Abstract
Inbreeding depression results from mating among genetically related individuals and impairs reproductive success. The decrease in male mating success is usually attributed to an impact on multiple fitness-related traits that reduce the general condition of inbred males. Here, we find that the production of the male sex pheromone is reduced significantly by inbreeding in the butterfly Bicyclus anynana. Other traits indicative of the general condition, including flight performance, are also negatively affected in male butterflies by inbreeding. Yet, we unambiguously show that only the production of male pheromones affects mating success. Thus, this pheromone signal informs females about the inbreeding status of their mating partners. We also identify the specific chemical component (hexadecanal) probably responsible for the decrease in male mating success. Our results advocate giving increased attention to olfactory communication as a major causal factor of mate-choice decisions and sexual selection.
Keywords: inbreeding depression, male sex pheromone, mating success, flight performance, female choice
1. Introduction
Inbreeding depression results from mating among genetically related individuals, which increases the impact of recessive detrimental alleles and/or the loss of advantageous heterozygosity. This occurs in wild animal and plant populations as well as in humans, and is covered by an extensive and diverse literature [1]. Inbreeding can decrease male courtship and mating success [2–4], and it is usually considered to involve an impairment of multiple fitness-related traits that reduce the general condition of inbred individuals [5–7]. However, the reduced mating success of inbred males could also be a consequence of strong selection on females to avoid mating with an inbred male. This is especially relevant if females gain direct benefits through appropriate mate choice (parental care, territory defences and nuptial gifts) [8–10] or if they suffer direct costs should they mate with an inbred male (decreased offspring viability and fertility) [11,12].
Inbreeding depression of secondary sexual traits may decrease male attractiveness, which could then inform females about the inbred status of their potential mating partners. Indeed, the phenotypic variation of sexually selected male traits, such as song repertoire in birds and coloration patterns in fish, is often found to be strongly correlated to inbreeding status [7,13–16]. These correlations suggest that the effect of inbreeding on such male traits may cause the reduction in mating success of inbred males. However, this relationship has rarely been demonstrated empirically, and it remains unclear whether male secondary sexual traits (and which ones) could be causally responsible for the reduced mating success of inbred males.
In this regard, females of the cricket Teleogryllus commodus detect and reject inbred males based on the reduced acoustic signals that they produce compared with outbred males [16,17]. In other case studies, females detect the genetic quality [18–21] or the heterozygosity level [22] of their potential mating partners through the assessment of their olfactory cues. Female mice, for instance, assess male heterozygosity directly through male scent [23]. The diversity of major urinary proteins (MUPs) and heterozygosity at specific major histocompatibility complex loci appear to be responsible for the variation in female preferences and male mating success in this species [23,24]. Similarly, Pölkki et al. [25] recently demonstrated that females of the mealworm beetle Tenebrio mollitor were more attracted to the odour produced by outbred males than to that of inbred males [25]. These studies suggest that the lowered mating success of inbred males may be a direct result of the lowered quality of the olfactory cues used by females to assess males. Yet, in all these studies, the identity of the behaviourally active pheromone component (or components) that signals the heterozygosity level of males remains unknown [23,25,26].
In the tropical butterfly Bicyclus anynana (Butler, 1879), inbreeding significantly reduces male mating success in free-flying populations [3]. However, the precise traits responsible for the decrease in mating success of inbred males have not been identified. A surprisingly high genetic load has been found for several traits closely related to fitness in this species, and inbreeding depression was shown to affect males more than females [11,12,27]. Male sterility contributes disproportionately to inbreeding depression as about half the sons from brother–sister matings are sterile, whereas female fertility is largely unaffected by inbreeding [12]. Females could therefore significantly reduce the probability of producing completely unviable egg batches if they are able to detect and reject inbred males. Brakefield & Reitsma [28] and Windig et al. [29] have studied the population biology of Bicyclus in the field, especially the closely related species Bicyclus safitza, over transitions from wet to dry seasons at a forest-edge site in Malawi [28,29]. The adults are rather sedentary and long-lived, and the population structure consists of an unstable mosaic of localized high concentrations of butterflies. Inbreeding significantly increased local extinction risk in natural populations of another butterfly [30] and may also affect some natural populations of B. anynana.
In B. anynana, close-range courtship by males is important to their mating success, and the transfer of a male sex pheromone (MSP) from exposed wing androconia on to the antennae of females is essential to male mating success [31,32]. Furthermore, using gas-chromatography coupled to electro-antennogram (GC–EAD) experiments, we established that female antennae contain olfactory receptors for three components present on male wings, forming the B. anynana MSP: Z9–14:OH (MSP1), 16:Ald (MSP2) and 6,10,14-trime-15-2-ol (MSP3) [32]. Variation in MSP production has been found between courting males of different age classes. Females prefer to mate with mid-aged over younger males, and the pheromone composition seems sufficient to explain this preference [33].
Here, we demonstrate that inbreeding reduces several male traits in B. anynana, including MSP production and flight performance. However, impairing the antennal perception of females was sufficient to restore the mating success of inbred males to that of outbred males. Such females were thus able to detect any other male trait, but not their volatile scent, showing that the male scent alone is probably responsible for the decreased mating success of inbred males. Moreover, we specifically identified MSP1 ((Z)-9-tetradecenol) and MSP2 (hexadecanal) as the most likely cues used by females to assess the quality of males.
2. Material and methods
(a). Insect rearing
The butterflies originated from the Leiden University stock population that was founded in 1988 from over 80 gravid females. Several hundred individuals are reared in each generation such that high levels of heterozygosity are maintained [34,35]. All larvae were fed on maize plants Zea mays in a climate cell at 27°C, 70 per cent RH, 12 L : 12 D [36].
