Abstract
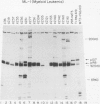
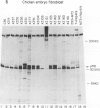
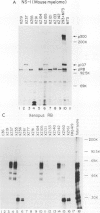
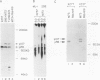
Even though the retinoblastoma gene is one of the best-studied tumor suppressor genes, little is known about its functional role. Like all tumor suppressor gene products, the retinoblastoma protein (pRB) is thought to inhibit some aspect of cell proliferation. It also appears to be a cellular target of several DNA tumor virus-transforming proteins, such as adenovirus E1A, human papillomavirus E7, or simian virus 40 large T antigen. To help in the analysis of pRB, we have prepared a new set of anti-human pRB monoclonal antibodies. In addition to being useful reagents for the study of human pRB, these antibodies display several unexpected properties. They can be used to distinguish different subsets of the pRBs on the basis of their phosphorylation states. Some are able to recognize pRB homologs in other species, including mice, chickens, and members of the genus Xenopus. In addition, some of these antibodies can bind directly to other cellular proteins that, like pRB, were originally identified through their association with adenovirus E1A. These immunologically cross-reactive proteins include the p107 and p300 proteins, and their recognition by antibodies raised against pRB suggests that several members of the E1A-targeted cellular proteins form a structurally and functionally related family.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. M. Molecular themes in oncogenesis. Cell. 1991 Jan 25;64(2):235–248. doi: 10.1016/0092-8674(91)90636-d. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bookstein R., Rio P., Madreperla S. A., Hong F., Allred C., Grizzle W. E., Lee W. H. Promoter deletion and loss of retinoblastoma gene expression in human prostate carcinoma. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7762–7766. doi: 10.1073/pnas.87.19.7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookstein R., Shew J. Y., Chen P. L., Scully P., Lee W. H. Suppression of tumorigenicity of human prostate carcinoma cells by replacing a mutated RB gene. Science. 1990 Feb 9;247(4943):712–715. doi: 10.1126/science.2300823. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Figge J., Shew J. Y., Huang C. M., Lee W. H., Marsilio E., Paucha E., Livingston D. M. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988 Jul 15;54(2):275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- Dunn J. M., Phillips R. A., Zhu X., Becker A., Gallie B. L. Mutations in the RB1 gene and their effects on transcription. Mol Cell Biol. 1989 Nov;9(11):4596–4604. doi: 10.1128/mcb.9.11.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson N., Buchkovich K., Whyte P., Harlow E. The cellular 107K protein that binds to adenovirus E1A also associates with the large T antigens of SV40 and JC virus. Cell. 1989 Jul 28;58(2):249–255. doi: 10.1016/0092-8674(89)90839-8. [DOI] [PubMed] [Google Scholar]
- Dyson N., Howley P. M., Münger K., Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989 Feb 17;243(4893):934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- Ewen M. E., Ludlow J. W., Marsilio E., DeCaprio J. A., Millikan R. C., Cheng S. H., Paucha E., Livingston D. M. An N-terminal transformation-governing sequence of SV40 large T antigen contributes to the binding of both p110Rb and a second cellular protein, p120. Cell. 1989 Jul 28;58(2):257–267. doi: 10.1016/0092-8674(89)90840-4. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Horowitz J. M., Gerber M. R., Wang X. F., Bogenmann E., Li F. P., Weinberg R. A. Deletions of a DNA sequence in retinoblastomas and mesenchymal tumors: organization of the sequence and its encoded protein. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9059–9063. doi: 10.1073/pnas.84.24.9059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano A., McCall C., Whyte P., Franza B. R., Jr Human cyclin A and the retinoblastoma protein interact with similar but distinguishable sequences in the adenovirus E1A gene product. Oncogene. 1991 Mar;6(3):481–485. [PubMed] [Google Scholar]
