Abstract
Many insects possess adhesive organs that can produce extreme attachment forces of more than 100 times body weight but they can rapidly release adhesion to allow locomotion. During walking, weaver ants (Oecophylla smaragdina) use only a fraction of their maximally available contact area, even upside-down on a smooth surface. To test whether the reduced contact area makes the ants more susceptible to sudden and unexpected detachment forces, for example, by rain or wind gusts, we investigated the reaction of untethered ants to rapid horizontal displacements of the substrate. High-speed video recordings revealed that the pad's contact area could more than double within the first millisecond after the perturbation. This contact area expansion is much faster than any neuromuscular reflex and therefore represents a passive ‘preflex’, resulting from the mechanical properties and geometrical arrangement of the (pre-)tarsus. This preflex reaction protects ants effectively against unexpected detachment, and allows them to use less contact area during locomotion. Contact area expanded most strongly when the substrate displacement generated a pull along the axis of the tarsus, showing that the ants' preflex is direction-dependent. The preflex may be based on the ability of Hymenopteran adhesive pads to unfold when pulled towards the body. We tested Indian stick insects (Carausius morosus), which have smooth pads that lack this motility. Similar to the ants, they showed a rapid and direction-dependent expansion of the contact area mainly in the lateral direction. We propose that the preflex reaction in stick insects is based on the reorientation of internal cuticle fibrils in a constant-volume system, whereas the ants' pad cuticle is probably not a hydrostat, and pad extension is achieved by the arcus, an endoscelerite of the arolium.
Keywords: preflex, reflex, neuromuscular control, safety mechanism, insect
1. Introduction
Many insects use adhesive pads on their feet to climb over smooth surfaces. If required, some insects can produce impressive forces, exceeding many times their body weight [1–3]. However, during locomotion, their feet have to be able to attach and detach rapidly. Controllability is one of the most remarkable properties of animal adhesive organs, but the detailed mechanisms involved are still largely unknown. We showed recently how weaver ants (Oecophylla smaragdina) can control attachment by contracting their claw flexor muscle to move the adhesive pad (arolium) [4–6]. The Hymenopteran insects (sawflies, bees, wasps and ants) bear an intricate mechanism to unfold and expand the arolium to make contact to the substrate [4,7–9]. Responsible for this action is the tri-partite claw flexor muscle, situated in the femur and tibia of the insects' leg, which controls the movement of the claws via a long tendon running through the leg [7,10]. The arolium is coupled to the claws and moves with them [4], but the mechanical design of the pretarsus still allows some independent control of claw and arolium use depending on surface roughness [5].
The arolium in ants can be unfolded even without any action of the claw flexor muscle [4]. In severed legs of ants, a short proximal pull on the leg, with the pad partly in contact with the substrate, leads to an expansion of the arolium and to a strong increase in contact area. Many other animals also use a proximal pull of their legs to switch their adhesive pads from a default ‘non-sticky’ state to an adhered state; examples include both animals with hairy pad structures such as spiders and geckos [11–13], and animals with smooth pads such as bush crickets and tree frogs [14,15].
This mechanism usually involves an active pull of the leg towards the body. However, it also works when the animal is moved by external forces or when the substrate is moved away from the body; hence, no active movement by the animal is required. Such a passive switch of the pads to the ‘adhesive state’ has the potential to occur faster than a neuronal reflex. Brown & Loeb [16] coined the term ‘preflex’ to describe such passive, mechanical responses of the musculoskeletal system. For insects living high up in the canopy, an extremely rapid increase in adhesion could provide an important ‘safety mechanism’, allowing them to react almost instantaneously to unpredictable perturbations, caused by rain drops or wind gusts.
Here, we test (i) whether a fast shear movement of the walking substrate can elicit a rapid increase of the pad contact area, and (ii) how this reaction is affected by the direction of the shear movement. We tested two insects with different types of smooth pads, ants (O. smaragdina) with unfoldable pads and stick insects (Carausius morosus) that lack this folding mechanism.
