Abstract
High relatedness among interacting individuals has generally been considered a precondition for the evolution of altruism. However, kin-selection theory also predicts the evolution of altruism when relatedness is low, as long as the cost of the altruistic act is minor compared with its benefit. Here, we demonstrate evidence for a low-cost altruistic act in bacteria. We investigated Escherichia coli responding to the attack of an obligately lytic phage by committing suicide in order to prevent parasite transmission to nearby relatives. We found that bacterial suicide provides large benefits to survivors at marginal costs to committers. The cost of suicide was low, because infected cells are moribund, rapidly dying upon phage infection, such that no more opportunity for reproduction remains. As a consequence of its marginal cost, host suicide was selectively favoured even when relatedness between committers and survivors approached zero. Altogether, our findings demonstrate that low-cost suicide can evolve with ease, represents an effective host-defence strategy, and seems to be widespread among microbes. Moreover, low-cost suicide might also occur in higher organisms as exemplified by infected social insect workers leaving the colony to die in isolation.
Keywords: altruistic suicide, host defence, parasite transmission, structured population, Hamilton's rule, kin selection
1. Introduction
Kin-selection theory [1] predicts that an altruistic behaviour is favoured if its fitness cost (c) to the actor is outweighed by its fitness benefit (b) to the recipient times the genetic relatedness (r at the cooperative trait) between the actor and the recipient (rb > c, Hamilton's rule). Consistent with theory, the importance of relatedness for the evolution of altruism has been demonstrated in numerous studies [2]. It most clearly emerged in comparative work showing that high relatedness was key for the evolution of reproductive altruism in social insects [3,4], birds [5] and mammals [6]. However, Hamilton's rule can also predict the evolution of altruism when relatedness is low, for example, in situations where the cost of altruism is minor compared with its benefit. In the extreme case where cost approaches zero, Hamilton's rule reduces to rb > 0, such that altruism can be favoured even when r tends towards zero.
Here, we demonstrate evidence of such a low-cost altruistic act in Escherichia coli bacteria, which express an abortive infection system (Abi) that responds to lethal phage attacks by causing infected cells to die together with the infecting phage. From an evolutionary perspective, the expression of an Abi system could reflect an altruistic act that allows infected bacteria to commit suicide in order to prevent parasite transmission to nearby relatives. Bacteria possess a number of Abi mechanisms, which usually involve joint actions by a prophage (a phage integrated into the bacterial genome) or a plasmid that encodes the genes for the abortive infection, and the bacterium, which transcribes and synthesizes the abortive machinery [7–11]. Important to note is that in many cases the interests of the bacterium and an already resident prophage become completely aligned upon obligately lytic phage infection (i.e. infection by phages that do not insert into the bacterial genome, but kill the bacterium instantly), such that they can be regarded as one genetic entity [12].
Although Abi systems have long been considered by some to reflect altruistic acts [8], a rigorous test involving the measurement of parameters of Hamilton's rule is lacking. We accomplished this by measuring the benefit and cost of suicide, as well as the effect of relatedness on suicide in E. coli carrying the prophage λ (henceforth E. coli λ), which encodes the Rex abortive infection system [13]. The Rex system codes for an intracellular sensor (RexA) that is activated upon infection by the obligately lytic phage T4rII [14]. RexA then activates a membrane-anchored ion channel (RexB), which immediately results in the drop of the membrane potential and the cellular ATP levels, finally leading to the death of the bacterium, its prophage and the infecting phage [8]. To assess the benefit of the Rex system, we infected altruistic E. coli λ cells with phage T4rII in liquid-shaken medium and compared their fitness with an infected, non-altruistic E. coli strain (carrying the control prophage HK97, henceforth E. coli HK97). To measure intrinsic (maintenance) and extrinsic (inefficiency) costs of the Rex system, we competed E. coli λ against E. coli HK97 in the absence and presence of phages. In both types of competition assays, costs of suicide should manifest in E. coli HK97 outcompeting E. coli λ. Finally, we manipulated relatedness at the altruistic trait by competing E. coli λ against E. coli HK97 in medium containing different amounts of agarose. Bacterial dispersal is limited in more solid medium [15,16], which increases relatedness among interacting individuals (i.e. Rex-carriers interact more often among themselves than expected based on their relative frequency in the population).
