Abstract
Sperm production is a key male reproductive trait and an important parameter in sperm competition theory. Under sperm competition, paternity success is predicted to depend strongly on male allocation to sperm production. Furthermore, because sperm production is inherently costly, individuals should economize in sperm expenditure, and conditional adjustment of the copulation frequency according to their sperm availability may be expected. However, experimental studies showing effects of sperm production on mating behaviour and paternity success have so far been scarce, mainly because sperm production is difficult to manipulate directly in animals. Here, we used phenotypic engineering to manipulate sperm-production rate, by employing dose-dependent RNA interference (RNAi) of a spermatogenesis-specific gene, macbol1, in the free-living flatworm Macrostomum lignano. We demonstrate (i) that our novel dose-dependent RNAi approach allows us to induce high variability in sperm-production rate; (ii) that a reduced sperm-production rate is associated with a decreased copulation frequency, suggesting conditional adjustment of mating behaviour; and (iii) that both sperm production and copulation frequency are important determinants of paternity success in a competitive situation, as predicted by sperm competition theory. Our study clearly documents the potential of phenotypic engineering via dose-dependent RNAi to test quantitative predictions of evolutionary theory.
Keywords: phenotypic engineering, RNA interference, sperm production, sperm competition, copulation frequency, simultaneous hermaphrodite
1. Introduction
Male allocation towards sperm production is a key parameter in sperm competition theory. When several sperm donors copulate with the same recipient, this can lead to sperm competition between their ejaculates [1], an important aspect of post-copulatory sexual selection [2,3]. With increasing levels of sperm competition, higher sperm production is predicted, since the paternity share will depend, at least partly, on the relative amounts of sperm transferred by each donor [3,4]. Supporting this prediction, many interspecific comparative studies have shown a positive correlation between relative testis size (a common morphological proxy for sperm production) and mating system (a proxy for sperm competition level) in a large range of animal taxa (e.g. [5–10]; reviewed in [11]). In contrast, direct empirical evidence for the selective pressure on sperm production under sperm competition is less abundant. While there are a fair amount of studies showing a positive correlation between intraspecific variation in relative testis size and sperm transfer and/or paternity success [12–16], few have experimentally manipulated sperm production. These include experimental evolution studies where individuals were kept under conditions with more or less sperm competition over many generations, leading to the expected changes in relative testis size and paternity success [17–20]. However, this approach does not disentangle effects of potentially correlated changes in other traits.
Moreover, there is considerable evidence to suggest that costs associated with sperm production are substantial [21–24], and theory and empirical studies suggest that, to lower the risk of sperm depletion, individuals should be economical about their sperm allocation and, when possible, conditionally adjust it depending on factors such as sperm competition level and the mate's quality and mating history (reviewed in [11,25]). Furthermore, individuals may be expected to conditionally adjust their copulation frequency depending on the amount of all ejaculate components they have available at a given time [26] (in this study, we focus on sperm). Studying the direct link between the amount of own sperm and the resulting adjustment of mating behaviour has, however, been challenging so far, since manipulation of sperm reserves has generally been achieved by manipulating the mating history [27–29], potentially affecting other traits and possibly confounding the effect of the own sperm availability.
The scarcity of studies directly manipulating sperm production is probably owing to animal testes usually being internal and thus difficult to assess, let alone manipulate in vivo (but see [30]). However, manipulating phenotypic traits and examining the link between their values and their fitness effects is a powerful approach in evolutionary biology, and has yielded capital insights into the function of a broad range of different traits [31–34]. Here, we have established a method to experimentally and quantitatively manipulate sperm-production rate in the free-living flatworm Macrostomum lignano, and used it to test two predictions from sperm competition theory: that copulation frequency may be adjusted based on the availability of ejaculate components [26] (i.e. sperm in this study), and that paternity success should scale positively with sperm production [3,4].
Macrostomum lignano is an excellent model in developmental [35,36] and evolutionary biology [37,38] that permits powerful experimental approaches to study reproduction. For example, its transparency allows measuring sperm-production rate in vivo [39] and molecular genetic tools allow manipulating organ-specific gene expression [40,41]. Moreover, worms can strategically adjust copulation frequency based on the quality of mating partners, copulating more frequently with well-fed partners [42]. Here, we experimentally manipulated sperm-production rate using a novel dose-dependent RNA interference (RNAi) gene knock-down approach. Specifically, we modulated the expression of macbol1, an essential gene for spermatogenesis in M. lignano [35], and were able to show that double-stranded RNA (dsRNA) suppressed sperm-production rate in a dose-dependent manner, leading to different amounts of sperm ready to be donated. Moreover, we found that copulation frequency was associated with sperm-production rate, suggesting conditional adjustment of the mating behaviour. Using microsatellite paternity analysis, we could then show that sperm-production rate and copulation frequency affected paternity success. To our knowledge, this is the first study to use quantitative phenotypic engineering to study the link between sperm-production rate and both mating behaviour and paternity success.
2. Material and methods
(a). Study organism
Macrostomum lignano (Macrostomorpha, Platyhelminthes) is an outcrossing simultaneous hermaphrodite [37] from the interstitial sand fauna of the Northern Adriatic [43]. It is transparent, allowing non-invasive measurements of body, testis, ovary and seminal vesicle size [37]. Copulation is reciprocal, with mutual insertion of the copulatory stylets into the partner's female gonopore [44]. In the laboratory, worms are kept in Guillard's f/2 medium [45] in glass Petri dishes and fed with the diatom Nitzschia curvilineata, at 20°C on a 14 : 10 light/dark cycle [46].
(b). Experiment's rationale
To phenotypically engineer the sperm-production rate, we modulated the expression of the testis-specific macbol1 gene with a novel dose-dependent RNAi approach. macbol1, a boule gene belonging to the DAZ gene family [47,48], has testis-specific expression, and its complete RNAi knock-down results in arrested spermatogenesis, leading to an empty seminal vesicle and male sterility [35]. Varying the dsRNA dose produces intermediate macbol1 RNAi phenotypes, allowing us to engineer a broad range of sperm-production rates (see §2d). To quantify these different rates, we examined changes in seminal vesicle size, which reflects the amount of sperm contained in it and changes in response to different sperm-production rates [39]. As germ cells that do not complete spermatogenesis do not reach the seminal vesicle [35,40], changes in seminal vesicle size caused by the macbol1 RNAi treatment can be attributed to differences in the amount of fully differentiated sperm produced, thus providing a proxy for sperm-production rate (i.e. a higher sperm-production rate leads to a larger increase in seminal vesicle size; see §2e). Finally, to test the consequences of this experimentally induced variation in sperm-production rate, we performed sperm competition experiments, using competitors as first sperm donors and focals as second sperm donors, always facing sperm competition to sire the eggs of recipients (see §2c,f). In M. lignano, second male paternity success (P2) has a mean P2-value of 0.64 (P. Sandner, D. B. Vizoso, T. Janicke & L. Schärer 2010, unpublished data). Paternity was assessed with a microsatellite marker (see §2g). The experiment's timeline is shown in the electronic supplementary material (figure S1).
