Abstract
Plant volatiles serve as key foraging and oviposition cues for insect herbivores as well as their natural enemies, but little is known about how genetic variation within plant populations influences volatile-mediated interactions among plants and insects. Here, we explore how inbred and outbred plants from three maternal families of the native weed horsenettle (Solanum carolinense) vary in the emission of volatile organic compounds during the dark phase of the photoperiod, and the effects of this variation on the oviposition preferences of Manduca sexta moths, whose larvae are specialist herbivores of Solanaceae. Compared with inbred plants, outbred plants consistently released more total volatiles at night and more individual compounds—including some previously reported to repel moths and attract predators. Female moths overwhelmingly chose to lay eggs on inbred (versus outbred) plants, and this preference persisted when olfactory cues were presented in the absence of visual and contact cues. These results are consistent with our previous findings that inbred plants recruit more herbivores and suffer greater herbivory under field conditions. Furthermore, they suggest that constitutive volatiles released during the dark portion of the photoperiod can convey accurate information about plant defence status (and/or other aspects of host plant quality) to foraging herbivores.
Keywords: horsenettle, inbreeding, tobacco horn worm, night-time volatiles, oviposition, repellants
1. Introduction
Olfaction is a key sensory modality for most insects, and volatile organic compounds emitted by plants serve as foraging cues for both insect herbivores and their natural enemies [1]. A major focus of past research on the role of plant volatiles as info-chemicals has been on understanding how quantitative and qualitative changes in volatile emissions induced by herbivory, pathogen infection and other environmental stressors convey information about plant status to—and consequently influence the behaviour of—insects and other organisms [2–7]. The exploitation of herbivore-induced plant volatiles by insect predators and parasitoids, for example, is widespread and well documented [2,8,9]. Induced plant volatiles are also known to guide foraging and oviposition by insect herbivores [10], in some cases serving as aggregation cues [11] but in others eliciting aversive responses that probably reflect reduced host plant quality resulting from the presence of competitors and prior induction of plant defences [12,13].
Despite the greater focus on induced plant volatiles, constitutive volatile emissions are also known to play important roles in mediating interactions between plants and insects. The composition of constitutive volatile blends emitted by different plant species and tissues can vary in systematic ways [10,14]. Thus, constitutive plant volatile emissions can provide host-location cues for insect herbivores that may convey information about plant identity and ecologically relevant aspects of the plant phenotype. It is reasonable to assume that such interactions frequently entail the exploitation by herbivores of emissions that serve no adaptive signalling function for the plant but rather derive as byproducts of plants’ normal physiological activities [5,15,16]. However, it is also possible that constitutive emissions may in some cases play an active signalling function. For example, emissions that provide accurate information regarding plant defence status or nutritional quality could mediate herbivore choices among potential hosts.
Despite the well-established role of plant volatiles as foraging cues for insects, we know relatively little about how these cues, or the interactions they mediate, are influenced by intraspecific genetic variation among plants or other population-level processes such as population genetic structure and mating systems. Several greenhouse and growth chamber studies of cultivated species have reported differences among cultivars/varieties in volatile production [17,18], but only a few studies have examined variation for volatile production within non-cultivated species [19–21]. To address this gap in our current knowledge, we have begun investigating the population ecology and evolution of volatile-mediated plant–insect interactions in horsenettle (Solanum carolinense L.) and, in particular, the ways in which such interactions are impacted by inbreeding. Inbreeding is common in flowering plants [22] and increases homozygosity in resulting offspring, thereby exposing deleterious recessive alleles to selection while decreasing the contribution of overdominance to fitness [23]. Consequently, selfed progeny often exhibit reduced fitness relative to outbred progeny (i.e. inbreeding depression). Although investigators have only recently begun to document the impacts of inbreeding on plant–insect interactions, several recent studies show that inbred plants suffer higher levels of herbivory than outbred plants [24,25], and that herbivores develop more rapidly on inbred plants [26,27]. The mechanisms underlying such effects are not well studied, but may include indirect effects mediated by a general reduction in vigour associated with inbreeding [23,24,28], as well as the direct disruption of plant defences—for example, through the effects of deleterious recessives on the expression of genes involved in plant defence pathways [29,30].
Our previous work on horsenettle has documented significant effects of inbreeding on plant defences against insect herbivores, including alteration of constitutive and induced volatile emissions and the plant–insect interactions they mediate [16]. For example, we observed higher levels of constitutive volatile emissions from inbred plants (relative to outbred plants) under field conditions, which appeared to mediate increased recruitment of insect herbivores. By contrast, volatile induction in response to insect feeding was attenuated in inbred plants, which consequently recruited fewer predators and parasitoids than herbivore-damaged outbred plants [16]. These findings suggest that the overall volatile signalling phenotype of horsenettle is compromised by inbreeding, consistent with a previous observation of dramatic impacts of inbreeding on plant fitness and plant susceptibility to herbivore damage in the field [31].
Building on this work, the current study investigates the behavioural responses and oviposition preferences of a night-flying, Solanaceae-specialist lepidopteran (Manduca sexta L.) to the night-time volatile emissions of inbred and outbred horsenettle plants from three maternal families. We previously demonstrated that the performance of M. sexta larvae is significantly enhanced on inbred relative to outbred horsenettle plants [27], also work with other plant and insect species has shown that plant-derived volatile cues can exhibit substantial variation between day and night, and that night-active insect herbivores are particularly responsive to volatile profiles emitted during the dark phase of the photoperiod [12,32–34]. The current study explores how the night-time volatile profiles—and the plant–herbivore interactions they mediate—are influenced by genotypic variation among individual plants and inbreeding.
2. Material and methods
(a). The study system
Solanum carolinense is an herbaceous perennial weed that inhabits agricultural fields, crop pastures and wastelands throughout southeastern Canada and the central and eastern United States [35]. Once established, S. carolinense spreads via horizontal, rhizome-like roots that extend up to 1 m from the parent stem [36]. Though self-incompatibility (SI) is uncommon in weeds [37,38], horsenettle exhibits a typical solanaceous-type ribonuclease-mediated gametophytic SI system [39,40]. However, the SI system in S. carolinense is leaky, and the likelihood of selfing is influenced by flower age, prior fruit production [41,42], and the presence of certain S alleles [42]. Horsenettle exhibits a variety of defence traits (spines, stellate trichomes and toxic glycoalkaloids) [43–45] and is attacked by many important solanaceae-specialist herbivores and pathogens, which also attack related crops in the genus Solanum (e.g. tomato and potato) [46–48]. Tobacco hornworm larvae (M. sexta) have been observed feeding on S. carolinense within the area from which our laboratory populations are collected [31,46].