2. Material and methods
(a). Strains
We used E. coli str. K-12 substr. MG1655 in all experiments. Two strains carrying different prophages were constructed by infection with either temperate phage λ PaPa or HK97 (henceforth referred to as E. coli λ and E. coli HK97). Escherichia coli λ expresses the suicide trait, whereas E. coli HK97 does not. Because both prophages belong to the same immunity group, they do not transmit in a co-culture, and hence the phenotypic difference between the two E. coli strains is reduced to the suicide trait. Both strains were marked with plasmids pGFP and pDsRed carrying genes for fluorescent proteins under an inducible promoter. Specifically, the GFPmut3 variant from plasmid pMA3 and a fast-maturing DsRed1 from pCLR7 were PCR-amplified with primers containing KpnI and HindIII restriction sites (GFPmut3) or BamHI HindIII restriction sites (DsRed), and cloned into pGDR11,2 a pQE31-based vector with an isopropyl β-d-1-thiogalactopyranoside (IPTG)-inducible promoter. Colonies that appeared bright green or red with 100 µM IPTG were picked, and the insert was verified by digestion and PCR. Primer sequences (5′–3′) for PCR verification were AGGTACCTAGTAAAGGAGAAGAACTTTT (5′-GFP), GATAAGCTTTTATTTGTAGAGCTCATCCA (3′-GFP), CGCGGATCCTGCATCCACCGAGGACGTCAT (5′-DsRed), GGGAAGCTTCTACAGGAACAGGTGGTGGC (3′-DsRed).
We used phage T4rII, a mutant strain of phage T4 that is susceptible to the Rex system of λ lysogens of E. coli [14]. The strain was plated on a lawn of E. coli, a single plaque was cored and used to obtain a high-titer stock via plate lysis. The stock was stored in sodium, magnesium, gelatin buffer (0.1 M NaCl, 8 mM MgSO4, 50 mM Tris–HCl pH 7.5, 0.01% gelatin) at 4°C. A drop of chloroform was added to prevent contamination by other micro-organisms.
(b). Experimental conditions
We carried out all experiments in lysogeny broth (10 g l−1 tryptone, 5 g l−1 yeast extract, 10 g l−1 NaCl; Sigma, Switzerland) supplemented with 100 μg ml−1 ampicillin (Sigma) to prevent plasmid loss and 200 μM IPTG (Sigma) to induce fluorescent proteins. Where indicated, agarose (Invitrogen) was added to impose population structure. All experiments were initiated using overnight cultures of E. coli λ and E. coli HK97.
To assess the benefit of host suicide, monocultures of E. coli λ and E. coli HK97 (5 × 106 cells) were infected with 0, 5, 50, 5 × 102, 5 × 103, 5 × 104, 5 × 105, 5 × 106, 5 × 107 or 5 × 108 pfu of phage T4rII in 100 µl medium in 96-well plates. All treatments were replicated four times; controls without T4rII were replicated eight times. Cultures were incubated in a plate reader (Molecular Devices, Sunnyvale, CA, USA) at 37°C and shaken at regular intervals (2 min), at which optical densities (600 nm) were measured. Here, we used strains carrying pGFP.
To estimate intrinsic (maintenance) costs of encoding the suicide system, we mixed E. coli λ and E. coli HK97 in a 50 : 50 ratio (5 × 105 cells in total) in 500 µl medium in a 96-deep well plate in the absence of T4rII. Cultures were replicated 24 times for each marker combination, whereby each culture was based on an independent overnight culture. Cultures were incubated overnight at 37°C in an orbital shaker (400 r.p.m., a glass bead was added to each well to ensure proper mixing). Frequencies of both strains were measured before and after overnight growth using flow cytometry and used to calculate fitness of E. coli λ relative to E. coli HK97 for every replicate.
The Rex system might also incur extrinsic costs, for example because of mistakes (e.g. mortality rate upon T4rII infection might be lower than 100%, such that suicide is sometimes in vain resulting in the loss of direct reproduction). We estimated putative extrinsic costs of suicide in well-shaken medium. Here, the relatedness among interacting individuals is expected to be low, and any costs associated with suicide should result in the relative increase in E. coli HK97, which does not pay the cost, but still benefits from suicide (see [17,18] for theory). Specifically, we mixed E. coli λ and E. coli HK97 in various frequencies (5 × 105 cells in total) in 100 µl medium in the presence (104 pfu, multiplicities of infection (MOI) = 0.02) or absence of T4rII. The volumetric mixing frequencies of E. coli λ were 10, 50, 90, 95, 99 and 99.9 per cent, which corresponded to the actual mixing frequencies of 18.6, 66.3, 95.7, 98.0, 99.1 and 99.8 per cent (carrying pGFP), and 11.5, 51.0, 90.5, 95.5, 98.4 and 99.5 per cent (carrying pDsRed) assessed by flow cytometry. All treatments were replicated four times for both marker combinations. Cultures were incubated for 8 h in a plate reader at 37°C and shaken in 2 min intervals, after which optical densities (600 nm) were measured. Final frequencies of both strains were measured using flow cytometry.
