Abstract
Sexually selected ornaments and weapons are among nature's most extravagant morphologies. Both ornaments and weapons improve a male's reproductive success; yet, unlike ornaments that need only attract females, weapons must be robust and functional structures because they are frequently tested during male–male combat. Consequently, weapons are expected to be particularly costly to bear. Here, we tested the aerodynamic costs of horns in the giant rhinoceros beetle, Trypoxylus dichotomus. We predicted that the long, forked head horn would have three main effects on flight performance: increased body mass, an anterior shift in the centre of mass and increased body drag. We found that the horns were surprisingly lightweight, and therefore had a trivial effect on the male beetles' total mass and mass distribution. Furthermore, because beetles typically fly at slow speeds and high body angles, horns had little effect on total body drag. Together, the weight and the drag of horns increased the overall force required to fly by less than 3 per cent, even in the largest males. Because low-cost structures are expected to be highly evolutionarily labile, the fact that horns incur very minor flight costs may have permitted both the elaboration and diversification of rhinoceros beetle horns.
Keywords: rhinoceros beetles, horns, aerodynamic costs, sexual selection
1. Introduction
From foraging for food to avoiding predators, to finding and securing mates, locomotion is critical to the survival and reproductive success of nearly all animals. Selection for efficient locomotion, particularly among animals that fly, favours streamlined body forms, yet competition for mates has driven the evolution of an array of flashy ornaments and exaggerated weapons that often make males anything but streamlined [1,2]. Thus, the elaborate morphologies that are favoured by sexual selection typically oppose the simple, streamlined morphologies favoured by natural selection, which has led to a number of compensatory changes to ameliorate the costs of bearing large, sexually selected traits [3–5].
For example, the outermost feathers of long-tailed, sexually dimorphic male birds are often narrowed at the tips to help offset the aerodynamic drag that these tail ornaments accrue [6,7]. Similarly, in sexually dimorphic stalk-eyed flies, males have significantly longer eye spans than females, yet overall head mass does not differ because males have smaller eye bulbs and thinner eye stalks [8–10]. In contrast to ornaments (e.g. eye stalks and tail streamers), however, which merely need to be attractive to females, sexually selected weapons must be robust and functional structures because they are often tested during male–male combat [11–15]. Consequently, structural modifications that reduce the costs of carrying large weapons may not be favoured, if these changes compromise the weapons' structural integrity and performance during fights. Sexually selected weapons are therefore expected to be particularly costly to bear; yet we still know surprisingly little about the costs of carrying these exaggerated structures.
Here, we investigate the aerodynamic costs of elaborate horns in a large Asian rhinoceros beetle, Trypoxylus dichotomus. Rhinoceros beetles are an ideal system for investigating the costs of carrying sexually selected weapons because of the impressive size of their horns: in some species, the length of the horn is greater than the length of the rest of the body [16,17]. Moreover, flying is the primary mode of locomotion for rhinoceros beetles, with beetles flying to and from resource sites each night [18,19]. Recent studies have begun to examine the effects of horns on the flight performance of rhinoceros beetles in the field [20,21], yet no studies to date have examined the specific aerodynamic effects of beetle horns, or of any other sexually selected weapon.
We predicted that the long, forked head horn of male T. dichotomus would have three primary effects on the beetles' flight performance. First, we expected that the large horns would significantly increase total body mass, thereby increasing the amount of lift required to support the beetle's weight to stay aloft [22–24]. Second, we expected that the long horns extending forward from the head would significantly shift the centre of mass forward, which could alter stability and manoeuvrability [25,26]. And third, we expected that the pitchfork-like head horn would significantly increase total body drag, thereby increasing the amount of thrust required to drive the beetle forward [22–24]. We present a set of experiments that investigate the consequences of horns on the beetles' total body mass, mass distribution and aerodynamic drag, and then discuss how these three factors contribute to the overall cost to fly with an elaborate sexually selected horn.
2. Material and methods
Trypoxylus dichotomus is a large Asian rhinoceros beetle with a wide variation in body and horn size. Large males have a branched head horn that can reach nearly two-thirds the length of their body and a smaller thoracic horn; small males have a short head horn and tiny thoracic horn; and females are hornless. Males use their horns to pry rival males away from wounds or sap sites on trees where females come to feed [27,28]. Males with the longest horns are more likely to win fights and gain access to these sap sites, and to achieve higher mating success [29,30].