(b). Plant materials
Rootstocks were collected from a field population near State College, Pennsylvania and grown in a greenhouse in 4 l pots (16 L : 8 D; 25°C : 22°C, respectively, 65% relative humidity (RH)) [42]. After a six to eight week cold treatment, each root was divided into pieces, which were replanted in 4 l pots to re-sprout. Flowers produced on one ramet from each of the original 16 field-collected plants were outcrossed, while flowers from a second ramet from each of the 16 original genets were self-pollinated (inbred) until a total of 40 flowers per ramet were pollinated. A sample of the resulting seeds from self- and cross-pollinations were germinated and grown in the greenhouse.
Horizontal roots from one inbred and one outbred plant from three of the original 16 maternal families (designated B1, B3 and B4) were allowed to sprout in flatbeds, containing a peat-based, general-purpose potting soil (Pro-Mix, Premier Horticulture Inc., Quakertown, PA, USA), which were maintained in a growth chamber (conditions as above) and watered on alternate days. After 10–15 days, the sprouts were transplanted into 2 l pots and moved to an insect-free greenhouse. Plants used in all experiments were six to eight weeks old and had not yet flowered.
(c). Rearing of Manduca sexta
Eggs obtained from the Carolina Biological Supply (NC) were hatched on moist filter paper, and the larvae were reared on artificial casein diet in plastic containers inside a growth chamber set to a 16 L : 8 D photoperiod, 25°C day and 22°C night temperatures, respectively, and 65% RH. Larvae that pupated were sexed and stored in a bin of shredded paper in the dark. After eclosion, one male and one female moth were moved to a cage (0.25 m3) and provided with a dilute Gatorade solution as a food source.
(d). Volatile collections
We collected volatiles from 12 inbred and 12 outbred ramets (four ramets of inbred and outbred plants from each of the three maternal families). Collections occurred on four successive nights, with one inbred and one outbred plant from each family represented each night, and were conducted in a greenhouse with no supplemental lighting using a push–pull collection system (Analytical Research Systems, Gainesville, FL, USA (see [16] for full description). Each volatile trap collected headspace volatiles for a maximum of 4 h (to prevent break-through loss of small molecular weight compounds) from 22.00 to 02.00 (trap 1) and 02.00 to 06.00 (trap 2). After the collections, the traps were eluted with 150 µl methylene chloride, plus 5 µl of a mix containing the internal standards n-octane (40 ng µl−1) and nonyl-acetate (80 ng µl−1). Samples were injected in 1 μl aliquots into an Agilent model 6890 gas chromatograph fitted with a flame ionization detector, and then quantified (for details see [16]). For the final analysis, the two time points (22.00–02.00 and 02.00–06.00) were summed for each replicate. Volatile amounts were corrected for plant dry weight in order to account for slight differences in plant size among replicates. The data from the four nights were pooled for each inbred and outbred genet from each of the three maternal families for the final analysis. Total volatiles were analysed using ANOVA (using log-transformed values) with breeding (fixed), family (random) and family × breeding as the terms in the model (Minitab v. 14). To examine qualitative differences in herbivore-induced blends, we employed principal component analysis (PCA; with each volatile as a variable in the analysis), followed by MANOVA (with terms as for total volatiles ANOVA) using rank-transformed scores (newly generated orthogonal data values) for components 1 and 2 (Minitab v. 14). This multivariate approach has been used previously to analyse volatile emissions of horsenettle [46] and is appropriate for volatile data where individual compounds are likely to be correlated with one another. To complement the multivariate analysis (compound loading plots, see figure 1) and further explore the contributions of individual compounds to differences in the blend, we also examined compound abundance by breeding type. A compound was considered elevated if means were separated by 1.5 times each standard error value (lower compound plus 1.5 × s.e. versus higher compound minus 1.5 × s.e.). Our inclusion of the additional separation criteria of half of each s.e. interval in addition to a full s.e. interval results in a more stringent comparison of means relative with other studies [6,16], which used simple non-overlap of s.e. to describe upregulation of individual compounds arising from common biosynthetic pathways.
Figure 1.
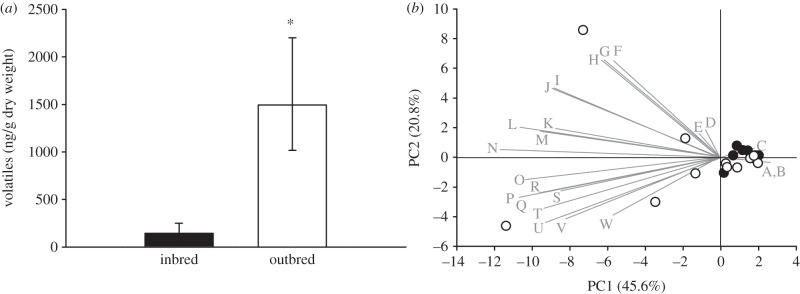
Night-time volatile emissions from horsenettle plants. (a) Mean total volatiles ± s.e. emitted from inbred and outbred plants (The asterisk indicates significant difference at p < 0.05); (b) PCA output showing a scatter plot of component-one (x) and component-two (y) scores for each replicate plant overlaid on loading plots of the different compounds (variables in the PCA) composing the volatile blend (grey lines). Letters stand for compounds (full names in table 3), open symbols are outbred plants, and black symbols are inbred plants. Percentages next to the axis labels indicate the amount of variation explained by that component.
(e). Oviposition trials
Oviposition choice tests were performed with uncovered plants (moths allowed to contact leaf surface) and plants covered with a green mesh fabric. For the uncovered plants, 4 six-week-old horsenettle plants (two ramets from one inbred progeny and two ramets from one outbred progeny from one maternal family) were used for each trial. For each of the three families, the oviposition preference of six female moths was examined for inbred versus outbred plants in a large mesh cage (1 m3), with a new set of plants used for each moth. Plants were placed on diagonally opposite corners of each cage in a climate controlled room under a 16 L : 8 D cycle (see the electronic supplementary material, figure S1). For each trial, a pair of Manduca adults (one male and one female) was placed into the centre of the cage on the day of the experiment. An Erlenmeyer flask with 50 per cent lemon-flavour Gatorade solution was placed at the centre of the cage as a food source. During the next day, eggs were carefully counted and removed (see the electronic supplementary material, figure S2 and video S1). Plants were watered each day after egg counting, and the trial continued for a total of four nights for each moth. Data (total number of eggs laid on each breeding treatment) were analysed separately using χ2-tests (Minitab v. 14). We also performed a ‘no-choice’ experiment in which moths were presented with only inbred or only outbred ramets from each of the three maternal families (each breeding × family combination represented by three sets of plants and three separate moths). The total number of eggs deposited per moth on each breeding treatment over four consecutive days was analysed using a paired t-test (Minitab v. 14).
