Abstract
Many short-lived desert organisms remain in diapause during drought. Theoretically, the cues desert species use to continue diapause through drought should differ depending on the availability of critical resources, but the unpredictability and infrequent occurrence of climate extremes and reduced insect activity during such events make empirical tests of this prediction difficult. An intensive study of a diverse bee–plant community through a drought event found that bee specialists of a drought-sensitive host plant were absent in the drought year in contrast to generalist bees and to specialist bees of a drought-insensitive host plant. Different responses of bee species to drought indicate that the diapause cues used by bee species allow them to reliably predict host availability. Species composition of the bee community in drought shifted towards mostly generalist species. However, we predict that more frequent and extended drought, predicted by climate change models for southwest North America, will result in bee communities that are species-poor and dominated by specialist species, as found today in the most arid desert region of North America.
Keywords: pollination, diapause, host specialization, climate change, Apiformes
1. Introduction
Despite how infrequently and unpredictably droughts occur, long-term studies have shown they present strong selective challenges to desert organisms and can be powerful agents of natural selection [1,2]. Examples are taxonomically widespread and include desert insects and annual plants that remain in prolonged metabolic quiescence in years when conditions are less than optimal [3] and Galapagos Islands finches whose beak morphology has evolved rapidly in response to drought-mediated selection associated with food resources [1]. Droughts have changed the species composition of vertebrate and plant communities rapidly, and the changes from these severe, episodic events have persisted for decades ([4] references in [5]). However, how communities of short-lived insects respond to drought has been examined only anecdotally. This omission is notable given the diversity of insect species, their role as consumers (herbivores and pollinators) and as keystone organisms involved in the maintenance and composition of plant communities. Furthermore, the range of ecological and life-history tactics represented among insects provide an opportunity to identify key biological features which can then be used to understand how community composition will respond to the increased drought intensity and frequency predicted by climate change models in southwestern North America [6].
A severe drought throughout western North America occurred during a multi-year study of bee and flowering plant communities in the northeastern Chihuahuan Desert. In response to below normal precipitation, bee occurrence was sporadic and most of the plants that did flower were patchily distributed with few flowers. Despite unusually low species richness and abundance, we were able to quantify the effects of drought on flowering plants and bees because: (i) collections had been made in years before and after the drought when precipitation was closer to the long-term average, and (ii) bees were sampled using pan traps in all 3 years in addition to collections taken at flowers. Pan traps provide unusual insight into bee activity because they attract bees when flowers are unavailable and can potentially capture both local individuals and transient bees in search of floral resources. These data allowed us to quantify how an extremely diverse [7] bee community responded to severely dry conditions and how this response corresponded to that of the flowering plant community.
Most desert bees worldwide nest in the ground and are solitary, and many can remain in extended diapause. Record durations are an Australian species that emerged after 10 years [8] and a Pakistani species that emerged after 7 years [9]. Some bee species from all continents with deserts are known to be able to delay emergence for at least 1 year (reviewed in [10]), although the cues bees use to delay emergence are poorly understood. In the best-documented case of bees and diapause, more individuals continued development to the adult stage when they were experimentally exposed to high moisture levels than nest-mates that were kept dry, suggesting precipitation cued environmental suitability [10]. All other reports of delayed emergence patterns have been based on single observations of individuals emerging in the laboratory, from observations made at host flowers in drought and non-drought years, or after unusual precipitation events ([11] references in [10,12]).
In the northwestern Chihuahuan desert, plant species vary in the amount of rainfall that triggers flowering and bee species vary in their dependence on floral host species from specialists that collect pollen from one or a few plant species (hereafter, specialist bee species) to generalists that collect pollen from many plant species (hereafter, generalist bee species). Of the few plants that did flower during the drought year, two plant species (creosote bush (Larrea tridentata) and mesquite tree (Prosopis velutina)) were of particular interest because they are highly bee-attractive and are the sole pollen hosts for a species-rich group of specialist bees. These two plant species also differ in how much precipitation they require to initiate flower development. The creosote bush requires one rainfall event of at least 13 mm to prompt bloom in the spring [13]. Mesquite trees flower independently of rain because they have roots that extend to permanently saturated soils. We intensively sampled bees at these two plant species to test the hypothesis that the specialist bee species of the creosote bush would not be active in the drought year and that the specialist bee species of mesquite would be active. If specialist bees of the creosote bush were not active, and those of mesquite were active, this would be evidence that specialist species use specific cues or environmental thresholds that predict the availability of their host, not more general cues that predict flowering across the whole plant community.
