Abstract
Insect osmoregulation is subject to highly sophisticated endocrine control. In Drosophila, both Drosophila kinin and tyramine act on the Malpighian (renal) tubule stellate cell to activate chloride shunt conductance, and so increase the fluid production rate. Drosophila kinin is known to act through intracellular calcium, but the mode of action of tyramine is not known. Here, we used a transgenically encoded GFP::apoaequorin translational fusion, targeted to either principal or stellate cells under GAL4/UAS control, to demonstrate that tyramine indeed acts to raise calcium in stellate, but not principal cells. Furthermore, the EC(50) tyramine concentration for half-maximal activation of the intracellular calcium signal is the same as that calculated from previously published data on tyramine-induced increase in chloride flux. In addition, tyramine signalling to calcium is markedly reduced in mutants of NorpA (a phospholipase C) and itpr, the inositol trisphosphate receptor gene, which we have previously shown to be necessary for Drosophila kinin signalling. Therefore, tyramine and Drosophila kinin signals converge on phospholipase C, and thence on intracellular calcium; and both act to increase chloride shunt conductance by signalling through itpr. To test this model, we co-applied tyramine and Drosophila kinin, and showed that the calcium signals were neither additive nor synergistic. The two signalling pathways thus represent parallel, independent mechanisms for distinct tissues (nervous and epithelial) to control the same aspect of renal function.
Keywords: Malpighian tubule, insect physiology, ion transport, endocrinology, aequorin, calcium
1. Introduction
Insect Malpighian tubules play key roles in ion transport and excretion [1], immune function [2,3] and xenobiotic detoxification [4,5]. Because of these multiple roles, they are also important both in sensing and in mounting a homeostatic response to stress [6–12]. They even show positional and gender-specific asymmetry in function [13]. Their neuroendocrine control is appropriately sophisticated, and well reviewed elsewhere [1,14,15].
The Drosophila melanogaster tubule is an excellent model for insect tubules, particularly of Diptera, which segregate their transport function into two specialized cell types [16]. Active cation transport is energized by an apical plasma membrane H+ V-ATPase, which drives alkali metal–proton exchange to produce a net transport of potassium or sodium, so increasing the transepithelial potential (TEP) [1]. Several neuropeptides have been linked to activation of the principal cell: the diuretic hormones DH31 [17] and DH44 [18], which both act through cyclic AMP; CAPA [19], acting through calcium; and Nplp1-4, an ‘orphan’ peptide [20] that was recently shown to activate a receptor guanylate cyclase [10]. Activation of the principal cell alone produces a modest increase in fluid secretion, because the resting chloride conductance is relatively low.
Stellate cells are activated by Drosophila kinin, or Drosokinin (NSVVLGKKQRFHSWGamide) [21], a member of a neuropeptide family found in most insects [22,23], which signals through a canonical G-protein coupled receptor (GPCR) to raise intracellular calcium [24], and thence to rapidly increase the chloride shunt conductance, effectively removing the ‘brake’ on active cation pumping, resulting in a rapid collapse of TEP and concomitant increase in fluid secretion [25,26].
Recently, it has become clear that tyramine is a second agonist for the stellate cell [6,27,28]. Like Drosophila kinin, it signals through a canonical GPCR and acts to collapse the TEP, and so increase fluid secretion. It is thus of great interest to establish whether tyramine acts through intracellular calcium, and whether the Drosophila kinin and tyramine signals interact in any way. This is particularly straightforward to address in Drosophila, with ready availability of classical mutants, and powerful transgenics—indeed the first report of the use of a genetically encoded calcium sensor in animals was in Drosophila [29]. Here, as well as demonstrating that tyramine does indeed signal through intracellular calcium in only the stellate cells, we report the use of an improved calcium sensor in tubules that is based on a translational fusion of the two jellyfish photoproteins apoaequorin and green fluorescent protein (GFP), resulting in markedly improved sensitivity [30,31].
2. Material and methods
(a). Drosophila maintenance
Drosophila were kept at 25°C, 12 : 12 h photoperiod and 45–55 per cent relative humidity, and raised on standard Drosophila medium, as described previously [32].
(b). Generation of calcium reporter flies
We have previously described the use of quantitative reporters based on transgenic aequorin [29], as well as imaging reporters based on pericam [33]; here, we generated flies transgenic for a calcium reporter based on a translational fusion of GFP and apoaequorin, under control of the UAS control region (‘UAS-GFP::aeq’) by cloning a synthetic cDNA into the transformation vector pP{UAST} and germ-line transforming Drosophila according to standard protocols. As reported elsewhere, we found that such a reporter shows greatly increased stability and luminescence [30], allowing superior real-time recordings to be obtained with less tissue in each sample.