Beetles were purchased as final instar larvae from a commercial insect distributor (Yasaka Dabuto Kuwagata World, Japan) and reared to adulthood at the University of Montana. Horn lengths of all males and prothorax widths (a standard measure of body size [31]) of both males and females were measured to the nearest 0.01 mm with dial callipers.
The mass contribution of the horns was measured by placing males (n = 91) in airtight containers, euthanizing them by freezing and weighing each beetle to the nearest 0.001 g with an analytical balance. The head and thoracic horns were removed using nail clippers, and then the horns and hornless body were re-weighed. Horns were hollow, air-filled and surprisingly lightweight. We therefore measured the relative moisture content of the horns and three additional body parts—the legs, elytra and head/pterothorax (hereafter referred to as the thorax)—in order to further investigate potential differences in the composition of the horns. Relative moisture content was measured by severing and weighing each body part, drying the samples to a constant mass for at least 72 h and then re-weighing them dry. Relative moisture content was calculated as the difference between wet and dry masses, divided by the original wet mass.
A plumb line method was used to measure the beetles' centre of mass [24]. Freeze-euthanized beetles (males, n = 56; females, n = 40) were pinned dorsoventrally through the anterior distal corner of their right elytron and balanced on two horizontal bars. A digital photograph of each beetle was taken after it had settled. The pin was then removed and reinserted through the outer corner of the left elytron. The beetle was balanced again on the bars and a second photograph was taken. A line of gravity was drawn on each photograph through the suspension point, and the two photographs were superimposed. The centre of mass was defined by the intersection of the two lines of gravity, and measured as a fractional position along the anteroposterior body axis between the clypeus (position = 0) and the tip of the abdomen (position = 1).
Total body drag was measured by mounting dried specimens onto a custom-made, calibrated force transducer (see [32] for details). Because we were interested in the aerodynamic effects of horns, we dried the beetles with their elytra closed and legs removed, which minimized the effects of other body parts on flow dynamics. Drag was measured on 10 large males, 10 small males and 8 females. To determine the relative contribution of the horns to drag, half of the males (five large and five small) were measured both before and after the head horn and thoracic horn were removed. Drag was measured for each beetle in a variable speed wind tunnel (see [33] for details) at body angles of attack relative to flight direction (hereafter referred to as body angle) ranging from 0° to 90°, and wind speeds ranging from 0.5 to 8 m s−1. Throughout this study, ‘wind speed’ refers to equivalent wind speed, which is the true wind speed at sea level that would produce the same dynamic pressure given the observed air pressure. We preferred to adjust equivalent wind speed in our experiments in order to control for daily fluctuations in temperature and barometric pressure, and because it is equivalent wind speed, rather than true wind speed, that determines the magnitudes of forces acting on a flying beetle [34]. Typical flight speeds were determined previously [21] using a high-performance speed sensor on free-flying beetles in the field. Typical body angles were measured by filming beetles flying down the laboratory hallway at 500 frames per second using a high-speed video camera (Photron SA3).
To control for the large variation in size among individuals, drag measurements were converted to drag coefficients (CD),
where D is the measured drag, ρ is air density, S is frontal surface area and u is wind speed. Drag coefficients of manipulated males are based on frontal surface areas after the horns were removed. Frontal surface areas were measured using imaging software (ImageJ v. 1.41, National Institutes of Health) from digital photographs of the beetles at 0° body angle. Our results are qualitatively the same when the drag coefficients were calculated using the frontal surface area specific to each body angle (see [35] for rationale for keeping frontal surface area constant).
Finally, we used two-dimensional particle image velocimetry (PIV) to visualize the effects of horns on the near-wake fluid dynamics of beetle specimens mounted in the wind tunnel with body angles of 50° and wind speeds of 3 m s−1. Details on our PIV system are described elsewhere [32]. In brief, we seeded the air using submicron-sized olive oil particles, positioned the laser to illuminate a parasagittal slice just off the beetle's midline and placed the camera perpendicular to the planar illumination field. We used cross-correlation of paired images (elapsed time between images = 210 μs) and adaptive multi-pass processing to calculate particle velocity. We computed average particle velocity and vorticity using 50 sequential image pairs. To reduce surface reflections, beetle specimens were coated with a thin layer of rhodamine B dissolved in acrylic lacquer [36]. To quantify the effect of horns on the near-wake flow field, we measured average and minimum horizontal and vertical velocities 1.5 body lengths behind each beetle.