Bee data over 3 years from standardized net sampling of floral communities, from pan traps and from flowers of the creosote bush and mesquite, allowed us to test the hypothesis that in the drought year: (i) the number of flowering plant species and flowers per plant, and the composition and abundance of bee species would be reduced compared to years with rain that was closer to normal; (ii) generalist, and those specialist bees of plants that bloom regardless of rainfall amount, would be active in drought years; and (iii) specialist bees of plants that received insufficient rainfall for bloom would remain in diapause. Tests of these hypotheses extend findings based on biogeographic patterns that had suggested that one ecological advantage of host plant specialization among desert bees is the ability to reliably predict host bloom [14], and provide a basis to predict that bee communities will shift towards a greater proportion of specialist species with the greater drought intensification and frequency expected for the southwestern North America by climate change models.
2. Material and methods
The study was carried out in the San Bernardino Valley Sonora, Mexico and Arizona, USA, a valley in the Chihuahuan Desert that runs north-south across the Mexico–United States border. Elevation in the study area is approximately 1070 m, and climate is xeric temperate with an average precipitation of 360 mm yr–1. The dominant vegetation association in the study area is Chihuahuan desert scrub [15], with some intact grassland remaining and bands of riparian vegetation along streambeds [7]. Monthly precipitation totals from September 2003 to March 2007 were obtained from the Glenn weather station 3–4 km north of the study area (www.rmrs.nau.edu/weather/stations/glenn/).
The bee fauna of the San Bernardino Valley is more species-rich than reported from any other comparably sized area in the world, with few social (3%), and many cleptoparasitic (17%) and solitary (79%) species [7]. Of the solitary pollen-collecting species, 30–35% are floral host specialists that collect pollen from only one or several closely related plant species.
(a). Plant sampling of communities and focal species
In the drought year, plants in bloom were patchily distributed and uncommon. Two approaches were used to characterize the year-to-year magnitude of change in the flowering plant community. First, flowering plant richness from hectare plots (100 × 100 m) was recorded. The number of hectares sampled was 10 in 2005, 27 in 2006 and 16 in 2007. Mean numbers of plant species on plots each year were compared by one-way ANOVA after data were square root transformed. All plant species in flower were identified, and flowers per plant species were counted in each hectare. Second, we compared numbers of species from lists of all plants at which bees were collected between 1 April and 14 May in the year before (2005), during (2006) and after (2007) the drought.
The creosote bush and mesquite tree were two of the most bee-attractive plant species in our study area [16] with unusually diverse pollen-specialist bee faunas (n = 6 and 7 specialist bee species for the creosote bush and mesquite tree, respectively, in our study area) and were the two most common plant species in bloom during the drought. We estimated the proportion of individuals of these two species that had flowered along transects we established near the end of the bloom period in 2006 (8–12 May). Nine transects were established in total, three each in desert scrub, grassland and riparian habitat. Transects were straight lines that continued until the bloom status of 200 creosote bushes had been scored. Plants were scored as having bloom if they had flowers, buds or fruits, and the number was counted of each phenological reproductive stage. Mesquite trees were scored for bloom status on the same transects used for the creosote bush, but fewer were encountered because these are larger and less densely distributed than the creosote bush. No mesquite trees occurred on three of the nine transects. Overall, 1800 creosote bushes and 323 mesquite trees were surveyed.
(b). Bee community sampling
Sites sampled for bees by netting were the same 100 × 100 m plots described above in the section on flowering plant communities. Bees were sampled the day following flowering plant measurements. In 2005 and 2007, net sampling was done by up to six people. In 2006, reduced bloom allowed all sites to be sampled by one or two collectors. Each site was net surveyed one to three times per season. Net samples were taken at different sites each year because of high local spatial and temporal variation in flowering and were at different locations than where pan trap samples were taken (see below).
Bee sampling by net was standardized as follows: every plant species at each site was sampled for 2 h per day broken up into six 20 min sessions, three in the first half and three in the latter half of the bee activity period. Early and late sampling periods varied between 09.00–11.30 h and 11.30–14.00 h depending on temperature. Two to five researchers (depending on the number of co-flowering plant species in each plot) netted in the plot simultaneously, each focused on a different plant species for 20 min. After each 20 min period, a researcher shifted to a different plant species to avoid bias owing to collector skill on the estimates of bee species richness and abundance. Researchers moved through the site during the 20 min sampling period and collected any floral visitors on the current target plant species. We made more 2-hour samples of bees at creosote bush and mesquite flowers in 2006 than in the other 2 years, because during the drought these were often the only plants in bloom.
Bees were sampled with pan traps at 27 permanent plots (1 ha) that had been established in 2000 and sampled for bees each year since. Pan traps sample a somewhat different bee fauna [17,18] than net sampling but have the advantage of capturing bees even when flowers are absent and at a rate that is independent of collector ability. Plots were established in desert scrub, grassland and riparian habitats and were all within a 10 km2 area [19]. At each site, three yellow, three white and three fluorescent blue pan traps that we had filled with soapy water were placed ca 3 m apart along a linear transect. Pan traps stayed out for 3–5 h beginning between 08.30 and 10.00 h. All 27 sites were sampled in 2005, 2006 and 2007. Sites were sampled three times in 2006 (8–11 April, 25–27 April and 11–13 May) and sampled once in 2005 (19–21 April) and in 2007 (18–22 April). For abundance and composition of bees in pan traps, we compared 2005 and 2007 samples with the most similar date a pan trap collection was made in 2006 (25–27 April).
