Abstract
Signals relevant to different sets of receivers in different contexts create a conflict for signal design. A classic example is vocal alarm signals, often used both during intraspecific and interspecific interactions. How can signals alert individuals from a variety of other species in some contexts, while also maintaining efficient communication among conspecifics? We studied heterospecific responses to avian alarm signals that drive the formation of anti-predator groups but are also used during intraspecific interactions. In three species-rich communities in the western Himalayas, alarm signals vary drastically across species. We show that, independently of differences in their calls, birds respond strongly to the alarm signals of other species with which they co-occur and much more weakly to those of species with which they do not co-occur. These results suggest that previous exposure and learning maintain heterospecific responses in the face of widespread signal divergence. At an area where only two species regularly interact, one species' calls incorporate the call of the other. We demonstrate experimentally that signal copying allows strong responses even without previous exposure and suggest that such hybrid calls may be especially favoured when pairwise interactions between species are strong.
Keywords: anti-predator behaviour, communication, interspecific interactions, learning, passerine birds
1. Introduction
Signals evolve to maximize the beneficial responses of receivers. However, when the same signal is used in multiple contexts, a conflict for signal design may arise. This conflict should be most pronounced when contexts are relevant to distinct sets of receivers, such as for signals that are relevant to conspecifics in some contexts, but heterospecifics in others. Signals used in intraspecific communication are generally under selection to diverge across species [1], but differences across species may weaken heterospecific recognition [2]. When the responses of heterospecifics are neither beneficial nor costly for signallers, such as for the breeding songs of many species, this tradeoff may be unimportant, freeing signals to diverge along species-specific lines [1]. In contrast, when heterospecific responses are beneficial and aligned with conspecific selection pressures, such as during interactions between pairs of species that are interspecifically territorial, their signals may converge [3–6]. Here, we address the problem of how communication arises in an intermediate situation; i.e. when communication with heterospecifics is beneficial in some, but not all contexts.
We studied heterospecific communication during the formation of avian mobs. Mobbing is an anti-predator defence in which a group of individuals collectively attack a predator to drive it away [7]. Many birds produce loud vocalizations during mobbing that attract additional mobbers from multiple species also threatened by the predator [7–9]. Large mobs are more effective than small mobs at driving off predators [10–12], implying that interspecific recognition is mutually beneficial to signallers and receivers [8,13]. However, calls used during mobbing may also be used in multiple intraspecific interactions [14], such as territorial interactions during the non-breeding season [15] or flock cohesion [16,17], leading to at least some divergence across species ([18,19]; figure 1]). Species-specific selection pressures may lead to divergence across species because of stochastic processes [21], species-specific environmental adaptation [14,22] and constraints due to body size or divergence in other vocal signals [23]. Likewise, if the same calls are used among different sets of heterospecifics, such as during the breeding and the non-breeding seasons, selection for heterospecific responses may lead to divergence as a result of different tradeoffs for different species.
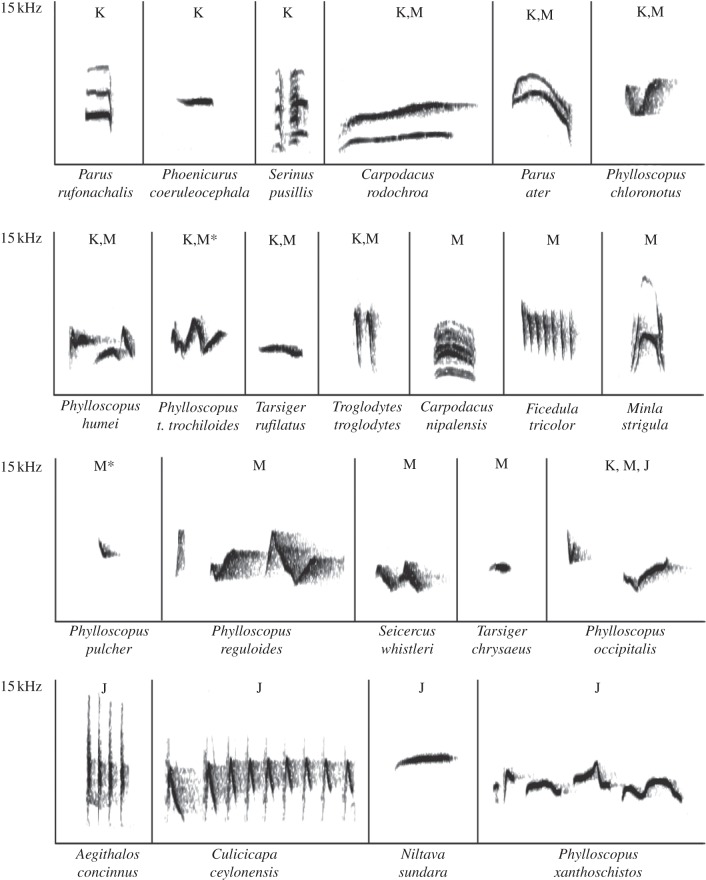
Figure 1.
