Abstract
The distribution of rainforest in many regions across the Earth was strongly affected by Pleistocene ice ages. However, the extent to which these dynamics are still important for modern-day biodiversity patterns within tropical biodiversity hotspots has not been assessed. We employ a comprehensive dataset of Madagascan palms (Arecaceae) and climate reconstructions from the last glacial maximum (LGM; 21 000 years ago) to assess the relative role of modern environment and LGM climate in explaining geographical species richness patterns in this major tropical biodiversity hotspot. We found that palaeoclimate exerted a strong influence on palm species richness patterns, with richness peaking in areas with higher LGM precipitation relative to present-day even after controlling for modern environment, in particular in northeastern Madagascar, consistent with the persistence of tropical rainforest during the LGM primarily in this region. Our results provide evidence that diversity patterns in the World's most biodiverse regions may be shaped by long-term climate history as well as contemporary environment.
Keywords: Arecaceae, last ice age, Madagascar, palms, palaeoclimate, species richness
1. Introduction
The underlying drivers of species richness have eluded scientists for decades [1]. Contemporary environment is commonly thought to govern large-scale patterns in species richness, and consequently most studies have highlighted its role, with climate and productivity identified as the strongest drivers of richness patterns (e.g. [2,3]). However, species richness patterns are also shaped by the interaction of such contemporary environmental drivers with the long-term evolutionary–historical processes that generate species richness [4]. Notably, palaeoclimate has received increasing attention as an historical driver of current richness patterns [5–7]. The Earth's climate has undergone major changes in the past, with massive oscillations between cold glacial and warm interglacial periods during the last 2.6 Myr (the Quaternary; [8]). The well-established climatic stability hypothesis [9–11] proposes that climatically stable areas give rise to higher species richness [10] and higher endemism [11]. Notably, a recent global-scale study has linked endemism in birds, mammals and amphibians to low Late Quaternary climate displacement rates [12].
In general, the tropics are considered to be more climatically stable than higher latitudes, as they were not directly subjected to massive Quaternary glaciations [8]. However, Quaternary and older climatic changes have also occured in the tropics, affecting the distribution of vegetation [13–15]. Both cooling and precipitation changes (often drying) and low CO2 levels associated with glacial periods have been important, causing contractions of wet lowland forest [16], the extent of which remain the subject of ongoing debate [17,18]. Remnant rainforest areas have been hypothesized as glacial refugia for many organisms, for example, in Africa [19–21] and the New World [22]. Empirical studies have provided indirect or less commonly direct evidence that species distributions and endemism patterns can indeed be associated with Quaternary-scale habitat stability in tropical areas [7,23,24]. Today, vegetation distribution in the tropics is primarily controlled by precipitation [25], which also plays a particularly important role as the main driver of species richness at low latitudes [3]. Nevertheless, the importance of palaeoclimate change for tropical species richness and endemism patterns, in particular the role played by precipitation change, still remains poorly understood and controversial.
Madagascar is among the most important biodiversity hotspots [26]. The origin of Madagascar's biotic diversity and endemism has stimulated intense scientific interest [27–29]. Madagascar's high species richness and endemism rate is commonly attributed to the long isolation of the island from other landmasses (88 Myr; [30]), and notably biome age within Madagascar has been linked to plant endemism levels [31]. The tropical rainforest in eastern Madagascar harbours most of Madagascar's biodiversity [32], parts of which have been identified as refugia during the last glacial maximum (LGM) [33]. The age of Madagascar's eastern rainforests, which have existed since the Eocene [31], coupled with high geological and topographic complexity [34], is thought to explain patterns of micro-endemism and radiations in numerous taxa [35]. However, at this point, little consensus exists regarding general drivers of species richness and endemism patterns in Madagascar. Furthermore, all previous studies are based on fauna, leaving a major knowledge gap concerning Madagascar's species-rich and highly endemic flora.
Here, we present, to our knowledge, the first study assessing the drivers of Madagascan plant species richness and endemism patterns, with special emphasis on the potential role of palaeoclimate. We focus on a keystone plant family that has diversified in the rainforest biome since its earliest origin, the palms (Arecaceae; [36]). Madagascar is among the most palm-rich islands in the world [37]. Its palm diversity exceeds that of the whole African continent threefold [37] with 195 native species, of which 192 are endemic and over 90 per cent are placed within two genera, Dypsis (159 species) and Ravenea (18 species) [38]. New species of palms continue to be discovered at a high rate in Madagascar, although novelties tend to be both rare and threatened with extinction [39]. Over 90 per cent of Madagascan palms occur in rainforest, a figure that matches global estimates of palm biome preference [36], consistent with the important role of precipitation as an environmental driver of palm distribution and diversity patterns in other areas [6,40–42]. Palaeoclimate has been found to be an important driver of palm richness and endemism patterns globally and in the New World [6,43], but no previous study to our knowledge has addressed this issue for Madagascan palms, or indeed any other component of the island's biota. Here, we examine specifically: (i) if LGM climate effects are still discernible in palm species richness patterns on Madagascar, and (ii), if yes, what is the relative importance of LGM precipitation (reflecting the general importance of precipitation for diversity gradients at low latitudes) and temperature (as seen at high latitudes)? We built on our long-term research efforts on Madagascan palm diversity [39,44] to develop and evaluate carefully a methodology to estimate spatial patterns in palm species richness using species distribution modelling [45] to estimate the species ranges.
2. Material and methods
(a). Palm distributions
Distributional data for 193 native species came from a comprehensive database of Madagascan palms developed by the Royal Botanic Gardens, Kew (figure 1a; electronic supplementary material, appendix A and table S1). Distribution models for 83 species (those with greater than or equal to 5 geographically unique locality records at 30″ resolution (approx. 1 × 1 km resolution)) were created using Maxent [46]. The modelling was implemented using three sets of predictors: (i) 27 environmental predictors, (ii) 27 environmental predictors and a balancing number of spatial filters, and (iii) 10 environmental predictors and a balancing number of spatial filters, following Blach-Overgaard et al. [41] (see electronic supplementary material, appendix B and table S2). Species richness maps were constructed by summing up the estimated distributions of the 193 palm species, i.e. the estimated distributions for the 83 successfully modelled species made into binary predictions using the minimum training presence threshold (MIN; the lowest predicted value of probability of occurrence coinciding with any site, where a species has been detected) and maximum training specificity plus sensitivity threshold (MAX; the threshold at which the sum of sensitivity and specificity is maximized) [47] and the observed localities for the remaining 110 species (see the electronic supplementary material, appendix B) resulting in six different estimated species richness patterns. To assess the influence of sample size, we also constructed richness maps by summing up only modelled distributions for species with greater than or equal to 10 (n = 46) geographically unique locality records, using the observed locations for the remaining 147 species. We used modelled distributions for species with sufficient presence data as observed localities only partially represent widespread species' actual distributions, leading to an underestimation of species richness. As a final step, to assess the relative accuracy of the six species richness estimates we compared them with the observed species richness in 23 well-surveyed localities across Madagascar, where the local palm flora has been comprehensively sampled (figure 1a). The evaluation was carriedout using Pearson's correlation r and ANOVA tests. The four richness estimates based on modelling involving spatial filters deviated the least from that observed at the 23 well-sampled sites, in terms of both general richness level and spatial patterning (see the electronic supplementary material, tables S3–S5). Using the MIN threshold tended to provide slightly better estimates than the MAX threshold; thus, we used the estimates based on the former for the species richness modelling. Given the small differences in predictive ability and richness estimates for the two environment-filter distribution models using the MIN threshold (see the electronic supplementary material, table S6), we used richness estimates from both models for the richness analyses, reporting the results of the model with 10 environmental predictors in the main text and those of the model with 27 environmental predictors in the electronic supplementary material, appendix B.