(c). Functional groups
Plants were sorted into two groups, annuals/biannuals and perennials to examine whether propensity to flower in response to drought differed among these life-history groups. Both annuals and biannuals are shallow-rooted so were expected to respond similarly to precipitation. All plant species were identified to species except for Sphaeralcea spp. (Malvaceae), a species complex (Sphaeralcea laxa, Sphaeralcea angustifolia and potential hybrids) that could not confidently be assigned to species during field surveys. All have very similar flower colour and floral morphology and attract similar floral visitors so were combined for analyses. Plant identifications were confirmed by Dr Van Devender and vouchers deposited at the University of Arizona herbarium (Tucson, AZ, USA).
Bees were sexed and identified to species by the first author and categorized as social or solitary, specialist or generalist, and pollen-collecting or cleptoparasitic. Information on host breadth for bees came primarily from Krombein et al. [20] and nesting preference from Krombein et al. [20] and Krombein [21]. Species for which relevant life-history information was unknown were included if all other species in the taxonomic group shared the same biological characteristic. For example, we categorized all species of Perdita and Calliopsis as oligolectic because most species in these genera are specialists. Species were not used in the analyses if relevant life-history information were not known, or if they were in groups with, for example, both oligolectic and polylectic species. The small proportion of social species in the bee fauna prevented us from including this category in the analyses. Specimens will be deposited in museums at the University of Kansas (Lawrence) and National Autonomous University of Mexico (Mexico City).
We tested whether generalist and specialist bee species differed in their response to drought by including only abundant species, which we defined as those represented by 20 individuals or more from pan traps and by net sampling from 1 ha plots between 1 April and 18 May 2006, 2007 and 2008. Most abundant species were collected in the 2 years when rain was closer to the long-term average. By this criterion, there were 18 specialist and 29 generalist species. Species of Lasioglossum (Dialictus) could not be identified to the species level and were excluded from the analysis. The honeybee (Apis mellifera) is the only introduced bee species in this fauna and was excluded. We compared by a χ2-test if the proportions of abundant species not active in drought differed among specialist and generalist bees. Changes in the abundance and species richness of generalists and specialist bee species visiting creosote bushes and mesquite were tested with a nested ANOVA that had as the measurement variable, abundance or species richness, and effects as year, host breadth and host with host breadth and host nested within a year. All ANOVA analyses were carried out after data were square root transformed.
3. Results
Monthly precipitation amounts in the six months (October–March) prior to spring 2006 averaged 2.8 mm (±2.3 s.d.) rain per month, much below the same months in 2005 (39.6 ± 25.6 s.d.) and 2007 (18.5 ± 17.6 s.d.) and the long-term regional average (see the electronic supplementary material, figure S1).
(a). Flowering plant community response to drought
In spring 2006, most perennial and biannual plant species, including cacti, had no flowers and few rainfall-dependent annual species germinated. Mean numbers of blooming plant species in 1 ha plots was less than 1.6 in spring, 2006 in contrast to 11 and 5.8 in the same period of the year before and after, respectively (F2,22 = 13.42, p = 0.00016; figure 1). The list of flowering plant species from which bees were collected in the drought year was reduced fourfold or more from other years, and conspicuously absent during the drought were flowers on annual species (see figure 2 and electronic supplementary material, table S1). Only 5 per cent of 1800 creosote bushes we surveyed along nine transects had one or more flowers in 2006 in contrast with 93 per cent of 323 mesquite trees.
Figure 1.
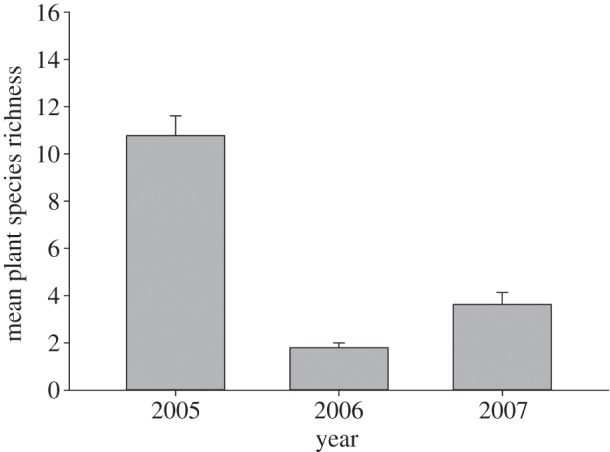
Mean number of plant species in flower in 1 ha plots. Samples were taken between 1 April and 18 May in all 3 years; 2006 was the drought year and 2005 and 2007 were years when rains were closer to the long-term average. Plots were established where plants were in flower so overestimate numbers that would be found if plots were randomly placed throughout the study area. Numbers of hectares sampled are 10 in 2005, 15 in 2006 and 19 in 2007.