(c). Real-time intracellular calcium assays
Assays were as described earlier [29]. Briefly, week-old adult flies were anaesthetised by chilling on ice for a few minutes, then tubules dissected in Schneider's culture medium (except as described below). Where reduced tyrosine or tyramine levels were required, tissues were dissected and assayed in standard Drosophila saline [32], which does not contain these compounds. Depending on the experiment, tubules expressed UAS-GFP::aeq, driven by GAL4 lines c42 (specific to principal cells in the main segment) or c724 (specific to stellate cells).
Tubules were incubated in the dark with coelenterazine to reconstitute active aequorin, then real-time luminescence measured in a Berthold luminometer. After establishing a stable baseline, tyramine or Drosophila kinin was applied through injectors, and response was followed up for a further period. At the end of the experiment, undischarged aequorin was measured by permeabilizing the cells with Triton X-100 in the presence of excess calcium. Instantaneous real-time calcium values throughout the experiment were then back-calculated with an in-house Perl routine, based on standard methods [34].
(d). Statistics
Data are plotted as mean ± s.e.m. Where needed, data were compared using Student's t-test, taking p = 0.05 (two-tailed) as the critical value. For EC50 values, best fit was calculated by least-squares nonlinear fit (GraphPad Prism), and the resulting log(EC50) values compared with a t-test.
3. Results and discussion
The action of tyramine is to collapse the TEP across the tubule by rapidly increasing the chloride shunt conductance, and thus to stimulate KCl transport and fluid production [27]. These are the same actions ascribed to the neuropeptide Drosophila kinin, which has been shown to act to raise intracellular calcium only in stellate cells [24,35], implying that the chloride shunt conductance route is controlled by these cells. Consistent with this, the Drosophila kinin receptor is found in stellate cells in Drosophila [24], Anopheles [35] and Aedes [36]. Accordingly, tyramine was applied to tubules transgenic for the enhanced aequorin::GFP fusion, which provides a sensitive, real-time, absolute measurement of intracellular calcium (figure 1). When GFP::Aeq was driven in principal cells, no response to tyramine was seen; but when driven in stellate cells, a prominent, rapid calcium rise was observed, as previously documented for Drosophila kinin. Therefore, tyramine, like Drosophila kinin, acts to raise intracellular calcium in only stellate cells.
Figure 1.
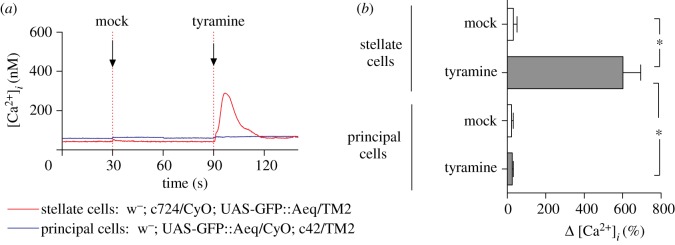
Tyramine acts to raise intracellular calcium in stellate, but not in principal cells. (a) Representative experiment, in which an apoaequorin::eGFP fusion was expressed in principal cells by crossing to the c42 GAL4 driver (blue), or only in stellate cells, by crossing to the c724 GAL4 driver (red). A mock injection before the addition of the secretagogue (at 5 × 10−8 M) allows any injection artefact to be estimated; in this case, it was negligible. (b) Summary of peak responses from three such experiments. Significant differences are marked with an asterisk.
The tyramine response was concentration-dependent (figure 2), with an EC50 of 1.77 × 10−8 M (figure 3a). To test whether this was relevant to the functional endpoint of elevated shunt conductance, this value was compared with the EC50 for chloride shunt conductance activation, assayed as a change in TEP [27]. No formal EC50 was reported in this paper; accordingly, the original data were re-measured and re-plotted (figure 3b) to obtain an EC50 of 1.6 × 10−8 M. These two values do not differ significantly (p = 0.83). Therefore, the concentration dependence of tyramine-induced elevation of intracellular calcium is exactly compatible with an action on chloride shunt conductance.
Figure 2.
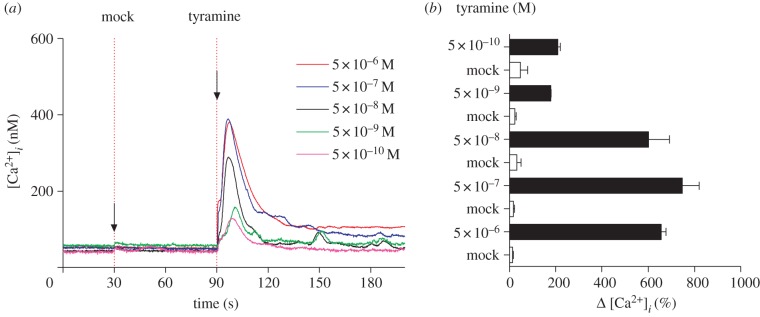
Concentration dependence of tyramine activation of intracellular calcium in stellate cells. (a) Typical responses to varying concentrations of tyramine, injected at 90 s. (b) Mean response across a range of concentrations, compared to corresponding mock injections (n = 3 except for n = 2 at 5×10−10 M).