2. Material and methods
(a). Study animals
Weaver ants (O. smaragdina) were kept in plastic containers in a temperature-regulated room (25−28°C on a 12 L : 12 D cycle) and fed with a mixture of honey and water (1 : 1) as well as dead insects ad libitum. For the experiments, we used medium-sized ant workers (6.5±2.1 mg; all such values given in the text represent mean±standard deviation). Stick insects (C. morosus) were kept at room temperature (on a 12 L : 12 D cycle) in a cage with freshly cut bramble leaves; they were sprayed three times per week with water. We used first and second instars (7.4±1.1 mg), as they were closest to the size of the ants.
(b). Rapid displacement experiment
The insects were placed on the underside of an open polystyrene Petri dish and were allowed to walk freely in an upside-down orientation. The Petri dish had a small rectangular cut-out where its surface was replaced by a movable glass coverslip (ca 0.1 × 5 × 5 mm). The coverslip was glued onto a double spring cantilever beam (figure 1). The two-cantilever beam construction minimized tilt of the glass coverslip when pushed to the side, important for visualizing the contact area.
Figure 1.
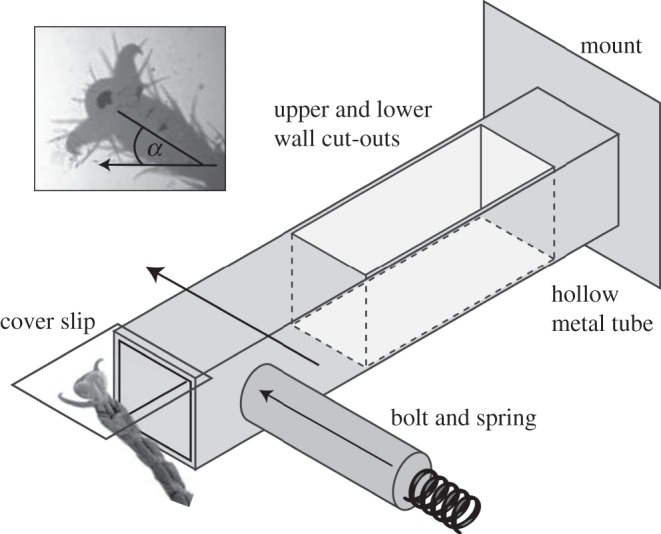
Set-up to test the reaction of insect adhesive pads to a rapid horizontal displacement. The displacement is produced by a spring-loaded bolt pushing against the side of a double cantilever beam. The inset shows a stick insect pad in contact with the substrate. The direction of the displacement is shown by the arrow; the angle α denotes the orientation of the tarsus relative to it. Note that elements are not drawn to scale.
Whenever the insect placed one of its feet on the platform, a spring mechanism was triggered, releasing a bolt that pushed against the cantilever, eliciting a horizontal movement of the platform attached to the beam. The movement of the platform was stopped by a fixed bolt on the opposite side that could be adjusted to limit the displacement to a fixed distance. The coverslip was displaced by 733±437 μm within 1.72±1.0 ms (with a maximum velocity of 0.51±0.22 ms−1). This displacement corresponded to approximately 10–14 times the measured proximal–distal length of the adhesive contact area (ants: 75±16 μm, stick insects: 49±7 μm). Individual legs of 12 ants and 17 stick insects were studied, resulting in a total of 36 and 68 trials for ants and stick insects, respectively. Each trial represents a different leg; not more than four legs were tested from the same animal.
The contact area of the adhesive pads was visualized in reflected light and 5× magnification using a compound microscope (Leica DMR-HC) and recorded with a high-speed video camera (Redlake PCI 1000 B/W and NAC HotShot 1280 cameras, frame rate 1 and 2 kHz, respectively). Contact area (appearing dark on a bright background) was measured for each frame using thresholding routines in Matlab (Mathworks Corp., USA). The lateral displacement of the walking substrate was tracked by manually digitizing little spots on the coverslip surface. During the rapid movement of the glass substrate, the adhesive contact area was often blurred or out of focus; we therefore quantified contact area in the first analysable frame after the displacement, corresponding to an average post-event time of 1.75 ms.