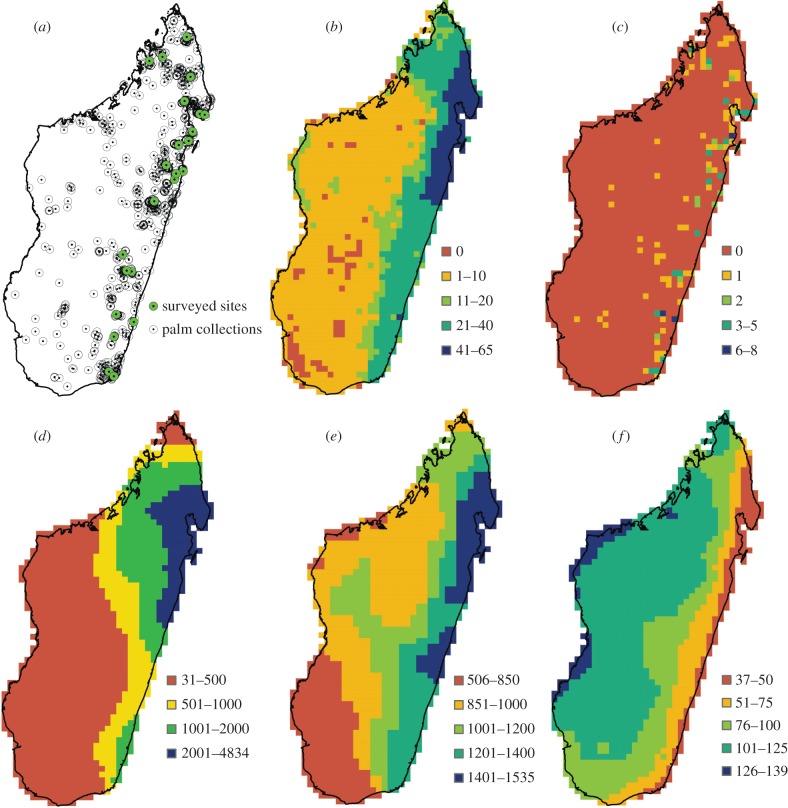
Figure 1.
Palm species occurrences and richness patterns and their most important palaeoclimatic and present-day drivers across Madagascar drawn at 0.2° resolution. (a) Palm collections across Madagascar. Green circles indicate the well-surveyed sites for palms on Madagascar (23) and open circles indicate sites where other palm collections have been made. (b) Total species richness (number of species), (c) rare palm species richness (number of species), (d) LGM precipitation anomaly (mm), (e) actual evapotranspiration (mm), (f) precipitation seasonality (coefficient of variation).
(b). Species richness analyses
Total species richness estimates were obtained by summing individual species distributions corresponding to the predictions used in the best species richness representations in comparison with the well-surveyed sites (see the electronic supplementary material, appendix B and tables S3–S5) at two different spatial scales, namely at 0.2° (approx. 22 × 22 km; n = 1395 cells) and 0.5° (approx. 52 × 52 km; n = 249 cells; only given in the electronic supplementary material). We subsequently extracted the rare-species richness fraction corresponding to the rarest 51.3 per cent, i.e. species with less than five occurrence records, thus species not modelled in Maxent (n = 99). We assessed the potential role of palaeoclimate for spatial variation in palm species richness in Madagascar by including measures of climate change since the LGM (21 000 years before present; [48]). We used the ensemble mean of two palaeoclimatic reconstructions, namely the Community Climate System Model v. 3 (CCSM3) and the Model for Interdisciplinary Research on Climate v. 3.2 (MIROC3.2) obtained from the Worldclim dataset provided by the Palaeoclimate Modelling Intercomparison Project Phase II (PMIP2) [48]. The palaeoclimatic predictors were computed as anomalies between precipitation and temperature from the LGM and present-day annual precipitation and mean annual temperature from the Worldclim dataset [49] at 2.5′ (i.e. LGM minus present) resulting in two predictors, namely LGM temperature (TEMPLGM) and precipitation (PRECLGM) anomaly (see the electronic supplementary material, appendix C). In addition, we also computed the anomalies for the two palaeoclimatic reconstructions separately and used these in supplementary separate richness analyses. We interpreted the palaeoclimate anomalies as representative for the general spatial pattern in glacial–interglacial climate oscillations during the Quaternary [11]. Present-day environment was represented by 10 predictor variables (for computations and sources, see electronic supplementary material, appendix C): annual precipitation (PREC) and mean annual temperature (TEMP), precipitation seasonality (PSEA), actual (AET) and potential evapotranspiration (PET), topographic range (TOPO), geological heterogeneity (GEOL), vegetation-type heterogeneity (VEG), the human influence index (HII) and average historical human population density (HPD) per square kilometre for AD 0, AD 500, AD 1000 and AD 1500. The species richness data are deposited in the Dryad Repository: http://dx.doi.org/10.5061/dryad.3df12.
Bivariate correlations between all potential explanatory variables for the richness analyses and the richness estimates were computed using the package Modttest in R [50]. Significance of the correlations was assessed using Dutilleul's method to estimate the number of degrees of freedom to account for spatial autocorrelation [51]. Multicollinearity may compromise multiple regressions and was assessed by computing variance inflation factors (VIFs) for each explanatory variable in the full set providing VIFs ≤ 6.2 at 0.2° and 7.3 at 0.5°. VIF < 10 usually indicates that multicollinearity is not a problem [52].