Figure 3.
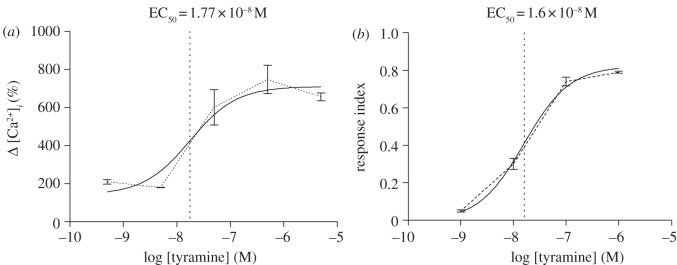
The EC50 for tyramine activation of stellate cell intracellular calcium matches that calculated for activation of chloride conductance. (a) Data from figure 2 were re-plotted as a standard semi-log dose–response curve, and a curve (solid line) fitted to the original data (dotted line) using GraphPad Prism. (b) Data were re-measured from fig. 2c of [4], and re-plotted as in (a).
Both Drosophila kinin and tyramine signal through distinct GPCRs (lkr and CG7431, respectively [24,37,38]), but use the same downstream messenger. It was therefore of interest to establish whether tyramine signals through phospholipase C (PLC) and inositol trisphosphate (IP3), as has previously been established for Drosophila kinin [39]. This was tested using well-known mutants for the widely expressed PLC, no receptor potential A (norpA), and for the only InsP3 receptor gene, itpr. NorpA nulls are viable, because there is a second PLC in Drosophila (Plc21C), so using the null norpA24 it was possible to study the calcium response in tubules with 2, 1 or 0 working copies of norpA (figure 4a). As can be seen, reduction in the number of copies of norpA produced a corresponding reduction in calcium response, as previously shown for the neuropeptide Drosophila kinin [39]. PLC acts to liberate InsP3, which classically acts on its cognate receptor in the endoplasmic reticulum to produce a rapid calcium pulse, which typically triggers further calcium entry into the cell. As iptr is a single copy gene in Drosophila, nulls are lethal [40]—perhaps surprisingly as late as the pupal stage—and so the impact of itpr was assessed in feeding third instar larvae (figure 4b). As can be seen, in itpr1664/itpr1664 hypomorphs, the calcium response was attenuated. Therefore, although the tyramine and Drosophila kinin signals originate from different sources and act on distinct receptors, their downstream signalling through NorpA, Itpr and Ca2+i is indistinguishable.
Figure 4.
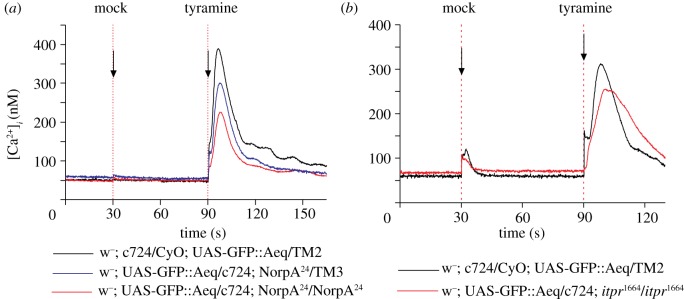
Like Drosophila kinin, tyramine calcium signalling is mediated by phospholipase C and the IP3 receptor. (a) Comparison of calcium responses in lines carrying 2, 1 or 0 copies of NorpA, the major phospholipase C of tubules. Typical traces. (b) Comparison of itpr1664/itpr1664 homozygous mutant flies with wild type. Note that, because of extensive pupal lethality of itpr mutants, these experiments were performed on feeding third instar larvae. Each trace is the average of three independent replicates.
Is parallel activation of the Drosophila kinin and tyramine pathways synergistic? As both act through the same second messenger, this would not be expected; and indeed (figure 5), the calcium response to tyramine and Drosophila kinin combined is not significantly greater than to either secretagogue separately, at either high or submaximal concentrations of the two agonists. Indeed, there is little evidence for additivity in the signals, implying that the two pathways converge on a limiting downstream component.
Figure 5.