To test whether the pad's reaction is direction-dependent, we restrained the insects to be able to vary the orientation of the tarsus relative to the direction of the displacement. The body of an ant was held with self-closing forceps at the petiolus. One of the hind legs was fixed to a stiff wire using a droplet of melted wax. Stick insects were enclosed in a narrow hollow square metal tube so that either front or hind legs protruded from the end. The leg was immobilized as for the ants.
The insect leg could be positioned and oriented relative to the pulling direction using a micro-manipulator. The angle of the leg was varied from 0°, corresponding to a pull of the leg towards the body, to 180°, corresponding to a push away from it. The angle was measured in the plane of the coverslip between the projection of the tarsus and the direction of the displacement (see inset in figure 1).
3. Results
(a). Passive preflex
The footpads of ants and stick insects were typically not detached by the rapid displacement of the surface but followed the movement of the glass coverslip, where we observed only a small amount of sliding, corresponding to approximately 14–22% of the proximal–distal length of the adhesive pad. However, both ants and stick insects showed extremely rapid changes of the contact area as a result of the perturbation (figure 2 and electronic supplementary material, video clip S1).
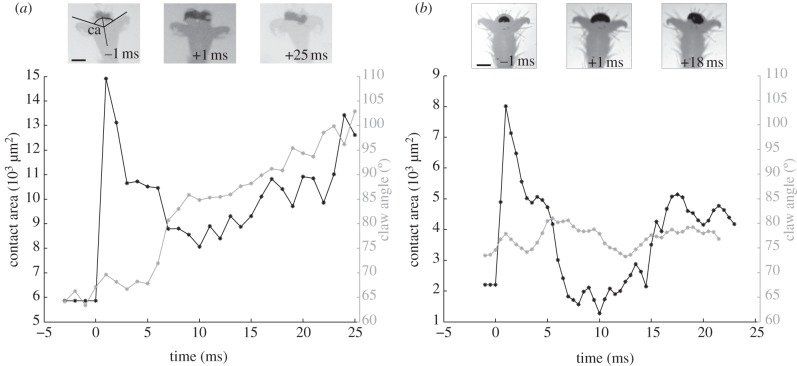
Figure 2.
Adhesive contact area increase for pads of (a) weaver ants and (b) stick insects in response to a rapid displacement of the substrate. In both insects, the contact area more than doubled within the first millisecond of the displacement. A delayed active reaction is visible in the ant by the flexion of the claws. Insets show frames from high-speed recordings; the contact zones are visible as dark areas. The claw angle (ca) was measured as the average of both angles of the distal claw edges to the longitudinal axis of the tarsus. (a,b) Scale bars, 100 μm.
When the tarsi were aligned with the direction of the displacement (0°), the contact area of both ants and stick insects could more than double within the first millisecond after the perturbation (figure 2 and electronic supplementary material, video clip S1). Summarizing all trials where the displacement was approximately aligned with the tarsus (0−45°), the contact area increased on average by a factor of 1.49±0.63 in ants, and by a factor of 1.65±1.02 in stick insects (figure 3). This increase was significant in both insects (paired t-test for ants: t =−3.38, d.f. = 42, p < 0.01; for stick insects: t =−2.54, d.f. = 67, p < 0.05).
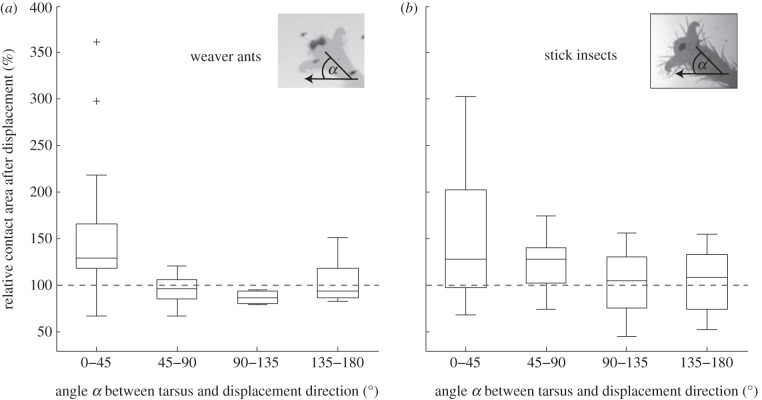
Figure 3.