To assess the effects of olfactory cues on moth oviposition preferences in the absence of other cues, we repeated the choice tests (as above) using plants covered in four layers of green bridal veil which allowed emission of volatiles while obscuring visual cues—especially as the assays were conducted in darkness—and preventing direct contact with the plants (see the electronic supplementary material, figure S3 and video S2). This experiment was carried out using three moths per maternal family. Thus, the lower overall egg counts in these assays relative to the previous experiment reflect the smaller number of moths used. The distribution of eggs laid across the two breeding treatments was analysed as for the previous set of choice tests. Since plant volatile emissions are a function of total leaf area, we also measured the fresh weight of leaves from plants used in oviposition experiments (three plants of each breeding type from each family). The leaves were removed and weighed after oviposition and the data were analysed using a paired t-test (Minitab v. 14).
We also made behavioural observations to determine whether female Manduca moths spent different amounts of time flying near or contacting inbred and outbred plants. These observations were carried out in dim light just sufficient for visual observation (accomplished by allowing light infiltration from an adjacent room through a slightly open door). Using a stopwatch, we recorded time spent hovering near or laying eggs on plants of each breeding type during bouts of oviposition activity (‘oviposition events’). Individual oviposition events were clearly delimited by intervening periods of prolonged resting/feeding lasting several minutes. Within individual events, moths would sometimes alight briefly (for a few seconds) on the floor or walls of the cage; these brief resting periods were not timed. Each moth was observed until four distinct oviposition events were completed. To evaluate the resulting data, we used repeated measures ANOVA with breeding (fixed) and oviposition event (the time component), moth, family and family × breeding as random factors (Minitab v. 14).
(f). Raw data
The raw data collected from the experiments have been deposited in ScholarSphere, a secure repository operated by The Pennsylvania State University. The files can be accessed at https://scholarsphere.psu.edu/files/gf06g267d.
3. Results
(a). Volatile collections
Outbred plants exhibited significantly higher total night-time volatile emissions than inbred plants (figure 1a and table 1). This pattern was consistent across all three families despite among-family variation (see the electronic supplementary material, figure S4 and table S1). In the PCA, the first two components explained over 66 per cent of the variation in volatile blend (figure 1b), and there is significant separation of data points only by breeding according to these two axes (family and family × breeding are not significant; table 2). Of 23 compounds observed in all families, 17 were elevated in blends emitted by outbred plants relative to inbred plants (table 3), and no compounds were elevated in inbred plant blends relative to outbred blends. Of the 10 most abundant compounds, eight were elevated in outbred plants, with seven of these being terpenes. Additionally, the 10 most abundant compounds all had strong loadings along PC1 (values further from the origin along the x-axis), and several, including indole, tridecatetraene and methyl salicylate, also had moderately strong loadings along PC2 (values further from the origin along the y-axis). This indicates that these compounds, 80 per cent of which were elevated in outbred blends, also contributed the most to explaining variation along the two PC axes. Additionally, four compounds occurring as minor constituents of the overall blend were only detected in outbred plants (methyl benzoate, 1-butanol 3-methyl acetate, myrcene and an unknown compound).
Table 1.
Analysis of variance table for the total volatiles emitted over 8 h (night) from inbred and outbred plants. (Italics denote p-values of <0.05. MS, mean square.)
| source of variation | d.f. | MS | F | p-value |
|---|---|---|---|---|
| family (random) | 2 | 1.51 | 3.85 | 0.20 |
| breeding (fixed) | 1 | 4.48 | 22.89 | 0.04 |
| family × breeding | 2 | 0.39 | 0.79 | 0.46 |
| error | 18 | 4.45 |
Table 2.
Multivariate analysis of variance table for PC1 and PC2 scores generated from a PCA performed on the full volatile blend. (Italics denote p-values of <0.05.)
| source of variation | Wilks’ λ | d.f. | F | p-value |
|---|---|---|---|---|
| breeding (fixed) | 0.0017 | 2, 1 | 298.268 | 0.041 |
| family (random) | 0.0023 | 4, 2 | 9.732 | 0.095 |
| breeding × family | 0.827 | 4, 34 | 0.844 | 0.507 |
Table 3.
Mean and s.e. values for night-time volatiles emitted by inbred and outbred horsenettle plants.
| compounda,b | inbred mean | inbred s.e. | outbred mean | outbred s.e. |
|---|---|---|---|---|
| (J) Nonatriene (homoterpene) | 90.97 | 42.38 | 786.88 | 361.54 |
| (U) Tridecatetraene (homoterpene) | 126.76 | 60.65 | 766.77 | 305.75 |
| (H) E-beta-ocimene (monoterpene) | 59.1 | 21.82 | 505.29 | 346.9 |
| (V) Methyl salicylate (aromatic) | 34.55 | 21.94 | 294.83 | 124.63 |
| (M) Beta-springene (diterpene) | 21.08 | 10.64 | 107.54 | 27.41 |
| (S) Z-3-hexenyl acetate (GLV) | 11.05 | 6.55 | 100.64 | 27.56 |
| (T) Nerolidol (sesquiterpene) | 11.92 | 9.79 | 79.73 | 30.42 |
| (G) Indole (aromatic) | 6.33 | 3.44 | 69.76 | 54.55 |
| (P) Alpha-selinene (sesquiterpene) | 4.57 | 2.42 | 52.05 | 26.91 |
| (Q) Elemene (sesquiterpene) | 4.46 | 2.27 | 48.42 | 24.21 |
| (I) Linalool (monoterpene) | 5.19 | 2.28 | 24.1 | 9.44 |
| (A) E-2-hexenal (GLV) | 0.51 | 0.51 | 6.79 | 3.15 |
| (B) Z-3-hexen-1-ol (GLV) | 0.51 | 0.51 | 6.79 | 3.15 |
| (W) beta-selinene (sesquiterpene) | 1.74 | 1.44 | 6.2 | 2.18 |
| (K) alpha-farnesene (sesquiterpene) | 1.68 | 0.95 | 5.87 | 2.16 |
| (D) Caryophyllene (sesquiterpene) | 0.71 | 0.39 | 3.64 | 1.29 |
| (N) Aromadendrene (sesquiterpene) | 0.18 | 0.18 | 3.27 | 1.49 |
| (O) Methyl benzoate (aromatic) | 0 | 0 | 2.46 | 1.71 |
| (R) unknown | 0 | 0 | 1.95 | 1.71 |
| (L) 1-butanol, 3-methyl-, acetate (GLV) | 0 | 0 | 1.56 | 1.05 |
| (C) Cyclopentene, 1,2,3,4,5-pentamethyl- (GLV) | 1.54 | 1.34 | 1.56 | 0.66 |
| (E) Z-jasmone (aromatic) | 1.44 | 1.44 | 1.41 | 0.85 |
| (F) Myrcene (monoterpene) | 0 | 0 | 0.49 | 0.49 |
aItalic names indicate mean separation by greater than 1.5 s.e. values (lower value plus 1.5 times s.e. versus higher value minus 1.5 times s.e.).
