cotton than on the non-Bt cotton plants (F3,69 = 6.22; p < 0.001; figure 1b). The median of hind-tibia length (which was used as an indicator of aphid size) ranged from 0.28 to 0.30 mm and did not differ among the different plant treatments (χ26 = 4.11, p = 0.66).
(b). Field experiment
Bt plants experienced significantly less damage than non-Bt plants, when plants were artificially infested with S. exigua (repeated measures ANOVA; plant type: F1,2 = 997.56, p = 0.001; plant type × time interaction: F5,10 = 131.10; p < 0.0001) (see the electronic supplementary material, figure S1). As a consequence, the total terpenoid concentrations measured in leaf samples collected in late July were lower in infested Bt plants (12 149 ± 260.1 ng g–1 dry weight (dw) leaf) than in infested non-Bt plants (14 949 ± 542.9 ng g–1 dw leaf) (t-test; t2 = 4.65, p = 0.043). This difference was mainly because of an increased gossypol concentration (table 3).
Table 3.
Terpenoid levels (ng mg–1 dw) in Bt and non-Bt cotton in the field experiment. (Terpenoid concentration (mean±s.e.; n = 2) in the youngest fully developed leaf of Bt and non-Bt cotton plants collected in the field on 20 July 2009. HGQ, hemigossypolone; G, gossypol; H1–H4, heliocide 1–4. Spodoptera exigua infested plants: plants that were artificially infested with S. exigua larvae. Naturally infested plants: plants that were exposed to natural infestation by herbivores.)
| treatment | terpenoids |
t-test |
concentration (ng mg–1 dw±s.e.) |
||
|---|---|---|---|---|---|
| t | p-value | Bt | non-Bt | ||
| S. exigua infested plants | HGQ | 0.35 | 0.75 | 3255±1039.2 | 3799±1155.0 |
| G | 6.88 | 0.02 | 5687±178.1 | 7476±189.6 | |
| H1/H4 | 0.51 | 0.66 | 842±153.1 | 949±140.8 | |
| H2/H3 | 0.68 | 0.56 | 2363±448.1 | 2723±281.7 | |
| total | 4.65 | 0.043 | 12149±260.1 | 14949±542.9 | |
| naturally infested plants | HGQ | 0.18 | 0.88 | 2193±506.9 | 2336±634.3 |
| G | 0.75 | 0.53 | 4370±698.0 | 4960±364.0 | |
| H1/H4 | 0.41 | 0.72 | 623±50.8 | 654±57.6 | |
| H2/H3 | 0.26 | 0.82 | 1798±153.5 | 1864±193.5 | |
| total | 0.59 | 0.62 | 8986±1409.3 | 9815±19.2 | |
In the case of naturally infested plants, however, the damage between Bt and non-Bt plots did not differ significantly (repeated measures ANOVA; plant type: F1,2 = 1.01, p = 0.42; plant type × time interaction: F5,10 = 1.76; p = 0.21) (see the electronic supplementary material, figure S1). As a consequence, the total terpenoid concentrations measured in leaf samples collected in late July did not differ between plant types (Bt cotton: 8990 ± 1405.6 ng g–1 dw leaf; non-Bt cotton: 9815 ± 19.2 ng g–1 dw leaf) (t-test; t2 = 0.59, p = 0.62; table 3).