The influence of the potential drivers on the species richness estimates was then tested using regression modelling. Regression modelling was first implemented using ordinary least squares (OLS) multiple linear regression, starting with a model using all the predictors. Given the spatial nature of the data, residuals were checked for spatial autocorrelation by computing Moran's I correlograms. If the OLS residuals showed non-negligible spatial autocorrelation (Moran's I > 0.100 for any distance class, using 21 distance classes with approximately equal frequency), indicating violation of statistical independence among the data points, regression modelling was then implemented using simultaneous autoregressive (SAR) modelling, which has been shown to be one of the best performing spatial regression techniques [53]. Spatial effects are here incorporated directly into model residual structure. As a result, the overall residuals will be by definition spatially autocorrelated, but they can be decomposed into a spatial component and an error component [54]. The latter was again checked for spatial autocorrelation by computing Moran's I correlograms. The spatial autoregressive parameter (rho) determines the strength of the spatial effect and was estimated empirically. In the SAR model, the matrix W contains neighbour weights (wij), which indicate the relationships among spatial units. The elements wij are given as an inverse function of geographical distance (dij), given by wij = 1/dijalpha. Alpha was first set to 1, but increased to higher values if necessary to obtain Moran's I ≤ 0.100 in all distance classes for the error term. After obtaining a full model with negligible spatial autocorrelation in the error term, we then obtained a minimum adequate model by sequentially removing the most non-significant term until only significant terms remained in the model. This model simplification did not cause other than minor changes in coefficient estimates and explanatory power (R2), and spatial autocorrelation remained negligible. For SAR models, two R2's are reported: one accounting for the effect of the predictors free of space (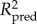 ), and one also taking into account the spatial structure modelled
), and one also taking into account the spatial structure modelled 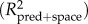 . For OLS models, both the standard R2
. For OLS models, both the standard R2
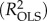 and the adjusted R2
and the adjusted R2
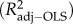 , with the latter adjusting for the number of predictors, are reported.
, with the latter adjusting for the number of predictors, are reported.
Residual plots of all regressions were inspected to check for nonlinearity and normality (residuals for overall richness estimates were all fine, while those for rare palms are somewhat skewed reflecting the high skewness of the rare palms variables). All regression analyses were computed in SAM v. 4.0 [55].
3. Results
All richness measures were positively correlated to the LGM precipitation anomaly, annual precipitation and actual evapotranspiration, and negatively correlated to precipitation seasonality, the correlations being strong for overall species richness and moderate for rare palm species richness (at both resolutions: table 1 and figure 1b–f; see also electronic supplementary material, tables S7 and S8). Additionally, there were moderate, positive correlations with topographic range for all richness patterns and with human influence for overall richness (table 1; electronic supplementary material, tables S7 and S8). The other predictors did not have significant correlations to the species richness estimates (table 1; electronic supplementary material, tables S7 and S8).
Table 1.
Pearson correlations (r) between 0.2° palm species richness on Madagascar and its potential drivers, with significance computed using Dutilleul's method [51] to estimate the number of degrees of freedom (accounting for spatial autocorrelation). (n = 1387 0.2° cells. The asterisks indicate significant levels. *p < 0.05; **p < 0.01; ***p < 0.001; n.s., not significant. Total species richness is based on the parsimonious complex model (see §2). PRECLGM, LGM precipitation anomaly; TEMPLGM, LGM temperature anomaly; PREC, annual precipitation; TEMP, annual mean temperature; PSEA, precipitation seasonality; AET, actual evapotranspiration; PET, potential evapotranspiration; TOPO, topographic range; VEG, vegetation-type heterogeneity; GEOL, geological heterogeneity; TOPO, topographic range; HII, human influence; HPH, historical population density.)
| variable | richnesstotalr | richnessrare r |
|---|---|---|
| PRECLGM | 0.809*** | 0.260*** |
| TEMPLGM | −0.317 (n.s.) | −0.118 (n.s.) |
| PREC | 0.711** | 0.249*** |
| TEMP | −0.265 (n.s.) | −0.074 (n.s.) |
| PSEA | −0.767*** | −0.272*** |
| AET | 0.821*** | 0.257*** |
| PET | 0.100 (n.s.) | 0.027 (n.s.) |
| TOPO | 0.259* | 0.123* |
| VEG | 0.089 (n.s.) | 0.011 (n.s.) |
| GEOL | −0.018 (n.s.) | −0.024 (n.s.) |
| HII | 0.357* | 0.069 (n.s.) |
| HPH | 0.264 (n.s.) | 0.058 (n.s.) |
OLS regression models with the full set of predictors had strong residual spatial autocorrelation for overall richness estimates (see the electronic supplementary material, table S9), but negligible residual spatial autocorrelation for rare palm species richness (see the electronic supplementary material, table S10). Hence, final models were fitted for overall richness using SAR, successfully removing spatial autocorrelation from the error residual structure (see the electronic supplementary material, table S10). The final minimum adequate models had strong explanatory power for overall richness and consistently indicated a strong, positive effect of PRECLGM as the strongest effect, with additional strong, positive effects of AET and TEMPLGM, a strong negative effect of PSEA, and moderate to weak effects of the remaining predictors (table 2 and figure 2; see also electronic supplementary material, table S11 and figure S1). For rare palm species, the strongest predictors were PSEA with a moderate, negative effect and PREC with a moderate, positive effect, with additional positive effects of PRECLGM and TOPO, but explanatory power was low (table 2). Similar results were provided by models using the full set of predictors as well as the OLS models for overall richness (see the electronic supplementary material, tables S9 and S10). For total richness, PRECLGM and AET were also consistently the strongest predictors at 0.5° resolution (see the electronic supplementary material, tables S12–S14) and for the richness estimates based on modelled distributions for species with at least 10 records and the observed localities for the remaining species (see the electronic supplementary material, table S15).
Table 2.
Final minimum adequate model (backward elimination of non-significant predictors (least significant first removed)) constructed to explain 0.2° palm species richness on Madagascar for total and rare species. (Alpha kept as in the final full model (see the electronic supplementary material, table S10). Only the OLS model for richnessrare−0.2° was run as Moran's I < 0.100 for OLS regression residuals in all distance classes. Standardized predictors are given. n = 1387 0.2° cells. The asterisks indicate significant levels. *p < 0.05; **p < 0.01; ***p < 0.001. Dashes (—) denote predictor not selected.)
| richnesstotal |
richnessrare |
|||
|---|---|---|---|---|
| variable | beta | p-value | beta | p-value |
| PRECLGM | 0.470 | *** | 0.093 | * |
| TEMPLGM | 0.294 | *** | — | — |
| PREC | −0.074 | * | 0.107 | ** |
| TEMP | −0.144 | *** | — | — |
| PSEA | −0.291 | *** | −0.169 | *** |
| AET | 0.298 | *** | — | — |
| PET | — | — | — | — |
| TOPO | 0.094 | *** | 0.081 | ** |
| VEG | 0.071 | *** | — | — |
| GEOL | 0.033 | *** | — | — |
| HII | 0.032 | *** | — | — |
| HPH | — | — | −0.068 | * |
| rho/alpha | 0.993 | /2.5 | — | — |
| F | 1001.9 | *** | 31.7 | *** |
| R2pred+space | 0.884 | — | — | |
| R2pred/R2OLS | 0.879 | /0.902 | — | 0.103a |
| Moran's Ib | 0.064 | 0.011 | ||
aR2adj−OLS = 0.100. Total species richness based on the parsimonious complex model (see §2). For predictor abbreviations, see table 1.
bMaximum |Moran's I| for the SAR error residuals, or richnessrare OLS regression residuals.