(c). Experimental animals
To minimize undesired genetic variation, we used fixed genotypes for the focals, recipients and competitors, using our established inbred lines (DV lines, with over 40 generations of inbreeding; D. B. Vizoso 2010, unpublished data). Recipients were the offspring of DV13 fathers and DV8 mothers (crossed on day 1) and were individually distributed on day 15 into 24-well plates (Techno Plastic Products, Switzerland; electronic supplementary material, figure S1). Focals had DV71 fathers and DV28 mothers (crossed on day 5) and were individually distributed on day 12 (1 day old) into 60-well microtest plates (Greiner Bio-One, Germany), and immediately submitted to the RNAi treatment. Competitors were pure DV69 worms (the intended cross between DV69 and DV3 was unsuccessful), collected on day 24 as adults from a mass culture and thereafter kept individually until they were paired with the recipients. While line DV69 may have been a weak competitor owing to inbreeding, these worms were clearly able to sire viable offspring in the recipients (see §3).
(d). RNAi treatment
We used seven doses for the RNAi treatment. The control dose (D1) did not contain any dsRNA and was expected to produce a large seminal vesicle filled with sperm. In the highest dose (D7), the dsRNA concentration was 4.85 ng µl−1, and was expected to produce an empty and small seminal vesicle [35]. The intermediate five doses were made in a 2.3× dilution series (i.e. D6, 2.11 ng µl−1; D5, 0.917 ng µl−1; D4, 0.399 ng µl−1; D3, 0.173 ng µl−1; D2, 0.0754 ng µl−1) and were expected to produce intermediate seminal vesicle sizes. The dsRNA probe was synthesized in vitro as previously described [35] (see also ‘macbol1 dsRNA synthesis’ in the electronic supplementary material).
During RNAi treatment, focals were kept individually in 60-well microtest plates (their positions spatially balanced for dose) in 10 µl of dsRNA solution (in f/2 with 50 µg ml−1 of kanamycin and ampicillin [41]) with ad libitum diatoms, and transferred daily into a new well with fresh dsRNA solution and diatoms. The initial sample size was n = 168 (24 replicates for each of the seven dsRNA doses).
(e). Morphological measurements
On days 22, 25 and 28 (i.e. when the focals were 11, 14 and 17 days old, respectively), we measured the focals' seminal vesicle size to confirm successful manipulation of the sperm-production rate. Having grown up in isolation, seminal vesicle size should reflect the complete sperm production of these worms up to that point. We also measured body, testis and ovary size, which might be important factors for paternity success. The measurements were performed as previously described [37]. Briefly, we took images of worms relaxed with MgCl2 and squeezed dorsoventrally using a Leica DM 2500 microscope (Leica Microsystems, Germany), a digital video camera (DFK 41BF02, The Imaging Source, Germany) and the software BTV Pro v. 6.0b1 (http://www.bensoftware.com). Body size was photographed at 40× magnification, and testis, ovary and seminal vesicle size at 400× magnification. We measured the area of the traits using ImageJ v. 1.37v (http://rsb.info.nih.gov/ij). For testis and ovary size, we used the sum of both left and right testes (or ovaries). We visually scored the seminal vesicle's fill grade (see §2h).
(f). Sperm competition experiment
On days 29 and 30 (i.e. focals were 18 or 19 days old, recipients 22 or 23 days old and competitors two months old), we performed the sperm competition experiment. Copulations were set up and filmed in mating chambers as previously described [44], pairing worms in 4 µl of f/2 medium in mating chambers. Each chamber contained 14 drops, spatially balanced for dose. Recipients were first paired with a competitor for 2 h, immediately recovered, and (within 25 minutes) then paired with a focal for an additional 2 h. Recipients had been colourized in f/2 medium with a red food colourant (6.25 mg ml−1, E124, Werner Schweizer AG, Switzerland) for 12 h prior to mating, to visually distinguish them. We filmed the mating chambers using digital video cameras (DFK 31BF03, The Imaging Source, Germany or DFW-X700, Sony, Japan) and the software SecuritySpy v. 2.0.3 (http://www.securityspy.com), producing time-lapse movies of 1 frame per second. We scored the copulation frequency and the copulation duration by visual frame-by-frame analysis [44].
(g). Paternity analysis
After the sperm competition experiment, the recipients were kept isolated in 24-well plates. We fixated the recipients' offspring when about 7 days old (day 40 onwards) in 75 per cent ethanol and stored them at −20°C until genotyping. After evaporating the ethanol, we extracted DNA by adding MgCl2-free PCR buffer containing 0.5 µg µl−1 of Proteinase K (Sigma Aldrich, USA), breaking up tissue by freezing at −80°C for 1 h, digesting at 50°C for 1 h and inactivating the Proteinase K at 95°C for 15 min. Using this extraction, we amplified the microsatellite locus Macro21 by PCR with a fluorescent conjugated forward primer 5′-TTC ATC AAC ATC AGC CTT ATC C-3′ and a reverse primer 5′-CTG CTG CTG AGG TGT TTG G-3′. The PCR conditions were 15 min at 95°C, 35 cycles of 30 s at 94°C, 90 s 53°C and 60 s at 72°C, and 30 min at 60°C. We performed genotyping using an AB3130xl Genetic Analyzer (Applied Biosystems, USA) and the software Genemapper v. 4.0 (Applied Biosystems). Competitors and recipients were chosen to be monoallelic at the Macro21 locus (allele size 90 bp), while focals never carried that allele (they had alleles of 87 bp and/or 97 bp). Therefore, paternity of offspring carrying alleles other than the 90 bp allele could be unequivocally assigned to the focal (assuming no genotyping errors or mutations).
(h). Statistical analyses
We tested if the dsRNA doses successfully manipulated sperm-production rate, as indicated by changes in seminal vesicle size from the second to the third measurement, using a repeated-measures ANOVA with seminal vesicle size as response variable and dsRNA dose as fixed factor. We excluded the first measurement, because about 20 per cent of the worms were still immature and lacked mature copulatory stylets. When the seminal vesicle was absent its size was considered zero (two of 147 individuals in the second measurement). Generally, empty seminal vesicles are very small, but their size is not zero. Therefore, we also assessed the seminal vesicles' fill grade at the third measurement, blind with respect to the treatment group. We call it ‘full’ if both compartments (the true and the false seminal vesicle [43]; electronic supplementary material, figure S2) were densely packed with sperm, ‘fairly full’ if individual sperm were distinguishable in both, ‘half full’ if only the true seminal vesicle contained ample sperm, ‘fairly empty’ if only a few sperm were seen and ‘empty’ if both were empty. We tested whether dsRNA dose affected the fill grade using Pearson's χ2-test.