Effect of leg orientation (angle α between the tarsus and the substrate's displacement direction, shown by the arrow) on the change in contact area. Contact areas were measured immediately before, and in the first frame after the displacement that could be analysed (average 1.75 ms after the displacement). In both insects, contact area increased most strongly when the legs were approximately aligned with the displacement. ‘100%’ denotes no change of contact area.
Contact area increased mainly by lateral expansion. In both ants and stick insects, we observed a significantly stronger relative change in width than in proximal–distal length (ants: width 1.7±2.1-fold, length 0.9±0.3-fold, Wilcoxon signed-rank test: n = 34, z = –3.3, p < 0.001; stick insects: width 1.5±0.5-fold, length 1.1±0.3-fold, Wilcoxon signed-rank test: n = 45, z =−3.9, p < 0.001). The increase in contact area was observed in the first frame that could be analysed after the perturbation (1.75±0.98 ms); in many cases, the contact area reaction occurred within less than 1 ms of the perturbation. Such a rapid change would be impossible with a neuromuscular reflex; the short timescale, therefore, confirms the passive nature of the reaction. Probably as a result of the backlash caused by the insect's inertia 2–3 ms after the perturbation, the contact area typically decreased again, sometimes leading to a detachment of the foot. In most cases, the contact area increased again after approximately 10–15 ms. In the ants, this increase coincided with a flexion of the claws from 81±10° (before the perturbation) to 86±9° (20 ms after the perturbation; t-test: n = 24, t = –2.3, p < 0.01), indicating an active response of the claw flexor muscle (figures 1 and 4a). This reaction was less pronounced and not significant in the stick insects (t-test, n = 31, t = −0.6, p > 0.05; figure 4b).
Figure 4.
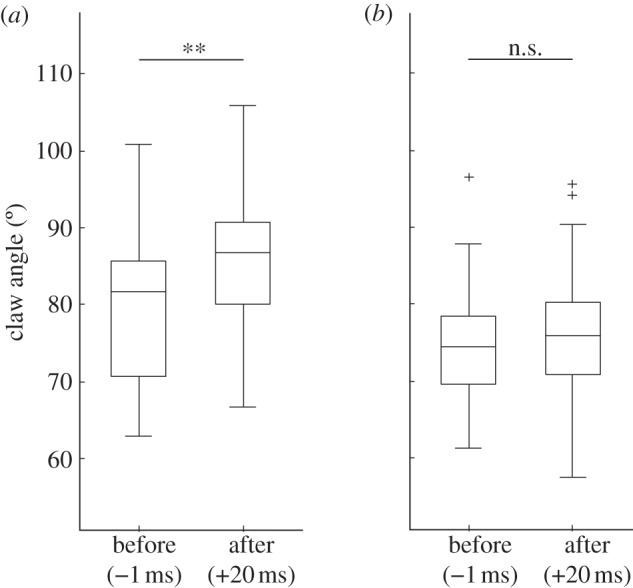
Claw flexion of (a) weaver ants and (b) stick insects, 1 ms before and 20 ms after the perturbation. n.s., not significant, **p < 0.01.
(b). Effect of tarsus orientation
To test whether and how the direction of the displacement affects the pad's reaction, we tested different displacement directions on restrained insects. For both insect species, the strongest increase in contact area was observed when the tarsi were approximately aligned with the direction of the displacement (0−45°, i.e. ‘pulling’ direction; figure 3). The strength of the preflex decreased for larger angles (i.e. movements in the transverse or ‘pushing’ direction; Spearman correlation coefficient for ants: n = 43, ρ =−0.46, p < 0.01; for stick insects: n = 68, ρ =−0.34, p < 0.01). In ants (figure 3a), hardly any contact area increase was observed when the tarsus was not aligned to the pull. In stick insects, however, a preflex reaction could sometimes be elicited even for larger angles, where the tarsus was no longer aligned with the displacement (see values above 100% in figure 3b).