bLetters correspond to those in figure 1b.
(b). Oviposition assays
When allowed to choose among uncovered plants, M. sexta females consistently preferred inbred over outbred plants, across all maternal families (χ21 = 369.08, p < 0.001; figure 2a, electronic supplementary material, figure S5a and movie S1). This preference persisted when plants were covered with green bridal veil (χ21 = 215.10, p < 0.001; figure 2b; electronic supplementary material, figure S5b and movie S2). We found that moths readily oviposited onto the bridal veil and did not appear to make any extra effort to contact the leaf surface underneath the veil for oviposition (see the electronic supplementary material, figure S3). In no-choice assays, moths laid slightly, but not significantly, more eggs on inbred plants (χ21 = 2.95, p = 0.08; electronic supplementary material, figure S6).
Figure 2.
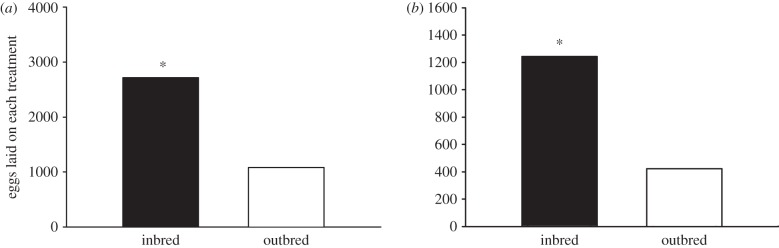
Oviposition choice by adult female M. sexta. (a) Number of eggs laid on inbred and outbred uncovered plants. (b) Number of eggs laid on inbred and outbred plants covered with bridal veil. (The asterisk indicates significant difference at p < 0.05.)
Our behavioural analysis showed that moths spent significantly more time hovering near and contacting inbred plants than outbred plants (figure 3; electronic supplementary material, table S2). Time spent was affected by maternal family, but was independent of the moth and individual oviposition event (see the electronic supplementary material, table S2 and figure S7). Leaf-area analyses between inbred and outbred plants after oviposition demonstrated no significant difference (paired t-test; t-value = −1.56, p = 0.138), suggesting that observed patterns of oviposition behaviour reflect breeding-specific differences in volatile emissions rather than differences in plant size.
Figure 3.
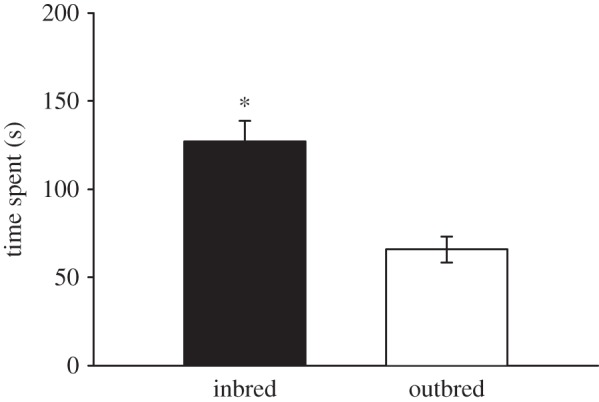
Mean time spent ± s.e. on each breeding treatment during oviposition choice tests on uncovered plants ((*) indicates significant difference at p < 0.05).
4. Discussion
Our data reveal a clear preference of ovipositing M. sexta for inbred (relative to outbred) horsenettle plants (figure 2) that appears to be driven by breeding-specific differences in plant volatile emissions (figure 1 and table 3). In choice assays, female moths laid far more eggs on inbred than outbred plants, even when plants were covered in bridal veil cloth that minimized visual cues and prevented moths from contacting the plants (figure 2). This result is consistent with our previous reports of increased herbivore recruitment to inbred plants in the field [16,49] and with the general expectation that the disruption of adapted plant phenotypes by inbreeding can increase susceptibility to herbivory [24,26].