We examined whether dsRNA dose affected the amount of sperm that the worms had immediately before the sperm competition experiment, as indicated by seminal vesicle size at the time of the third measurement, as well as other morphological traits, such as body, testis and ovary size. Moreover, we tested if dsRNA dose also affected mating behaviour, performing linear regression analyses of the focals' copulation frequency and duration onto the dsRNA dose (as a continuous variable), which was the loge-transformed dsRNA concentration. As the control's concentration (D1) was 0 ng µl−1, we added 0.1 to all the concentrations before loge-transformation. In this analysis, seminal vesicle size was also loge-transformed to improve the distribution of the data. Note that the previous analysis was used to confirm that the RNAi treatment worked as intended, by testing whether the increase of seminal vesicle size (our estimate of sperm-production rate) is different between dsRNA dose treatments, while the analysis conducted here describes how the final phenotype of the focals in the sperm competition experiment was affected as a function of dsRNA dose. We then include those phenotypes as predictors in subsequent analysis to examine their effects on paternity success.
We examined if the focals' copulation frequency correlated with seminal vesicle size, which would suggest that copulation frequency may be conditionally adjusted as sperm production changes. As we paired the same recipients with both the competitors and the focals, we also examined the correlation between the copulation frequencies of focals and competitors, as they are potentially dependent (although each copulation trial occured independently with no direct interaction between focals and competitors).
Finally, we examined the effect of the predictor traits on paternity success. Here, we fitted generalized linear models (GLMs) using a logit-link function. Logistic regression-based GLMs are the standard statistical tool for handling proportional data such as paternity success [49,50], especially if the data include many zeroes and ones, as is the case here. We used quasi-GLMs to correct for overdispersion [50,51]. We handled the number of offspring sired by the focals and the competitors as a two-vector response to take into account the accuracy with which paternity success could be estimated. For model selection, we first fitted a full model, including all morphological or behavioural traits of the focals that were affected by dsRNA dose (see §3). In addition, we also included information on the copulation frequency of the competitors, which could potentially have influenced the focals' paternity success. As we found a significant negative correlation between the copulation frequency of the focals and the competitors (see §3), we here used the difference and the sum of these two variables in the analysis (called delta copulation frequency and total copulation frequency, respectively), which are uncorrelated. In summary, the full model contained the seminal vesicle size (at the third measurement), the delta copulation frequency and the total copulation frequency (see full model in table 1). Then, by stepwise deletion of non-significant parameters, we selected a reduced model containing only the parameters with significant effects on paternity success. To statistically test the effects of these terms in the reduced model, we performed an analysis of deviance with F-statistics, as recommended when using a quasi-GLM [51,52].
Table 1.
Model selection of the effects on paternity success of the predictor traits seminal vesicle size, delta copulation frequency and total copulation frequency.
| source | estimate | s.e. | d.f. | F-value | p-value |
|---|---|---|---|---|---|
| full model | |||||
| seminal vesicle size | 4.60 × 10–4 | 9.91 × 10–5 | 1, 63 | 50.89 | <0.001 |
| delta copulation frequency | 0.0369 | 0.0174 | 1, 62 | 4.88 | 0.031 |
| total copulation frequency | −0.0014 | 0.0206 | 1, 61 | 0.0046 | 0.946 |
| reduced model | |||||
| seminal vesicle size | 4.58 × 10–4 | 9.67 × 10–5 | 1, 63 | 51.67 | <0.001 |
| delta copulation frequency | 0.0371 | 0.0171 | 1, 62 | 4.95 | 0.030 |
Initial sample size was n = 168 (24 replicates for each of the seven dsRNA doses), but 21 worms (balanced over the treatment groups) were excluded because they did not mature or died during the experiment. The effect of dsRNA doses on sperm-production rate and seminal vesicle's fill grade was thus tested on 147 individuals. A further 82 replicates were excluded from the other tests (four were lost during experiment, in 61 either the focal or its competitor did not copulate and in 17 the recipients produced none or only one offspring) for a final sample size of 65 (D1, n = 8; D2, n = 11; D3, n = 10; D4, n = 12; D5, n = 8; D6, n = 9; D7, n = 7).
We performed the statistical analyses using JMP v. 8.0.2 (SAS Institute, USA) and R v. 2.10.1 (R Development Core Team, Austria). The data of this study are available in the electronic supplementary material.
3. Results
At the highest dose, macbol1 RNAi generally produced empty seminal vesicles (see the electronic supplementary material, figure S2), as expected [35]. Sperm-production rate was strongly affected by dsRNA dose, as indicated by the significant treatment and time effects on seminal vesicle size between the second and third measurement (figure 1a; repeated-measures ANOVA: treatment, F6,140 = 31.41, p < 0.0001; time, F1,140 = 24.05, p < 0.0001; time × treatment, F6,140 = 11.41, p < 0.0001). Specifically, seminal vesicles were significantly smaller at higher dsRNA doses and generally grew over time owing to the accumulation of produced sperm, but this increase occured only at the lower dsRNA doses, as indicated by the significant interaction term. The seminal vesicle's fill grade was also affected by dsRNA dose (figure 1b), with worms from higher doses having emptier seminal vesicles more often (Pearson's χ2-test: χ2 = 99.47, d.f. = 24, p < 0.0001).
Figure 1.
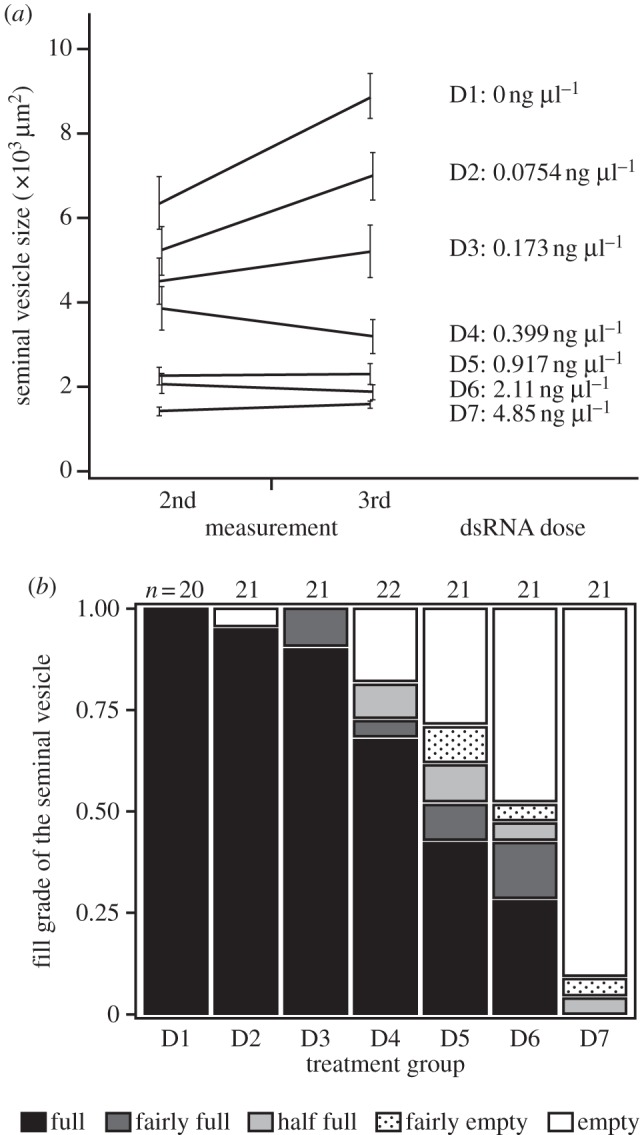
Effect of the dsRNA dose on sperm production. (a) Change in seminal vesicle size from the second to the third measurement. Seminal vesicle size at lower dsRNA doses (ng µl−1) increased quicker than at higher doses. Error bars indicate s.e. (b) The seminal vesicle's fill grade at the third measurement. At the lowest dsRNA dose, seminal vesicles were filled with sperm and gradually became emptier at higher doses. n = 147.