3. Results
The horns of male T. dichotomus made a very minor contribution to the beetles' total body mass. Relative horn mass ranged between 0.5 and 2.5 per cent (1.5 ± 0.6%, mean ± s.d.). The horns were significantly drier than other body parts (ANOVA: F3,96 = 1156.7, p < 0.001): relative moisture content was 63.8 ± 1.7 per cent in thoraces, 54.2 ± 2.0 per cent in legs and 39.8 ± 2.1 per cent in elytra, but only 25.7 ± 3.6 per cent in horns.
The centre of mass was significantly closer to the head in males compared with females (Welch's t-test: t = 10.35, d.f. = 66.57, p < 0.001); the fractional position was 0.48 ± 0.03 in males and 0.57 ± 0.05 in females. There was no relationship between centre of mass and body size in females (R2 = 0.03, F1,38 = 1.18, p = 0.29), but there was a significant correlation in males (figure 1; R2 = 0.34, F1,54 = 28.42, p < 0.001); the centre of mass was more anterior in large males than small males. However, the horns themselves had a trivial effect on the centre of mass. The centre of mass was significantly closer to the head in males with their horns intact compared with hornless males (paired t-test: t = −5.86, d.f. = 55, p < 0.001), but horn removal shifted the centre of mass by only 1.7 per cent. Furthermore, there was no relationship between body size and the change in centre of mass between intact and hornless males (R2 = 0.001, F1,54 = 0.05, p = 0.82), indicating that the long horns of large males did not shift the centre of mass more than the short horns of small males.
Figure 1.
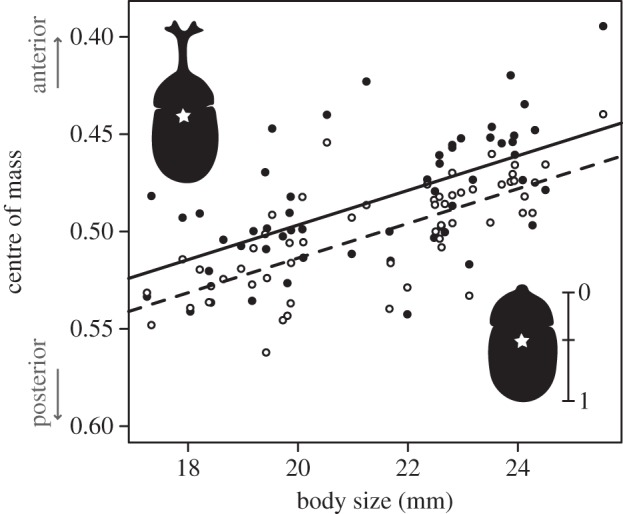
Relationships between body size and centre of mass for males with horns (black circles, solid line) and without their horns (white circles, dashed line). The centre of mass was significantly closer to the head in large males than small males (R2 = 0.34, F1,54 = 28.42, p < 0.001), and in horned males compared with hornless males (T = −5.86, p < 0.001). Horn removal, however, resulted in only a 1.7 per cent shift in the centre of mass.
Beetles flew at slow speeds and high body angles (see the electronic supplementary material, movie S1). Typical flight speeds were between 1 and 4 m s−1 (2.27 ± 0.44 m s−1), and body angles ranged between 30° and 85° (54 ± 12°). As expected from aerodynamic theory, drag coefficients increased at higher body angles (figure 2). However, drag coefficients at body angles from 0° to 90° did not differ significantly between males with and without their horns (repeated-measures ANOVA: F1,96 = 2.29, p = 0.13). Intact males had slightly higher drag coefficients compared with hornless males at shallow body angles (less than 30°), but they actually had slightly lower drag coefficients at high body angles (greater than 30°); this is the opposite of what we would expect if horns significantly increased body drag. Moreover, at the beetles' typical flight speed (3 m s−1) and body angle (50°), drag coefficients did not differ among large males with and without their horns, small males with and without their horns, and naturally hornless females (ANOVA: F1,35 = 2.26, p = 0.14).
Figure 2.