Figure 2.
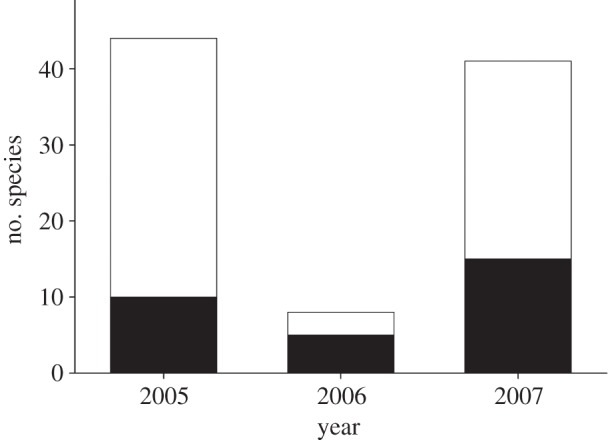
Number of flowering plant species on which bees were captured between 1 April and 10 June in 3 years. The number of annual plant species (white bars) that flowered changed much more between years than the number of perennial plant species (filled bars).
(b). Bee community response to drought
Between late March and mid-May, the number of bee collections taken by net (collection = plant species/site/day) and pan traps (collection = site/day) totalled 393 in 2005, 232 in 2006 and 272 in 2007; however, collection intensity per plant in flower was greater in 2006 given that overall flowering was greatly diminished and only 10 plant species that attracted bees were in flower, while in 2005 and 2007 we sampled bees at 46 and 40 plant species, respectively (see figure 2 and electronic supplementary material, tables S1 and S2). The greater number of collections per plant species and the decreased number of flowers per plant on most of these species in the drought year than in other years suggests that the bee catch in the drought is a better estimate of richness and relative abundance than in other years, and that these data represent a conservative estimate of the response by the bee community to drought.
A total of 8704 bees and 238 species (see the electronic supplementary material, table S2) were collected by pan trap and net sampling over this study. One-way ANOVA tests of year-to-year changes in mean bee species richness and abundance were significantly different in samples made by net and pan trap (d.f. = 2, p < 0.0001 for all four comparisons), and in all four of these comparisons (species richness and abundance in net and pan trap samples) there were significantly fewer bee species and individuals in the drought year (2006) than in the years before and after (all comparisons tested for significance at p < 0.05 using Tukey post-hoc comparison). For brevity, only the abundance data from pan trap samples are shown (figure 3). Many collections by net in the drought year resulted in none or very few specimens all day. Pan trap collections yielded more species and individuals than net samples, which allowed us to test whether pollen-specialist bee species responded to drought differently than generalist bee species. Pan traps sampled fewer specialist bee species (F2,83 = 46.12, p < 0.0001) and individuals (F2,83 = 20.87, p < 0.0001) during the drought. A post-hoc Tukey test for all comparisons showed significant differences at p < 0.05 among the drought year and the years before and after. Numbers of generalist bee species did not change among years (F2,83 = 1.12, p < 0.331), but numbers of individuals was greater the year after the drought (F2,83 = 5, p = 0.0089; post-hoc Tukey's test p < 0.05). For the most abundant specialist (n = 18) and generalist bee species (n = 28), the proportion of specialist bee species that were entirely absent from drought year samples was significantly more than the proportion of generalist bee species absent (seven of 18 specialists but only four of 28 generalists were absent; χ21 = 31.72, p = 0.0001; electronic supplementary material, table S1). All six of the specialist bee species of mesquite, the plant that bloomed despite minimal precipitation, were collected in the drought year and two of these specialist bee species were among the most common bees captured (see the electronic supplementary material, table S3). The five most abundant bee species in 2006 from our combined standardized net and pan trap samples were comprised of two specialist and three pollen generalist bee species (see the electronic supplementary material, table S3).
Figure 3.
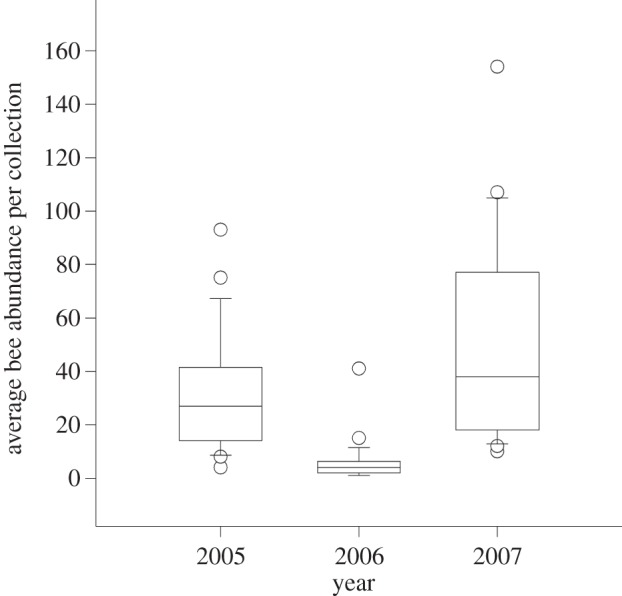
Average bee abundance from pan traps in the springs of 2005, 2006 and 2007 shown as a box plot. Box plots display data divided into quartiles. The box encompasses 25–75% of the data and lines above and below the box extend to the upper and lower limits. The line through the middle of each box is the median value and dots represent outlier data points.