Figure 2.
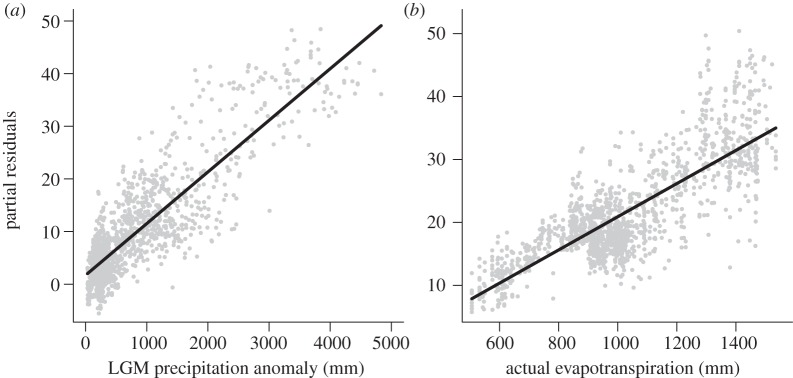
Partial residual plots showing the effect of (a) LGM precipitation anomaly (PRECLGM) and (b) actual evapotranspiration (AET) on total species richness when all other predictors in the final minimum adequate model (table 2) are accounted for statistically at 0.2° (see also electronic supplementary material, figure S1). Black lines are linear fits.
Considering the two palaeoclimatic reconstructions separately, the CCSM3 simulations provided similar results as the ensemble mean for overall richness, while the MIROC3.2 simulations differed, with TEMPLGM not being selected in the final model and PRECLGM showing a weaker, negative effect. However, the latter model also had a much lower explanatory power than that of the ensemble mean and the CCSM3 (see the electronic supplementary material, table S16).
4. Discussion
The results presented here provide, to our knowledge, the first direct empirical evidence that palaeoclimatic legacies are important for geographical species richness patterns within a tropical biodiversity hotspot. More specifically, we find that higher LGM precipitation relative to present-day in northeastern Madagascar is a major factor in the high palm species richness in this region. In line with the general importance of precipitation for diversity patterns at low latitudes [3] and for glacial–interglacial vegetation dynamics in tropical lowland areas (e.g. [13]), we also find a stronger effect of LGM precipitation than temperature, in contrast with previous high-latitude and global findings [5,11,12].
Peaks in palm species richness, including the presence of rare local endemics in Madagascar, occur in the northeastern part of the island, extending from Toamasina region to the Masoala Peninsula and Marojejy mountains (figure 1b,c). These patterns coincide with areas where LGM precipitation has been highest relative to today (figure 1d), and we found this aspect of palaeoclimate to be the strongest driver compared with present-day environment for overall species richness and also having a moderate effect on the rare-species richness pattern (table 2). In addition, we note that correlations between the richness estimates and the actual values for precipitation at the LGM rather than the anomaly also were positive, the correlations being strong for overall richness and moderate for rare-species richness (see the electronic supplementary material, table S17). For all richness estimates, the correlations to actual values for precipitation at the LGM were higher than their correlations to present-day precipitation (table 1; electronic supplementary material, table S7 and S17). Hence, our results indicate that palm species richness on Madagascar is not only determined by contemporary factors, but also tend to peak where rainforest was most likely to persist through glacial periods. We note that precipitation varied more strongly across Madagascar during the LGM (s.d. =±1366.4 mm) than currently (s.d. =±572.4 mm). Our results add to the growing evidence that palaeoclimate is important in shaping current biodiversity patterns [5,11,12]. Specifically, our results are in line with studies from Africa and other tropical regions that have found palaeoclimate to be important for the persistence and diversification of lineages during the massive global climatic vicissitudes of the last few million years [7,23,24]. In addition, other studies of palms have also shown that long-term habitat stability and palaeoclimate specifically have a determining role for palm diversity patterns both in other tropical regions and globally [6,43]. Our results are also congruent with empirical analyses of the patterns of endemism in different groups of animals in Madagascar, which have also identified the northeastern region as harbouring the highest endemic species richness [29,33,56]. Owing to these biogeographic patterns, parts of north and northeastern Madagascar have been hypothesized to have acted as refugia during the Pleistocene [33,35], although direct evidence for this is lacking. In this study, we focused on species richness as a key facet of macroecological patterns [1], but alternatively one could also have looked at the importance of palaeoclimate for the distribution of individual species [57]. However, as a majority of the Madagascan palm species have small to very small ranges, sample size will be too low to implement such an approach for most species (cf. [57]).
The stronger importance of LGM precipitation relative to LGM temperature is contrary to a number of previous studies [5,6,12], probably reflecting the fact that Madagascar is a tropical region, while most of these studies have focused on the temperate zone or global patterns, and thus include areas that have been influenced by the massive high-latitude temperature shifts. The high importance of LGM precipitation documented in this study is consistent with large-scale vegetation-type distributions in the tropics being strongly controlled by precipitation (e.g. [25]), and with water availability predominating over temperature in controlling species diversity patterns in the tropics and subtropics [3]. For continental-scale assessments of palms specifically, there is also evidence that species distribution and diversity patterns are more strongly driven by precipitation than temperature [40–42]. It is well known that the dry and cold conditions during the Pleistocene glacial periods, including the LGM have caused moderate to drastic changes in tropical rainforest distributions [13,14]; notably, rainforests in Africa retracted to fairly small regions where water availability remained sufficient, i.e. rainforest refugia [14,15,20]. Pollen-based palaeoecological reconstructions and biome modelling indicate LGM persistence of rainforest in the northeast of Madagascar [16,58]. The relative LGM precipitation anomaly pattern according to the mean of the two palaeoclimatic reconstructions used in this study, and CCSM3 alone (but not MIROC3.2) is consistent with this pattern. Interpreting the LGM-to-present anomalies as representative for the general pattern of the Quaternary glacial–interglacial climate oscillations (cf. [11]), the LGM predictions used here generally suggest higher precipitation in Madagascar during glacial periods, with the greatest increase in the northeast (figure 1d). We interpret this pattern as indicating the relative wetness change during the Quaternary, with high values indicating where rainforest has had the greatest possibilities for persistence through glacials. The low CO2 concentrations during glacial periods would have increased plant–water requirements, and thus changed vegetation–precipitation relationships [59]. Hence, relatively higher precipitation levels—as reconstructed for the northeast of Madagascar—may have been required for maintaining rainforest through glacial periods. Therefore, the northeast has probably acted as the major rainforest refugium in Madagascar during glacials, as supported also by phylogeographic studies [60]. In the light of this, our results show that this refuge pattern has left an important imprint on current patterns of palm species richness in this tropical biodiversity hotspot. Similarly, some African palm species have distributions that are still associated with rainforest refugia [41], and small-range palm species in Western Amazonia are also more frequent in rainforest refuge areas [43]. A slightly different interpretation of the observed patterns could be as legacy effect with species richness having built up in areas with the highest long-term precipitation and thus highest productivity and thus is not in equilibrium with modern climate [61]. At present, we do not have data to assess if glacial precipitation conditions are indeed more representative of the long-term Late Cenozoic climate on Madagascar than present-day conditions.