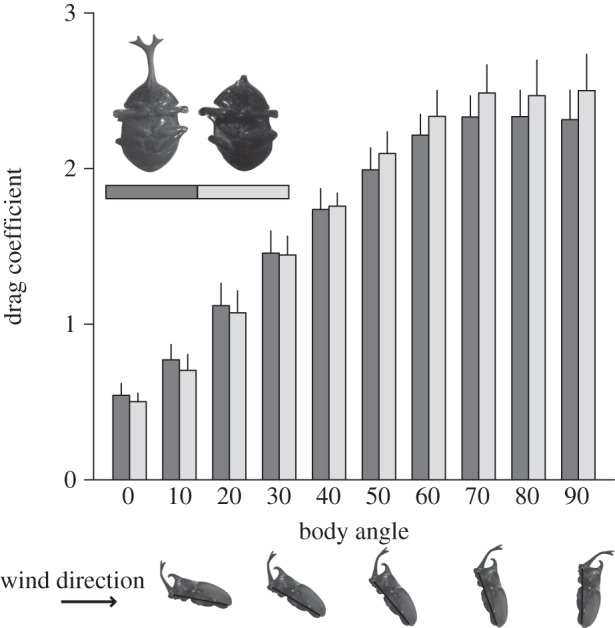
Drag coefficient as a function of body angle for males with horns (dark grey/light grey bars) and without their horns (open bars). Drag coefficients increased with increasing body angle, but did not differ between males with and without their horns (repeated-measures ANOVA: F1,96 = 2.29, p = 0.13). Inset: representative frontal pictures of an intact and hornless male at 50° body angle.
Visualizations of the airflow around mounted beetles using PIV (figure 3) provided further evidence that the beetles generate substantial drag during flight, but that the horns have a relatively small effect. The bodies of horned males generated a wide, drag-based wake with strong vortex shedding dorsally and ventrally (figure 3a), as expected for a non-streamlined body at moderate Reynolds numbers (Re ≈ 10 500). There was a large momentum deficit in the wake of the male's body, indicating nearly complete flow separation; average horizontal velocity in the near-field wake was only 70 per cent of the free-stream velocity, and minimum horizontal velocity was only 27 per cent of free-stream flow.
Figure 3.
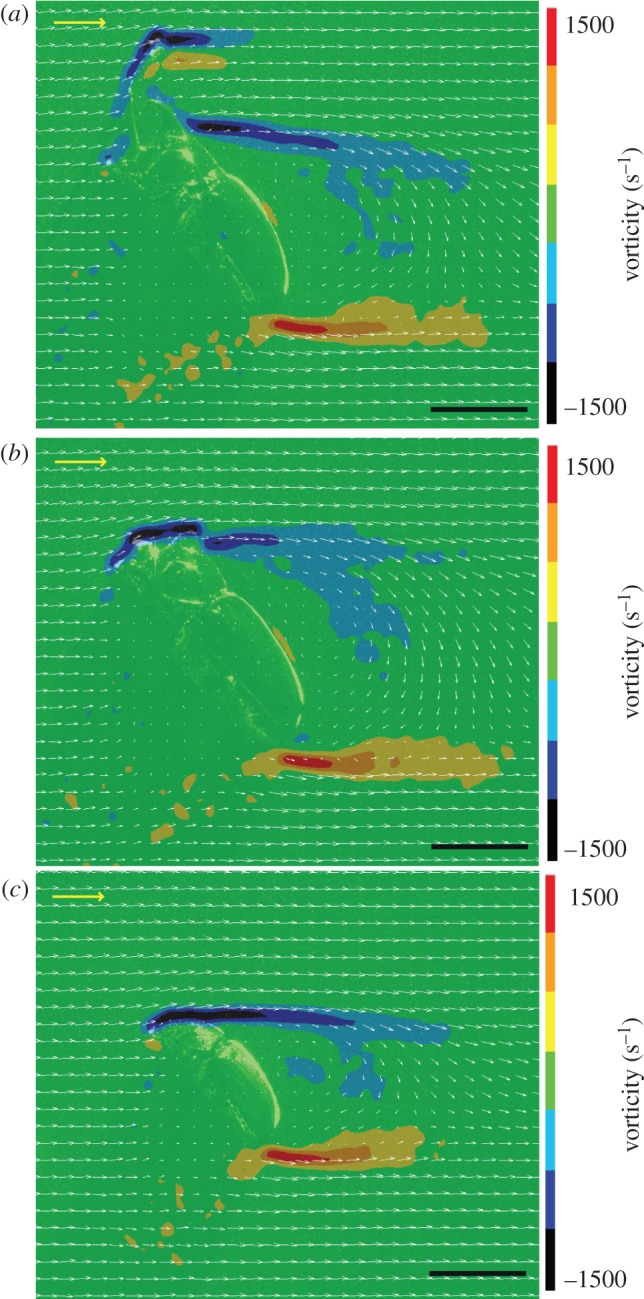
Time-averaged flow fields of a parasagittal PIV sample around mounted beetles in a wind tunnel. Wind speed = 3 m s−1 and body angle = 50°. White vectors in the foreground indicate average velocity; coloured background indicates average vorticity. (a) Large males with their horns, (b) without their horns and (c) naturally hornless females all generated drag-based wakes with complete flow separation. Horns also produced a drag wake, but their contribution to overall drag was small compared with the rest of the body. All beetles generated a slight net downward induced flow, indicating a small amount of body lift production. Yellow scale vector, 10 m s−1; black scale bar, 20 mm.