Standardized net samples at mesquite and creosote bush flowers found approximately similar numbers of bee species at both plant species in most years (figure 4), although more individuals were generally collected during samples at mesquite than at creosote bushes (figure 5). When generalist and specialist bee species were compared, generalist bee species richness was high at creosote bushes and mesquite in all 3 years as was mesquite specialist bee species richness (figure 4). By contrast, species richness and abundance of specialist bee species on the creosote bush was significantly lower in the drought year than in the non-drought years (figures 4 and 5). A nested least-squares model showed the differences for species richness were significant among years (F2 = 13.402, p < 0.0001), host within years (F3 = 8.015, p < 0.0001) and host breadth (specialist and generalist) within years and host (F6 = 8.12, p < 0.0001). A nested least-square model testing abundance also showed the differences were significant among years (F2 = 11.952, p < 0.0001), host within years (F3 = 12.475, p < 0.0001) and host breadth (specialist and generalist) (F6 = 36.945, p = 0.0034).
Figure 4.
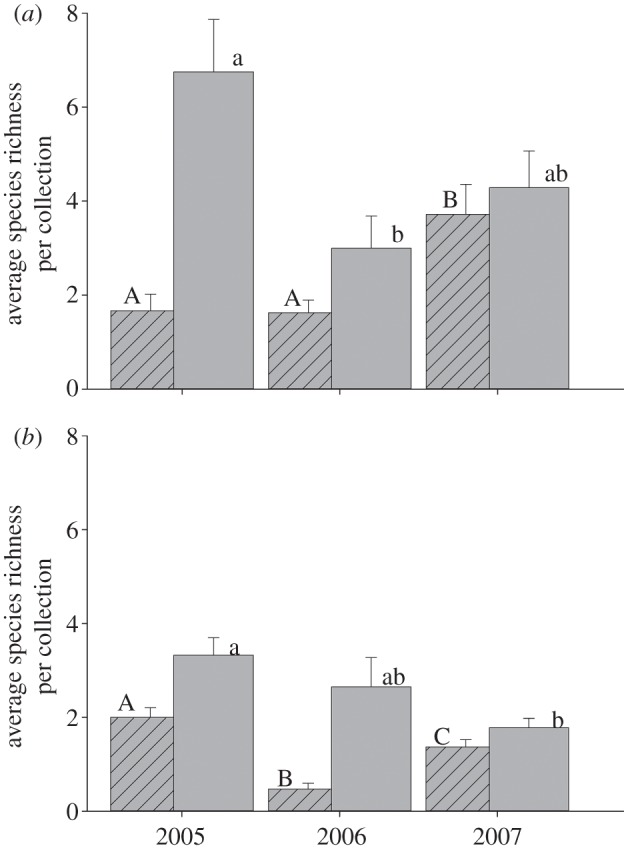
Mean bee species richness from 2 h standardized collections of bees from flowers of (a) mesquite (Prosopis velutina) and (b) the creosote bush (Larrea tridentata). Richness of generalist bee species (bars without stripes) at flowers of mesquite and creosote bushes was high in all 3 years. Richness of mesquite specialist bee species (bars with stripes) was similar in the drought year (2006) to the years before and after, in contrast to specialist bee species on the creosote bush, which decreased dramatically in the drought year compared to the non-drought years. Letters above columns indicate if differences were significant by a Tukey post-hoc comparison.
Figure 5.