Independent F0, F0.25 and F0.375 families were reared simultaneously following Joron & Brakefield [3]. In short, we used a staggered breeding programme based on the wild-type outbred stock population to produce, at single time periods, a large number of butterfly families, each with one of the following three inbreeding coefficients: F = 0 (F0), F = 0.25 (F0.25) and F = 0.375 (F0.375). Mating pairs were drawn randomly from the outbred stock population and their offspring were reared in individual net sleeves to generate independent families with an inbreeding coefficient of F = 0. To cope with known deleterious effects of inbreeding on egg hatching rate and juvenile survival [12,14], six brother–sister mating pairs were drawn from each F0 family. Per family, only one pair that produced more than 40 hatched eggs was selected to produce the next level of inbreeding. Similarly, brother–sister mating pairs were drawn from the F0.25 lines to produce the F = 0.375 inbred level. This procedure minimizes the deleterious effects of inbreeding observed in our study as only mating pairs least affected by inbreeding were used to produce the next level of inbreeding. Two independent sets of inbred butterflies were produced, in 2008 and in 2010, respectively. In 2008, 20 independent F0 (10), F0.25 (8) and F0.375 (2) family-lines were reared, whereas in 2010 the dataset was composed of 30 independent F0 (10), F0.25 (10) and F0.375 (10) family-lines.
(b). Quantification of male sex pheromone production
Males from each family were sacrificed either on the third (2008) or on the sixth (2010) day after eclosion, 7 h after lights on, and stored in a −80°C freezer. The MSP components were extracted by soaking one fore- and one hind-wing of each individual in 500 μl of hexane with palmitic acid (3 ng µl−1) added as an internal standard. GC analyses were conducted independently for the 2008 and 2010 datasets, and in several separate subsets for each of which individuals of one of the three levels of inbreeding were randomly chosen. In 2008, the extracts were analysed on a Hewlett-Packard 6890 series II GC equipped with flame-ionization detector and interfaced with a HP-6890 series integrator. The carrier gas was nitrogen with the injector temperature set at 240°C and the detector temperature at 250°C. A HP-1 column was used, and the oven temperature was increased from the initial temperature of 50°C to a final temperature of 295°C at a rate of 15°C min−1, which was maintained for 6 min [32]. In 2010, fast GC analyses were conducted on a Thermo ultra-fast trace GC gas chromatograph operated with a split/splitless injector and a Thermo AS 3000 autosampler (Thermo Electron Corp., Interscience, Louvain-la-Neuve, Belgium). The temperature programme was as follows: initial temperature at 40°C, held for 0.1 min, ramp 1 at 150°C min−1 to 150°C and ramp 2 at 40°C min−1 to 300°C. The injector temperature was set at 240°C and the detector temperature at 250°C (see [37] for more details).
(c). Quantification of flight performance and relative thorax mass
We tested the effect of inbreeding on flight performance as an indicator of the general condition and chasing ability of males. Flight performance was assessed using the dataset from 2010. From each level of inbreeding, 10 independent families were available simultaneously. Males were collected from each family (n = 6–12 individuals per family), and on the third day after eclosion, each male was placed individually in a net cage (diameter: 60 × 70 cm). After a short adaptation period, the netting cage was gently touched and the vibrations motivated the butterfly to take flight. Every time the male alighted, it was immediately stimulated to take off again. The flight impairment index (FII) was taken as the number of times the butterfly was forced to take off during 2 min of forced flight [38]. We interpret an elevated FII to reflect the inability of the male to engage in longer flight bouts. Males that refused to fly for 2 min (n = 9), died during the experiment (n = 9) or had extremely worn wings (n = 18) were excluded from the dataset (n = 313). To assess the relative investment in thoracic muscles, the thorax, abdomen and head were dried to constant mass (60°C for 24 h) and weighed to the nearest 0.01 mg separately using an electronic microbalance (Sartorius Research RC 210D).
(d). Male mating success in semi-natural conditions
To test the role of inbreeding and MSP perception by females in male mating success, we performed replicated mating competition experiments in semi-natural conditions, using equal-sized groups of males of the three levels of inbreeding. As described earlier, F0 males were from the stock population, whereas F0.25 and F0.375 males originated from 12 and 9 independent families, respectively. The experiments were performed in a large flight cage (3.1 × 2.5 × 2.3 m3) set up in a climate cell, allowing the males to express their natural mate location and courtship behaviour (visual recordings of this experiment and Bicyclus anynana courtship are available at http://www.youtube.com/watch?v=HZULH7y50g4). Matings were tracked using ‘rodent-tracking’ fluorescent dust on the genitalia of males [3]. A different colour was used for each inbreeding class and we switched the colours used for each cohort between trials. In each trial, the age distribution of the males was between 5 and 7 days old for each inbreeding level.
Males of each inbreeding level competed for mating with virgin, outbred 3- to 5-day old blocked and control females. To suppress the ability to perceive odours, a transparent nail polish (Rimmel London 60 Seconds Nail Polish, Clear 740) was applied onto the antennae of the females that formed the blocked group on the day prior to release. The control females received nail polish on the anterior wings, immediately behind the antennae (note that both groups of females received nail polish in similar amounts so that any influence of the nail polish on female and male behaviour applied to all groups equally). Males were introduced into the flight cage and left to adapt and interact with one another for 2 h. Blocked and control females were then released in numbers corresponding to a three to one male-to-female ratio. A second group of females, half the size of the first group, was released 6 h later. On the following morning, the females were inspected for fluorescence under ultraviolet illumination to assess the group identity of their male mating partner. Double fluorescence could be detected and was scored as 0.5 : 0.5 for the two male groups concerned. Six replicate competition experiments were performed sequentially.
(e). Statistical analyses
To test for differences in MSP production between males in relation to the level of inbreeding, we performed a multivariate analysis of variance (MANOVA) with MSP1, MSP2 and MSP3 titres as dependent variables and the inbreeding coefficient as a fixed variable. The F0.25 and F0.375 levels of inbreeding were pooled in the ‘3-day’ (2008) dataset because of insufficient replication at the family level, but were kept separate for the ‘6-day’ (2010) dataset. To identify which part of the MSP perfume was affected by inbreeding, we performed univariate ANOVAs for each MSP titre, of which p-values were corrected for multiple testing using the Bonferroni procedure.
The effects of inbreeding on FII and relative thorax mass were analysed using one-way ANOVA with the inbreeding coefficient as the independent variable. The residuals of a linear model with thorax dry mass as the dependent variable and the remaining dry mass (head and abdomen) as the independent variable were used as a measure of the relative thorax mass (r2 = 0.23).