Habitat stability is thought to favour high speciation as well as low-extinction rates—allowing the accumulation of ancient lineages [23]. Palms have been found to exhibit higher diversification rates in warm and wet environments, such as the rainforest [62]. Thus, glacial rainforest refugia on Madagascar may have functioned both as low-extinction safe-havens and potentially as speciation pumps for palms, provided these refugia have persisted throughout the Quaternary. Supporting this interpretation, the northeast of Madagascar includes both clear palaeoendemics such as the monotypic genera Lemurophoenix, Satranala and Voanioala as well as many endemics from the species-rich genera Dypsis and Ravenea. Over 90 per cent of species diversity of Madagascar palms falls within these two genera, which are both estimated to have diverged from their closest relatives relatively recently compared with other palm genera (ca 13 Ma; [36]) and may have undergone rapid radiations, especially Dypsis [63]. Glacial refuge locations have been proposed to explain patterns of micro-endemism on Madagascar [28], notably the habitat isolation caused by aridification of low-elevation river catchments during Quaternary glacial periods will have acted as barriers to gene flow, and consequently would have stimulated speciation of local endemics that are now concentrated mainly in these refuge sites [28]. However, we do not yet understand the evolutionary history of Madagascar's palms well enough to assess how much speciation has occured during the Quaternary.
Regarding the data for this study, we considered the uncertainties in palaeoclimatic simulations of precipitation by attempting to minimize them using the ensemble mean of two simulation models, as well as testing the effects of the individual simulation models CCSM3 and MIROC3.2. The strong LGM precipitation anomaly link to palm richness for the ensemble-mean reconstruction was similarly found for CCSM3, but not MIROC3.2 (see the electronic supplementary material, table S16). The ensemble-mean and CCSM3 precipitation patterns capture the peak in palm species richness in northeast Madagascar and as mentioned above this is consistent with the LGM presence of rainforest habitat in the northeast according to palaeoecological reconstructions and biome modelling [16,58]. The MIROC3.2 reconstruction fails to do this, and as a result provides a much weaker explanatory model for the palm species richness patterns (table 2; electronic supplementary material, tables S11 and S16).
While palaeoclimate emerged as a strong driver of Madagascan palm species richness patterns, current environment was also important, with palm species richness increasing with current water-energy availability (AET; total richness) or increasing levels of present-day precipitation (rare species), and in areas with less seasonality in precipitation. More weakly, palm species richness also increased in environmentally heterogeneous areas, notably in relation to topographic heterogeneity. Analogously, annual precipitation and topographic range have been found to be important for palm species richness patterns in the New World [40] and globally ([6], see [42] for a review). There was also a very weak positive effect of human influence, which may reflect that suitable palm habitats are also preferred sites for human settlements.
Here, we have presented, to our knowledge, the first quantitative assessment of the role of palaeoclimate for diversity patterns on Madagascar, and the first spatial study of plant species richness drivers in this tropical biodiversity hotspot. Notably, our findings provide, to the best of our knowledge, the first example of palaeoclimate as the main driver of species richness patterns within a tropical biodiversity hotspot. Owing to their association with tropical rainforest, Madagascan palms are sensitive to changes in precipitation, as indicated in this study (see also [42]). The strong roles of long-term precipitation changes and present-day precipitation for palm species richness are therefore a matter of concern given the projected future climate changes on Madagascar. It is predicted that temperatures will rise in Madagascar, as elsewhere, and precipitation will become more erratic in the future [64], with the fragmented eastern forests being especially prone to future drying [65]. The potential threat from future climate change for palms is likely to be even more severe when combined with land-use changes. Primary vegetation has disappeared in many areas of the island, including in the important glacial refuge region in the northeast. Rainforest as well as other forest types in Madagascar are highly fragmented owing to extensive deforestation [66,67] and has been reduced to perhaps less than 25 per cent of its original extent (J. Moat 2012, unpublished data). As a result, some species are already locally extinct while others are known only from historical data [68,69]. With growing population rates and demand for agricultural land [70], it is likely that the biodiversity of Madagascar will become in double jeopardy because of climate change and habitat loss.
Acknowledgements
We thank the herbaria of the Royal Botanic Gardens Kew, Missouri Botanical Garden, Musée National d'Histoire Naturelle in Paris, New York Botanical Garden, Aarhus University and Parc Botanique et Zoologique de Tsimbazaza for sharing data and providing access to specimens. We further acknowledge the international modelling groups for providing the LGM data for analysis and the Laboratoire des Sciences du Climat et de l'Environnement (LSCE) for collecting and archiving them. The PMIP2 Data Archive is supported by CEA, CNRS and the Programme National d'Etude de la Dynamique du Climat (PNEDC). M.R. wishes to thank the Global Biodiversity Information and Facility (GBIF) for the opportunity to participate in the third ecological niche modelling workshop in India, 2006. This work was funded by the Threatened Plants Appeal at the Royal Botanic Gardens, Kew, the Bentham-Moxon Trust and a grant from the Danish Council for Independent Research—Natural Sciences (grant nos 272–07-0242 and 12-125079 to J.-C.S.). We thank Lindell Bromham and two anonymous reviewers for their helpful comments.