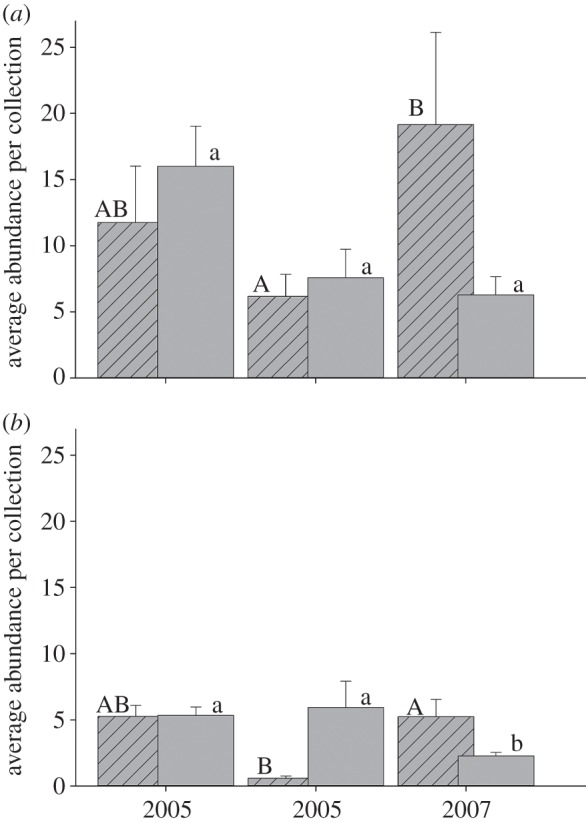
Mean bee abundance from 2 h standardized collections at flowers of (a) mesquite (P. velutina) and (b) the creosote bush (L. tridentata). Numbers of generalist bees (bars without stripes) at mesquite and creosote bushes was high in all 3 years. Abundance of mesquite specialist bee species (bars with stripes) was similar in the drought year (2006) to the years before and after, in contrast to creosote bush specialist bees which were much less abundant in the drought year than in the non-drought years. Letters above columns indicate if differences were significant by a Tukey post-hoc comparison.
4. Discussion
Flowering of most plants and activity of most bees dropped substantially or completely in response to drought. The average number of flowering plant species in 1 ha net plots was much less in the drought year (figure 1) than in the years before or after when precipitation had been closer to the long-term average (see the electronic supplementary material, figure S1). The plants on which we collected bees during the drought were mostly perennial species, in contrast with non-drought years when many bees were collected on annuals (figure 2). The absence of flowering annual species further indicated that rain over the previous winter had been insufficient for many species to initiate germination and flower.
Many bees remained in diapause through the drought. Mean number of individuals in 1 ha plots was less than 2 in 2006, a decline of more than 10-fold from non-drought years (figure 3). Some generalist bee species remained in diapause during the drought, but a much greater proportion of specialists did so than generalists (see figure 4 and electronic supplementary material, table S3). Among specialist bee species, those with host plants that bloom with minimal or no rainfall were more likely to emerge in dry conditions than were specialist bee species that visit host plants which require adequate rain (figures 4 and 5). Differences among those specialist bee species that emerged and those that did not during the drought are further evidence of the importance of host availability when environmental conditions are suboptimal. At creosote bush flowers, a plant species that requires rainfall to bloom, specialist bee species were largely absent in the drought year, whereas generalist bees on flowers of the same plant species were as common in the drought year as they were in one or both years with rainfall amounts close to the long-term average (figure 5). By contrast, at mesquite flowers, a plant that blooms independently of rainfall, abundance and species richness of specialist and generalist bee species in the drought year were comparable to non-drought years (figures 4 and 5). Differences in the activity pattern of generalist and specialist bee species in drought and non-drought years indicate that the propensity to remain in diapause during drought was related more closely to the probability that their host bloomed than if the host was an annual or perennial plant species. Bee species that emerged from diapause despite a drought were those that were generalists and specialists of plant species that bloom regardless of precipitation. Bee species that continued diapause for another year were those that were specialists of plant species that require precipitation for bloom. Differences in the proportions of generalist and specialist bee species that were absent or rare in drought indicate that, as bee species become more specialized to a limited suite of floral resources, there is selection to reliably predict when their specific host blooms. This finding is probably a general one that applies to many desert organisms, especially those that are short-lived such as most insects.
The increased patchiness of flowering during drought resulted in more comprehensive sampling of both plant and bees during drought years than during the non-drought years, however, for each of our sampling protocols this bias was skewed against our hypotheses. For example, the 1 ha plots used to measure flowering in the San Bernardino Valley were chosen each year for high flowering plant diversity. These sites therefore overestimate mean flowering plant richness and density for the study area, but the overestimate is larger in the drought year when bloom was minimal and flowering plants were very patchily distributed. Similarly, the list of plant species from which bees were collected in drought were more complete than in non-drought years, because fewer flowering plants in drought allow for a greater proportion of species to be sampled. Our bee sampling protocols also generated data that conservatively test our hypotheses given that in the drought year, flowering plants were extremely clumped and had reduced flowering which allowed us to sample a greater proportion of the bee community, because our collections in the drought encompassed a greater proportion of the available floral resources than they did in years with greater precipitation. Finally, bee samples taken from pan traps vary from low attractiveness when flowers are locally abundant to high attractiveness when flowers are locally scarce or absent [12,22]. In this study, pan traps would have been more attractive during the drought year than the years before and after. Taken together, sampling biases for collection protocols of flowering plants and bees used in this study were probably large but favoured overestimates of plants and bee richness and abundance in the drought year. It is likely that the reduction in flowering and activity in the plant and bee communities in response to the drought over spring 2006, was much greater than our data indicate.