4. Discussion
Our results show that ants and stick insects react to sudden displacements of the walking substrate by an increase of their adhesive pad contact area. We could distinguish between an extremely fast, mechanical reaction (preflex) and a delayed reaction of the claw flexor muscle (reflex).
The mechanical reaction has the obvious advantage that it is not constrained by the delays inherent in the transmission of neuronal signals and the activation of muscle. Previous studies on insects suggest that the minimum delay between a perturbation and a muscular reaction is of the order of 5–15 ms (found in locusts and cockroaches; [17,18]) and greater than 40 ms for humans [19]. Delays of less than 1 ms between stimulus and reaction are clearly impossible for neuromuscular responses. Even when signals are transmitted mechanically from the periphery to mechanosensors closer to the body and thus closer to the central nervous system (as has been demonstrated for locust legs; [17]), the delays of efferent signals and muscle activation are still one order of magnitude larger than the reaction time observed in our experiments. The usage of a mechanical control system instead of a neuromuscular one may help to simplify neural control. When animals replace muscles by mechanical elements, they can save energy, and make limbs more lightweight [20,21]. The insect's tarsus is a prime example of an efficient, lightweight structure [22]: the claw flexor muscle is located in the tibia and femur, far away from the foot tip. Moreover, the claw flexor muscle has no antagonist, and the claws return to their extended position by recoil of elastic cuticle that may contain resilin [23]. As a result, the tarsus does not contain any intrinsic muscles and thus has a reduced inertia, which is essential for rapid leg cycling. The design of the pretarsus not only enables efficient movement but also includes a purely mechanical control of arolium/claw use on surfaces of different roughness [5].
Our results show that the adhesive preflex reaction is direction-dependent for both ants and stick insects. However, we found a stronger direction dependency for the ants. The preflex reaction in stick insects may be based on the reorientation of internal cuticle fibrils of the pad [24]. A pull towards the body likely changes the fibril angle and thereby reduces the cuticle's thickness, which, in turn, may cause a lateral expansion of the adhesive contact area. This hypothetical mechanism requires that the smooth-pad cuticle represents a constant-volume system. The morphology of the pad cuticle in stick insects is consistent with this idea, because the cuticle is bordered on the inside by the epidermis and laterally by much thinner areas of cuticle [25]. By contrast, the smooth arolium cuticle in ants and other Hymenoptera is probably not a hydrostat, because it is not isolated by the epidermis, and adjoins a large, fluid-filled compartment inside the arolium [4]. Here, lateral expansion of the contact area is achieved in a completely different way, via a U-shaped endoscelerite of the arolium, the arcus, which helps to translate a pull along the leg into a lateral expansion [4,5,9,26]. Our results suggest that the ants' unfolding mechanism is more confined to purely proximal pulls, whereas the stick insects' mechanism can be triggered for a wider range of pulling angles.
Interestingly, neither ants nor stick insects showed a clear reduction in contact area for pure pushes (180°), although this seems to be expected from their direction-dependent properties. However, separating (peeling) a large contact zone from a surface may be an inherently slower process than the formation of new contact area. Thus, at least for the smooth pads investigated here, detachment during pushes appears to be slower than the extremely rapid attachment during pulls.
Insects may use the preflex as a safety mechanism for unpredictable and fast perturbations, in order to prevent detachment from the substrate. The direction dependency of the preflex suggests that it should only protect the insect against perturbations acting in one specific direction. However, as the six legs of insects are spread out more or less radially, a perturbation will almost always elicit a full preflex in one or several legs. This may also compensate potential detachment of individual legs because of a backlash as observed in our experiments. If an insect gets pulled off the surface perpendicularly, all legs would be subject to a centripetal pull and would therefore be in the right orientation for the preflex reaction.