It is notable, however, that the oviposition preferences observed here appear to be caused by a reduction of volatile emissions from inbred plants during the dark phase of the photoperiod, including a marked reduction in the emission of specific monoterpenes (linalool and a marginal reduction in E-beta-ocimene) and sesquiterpenes (nerolidol, alpha-selinene, elemene, caryophyllene and aromadendrene; figure 1b and table 3), which are frequent components of herbivore-induced volatile blends that have previously been reported to deter herbivores [12,50] and attract predators and parasitoids—although the amounts released constitutively in our night-time collections (even by outbred plants) are much lower than those typically released during the day in response to insect feeding (by the same plant genotypes at the same developmental stage) [16]. Linalool, in particular, has previously been reported in response to feeding by Manduca larvae on Nicotiana [51] and has been shown to elicit a significant response in the projection neurons of female-specific glomeruli in this moth [34,52]. It has also been implicated as a cue for parasitic wasps foraging for Manduca caterpillars [53–55]. In addition to mono- and sesquiterpenes, our data also indicate that two homoterpenes, nonatriene (4,8-dimethyl-1,3,7-nonatriene) and tridecatetraene (3E,7E)-4,8,12-trimethyl-1-3-7-11-tridecatetraene), were elevated in the night-time emissions of outbred plants relative to inbred plants (figure 1b and table 3). These compounds have also been shown to act as oviposition deterrents for other insects [56]. For example, constitutive volatile emissions from Melinis minutiflora, including nonatriene and the sesquiterpene beta-caryophyllene, are repellent to gravid stem-borer females of Busseola fusca (Lepidoptera, Noctuidae) and Chilo partellus (Lepidoptera, Pyralidae), facilitating the use of this plant as the ‘push’ component of a model ‘push–pull’ agricultural system where it is inter-planted with valuable crop species [57,58]. In horsenettle, we recently reported that linalool and E-beta-ocimene were upregulated above constitutive levels (in day-time volatile profiles) following damage by M. sexta larvae, along with nonatriene, which was significantly induced only in outbred plants. In a field experiment testing the consequences of this variation in volatile blends among inbred and outbred plants, we found that fewer herbivores were recruited to the odours of damaged plants in the field [16]. Thus, it appears that horsenettle plants constitutively emit a number of compounds during the dark phase of the photoperiod that have previously been shown to have repellent effects on insect herbivores, including M. sexta females, and many of which are known to be induced or upregulated by insect feeding damage. Furthermore, we observe an overall reduction of volatile emissions (particularly for inbred plants) and emission of fewer volatile compounds at night relative to the day-time volatile emissions previously described for each of these maternal families [16].
The strong preference of female moths for odours of inbred plants despite their attenuated volatile emissions provides some support for the hypothesis that the constitutive volatile emissions released by horsenettle plants during the dark phase of the photoperiod may convey accurate information about plant defence capabilities—or other features of host plant quality for the herbivores—and suggests that these repellent cues are compromised by inbreeding. This is consistent with our previous findings suggesting that the overall plant resistance phenotype is compromised in inbred plants [16,31,49]. We have, furthermore, previously reported significantly enhanced performance of M. sexta larvae on inbred relative to outbred plants [27], suggesting that the oviposition preferences observed in the current study reflect an accurate assessment of host plant quality on the basis of olfactory cues.
Despite the fact that both our current and past findings reveal a consistent pattern of increased herbivore recruitment to and colonization of inbred plants, the current findings contrast strongly with our previous finding that constitutive volatile emissions of inbred plants were elevated during the light phase of the photoperiod [16]. The pattern observed during the daytime was driven to a large extent by overall higher emissions of green leaf volatiles (6-carbon alcohols, aldehydes and esters), which are attractive to herbivores [59,60] and to a lesser extent by specific terpenes (including myrcene and beta-pinene), which have also been shown to attract herbivores in other studies [61,62]. By contrast, at night, overall constitutive emissions of inbred plants are reduced relative to outbred plants (figure 1a), with this difference being driven by increased emissions of terpene compounds from outbred plants (figure 1b and table 3). Synthesis of mono- and sesquiterpenes is often light-dependent (through photosynthesis-driven production of the C-5 isoprene units that serve as precursors). However, studies have shown that storage of monoterpenes in both aqueous and lipid-rich cellular environments is possible, even in the absence of specific storage structures such as glandular trichomes or resin ducts (not present in horsenettle) [63,64]. Furthermore, significant evidence exists for night-time emission of sesquiterpenes, which constitute the majority of the biologically relevant molecules emitted in lower amounts by inbred plants in our study (e.g. [12], reviewed in [65]). Therefore, it is likely that the maintenance of consistent levels of constitutive terpene emissions by outbred plants during the dark phase, with a concurrent reduction in constitutive release of these compounds by inbred plants, may represent breeding-specific differences in the capacity for terpene storage and release (mono- and sesquiterpenes) or synthesis under light-limited conditions (sesquiterpenes). Our previous work examining herbivore-induced volatile emissions for these same genotypes under light conditions clearly indicates that inbred plants are impaired in their capacity to synthesize terpenes, lending support to the hypothesis that other aspects of terpene regulation may be similarly disrupted [16]. Thus, the impacts of inbreeding on horsenettle volatile emissions may have the effect of increasing the recruitment of herbivores to inbred plants both during the day and at night (consistent with the expectation of a general disruption of the adapted plant phenotype), and may in both instances be related to the functionality of the terpene biosynthetic machinery.
5. Conclusions
Although several recent studies have shown that inbreeding reduces plant resistance to herbivores and that herbivores feeding on inbred plants outperform herbivores feeding on outbred plants [24–26,65], the mechanisms underlying the reduced resistance have not been examined. Our current findings demonstrate that inbreeding alters the nocturnal emission of volatiles in S. carolinense and that female M. sexta moths can distinguish between the volatile blends produced by inbred and outbred plants and preferentially oviposit on inbred plants. To our knowledge, this is the first study to demonstrate that intraspecific variation in constitutively emitted nocturnal volatiles influences herbivore oviposition behavior. This study also suggests that the reduced resistance of inbred plants is apparent to at least some herbivores before they land on the plant. Given the strong possibility that disruption of terpene production and/or storage is related to the behavioural patterns we have observed, future work should focus on understanding how inbreeding disrupts the production of terpene synthase enzymes and other constitutive chemical and physical defences (e.g. transcriptome analysis), a task that is now feasible owing to the recent successful hybridization of horsenettle transcripts with commercially available tomato microarray chips [49].
Acknowledgements
The authors thank T. Omeis, S. Diloreto and the staff at the Buckhout Greenhouse for the cultivation of the plants; J. Saunders and E. Smyers for logistical support; J. Mena-Ali and C. M. Delphia for plant materials. This research was supported by National Science Foundation grant no. DEB1050998 and by the David and Lucile Packard Foundation.