Significance of the results of the behavioural experiments was assessed using G-tests. In each replicate, we determined whether the mating distribution deviated from a 1 : 1 : 1 ratio. We tested for heterogeneity among replicates using a Pearson's chi-squared test before performing the G-test analyses on the pooled dataset. A Pearson's chi-squared test was also used to investigate whether the overall distribution of matings was different between control and blocked female treatments. All statistical analyses were performed with the R language and environment for statistical computing and graphics (v. 2.8.1; http://www.r-project.org).
3. Results
(a). Inbreeding reduces the production of the male sex pheromone
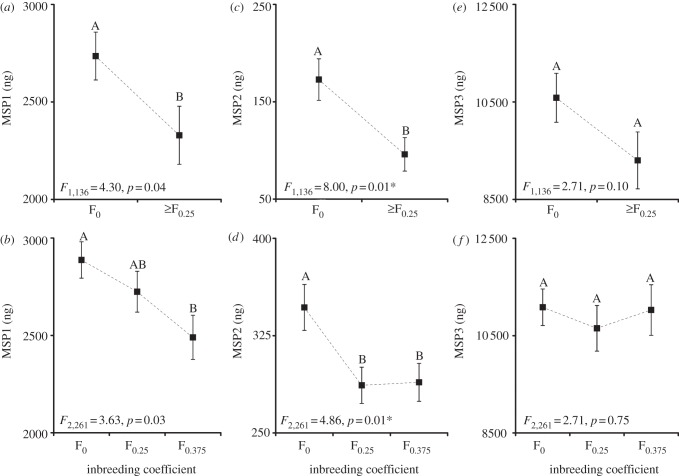
We tested whether reduced mating success of inbred males in B. anynana was explained by differences in the production of their olfactory signal. The production of MSP was measured in two independent datasets of 3-day-old (2008 dataset) and 6-day-old (2010 dataset) males from F0, F0.25 and F0.375 families. The MANOVAs revealed significant differences in MSP production between levels of inbreeding in both the 3-day-old (Wilks's λ = 0.93, F = 3.43, d.f. = 3 and 134, p < 0.02) and the 6-day-old males (Wilks's λ = 0.87, F = 6.14, d.f. = 6 and 518, p < 0.0001). We next examined the effect of inbreeding on the individual components of the MSP using separate ANOVA tests (figure 1). The production of MSP1 and MSP2 titres was significantly reduced in both 3- and 6-day-old inbred males, whereas MSP3 titres were not affected by inbreeding. Only one compound, MSP2, remained significantly affected by inbreeding in both datasets after correction for multiple testing (figure 1). It is noteworthy that the production of MSP2 titre decreased significantly at the first level of inbreeding (F0.25), but was then unaffected by further inbreeding. Mean MSP2 titres of both F0.25 and F0.375 males were about 17 per cent lower than for outbred males (figure 1d).
Figure 1.
The effect of inbreeding on the male sex pheromone of B. anynana. Inbreeding significantly affects MSP1 (a,b) and MSP2 (c,d) but not MSP3 (e,f) titres at the ages of 3 days (a,c,e) and 6 days (b,d,f), respectively. F- and p-values of the univariate ANOVAs are given at the bottom of each panel. Tukey's honest significant differences are indicated by different letters to specify the significant differences between the levels of inbreeding. Error bars represent ±1 s.e. with n = 138 for 3-day-old males (2008 dataset) and n = 264 for 6-day-old males (2010 dataset). Only one compound, MSP2, remained significantly affected by inbreeding in both datasets after Bonferroni correction for multiple testing (*).
Body size is known to affect MSP production [33]. However, the differences in MSP production remained significant when a principal component measure of body size (PC1) was included as covariate (see the electronic supplementary material section on MANCOVA), thus inbreeding level reduced MSP production independently of body size. When accounting for body size (ANCOVAs; electronic supplementary material, table S2), again, only the MSP2 titre was decreased significantly by inbreeding in the 6-day-old males (after correcting for multiple testing; 2010 dataset). However, in the 3-day-old males (2008 dataset), the MSP1 but not MSP2 titre was significantly reduced by inbreeding (after correcting for multiple testing; electronic supplementary material, table S2).
(b). Inbreeding also reduces the general condition of males
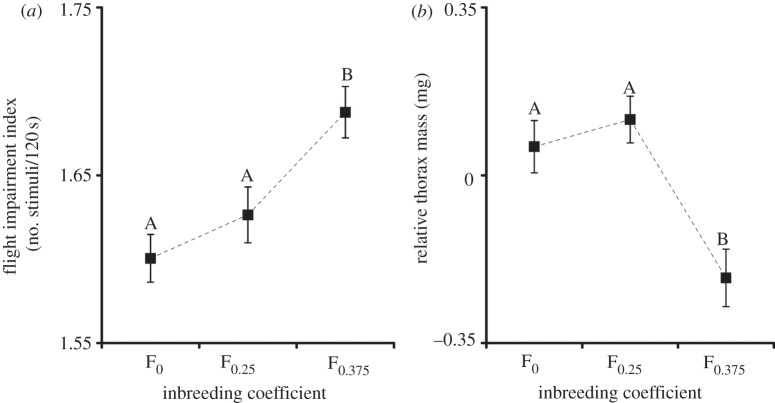
Bicyclus anynana males spend most of their active time attempting to locate mates and chasing females by repeated alighting and take-off [28]. It has thus been suggested that reduced flight performance could be a critical trait responsible for reduced mating success of inbred males in this species [3]. The FII increased significantly with inbreeding (one-way ANOVA; F = 7.84, d.f. = 2 and 310, p < 0.001); outbred males required 42.14 (±1.28, s.e.) stimuli resulting in an average length of the flight bouts of 2.85 s. The flight bouts of the severely inbred males were about 18 per cent shorter as they were stimulated to take off 51.37 (±1.71) times on average (figure 2a). Note that an elevated FII reflects the inability of the male to engage in longer flight bouts and that this trait can be used as a proxy of the general condition and chasing ability of males [38]. Severe inbreeding (F0.375) also had a significant effect on the relative thorax mass of B. anynana males (one-way ANOVA; F = 10.51, d.f. = 2 and 310, p < 0.001) with the inbred males displaying a thorax mass, on average, 5.4 per cent smaller than that of outbred males (figure 2b). Interestingly, at a less severe level of inbreeding (F0.25), neither FII nor relative thorax mass was significantly reduced compared with those of outbred males (Tukey's honest significant differences tests, p > 0.4; figure 2a,b).