Models of climate change for western North America predict that both frequency and duration of droughts will increase [6,23,24] and long-term studies have documented cases where drought has fundamentally altered plant community composition [4,25]. How such events change the insect communities that depend on these plants is unknown. Given the caveat that our data were from 1 year of drought, the empirical findings from this study provide useful insight into how climate change may affect pollinator communities and, possibly, the plants that depend on them. We find that for a species rich, ecologically diverse, pollinator community there is considerable variation among species in their ability to undergo diapause under adverse conditions. In the short term, this variation shifts bee species composition during drought years towards pollen generalists that forage on the few plant species in flower and toward specialists whose hosts bloom independently of annual rainfall. In our study area, this latter group consisted of only specialist species of mesquite. However, if drought intensity increases with climate change, declining groundwater levels during drought years may not support flowering by any plants, including those with deep roots. This should lead to communities composed predominately, if not completely, of plant species that bloom in response to adequate rain and of those bee species that are specialists and have the ability to undergo long-term facultative diapause. Pollen generalist bee species that are unable to predict years when bloom is poor or absent presumably suffer diminished reproductive success during droughts and would be extirpated or rare in these communities if these conditions persist. Although needed is more study of diapause dynamics of desert bees, such a pattern would be consistent with the bee species composition in the hottest, driest North American deserts where winter rainfall is least predictable [26]. There the spring-active bee fauna is dominated by specialist bee species and generalist bee species are rare [13]. The ability to respond appropriately to fluctuating resource availability induced by drought may account for why the proportion of specialist bee species is higher in xeric areas of the Eastern and Western Hemisphere, and why some groups endemic to these xeric areas are largely or entirely composed of specialist bee species [27,28].
Acknowledgements
Long-term support from Josiah and Valer Austin (Cuenca de Los Ojos Foundation) and William Radke (US Fish and Wildlife Service), and excellent assistance from Jose Arturo Romero and Esther Julier made this study possible. National Science Foundation grant no. DEB 0418871 provided financial support. Bryan Danforth and John Jaenike made helpful comments on an earlier version of this manuscript.
References
- 1.Boag PT, Grant PR. 1981. Intense natural selection in a population of Darwin's finches (Geospizinae) in the Galapagos. Science 214, 82–85 10.1126/science.214.4516.82 (doi:10.1126/science.214.4516.82) [DOI] [PubMed] [Google Scholar]
- 2.Wilkelski M, Trillmich F. 1997. Body size and sexual dimorphism fluctuate as result of opposing natural and sexual selection: an island comparison. Evolution 51, 922–936 10.2307/2411166 (doi:10.2307/2411166) [DOI] [PubMed] [Google Scholar]
- 3.Venable DL. 2007. Bet hedging in a guild of desert annuals . Ecology 88, 1086–1090 10.1890/06-1495 (doi:10.1890/06-1495) [DOI] [PubMed] [Google Scholar]
- 4.Turner RM. 1990. Long-term vegetation change at a fully protected Sonoran Desert site. Ecology 71, 461–477 10.2307/1940301 (doi:10.2307/1940301) [DOI] [Google Scholar]
- 5.Smith MD. 2011. The ecological perspective on extreme climatic events: a synthetic definition and framework to guide future research. J. Ecology 99, 656–663 10.1111/j.1365-2745.2011.01798.x (doi:10.1111/j.1365-2745.2011.01798.x) [DOI] [Google Scholar]
- 6.Easterling DR, Meehl GA, Parmesan C, Changnon SA, Karl TR, Mearns LO. 2000. Climate extremes: observations, modeling and impacts. Science 289, 2068–2074 10.1126/science.289.5487.2068 (doi:10.1126/science.289.5487.2068) [DOI] [PubMed] [Google Scholar]
- 7.Minckley RL. 2008. Faunal composition and species richness differences of bees (Hymenoptera: Apiformes) from two North American regions. Apidologie 39, 176–188 10.1051/apido:2007062 (doi:10.1051/apido:2007062) [DOI] [Google Scholar]