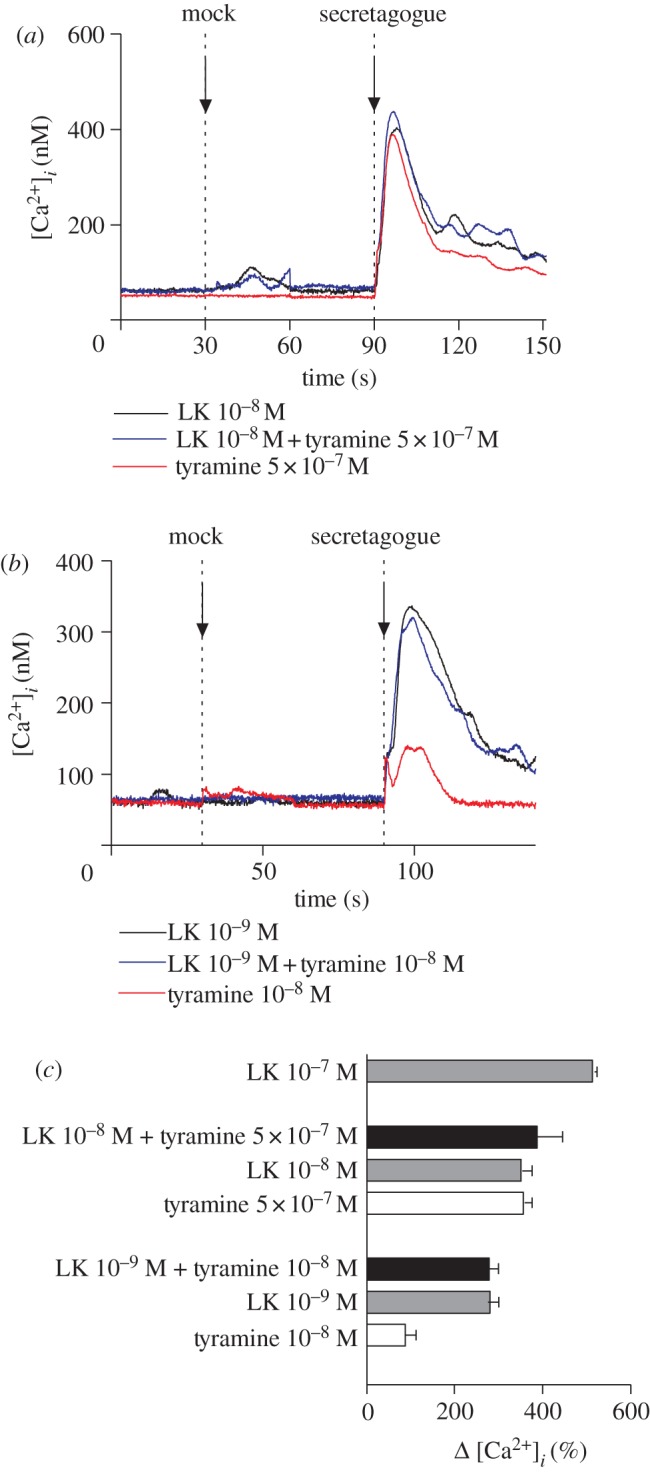
Tyramine calcium signalling in stellate cells is not synergistic to that of Drosophila kinin. (a) Traces from experiment with high concentrations of kinin and tyramine. (b) Traces from experiment with lower concentrations of kinin and tyramine. (c) Peak increases in calcium signals (relative to basal) observed in A and B, with a saturating concentration of kinin (10−7 M) for reference. Tubules from adult c724>GFP::aeq flies were dissected and exposed to Drosophila kinin, tyramine or both at the point indicated, and responses compared with mock injections at 30 s. Typical traces.
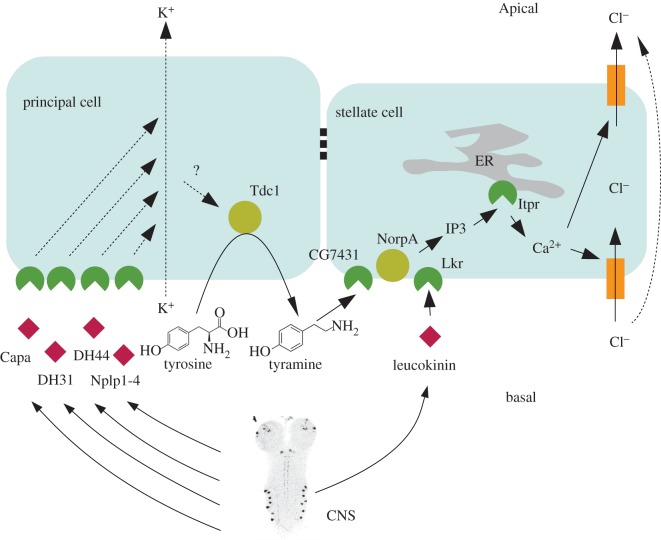
Overall, then, an intriguing model has been demonstrated, in which two distinct secretagogues with two different origins within the organism elicit responses which are indistinguishable downstream, with both acting through PLC and InsP3 to elevate intracellular calcium, and thence to trigger a massive and rapid increase in the chloride shunt conductance. At first sight, such a system would seem to defy Occam's razor; why should such independent pathways exist? The solution proposed by Blumenthal [28] is based on the origins of the two signals (figure 6). Drosophila kinin is a bona fide neuropeptide, which has been mapped to neurosecretory cells in the CNS and peripheral tissues [42–45]. It thus provides a clear route through which the CNS controls diuresis. In contrast, tyramine is generated from tyrosine by the action of tyrosine decarboxylase, which is found in the adjacent principal cells within the tubule itself [28]. The principal cells are themselves under neuroendocrine control, from both the CNS and neurosecretory cells in the midgut [46], and are the sites for active cation transport. The parallel activation model would thus allow the cation pumping cell (which sets up the TEP gradient for chloride) to influence the conductance of the chloride shunt pathway directly, and so produce efficient diuresis. So the potential exists for neuroendocrine stimulation of the principal cell, by any of the neuropeptides DH31, DH44, CAPA or Nplp1-4, to not only increase the driving force for chloride (by pumping cations to the lumen), but also to increase the conductance for chloride simultaneously. While further work is needed, such a mechanism would be parsimonious, as increasing active transport of cations without increasing the chloride shunt conductance necessary for fluid secretion would be energetically wasteful. With two secretagogues with very different threshold concentrations, there is also the scope to tune the system over a broad range of inputs.