Spectrograms of the mobbing calls of species included in the study. Species nomenclature follows [20]). Letters indicate sites (J, Jagatsukh; K, Keylong; M, Manali) at which a species breeds. Asterisks (*) denote species that breed also at the higher elevation location at Manali (3450–3600 m). The x-axes represent 2 s.
Although the simplest pathway to recognition across species is if the signals of each interacting species are identical [2], recognition in the face of call divergence can be maintained through either similarity of some call features or learning. For example, despite divergence as a result of selection in other contexts, calls might include widely recognizable features [24], meaning calls are ‘similar enough’ across species for receivers to recognize them [25,26]. However, if divergence is too great for automatic recognition, repeated exposure could allow receivers to learn to associate the calls of other species with an appropriate alarm response [27,28]. Learning requires previous experience and, therefore, time to build up responses to the calls of another species [29], which may cause costly delays in alarm situations. As a result, when efficient responses of particular species are critical, convergence or mimicry can arise, allowing communication even with naive individuals from another species [30–32]. Convergence should be most likely among small numbers of closely interacting species living in similar acoustic environments, such that adaptations for species-specific and heterospecific communication are more aligned [3–6].
We evaluated the form and perception of signals in seasonal, multi-species communities of birds at three sites in the western Himalayas of India (figure 2). We studied 22 species that regularly mob. First, we recorded the composition of mobs elicited in response to presentations of taxidermied predator mounts. Second, we considered the role of similarity on recognition by evaluating whether birds' responses to the calls of other species were related to the acoustic similarity of their calls. Third, we evaluated the role of previous exposure and learning by comparing responses of birds at all three of our sites to the playback of calls of both co-occurring and non-co-occurring species. Fourth, we discovered, at a high-elevation area at which only two species regularly form mobs, one species sometimes incorporates the calls of a second species into its own. We tested whether call incorporation increased responses of the other species, despite their calls being otherwise dissimilar.
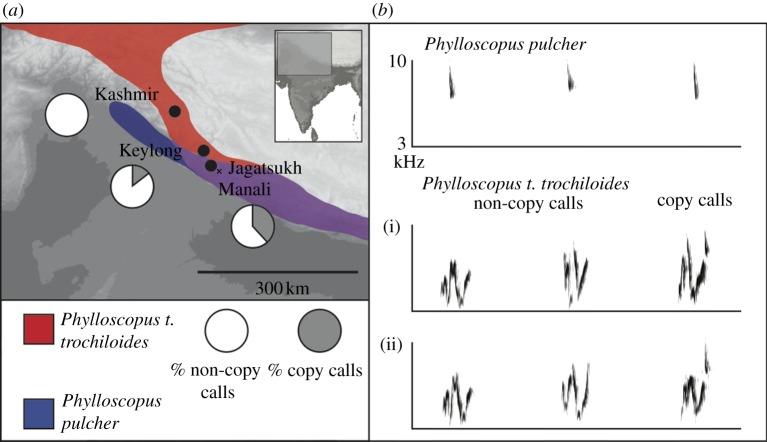
Figure 2.
(a) Field sites in the Indian Himalayas included in the study. Shading indicates 1000 m elevation bands. Manali is divided into two areas: a lower elevation area (3100–3450 m) with 15 commonly mobbing species and a higher elevation area (more than 3450 m), with only two commonly mobbing species, Phylloscopus t. trochiloides and Phylloscopus pulcher. Breeding ranges of these two species are given in colour (modified from [20]), showing the percentage of calls used by P. t. trochiloides that copy the call of P. pulcher (high-elevation Manali: 41%; Keylong: 13%; Pakistan: 0%, [21]). (b) Representative mobbing calls produced by P. pulcher and P. t. trochiloides. Phylloscopus trochiloides trochiloides individuals (i) and (ii) produce both non-copying calls and calls that incorporate the call of P. pulcher.
2. Material and methods
(a). System
We studied mobbing behaviour among breeding songbirds in Himachal Pradesh, India in May and June, 2008–2011, at three sites: Manali Wildlife Sanctuary (Manali, 32°25′ N, 77°15′ E, between 3100 and 3600 m, [33]), Jagatsukh Forest (Jagatsukh, 32°11′ N, 77°12′ E, between 2200 and 2500 m); Mooling Forest near Keylong (Keylong, 32°50′ N, 76°98′ E, between 3400 and 3600 m; figure 2). Owls (notably Glaucidium brodei and Strix aluco) and hawks (notably Accipiter nisus) are present at each site [20]. We observed small songbirds mobbing hawks and owls in natural situations and were able to easily induce mobs on taxidermied models of both hawks and owls. Although the sites are geographically close (approx. 40 km), they contain distinct species assemblages (figure 1). We studied all species at each site that regularly participated in mobs (22 species from 12 genera; figure 1). Seven of these species are found only at Manali, four species only at Jagatsukh, three species only at Keylong, while the other eight occur at least two of the three sites (figure 1). For some analyses, Manali was subdivided into a species-rich lower elevation area (3100–3450 m) and a species-poor higher elevation area (3450–3600 m), at which only two species, Phylloscopus trochiloides (subspecies trochiloides [20]) and Phylloscopus pulcher, regularly participate in mobs. Both species also occur at the lower elevation area, but the other species rarely participate in mobs above 3450 m (see below).