- Harbour J. W., Lai S. L., Whang-Peng J., Gazdar A. F., Minna J. D., Kaye F. J. Abnormalities in structure and expression of the human retinoblastoma gene in SCLC. Science. 1988 Jul 15;241(4863):353–357. doi: 10.1126/science.2838909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Franza B. R., Jr, Schley C. Monoclonal antibodies specific for adenovirus early region 1A proteins: extensive heterogeneity in early region 1A products. J Virol. 1985 Sep;55(3):533–546. doi: 10.1128/jvi.55.3.533-546.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Whyte P., Franza B. R., Jr, Schley C. Association of adenovirus early-region 1A proteins with cellular polypeptides. Mol Cell Biol. 1986 May;6(5):1579–1589. doi: 10.1128/mcb.6.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensel C. H., Hsieh C. L., Gazdar A. F., Johnson B. E., Sakaguchi A. Y., Naylor S. L., Lee W. H., Lee E. Y. Altered structure and expression of the human retinoblastoma susceptibility gene in small cell lung cancer. Cancer Res. 1990 May 15;50(10):3067–3072. [PubMed] [Google Scholar]
- Horowitz J. M., Park S. H., Bogenmann E., Cheng J. C., Yandell D. W., Kaye F. J., Minna J. D., Dryja T. P., Weinberg R. A. Frequent inactivation of the retinoblastoma anti-oncogene is restricted to a subset of human tumor cells. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2775–2779. doi: 10.1073/pnas.87.7.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz J. M., Yandell D. W., Park S. H., Canning S., Whyte P., Buchkovich K., Harlow E., Weinberg R. A., Dryja T. P. Point mutational inactivation of the retinoblastoma antioncogene. Science. 1989 Feb 17;243(4893):937–940. doi: 10.1126/science.2521957. [DOI] [PubMed] [Google Scholar]
- Hu Q. J., Dyson N., Harlow E. The regions of the retinoblastoma protein needed for binding to adenovirus E1A or SV40 large T antigen are common sites for mutations. EMBO J. 1990 Apr;9(4):1147–1155. doi: 10.1002/j.1460-2075.1990.tb08221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. J., Yee J. K., Shew J. Y., Chen P. L., Bookstein R., Friedmann T., Lee E. Y., Lee W. H. Suppression of the neoplastic phenotype by replacement of the RB gene in human cancer cells. Science. 1988 Dec 16;242(4885):1563–1566. doi: 10.1126/science.3201247. [DOI] [PubMed] [Google Scholar]
- Huang S., Wang N. P., Tseng B. Y., Lee W. H., Lee E. H. Two distinct and frequently mutated regions of retinoblastoma protein are required for binding to SV40 T antigen. EMBO J. 1990 Jun;9(6):1815–1822. doi: 10.1002/j.1460-2075.1990.tb08306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin W. G., Jr, Ewen M. E., Livingston D. M. Definition of the minimal simian virus 40 large T antigen- and adenovirus E1A-binding domain in the retinoblastoma gene product. Mol Cell Biol. 1990 Jul;10(7):3761–3769. doi: 10.1128/mcb.10.7.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. Y., To H., Shew J. Y., Bookstein R., Scully P., Lee W. H. Inactivation of the retinoblastoma susceptibility gene in human breast cancers. Science. 1988 Jul 8;241(4862):218–221. doi: 10.1126/science.3388033. [DOI] [PubMed] [Google Scholar]
- Marshall C. J. Tumor suppressor genes. Cell. 1991 Jan 25;64(2):313–326. doi: 10.1016/0092-8674(91)90641-b. [DOI] [PubMed] [Google Scholar]