The male's horn also produced a drag-based wake, but the contribution of the horn was small compared with the rest of the body: the horn's wake width was only 20 per cent of the width of the body wake. Moreover, the loss of horns did not substantially change the flow field. Males without their horns (figure 3b) and naturally hornless females (figure 3c) also produced a fully separated, drag-based wake, and the momentum deficit in the wake of hornless males and females was similar to that of the intact males: minimum horizontal velocity was 31 per cent of free-stream flow in the wake of hornless males and 30 per cent in females. Furthermore, even in the hornless males and females, the air stream detached from the surface of the beetles at the maximum width of the body, as expected for a non-streamlined object. As a result, the lack of horns did not delay flow separation or help streamline the beetle's body. There was, however, a slight net downward induced flow for all beetles. Average vertical velocity in the near-field wake was −0.62 m s−1 for horned males, −0.52 m s−1 for hornless males and −0.48 m s−1 for females. These results indicate that, despite the non-streamlined bodies and high body angles, beetles produced a small amount of body lift [32].
4. Discussion
The horns of rhinoceros beetles are among the most elaborate traits found in nature, and we intuitively expect these structures to impair locomotion. However, our data show that the aerodynamic costs of bearing large horns are exceptionally small and probably biologically negligible. In particular, the drag coefficients and patterns of airflow around beetles were essentially the same for males flying with and without their horns. Drag increases exponentially with flight speed [35], so minimizing drag may be relatively unimportant for beetles that typically fly at slow speeds. Furthermore, because beetles fly at very high body angles, the large projected area and prominent flow separation in the wake of the beetle's body swamp the relatively small drag contribution from the horn.
Large horns also contributed surprisingly little to the beetle's total body mass. Even in the largest males, horns represented only 2.5 per cent of the beetle's total mass. While the centre of mass was closer to the head in large males compared with small males, this difference did not stem from the disproportionately long horns of large males. Rather, the centre of mass appears to be shifted towards the head in large males because they have larger prothoracic muscles (E. L. McCullough 2010, unpublished data), which are critical for generating sufficient torque to dislodge rivals off the trunks and branches of trees during male–male combat [27,30,37].
In order to fly at a constant velocity, an animal must generate enough lift to overcome its body weight, and enough thrust to overcome its body drag. Horns represent a very small increase in total body weight and body drag, and therefore result in a trivial increase in the overall force required to fly. Among the largest males, less than 3 per cent more force is required to fly with a horn compared with without a horn; among the smallest males, the added force requirement is less than 2 per cent.
Many insects are able to carry loads substantially heavier than their body weight [38]. For example, vespid wasps often carry animal prey that weigh 20–70 per cent of their body mass [39,40], and foraging bumble-bees may return to the nest with nectar and pollen loads that double their body mass [41]. As a result, it is unlikely that the beetles are significantly burdened by their lightweight horns. Moreover, because the horns of T. dichotomus are among the largest found in rhinoceros beetles, and are the only horns with such a broad, forked tip [17], it is unlikely that aerodynamic costs will be significant in other species with smaller horns.