The dsRNA dose significantly affected seminal vesicle size at the third measurement (figure 2a), presumably as a result of a reduced sperm-production rate at higher dsRNA doses (figure 1a). The dsRNA dose did not significantly affect body, testis or ovary size (linear regressions: body size, slope = −1221.1, t = −0.17, p = 0.87, R2 < 0.01; testis size, slope = 763.2, t = 1.94, p = 0.057, R2 = 0.06; ovary size, slope = −100.2, t = −0.35, p = 0.73, R2 < 0.01). The dsRNA dose also did not affect the focals' average copulation duration (linear regression: slope = 0.13, t = 0.52, p = 0.60, R2 < 0.01), but did significantly affect the focals' copulation frequency (figure 2b). This leads to a significant positive correlation between seminal vesicle size and copulation frequency (figure 3), although this correlation only explained 16 per cent of the variation. The focals' copulation frequency was also negatively correlated with the competitors' copulation frequency (Pearson's correlation: r = −0.27, t = −2.20, p < 0.001; electronic supplementary material, figure S3).
Figure 2.
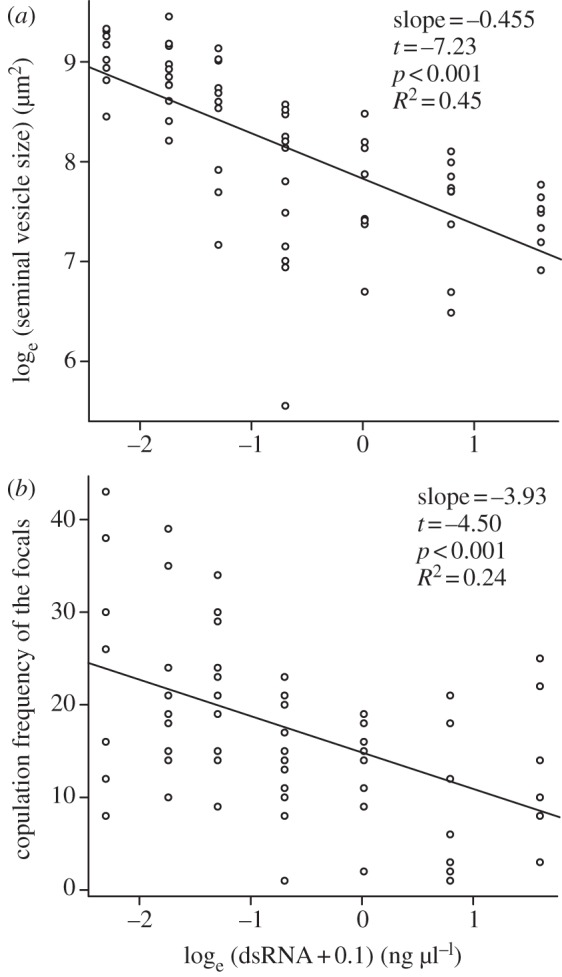
Effects of the dsRNA dose on final seminal vesicle size and copulation frequency. (a) The focals' seminal vesicle size at the third measurement. (b) The focals' copulation frequency during the sperm competition experiment. n = 65.
Figure 3.
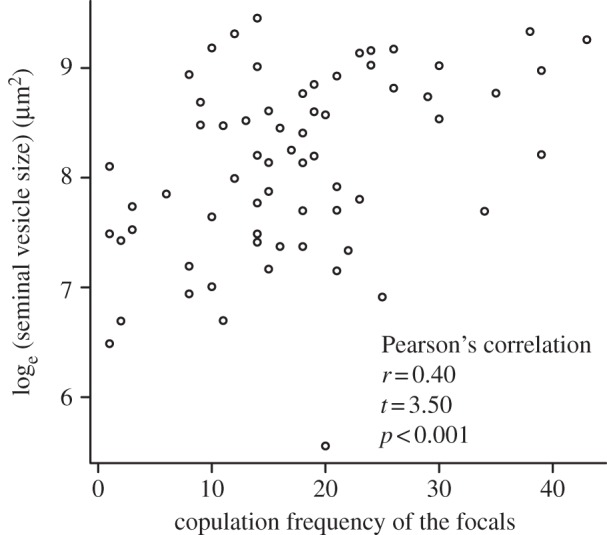
Correlation between the focals' copulation frequency and seminal vesicle size. n = 65.
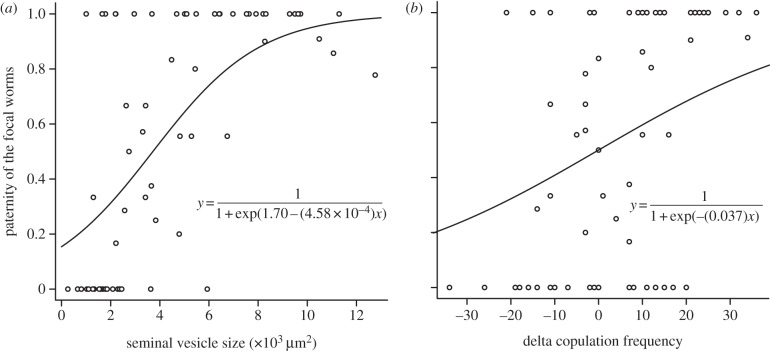
The primary predictors for paternity success identified by the reduced model were seminal vesicle size and delta copulation frequency, both of which had a positive relationship with paternity success (see reduced model in table 1 and figure 4).
Figure 4.
Effects of the phenotypic predictors on paternity success. (a) Effect of seminal vesicle size on paternity success. (b) Effect of delta copulation frequency on paternity success. Coefficients used for each fitted curve are derived from the reduced model presented in table 1. n = 65.
4. Discussion
(a). Phenotypic engineering of sperm-production rate
Our novel experimental approach, a dose-dependent RNAi gene knock-down, succeeded in producing considerable variation in sperm-production rate, as reflected by different rates of increase in seminal vesicle size over time at different dsRNA doses. Moreover, our approach allowed us to manipulate the number of sperm available to sperm donors (estimated as seminal vesicle size at the third measurement). The obtained broad variation may be difficult to achieve with approaches such as artificial selection [53,54], experimental evolution [17–19] or phenotypic plasticity [37,39,55] (reviewed in [56]).