Aphids began to appear in mid-June. Aphid density peaked around mid-July, after which the population dropped (figure 3). A quick collapse of the A. gossypii population on cotton is a typical pattern in the Southeastern USA and is caused by epizootics of the entomopathogenic fungus Neozygites fresenii (Nowakowski) (Entomophthorales: Neozygitaceae) [23]. During the observation period, aphid populations developed differently in plots with Bt cotton than in those with non-Bt cotton when the plants were artificially infested with S. exigua (repeated measures ANOVA; plant type: F1,2 = 3.14; p = 0.22; plant type × time interaction: F12,24 = 4.52; p = 0.001) (figure 3) or when naturally infested (plant type: F1,2 = 9.12; p = 0.09; plant type × time interaction: F12,24 = 2.7; p = 0.024; figure 3). Aphids were significantly more abundant on naturally infested Bt plants compared with non-Bt plants on 13, 16 and 20 July. Likewise, aphids were more abundant on artificially infested Bt plants on 13 and 20 July. Terpenoid concentrations in leaf samples collected on 20 July were not positively correlated with leaf damage recorded on 15 July (naturally infested plants: τ = 0.033; p = 0.67, n = 79; S. exigua infested plants: τ = 0.082; p = 0.47, n = 39). The cumulative aphid abundance until 20 July was negatively correlated with the plant damage on 15 July for naturally infested (τ =−0.178; P = 0.020, n = 79) and S. exigua infested plants (τ =−0.22; p = 0.051, n = 39). No correlation between aphid populations and terpenoid concentrations of the plants was detected (naturally infested plants: τ = −0.10; p = 0.20, n = 80; S. exigua infested plants: τ =−0.09; p = 0.39, n = 40).
Figure 3.
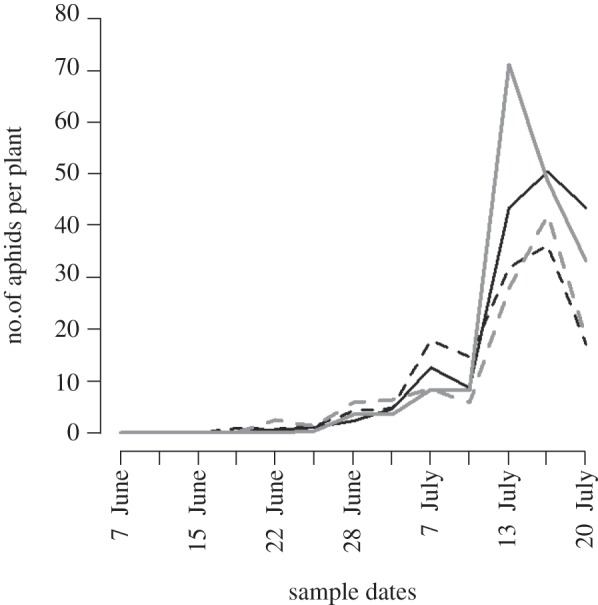
Aphid numbers on Bt (solid lines) and non-Bt (dashed lines) cotton plants in the field experiment. Values, which are the means of two replicate plots, differed significantly during the observation period in Bt versus non-Bt cotton that were infested with Spodoptera exigua larvae (plant type × time interaction: p = 0.001) and in Bt versus non-Bt cotton that were naturally infested by herbivores (plant type × time interaction: p = 0.024) according to repeated measures ANOVA. Grey lines indicate plants that were artificially infested with S. exigua larvae, while black lines represent plants that were naturally infested by herbivores.
4. Discussion
Our results show that effective suppression of target herbivores by Bt cotton translates into a decrease in the level of induced terpenoids, leaving plants more susceptible to the cotton aphid A. gossypii, a herbivore not targeted by the Bt toxins. This effect was very pronounced under protected glasshouse conditions but also visible in the field, although to a much smaller degree.
That prior herbivory renders plants more resistant to subsequent attack has been well established for a number of non-GE systems [15,24,25] and is commonly referred to as indirect plant-mediated competition. Some studies could link plant-mediated competition to the well-documented induction of plant secondary metabolites in response to herbivory [26]. Wild radish plants damaged by Pieris rapae (L.) (Lepidoptera: Pieridae) show increased glucosinolate levels corresponding with adverse effects on Lepidoptera, aphids and a leafminer [27]. In cotton, a number of studies have linked secondary metabolite induction to reduced fitness for the inducing herbivore [13,15,28]. Induction of terpenoids in cotton has also been reported in relation to plant-mediated competition, both between root- and shoot-feeding herbivores [15], and between the spider mite Tetranychus urticae (Koch) (Acari: Tetranychidae) and the fungal pathogen Verticillium dahliae (Kleb.) (Ascomycetes: Hypocreales) sharing cotton as a host plant [29]. In addition to conferring resistance against insect herbivores, cotton terpenoids act on a broad range of other organisms, including pathogens and nematodes [30–32]. The reported differences in induced terpenoid levels between Bt and non-Bt cotton are, therefore, likely to affect these organisms as well.