References
- 1.Turner JRG. 2004. Explaining the global biodiversity gradient: energy, area, history and natural selection. Basic Appl. Ecol. 5, 435–448 10.1016/j.baae.2004.08.004 (doi:10.1016/j.baae.2004.08.004) [DOI] [Google Scholar]
- 2.Field R, et al. 2009. Spatial species-richness gradients across scales: a meta-analysis. J. Biogeogr. 36, 132–147 10.1111/j.1365-2699.2008.01963.x (doi:10.1111/j.1365-2699.2008.01963.x) [DOI] [Google Scholar]
- 3.Hawkins BA, et al. 2003. Energy, water, and broad-scale geographic patterns of species richness. Ecology 84, 3105–3117 10.1890/03-8006 (doi:10.1890/03-8006) [DOI] [Google Scholar]
- 4.Ricklefs RE. 2004. A comprehensive framework for global patterns in biodiversity. Ecol. Lett. 7, 1–15 10.1046/j.1461-0248.2003.00554.x (doi:10.1046/j.1461-0248.2003.00554.x) [DOI] [Google Scholar]
- 5.Svenning J-C, Skov F. 2007. Ice age legacies in the geographical distribution of tree species richness in Europe. Global Ecol. Biogeogr. 16, 234–245 10.1111/j.1466-822x.2006.00280.x (doi:10.1111/j.1466-822x.2006.00280.x) [DOI] [Google Scholar]
- 6.Kissling WD, Baker WJ, Balslev H, Barfod AS, Borchsenius F, Dransfield J, Govaerts R, Svenning J-C. 2012. Quaternary and pre-Quaternary historical legacies in the global distribution of a major tropical plant lineage. Global Ecol. Biogeogr. 21, 909–921 10.1111/j.1466-8238.2011.00728.x (doi:10.1111/j.1466-8238.2011.00728.x) [DOI] [Google Scholar]
- 7.Carnaval AC, Moritz C. 2008. Historical climate modelling predicts patterns of current biodiversity in the Brazilian Atlantic forest. J. Biogeogr. 35, 1187–1201 10.1111/j.1365-2699.2007.01870.x (doi:10.1111/j.1365-2699.2007.01870.x) [DOI] [Google Scholar]
- 8.Ruddiman WF. 2001. Earth's climate: past and future, 2nd edn New York, NY: W. H. Freeman and Company [Google Scholar]
- 9.Pianka ER. 1966. Latitudinal gradients in species diversity: a review of concepts. Am. Nat. 100, 33–46 10.1086/282398 (doi:10.1086/282398) [DOI] [Google Scholar]
- 10.Dynesius M, Jansson R. 2000. Evolutionary consequences of changes in species’ geographical distributions driven by Milankovitch climate oscillations. Proc. Natl Acad. Sci. USA 97, 9115–9120 10.1073/pnas.97.16.9115 (doi:10.1073/pnas.97.16.9115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jansson R. 2003. Global patterns in endemism explained by past climate change. Proc. R. Soc. Lond. B 270, 583–590 10.1098/rspb.2002.2283 (doi:10.1098/rspb.2002.2283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandel B, Arge L, Dalsgaard B, Davies RG, Gaston KJ, Sutherland WJ, Svenning J-C. 2011. The influence of late Quaternary climate-change velocity on species endemism. Science 334, 660–664 10.1126/science.1210173 (doi:10.1126/science.1210173) [DOI] [PubMed] [Google Scholar]
- 13.Anhuf D, et al. 2006. Paleo-environmental change in Amazonian and African rainforest during the LGM. Palaeogeogr. Palaeoclimatol. Palaeoecol. 239, 510–527 10.1016/j.palaeo.2006.01.017 (doi:10.1016/j.palaeo.2006.01.017) [DOI] [Google Scholar]
- 14.Plana V. 2004. Mechanisms and tempo of evolution in the African Guineo-Congolian rainforest. Phil. Trans. R. Soc. Lond. B 359, 1585–1594 10.1098/rstb.2004.1535 (doi:10.1098/rstb.2004.1535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamilton AC, Taylor D. 1991. History of climate and forests in tropical Africa during the last 8 million years. Clim. Change 19, 65–78 10.1007/BF00142215 (doi:10.1007/BF00142215) [DOI] [Google Scholar]
- 16.Harrison SP, Prentice CI. 2003. Climate and CO2 controls on global vegetation distribution at the last glacial maximum: analysis based on palaeovegetation data, biome modelling and palaeoclimate simulations. Global Change Biol. 9, 983–1004 10.1046/j.1365-2486.2003.00640.x (doi:10.1046/j.1365-2486.2003.00640.x) [DOI] [Google Scholar]
- 17.Bush MB, De Oliveira PE, Colinvaux PA, Miller MC, Moreno JE. 2004. Amazonian paleoecological histories: one hill, three watersheds. Palaeogeogr. Palaeoclimatol. Palaeoecol. 214, 359–393 10.1016/j.palaeo.2004.07.031 (doi:10.1016/j.palaeo.2004.07.031) [DOI] [Google Scholar]
- 18.Hooghiemstra H, van der Hammen T. 1998. Neogene and Quaternary development of the neotropical rain forest: the forest refugia hypothesis, and a literature overview. Earth Sci. Rev. 44, 147–183 10.1016/S0012-8252(98)00027-0 (doi:10.1016/S0012-8252(98)00027-0) [DOI] [Google Scholar]
- 19.Hamilton AC. 1981. The Quaternary history of African forests: its relevance to conservation. Afr. J. Ecol. 19, 1–6 10.1111/j.1365-2028.1981.tb00647.x (doi:10.1111/j.1365-2028.1981.tb00647.x) [DOI] [Google Scholar]
- 20.Maley J. 1991. The African rain forest vegetation and paleoenvironments during late Quaternary. Clim. Change 19, 79–98 10.1007/BF00142216 (doi:10.1007/BF00142216) [DOI] [Google Scholar]
- 21.Mayr E, O'Hara RJ. 1986. The biogeographic evidence supporting the Pleistocene forest refuge hypothesis. Evolution 40, 55–67 10.2307/2408603 (doi:10.2307/2408603) [DOI] [PubMed] [Google Scholar]
- 22.De Mello Martins F. 2011. Historical biogeography of the Brazilian Atlantic forest and the Carnaval–Moritz model of Pleistocene refugia: what do phylogeographical studies tell us? Biol. J. Linnean Soc. 104, 499–509 10.1111/j.1095-8312.2011.01745.x (doi:10.1111/j.1095-8312.2011.01745.x) [DOI] [Google Scholar]
- 23.Fjeldså J, Lovett JC. 1997. Geographical patterns of old and young species in African forest biota: the significance of specific montane areas as evolutionary centres. Biodivers. Conserv. 6, 325–346 10.1023/A:1018356506390 (doi:10.1023/A:1018356506390) [DOI] [Google Scholar]