The RNAi treatment could potentially have affected traits other than the targeted sperm-production rate. While our data suggest that none of the other morphological traits was significantly affected by the RNAi treatment, testis size in fact tended to be larger at higher doses. As the macbol1 gene acts during meiosis, the pre-meiotic proliferation of the male germ line seemed not to be halted by the RNAi treatment, leading to some accumulation of malformed sperm in the testis, presumably because immature or malformed sperm are not transferred to the seminal vesicle [35]. One might have expected that knocking down a testicular gene would lead to smaller testes, which we clearly did not observe here. To achieve this, earlier-acting testicular genes should be targeted. We cannot exclude the possibility that macbol1 RNAi affected other unmeasured traits of sperm competitiveness such as sperm morphology, mobility or viability. It appears, however, that fertilization ability was not strongly affected, as offspring were successfully sired by focals of each treatment group, including the highest dsRNA dose.
In summary, we show that phenotypic engineering via dose-dependent RNAi allows us to quantitatively manipulate a specific trait—sperm-production rate—and thus to disentangle its fitness effects from those of other traits. Dose-dependent RNAi may therefore be a powerful approach to study not only the consequences of variation in sperm production, but also those of other traits that may otherwise be difficult to manipulate quantitatively [57]. One potentially interesting application would be experimental tests of sex allocation theory. In sex allocation theory, how much fitness is obtained for a given level of resource investment into male and female function is important to predict an individual's optimal sex allocation. In simultaneous hermaphrodites, the fitness returns for increased male allocation are predicted to saturate under many conditions [56,58,59]. However, experimental evidence in animals is currently scarce because quantitative manipulation of sex allocation has been difficult to achieve [56]. While dose-dependent RNAi of macbol1 does not allow one to manipulate sex allocation directly (as ovary size was unaffected by the manipulation), we argue that it does manipulate the most important outcome of a change in male allocation: sperm-production rate (but see also [60]). This approach could therefore allow us to describe the shape of the male fitness gain curve under different conditions by mimicking the outcome of varied male allocation.
(b). Conditional adjustment of mating behaviour
The dsRNA dose significantly affected copulation frequency, with focals subjected to lower doses (and thus having larger seminal vesicles) copulating more frequently than those from higher doses. Considering that the macbol1 gene shows male-gamete-specific expression and acts during male meiosis [35], it seems unlikely that its decreased gene expression would directly affect mating behaviour. The observed changes in copulation frequency are therefore more likely to be a consequence of the change in sperm production, suggesting a conditional adjustment of mating behaviour based on sperm availability (possibly perceived as a differentially filled seminal vesicle). Unexpectedly, we also found a significant negative correlation between the focals' and the competitors' copulation frequency. One possible explanation for this is the influence of previous mating history on mating strategy within the recipients. That is, the more the recipients copulated with the competitors, the lower their mating motivation may have been when copulating with the focals, which would match recent findings about differences in mating motivation between virgin and sexually experienced worms [61].
Although we found a positive effect on paternity success of the focal copulating more frequently than the competitor (i.e. the effect of delta copulation frequency), a conditional reduction of copulation frequency may still be beneficial, especially when the amount of available sperm is limited. For example, by reducing copulation frequency, individuals may save and/or replenish sperm for novel and/or better mates. A decline in sexual motivation towards a familiar mating partner, or Coolidge effect [62,63], has been widely reported in animals [64,65], including the simultaneously hermaphroditic pond snail [66]. In our study, the focals were allowed to copulate only with one recipient. It might be possible that copulation frequency was more severely reduced in focals with limited sperm availability, because inseminating the same partner would only provide diminishing marginal fitness returns, and thus be costlier for such worms. It would be intriguing to examine if adding new mates would restore decreased copulation frequencies in a sperm-availability-dependent manner. A change in copulation frequency could in theory be caused by differential sperm-production rates (i.e. rate of sperm replenishment) and/or differential amounts of sperm stored in the seminal vesicle, but we can not distinguish these two possibilities in this study since both factors were manipulated non-independently.
(c). Paternity success increases with sperm-production rate
Our results suggest that sperm-production rate, as well as delta copulation frequency, had a positive relationship with paternity success, as predicted by sperm competition theory. Sperm competition is a common phenomenon and an important aspect of post-copulatory sexual selection [2,3]. Although many comparative studies have suggested that increased sperm competition favours an increased expenditure on sperm production, as assessed by testis size [5–10], only a few studies have tested the effect of sperm-production rate on paternity success within a single species. For example, testicular circumference of Soay sheep [12] and external testis length of yellow-pine chipmunk [13], both proxies for testis size, positively correlate with paternity success. However, these results may need to be interpreted with some caution, because the testes of mammals also produce hormones that can affect other traits, such as behaviour or ornaments [67]. Moreover, very few studies have manipulated sperm production experimentally to look at its effect on sperm competitiveness. In some studies, gonad size was manipulated indirectly via experimental evolution regimes (e.g. monogamous and polygamous selection [17,19]) or genetic manipulation [68], with mixed results. Male yellow dung flies from polygamous lines had larger testes and sired more offspring than males from monogamous lines [17], while the smaller testes of monogamously selected male fruit flies did not lead to a consistent reduction in competitiveness [19]. However, experimental evolution can potentially affect other traits influencing paternity success, which would confound the actual fitness effects of the trait in question. In our study, we directly manipulated one particular gene involved in spermatogenesis in individuals that otherwise had an identical genetic background, which has been suggested as a powerful way of studying traits [57]. Moreover, our approach left the decision on sperm allocation to the focals, as opposed to sperm number manipulations prior to artificial fertilization [69]. Our results thus represent some of the most direct evidence that higher sperm production is indeed beneficial for sperm competitiveness and paternity success in copulating animals. Our technique offers considerable promise to test more quantitative aspects of sperm competition theory.
As a corollary, we found that, even when taking the effect of seminal vesicle size into account, the delta copulation frequency also had a positive effect on paternity success. This suggests that sperm displacement occurs during copulation by physically removing some sperm from the recipient in each mating, gradually reducing the proportion of rival sperm. Sperm displacement is certainly possible given the anatomy of this species, because previously received sperm in the female storage organ is within reach of the copulatory stylet [70]. Recently established GFP-positive transgenic lines in M. lignano [71] may in future allow us to examine such a possibility by combining it with RNAi, and by observing the dynamics of sperm transfer and removal during sperm competition.
5. Conclusions
We successfully manipulated sperm-production rate using dose-dependent RNAi and provide clear experimental evidence for a key prediction of sperm competition theory that higher sperm-production rate is beneficial for paternity success. Moreover, our study indicates that, although copulation frequency is an important factor for gaining paternity success, it may be adjusted based on the availability of own sperm. Our study shows that phenotypic engineering of sperm-production rate is a powerful tool for detailed quantitative experimental studies on predictions of evolutionary theory.
Acknowledgements
We thank two anonymous reviewers for their comments and suggestions, Tim Janicke, Peter Sandner, Lucas Marie-Orleach, Steve Ramm, Tommaso Pizzari, Pau Carazo, Grant McDonald, Cedric Tan, Aitor Alvarez Fernández, Eleanor Bath and Jen Perry for fruitful discussions, Matthew Hall, David Duneau and Patrice David for statistical help, Nadia Riebli and Tobias Suter for help with a pilot project on the dose-dependent RNAi, Karen Haag and Jean-Claude Walser for advice on molecular work, and Jürgen Hottinger, Brigitte Aeschenbach, Urs Stiefel, Victor Mislin and Lukas Zimmerman for laboratory and IT support. This study was funded by grants from the Swiss National Science Foundation to L.S. (grant nos. 3100A0–113708 and 3100A0–127503), a Swiss Government Scholarship for Foreign Students to K.S. and Austrian Science Fund (FWF) to P.L. (grant no. 18099).