Emergent pest problems in crops expressing Bt toxins have been attributed to the reduced use of broad-spectrum insecticides [1], reduced competition from pests targeted by the Bt trait, or unintended transformation-related effects. In the latter case, the transformation process may generate inadvertent changes in the plant, rendering it more susceptible to non-target pests, as has been suggested for aphids on Bt-transgenic maize [33,34]. However, we found no evidence of such unintended transformation-related effects in our glasshouse experiment because the increases in aphid numbers and the size of the aphids in the absence of caterpillars were similar on the Bt-transgenic cotton and its non-transformed comparator, confirming earlier studies with other Bt cotton events [35]. Furthermore, the Bt and non-Bt cotton plants did not differ in their constitutive levels of terpenoid expression, and both plant types showed equal levels and comparable patterns of terpenoid induction in response to treatment with JA.
Indirect plant-mediated herbivore–herbivore competition can be based on a range of herbivore-induced changes other than induced secondary metabolites. These include changes in plant morphology or overall resource quantity [25,36]. There is evidence that release from such exploitative (or resource) competition in Bt cotton may also contribute to emergent pest development. Protection from lepidopteran damage significantly reduces damaged squares and flowers in Bt cotton [37], which benefits stink bugs and plant bugs that feed on these structures [38,39]. In addition, there is evidence that the herbivores also profit from reduced physical interaction with the Bt-targeted caterpillars [39].
In the field, the results from the glasshouse experiment could only partly be confirmed. Artificial infestation with S. exigua larvae caused larger damage to non-Bt plants compared with Bt plants and consequently led to a higher terpenoid concentration in the non-Bt plants. This difference in damage and terpenoid concentration, however, was not visible for naturally infested Bt and non-Bt cotton. Nevertheless, the development of the aphid population differed between Bt and non-Bt plants in both cases, for artificially and naturally infested plants. The fact that cumulative aphid populations were not correlated with the amount of terpenoids measured in the cotton plants at the termination of the experiment indicates that, under field conditions, factors other than terpenoids also impact the aphid populations. These factors include other chemical compounds or morphological changes of the plant that affect the attractiveness or nutritional quality of the plant [40,41], and activity of natural enemy species, especially coccinellid species later in the season [23]. Additionally, a lack of correlation could arise from terpenoid induction changes over time [16,17], making it difficult to identify the point in time at which the altered terpenoid levels translate into detectable changes in aphid populations.
There was also a lack of correlation between leaf damage and terpenoid concentration in the field, which might be caused by the large complex of pest species that attack cotton. Cotton plants can react very differently to herbivory by even closely related pest species and depending on the part of the plant on which they feed [10,40]. Therefore, the measurement of leaf damage alone might not be sufficient to adequately predict the degree of induced resistance in a cotton plant.
Herbivore–herbivore interactions mediated by induced plant compounds have not been previously considered as a mechanism to explain the increased susceptibility of Bt-transgenic crops to pests that are insensitive to the Bt trait. Interactions between introduced insecticidal Cry toxins and inherent resistance mechanisms have only been discussed in the context of improving the insect resistance of Bt cotton [42,43] and reducing the risk of resistance evolution in the target pests by increasing the fitness costs associated with resistance to Bt [2,44]. The interaction between introduced insecticidal Cry toxins and induced levels of secondary metabolites demonstrated in our study may also help explain results from studies showing inconsistencies between levels of Cry toxin in the plant tissues and survival of Bt-susceptible herbivores [19,45–47].