(b). Call recordings and analysis
We presented taxidermied mounts of hawk (two mounts) and owl (one mount) species 1 m from 59 nests of 11 species (67 total presentations) and noted the responses of nest owners, additional conspecifics and heterospecifics (see the electronic supplementary material). Using these presentations, we recorded mobbing calls of the nest owners as well as other species that joined an existing mob. We recorded all calls from a distance of 1–5 m with a Sennheiser ME66 microphone into a Marantz PMD660 digital recorder. Recordings were in 16-bit PCM WAV format, sampled at 44.1 kHz. Each contained at least 1 min of continuous, high-quality calls (see the electronic supplementary material).
We obtained measurements of call similarity by measuring spectrograms in raven (v. 1.3) [34], following established methods [35,36]. For each call type, we made 10 acoustic measurements: (i) call duration (s), (ii) low frequency (Hz), (iii) high frequency (Hz), (iv) frequency bandwidth or range (Hz), (v) centre frequency (the frequency at which half the call's acoustic energy is below; Hz), (vi) first quartile frequency (the frequency at which 25% of the call's acoustic energy is below; Hz), (vii) third quartile frequency (the frequency at which 75% of the call's acoustic energy is below; Hz), (viii) peak frequency or frequency at which the amplitude of the call is highest, (ix) number of elements or discrete continuous sounds, that make up each call, log transformed, (x) number of changes in frequency modulation, or how many times the frequency trace switches from increasing to decreasing or vice-versa, log transformed (see the electronic supplementary material, figure S1). We extracted four principal components from the resulting correlation matrix of all measurements, which together explained 93 per cent of the total variation in the data (see the electronic supplementary material, table S1). Our measure of difference between calls of different species is the Euclidean distance of the scores for these four principal components (see the electronic supplementary material, table S2). We repeated all analyses excluding the six species for which we recorded only one individual. The results for this partial dataset were consistent with the results for the entire dataset.
(c). Playback experiment: responses to familiar and unfamiliar calls
We compared the response of birds to calls of both familiar (i.e. co-occurring) and unfamiliar species (i.e. present only at another site, as well as New World species; electronic supplementary material, table S3 and figure S2) by playing back recordings of the species along transects at Manali (lower elevation location only), Jagatsukh and Keylong. We measured the responses of birds to playbacks of mobbing calls on a four point scale: 0 (did not approach to within 10 m of the speaker), 1 (in the area longer than 1 min or approached within 10 m), 2 (in the area longer than 1 min and approached within 10 m) or 3 (in the area longer than 1 min, approached within 10 m, and engaged in typical mobbing behaviours; modified from [37]). To control for the fact that, given a bird responded, subsequent birds could be responding to the initial responder's behaviour, we considered only the first heterospecific individual to respond to the playback and excluded the six (11%) of 54 trials in which a conspecific responded first. Additionally, we measured the total number of individuals to respond. We used the species being broadcast as the unit of replication and averaged repeated tests from the same species. Thus, there were 18 replicates of the familiar treatment (i.e. each species call was broadcast in the locality in which it occurs and, when a species occurs in more than one locality, responses were averaged), 11 replicates of the unfamiliar treatment and nine replicates of the New World species treatment.
(d). Role of call similarity on responses
We compared the acoustic similarity of a species pairs' calls with how strongly they responded to one another for all pairwise interactions in which one species was the first to respond to the playback of another (n = 45 interactions). Response strengths from multiple interactions between the same species pair (with either species as signaller or receiver) were averaged. We computed the correlation between average response and call distance across all 45 interactions and determined the percentage of correlations from 1000 random permutations of the data that were larger, using R (v. 2.12.1) [38].
(e). Role of call incorporation on responses
As we demonstrate below, some calls of P. t. trochiloides incorporate P. pulcher calls, whereas others do not. We compared responses to these calls (six recordings from six different individuals with non-copying calls edited out) as well as recordings of P. t. trochiloides calls that do not include P. pulcher calls (six recordings from the same six individuals with copying calls edited out; see the electronic supplementary material). In 2008, we broadcast each recording along transects at the high-elevation area at Manali and noted the response strength of P. pulcher individuals (see the electronic supplementary material). Additionally, in 2011, we played back recordings of Phylloscopus humei (n = 5) and P. t. trochiloides (n = 5) at Jagatsukh, where neither species occurs. We noted responses of Phylloscopus occipitalis, a common species at Jagatsukh, to these recordings as well as to altered recordings that were edited in raven (v. 1.3) such that the calls of P. humei and P. t. trochiloides ‘incorporated’ the call of P. occipitalis at their end (n = 5 for each species; electronic supplementary material, figure S3), somewhat similarly to the way P. t. trochiloides naturally incorporates the call of P. pulcher.