Figure 2.
The effect of inbreeding on the general condition of butterflies. (a) Flight impairment index and (b) relative thorax mass are significantly affected by severe (F0.375) inbreeding. Error bars represent ±1 s.e. with n = 313. Tukey's honest significant differences are indicated by different letters to specify the significant differences between the levels of inbreeding.
(c). Females need to perceive the male sex pheromone to reject inbred males
It was previously shown that the reproductive success of inbred males is already significantly affected at the intermediate level of inbreeding, F0.25 [3] (cf. figure 3a). This suggests that the reduction in flight performance with inbreeding may not be the major trait responsible for reduced reproductive success of inbred males in this species, as we find no differences in flight ability traits between F0 and F0.25 males (figure 2). However, because MSP2 production was already significantly reduced at the intermediate level of inbreeding (F0.25), we hypothesized that impaired MSP production may be causally involved in the reduced mating success of inbred males. To test this hypothesis, we performed replicated trials involving competition between males of the three levels of inbreeding for mating with virgin, outbred females. We manipulated the ability of one-half of the females to perceive odours by covering their antennae with a transparent nail polish (blocked females) [31]. Such females were thus able to detect any other male trait but not their scent from volatiles. Control females, on the other hand, retained their ability to detect the MSP.
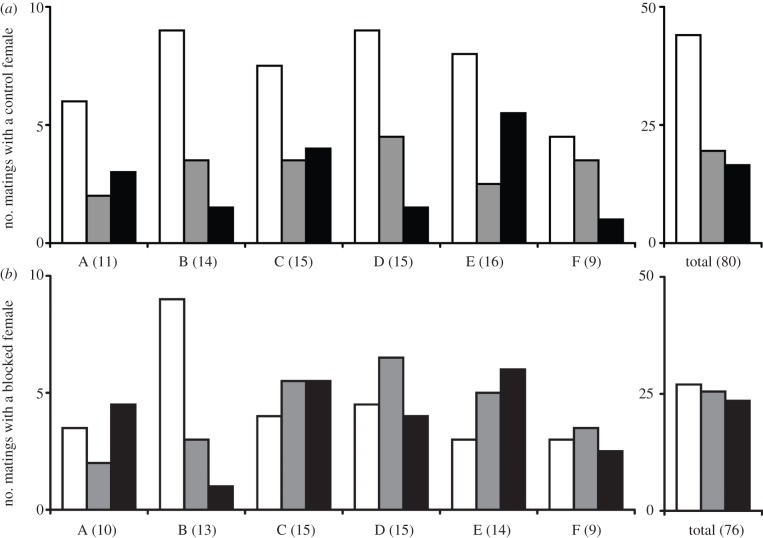
Figure 3.
The mating success of inbred males of B. anynana depends on the ability of females to perceive their MSP. (a) Male mating success with control females: inbred males have a lower mating success than outbred males. (b) Male mating success with blocked females: females with suppressed ability to perceive odours mate similarly with both inbred and outbred males (except in replicate B; excluding replicate B from the statistical analyses improves the quality of our results). White bars, F0 outbred males; grey bars, F0.25 inbred males; black bars, F0.375 inbred males. Six replicate behavioural experiments were performed sequentially (A to F). Three equal-sized cohorts (12 to 20 individuals depending on the replicate) of F0, F0.25 and F0.375 males competed for matings with blocked and control females. In brackets is given the total number of successful matings for each replicate. Right-hand panels show the total male mating success with control and blocked females once the six replicates are pooled, as no heterogeneity among replicates (p = 0.82 and p = 0.37) was observed for control and blocked females, respectively. Importantly, control and blocked females were courted simultaneously by the same groups of both inbred and outbred males in each replicate (A to F), which rules out the possibility that some changes in the experimental set-up could be responsible for the observed differences in male mating success.
The mating rate of both types of female was high (94% and 98% for blocked and control females, respectively) and did not differ (χ2 = 1.26, d.f. = 1, p = 0.26), showing that the willingness of females to mate was similar despite the difference in antennal treatment. In addition, the frequency of multiple matings did not differ between female treatments (9% and 11% for blocked and control females, respectively; χ2 = 0.14, d.f. = 1, p = 0.70; see the electronic supplementary material, table S3).
When competing for control females, outbred (F0) males showed a strong and repeatable advantage in mating success over either class of inbred males (figure 3a; no heterogeneity among trials: χ2 = 5.98, d.f. = 10, p = 0.82; on the pooled dataset: F0 : F0.25 comparison, G = 9.70, d.f. = 1, p < 0.05; F0 : F0.375 comparison, G = 12.97, d.f. = 1, p < 0.001). The mating success of F0.25 and F0.375 inbred males was respectively 24 per cent and 21 per cent, whereas outbred males achieved 55 per cent of all matings (figure 3a; overall F0 : F0.25 : F0.375 comparison, G = 16.02, d.f. = 2, p < 0.001).
By contrast, inbred males obtained as many matings as outbred males when blocked females were used that were no longer able to detect MSP (figure 3b; no heterogeneity among trials: χ2 = 10.78, d.f. = 10, p = 0.37; on the pooled dataset: F0 : F0.25 comparison, G = 0.04, d.f. = 1, p = 0.84; F0 : F0.375 comparison, G = 0.24, d.f. = 1, p = 0.62). Each class of males achieved about one-third of their matings with a blocked female (36%, 34% and 31% for F0, F0.25, F0.375 males, respectively; figure 3b; overall F0 : F0.25 : F0.375 comparison, G = 0.24, d.f. = 2, p = 0.88). Critically, the deleterious effect of inbreeding on mating success differed between the female treatments (χ2 = 6.00, d.f. = 2, p < 0.05 between blocked and control females), indicating that the decrease in mating success of inbred males can be restored by manipulating the female ability to detect their MSP.