Even though the aphids in our study performed better on Bt-transgenic than on caterpillar-damaged non-Bt cotton plants, aphid outbreaks are not more common in commercial Bt cotton fields than in non-Bt cotton fields. This may be because herbivore populations may be suppressed by enhanced survival of natural enemies in Bt cotton as a result of reduced use of insecticides against target Lepidoptera [1,48,49] or because generalist predators feed more on aphids in the absence of caterpillars. This would generate increased top-down control of Bt-insensitive pests which could offset enhanced aphid performance [50]. For mirids, stinkbugs and other herbivores that lack effective natural enemies, this counteracting mechanism is apparently insufficient to prevent non-target pests from developing pest status [1,6].
In addition to being relevant to pest control with Bt crop plants, the mechanism reported here is likely to be relevant to other control methods that provide a comprehensive suppression of one group of herbivores. The common phenomenon that (secondary) pest levels increase following insecticide treatments [51] could also be partly attributed to lower levels of induced resistance resulting from the reduced herbivory by primary pests. The plant resistance-mediated interaction reported here probably pertains to a broad range of agricultural crops expressing induced resistance. It follows that these findings could have wide-reaching implications for interactions between plants and associated organisms.
Acknowledgements
This research was supported by the Swiss National Science Foundation grant no. 31003A-120477. The primary data set used for statistical analyses of this paper is available at the Dryad data repository. Accessible via this doi:10.5061/dryad.t815g.
References
- 1.Naranjo SE. 2011. Impacts of Bt transgenic cotton on integrated pest management. J. Agric. Food. Chem. 59, 5842–5851 10.1021/jf102939c (doi:10.1021/jf102939c) [DOI] [PubMed] [Google Scholar]
- 2.Carrière Y, Ellers–Kirk C, Sisterson M, Antilla L, Whitlow M, Dennehy TJ, Tabashnik BE. 2003. Long-term regional suppression of pink bollworm by Bacillus thuringiensis cotton. Proc. Natl Acad. Sci. USA 100, 1519–1523 10.1073/pnas.0436708100 (doi:10.1073/pnas.0436708100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu KM, Lu YH, Feng HQ, Jiang YY, Zhao JZ. 2008. Suppression of cotton bollworm in multiple crops in China in areas with Bt toxin-containing cotton. Science 321, 1676–1678 10.1126/science.1160550 (doi:10.1126/science.1160550) [DOI] [PubMed] [Google Scholar]
- 4.Kennedy GG. 2008. Integration of insect-resistant genetically modified crops within IPM programs. In Integration of insect-resistant genetically modified crops within IPM programs (eds Romeis J, Shelton AM, Kennedy GG.), pp. 1–26 Berlin, Germany: Springer [Google Scholar]
- 5.Greene JK, Turnipseed SG, Sullivan MJ, May OL. 2001. Treatment thresholds for stink bugs (Hemiptera: Pentatomidae) in cotton. J. Econ. Entomol. 94, 403–409 10.1603/0022-0493-94.2.403 (doi:10.1603/0022-0493-94.2.403) [DOI] [PubMed] [Google Scholar]