Figure 6.
A possible model for the parallel activation of the stellate cell by tyramine and Drosophila kinin. Neuropeptides (red diamonds) from the CNS, or neuroendocrine cells in the gut, activate electrogenic cation pumping in the principal cell, or shunt conductance in the stellate cell. As tyrosine decarboxylase 1 is located in the principal cell, there is also the potential for principal cell secretagogues to influence Tdc1 activity, and thence tyramine production. The image of the CNS (in fact stained for Drosophila kinin) is taken from [41].
This pathway should be seen in the context of multiple opportunities for cross-talk in the control of the insect renal system. For example, although central control of renal function is widely studied, there are neurosecretory cells in the midgut which contain—and so may co-release—several pairs of neuropeptides that are known to act on the tubule; for example, kinin and DH31, or short neuropeptide F and DH31 [46]. In Locusta [47] and Rhodnius [48], the DH44 and kinin homologues co-localize in the same abdominal neurosecretory cells. Within the CNS, the Drosophila kinin receptor is known to be expressed on the neurosecretory cells that express DH44 [18,24]. In small animals, scaling arguments suggest that ion and water homeostasis are critical for survival, so perhaps it is not surprising that such a complex network of signals can interact to optimize the response of the renal tubule from moment to moment.
Acknowledgements
This work was funded by the UK Biotechnology and Biological Sciences Research Council (BBSRC), and by general funds of the University of Glasgow. Work in the laboratory of M.N. is supported in part by the National Institute of Neurological Disorders and Stroke, National Institutes of Health (NIH; R01NS055035, R01NS058443, R21NS058330), and National Institute of General Medical Sciences (R01GM098931).
References
- 1.Beyenbach KW, Skaer H, Dow JAT. 2010. The developmental, molecular, and transport biology of Malpighian tubules. Annu. Rev. Entomol. 55, 351–374 10.1146/annurev-ento-112408-085512 (doi:10.1146/annurev-ento-112408-085512) [DOI] [PubMed] [Google Scholar]
- 2.Kaneko T, et al. 2006. PGRP-LC and PGRP-LE have essential yet distinct functions in the Drosophila immune response to monomeric DAP-type peptidoglycan. Nat. Immunol. 7, 715–723 10.1038/ni1356 (doi:10.1038/ni1356) [DOI] [PubMed] [Google Scholar]
- 3.McGettigan J, et al. 2005. Insect renal tubules constitute a cell-autonomous immune system that protects the organism against bacterial infection. Insect Biochem. Mol. Biol. 35, 741–754 10.1016/j.ibmb.2005.02.017 (doi:10.1016/j.ibmb.2005.02.017) [DOI] [PubMed] [Google Scholar]
- 4.Chahine S, O'Donnell MJ. 2011. Interactions between detoxification mechanisms and excretion in Malpighian tubules of Drosophila melanogaster. J. Exp. Biol. 214, 462–468 10.1242/jeb.048884 (doi:10.1242/jeb.048884) [DOI] [PubMed] [Google Scholar]
- 5.Yang J, McCart C, Woods DJ, Terhzaz S, Greenwood KG, ffrench-Constant RH, Dow JAT. 2007. A Drosophila systems approach to xenobiotic metabolism. Physiol. Genomics 30, 223–231 10.1152/physiolgenomics.00018.2007 (doi:10.1152/physiolgenomics.00018.2007) [DOI] [PubMed] [Google Scholar]
- 6.Blumenthal EM. 2005. Modulation of tyramine signaling by osmolality in an insect secretory epithelium. Am. J. Physiol. Cell Physiol. 289, C1261–C1267 10.1152/ajpcell.00026.2005 (doi:10.1152/ajpcell.00026.2005) [DOI] [PubMed] [Google Scholar]
- 7.Davies SA, Overend G, Sebastian S, Cundall M, Cabrero P, Dow JAT, Terhzaz S. 2012. Immune and stress response ‘cross-talk’ in the Drosophila Malpighian tubule. J. Insect Physiol. 58, 488–497 10.1016/j.jinsphys.2012.01.008 (doi:10.1016/j.jinsphys.2012.01.008) [DOI] [PubMed] [Google Scholar]