4. Discussion
(a). Reduction in male sex pheromone titre affects mating success of inbred males
The impairment of reproductive success in inbred males is usually attributed to cumulative effects on multiple fitness-related traits, which together reduce their general condition [6,7,39–42]. These traits may include flight performance (this study) [2] and sperm production [43,44]. Indeed, our results clearly demonstrate a reduction in flight performance and in thorax mass of inbred males. Recent studies revealed that relative thorax mass correlates with flight capacity in butterflies and other insects [45,46], and with the ability to withstand flight stress in B. anynana [38]. Our results support the view that a lower relative thorax mass, indicating less developmental allocation to thoracic muscles [47], accounts for reduced flight performance in severely inbred B. anynana males. However, flight performance was not significantly reduced in F0.25 compared with F0 males and can thus not account for their reduced mating success. By contrast, the MSP production of inbred males was already significantly reduced in F0.25 inbred males. We also showed in a large-scale behavioural experiment that the difference in mating success between inbred and outbred males disappeared for ‘blocked’ females for which only the antennal perception was impaired. Blocked females were indeed able to detect any male trait except their scent. The semi-natural set-up we used for this experiment ensured that males flew freely while courting females. Other male traits impaired by inbreeding, including flight performance, were detectable for females but did not reduce the mating success of inbred males once scent perception was impaired in females. The most parsimonious explanation of our results is thus that the male scent alone causes female rejection of inbred males. This is the first study, to the best of our knowledge, that identifies which male trait, among those affected by inbreeding, is causally responsible for the reduced mating success of inbred males. In addition, this result highlights that such manipulation of female antennal reception can elegantly complement behavioural experiments that manipulate male scent production in butterflies, as conducted previously in B. anynana [32].
(b). Inbreeding information is provided via specific chemical components
Several lines of evidence allow us to specify which component of the MSP is likely to be the key determinant of the reduced mating success of males. GC–EAD experiments previously showed that three male wing components (MSP1, MSP2 and MSP3) have olfactory receptors in female antennae [32]. Other chemicals relevant in insect interactions, such as cuticular hydrocarbons, cannot be responsible for the reduced mating success of inbred males in our behavioural experiments. Receptors for such non-volatile chemicals are found in the proboscis or legs of various insects but not in the antennae [48–50].
In both the 3-day-old and 6-day-old dataset, MSP2 titre is consistently reduced by inbreeding. Crucially, MSP2 is the only MSP component to show a significant reduction in F0.25 inbred males, and thus was the only MSP component giving females the potential to distinguish between outbred and F0.25 inbred males. It is noteworthy that the 3-day-old and 6-day-old datasets were produced independently (by different researchers in different labs in different years and using different gas chromatograph analysis settings; see §2), and were drawn from a large number of families sampled randomly from the wild-type stock population in which high levels of heterozygosity have been maintained [28,34,35]. Therefore, finding a similar reduction in MSP2 titre for these two datasets provides strong support that the MSP2 titre is consistently affected by inbreeding and can thus serve as a stable male signal of inbreeding status or as a general indicator of quality in B. anynana.
In this context, we recently found that MSP2 titre probably determines the preference of females for males of intermediate age compared with younger individuals [33], and MSP2 production is thus likely to be under strong selection. Such directional sexual selection for increasing MSP2 titre is expected to deplete the trait for additive genetic variation, and genetic variance is thus expected to be mostly composed of dominant and epistatic interactions [40,51]. This is in agreement with our previous observations that MSP2 titre displayed no additive genetic variance, in contrast to MSP1 and MSP3 titres [33]. It also explains why MSP2 is sensitive to inbreeding depression through homozygosity of recessive deleterious alleles. As a consequence, intriguingly, the same signal, MSP2 titre, can be used by B. anynana females to assess both the age and inbreeding status of potential mating partners.
Although multiple traits may be used by females to assess male quality [52] (see also [53]), here we suggest that a single olfactory trait, namely changes in MSP composition, can inform females about several aspects of male quality (i.e. age, inbreeding). Overall, our results indicate that the MSP2 titre is the most likely candidate trait determining B. anynana male mating success in these experiments.
(c). Selection on female mate-choice strategies for the avoidance of inbred males
It could be that inbred males suffer reduced mating success simply because they do not produce levels of MSP that make them attractive. This could, for instance, be a direct consequence of reduced body size. MSP titres are indeed associated with size and age, and females may avoid inbred males as a by-product of other aspects of sexual selection (i.e. preference for larger size or older males that are known to produce more MSPs [33]).
However, there are several reasons why we think that females are able to use the MSP specifically to avoid mating with inbred males. First, during our behavioural experiments, we controlled for the effect of age on MSP production by releasing males from the same age classes into the competition cage. Thus, male age cannot explain differential mating success between inbred and outbred males. Second, in both datasets (2008 and 2010), inbreeding did not affect male body size of the males (see the electronic supplementary material, section MANCOVA), so females could not identify inbred males based on their size. So, although MSP production is dependent on body size, age and inbreeding level, our experiments showed that the MSP titres indicate inbreeding level independently of male body size or age, and that variation in MSP titres accounts for female preference.
There are important reasons why females should avoid inbred males in B. anynana. Inbreeding affects males disproportionately to females as about 50 per cent of the sons from a brother–sister mating are completely sterile [12]. Consequently, females mating with an inbred male have a very high chance that the eggs that they produce will be completely inviable. Females that have some ability to detect and reject these sterile inbred males will increase their fitness dramatically in the presence of such males.
Our observations indicate that B. anynana females are under selection to avoid mating with inbred males. However, it remains an open question whether the avoidance of inbred males evolved directly or indirectly via the MSP2 titre levels of B. anynana males. Our previous data [33] strongly indicate that MSP2 production is under strong sexual selection. As a consequence of such directional selection, MSP2 titres are likely to be sensitive to inbreeding depression. Thus, strong sexual selection for increased MSP2 production has the potential to simultaneously result in the avoidance of inbred males as mating partners. Further research in this species should focus on whether these two aspects of selection—sexual selection for high MSP2 levels and the avoidance of inbreeding depression—have reinforced each other.
5. Conclusions and perspectives
We have shown that the olfactory signal of inbred males was the sole trait causally responsible for their decreased mating success. The high level of sterility of inbred males could account for female choosiness in B. anynana [12] (see §1). These results indicate that it is now time to investigate whether and how olfactory traits, such as MSP and MUP isoforms, can help to account for reduced mating success observed for inbred males. These findings underscore the importance of increased research effort in the field of olfactory communication as a major, but so far underestimated, causal factor in sexual selection [54,55]. This could be particularly important in the conservation of endangered species for which their long-term survival depends on the successful breeding of populations of comparatively small size and with elevated levels of inbreeding [56].