3. Results
(a). Mob formation depends on heterospecifics
Birds initiating mobs on taxidermied predator mounts at their nests regularly attracted individuals from other species (n = 67 mobs at 59 nests, 56 contained at least one heterospecific), while responses by conspecifics were rare and only four mobs contained conspecifics in addition to the breeding pair (Fisher's exact test, p < 0.0001; electronic supplementary material, table S4 for a breakdown by species). Moreover, heterospecifics responded in larger numbers (3.76 individuals±0.42 s.e. versus 0.06 ± 0.03 extra-pair conspecifics, paired t58 = 9.10, p < 0.0001). The paucity of conspecific responders was almost certainly due both to the distance between adjacent nests and territorial interactions between conspecifics. All four mobs containing conspecifics beyond the pair of nest owners occurred at the nests of P. humei at Keylong, where their nests are much more densely spaced than at Manali (D.W. 2009, personal observation). These additional conspecifics were sometimes attacked and chased by the nest owners, implying a cost to responding to conspecific mobbing calls.
(b). Mobbing calls and heterospecific responses
We analysed the acoustic characteristics of mobbing calls from all 22 Indian species included in this study (figure 1; electronic supplementary material, table S2). The spectrographic characteristics of calls differ much more among species than within species (mean Euclidean distance between species pairs: 3.6±0.54 s.d., within species: 1.1±1.11). Peak frequency is highly variable across species (range approx. 2000–7000 Hz), as is bandwidth (750–10 000 Hz).
Responses to other species' mobbing calls are not based on acoustic similarity. Excluding the few conspecific responses, the similarity of two species' calls was not significantly correlated with how strongly they responded to each other (n = 45 pairwise interactions for which we have both a measure of response strength and call similarity, r = 0.11; p = 0.24 by a permutation test; electronic supplementary material, table S5). For example, the calls of P. pulcher are dissimilar from the calls of Carpodacus rodochroa (4.23 Euclidean distance) and similar to those of Phylloscopus chloronotus (1.41 Euclidean distance), but P. pulcher responded with equal strength to both species (see the electronic supplementary material, table S5).
Responses to mobbing calls are stronger when the mobbing call is from a co-occurring species (table 1). Approximately, three times as many individuals responded to playbacks of familiar, co-occurring species than to playbacks of unfamiliar species, and the individuals that responded to the playback first did so more than three times stronger to playbacks of familiar species (table 1). The weak responses to calls of unfamiliar species were similar to those given to calls of New World species (table 1). In general, birds responding to the calls of unfamiliar species left after less than 1 min without engaging in typical mobbing behaviours.
Table 1.
Responses to experimental playbacks.
| playback treatment | number of heterospecific responders (mean ± s.e.) | response strength of first responder (mean ± s.e.) |
|---|---|---|
| familiar heterospecifics (n = 18 species) | 5.42 ± 0.48a | 2.61 ± 0.14b |
| unfamiliar heterospecifics (n = 11 species) | 1.72 ± 0.39a | 0.80 ± 0.15b |
| New World heterospecifics (n = 9 species) | 1.02 ± 0.33a | 0.69 ± 0.26b |
a,bSignificantly different means (p < 0.05) by post-hoc Tukey tests.
(c). Mobs with two interacting species
At elevations between 3450 and 3600 m at Manali, only two species, P. t. trochiloides and P. pulcher, regularly come together to form small mobs. All 16 mobs we have studied above 3450 m contained individuals from both of these species. Four contained only these species, while 11 of the other 12 mobs were joined by one extra species and one mob by two extra species. The identity of these additional species was variable and the most frequent additional participant, Tarsiger chrysaeus, was present only in three mobs. The calls of P. t. trochiloides and P. pulcher are highly dissimilar (4.18 Euclidean distance; figure 2, electronic supplementary material, S5). Phylloscopus pulcher calls are stereotyped, but P. t. trochiloides individuals use roughly seven call variants each (n = 17 individuals; 6.9±1.64 s.d. call variants per individual), which can be readily classified by their spectrograms (figure 2). Most of these calls are shared across individuals (of the 52 total call variants in the dataset and 43 are shared by at least two individuals). The importance of considering these variants is that some of the calls incorporate the call of P. pulcher (figure 2), with which P. t. trochiloides regularly co-occurs at elevations above 3450 m in the Himalayas [20]. The copying is essentially perfect: we compared the acoustic structure of P. pulcher calls (n = 4) with the copying portion of P. t. trochiloides (n = 4) calls and found the acoustic measurements to be highly similar (the Euclidean distance between P. pulcher and its P. t. trochiloides copy averaged 0.58, while the difference between different P. pulcher individuals averaged 0.56).
Where P. pulcher and P. t. trochiloides co-occur at high elevations at Manali, individual P. t. trochiloides (n = 10) use a greater percentage of call variants incorporating P. pulcher calls than at Keylong (n = 7), where P. pulcher is absent (41±3% s.e. versus 13±6%; t-test on arcsin square-root proportions: t15 = 4.28, p = 0.0007; figure 2). At Manali, playback of P. t. trochiloides calls regularly attracted at least one P. pulcher individual (8/12 mobbing call playbacks), but P. pulcher individuals responded strongly (response strength = 3) to playbacks of P. t. trochiloides calls that included a copy of a P. pulcher call, but weakly (response strength < 3) to playbacks that did not (table 2).