- 8.Huang X, Huang Y, Chinnappan R, Bocchini C, Gustin MC, Stern M. 2002. The Drosophila inebriated-encoded neurotransmitter/osmolyte transporter: dual roles in the control of neuronal excitability and the osmotic stress response. Genetics 160, 561–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naikkhwah W, O'Donnell MJ. 2011. Salt stress alters fluid and ion transport by Malpighian tubules of Drosophila melanogaster: evidence for phenotypic plasticity. J. Exp. Biol. 214, 3443–3454 10.1242/jeb.057828 (doi:10.1242/jeb.057828) [DOI] [PubMed] [Google Scholar]
- 10.Overend G, et al. 2012. The receptor guanylate cyclase Gyc76C and a peptide ligand, NPLP1-VQQ, modulate the innate immune IMD pathway in response to salt stress. Peptides 34, 209–218 10.1016/j.peptides.2011.08.019 (doi:10.1016/j.peptides.2011.08.019) [DOI] [PubMed] [Google Scholar]
- 11.Stergiopoulos K, Cabrero P, Davies SA, Dow JAT. 2009. Salty dog, an SLC5 symporter, modulates Drosophila response to salt stress. Physiol. Genomics 37, 1–11 10.1152/physiolgenomics.90360.2008 (doi:10.1152/physiolgenomics.90360.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terhzaz S, Cabrero P, Chintapalli VR, Davies SA, Dow JAT. 2010. Mislocalization of mitochondria and compromised renal function and oxidative stress resistance in Drosophila SesB mutants. Physiol. Genomics 41, 33–41 10.1152/physiolgenomics.00147.2009 (doi:10.1152/physiolgenomics.00147.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chintapalli VR, Terhzaz S, Wang J, Al Bratty M, Watson DG, Herzyk P, Davies SA, Dow JAT. 2012. Functional correlates of positional and gender-specific renal asymmetry in Drosophila. PLoS ONE 7, e32577. 10.1371/journal.pone.0032577 (doi:10.1371/journal.pone.0032577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coast G. 2007. The endocrine control of salt balance in insects. Gen. Comp. Endocrinol. 152, 332–338 10.1016/j.ygcen.2007.02.018 (doi:10.1016/j.ygcen.2007.02.018) [DOI] [PubMed] [Google Scholar]
- 15.Dow JAT, Davies SA. 2003. Integrative physiology and functional genomics of epithelial function in a genetic model organism. Physiol. Rev. 83, 687–729 [DOI] [PubMed] [Google Scholar]
- 16.Dow JAT. 2011. The versatile stellate cell–more than just a space-filler. J. Insect Physiol. 58, 467–472 10.1016/j.jinsphys.2011.12.003 (doi:10.1016/j.jinsphys.2011.12.003) [DOI] [PubMed] [Google Scholar]
- 17.Coast GM, Webster SG, Schegg KM, Tobe SS, Schooley DA. 2001. The Drosophila melanogaster homologue of an insect calcitonin-like diuretic peptide stimulates V-ATPase activity in fruit fly Malpighian tubules. J. Exp. Biol. 204, 1795–1804 [DOI] [PubMed] [Google Scholar]
- 18.Cabrero P, Radford JC, Broderick KE, Costes L, Veenstra JA, Spana EP, Davies SA, Dow JAT. 2002. The Dh gene of Drosophila melanogaster encodes a diuretic peptide that acts through cyclic AMP. J. Exp. Biol. 205, 3799–3807 [DOI] [PubMed] [Google Scholar]
- 19.Kean L, Cazenave W, Costes L, Broderick KE, Graham S, Pollock VP, Davies SA, Veenstra JA, Dow JAT. 2002. Two nitridergic peptides are encoded by the gene capability in Drosophila melanogaster. Am. J. Physiol. Regul. Integr. Comp. Physiol. 282, R1297–R1307 [DOI] [PubMed] [Google Scholar]
- 20.Baggerman G, Cerstiaens A, De Loof A, Schoofs L. 2002. Peptidomics of the larval Drosophila melanogaster central nervous system. J. Biol. Chem. 277, 40 368–40 374 10.1074/jbc.M206257200 (doi:10.1074/jbc.M206257200) [DOI] [PubMed] [Google Scholar]
- 21.Terhzaz S, O'Connell FC, Pollock VP, Kean L, Davies SA, Veenstra JA, Dow JAT. 1999. Isolation and characterization of a leucokinin-like peptide of Drosophila melanogaster. J. Exp. Biol. 202, 3667–3676 [DOI] [PubMed] [Google Scholar]