Acknowledgements
We are grateful to C. Desmet and two anonymous reviewers for suggestions that greatly improved our manuscript, and to K. Koops, M. Lavrijsen, D. Hallesleben and M. van Eijk for the maintenance of laboratory butterflies, plant rearing and technical support. This work was supported by the EU-funded Network of Excellence LifeSpan (FP6 036894), the European Research Council grant EMARES (250325), a Marie Curie International European Fellowship to C.M.N. (FP6-2005-Mobility-5 nr039083), the Belgian ‘Fonds National de la Recherche Scientifique’ (FRFC 2.4600.10 and FRFC 24560.11) and by UCL (grant no. ARC 10/15-031). This study is publication BRC233 of the Biodiversity Research Centre (Université catholique de Louvain). E.v.B., P.M.B., B.J.Z. and C.M.N. conceived and designed the experiments; E.v.B. and S.H. performed the experiments; E.v.B. analysed the data; C.M.N. and B.J.Z. contributed reagents/materials/analysis tools; E.v.B. and C.M.N. wrote the manuscript; and P.M.B. and B.J.Z. contributed to the writing. Data are deposited in the Dryad repository (doi:10.5061/dryad.g6j76).
References
- 1.Charlesworth D, Willis JH. 2009. The genetics of inbreeding depression. Nat. Rev. Genet. 10, 783–796 10.1038/nrg2664 (doi:10.1038/nrg2664) [DOI] [PubMed] [Google Scholar]
- 2.Miller PS, Glasner J, Hedrick PW. 1993. Inbreeding depression and male-mating behavior in Drosophila melanogaster. Genetica 88, 29–36 10.1007/bf02424449 (doi:10.1007/bf02424449) [DOI] [PubMed] [Google Scholar]
- 3.Joron M, Brakefield PM. 2003. Captivity masks inbreeding effects on male mating success in butterflies. Nature 424, 191–194 10.1038/nature01713 (doi:10.1038/nature01713) [DOI] [PubMed] [Google Scholar]
- 4.Mariette M, Kelley JL, Brooks R, Evans JP. 2006. The effects of inbreeding on male courtship behaviour and coloration in guppies. Ethology 112, 807–814 10.1111/j.1439-0310.2006.01236.x (doi:10.1111/j.1439-0310.2006.01236.x) [DOI] [Google Scholar]
- 5.Meagher S, Penn DJ, Potts WK. 2000. Male–male competition magnifies inbreeding depression in wild house mice. Proc. Natl Acad. Sci. USA 97, 3324–3329 10.1073/pnas.060284797 (doi:10.1073/pnas.060284797) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keller LF, Waller DM. 2002. Inbreeding effects in wild populations. Trends Ecol. Evol. 17, 230–241 10.1016/s0169-5347(02)02489-8 (doi:10.1016/s0169-5347(02)02489-8) [DOI] [Google Scholar]
- 7.Bolund E, Martin K, Kempenaers B, Forstmeier W. 2010. Inbreeding depression of sexually selected traits and attractiveness in the zebra finch. Anim. Behav. 79, 947–955 10.1016/j.anbehav.2010.01.014 (doi:10.1016/j.anbehav.2010.01.014) [DOI] [Google Scholar]
- 8.Thornhill R. 1976. Sexual selection and paternal investment in insects. Am. Nat. 110, 153–163 10.1086/283055 (doi:10.1086/283055) [DOI] [Google Scholar]
- 9.Cote IM, Hunte W. 1989. Male and female mate choice in the redlip blenny: why bigger is better. Anim. Behav. 38, 78–88 10.1016/s0003-3472(89)80067-3 (doi:10.1016/s0003-3472(89)80067-3) [DOI] [Google Scholar]
- 10.Boggs CL, Gilbert LE. 1979. Male contribution to egg-production in butterflies: evidence for transfer of nutrients at mating. Science 206, 83–84 10.1126/science.206.4414.83 (doi:10.1126/science.206.4414.83) [DOI] [PubMed] [Google Scholar]
- 11.Saccheri IJ, Brakefield PM, Nichols RA. 1996. Severe inbreeding depression and rapid fitness rebound in the butterfly Bicyclus anynana (Satyridae). Evolution 50, 2000–2013 10.2307/2410758 (doi:10.2307/2410758) [DOI] [PubMed] [Google Scholar]
- 12.Saccheri IJ, Lloyd HD, Helyar SJ, Brakefield PM. 2005. Inbreeding uncovers fundamental differences in the genetic load affecting male and female fertility in a butterfly. Proc. R. Soc. B 272, 39–46 10.1098/rspb.2004.2903 (doi:10.1098/rspb.2004.2903) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aspi J. 2000. Inbreeding and outbreeding depression in male courtship song characters in Drosophila montana. Heredity 84, 273–282 10.1046/j.1365-2540.2000.00655.x (doi:10.1046/j.1365-2540.2000.00655.x) [DOI] [PubMed] [Google Scholar]
- 14.van Oosterhout C, Trigg RE, Carvalho GR, Magurran AE, Hauser L, Shaw PW. 2003. Inbreeding depression and genetic load of sexually selected traits: how the guppy lost its spots. J. Evol. Biol. 16, 273–281 10.1046/j.1420-9101.2003.00511.x (doi:10.1046/j.1420-9101.2003.00511.x) [DOI] [PubMed] [Google Scholar]
- 15.Reid JM, Arcese P, Cassidy A, Marr AB, Smith JNM, Keller LF. 2005. Hamilton and Zuk meet heterozygosity? Song repertoire size indicates inbreeding and immunity in song sparrows (Melospiza melodia). Proc. R. Soc. B 272, 481–487 10.1098/rspb.2004.2983 (doi:10.1098/rspb.2004.2983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drayton JM, Milner RNC, Hunt J, Jennions MD. 2010. Inbreeding and advertisement calling in the cricket Teleogryllus commodus: laboratory and field experiments. Evolution 64, 3069–3083 10.1111/j.1558-5646.2010.01053.x (doi:10.1111/j.1558-5646.2010.01053.x) [DOI] [PubMed] [Google Scholar]