References
- 1.Parker GA. 1970. Sperm competition and its evolutionary consequences in the insects. Biol. Rev. 45, 525–567 10.1111/j.1469-185X.1970.tb01176.x (doi:10.1111/j.1469-185X.1970.tb01176.x) [DOI] [Google Scholar]
- 2.Birkhead TR, Pizzari T. 2002. Postcopulatory sexual selection. Nat. Rev. Genet. 3, 262–273 10.1038/nrg774 (doi:10.1038/nrg774) [DOI] [PubMed] [Google Scholar]
- 3.Parker GA. 1998. Sperm competition and the evolution of ejaculates: towards a theory base. In Sperm competition and sexual selection (eds Birkhead TR, Møller AP.), pp. 3–54 London, UK: Academic Press [Google Scholar]
- 4.Parker GA. 1990. Sperm competition games: raffles and roles. Proc. R. Soc. Lond. B 242, 120–126 10.1098/rspb.1990.0114 (doi:10.1098/rspb.1990.0114) [DOI] [Google Scholar]
- 5.Stockley P, Gage MJG, Parker GA, Møller AP. 1997. Sperm competition in fishes: the evolution of testis size and ejaculate characteristics. Am. Nat. 149, 933–954 10.1086/286031 (doi:10.1086/286031) [DOI] [PubMed] [Google Scholar]
- 6.Pitcher TE, Dunn PO, Whittingham LA. 2005. Sperm competition and the evolution of testes size in birds. J. Evol. Biol. 18, 557–567 10.1111/j.1420-9101.2004.00874.x (doi:10.1111/j.1420-9101.2004.00874.x) [DOI] [PubMed] [Google Scholar]
- 7.Møller AP, Briskie JV. 1995. Extra-pair paternity, sperm competition and the evolution of testis size in birds. Behav. Ecol. Sociobiol. 36, 357–365 10.1007/BF00167797 (doi:10.1007/BF00167797) [DOI] [Google Scholar]
- 8.Møller AP. 1991. Sperm competition, sperm depletion, paternal care, and relative testis size in birds. Am. Nat. 137, 882–906 10.1086/285199 (doi:10.1086/285199) [DOI] [Google Scholar]
- 9.Harcourt AH, Harvey PH, Larson SG, Short RV. 1981. Testis weight, body-weight and breeding system in primates. Nature 293, 55–57 10.1038/293055a0 (doi:10.1038/293055a0) [DOI] [PubMed] [Google Scholar]
- 10.Ginsberg JR, Rubenstein DI. 1990. Sperm competition and variation in zebra mating-behavior. Behav. Ecol. Sociobiol. 26, 427–434 10.1007/BF00170901 (doi:10.1007/BF00170901) [DOI] [Google Scholar]
- 11.Parker GA, Pizzari T. 2010. Sperm competition and ejaculate economics. Biol. Rev. 85, 897–934 [DOI] [PubMed] [Google Scholar]
- 12.Preston BT, Stevenson IR, Pemberton JM, Coltman DW, Wilson K. 2003. Overt and covert competition in a promiscuous mammal: the importance of weaponry and testes size to male reproductive success. Proc. R. Soc. Lond. B 270, 633–640 10.1098/rspb.2002.2268 (doi:10.1098/rspb.2002.2268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulte-Hostedde AI, Millar JS. 2004. Intraspecific variation of testis size and sperm length in the yellow-pine chipmunk (Tamias amoenus): implications for sperm competition and reproductive success. Behav. Ecol. Sociobiol. 55, 272–277 10.1007/s00265-003-0707-z (doi:10.1007/s00265-003-0707-z) [DOI] [Google Scholar]
- 14.Janicke T, Schärer L. 2009. Determinants of mating and sperm-transfer success in a simultaneous hermaphrodite. J. Evol. Biol. 22, 405–415 10.1111/j.1420-9101.2008.01660.x (doi:10.1111/j.1420-9101.2008.01660.x) [DOI] [PubMed] [Google Scholar]
- 15.Bercovitch FB, Nurnberg P. 1996. Socioendocrine and morphological correlates of paternity in rhesus macaques (Macaca mulatta). J. Reprod. Fertil. 107, 59–68 10.1530/jrf.0.1070059 (doi:10.1530/jrf.0.1070059) [DOI] [PubMed] [Google Scholar]
- 16.Awata S, Heg D, Munehara H, Kohda M. 2006. Testis size depends on social status and the presence of male helpers in the cooperatively breeding cichlid Julidochromis ornatus. Behav. Ecol. 17, 372–379 10.1093/beheco/arj043 (doi:10.1093/beheco/arj043) [DOI] [Google Scholar]
- 17.Hosken DJ, Garner TWJ, Ward PI. 2001. Sexual conflict selects for male and female reproductive characters. Curr. Biol. 11, 489–493 10.1016/S0960-9822(01)00146-4 (doi:10.1016/S0960-9822(01)00146-4) [DOI] [PubMed] [Google Scholar]
- 18.Hosken DJ, Ward PI. 2001. Experimental evidence for testes size evolution via sperm competition. Ecol. Lett. 4, 10–13 10.1046/j.1461-0248.2001.00198.x (doi:10.1046/j.1461-0248.2001.00198.x) [DOI] [Google Scholar]
- 19.Pitnick S, Miller GT, Reagan J, Holland B. 2001. Males’ evolutionary responses to experimental removal of sexual selection. Proc. R. Soc. Lond. B 268, 1071–1080 10.1098/rspb.2001.1621 (doi:10.1098/rspb.2001.1621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simmons LW, Garcia-Gonzalez F. 2008. Evolutionary reduction in testes size and competitive fertilization success in response to the experimental removal of sexual selection in dung beetles. Evol. Int. J. Organ. Evol. 62, 2580–2591 10.1111/j.1558-5646.2008.00479.x (doi:10.1111/j.1558-5646.2008.00479.x) [DOI] [PubMed] [Google Scholar]
- 21.Dewsbury DA. 1982. Ejaculate cost and male choice. Am. Nat. 119, 601–610 10.1086/283938 (doi:10.1086/283938) [DOI] [Google Scholar]
- 22.Nakatsuru K, Kramer DL. 1982. Is sperm cheap? Limited male-fertility and female choice in the lemon tetra (Pisces, Characidae). Science 216, 753–755 10.1126/science.216.4547.753 (doi:10.1126/science.216.4547.753) [DOI] [PubMed] [Google Scholar]
- 23.Van Voorhies WA. 1992. Production of sperm reduces nematode life-span. Nature 360, 456–458 10.1038/360456a0 (doi:10.1038/360456a0) [DOI] [PubMed] [Google Scholar]
- 24.Olsson M, Madsen T, Shine R. 1997. Is sperm really so cheap? Costs of reproduction in male adders, Vipera berus. Proc. R. Soc. Lond. B 264, 455–459 10.1098/rspb.1997.0065 (doi:10.1098/rspb.1997.0065) [DOI] [Google Scholar]
- 25.Wedell N, Gage MJG, Parker GA. 2002. Sperm competition, male prudence and sperm-limited females. Trends Ecol. Evol. 17, 313–320 10.1016/S0169-5347(02)02533-8 (doi:10.1016/S0169-5347(02)02533-8) [DOI] [Google Scholar]