- 22.Hayes TK, Pannabecker TL, Hinckley DJ, Holman GM, Nachman RJ, Petzel DH, Beyenbach KW. 1989. Leucokinins, a new family of ion transport stimulators and inhibitors in insect Malpighian tubules. Life Sci. 44, 1259–1266 10.1016/0024-3205(89)90362-7 (doi:10.1016/0024-3205(89)90362-7) [DOI] [PubMed] [Google Scholar]
- 23.Schooley DA, Horodyski FM, Coast GM. 2011. Hormones controlling homeostasis in insects. In Insect endocrinology (ed. Gilbert L. I.), pp. 366–429 Oxford, UK: Elsevier [Google Scholar]
- 24.Radford JC, Davies SA, Dow JAT. 2002. Systematic G-protein-coupled receptor analysis in Drosophila melanogaster identifies a leucokinin receptor with novel roles. J. Biol. Chem. 277, 38 810–388 17 10.1074/jbc.M203694200 (doi:10.1074/jbc.M203694200) [DOI] [PubMed] [Google Scholar]
- 25.O'Donnell MJ, Dow JAT, Huesmann GR, Tublitz NJ, Maddrell SHP. 1996. Separate control of anion and cation transport in malpighian tubules of Drosophila melanogaster. J. Exp. Biol. 199, 1163–1175 [DOI] [PubMed] [Google Scholar]
- 26.O'Donnell MJ, Rheault MR, Davies SA, Rosay P, Harvey BJ, Maddrell SHP, Kaiser K, Dow JAT. 1998. Hormonally controlled chloride movement across Drosophila tubules is via ion channels in stellate cells. Am. J. Physiol. 274, R1039–R1049 [DOI] [PubMed] [Google Scholar]
- 27.Blumenthal EM. 2003. Regulation of chloride permeability by endogenously produced tyramine in the Drosophila Malpighian tubule. Am. J. Physiol. Cell Physiol. 284, C718–C728 [DOI] [PubMed] [Google Scholar]
- 28.Blumenthal EM. 2009. Isoform- and cell-specific function of tyrosine decarboxylase in the Drosophila Malpighian tubule. J. Exp. Biol. 212, 3802–3809 10.1242/jeb.035782 (doi:10.1242/jeb.035782) [DOI] [PubMed] [Google Scholar]
- 29.Rosay P, Davies SA, Yu Y, Sozen MA, Kaiser K, Dow JAT. 1997. Cell-type specific calcium signalling in a Drosophila epithelium. J. Cell Sci. 110, 1683–1692 [DOI] [PubMed] [Google Scholar]
- 30.Gorokhovatsky AY, Marchenkov VV, Rudenko NV, Ivashina TV, Ksenzenko VN, Burkhardt N, Semisotnov GV, Vinokurov LM, Alakhov YB. 2004. Fusion of Aequorea victoria GFP and aequorin provides their Ca2+-induced interaction that results in red shift of GFP absorption and efficient bioluminescence energy transfer. Biochem. Biophys. Res. Commun. 320, 703–711 10.1016/j.bbrc.2004.06.014 (doi:10.1016/j.bbrc.2004.06.014) [DOI] [PubMed] [Google Scholar]
- 31.Martin JR, Rogers KL, Chagneau C, Brulet P. 2007. In vivo bioluminescence imaging of Ca signalling in the brain of Drosophila. PLoS ONE 2, e275. 10.1371/journal.pone.0000275 (doi:10.1371/journal.pone.0000275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dow JAT, Maddrell SHP, Görtz A, Skaer NV, Brogan S, Kaiser K. 1994. The Malpighian tubules of Drosophila melanogaster: a novel phenotype for studies of fluid secretion and its control. J. Exp. Biol. 197, 421–428 [DOI] [PubMed] [Google Scholar]
- 33.Terhzaz S, Southall TD, Lilley KS, Kean L, Allan AK, Davies SA, Dow JAT. 2006. Differential gel electrophoresis and transgenic mitochondrial calcium reporters demonstrate spatiotemporal filtering in calcium control of mitochondria. J. Biol. Chem. 281, 18 849–18 858 10.1074/jbc.M603002200 (doi:10.1074/jbc.M603002200) [DOI] [PubMed] [Google Scholar]
- 34.Button D, Eidsath A. 1996. Aequorin targeted to the endoplasmic reticulum reveals heterogeneity in luminal Ca++ concentration and reports agonist-induced or IP3-induced release of Ca++. Mol. Biol. Cell 7, 419–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radford JC, Terhzaz S, Cabrero P, Davies SA, Dow JAT. 2004. Functional characterisation of the Anopheles leucokinins and their cognate G-protein coupled receptor. J. Exp. Biol. 207, 4573–4586 10.1242/jeb.01317 (doi:10.1242/jeb.01317) [DOI] [PubMed] [Google Scholar]