To measure the fitness consequences of host suicide in structured environments, we mixed strains (105 cells) in volumetric frequencies of 0.1 per cent and 50 per cent E. coli λ (actual frequencies assessed by flow cytometry were 0.38 per cent and 46.6 per cent, carrying pGFP; and 0.56 per cent and 43.0 per cent, carrying pDsRed) in 20 µl medium. The mix was supplemented with 0, 103, 104, 105, 106 and 107 pfu of phage T4rII, and then added on top of 1 ml medium containing 0.4 per cent agarose in 24-well plates. All treatments were replicated four times for both marker combinations. Cultures were incubated at 37°C overnight, followed by strain frequency estimation using flow cytometry.
To measure the fitness consequences of host suicide in environments with reduced population structure, the same 50 : 50 mix as above was used. Cells (105) in 20 µl medium were supplemented with 0 or 104 pfu of phage T4rII and added into 1 ml medium containing 0, 0.025, 0.05, 0.1, 0.2 and 0.4 per cent agarose in 24-well plates. All treatments were replicated four times for both marker combinations. Cultures were incubated at 37 °C overnight in an orbital shaker (250 r.p.m.), followed by strain frequency estimation using flow cytometry. Because cultures grown with T4rII in 0 per cent agarose went extinct, no frequencies could be measured at this agarose concentration.
(c). Flow cytometry
Following competition, bacterial co-cultures were diluted in phosphate-buffered saline (Sigma), and 20 000 cells per sample were analysed (FACSCalibur, BD Biosciences, Franklin Lakes, NJ, USA). Samples were excited at a wavelength of 488 nm, whereas emission was detected at 510 (GFP) and 560 nm (DsRed). The use of GFP and DsRed is ideal here, because both markers can be excited with the same wavelength. Data were filtered by gating based on forward and side scatter, after which cells were counted based on fluorescence intensity using WEASEL v. 3.0.1 (Walter and Eliza Hall Institute of Medical Research, Parkville, Victoria, Australia).
(d). Statistical analysis
All statistical analyses were carried out with R v. 2.15.0. [19]. Maximum doubling times were estimated by fitting a Gompertz growth model [20] to the monoculture growth data obtained during the first 180 min following incubation. Doubling times were compared across treatments with a one-way analysis of variance (ANOVA), including the dose of phage T4rII as a discrete explanatory variable. Post hoc pairwise comparisons were carried out with Tukey's honestly significant difference (HSD) test (95% confidence interval, CI).
We first calculated the Malthusian fitness (m) of E. coli λ as m = log([fE. coli λ end · fE. coli HK97 start]/[fE. coli λ start · fE. coli HK97 end]) [21,22], where f with the respective subscript is the frequency of E. coli λ or E. coli HK97 at the beginning or the end of the competition in the same replicate. Fitness is thus based on the shift in strain frequencies that occurred during the time of competition, with fitness values of m > 0 or m < 0 signifying competitive advantages of E. coli λ or E. coli HK97, respectively.
Analysis of this first measure of fitness revealed significant effects of the marker plasmids in the absence of the phage. Strains with pGFP generally performed better than their competitors with pDsRed (average m of E. coli λ pGFP±95% CI: 1.06±0.82; E. coli λ pDsRed: −0.95±0.78; Student's t-test, t46 = 17.2, p < 0.0001), suggesting that pDsRed bears higher costs. However, this effect was consistent and independent of the strain itself: m of E. coli λ pGFP did not significantly differ from the absolute |− m| of E. coli λ pDsRed (Student's t-test, t46 = 0.95, p = 0.35). To control for this marker effect in the presence of the phage, we used a modified formula for m, which compared the final frequencies of E. coli λ (carrying a given plasmid marker) between competitions with (fE. coli λ treat) and without (fE. coli λ ctrl) phage T4rII: m = log([fE. coli λ treat · fE. coli HK97 ctrl]/[fE. coli λ ctrl · fE. coli HK97 treat]). Because replicates in treatment and control competitions were not paired, the final frequency in each treatment replicate was compared with the average final frequency across all control replicates.
We used Student's t-tests to compare fitness estimates or to test whether they were significantly different from 0. Furthermore, we fitted full factorial ANOVAs to fitness values (response variable), including the factors ‘dose of T4rII’ (continuous), ‘initial frequency of E. coli λ’ (discrete), ‘agarose concentration’ (continuous) and ‘marker colour’ (discrete). Our data is deposited at Dryad: doi:10.5061/dryad.b1q2n.