References
- 1.Bruce TJA, Wadhams LJ, Woodcock CM. 2005. Insect host location: a volatile situation. Trends Plant Sci. 10, 269–274 10.1016/j.tplants.2005.04.003 (doi:10.1016/j.tplants.2005.04.003) [DOI] [PubMed] [Google Scholar]
- 2.Turlings TCJ, Tumlinson JH, Lewis WJ. 1990. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 250, 1251–1253 10.1126/science.250.4985.1251 (doi:10.1126/science.250.4985.1251) [DOI] [PubMed] [Google Scholar]
- 3.Paré PW, Tumlinson JH. 1997. De novo biosynthesis of volatiles induced by insect herbivory in cotton plants. Plant Physiol. 114, 1161–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farmer EE. 2001. Surface-to-air signals. Nature 411, 854–856 10.1038/35081189 (doi:10.1038/35081189) [DOI] [PubMed] [Google Scholar]
- 5.Dudareva NF, Negre DA, Nagegowda I, Orlova I. 2006. Plant volatiles: recent advances and future perspectives. Crit. Rev. Plant Sci. 25, 417–440 10.1080/07352680600899973 (doi:10.1080/07352680600899973) [DOI] [Google Scholar]
- 6.Mauck KE, De Moraes CM, Mescher MC. 2010. Deceptive chemical signals induced by a plant virus attract insect vectors to inferior hosts. Proc. Natl Acad. Sci. USA 107, 3600–3605 10.1073/pnas.0907191107 (doi:10.1073/pnas.0907191107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shapiro L, De Moraes CM, Stephenson AG, Mescher MC. 2012. Pathogen effects on vegetative and floral odours mediate vector attraction and host exposure in a complex pathosystem. Ecol. Lett. 15, 1430–1438 10.1111/ele.12001 (doi:10.1111/ele.12001) [DOI] [PubMed] [Google Scholar]
- 8.Dickie M, Van Loon J. 2000. Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context. Entomol. Exp. Appl. 97, 237–249 10.1046/j.1570-7458.2000.00736.x (doi:10.1046/j.1570-7458.2000.00736.x) [DOI] [Google Scholar]
- 9.De Moraes CM, Lewis WJ, Paré PW, Alborn HT, Tumlinson JH. 1998. Herbivore-infested plants selectively attract parasitoids. Nature 393, 570–573 10.1038/31219 (doi:10.1038/31219) [DOI] [Google Scholar]
- 10.Arimura G, Matsui K, Takabayashi J. 2009. Chemical and molecular ecology of herbivore-induced plant volatiles: proximate factors and their ultimate functions. Plant Cell Physiol. 50, 911–923 10.1093/pcp/pcp030 (doi:10.1093/pcp/pcp030) [DOI] [PubMed] [Google Scholar]
- 11.Hammons DL, Kurtural SK, Newman MC, Potter DA. 2009. Invasive Japanese beetles facilitate aggregation and injury by a native scarab pest of ripening fruits. Proc. Natl Acad. Sci. USA 106, 3686–3691 10.1073/pnas.0811097106 (doi:10.1073/pnas.0811097106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Moraes CM, Mescher MC, Tumlinson JH. 2001. Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 410, 577–580 10.1038/35069058 (doi:10.1038/35069058) [DOI] [PubMed] [Google Scholar]
- 13.Gatehouse JA. 2002. Plant resistance towards insect herbivores: a dynamic interaction. New Phytol. 156, 145–169 10.1046/j.1469-8137.2002.00519.x (doi:10.1046/j.1469-8137.2002.00519.x) [DOI] [PubMed] [Google Scholar]
- 14.Paré PW, Tumlinson JH. 1999. Plant volatiles as a defense against insect herbivores. Plant Physiol. 121, 325–331 10.1104/pp.121.2.325 (doi:10.1104/pp.121.2.325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pickersky E, Gershenson J. 2002. The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr. Opin. Plant Biol. 5, 237–243 10.1016/S1369-5266(02)00251-0 (doi:10.1016/S1369-5266(02)00251-0) [DOI] [PubMed] [Google Scholar]
- 16.Kariyat RR, Mauck KE, De Moraes CM, Stephenson AG, Mescher MC. 2012. Inbreeding alters volatile signalling phenotypes and influences tri-trophic interactions in horsenettle (Solanum carolinense L.). Ecol. Lett. 15, 301–309 10.1111/j.1461-0248.2011.01738.x (doi:10.1111/j.1461-0248.2011.01738.x) [DOI] [PubMed] [Google Scholar]
- 17.Loughrin JH, Manukian A, Heath RR, Tumlinson JH. 1995. Volatiles emitted by different cotton varieties damaged by feeding beet armyworm larvae. J. Chem. Ecol. 21, 1217–1227 10.1007/BF02228321 (doi:10.1007/BF02228321) [DOI] [PubMed] [Google Scholar]
- 18.Takabayashi J, Dicke M, Posthumus MA. 1991. Variation in composition of predator-attracting allelochemicals emitted by herbivore-infested plants: relative influence of plant and herbivore. Chemoecology 2, 1–6 10.1007/BF01240659 (doi:10.1007/BF01240659) [DOI] [Google Scholar]
- 19.Glawe GA, Zavala JA, Kessler A, Van Dam NM, Baldwin IT. 2003. Ecological costs and benefits correlated with trypsin protease inhibitor production in Nicotiana attenuata. Ecology 84, 79–90 10.1890/0012-9658(2003)084[0079:ECABCW]2.0.CO;2 (doi:10.1890/0012-9658(2003)084[0079:ECABCW]2.0.CO;2) [DOI] [Google Scholar]
- 20.Halitschke R, Kessler A, Kahl J, Lorenz A, Baldwin IT. 2000. Ecophysiological comparison of direct and indirect defenses in Nicotiana attenuata. Oecologia 124, 408–417 10.1007/s004420000389 (doi:10.1007/s004420000389) [DOI] [PubMed] [Google Scholar]
- 21.Hare JD. 2007. Variation in herbivore and methyl jasmonate-induced volatiles among genetic lines of Datura wrightii. J. Chem. Ecol. 33, 2028–2043 10.1007/s10886-007-9375-1 (doi:10.1007/s10886-007-9375-1) [DOI] [PubMed] [Google Scholar]
- 22.Barrett S, Eckert C. 1990. Variation and evolution of mating systems in seed plants. In Biological approaches and evolutionary trends in plants (ed. Kawano S.), pp. 229–254 New York, NY: Academic Press Limited [Google Scholar]