- 36.Lu HL, Kersch C, Pietrantonio PV. 2011. The kinin receptor is expressed in the Malpighian tubule stellate cells in the mosquito Aedes aegypti (L.): a new model needed to explain ion transport? Insect Biochem. Mol. Biol. 41, 135–140 10.1016/j.ibmb.2010.10.003 (doi:10.1016/j.ibmb.2010.10.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cazzamali G, Klaerke DA, Grimmelikhuijzen CJ. 2005. A new family of insect tyramine receptors. Biochem. Biophys. Res. Commun. 338, 1189–1196 10.1016/j.bbrc.2005.10.058 (doi:10.1016/j.bbrc.2005.10.058) [DOI] [PubMed] [Google Scholar]
- 38.Herman AM, Blumenthal EM. 2006. Identification of the tyramine receptor in the Drosophila Malpighian tubule. FASEB J. 20, A345–A346 [Google Scholar]
- 39.Pollock VP, Radford JC, Pyne S, Hasan G, Dow JAT, Davies SA. 2003. NorpA and itpr mutants reveal roles for phospholipase C and inositol (1,4,5)-trisphosphate receptor in Drosophila melanogaster renal function. J. Exp. Biol. 206, 901–911 10.1242/jeb.00189 (doi:10.1242/jeb.00189) [DOI] [PubMed] [Google Scholar]
- 40.Venkatesh K, Hasan G. 1997. Disruption of the IP3 receptor gene of Drosophila affects larval metamorphosis and ecdysone release. Curr. Biol. 7, 500–509 10.1016/S0960-9822(06)00221-1 (doi:10.1016/S0960-9822(06)00221-1) [DOI] [PubMed] [Google Scholar]
- 41.Benito-Sipos J, Estacio-Gomez A, Moris-Sanz M, Baumgardt M, Thor S, Diaz-Benjumea FJ. 2010. A genetic cascade involving klumpfuss, nab and castor specifies the abdominal leucokinergic neurons in the Drosophila CNS. Development 137, 3327–3336 10.1242/dev.052233 (doi:10.1242/dev.052233) [DOI] [PubMed] [Google Scholar]
- 42.Cantera R, Nassel DR. 1992. Segmental peptidergic innervation of abdominal targets in larval and adult dipteran insects revealed with an antiserum against leucokinin I. Cell Tissue Res. 269, 459–471 10.1007/BF00353901 (doi:10.1007/BF00353901) [DOI] [PubMed] [Google Scholar]
- 43.de Haro M, Al-Ramahi I, Benito-Sipos J, Lopez-Arias B, Dorado B, Veenstra JA, Herrero P. 2010. Detailed analysis of leucokinin-expressing neurons and their candidate functions in the Drosophila nervous system. Cell Tissue Res. 339, 321–336 10.1007/s00441-009-0890-y (doi:10.1007/s00441-009-0890-y) [DOI] [PubMed] [Google Scholar]
- 44.Herrero P, Magarinos M, Torroja L, Canal I. 2003. Neurosecretory identity conferred by the apterous gene: lateral horn leucokinin neurons in Drosophila. J. Comp. Neurol. 457, 123–132 10.1002/cne.10555 (doi:10.1002/cne.10555) [DOI] [PubMed] [Google Scholar]
- 45.Lopez-Arias B, Dorado B, Herrero P. 2011. Blockade of the release of the neuropeptide leucokinin to determine its possible functions in fly behavior: chemoreception assays. Peptides 32, 545–552 10.1016/j.peptides.2010.07.002 (doi:10.1016/j.peptides.2010.07.002) [DOI] [PubMed] [Google Scholar]
- 46.Veenstra JA. 2009. Peptidergic paracrine and endocrine cells in the midgut of the fruit fly maggot. Cell Tissue Res. 336, 309–323 10.1007/s00441-009-0769-y (doi:10.1007/s00441-009-0769-y) [DOI] [PubMed] [Google Scholar]
- 47.Patel M, Chung JS, Kay I, Mallet AI, Gibbon CR, Thompson KS, Bacon JP, Coast GM. 1994. Localization of Locusta-DP in locust CNS and hemolymph satisfies initial hormonal criteria. Peptides 15, 591–602 10.1016/0196-9781(94)90081-7 (doi:10.1016/0196-9781(94)90081-7) [DOI] [PubMed] [Google Scholar]
- 48.Te Brugge VA, Nassel DR, Coast GM, Schooley DA, Orchard I. 2001. The distribution of a kinin-like peptide and its co-localization with a CRF-like peptide in the blood-feeding bug, Rhodnius prolixus. Peptides 22, 161–173 10.1016/S0196-9781(00)00373-9 (doi:10.1016/S0196-9781(00)00373-9) [DOI] [PubMed] [Google Scholar]