- 6.Lu Y, Wu K, Jiang Y, Xia B, Li P, Feng H, Wyckhuys KAG, Guo Y. 2010. Mirid bug outbreaks in multiple crops correlated with wide-scale adoption of Bt cotton in China. Science 328, 1151–1154 10.1126/science.1187881 (doi:10.1126/science.1187881) [DOI] [PubMed] [Google Scholar]
- 7.Wu K, Li W, Feng H, Guo Y. 2002. Seasonal abundance of the mirids Lygus lucorum and Adelphocoris spp. (Hemiptera: Miridae) on Bt cotton in northern China. Crop Prot. 21, 997–1002 10.1016/S0261-2194(02)00080-7 (doi:10.1016/S0261-2194(02)00080-7) [DOI] [Google Scholar]
- 8.Cui J, Xia J. 2000. Effects of Bt (Bacillus thuringiensis) transgenic cotton on the dynamics of pest population and their enemies. Acta Phytophyl. Sinica 27, 141–145 [Google Scholar]
- 9.Wilson FD, et al. 1992. Resistance of cotton lines containing a Bacillus thuringiensis toxin to pink bollworm (Lepidoptera: Gelechiidae) and other insects. J. Econ. Entomol. 85, 1516–1521 [Google Scholar]
- 10.Bezemer TM, Wahenaar R, Van Dam NM, van der Putten WH, Wäckers FL. 2004. Above- and below-ground terpenoid aldehyde induction in cotton, Gossypium herbaceum, following root and leaf injury. J. Chem. Ecol. 30, 53–67 10.1023/B:JOEC.0000013182.50662.2a (doi:10.1023/B:JOEC.0000013182.50662.2a) [DOI] [PubMed] [Google Scholar]
- 11.Opitz S, Kunert G, Gershenzon J. 2008. Increased terpenoid accumulation in cotton (Gossypium hirsutum) foliage is a general wound response. J. Chem. Ecol. 34, 508–522 10.1007/s10886-008-9453-z (doi:10.1007/s10886-008-9453-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stipanovic RD, Altman DW, Begin DL, Greenblatt GA, Benedict JH. 1988. Terpenoid aldehydes in upland cottons: analysis by aniline and HPLC methods. J. Agric. Food Chem. 36, 509–515 10.1021/jf00081a026 (doi:10.1021/jf00081a026) [DOI] [Google Scholar]
- 13.Alborn HT, Rose USR, McAuslane HJ. 1996. Systemic induction of feeding deterrents in cotton plants by feeding Spodoptera spp. larvae. J. Chem. Ecol. 22, 919–932 10.1007/BF02029945 (doi:10.1007/BF02029945) [DOI] [PubMed] [Google Scholar]
- 14.Agrawal AA, Karban R. 2000. Specificity of constitutive and induced resistance: pigment glands influence mites and caterpillars on cotton plants. Entomol. Exp. Appl. 96, 39–49 10.1023/A:1004073411100 (doi:10.1023/A:1004073411100) [DOI] [Google Scholar]
- 15.Bezemer TM, Wagenaar R, Van Dam NM, Wäckers FL. 2003. Interactions between above- and belowground insect herbivores as mediated by the plant defense system. Oikos 101, 555–562 10.1034/j.1600-0706.2003.12424.x (doi:10.1034/j.1600-0706.2003.12424.x) [DOI] [Google Scholar]
- 16.McAuslane HJ, Alborn HT, Thoth JP. 1997. Systemic induction of terpenoids aldehydes in cotton pigment glands by feeding of larval Spodoptera exigua. J. Chem. Ecol. 23, 2861–2879 10.1023/A:1022575313325 (doi:10.1023/A:1022575313325) [DOI] [Google Scholar]
- 17.Anderson DC, Jönsson M, Mörte U. 2001. Variation in damage to cotton affecting larval feeding preference of Spodoptera littoralis. Entomol. Exp. Appl. 101, 191–198 10.1046/j.1570-7458.2001.00903.x (doi:10.1046/j.1570-7458.2001.00903.x) [DOI] [Google Scholar]