Table 2.
Number of trials that led to typical mobbing behaviours (response strength = 3), with number of total trials in parentheses. Treatments were playbacks of heterospecific calls that do or do not incorporate a copy of the focal species' call. p-values are from Fisher's exact tests comparing a species' responses to playbacks with the copy and those without. Combined across all trials: p < 0.001. Phylloscopus trochiloides trochiloides call recordings targeting P. pulcher individuals were played back twice each. We include here the responses to the first replicate. The responses to the second replicate were similar: P. pulcher individuals never responded (response strength = 3) to P. trochiloides calls without a copy and responded on four occasions to calls with a copy (p = 0.06).
| target species |
P. trochiloides calls |
P. humei calls |
||||
|---|---|---|---|---|---|---|
| with copy | without copy | p | with copy | without copy | p-value | |
| P. pulchera | 5 (6) | 0 (6) | 0.015 | — | — | — |
| P. occipitalisb | 4 (5) | 0 (5) | 0.048 | 3 (5) | 0 (5) | 0.167 |
aPhylloscopus trochiloides trochiloides calls incorporating the call of P. pulcher were natural variants (figure 2).
bPlaybacks to P. occipitalis were digitally manipulated to incorporate P. occipitalis calls (see the electronic supplementary material, figure S3).
(d). Call incorporation increases responses to unfamiliar calls
To evaluate the role of signal incorporation in interspecific recognition on a naive species, we digitally altered the calls of P. humei and P. t. trochiloides to include the calls of P. occipitalis, a species that occurs at lower elevations (see the electronic supplementary material, figure S3). Phylloscopus occipitalis individuals did not respond to unaltered playbacks of the unfamiliar species but did respond strongly to playbacks that were altered to incorporate a copy of their own call (table 2).
4. Discussion
Responses among heterospecifics do not in general relate to similarity in their signals, implying that the presumably beneficial effect of signal similarity for heterospecific interactions is weak. With weak selection for similarity and use in a variety of contexts, mobbing calls have come to diverge across species. Divergence hinders recognition across species [2,39], but we show here that individuals respond strongly to the calls of other species as long as they co-occur. Heterospecific responses to alarm signals have been shown to depend on previous exposure in specific pairwise interactions in mammals [40] and birds [27,28], but this is the first demonstration of the importance of learning across entire assemblages of species.
We have not investigated how birds learn to recognize the calls of heterospecifics but found that birds responded, albeit weakly, to the calls of non-co-occurring species. It is likely these tentative responses to unfamiliar calls would be reinforced by the presence of a predator being mobbed at the sound's source, so that birds learn to associate the presence of a mob with the sound. This process has been demonstrated experimentally in golden-mantled ground squirrels, (Spermophilus lateralis) [29] and studies on the development of nestling recognition suggest that recognizing is an important aspect of learning even one's own species' call [41]. Work on captive populations has shown that birds mob novel objects after viewing the objects being mobbed by others [42]. Together, these results suggest that learned responses to predators and predator-relevant signals are common in nature [41,43]. The learning process may be particularly quick during stressful situations, such as during encounters with predators [44].
Even an efficient learning process necessarily introduces a time lag between when receivers first respond to signals tentatively and when they respond strongly [29]. As a result, any feature of a signal that speeds up recognition should be favoured [30]. In some cases, heterospecific alarm responses occur without previous exposure [2,24,26]. For example, the responses of superb fairy-wrens (Malurus cyaneus) to the alarm calls of allopatric species depend on the similarity of their calls [2]. An alternative is mimicry: for example, greater racket-tailed drongos (Dicrurus paradiseus) have been shown to mimic the calls of heterospecifics during temporary associations with them, which increases heterospecific responses [31,32]. We discovered that the calls of one species, P. t. trochiloides, incorporate the call of another, P. pulcher, at a species-poor location at which only these two species regularly participate in mobs. Call incorporation creates high call similarity between the two species—one species essentially responds to its own call—without which the calls of the two species are very different (figure 2). We demonstrate that experimental call incorporation increases the responses of a naive species even without previous exposure (table 2), suggesting that call incorporation bypasses or at least facilitates the learning process.
It is likely that call incorporation is a result of the interaction between the two species. Although they are members of the same genus, the last common ancestor to these two species was estimated to date > 12 Ma [45] and none of the other Phylloscopus species have acoustic features similar to the call of P. pulcher [20]. Call incorporation is mostly confined to where the two species overlap ([21]; this study) and occurs at relatively low frequency at Keylong, where P. pulcher is absent, but to which P. t. trochiloides individuals from Manali likely disperse. A special feature of the mobbing calls of P. t. trochiloides might facilitate the incorporation of another species' call. Unlike the other species we studied, individual P. t. trochiloides produce several discrete call variants that are used interchangeably (see the electronic supplementary material). Incorporation of P. pulcher calls in some of the variants need not negatively affect conspecific communication because other similar calls that do not include P. pulcher calls are part of every individual's repertoire. Phylloscopus pulcher calls, on the other hand, are simple and stereotyped (figure 2), suggesting that their communication system is less flexible than that of P. t. trochiloides, and that P. pulcher might, therefore, be less able to incorporate the calls of other species without compromising conspecific messages.