- 17.Bentsen CL, Hunt J, Jennions MD, Brooks R. 2006. Complex multivariate sexual selection on male acoustic signaling in a wild population of Teleogryllus commodus. Am. Nat. 167, E102–E116 10.1086/501376 (doi:10.1086/501376) [DOI] [PubMed] [Google Scholar]
- 18.Iyengar VK, Rossini C, Eisner T. 2001. Precopulatory assessment of male quality in an arctiid moth (Utetheisa ornatrix): hydroxydanaidal is the only criterion of choice. Behav. Ecol. Sociobiol. 49, 283–288 10.1007/s002650000292 (doi:10.1007/s002650000292) [DOI] [Google Scholar]
- 19.Rantala MJ, Jokinen I, Kortet R, Vainikka A, Suhonen J. 2002. Do pheromones reveal male immunocompetence? Proc. R. Soc. Lond. B 269, 1681–1685 10.1098/rspb.2002.2056 (doi:10.1098/rspb.2002.2056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez P, Martin J. 2005. Female Iberian wall lizards prefer male scents that signal a better cell-mediated immune response. Biol. Lett. 1, 404–406 10.1098/rsbl.2005.0360 (doi:10.1098/rsbl.2005.0360) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruther J, Matschke M, Garbe LA, Steiner S. 2009. Quantity matters: male sex pheromone signals mate quality in the parasitic wasp Nasonia vitripennis. Proc. R. Soc. B 276, 3303–3310 10.1098/rspb.2009.0738 (doi:10.1098/rspb.2009.0738) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ilmonen P, Stundner G, Thoss M, Penn DJ. 2009. Females prefer the scent of outbred males: good-genes-as-heterozygosity? BMC Evol. Biol. 9, 104. 10.1186/1471-2148-9-104 (doi:10.1186/1471-2148-9-104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thom MD, Stockley P, Jury F, Ollier WER, Beynon RJ, Hurst JL. 2008. The direct assessment of genetic heterozygosity through scent in the mouse. Curr. Biol. 18, 619–623 10.1016/j.cub.2008.03.056 (doi:10.1016/j.cub.2008.03.056) [DOI] [PubMed] [Google Scholar]
- 24.Thoss M, Ilmonen P, Musolf K, Penn DJ. 2011. Major histocompatibility complex heterozygosity enhances reproductive success. Mol. Ecol. 20, 1546–1557 10.1111/j.1365-294X.2011.05009.x (doi:10.1111/j.1365-294X.2011.05009.x) [DOI] [PubMed] [Google Scholar]
- 25.Pölkki M, Krams I, Kangassalo K, Rantala MJ. 2012. Inbreeding affects sexual signalling in males but not females of Tenebrio molitor. Biol. Lett. 8, 423–425 10.1098/rsbl.2011.1135 (doi:10.1098/rsbl.2011.1135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wyatt TD. 2009. Fifty years of pheromones. Nature 457, 262–263 10.1038/457262a (doi:10.1038/457262a) [DOI] [PubMed] [Google Scholar]
- 27.van Oosterhout C, Zijlstra WG, van Heuven MK, Brakefield PM. 2000. Inbreeding depression and genetic load in laboratory metapopulations of the butterfly Bicyclus anynana. Evolution 54, 218–225 [DOI] [PubMed] [Google Scholar]
- 28.Brakefield PM, Reitsma N. 1991. Phenotypic plasticity, seasonal climate and the population biology of Bicyclus butterflies (Satyridae) in Malawi. Ecol. Entomol. 16, 291–303 10.1111/j.1365-2311.1991.tb00220.x (doi:10.1111/j.1365-2311.1991.tb00220.x) [DOI] [Google Scholar]
- 29.Windig JJ, Brakefield PM, Reitsma N, Wilson JGM. 1994. Seasonal polyphenism in the wild: survey of wing patterns in 5 species of Bicyclus butterflies in Malawi. Ecol. Entomol. 19, 285–298 10.1111/j.1365-2311.1994.tb00420.x (doi:10.1111/j.1365-2311.1994.tb00420.x) [DOI] [Google Scholar]
- 30.Saccheri I, Kuussaari M, Kankare M, Vikman P, Fortelius W, Hanski I. 1998. Inbreeding and extinction in a butterfly metapopulation. Nature 392, 491–494 10.1038/33136 (doi:10.1038/33136) [DOI] [Google Scholar]
- 31.Costanzo K, Monteiro A. 2007. The use of chemical and visual cues in female choice in the butterfly Bicyclus anynana. Proc. R. Soc. B 274, 845–851 10.1098/rspb.2006.3729 (doi:10.1098/rspb.2006.3729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nieberding CM, et al. 2008. The male sex pheromone of the butterfly Bicyclus anynana: towards an evolutionary analysis. PLoS ONE 3, e2751. 10.1371/journal.pone.0002751 (doi:10.1371/journal.pone.0002751) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nieberding CM, Fischer K, Saastamoinen M, Allen CE, Wallin EA, Hedenström E, Brakefield PM. 2012. Cracking the olfactory code of a butterfly: the scent of ageing. Ecol. Lett. 15, 415–424 10.1111/j.1461-0248.2012.01748.x (doi:10.1111/j.1461-0248.2012.01748.x) [DOI] [PubMed] [Google Scholar]
- 34.Brakefield PM, El Filali E, van der Laan R, Breuker CJ, Saccheri IJ, Zwaan B. 2001. Effective population size, reproductive success and sperm precedence in: the butterfly, Bicyclus anynana, in captivity. J. Evol. Biol. 14, 148–156 10.1046/j.1420-9101.2001.00248.x (doi:10.1046/j.1420-9101.2001.00248.x) [DOI] [PubMed] [Google Scholar]
- 35.van 't Hof AE, Zwaan BJ, Saccheri IJ, Daly D, Bot ANM, Brakefield PM. 2005. Characterization of 28 microsatellite loci for the butterfly Bicyclus anynana. Mol. Ecol. Notes 5, 169–172 10.1111/j.1471-8268.2005.00870.x (doi:10.1111/j.1471-8268.2005.00870.x) [DOI] [Google Scholar]