- 18.Du L, Ge F, Zhu S, Parajulee MN. 2004. Effect of cotton cultivar on development and reproduction of Aphis gossypii (Homoptera: Aphididae) and its predator Propylaea japonica (Coleoptera: Coccinellidae). J. Econ. Entomol. 97, 1278–1283 10.1603/0022-0493-97.4.1278 (doi:10.1603/0022-0493-97.4.1278) [DOI] [PubMed] [Google Scholar]
- 19.Olsen KM, Daly JC, Finnegan EJ, Mahon RJ. 2005. Changes in Cry1Ac Bt transgenic cotton in response to two environmental factors: temperature and insect damage. J. Econ. Entomol. 98, 1382–1390 10.1603/0022-0493-98.4.1382 (doi:10.1603/0022-0493-98.4.1382) [DOI] [PubMed] [Google Scholar]
- 20.Walling LL. 2008. Avoiding effective defenses: strategies employed by phloem-feeding insects. Plant Physiol. 146, 859–866 10.1104/pp.107.113142 (doi:10.1104/pp.107.113142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benson CG, Wyllie SG, Leach DN, Mares CL, Fitt GP. 2001. Improved method for the rapid determination of terpenoid aldehydes in cotton. J. Agric. Food. Chem. 49, 2181–2184 10.1021/jf0010836 (doi:10.1021/jf0010836) [DOI] [PubMed] [Google Scholar]
- 22.Meyer R, Vorster S, Dubery IA. 2004. Identification and quantification of gossypol in cotton by using packed micro-tips columns in combination with HPLC. Anal. Bioanal. Chem. 380, 719–724 10.1007/s00216-004-2817-5 (doi:10.1007/s00216-004-2817-5) [DOI] [PubMed] [Google Scholar]
- 23.Abney MR, Ruberson JR, Herzog GA, Kring TJ, Steinkraus DC, Roberts PM. 2008. Rise and fall of cotton aphid (Hemiptera: Aphididae) populations in Southeastern cotton production systems. J. Econ. Entomol. 101, 23–35 10.1603/0022-0493(2008)101[23:RAFOCA]2.0.CO;2 (doi:10.1603/0022-0493(2008)101[23:RAFOCA]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 24.Anderson P, Padek MM, Wäckers FL. 2011. Root herbivory affects oviposition and feeding behavior of a foliar herbivore. Behav. Ecol. 22, 1272–1277 10.1093/beheco/arr124 (doi:10.1093/beheco/arr124) [DOI] [Google Scholar]
- 25.Kaplan I, Denno RF. 2007. Interspecific interactions in phytophagous insects revisited: a quantitative assessment of competition theory. Ecol. Lett. 10, 977–994 10.1111/j.1461-0248.2007.01093.x (doi:10.1111/j.1461-0248.2007.01093.x) [DOI] [PubMed] [Google Scholar]
- 26.Denno RF, Kaplan I. 2007. Plant-mediated interactions in herbivorous insects. In Ecological communities: plant mediation in indirect interaction webs (eds Ohgushi T, Craig TP, Price PW.), pp. 19–50 Cambridge, UK: Cambridge University Press [Google Scholar]
- 27.Agrawal AA. 1999. Induced responses to herbivory in wild radish: effects on several herbivores and plant fitness. Ecology 80, 1713–1723 10.1890/0012-9658(1999)080[1713:IRTHIW]2.0.CO;2 (doi:10.1890/0012-9658(1999)080[1713:IRTHIW]2.0.CO;2) [DOI] [Google Scholar]
- 28.McAuslane HJ, Alborn HT. 2000. Influence of previous herbivory on behavior and development of Spodoptera exigua larvae on glanded and glandless cotton. Entomol. Exp. Appl. 97, 283–291 10.1046/j.1570-7458.2000.00741.x (doi:10.1046/j.1570-7458.2000.00741.x) [DOI] [Google Scholar]
- 29.Karban R, Adamchak R, Schnathorst WC. 1987. Induced resistance and interspecific competition between spider mites and a vascular wilt fungus. Science 235, 678–680 10.1126/science.235.4789.678 (doi:10.1126/science.235.4789.678) [DOI] [PubMed] [Google Scholar]