Vocal mimicry in alarm contexts generally arises through copying a model individual [31,32,44,46]. Although we must leave open the possibility that P. t. trochiloides individuals incorporate P. pulcher calls through copying P. pulcher individuals, we suggest that this is unlikely because P. t. trochiloides calls incorporate P. pulcher calls in a stereotyped fashion—always at the end of their own calls and never in isolation (figure 2). Moreover, P. t. trochiloides individuals share call variants with their neighbours (figure 2; see the electronic supplementary material), including variants that incorporate P. pulcher calls. These results suggest that calls in P. t. trochiloides are learnt from conspecifics, similarly to songs in many avian species [22], and that both calls incorporating P. pulcher calls and those that do not may be learned from conspecifics.
Signal convergence may be particularly likely in pairs of strongly interacting species, as argued for the songs of some interspecifically territorial species pairs [3,5,6]. Phylloscopus trochiloides trochiloides and P. pulcher breed in very similar habitat and their nests are often in close-proximity, suggesting that interspecific communication in multiple types of interactions may be beneficial. No matter the mechanism explaining its origin and maintenance, our results suggest that call incorporation enhances interspecific responses and may be more likely to arise when interactions between species are particularly strong. The progression between group behaviours that benefit diverse participants and mutualistic interactions is continuous and slight changes in ecological conditions—such as along a species-diversity gradient as we have studied—could make evolution to facilitate interspecific recognition profitable when signallers depend on the responses of receivers from one other species.
Acknowledgements
We thank Emma Greig, Daizaburo Shizuka, Toshitaka Suzuki and Benjamin Taft for their help revising early versions of the paper, Joseph Tobias, Eben Goodale and one anonymous reviewer for greatly helpful comments and suggestions for how to think about call incorporation, and Chaman Lal and Jai Singh for help with fieldwork. Funded by a National Science Foundation grant to T.D.P. (www.nsf.gov), by a Frank M. Chapman Memorial Fund to D.W. (www.amnh.org), and by a National Science Foundation EAPSI grant to D.W. (www.nsf.gov/eapsi).
References
- 1.Marler P. 1957. Specific distinctiveness in the communication signals of birds. Behaviour 11, 13–39 10.1163/156853956X00066 (doi:10.1163/156853956X00066) [DOI] [Google Scholar]
- 2.Fallow PM, Gardner JL, Magrath RD. 2011. Sound familiar? Acoustic similarity provokes responses to unfamiliar heterospecific alarm calls. Behav. Ecol. 22, 401–410 10.1093/beheco/arq221 (doi:10.1093/beheco/arq221) [DOI] [Google Scholar]
- 3.Cody ML. 1969. Convergent characteristics in sympatric species: a possible relation to interspecific competition and aggression. Condor 71, 222–239 10.2307/1366300 (doi:10.2307/1366300) [DOI] [Google Scholar]
- 4.Grether GF, Losin N, Anderson CN, Okamoto K. 2009. The role of interspecific interference competition in character displacement and the evolution of competitor recognition. Biol. Rev. 84, 617–635 10.1111/j.1469-185X.2009.00089.x (doi:10.1111/j.1469-185X.2009.00089.x) [DOI] [PubMed] [Google Scholar]
- 5.Tobias JA, Seddon N. 2009. Signal design and perception in Hypocnemis antbirds: evidence for convergent evolution via social selection. Evolution 63, 3169–3189 10.1111/j.1558-5646.2009.00795.x (doi:10.1111/j.1558-5646.2009.00795.x) [DOI] [PubMed] [Google Scholar]
- 6.Laiolo P. 2012. Interspecific interactions drive cultural co-evolution and acoustic convergence in syntopic species. J. Anim. Ecol. 81, 594–604 10.1111/j.1365-2656.2011.01946.x (doi:10.1111/j.1365-2656.2011.01946.x) [DOI] [PubMed] [Google Scholar]
- 7.Curio E. 1978. The adaptive significance of avian mobbing. I. Teleonomic hypotheses and predictions. Zeitschr. Tierpsychol. 48, 184–202 10.1111/j.1439-0310.1978.tb00255.x (doi:10.1111/j.1439-0310.1978.tb00255.x) [DOI] [Google Scholar]