- 26.Reinhardt K, Naylor R, Siva-Jothy MT. 2011. Male mating rate is constrained by seminal fluid availability in bedbugs, Cimex lectularius. PLoS ONE 6, e22082. 10.1371/journal.pone.0022082 (doi:10.1371/journal.pone.0022082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemaître JF, Rigaud T, Cornet S, Bollache L. 2009. Sperm depletion, male mating behaviour and reproductive ‘time-out’ in Gammarus pulex (Crustacea, Amphipoda). Anim. Behav. 77, 49–54 10.1016/j.anbehav.2008.08.028 (doi:10.1016/j.anbehav.2008.08.028) [DOI] [Google Scholar]
- 28.Van Son TC, Thiel M. 2006. Mating behaviour of male rock shrimp, Rhynchocinetes typus (Decapoda : Caridea): effect of recent mating history and predation risk. Anim. Behav. 71, 61–70 10.1016/j.anbehav.2005.03.018 (doi:10.1016/j.anbehav.2005.03.018) [DOI] [Google Scholar]
- 29.Hettyey A, Vági B, Hévizi G, Török J. 2009. Changes in sperm stores, ejaculate size, fertilization success, and sexual motivation over repeated matings in the common toad, Bufo bufo (Anura: Bufonidae). Biol. J. Linnean Soc. 96, 361–371 10.1111/j.1095-8312.2008.01126.x (doi:10.1111/j.1095-8312.2008.01126.x) [DOI] [Google Scholar]
- 30.Hodgkin J, Barnes TM. 1991. More is not better: brood size and population-growth in a self-fertilizing nematode. Proc. R. Soc. Lond. B 246, 19–24 10.1098/rspb.1991.0119 (doi:10.1098/rspb.1991.0119) [DOI] [PubMed] [Google Scholar]
- 31.Andersson M. 1982. Female choice selects for extreme tail length in a widowbird. Nature 299, 818–820 10.1038/299818a0 (doi:10.1038/299818a0) [DOI] [Google Scholar]
- 32.Møller AP. 1988. Female choice selects for male sexual tail ornaments in the monogamous swallow. Nature 332, 640–642 10.1038/332640a0 (doi:10.1038/332640a0) [DOI] [Google Scholar]
- 33.Polak M, Rashed A. 2010. Microscale laser surgery reveals adaptive function of male intromittent genitalia. Proc. R. Soc. B 277, 1371–1376 10.1098/rspb.2009.1720 (doi:10.1098/rspb.2009.1720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maklakov AA, Arnqvist G. 2009. Testing for direct and indirect effects of mate choice by manipulating female choosiness. Curr. Biol. 19, 1903–1906 10.1016/j.cub.2009.08.058 (doi:10.1016/j.cub.2009.08.058) [DOI] [PubMed] [Google Scholar]
- 35.Kuales G, De Mulder K, Glashauser J, Salvenmoser W, Takashima S, Hartenstein V, Berezikov E, Salzburger W, Ladurner P. 2011. Boule-like genes regulate male and female gametogenesis in the flatworm Macrostomum lignano. Dev. Biol. 357, 117–132 10.1016/j.ydbio.2011.06.030 (doi:10.1016/j.ydbio.2011.06.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ladurner P, Egger B, De Mulder K, Pfister D, Kuales G, Salvenmoser W, Schärer L. 2008. The stem cell system of the basal flatworm Macrostomum lignano. In Stem cells: from hydra to man (ed. Bosch TCG.), pp. 75–94 Berlin, Germany: Springer [Google Scholar]
- 37.Schärer L, Ladurner P. 2003. Phenotypically plastic adjustment of sex allocation in a simultaneous hermaphrodite. Proc. R. Soc. Lond. B 270, 935–941 10.1098/rspb.2002.2323 (doi:10.1098/rspb.2002.2323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schärer L, Janicke T. 2009. Sex allocation and sexual conflict in simultaneously hermaphroditic animals. Biol. Lett. 5, 705–708 10.1098/rsbl.2009.0100 (doi:10.1098/rsbl.2009.0100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schärer L, Vizoso DB. 2007. Phenotypic plasticity in sperm production rate: there's more to it than testis size. Evol. Ecol. 21, 295–306 10.1007/s10682-006-9101-4 (doi:10.1007/s10682-006-9101-4) [DOI] [Google Scholar]
- 40.Sekii K, Salvenmoser W, De Mulder K, Schärer L, Ladurner P. 2009. Melav2, an elav-like gene, is essential for spermatid differentiation in the flatworm Macrostomum lignano. BMC Dev. Biol. 9, 62. 10.1186/1471-213X-9-62 (doi:10.1186/1471-213X-9-62) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfister D, De Mulder K, Hartenstein V, Kuales G, Borgonie G, Marx F, Morris J, Ladurner P. 2008. Flatworm stem cells and the germ line: developmental and evolutionary implications of macvasa expression in Macrostomum lignano. Dev. Biol. 319, 146–159 10.1016/j.ydbio.2008.02.045 (doi:10.1016/j.ydbio.2008.02.045) [DOI] [PubMed] [Google Scholar]
- 42.Janicke T, Kesselring H, Schärer L. 2012. Strategic mating effort in a simultaneous hermaphrodite: the role of the partner's feeding status. Behav. Ecol. Sociobiol. 66, 593–601 10.1007/s00265-011-1307-y (doi:10.1007/s00265-011-1307-y) [DOI] [Google Scholar]
- 43.Ladurner P, Schärer L, Salvenmoser W, Rieger RM. 2005. A new model organism among the lower Bilateria and the use of digital microscopy in taxonomy of meiobenthic Platyhelminthes: Macrostomum lignano, n. sp. (Rhabditophora, Macrostomorpha). J. Zool. Syst. Evol. Res. 43, 114–126 10.1111/j.1439-0469.2005.00299.x (doi:10.1111/j.1439-0469.2005.00299.x) [DOI] [Google Scholar]
- 44.Schärer L, Joss G, Sandner P. 2004. Mating behaviour of the marine turbellarian Macrostomum sp.: these worms suck. Mar. Biol. 145, 373–380 10.1007/s00227-004-1314-x (doi:10.1007/s00227-004-1314-x) [DOI] [Google Scholar]
- 45.Andersen RA, Berges JA, Harrison PJ, Watanabe MM. 2005. Recipes for freshwater and seawater media. In Algal culturing techniques (ed. Andersen RA.), pp. 429–538 Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 46.Rieger RM, Gehlen M, Haszprunar G, Holmlund M, Legniti A, Salvenmoser W, Tyler S. 1988. Laboratory cultures of marine Macrostomida (Turbellaria). Fortschr. Zool. 36, 523 [Google Scholar]