- 30.Mace ME, Stipanovic RD, Bell AA. 1985. Toxicity and role of terpenoid phytoalexins in Verticillium wilt resistance in cotton. Physiol. Plant Pathol. 26, 209–218 10.1126/science.235.4789.678 (doi:10.1126/science.235.4789.678) [DOI] [Google Scholar]
- 31.Puckhaber LS, Dowd MK, Stipanovic RD, Howell CR. 2002. Toxicity of (+) and (–) gossypol to the plant pathogen, Rhizoctonia solani. J. Agric. Food Chem. 50, 7017–7021 10.1021/jf0207225 (doi:10.1021/jf0207225) [DOI] [PubMed] [Google Scholar]
- 32.Veech JA. 1978. An apparent relationship between methoxy-substituted terpenoid aldehydes and the resistance of cotton to Meloidogyne incognita. Nematologica 24, 81–87 10.1163/187529278X00092 (doi:10.1163/187529278X00092) [DOI] [Google Scholar]
- 33.Faria CA, Wäckers FL, Pritchard J, Turlings TCJ. 2007. High susceptibility of Bt maize to aphids enhances the performance of parasitoids of lepidopteran pests. PLoS ONE 2, e600. 10.1371/journal.pone.0000600 (doi:10.1371/journal.pone.0000600) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lumbierres B, Albajes R, Pons X. 2004. Transgenic Bt maize and Rhopalosiphum padi (Hom., Aphididae) performance. Ecol. Entomol. 29, 309–317 10.1111/j.0307-6946.2004.00597.x (doi:10.1111/j.0307-6946.2004.00597.x) [DOI] [Google Scholar]
- 35.Lawo NC, Wäckers FL, Romeis J. 2009. Indian Bt cotton varieties do not affect the performance of cotton aphids. PLoS ONE 4, e4804. 10.1371/journal.pone.0004804 (doi:10.1371/journal.pone.0004804) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wäckers F, Romeis J, Van Rijn PCJ. 2007. Nectar and pollen feeding by insect herbivores and implications for multitrophic interactions. Annu. Rev. Entomol. 52, 301–323 10.1146/annurev.ento.52.110405.091352 (doi:10.1146/annurev.ento.52.110405.091352) [DOI] [PubMed] [Google Scholar]
- 37.Adamczyk JJ, Adams LC, Hardee DD. 2001. Field efficacy and seasonal expression profiles for terminal leaves of single and double Bacillus thuringiensis toxin cotton genotypes. J. Econ. Entomol. 94, 1589–1593 10.1603/0022-0493-94.6.1589 (doi:10.1603/0022-0493-94.6.1589) [DOI] [PubMed] [Google Scholar]
- 38.Whitehouse MEA, Wilson LJ, Constable GA. 2007. Target and non-target effects on the invertebrate community of Vip cotton, a new insecticidal transgenic. Aust. J. Agric. Res. 58, 273–285 10.1071/AR06100 (doi:10.1071/AR06100) [DOI] [Google Scholar]
- 39.Zeilinger AR, Olson DM, Andow DA. 2011. Competition between stink bug and heliothine caterpillar pests on cotton at within-plant spatial scales. Entomol. Exp. Appl. 141, 59–70 10.1111/j.1570-7458.2011.01165.x (doi:10.1111/j.1570-7458.2011.01165.x) [DOI] [Google Scholar]
- 40.Bi JL, Murphy JB, Felton GW. 1997. Antinutritive and oxidative components as mechanisms of induced resistance in cotton to Helicoverpa zea. J. Chem. Ecol. 23, 97–117 10.1023/B:JOEC.0000006348.62578.fd (doi:10.1023/B:JOEC.0000006348.62578.fd) [DOI] [Google Scholar]
- 41.Hegde M, et al. 2011. Identification of semiochemicals released by cotton, Gossypium hirsutum, upon infestation by the cotton aphid, Aphid gossypii. J. Chem. Ecol. 37, 741–750 10.1007/s10886-011-9980-x (doi:10.1007/s10886-011-9980-x) [DOI] [PubMed] [Google Scholar]