- 8.Klump GM, Shalter MD. 1984. Acoustic behaviour of birds and mammals in the predator context. I. Factors affecting the structure of alarm signals. II. The functional significance and evolution of alarm signals. Zeitschr. Tierpsychol. 66, 189–226 10.1111/j.1439-0310.1984.tb01365.x (doi:10.1111/j.1439-0310.1984.tb01365.x) [DOI] [Google Scholar]
- 9.Hurd CR. 1996. Interspecific attraction to the mobbing calls of black-capped chickadees (Parus atricapillus). Behav. Ecol. Sociobiol. 38, 287–292 10.1007/s002650050244 (doi:10.1007/s002650050244) [DOI] [Google Scholar]
- 10.Robinson SK. 1985. Coloniality in the yellow-rumped cacique (Cacicus cela) as a defense against nest predators. Auk 102, 506–519 [Google Scholar]
- 11.Flasskamp A. 1994. The adaptive significance of avian mobbing. V. An experimental test of the ‘move on’ hypothesis’. Zeitschr. Tierpsychol. 96, 322–333 10.1111/j.1439-0310.1994.tb01020.x (doi:10.1111/j.1439-0310.1994.tb01020.x) [DOI] [Google Scholar]
- 12.Krams I, Berzins A, Krama T. 2009. Group effect in nest defence behaviour of breeding pied flycatchers, Ficedula hypoleuca. Anim. Behav. 77, 513–517 10.1016/j.anbehav.2008.11.007 (doi:10.1016/j.anbehav.2008.11.007) [DOI] [Google Scholar]
- 13.Caro T. 2005. Antipredator defenses in birds and mammals. Chicago, IL: The University of Chicago Press [Google Scholar]
- 14.Marler P. 2004. Bird calls: a cornucopia for communication. In Nature’s music (eds Marler P, Slabbekoorn H.), pp. 132–177 San Diego, CA: Elsevier [Google Scholar]
- 15.Price TD. 1981. The ecology of the greenish warbler, Phylloscopus trochiloides, in its winter quarters. Ibis 123, 131–144 10.1111/j.1474-919X.1981.tb00920.x (doi:10.1111/j.1474-919X.1981.tb00920.x) [DOI] [Google Scholar]
- 16.Ficken MS, Ficken RW, Witkin SR. 1978. Vocal repertoire of the black-capped chickadee. Auk 95, 34–48 10.2307/4085493 (doi:10.2307/4085493) [DOI] [Google Scholar]
- 17.Bergmann HH. 1993. Der Buchfink: neues über einen bekannten Sänger. Weisbaden: Aula [Google Scholar]
- 18.Ficken MS, Popp J. 1996. A comparative analysis of passerine mobbing calls. Auk 113, 370–380 10.2307/4088904 (doi:10.2307/4088904) [DOI] [Google Scholar]
- 19.Jurisevic MA, Sanderson KJ. 1994. Alarm vocalizations in Australian birds: convergent characteristics and phylogenetic differences. Emu 94, 69–77 10.1071/MU9940067 (doi:10.1071/MU9940067) [DOI] [Google Scholar]
- 20.Rasmussen PC, Anderton JC. 2005. Birds of south Asia: the Ripley guide, vol. 2: attributes and status. Barcelona: Lynx Edicions [Google Scholar]
- 21.Irwin DE, Thimgan MP, Irwin JH. 2008. Call divergence is correlated with geographic and genetic distance in greenish warblers (Phylloscopus trochiloides): a strong role for stochasticity in signal evolution? J. Evol. Biol. 21, 435–448 10.1111/j.1420-9101.2007.01499.x (doi:10.1111/j.1420-9101.2007.01499.x) [DOI] [PubMed] [Google Scholar]
- 22.Bradbury JW, Vehrencamp SL. 1998. Principles of animal communication. Sunderland, MA: Sinauer Associates [Google Scholar]
- 23.Ryan MJ, Brenowitz EA. 1985. The role of body size, phylogeny, and ambient noise in the evolution of bird song. Am. Nat. 126, 87–100 10.1086/284398 (doi:10.1086/284398) [DOI] [Google Scholar]
- 24.Johnson FR, McNaughton EJ, Shelley CD, Blumstein DT. 2003. Mechanisms of heterospecific recognition in avian mobbing calls. Aust. J. Zool. 51, 577–585 10.1071/ZO03031 (doi:10.1071/ZO03031) [DOI] [Google Scholar]
- 25.Randler C. 2012. A possible phylogenetically conserved urgency response of great tits (Parus major) towards allopatric mobbing calls. Behav. Ecol. Sociobiol. 66, 675–681 10.1007/s00265-011-1315-y (doi:10.1007/s00265-011-1315-y) [DOI] [Google Scholar]
- 26.Fallow PM, Pitcher BJ, Magrath RD. 2013. Alarming features: birds use specific acoustic properties to identify heterospecific alarm calls. Proc. R. Soc. B 280, 20122539. 10.1098/rspb.2012.2539 (doi:10.1098/rspb.2012.2539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magrath RD, Pitcher BJ, Gardner JL. 2009. Recognition of other species’ aerial alarm calls: speaking the same language or learning another? Proc. R. Soc. B 276, 769–774 10.1098/rspb.2008.1368 (doi:10.1098/rspb.2008.1368) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magrath RD, Bennett TH. 2012. A micro-geography of fear: learning to eavesdrop on alarm calls of neighboring heterospecifics. Proc. R. Soc. B 279, 902–909 10.1098/rspb.2011.1362 (doi:10.1098/rspb.2011.1362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shriner WM. 1999. Antipredator responses to a previously neutral sound by free-living adult golden-mantled ground squirrels, Spermophilus lateralis (Sciuridae). Ethology 105, 747–757 10.1046/j.1439-0310.1999.00454.x (doi:10.1046/j.1439-0310.1999.00454.x) [DOI] [Google Scholar]