- 23.Charlesworth D, Charlesworth B. 1987. Inbreeding depression and its evolutionary consequences. Annu. Rev. Ecol. Syst. 18, 237–268 10.1146/annurev.es.18.110187.001321 (doi:10.1146/annurev.es.18.110187.001321) [DOI] [Google Scholar]
- 24.Stephenson AG, Leyshon B, Travers SE, Hayes CN, Winsor JA. 2004. Interrelationships among inbreeding, herbivory, and disease on reproduction in a wild gourd. Ecology 85, 3023–3034 10.1890/04-0005 (doi:10.1890/04-0005) [DOI] [Google Scholar]
- 25.Bello-Bedoy R, Núñez Farfán J. 2011. The effect of inbreeding on defence against multiple enemies in Datura stramonium. J. Evol. Biol. 24, 518–530 10.1111/j.1420-9101.2010.02185.x (doi:10.1111/j.1420-9101.2010.02185.x) [DOI] [PubMed] [Google Scholar]
- 26.Carr DE, Eubanks MD. 2002. Inbreeding alters resistance to insect herbivory and host plant quality in Mimulus guttatus (Scrophulariaceae). Evolution 56, 22–30 [DOI] [PubMed] [Google Scholar]
- 27.Delphia CM, Stephenson AG, De Moraes CM, Mescher MC. 2009. Inbreeding in horsenettle influences host–plant quality and resistance to herbivory. Ecol. Entomol. 34, 513–519 10.1111/j.1365-2311.2009.01097.x (doi:10.1111/j.1365-2311.2009.01097.x) [DOI] [Google Scholar]
- 28.Husband BC, Schemske DW. 1996. Evolution of the magnitude and timing of inbreeding depression in plants. Evolution 50, 54–70 10.2307/2410780 (doi:10.2307/2410780) [DOI] [PubMed] [Google Scholar]
- 29.Arimura G, Ozawa R, Shimoda T, Nishioka T, Boland W, Takabayashi J. 2000. Herbivoryinduced volatiles elicit defense genes in lima bean leaves. Nature 406, 512–515 10.1038/35020072 (doi:10.1038/35020072) [DOI] [PubMed] [Google Scholar]
- 30.Schnee C, Kollner TG, Held M, Turlings TCJ, Gershenzon J, Degenhardt J. 2006. The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proc. Natl Acad. Sci. USA 103, 1129–1134 10.1073/pnas.0508027103 (doi:10.1073/pnas.0508027103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kariyat RR, Scanlon SR, Mescher MC, De Moraes CM, Stephenson AG. 2011. Inbreeding depression in Solanum carolinense (Solanaceae) under field conditions and implications for mating system evolution. PLoS ONE 6, e28459. 10.1371/journal.pone.0028459 (doi:10.1371/journal.pone.0028459) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chamberlain K, Khan ZR, Pickett JA, Toshova T, Wadhams LJ. 2006. Diel periodicity in the production of green leaf volatiles by wild and cultivated host plants of stemborer moths, Chilo partellus and Busseola fusca. J. Chem. Ecol. 32, 565–77 10.1007/s10886-005-9016-5 (doi:10.1007/s10886-005-9016-5) [DOI] [PubMed] [Google Scholar]
- 33.Shiojiri K, Ozawa R, Takabayashi J. 2006. Plant volatiles, rather than light, determine the nocturnal behavior of a caterpillar. PLoS Biol. 4, e164. 10.1371/journal.pbio.0040164 (doi:10.1371/journal.pbio.0040164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reisenman CE, Riffell JA, Hildebrand JG. 2009. Neuroethology of oviposition behavior in the moth Manduca sexta. Ann. NY Acad. Sci. 1170, 462–467 10.1111/j.1749-6632.2009.03875.x (doi:10.1111/j.1749-6632.2009.03875.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Britton NL, Brown A. 1970. An illustrated flora of the northeastern United States and Canada, vol. 1 New York, NY: Dover Publications Inc [Google Scholar]
- 36.Ilnicki RD, Tisdell TF, Fertig SN, Furrer AH. 1962. Life history studies as related to weed control in the Northeast. 3. Horsenettle. Bulletin 368. Kingston, RI: University of Rhode Island Agricultural Experiment Station [Google Scholar]
- 37.Baker HG. 1955. Self-compatibility and establishment after ‘long distance’ dispersal. Evolution 9, 347–349 10.2307/2405656 (doi:10.2307/2405656) [DOI] [Google Scholar]
- 38.Byers DL, Meagher TR. 1992. Mate availability in small populations of plant species with homomorphic sporophytic self-incompatibility. Heredity 68, 353–359 10.1038/hdy.1992.50 (doi:10.1038/hdy.1992.50) [DOI] [Google Scholar]
- 39.Harden JW, Doerksen G, Herndon D, Hobson M, Thomas F. 1972. Pollination ecology and floral biology of four weedy genera in southern Oklahoma. Southwest Nat. 16, 403–412 10.2307/3670071 (doi:10.2307/3670071) [DOI] [Google Scholar]
- 40.Richman AD, Kao TH, Schaeffer SW, Uyenoyama MK. 1995. S-allele sequence diversity in natural populations of Solanum carolinense (Horsenettle). Heredity 75, 405–415 10.1038/hdy.1995.153 (doi:10.1038/hdy.1995.153) [DOI] [PubMed] [Google Scholar]
- 41.Travers SE, Mena-Alí JI, Stephenson AG. 2004. Plasticity in the self-incompatibility system of Solanum carolinense. Plant Spec. Biol. 19, 127–135 10.1111/j.1442-1984.2004.00109.x (doi:10.1111/j.1442-1984.2004.00109.x) [DOI] [Google Scholar]
- 42.Mena-Alí JI, Stephenson AG. 2007. Segregation analyses of partial self-incompatibility in self and cross progeny of Solanum carolinense reveal a leaky S-allele. Genetics 177, 501–510 10.1534/genetics.107.073775 (doi:10.1534/genetics.107.073775) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cipollini ML, Paulk E, Cipollini DF. 2002. Effect of nitrogen and water treatment on leaf chemistry in horsenettle (Solanum carolinense), and relationship to herbivory by flea beetles (Epitrix spp.) and tobacco hornworm (Manduca sexta). J. Chem. Ecol. 28, 2377–2398 10.1023/A:1021494315786 (doi:10.1023/A:1021494315786) [DOI] [PubMed] [Google Scholar]
- 44.Bassett IJ, Munro DB. 1986. The biology of Canadian weeds 78. Solanum carolinense L. and Solanum rostratum Dunal. Can. J. Plant. Sci. 66, 977–991 10.4141/cjps86-122 (doi:10.4141/cjps86-122) [DOI] [Google Scholar]