Rhinoceros beetles exhibit an impressive diversity in the shape, size, number and location of their horns [16,17]. We suspect that this morphological variation largely reflects species differences in the tactics used during male–male fights. Males fight on various substrates (e.g. broad tree trunks, narrow bamboo shoots and inside burrows), and wield their horns in different ways to pinch, push or pry their opponents away from valuable resource sites [13,14,28]. As a result, sexual selection may have favoured divergent horn designs, as different types of weapon are likely to perform best depending on where and how they are used.
Given that variations in the shape and size of horns appear to have very minor consequences on the beetle's flight performance, the exaggeration and diversification in horn morphology driven by male–male competition may have been largely unopposed by natural selection for efficient locomotion. Previous authors have similarly argued that the diversity of tail ornaments among birds may reflect the fact that tails are hidden by the wake of the body and thereby have minimal aerodynamic effects [42]. We suggest that, as in bird tail ornamentation, the large horns of rhinoceros beetles may effectively ‘hide’ from selective pressures because of the enormous wake of the body owing to the beetles' slow flight speeds and high body angles. However, whether the evolution of horns has been constrained by selection to minimize costs in other naturally selected tasks remains to be tested. Future studies are also necessary to determine if horns approach their mechanical limits during intense fights, and whether functional limitations play a role in determining the shape and size of the beetles' elaborate horns.
Acknowledgements
We thank Kael Melanson for his help analysing the beetles' centre of mass, and Cerisse Allen, Stacey Combes, Doug Emlen, Keaton Wilson and Art Woods for their comments on earlier drafts of this manuscript. Financial support for this project was provided by the Ford Foundation and the National Science Foundation (DGE-0809127 to E.L.M. and IOS-0919799 to B.W.T.).
References
- 1.Darwin C. 1871. The descent of man, and selection in relation to sex. London, UK: John Murray [Google Scholar]
- 2.Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press [Google Scholar]
- 3.Møller AP. 1996. The cost of secondary sexual characters and the evolution of cost-reducing traits. Ibis 138, 112–119 10.1111/j.1474-919X.1996.tb04317.x (doi:10.1111/j.1474-919X.1996.tb04317.x) [DOI] [Google Scholar]
- 4.Oufiero CE, Garland T. 2007. Evaluating performance costs of sexually selected traits. Funct. Ecol. 21, 676–689 10.1111/j.1365-2435.2007.01259.x (doi:10.1111/j.1365-2435.2007.01259.x) [DOI] [Google Scholar]
- 5.Husak JF, Swallow JG. 2011. Compensatory traits and the evolution of male ornaments. Behaviour 148, 1–29 10.1163/000579510X541265 (doi:10.1163/000579510X541265) [DOI] [Google Scholar]
- 6.Møller AP, De Lope F, Saino N. 1995. Sexual selection in the barn swallow Hirundo rustica. VI. Aerodynamic adaptations. J. Evol. Biol. 8, 671–687 10.1046/j.1420-9101.1995.8060671.x (doi:10.1046/j.1420-9101.1995.8060671.x) [DOI] [Google Scholar]
- 7.Møller AP, Hedenström A. 1999. Comparative evidence for costs of secondary sexual characters: adaptive vane emargination of ornamented feathers in birds. J. Evol. Biol. 12, 296–305 10.1046/j.1420-9101.1999.00034.x (doi:10.1046/j.1420-9101.1999.00034.x) [DOI] [Google Scholar]
- 8.Swallow JG, Wilkinson GS, Marden JH. 2000. Aerial performance of stalk-eyed flies that differ in eye span. J. Comp. Physiol. B 170, 481–487 10.1007/s003600000124 (doi:10.1007/s003600000124) [DOI] [PubMed] [Google Scholar]