- 30.Kostan KM. 2002. The evolution of mutualistic interspecific communication: assessment and management across species. J. Comp. Psychol. 116, 206–209 10.1037/0735-7036.116.2.206 (doi:10.1037/0735-7036.116.2.206) [DOI] [PubMed] [Google Scholar]
- 31.Goodale E, Kotagama SW. 2006. Context-dependent vocal mimicry in a passerine bird. Proc. R. Soc. B 273, 875–880 10.1098/rspb.2005.3392 (doi:10.1098/rspb.2005.3392) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodale E, Kotagama SW. 2006. Vocal mimicry by a passerine bird attracts other species involved in mixed-species flocks. Anim. Behav. 72, 471–477 10.1016/j.anbehav.2006.02.004 (doi:10.1016/j.anbehav.2006.02.004) [DOI] [Google Scholar]
- 33.Price T, Zee J, Jamdar K, Jamdar N. 2003. Bird species diversity along the Himalayas: a comparison of Himachal Pradesh with Kashmir. J. Bombay Nat. Hist. Soc. 100, 394–409 [Google Scholar]
- 34.Bioacoustics Research Program. 2008. Raven pro: interactive sound analysis software. Ithaca, NY: The Cornell Lab of Ornithology; See http://www.birds.cornell.edu/raven. [Google Scholar]
- 35.Weir JT, Wheatcroft D. 2010. A latitudinal gradient in rates of evolution of avian syllable diversity and song length. Proc. R. Soc. B 278, 1713–1720 10.1098/rspb.2010.2037 (doi:10.1098/rspb.2010.2037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weir JT, Wheatcroft D, Price TD. 2012. The role of ecological constraint in driving the evolution of song frequency across a latitudinal gradient. Evolution 66, 2773–2783 10.1111/j.1558-5646.2012.01635.x (doi:10.1111/j.1558-5646.2012.01635.x) [DOI] [PubMed] [Google Scholar]
- 37.Krams I, Krama T, Igaune K, Mänd R. 2008. Experimental evidence of reciprocal altruism in the pied flycatcher. Behav. Ecol. Sociobiol. 62, 599–605 10.1007/s00265-007-0484-1 (doi:10.1007/s00265-007-0484-1) [DOI] [Google Scholar]
- 38.R Development Core Team 2010. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 39.Charrier I, Sturdy CB. 2005. Call-based species recognition in black-capped chickadees. Behav. Process. 70, 271–281 10.1016/j.beproc.2005.07.007 (doi:10.1016/j.beproc.2005.07.007) [DOI] [PubMed] [Google Scholar]
- 40.Ramakrishnan U, Coss RG. 2000. Recognition of heterospecific alarm vocalizations by bonnet macaques (Macaca radiata). J. Comp. Psychol. 114, 3–12 10.1037/0735-7036.114.1.3 (doi:10.1037/0735-7036.114.1.3) [DOI] [PubMed] [Google Scholar]
- 41.Davies NB, Madden JJ, Butchart SMH. 2004. Learning fine-tunes a specific response of nestlings to the parental alarm calls of their own species. Proc. R. Soc. Lond. B 271, 2297–2304 10.1098/rspb.2004.2835 (doi:10.1098/rspb.2004.2835) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Curio E, Ernst U, Vieth W. 1978. The adaptive significance of avian mobbing. II. Cultural transmission of enemy recognition in blackbirds: effectiveness and some constraints. Zeitschr. Tierpsychol. 48, 184–202 10.1111/j.1439-0310.1978.tb00255.x (doi:10.1111/j.1439-0310.1978.tb00255.x) [DOI] [Google Scholar]
- 43.Davies NB, Welbergen JA. 2009. Social transmission of a host defense against cuckoo parasitism. Science 324, 1318–1320 10.1126/science.1172227 (doi:10.1126/science.1172227) [DOI] [PubMed] [Google Scholar]
- 44.Kelly LA, Healy SD. 2012. Vocal mimicry in bowerbirds is associated with an alarming context. J. Avian Biol. 43, 525–530 10.1111/j.1600-048X.2012.05863.x (doi:10.1111/j.1600-048X.2012.05863.x) [DOI] [Google Scholar]
- 45.Price TD. 2010. The roles of time and ecology in the continental radiation of the Old World leaf warblers (Phylloscopus and Seicercus). Phil. Trans. R. Soc. B 365, 1749–1762 10.1098/rstb.2009.0269 (doi:10.1098/rstb.2009.0269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kelley LA, Coe RL, Madden JR, Healy SD. 2008. Vocal mimicry in songbirds. Anim. Behav. 76, 521–528 10.1016/j.anbehav.2008.04.012 (doi:10.1016/j.anbehav.2008.04.012) [DOI] [Google Scholar]