- 8.Houston TF. 1991. Ecology and behaviour of the bee Amegilla dawsoni Rayment with notes on related species (Hymenoptera: Anthophoridae). Rec. West. Aust. Mus. 15, 535–554 [Google Scholar]
- 9.Rozen JG., Jr 1990. Pupa of the bee Pararhophites orobinus (Hymenoptera: Apoidea: Megachilidae). J. NY Entomol. Soc. 98, 379–382 [Google Scholar]
- 10.Danforth BN. 1999. Emergence dynamics and bet hedging in a desert bee, Perdita portalis. Proc. R. Soc. Lond. B 266, 1985–1994 10.1098/rspb.1999.0876 (doi:10.1098/rspb.1999.0876) [DOI] [Google Scholar]
- 11.Shinners LH. 1957. Scarcity of wild bees and wasps (Hymenoptera: Aculeata) in north central Texas in 1957. Southwestern Nat. 2, 129–131 10.2307/3669500 (doi:10.2307/3669500) [DOI] [Google Scholar]
- 12.Mayer C, Kuhlmann M. 2009. Synchrony of pollinators and plants in the winter rainfall area of South Africa-observations from a drought year. Trans. R. Soc. S. Afr. 59, 55–57 10.1080/00359190409519162 (doi:10.1080/00359190409519162) [DOI] [Google Scholar]
- 13.Bowers JE, Dimmitt MA. 1994. Flowering phenology of 6 woody plants in the northern Sonoran desert. Bull. Torrey Bot. Club. 121, 215–229 10.2307/2997177 (doi:10.2307/2997177) [DOI] [Google Scholar]
- 14.Minckley RL, Cane JH, Kervin L. 2000. Origins and ecological consequences of pollen specialization among desert bees. Proc. R. Soc. Lond. B 267, 265–271 10.1098/rspb.2000.0996 (doi:10.1098/rspb.2000.0996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown DE. 1994. Biotic communities: southwestern United States and northwestern Mexico. Salt Lake City, UT: University of Utah Press [Google Scholar]
- 16.Minckley RL, Roulston TH. 2006. Incidental mutualisms and pollen specialization among bees. In Plant–pollinator interactions: from specialization to generalization (eds Waser NM, Ollerton J.), pp. 69–98 Chicago, IL: University of Chicago Press [Google Scholar]
- 17.Roulston TH, Smith SA, Brewster AL. 2007. A comparison of pan trap and intensive net sampling techniques for documenting a bee fauna. J. Kans. Entomol. Soc. 80, 179–181 10.2317/0022-8567(2007)80[179:ACOPTA]2.0.CO;2 (doi:10.2317/0022-8567(2007)80[179:ACOPTA]2.0.CO;2) [DOI] [Google Scholar]
- 18.Grundel R, Frohnapple KJ, Jean RP, Pavlovic NB. 2011. Effectiveness of bowl trapping and netting for inventory of a bee community. Environ. Ent. 40, 374–380 10.1603/EN09278 (doi:10.1603/EN09278) [DOI] [Google Scholar]
- 19.Minckley RL. In press Trajectory and rate of desert vegetation response following cattle removal. In Biodiversity and management of the Madrean Archipelago: merging science and management in a rapidly changing world, (eds Gottfried GJ, Ffolliot PF, Gebow BS, Eskew LG.). Fort Collins, CO: USDA Forest Service. [Google Scholar]
- 20.Krombein KV, Hurd PD, Jr, Smith DR. 1979. Hymenoptera of America North of Mexico, vol. 3 Washington, DC: Smithsonian Institution Press [Google Scholar]
- 21.Krombein KV. 1967. Trap-nesting wasps and bees: life histories, nests and associates. Washington, DC: Smithsonian Press [Google Scholar]
- 22.Baum KA, Wallen KE. 2011. Potential bias in pan trapping as a function of floral abundance. J. Kans. Entomol. Soc. 84, 155–159 10.2317/JKES100629.1 (doi:10.2317/JKES100629.1) [DOI] [Google Scholar]
- 23.IPCC 2007. Climate change 2007: working group 1: the physical sciences basis. In Contribution of the working group 1 to the fourth assessment report of the intergovernmental panel on climate change (eds Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL.), pp. 996 Cambridge, UK: Cambridge University Press [Google Scholar]
- 24.Westerling AL, Hidalgo HG, Cayan DR, Swetnam TR. 2006. Warming and earlier spring increase Western U.S. forest wildfire activity. Science 313, 940–943 10.1126/science.1128834 (doi:10.1126/science.1128834) [DOI] [PubMed] [Google Scholar]
- 25.Miriti MN, Rodriguez-Buritica S, Wright SJ, Howe HF. 2007. Episodic death across species of desert shrubs. Ecology 88, 32–36 10.1890/0012-9658(2007)88[32:EDASOD]2.0.CO;2 (doi:10.1890/0012-9658(2007)88[32:EDASOD]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 26.Davidowitz G. 2002. Does precipitation variability increase from mesic to xeric biomes? Glob. Ecol. Biogeogr. 11, 143–154 10.1046/j.1466-822X.2002.00271.x (doi:10.1046/j.1466-822X.2002.00271.x) [DOI] [Google Scholar]
- 27.Michener CD. 1979. Biogeography of the bees. Ann. Miss. Bot. Gard. 66, 277–347 10.2307/2398833 (doi:10.2307/2398833) [DOI] [Google Scholar]
- 28.Michener CD. 2007. The bees of the world. Baltimore, MA: John Hopkins University Press [Google Scholar]