A rapid ‘safety’ mechanism as observed in our study may represent a general phenomenon among natural adhesive systems used for locomotion. It allows animals to minimize adhesive contact area during locomotion without increasing the risk of unwanted detachment, a principle that may find use in robotics. Further research will need to clarify how widespread such instantaneous attachment reactions are, and what detailed biomechanical adaptations are involved.
Acknowledgements
This study was supported by a grant from the Biotechnology and Biological Sciences Research Council (UK) awarded to W.F.
References
- 1.Federle W, Rohrseitz K, Hoelldobler B. 2000. Attachment forces of ants measured with a centrifuge: better ‘wax-runners’ have a poorer attachment to a smooth surface. J. Exp. Biol. 203, 505–512 [DOI] [PubMed] [Google Scholar]
- 2.Eisner T, Aneshansley DJ. 2000. Defense by foot adhesion in a beetle (Hemisphaerota cyanea). Proc. Natl Acad. Sci. USA 97, 6568–6573 10.1073/pnas.97.12.6568 (doi:10.1073/pnas.97.12.6568) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dixon AFG, Croghan PC, Gowing RP. 1990. The mechanism by which aphids adhere to smooth surfaces. J. Exp. Biol. 152, 243–253 [Google Scholar]
- 4.Federle W, Brainerd EL, McMahon TA, Hölldobler B. 2001. Biomechanics of the movable pretarsal adhesive organ in ants and bees. Proc. Natl Acad. Sci. USA 98, 6215–6220 10.1073/pnas.111139298 (doi:10.1073/pnas.111139298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Endlein T, Federle W. 2008. Walking on smooth or rough ground: passive control of pretarsal attachment in ants. J. Comp. Physiol. A 194, 49–60 10.1007/s00359-007-0287-x (doi:10.1007/s00359-007-0287-x) [DOI] [PubMed] [Google Scholar]
- 6.Federle W, Endlein T. 2004. Locomotion and adhesion: dynamic control of adhesive surface contact in ants. Arthropod Struct. Dev. 33, 67–75 10.1016/j.asd.2003.11.001 (doi:10.1016/j.asd.2003.11.001) [DOI] [PubMed] [Google Scholar]
- 7.Snodgrass R. 1956. Anatomy of the honey bee. Ithaca, NY: Comstock Publishing Associates [Google Scholar]
- 8.Frantsevich L, Gorb S. 2004. Structure and mechanics of the tarsal chain in the hornet, Vespa crabro (Hymenoptera: Vespidae): implications on the attachment mechanism. Arthropod Struct. Dev. 33, 77–89 10.1016/j.asd.2003.10.003 (doi:10.1016/j.asd.2003.10.003) [DOI] [PubMed] [Google Scholar]
- 9.Gladun D. 2008. Morphology of the pretarsus of the sawflies and horntails (Hymenoptera: Symphyta). Arthropod Struct. Dev. 37, 13–28 10.1016/j.asd.2007.04.002 (doi:10.1016/j.asd.2007.04.002) [DOI] [PubMed] [Google Scholar]
- 10.Radnikow G, Bässler U. 1991. Function of a muscle whose apodeme travels through a joint moved by other muscles: why the retractor unguis muscle in stick insects is tripartite and has no antagonist? J. Exp. Biol. 157, 87–99 [Google Scholar]
- 11.Hill D. 1977. The pretarsus of salticid spiders. Zool. J. Linn. Soc. 60, 319–338 10.1111/j.1096-3642.1977.tb00838.x (doi:10.1111/j.1096-3642.1977.tb00838.x) [DOI] [Google Scholar]
- 12.Autumn K, Liang Y, Hsieh ST, Zesch W, Chan W-P, Kenny TW, Fearing RS, Full RJ. 2000. Adhesive force of a single gecko foot-hair. Nature 405, 681–685 10.1038/35015073 (doi:10.1038/35015073) [DOI] [PubMed] [Google Scholar]