- 24.Anthony NM, et al. 2007. The role of Pleistocene refugia and rivers in shaping gorilla genetic diversity in central Africa. Proc. Natl Acad. Sci. USA 104, 20 432–20 436 10.1073/pnas.0704816105 (doi:10.1073/pnas.0704816105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greve M, Lykke AM, Blach-Overgaard A, Svenning J-C. 2011. Environmental and anthropogenic determinants of vegetation distribution across Africa. Global Ecol. Biogeogr. 20, 661–674 10.1111/j.1466-8238.2011.00666.x (doi:10.1111/j.1466-8238.2011.00666.x) [DOI] [Google Scholar]
- 26.Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature 403, 853–858 10.1038/35002501 (doi:10.1038/35002501) [DOI] [PubMed] [Google Scholar]
- 27.Pearson RG, Raxworthy CJ. 2009. The evolution of local endemism in Madagascar: watershed versus climatic gradient hypotheses evaluated by null biogeographic models. Evolution 63, 959–967 10.1111/j.1558-5646.2008.00596.x (doi:10.1111/j.1558-5646.2008.00596.x) [DOI] [PubMed] [Google Scholar]
- 28.Wilmé L, Goodman SM, Ganzhorn JU. 2006. Biogeographic evolution of Madagascar's microendemic biota. Science 312, 1063–1065 10.1126/science.1122806 (doi:10.1126/science.1122806) [DOI] [PubMed] [Google Scholar]
- 29.Wollenberg KC, Vieites DR, van der Meijden A, Glaw F, Cannatella DC, Vences M. 2008. Patterns of endemism and species richness in malagasy cophyline frogs support a key role of mountainous areas for speciation. Evolution 62, 1890–1907 10.1111/j.1558-5646.2008.00420.x (doi:10.1111/j.1558-5646.2008.00420.x) [DOI] [PubMed] [Google Scholar]
- 30.Storey M, Mahoney JJ, Saunders AD, Duncan RA, Kelley SP, Coffin MF. 1995. Timing of hot spot-related volcanism and the breakup of Madagascar and India. Science 267, 852–855 10.1126/science.267.5199.852 (doi:10.1126/science.267.5199.852) [DOI] [PubMed] [Google Scholar]
- 31.Wells NA. 2003. Some hypothesis on the Mesozoic and Cenozoic paleoenvironmental history of Madagascar. In The natural history of Madagascar (eds Goodman SM, Benstead JP.), pp. 16–33 Chicago, IL: Chicago Press [Google Scholar]
- 32.Dufils J-M. 2003. Remaining forest cover. In The natural history of Madagascar (eds Goodman SM, Benstead JP.), pp. 88–95 Chicago, IL: Chicago Press [Google Scholar]
- 33.Raxworthy CJ, Nussbaum RA. 1995. Systematics, speciation and biogeography of the dwarf chameleons (Brookesia, Reptilia, Squamata, Chamaeleontidae) of northern Madagascar. J. Zool. 235, 525–558 10.1111/j.1469-7998.1995.tb01767.x (doi:10.1111/j.1469-7998.1995.tb01767.x) [DOI] [Google Scholar]
- 34.Battistini R. 1996. Paléogéographie et variété des milieux naturels à Madagascar et dans les îles voisines: quelques données de base pour l’étude biogéographique de la région Malgache. In Biogéographie de Madagascar (ed. Lourenço WR.), pp. 1–17 Paris, France: Orstom [Google Scholar]
- 35.Vences M, Wollenberg KC, Vieites DR, Lees DC. 2009. Madagascar as a model region of species diversification. Trends Ecol. Evol. 24, 456–465 10.1016/j.tree.2009.03.011 (doi:10.1016/j.tree.2009.03.011) [DOI] [PubMed] [Google Scholar]
- 36.Couvreur TLP, Forest F, Baker WJ. 2011. Origin and global diversification patterns of tropical rain forests: inferences from a complete genus-level phylogeny of palms. BMC Biol. 9, 44. 10.1186/1741-7007-9-44 (doi:10.1186/1741-7007-9-44) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dransfield J, Uhl NW, Asmussen CB, Baker WJ, Harley MM, Lewis CE. 2008. Genera Palmarum: the evolution and classification of palms, 1st edn Richmond, UK: Royal Botanic Gardens, Kew [Google Scholar]
- 38.Govaerts R, Dransfield J, Zona SF, Hodel DR, Henderson A. 2012. World Checklist of Arecaceae. Kew, UK: Facilitated by the Royal Botanic Gardens; See http://apps.kew.org/wcsp/ (retrieved 11 April 2012) [Google Scholar]
- 39.Rakotoarinivo M, Dransfield J. 2010. New species of Dypsis and Ravenea (Arecaceae) from Madagascar. Kew Bull. 65, 279–303 10.1007/s12225-010-9210-7 (doi:10.1007/s12225-010-9210-7) [DOI] [Google Scholar]
- 40.Bjorholm S, Svenning J-C, Skov F, Balslev H. 2005. Environmental and spatial controls of palm (Arecaceae) species richness across the Americas. Global Ecol. Biogeogr. 14, 423–429 10.1111/j.1466-822x.2005.00167.x (doi:10.1111/j.1466-822x.2005.00167.x) [DOI] [Google Scholar]
- 41.Blach-Overgaard A, Svenning J-C, Dransfield J, Greve M, Balslev H. 2010. Determinants of palm species distribution across Africa: the relative roles of climate, non-climatic environmental factors, and spatial constraints. Ecography 33, 380–391 10.1111/j.1600-0587.2010.06273.x (doi:10.1111/j.1600-0587.2010.06273.x) [DOI] [Google Scholar]
- 42.Eiserhardt WL, Svenning J-C, Kissling WD, Balslev H. 2011. Geographical ecology of the palms (Arecaceae): determinants of diversity and distributions across spatial scales. Ann. Bot. 108, 1391–1416 10.1093/aob/mcr146 (doi:10.1093/aob/mcr146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kristiansen T, Svenning JC, Pedersen D, Eiserhardt WL, Grández C, Balslev H. 2011. Local and regional palm (Arecaceae) species richness patterns and their cross-scale determinants in the western Amazon. J. Ecol. 99, 1001–1015 10.1111/j.1365-2745.2011.01834.x (doi:10.1111/j.1365-2745.2011.01834.x) [DOI] [Google Scholar]
- 44.Dransfield J, Beentje H. 1995. The palms of Madagascar, 1st edn London, UK: Royal Botanic Gardens, Kew and The International Palm Society [Google Scholar]
- 45.Guisan A, Zimmermann NE. 2000. Predictive habitat distribution models in ecology. Ecol. Model. 135, 147–186 10.1016/S0304-3800(00)00354-9 (doi:10.1016/S0304-3800(00)00354-9) [DOI] [Google Scholar]