- 9.Ribak G, Swallow J. 2007. Free flight maneuvers of stalk-eyed flies: do eye-stalks affect aerial turning behavior? J. Comp. Physiol. B 193, 1065–1079 10.1007/s00359-007-0259-1 (doi:10.1007/s00359-007-0259-1) [DOI] [PubMed] [Google Scholar]
- 10.Worthington AM, Berns CM, Swallow JG. 2012. Size matters, but so does shape: quantifying complex shape changes in a sexually selected trait in stalk-eyed flies (Diptera: Diopsidae). Biol. J. Linn. Soc. 106, 104–113 10.1111/j.1095-8312.2011.01841.x (doi:10.1111/j.1095-8312.2011.01841.x) [DOI] [Google Scholar]
- 11.Geist V. 1966. The evolution of horn-like organs. Behaviour 27, 175–214 10.1163/156853966X00155 (doi:10.1163/156853966X00155) [DOI] [Google Scholar]
- 12.Crane J. 1975. Fiddler crabs of the world. Princeton, NJ: Princeton University Press [Google Scholar]
- 13.Eberhard WG. 1977. Fighting behavior of male Golofa porteri (Scarabeidae: Dynastinae). Psyche 84, 292–298 10.1155/1977/19030 (doi:10.1155/1977/19030) [DOI] [Google Scholar]
- 14.Eberhard WG. 1979. The function of horns in Podischnus agenor (Dynastinae) and other beetles. In Sexual selection and reproductive competition in insects, pp. 231–259 New York, NY: Academic Press [Google Scholar]
- 15.Emlen DJ. 2008. The evolution of animal weapons. Annu. Rev. Ecol. Evol. Syst. 39, 387–413 10.1146/annurev.ecolsys.39.110707.173502 (doi:10.1146/annurev.ecolsys.39.110707.173502) [DOI] [Google Scholar]
- 16.Arrow G. 1951. Horned beetles: a study of the fantastic in nature. The Hague, The Netherlands: Junk Publishers [Google Scholar]
- 17.Mizunuma T. 1999. Giant beetles. Tokyo, Japan: ESI Publishers [Google Scholar]
- 18.Beaudoin-Ollivier L, Bonaccorso F, Aloysius M, Kasiki M. 2003. Flight movement of Scapanes australis australis (Boisduval) (Coleoptera: Scarabaeidae: Dynastinae) in Papua New Guinea: a radiotelemetry study. Aust. J. Entomol. 42, 367–372 10.1046/j.1440-6055.2003.00369.x (doi:10.1046/j.1440-6055.2003.00369.x) [DOI] [Google Scholar]
- 19.McCullough E. 2013. Using radio telemetry to assess movement patterns in a giant rhinoceros beetle: are there differences among majors, minors, and females? J. Insect Behav. 26, 51–56 10.1007/s10905-012-9334-8 (doi:10.1007/s10905-012-9334-8) [DOI] [Google Scholar]
- 20.Hongo Y. 2010. Does flight ability differ among male morphs of the Japanese horned beetle Trypoxylus dichotomus septentrionalis (Coleoptera Scarabaeidae)? Ethol. Ecol. Evol. 23, 271–279 10.1080/03949370.2010.502322 (doi:10.1080/03949370.2010.502322) [DOI] [Google Scholar]
- 21.McCullough EL, Weingarden PR, Emlen DJ. 2012. Costs of elaborate weapons in a rhinoceros beetle: how difficult is it to fly with a big horn? Behav. Ecol. 23, 1042–1048 10.1093/beheco/ars069 (doi:10.1093/beheco/ars069) [DOI] [Google Scholar]
- 22.Rayner JMV. 1979. A vortex theory of animal flight. I. The vortex wake of a hovering animal. J. Fluid Mech. 91, 697–730 10.1017/S0022112079000410 (doi:10.1017/S0022112079000410) [DOI] [Google Scholar]
- 23.Rayner JMV. 1979. A vortex theory of animal flight. II. The forward flight of birds. J. Fluid Mech. 91, 731–763 10.1017/S0022112079000422 (doi:10.1017/S0022112079000422) [DOI] [Google Scholar]
- 24.Ellington CP. 1984. The aerodynamics of hovering insect flight. I-VI. Phil. Trans. R. Soc. Lond. B 305, 1–181 10.1098/rstb.1984.0049 (doi:10.1098/rstb.1984.0049) [DOI] [Google Scholar]
- 25.Taylor GK, Thomas ALR. 2002. Animal flight dynamics. II. Longitudinal stability in flapping flight. J. Theor. Biol. 214, 351–370 10.1006/jtbi.2001.2470 (doi:10.1006/jtbi.2001.2470) [DOI] [PubMed] [Google Scholar]