- 13.Autumn K, Hsieh ST, Dudek DM, Chen J, Chitaphan C, Full RJ. 2006. Dynamics of geckos running vertically. J. Exp. Biol. 209, 260–272 10.1242/jeb.01980 (doi:10.1242/jeb.01980) [DOI] [PubMed] [Google Scholar]
- 14.Gorb S, Jiao Y, Scherge M. 2000. Ultrastructural architecture and mechanical properties of attachment pads in Tettigonia viridissima (Orthoptera: Tettigoniidae). J. Comp. Physiol. A 186, 821–831 10.1007/s003590000135 (doi:10.1007/s003590000135) [DOI] [PubMed] [Google Scholar]
- 15.Hanna G, Barnes W. 1991. Adhesion and detachment of the toe pads of tree frogs. J. Exp. Biol. 155, 103–125 [Google Scholar]
- 16.Brown IE, Loeb GE. 2000. A reductionist approach to creating and using neuromusculoskeletal models. In Biomechanics and neural control of posture and movement (eds JM Winters, PE Crago), pp. 148–163 New York, NY: Springer [Google Scholar]
- 17.Höltje M, Hustert R. 2003. Rapid mechano-sensory pathways code leg impact and elicit very rapid reflexes in insects. J. Exp. Biol. 206, 2715–2724 10.1242/jeb.00492 (doi:10.1242/jeb.00492) [DOI] [PubMed] [Google Scholar]
- 18.Ridgel AL, Frazier SF, Zill SN. 2001. Dynamic responses of tibial campaniform sensilla studied by substrate displacement in freely moving cockroaches. J. Comp. Physiol. A 187, 405–420 10.1007/s003590100213 (doi:10.1007/s003590100213) [DOI] [PubMed] [Google Scholar]
- 19.Zehr EP, Stein RB. 1999. What functions do reflexes serve during human locomotion? Prog. Neurobiol. 58, 185–205 10.1016/S0301-0082(98)00081-1 (doi:10.1016/S0301-0082(98)00081-1) [DOI] [PubMed] [Google Scholar]
- 20.Gronenberg W. 1996. Fast actions in small animals: springs and click mechanisms. J. Comp. Physiol. A 178, 727–734 10.1007/BF00225821 (doi:10.1007/BF00225821) [DOI] [Google Scholar]
- 21.Alexander RM. 1988. Elastic mechanisms in animal movement Cambridge, UK: Cambridge University Press [Google Scholar]
- 22.Frazier SF, Larsen GS, Neff D, Quimby L, Carney M, DiCaprio RA, Zill SN. 1999. Elasticity and movements of the cockroach tarsus in walking. J. Comp. Physiol. 185, 157–172 10.1007/s003590050374 (doi:10.1007/s003590050374) [DOI] [Google Scholar]
- 23.Gorb SN. 1996. Design of insect unguitractor apparatus. J. Morphol. 230, 219–230 10.1002/(SICI)1097-4687(199611)230 (doi:10.1002/(SICI)1097-4687(199611)230) [DOI] [PubMed] [Google Scholar]
- 24.Dirks J-H, Li M, Kabla A, Federle W. 2012. In vivo dynamics of the internal fibrous structure in smooth adhesive pads of insects. Acta Biomater. 8, 2730–2736 10.1016/j.actbio.2012.04.008 (doi:10.1016/j.actbio.2012.04.008) [DOI] [PubMed] [Google Scholar]
- 25.Scholz I, Baumgartner W, Federle W. 2008. Micromechanics of smooth adhesive organs in stick insects: pads are mechanically anisotropic and softer towards the adhesive surface. J. Comp. Physiol. A 194, 373–384 10.1007/s00359-008-0314-6 (doi:10.1007/s00359-008-0314-6) [DOI] [PubMed] [Google Scholar]
- 26.Frantsevich L, Gorb S. 2002. Arcus as a tensegrity structure in the arolium of wasps (Hymentoptera: Vespidae). Zoology 105, 225–237 10.1078/0944-2006-00067 (doi:10.1078/0944-2006-00067) [DOI] [PubMed] [Google Scholar]