- Moran E. A region of SV40 large T antigen can substitute for a transforming domain of the adenovirus E1A products. Nature. 1988 Jul 14;334(6178):168–170. doi: 10.1038/334168a0. [DOI] [PubMed] [Google Scholar]
- Moran E., Zerler B., Harrison T. M., Mathews M. B. Identification of separate domains in the adenovirus E1A gene for immortalization activity and the activation of virus early genes. Mol Cell Biol. 1986 Oct;6(10):3470–3480. doi: 10.1128/mcb.6.10.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphree A. L., Benedict W. F. Retinoblastoma: clues to human oncogenesis. Science. 1984 Mar 9;223(4640):1028–1033. doi: 10.1126/science.6320372. [DOI] [PubMed] [Google Scholar]
- Münger K., Werness B. A., Dyson N., Phelps W. C., Harlow E., Howley P. M. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 1989 Dec 20;8(13):4099–4105. doi: 10.1002/j.1460-2075.1989.tb08594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissmann P. T., Simon M. A., Lee W. H., Slamon D. J. Studies of the retinoblastoma gene in human sarcomas. Oncogene. 1989 Jul;4(7):839–843. [PubMed] [Google Scholar]
- T'Ang A., Varley J. M., Chakraborty S., Murphree A. L., Fung Y. K. Structural rearrangement of the retinoblastoma gene in human breast carcinoma. Science. 1988 Oct 14;242(4876):263–266. doi: 10.1126/science.3175651. [DOI] [PubMed] [Google Scholar]
- Toguchida J., Ishizaki K., Sasaki M. S., Nakamura Y., Ikenaga M., Kato M., Sugimot M., Kotoura Y., Yamamuro T. Preferential mutation of paternally derived RB gene as the initial event in sporadic osteosarcoma. Nature. 1989 Mar 9;338(6211):156–158. doi: 10.1038/338156a0. [DOI] [PubMed] [Google Scholar]
- Varley J. M., Armour J., Swallow J. E., Jeffreys A. J., Ponder B. A., T'Ang A., Fung Y. K., Brammar W. J., Walker R. A. The retinoblastoma gene is frequently altered leading to loss of expression in primary breast tumours. Oncogene. 1989 Jun;4(6):725–729. [PubMed] [Google Scholar]
- Weichselbaum R. R., Beckett M., Diamond A. Some retinoblastomas, osteosarcomas, and soft tissue sarcomas may share a common etiology. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2106–2109. doi: 10.1073/pnas.85.7.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A. The retinoblastoma gene and cell growth control. Trends Biochem Sci. 1990 May;15(5):199–202. doi: 10.1016/0968-0004(90)90162-5. [DOI] [PubMed] [Google Scholar]
- Whyte P., Buchkovich K. J., Horowitz J. M., Friend S. H., Raybuck M., Weinberg R. A., Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988 Jul 14;334(6178):124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- Whyte P., Ruley H. E., Harlow E. Two regions of the adenovirus early region 1A proteins are required for transformation. J Virol. 1988 Jan;62(1):257–265. doi: 10.1128/jvi.62.1.257-265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte P., Williamson N. M., Harlow E. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989 Jan 13;56(1):67–75. doi: 10.1016/0092-8674(89)90984-7. [DOI] [PubMed] [Google Scholar]
- Yandell D. W., Campbell T. A., Dayton S. H., Petersen R., Walton D., Little J. B., McConkie-Rosell A., Buckley E. G., Dryja T. P. Oncogenic point mutations in the human retinoblastoma gene: their application to genetic counseling. N Engl J Med. 1989 Dec 21;321(25):1689–1695. doi: 10.1056/NEJM198912213212501. [DOI] [PubMed] [Google Scholar]
- Yee S. P., Branton P. E. Detection of cellular proteins associated with human adenovirus type 5 early region 1A polypeptides. Virology. 1985 Nov;147(1):142–153. doi: 10.1016/0042-6822(85)90234-x. [DOI] [PubMed] [Google Scholar]
- Yokota J., Akiyama T., Fung Y. K., Benedict W. F., Namba Y., Hanaoka M., Wada M., Terasaki T., Shimosato Y., Sugimura T. Altered expression of the retinoblastoma (RB) gene in small-cell carcinoma of the lung. Oncogene. 1988 Oct;3(4):471–475. [PubMed] [Google Scholar]