- 36.Brakefield PM, Beldade P, Zwaan BJ. 2009. The African butterfly Bicyclus anynana: a model for evolutionary genetics and evolutionary developmental biology. In Emerging model organisms: a laboratory manual (eds Behringer R, Johnson A, Krumlauf R.), pp. 291–329 Cold Spring Harbor, NY: CSHL Press; [DOI] [PubMed] [Google Scholar]
- 37.Heuskin S, Godina B, Leroy P, Capella Q, Wathelet JP, Verheggen F, Haubruge E, Lognay G. 2009. Fast gas chromatography characterisation of purified semiochemicals from essential oils of Matricaria chamomilla L. (Asteraceae) and Nepeta cataria L. (Lamiaceae). J. Chromatogr. A 1216, 2768–2775 10.1016/j.chroma.2008.09.109 (doi:10.1016/j.chroma.2008.09.109) [DOI] [PubMed] [Google Scholar]
- 38.Saastamoinen M, van der Sterren D, Vastenhout N, Zwaan BJ, Brakefield PM. 2010. Predictive adaptive responses: condition-dependent impact of adult nutrition and flight in the tropical butterfly Bicyclus anynana. Am. Nat. 176, 686–698 10.1086/657038 (doi:10.1086/657038) [DOI] [PubMed] [Google Scholar]
- 39.DeRose MA, Roff DA. 1999. A comparison of inbreeding depression in life-history and morphological traits in animals. Evolution 53, 1288–1292 10.2307/2640831 (doi:10.2307/2640831) [DOI] [PubMed] [Google Scholar]
- 40.Roff DA, Emerson K. 2006. Epistasis and dominance: evidence for differential effects in life-history versus morphological traits. Evolution 60, 1981–1990 10.1111/j.0014-3820.2006.tb01836.x (doi:10.1111/j.0014-3820.2006.tb01836.x) [DOI] [PubMed] [Google Scholar]
- 41.Kempenaers B. 2007. Mate choice and genetic quality: a review of the heterozygosity theory. Adv. Study Behav. 37, 189–278 10.1016/s0065-3454(07)37005-8 (doi:10.1016/s0065-3454(07)37005-8) [DOI] [Google Scholar]
- 42.Whitlock MC, Agrawal AF. 2009. Purging the genome with sexual selection: reducing mutation load through selection on males. Evolution 63, 569–582 10.1111/j.1558-5646.2008.00558.x (doi:10.1111/j.1558-5646.2008.00558.x) [DOI] [PubMed] [Google Scholar]
- 43.Roldan ERS, Cassinello J, Abaigar T, Gomendio M. 1998. Inbreeding, fluctuating asymmetry, and ejaculate quality in an endangered ungulate. Proc. R. Soc. Lond. B 265, 243–248 10.1098/rspb.1998.0288 (doi:10.1098/rspb.1998.0288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gage MJG, Surridge AK, Tomkins JL, Green E, Wiskin L, Bell DJ, Hewitt GM. 2006. Reduced heterozygosity depresses sperm quality in wild rabbits, Oryctolagus cuniculus. Curr. Biol. 16, 612–617 10.1016/j.cub.2006.02.059 (doi:10.1016/j.cub.2006.02.059) [DOI] [PubMed] [Google Scholar]
- 45.Dudley R. 2000. The biomechanics of insect flight: form, function, evolution. Princeton, NJ: Princeton University Press [Google Scholar]
- 46.Berwaerts K, van Dyck H, Aerts P. 2002. Does flight morphology relate to flight performance? An experimental test with the butterfly Pararge aegeria. Funct. Ecol. 16, 484–491 10.1046/j.1365-2435.2002.00650.x (doi:10.1046/j.1365-2435.2002.00650.x) [DOI] [Google Scholar]
- 47.Oostra V, de Jong MA, Invergo BM, Kesbeke F, Wende F, Brakefield PM, Zwaan BJ. 2011. Translating environmental gradients into discontinuous reaction norms via hormone signalling in a polyphenic butterfly. Proc. R. Soc. B 278, 789–797 10.1098/rspb.2010.1560 (doi:10.1098/rspb.2010.1560) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amrein H, Thorne N. 2005. Gustatory perception and behavior in Drosophila melanogaster. Curr. Biol. 15, R673–R684 10.1016/j.cub.2005.08.021 (doi:10.1016/j.cub.2005.08.021) [DOI] [PubMed] [Google Scholar]
- 49.Ebbs ML, Amrein H. 2007. Taste and pheromone perception in the fruit fly Drosophila melanogaster. Pflugers Arch. 454, 735–747 10.1007/s00424-007-0246-y (doi:10.1007/s00424-007-0246-y) [DOI] [PubMed] [Google Scholar]
- 50.Miyamoto T, Amrein H. 2008. Suppression of male courtship by a Drosophila pheromone receptor. Nat. Neurosci. 11, 874–876 10.1038/nn.2161 (doi:10.1038/nn.2161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lynch M, Walsh B. 1997. Genetics and the analysis of quantitative traits. Sunderland, MA: Sinauer Associates [Google Scholar]
- 52.Candolin U. 2003. The use of multiple cues in mate choice. Biol. Rev. 78, 575–595 10.1017/s1464793103006158 (doi:10.1017/s1464793103006158) [DOI] [PubMed] [Google Scholar]
- 53.Bretman A, Westmancoat James D, Gage Matthew JG, Chapman T. 2011. Males use multiple, redundant cues to detect mating rivals. Curr. Biol. 21, 617–622 10.1016/j.cub.2011.03.008 (doi:10.1016/j.cub.2011.03.008) [DOI] [PubMed] [Google Scholar]
- 54.Wyatt TD. 2003. Pheromones and animal behaviour: communication by smell and taste. New York, NY: Cambridge University Press [Google Scholar]
- 55.Coleman SW. 2009. Taxonomic and sensory biases in the mate-choice literature: there are far too few studies of chemical and multimodal communication. Acta Ethol. 12, 45–48 10.1007/s10211-008-0050-5 (doi:10.1007/s10211-008-0050-5) [DOI] [Google Scholar]
- 56.Campbell-Palmer R, Rosell F. 2011. The importance of chemical communication studies to mammalian conservation biology: a review. Biol. Conserv. 144, 1919–1930 10.1016/j.biocon.2011.04.028 (doi:10.1016/j.biocon.2011.04.028) [DOI] [Google Scholar]