- 26.Thomas ALR, Taylor GK. 2001. Animal flight dynamics. I. Stability in gliding flight. J. Theor. Biol. 212, 399–424 10.1006/jtbi.2001.2387 (doi:10.1006/jtbi.2001.2387) [DOI] [PubMed] [Google Scholar]
- 27.Siva-Jothy M. 1987. Mate securing tactics and the cost of fighting in the Japanese horned beetle, Allomyrina dichotoma L. (Scarabaeidae). J. Ethol. 5, 165–172 10.1007/BF02349949 (doi:10.1007/BF02349949) [DOI] [Google Scholar]
- 28.Hongo Y. 2003. Appraising behaviour during male–male interaction in the Japanese horned beetle Trypoxylus dichotomus septentrionalis (Kono). Behaviour 140, 501–517 10.1163/156853903322127959 (doi:10.1163/156853903322127959) [DOI] [Google Scholar]
- 29.Karino K, Niiyama H, Chiba M. 2005. Horn length is the determining factor in the outcomes of escalated fights among male Japanese horned beetles, Allomyrina dichotoma L. (Coleoptera: Scarabaeidae). J. Insect Behav. 18, 805–815 10.1007/s10905-005-8741-5 (doi:10.1007/s10905-005-8741-5) [DOI] [Google Scholar]
- 30.Hongo Y. 2007. Evolution of male dimorphic allometry in a population of the Japanese horned beetle Trypoxylus dichotomus septentrionalis. Behav. Ecol. Sociobiol. 62, 245–253 10.1007/s00265-007-0459-2 (doi:10.1007/s00265-007-0459-2) [DOI] [Google Scholar]
- 31.Emlen DJ. 1997. Alternative reproductive tactics and male-dimorphism in the horned beetle Onthophagus acuminatus (Coleoptera: Scarabaeidae). Behav. Ecol. Sociobiol. 41, 335–341 10.1007/s002650050393 (doi:10.1007/s002650050393) [DOI] [Google Scholar]
- 32.Tobalske B, Hearn J, Warrick D. 2009. Aerodynamics of intermittent bounds in flying birds. Exp. Fluids 46, 963–973 10.1007/s00348-009-0614-9 (doi:10.1007/s00348-009-0614-9) [DOI] [Google Scholar]
- 33.Tobalske BW, Puccinelli LA, Sheridan DC. 2005. Contractile activity of the pectoralis in the zebra finch according to mode and velocity of flap-bounding flight. J. Exp. Biol. 208, 2895–2901 10.1242/jeb.01734 (doi:10.1242/jeb.01734) [DOI] [PubMed] [Google Scholar]
- 34.Pennycuick C, Alerstam T, Hedenström A. 1997. A new low-turbulence wind tunnel for bird flight experiments at Lund University, Sweden. J. Exp. Biol. 200, 1441–1449 [DOI] [PubMed] [Google Scholar]
- 35.Vogel S. 1996. Life in moving fluids: the physical biology of flow. Princeton, NJ: Princeton University Press [Google Scholar]
- 36.Schröder A, Willert CE. 2008. Particle image velocimetry: new developments and recent applications. Berlin, Germany: Springer [Google Scholar]
- 37.Jarman GM, Hinton HE. 1974. Some defence mechanisms of the Hercules beetle, Dynastes hercules. J. Entomol. A 49, 71–80 10.1111/j.1365-3032.1974.tb00070.x (doi:10.1111/j.1365-3032.1974.tb00070.x) [DOI] [Google Scholar]
- 38.Dudley R. 2000. The biomechanics of insect flight: form, function, and evolution. Princeton, NJ: Princeton University Press [Google Scholar]
- 39.Archer M. 1977. The weights of forager loads of Paravespula vulgaris (Linn.) (Hymenoptera: Vespidae) and the relationship of load weight to forager size. Insectes Soc. 24, 95–102 10.1007/BF02223281 (doi:10.1007/BF02223281) [DOI] [Google Scholar]
- 40.Coelho JR, Hoagland J. 1995. Load-lifting capacities of three species of yellowjackets (Vespula) foraging on honey-bee corpses. Funct. Ecol. 9, 171–174 10.2307/2390561 (doi:10.2307/2390561) [DOI] [Google Scholar]
- 41.Heinrich B. 2004. Bumble-bee economics. Cambridge, MA: Harvard University Press [Google Scholar]
- 42.Clark CJ, Dudley R. 2009. Flight costs of long, sexually selected tails in hummingbirds. Proc. R. Soc. B 276, 2109–2115 10.1098/rspb.2009.0090 (doi:10.1098/rspb.2009.0090) [DOI] [PMC free article] [PubMed] [Google Scholar]