- 45.Cipollini ML, Levey DJ. 1997. Why are some fruits toxic? Glycoalkaloids in Solanum and fruit choice by vertebrates. Ecology 78, 782–798 [Google Scholar]
- 46.Delphia CM, Rohr JR, Stephenson AG, De Moraes CM, Mescher MC. 2009. Effects of genetic variation and inbreeding on volatile production in a field population of horsenettle. Int. J. Plant. Sci. 170, 12–20 10.1086/593039 (doi:10.1086/593039) [DOI] [Google Scholar]
- 47.Imura O. 2003. Herbivorous arthropod community of an alien weed Solanum carolinense L. Appl. Entomol. Zool. 38, 293–300 10.1303/aez.2003.293 (doi:10.1303/aez.2003.293) [DOI] [Google Scholar]
- 48.Wise MJ. 2007. The herbivores of Solanum carolinense (Horsenettle) in northern Virginia: natural history and damage assessment. Southeastern Nat. 6, 505–522 10.1656/1528-7092(2007)6[505:THOSCH]2.0.CO;2 (doi:10.1656/1528-7092(2007)6[505:THOSCH]2.0.CO;2) [DOI] [Google Scholar]
- 49.Kariyat RR, Mena-Alí J, Forry B, Mescher MC, De Moraes CM, Stephenson AG. 2012. Inbreeding, herbivory and the transcriptome of Solanum carolinense. Entomol. Exp. Appl. 144, 134–144 10.1111/j.1570-7458.2012.01269.x (doi:10.1111/j.1570-7458.2012.01269.x) [DOI] [Google Scholar]
- 50.Mihaliak CA. 1987. The influence of nitrate availability in production of plant carbon-based chemical defenses. PhD thesis, University of South Carolina, SC, USA [Google Scholar]
- 51.Effmert U, Dinse C, Piechulla B. 2008. Influence of green leaf herbivory by Manduca sexta on floral volatile emission by Nicotiana suaveolens. Plant Physiol. 146, 1996–2007 10.1104/pp.107.112326 (doi:10.1104/pp.107.112326) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reisenman CE, Christensen TA, Francke W, Hildebrand JG. 2004. Enantioselectivity of projection neurons innervating identified olfactory glomeruli. J. Neurosci. 24, 2602–2611 10.1523/JNEUROSCI.5192-03.2004 (doi:10.1523/JNEUROSCI.5192-03.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eller FJ, Tumlinson JH, Lewis WJ. 1988. Beneficial arthropod behavior mediated by airborne semiochemicals: source of volatiles mediating the host-location flight behavior of Microplitis croceipes (Cresson) (Hymenoptera: Braconidae), a parasitoid of Heliothis zea (Boddie) (Lepidoptera: Noctuidae). Environ. Entomol. 17, 745–753 [Google Scholar]
- 54.Turlings TCJ, Loughrin JH, McCall PJ, Röse USR, Lewis WJ, Tumlinson JH. 1995. How caterpillar-damaged plants protect themselves by attracting parasitic wasps. Proc. Natl Acad. Sci. USA 9, 4169–4174 10.1073/pnas.92.10.4169 (doi:10.1073/pnas.92.10.4169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Röse USR, Lewis WJ, Tumlinson JH. 1998. Specificity of systemically released cotton volatiles as attractants for specialist and generalist parasitic wasps. J. Chem. Ecol. 24, 303–319 10.1023/A:1022584409323 (doi:10.1023/A:1022584409323) [DOI] [Google Scholar]
- 56.Kang SH, Kim MK, Seo DK, Noh DJ, Yang JO, Yoon C, Kim GH. 2009. Comparative repellency of essential oils against Culex pipiens pallens (Diptera: Culicidae). J. Korean Soc. Appl. Biol. Chem. 52, 353–359 10.3839/jksabc.2009.063 (doi:10.3839/jksabc.2009.063) [DOI] [Google Scholar]
- 57.Khan ZR, et al. 1997. Intercropping increases parasitism of pests. Nature 388, 631–632 10.1038/41681 (doi:10.1038/41681) [DOI] [Google Scholar]
- 58.Khan ZR, Chiliswa P, Ampong-Nyarko K, Smart LE, Polaszek A, Wandera J, Mulaa MA. 1997. Utilisation of wild gramineous plants for the management of cereal stemborers in Africa. Insect Sci. Appl. 17, 143–150 10.1017/S1742758400022268 (doi:10.1017/S1742758400022268) [DOI] [Google Scholar]
- 59.Halitschke R, Stenberg JA, Kessler D, Kessler A, Baldwin IT. 2008. Shared signals -'alarm calls’ from plants increase apparency to herbivores and their enemies in nature. Ecol. Lett. 11, 24–34 [DOI] [PubMed] [Google Scholar]
- 60.Szendrei Z, Rodriguez-Saona C. 2010. A meta-analysis of insect pest behavioral manipulation with plant volatiles. Entomol. Exp. Appl. 134, 201–210 10.1111/j.1570-7458.2009.00954.x (doi:10.1111/j.1570-7458.2009.00954.x) [DOI] [Google Scholar]
- 61.Binder BF, Robbins JC. 1997. Effect of terpenoids and related compounds on the oviposition behavior of the European corn borer, Ostrinia nubilalis (Lepidoptera: Pyralidae). J. Agric. Food Chem. 45, 980–984 10.1021/jf960400z (doi:10.1021/jf960400z) [DOI] [Google Scholar]
- 62.Xiao Y, et al. 2012. Specific herbivore-induced volatiles defend plants and determine insect community composition in the field. Ecol. Lett. 15, 1130–1139 10.1111/j.1461-0248.2012.01835.x (doi:10.1111/j.1461-0248.2012.01835.x) [DOI] [PubMed] [Google Scholar]
- 63.Niinemets U, Reichstein M. 2002. A model analysis of the effects of nonspecific monoterpenoid storage in leaf tissues on emission kinetics and composition in Mediterranean sclerophyllous Quercus species. Glob. Biogeochem. Cycle 16, 1110. 10.1029/2002GB001927 (doi:10.1029/2002GB001927) [DOI] [Google Scholar]
- 64.Niinemets U, Loreto F, Reichstein M. 2004. Physiological and physicochemical controls on foliar volatile organic compound emissions. Trends Plant Sci. 9, 180–186 10.1016/j.tplants.2004.02.006 (doi:10.1016/j.tplants.2004.02.006) [DOI] [PubMed] [Google Scholar]
- 65.Hull-Sanders HM, Eubanks MD. 2005. Plant defense theory provides insight into interactions involving inbred plants and insect herbivores. Ecology 86, 897–904 10.1890/04-0935 (doi:10.1890/04-0935) [DOI] [Google Scholar]