- 46.Phillips SJ, Anderson RP, Schapire RE. 2006. Maximum entropy modeling of species geographic distributions. Ecol. Model. 190, 231–259 10.1016/j.ecolmodel.2005.03.026 (doi:10.1016/j.ecolmodel.2005.03.026) [DOI] [Google Scholar]
- 47.Jiménez-Valverde A, Lobo JM. 2007. Threshold criteria for conversion of probability of species presence to either-or presence–absence. Acta Oecol. 31, 361–369 10.1016/j.actao.2007.02.001 (doi:10.1016/j.actao.2007.02.001) [DOI] [Google Scholar]
- 48.Braconnot P, et al. 2007. Results of PMIP2 coupled simulations of the Mid-Holocene and Last Glacial Maximum. I. Experiments and large-scale features. Clim. Past 3, 261–277 10.5194/cp-3-261-2007 (doi:10.5194/cp-3-261-2007) [DOI] [Google Scholar]
- 49.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Clim. 25, 1965–1978 10.1002/joc.1276 (doi:10.1002/joc.1276) [DOI] [Google Scholar]
- 50.R Development Core Team 2012. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 51.Dutilleul P, Clifford P, Richardson S, Hemon D. 1993. Modifying the t-test for assessing the correlation between two spatial processes. Biometrics 49, 305–314 10.2307/2532625 (doi:10.2307/2532625) [DOI] [PubMed] [Google Scholar]
- 52.Quinn GP, Keough MJ. 2002. Experimental design and data analysis for biologists, 1st edn Cambridge, UK: Cambridge University Press [Google Scholar]
- 53.Dormann CF, et al. 2007. Methods to account for spatial autocorrelation in the analysis of species distributional data: a review. Ecography 30, 609–628 10.1111/j.2007.0906-7590.05171.x (doi:10.1111/j.2007.0906-7590.05171.x) [DOI] [Google Scholar]
- 54.Lichstein JW, Simons TR, Shriner SA, Franzreb KE. 2002. Spatial autocorrelation and autoregressive models in ecology. Ecol. Monogr. 72, 445–463 10.1890/0012-9615(2002)072[0445:SAAAMI]2.0.CO;2 (doi:10.1890/0012-9615(2002)072[0445:SAAAMI]2.0.CO;2) [DOI] [Google Scholar]
- 55.Rangel TF, Diniz JAF, Bini LM. 2010. SAM: a comprehensive application for spatial analysis in macroecology. Ecography 33, 46–50 10.1111/j.1600-0587.2009.06299.x (doi:10.1111/j.1600-0587.2009.06299.x) [DOI] [Google Scholar]
- 56.Vieites DR, Wollenberg KC, Andreone F, Köhler J, Glaw F, Vences M. 2009. Vast underestimation of Madagascar's biodiversity evidenced by an integrative amphibian inventory. Proc. Natl Acad. Sci. USA 106, 8267–8272 10.1073/pnas.0810821106 (doi:10.1073/pnas.0810821106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Normand S, Ricklefs RE, Skov F, Bladt J, Tackenberg O, Svenning J-C. 2011. Postglacial migration supplements climate in determining plant species ranges in Europe. Proc. R. Soc. B 278, 3644–3653 10.1098/rspb.2010.2769 (doi:10.1098/rspb.2010.2769) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prentice IC, Jolly D; BIOME 6000 participants 2000. Mid-Holocene and glacial-maximum vegetation geography of the northern continents and Africa. J. Biogeogr. 27, 507–519 10.1046/j.1365-2699.2000.00425.x (doi:10.1046/j.1365-2699.2000.00425.x) [DOI] [Google Scholar]
- 59.Gerhart LM, Ward JK. 2010. Plant responses to low [CO2] of the past. New Phytol. 188, 674–695 10.1111/j.1469-8137.2010.03441.x (doi:10.1111/j.1469-8137.2010.03441.x) [DOI] [PubMed] [Google Scholar]
- 60.Lamb JM, Naidoo T, Taylor PJ, Napier M, Ratrimomanarivo F, Goodman SM. 2012. Genetically and geographically isolated lineages of a tropical bat (Chiroptera: Molossidae) show demographic stability over the late Pleistocene. Biol. J. Linnean Soc. 106, 18–40 10.1111/j.1095-8312.2011.01853.x (doi:10.1111/j.1095-8312.2011.01853.x) [DOI] [Google Scholar]
- 61.Jetz W, Fine PVA. 2012. Global gradients in vertebrate diversity predicted by historical area-productivity dynamics and contemporary environment. PLoS Biol. 10, e1001292. 10.1371/journal.pbio.1001292 (doi:10.1371/journal.pbio.1001292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Svenning JC, Borchsenius F, Bjorholm S, Balslev H. 2008. High tropical net diversification drives the New World latitudinal gradient in palm (Arecaceae) species richness. J. Biogeogr. 35, 394–406 10.1111/j.1365-2699.2007.01841.x (doi:10.1111/j.1365-2699.2007.01841.x) [DOI] [Google Scholar]
- 63.Baker WJ, Couvreur TLP. 2013. Global biogeography and diversification of palms sheds light on the evolution of tropical lineages. II. Diversification history and origin of regional assemblages. J. Biogeogr. 40, 286–298 10.1111/j.1365-2699.2012.02794.x (doi:10.1111/j.1365-2699.2012.02794.x) [DOI] [Google Scholar]
- 64.Tadross M, Randriamarolaza L, Rabefitia Z, Zheng KY. 2008. Climate change in Madagascar; recent past and future. Washington, DC: World Bank [Google Scholar]
- 65.Hannah L, et al. 2008. Climate change adaptation for conservation in Madagascar. Biol. Lett. 4, 590–594 10.1098/rsbl.2008.0270 (doi:10.1098/rsbl.2008.0270) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harper GJ, Steininger MK, Tucker CJ, Juhn D, Hawkins F. 2007. Fifty years of deforestation and forest fragmentation in Madagascar. Environ. Conserv. 34, 325–333 10.1017/S0376892907004262 (doi:10.1017/S0376892907004262) [DOI] [Google Scholar]
- 67.Moat J, Smith P. 2007. Atlas of the vegetation of Madagascar, 1st edn Kew, UK: Royal Botanic Gardens [Google Scholar]
- 68.Prance GT, Beentje H, Dransfield J, Johns R. 2000. The tropical flora remains undercollected. Ann. Missouri Bot. Gard. 87, 67–71 10.2307/2666209 (doi:10.2307/2666209) [DOI] [Google Scholar]
- 69.Rakotoarinivo M, Dransfield J. In press The history of palm exploration in Madagascar. Plant Ecol. Evol. [Google Scholar]
- 70.Raik DB. 2007. Forest management in Madagascar: an historical overview. Madagascar Conserv. Dev. 2, 5–10 [Google Scholar]