- 47.Yen PH. 2004. Putative biological functions of the DAZ family. Int. J. Androl. 27, 125–129 10.1111/j.1365-2605.2004.00469.x (doi:10.1111/j.1365-2605.2004.00469.x) [DOI] [PubMed] [Google Scholar]
- 48.Shah C, VanGompel MJW, Naeem V, Chen YM, Lee T, Angeloni N, Wang Y, Xu EY. 2010. Widespread presence of human BOULE homologs among animals and conservation of their ancient reproductive function. Plos Genet. 6, e1001022. 10.1371/journal.pgen.1001022 (doi:10.1371/journal.pgen.1001022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krackow S, Tkadlec E. 2001. Analysis of brood sex ratios: implications of offspring clustering. Behav. Ecol. Sociobiol. 50, 293–301 10.1007/s002650100366 (doi:10.1007/s002650100366) [DOI] [Google Scholar]
- 50.Wilson K, Hardy ICW. 2002. Statistical analysis of sex ratios: an introduction. In Sex ratios (ed. Hardy ICW.), pp. 48–92 Cambridge, UK: Cambridge University Press [Google Scholar]
- 51.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with r (statistics for biology and health). Berlin, Germany: Springer [Google Scholar]
- 52.Crawley MJ. 2005. Statistics: an introduction using R. New York, NY: Wiley [Google Scholar]
- 53.Johnson RK, Eckardt GR, Rathje TA, Drudik DK. 1994. 10 generations of selection for predicted weight of testes in swine—direct response and correlated response in body-weight, backfat, age at puberty, and ovulation rate. J. Anim. Sci. 72, 1978–1988 [DOI] [PubMed] [Google Scholar]
- 54.Rathje TA, Johnson RK, Lunstra DD. 1995. Sperm production in boars after 9 generations of selection for increased weight of testis. J. Anim. Sci. 73, 2177–2185 [DOI] [PubMed] [Google Scholar]
- 55.Tan GN, Govedich FR, Burd M. 2004. Social group size, potential sperm competition and reproductive investment in a hermaphroditic leech, Helobdella papillornata (Euhirudinea: Glossiphoniidae). J. Evol. Biol. 17, 574–580 10.1111/j.1420-9101.2004.00692.x (doi:10.1111/j.1420-9101.2004.00692.x) [DOI] [PubMed] [Google Scholar]
- 56.Schärer L. 2009. Tests of sex allocation theory in simultaneously hermaphroditic animals. Evol. Int. J. Organ. Evol. 63, 1377–1405 10.1111/j.1558-5646.2009.00669.x (doi:10.1111/j.1558-5646.2009.00669.x) [DOI] [PubMed] [Google Scholar]
- 57.Tatar M. 2000. Transgenic organisms in evolutionary ecology. Trends Ecol. Evol. 15, 207–211 10.1016/S0169-5347(00)01834-6 (doi:10.1016/S0169-5347(00)01834-6) [DOI] [PubMed] [Google Scholar]
- 58.Charnov EL. 1982. The theory of sex allocation. Princeton, NJ: Princeton University Press [Google Scholar]
- 59.Petersen CW. 1991. Sex allocation in hermaphroditic seabasses. Am. Nat. 138, 650–667 10.1086/285240 (doi:10.1086/285240) [DOI] [Google Scholar]
- 60.Schärer L, Pen I. 2013. Sex allocation and investment into pre- and post-copulatory traits in simultaneous hermaphrodites: the role of polyandry and local sperm competition. Phil. Trans. R. Soc. B 368, 20120052. (doi:10.1098/rstb.2012.0052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marie-Orleach L, Janicke T, Schärer L. 2013. Effects of mating status on post-copulatory behaviour in a simultaneous hermaphrodite. Animal Behaviour 85, 453–461 [Google Scholar]
- 62.Dewsbury DA. 1981. Effects of novelty on copulatory behavior—the Coolidge effect and related phenomena. Psychol. Bull. 89, 464–482 10.1037/0033-2909.89.3.464 (doi:10.1037/0033-2909.89.3.464) [DOI] [Google Scholar]
- 63.Wilson JR, Kuehn RE, Beach FA. 1963. Modification in the sexual behavior of male rats produced by changing the stimulus female. J. Comp. Physiol. Psychol. 56, 636–644 10.1037/h0042469 (doi:10.1037/h0042469) [DOI] [PubMed] [Google Scholar]
- 64.Brown RE. 1974. Sexual arousal, the Coolidge effect and dominance in the rat (Rattus norvegicus). Anim. Behav. 22, 634–637 10.1016/S0003-3472(74)80009-6 (doi:10.1016/S0003-3472(74)80009-6) [DOI] [Google Scholar]
- 65.Steiger S, Franz R, Eggert AK, Müller JK. 2008. The Coolidge effect, individual recognition and selection for distinctive cuticular signatures in a burying beetle. Proc. R. Soc. B 275, 1831–1838 10.1098/rspb.2008.0375 (doi:10.1098/rspb.2008.0375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koene JM, Ter Maat A. 2007. Coolidge effect in pond snails: male motivation in a simultaneous hermaphrodite. BMC Evol. Biol. 7, 212. 10.1186/1471-2148-7-212 (doi:10.1186/1471-2148-7-212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moreira JR, Macdonald DW, Clarke JR. 1997. Correlates of testis mass in capybaras (Hydrochaeris hydrochaeris): dominance assurance or sperm competition? J. Zool. 241, 457–463 10.1111/j.1469-7998.1997.tb04837.x (doi:10.1111/j.1469-7998.1997.tb04837.x) [DOI] [Google Scholar]
- 68.Murray RL, Cutter AD. 2011. Experimental evolution of sperm count in protandrous self-fertilizing hermaphrodites. J. Exp. Biol. 214, 1740–1747 10.1242/jeb.053181 (doi:10.1242/jeb.053181) [DOI] [PubMed] [Google Scholar]
- 69.Boschetto C, Gasparini C, Pilastro A. 2011. Sperm number and velocity affect sperm competition success in the guppy (Poecilia reticulata). Behav. Ecol. Sociobiol. 65, 813–821 10.1007/s00265-010-1085-y (doi:10.1007/s00265-010-1085-y) [DOI] [Google Scholar]
- 70.Vizoso DB, Rieger G, Schärer L. 2010. Goings-on inside a worm: functional hypotheses derived from sexual conflict thinking. Biol. J. Linnean Soc. 99, 370–383 10.1111/j.1095-8312.2009.01363.x (doi:10.1111/j.1095-8312.2009.01363.x) [DOI] [Google Scholar]
- 71.Demircan T, De Mulder K, Simanov D, Glashauser J, Vizoso VB, Schärer L, Ladurner P, Berezikov E. In preparation. Stable transgenic lines of the regenerating flatworm Macrostomum lignano.