- 42.Anilkumar KJ, Sivasupramaniam S, Head G, Orth R, Van Santen E, Moar WJ. 2009. Synergistic interactions between Cry1Ac and natural cotton defenses limit survival of Cry1Ac-resistant Helicoverpa zea (Lepidoptera: Noctuidae) on Bt cotton. J. Chem. Ecol. 35, 785–795 10.1007/s10886-009-9665-x (doi:10.1007/s10886-009-9665-x) [DOI] [PubMed] [Google Scholar]
- 43.Mészáros A, Beuzelin JM, Stout MJ, Bommireddy PL, Riggio MR, Leonard BR. 2011. Jasmonic acid-induced resistance to the fall armyworm, Spodoptera frugiperda, in conventional and transgenic cottons expressing Bacillus thuringiensis insecticidal proteins. Entomol. Exp. Appl. 140, 226–237 10.1111/j.1570-7458.2011.01149.x (doi:10.1111/j.1570-7458.2011.01149.x) [DOI] [Google Scholar]
- 44.Williams JL, Ellers-Kirk C, Orth RG, Gassmann AJ, Head G, Tabashnik BE, Carrière Y. 2011. Fitness cost of resistance to Bt cotton linked with increased gossypol content in pink bollworm larvae. PLoS ONE 6, e21863. 10.1371/journal.pone.0021863 (doi:10.1371/journal.pone.0021863) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adamczyk JJ, Hardee DD, Adams LC, Sumerford DV. 2001. Correlating differences in larval survival and development of bollworm (Lepidoptera: Noctuidae) and fall armyworm (Lepidoptera: Noctuidae) to differential expression of Cry1A(c) δ-endotoxin in various plant parts among commercial cultivars of transgenic Bacillus thuringiensis cotton. J. Econ. Entomol. 94, 284–290 10.1603/0022-0493-94.1.284 (doi:10.1603/0022-0493-94.1.284) [DOI] [PubMed] [Google Scholar]
- 46.Gore J, Leonard BR, Adamczyk JJ. 2001. Bollworm (Lepidoptera: Noctuidae) survival on ‘Bollgard’ and ‘Bollgard II’ cotton flower bud and flower components. J. Econ. Entomol. 94, 1445–1451 10.1603/0022-0493-94.6.1445 (doi:10.1603/0022-0493-94.6.1445) [DOI] [PubMed] [Google Scholar]
- 47.Olsen KM, Daly JC, Holt HE, Finnegan EJ. 2005. Season–long variation in expression of cry1Ac gene and efficacy of Bacillus thuringiensis toxin in transgenic cotton against Helicoverpa armigera (Lepidoptera: Noctuidae). J. Econ. Entomol. 98, 1007–1017 10.1603/0022-0493-98.3.1007 (doi:10.1603/0022-0493-98.3.1007) [DOI] [PubMed] [Google Scholar]
- 48.Romeis J, Meissle M, Bigler F. 2006. Transgenic crops expressing Bacillus thuringiensis toxins and biological control. Nat. Biotech. 24, 63–71 10.1038/nbt1180 (doi:10.1038/nbt1180) [DOI] [PubMed] [Google Scholar]
- 49.Wolfenbarger LL, Naranjo SE, Lundgren JG, Bitzer RJ, Watrud LS. 2008. Bt crop effects on functional guilds of non–target Arthropods: a meta–analysis. PLoS ONE 3, e2118. 10.1371/journal.pone.0002118 (doi:10.1371/journal.pone.0002118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu Y, Wu K, Jiang Y, Guo Y, Desneux N. 2012. Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature 487, 362–365 10.1038/nature11153 (doi:10.1038/nature11153) [DOI] [PubMed] [Google Scholar]
- 51.Stoltz RL, Stern VM. 1978. Cotton arthropod food chain disruption by pesticides in the San Joaquin Valley, California. Environ. Entomol. 7, 703–707 [Google Scholar]