3. Results
(a). The benefit of host suicide
In liquid-shaken medium, we found that very low numbers of T4rII were sufficient to eradicate E. coli HK97 cultures (figure 1a). By contrast, E. coli λ exhibited unhindered growth that was not significantly different from an uninfected control culture (max. doubling time 24.65±6.92 min; mean±95% CI) at MOI (the phages-to-bacteria ratio) < 1 (at MOI = 10−6: 22.91±3.39, Tukey's HSD test, p > 0.99; 10−5: 22.98±6.23, p > 0.99; 10−4: 21.69±2.34, p = 0.94; 10−3: 21.91±3.38, p = 0.96; 10−2: 22.57±7.30, p = 0.99; 0.1: 26.93±13.95, p = 0.98; figure 1b). This demonstrates that host suicide clears the infection, thereby saving the clonal population from extinction. While at MOI = 1, growth was significantly delayed (44.74±14.24, p < 0.0001), we observed no growth at MOI > 1, a pattern compatible with population extinction occurring when all bacterial cells commit suicide upon simultaneous infection with T4rII (figure 1b).
Figure 1.
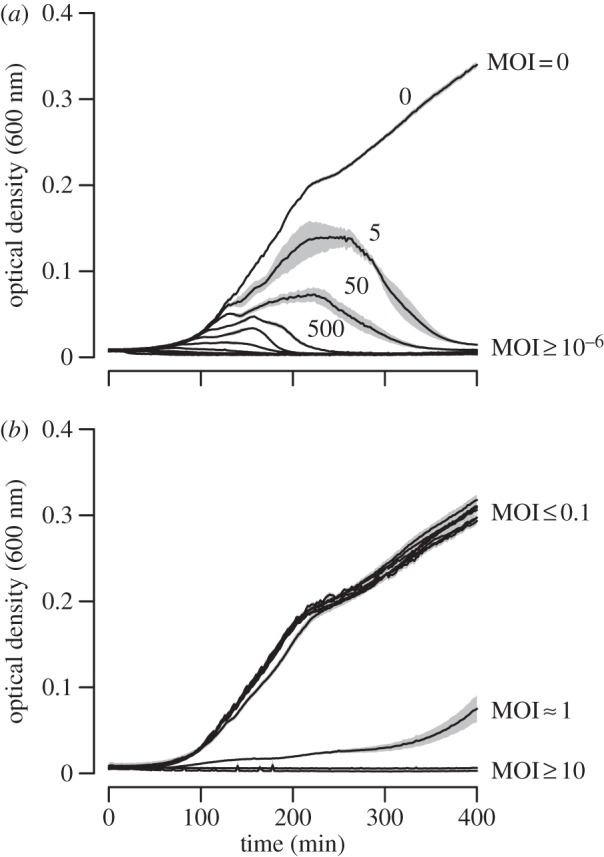
(a) Escherichia coli HK97 populations without a suicidal abortive system are completely eradicated within a short period of time even when infected with very low numbers (indicated above lines) of phage T4rII. (b) Escherichia coli λ populations with the Rex abortive system show unhindered growth when infected with a MOI ≤ 0.1, demonstrating the benefit of suicide in clonal populations. At a MOI ≈ 1, growth was delayed, and at higher MOIs, no growth occurred because T4rII simultaneously infected all bacteria, such that no survivors could benefit from suicide. Growth curves are means of four replicates (control treatment eight replicates) and shaded areas represent s.e.
(b). The cost of host suicide
In the absence of T4rII and taking marker effects into account, we found no evidence that the frequency of E. coli λ significantly changed during overnight competition against E. coli HK97 (average m of E. coli λ±95% CI = 0.11±0.32, t46 = 0.95, p = 0.35). This result indicates that intrinsic costs of maintaining the Rex system are negligible.
Our competition assays in the presence of T4rII also revealed no evidence for significant extrinsic costs. When E. coli λ was sufficiently frequent in a culture (>95%), its suicide cleared T4rII infections (figure 2a) and E. coli HK97 successfully hitchhiked along but never significantly increased in frequency (figure 2b). At an E. coli λ frequency of approximately 95 per cent, cultures entered a growth-arrest phase. In one of these cases (E. coli λ pGFP), E. coli λ significantly increased in frequency (figure 2b). This finding is compatible with previous observations that under certain conditions the Rex system induces dormancy instead of suicide [23,24] (see §4). By contrast, co-cultures went extinct when the initial E. coli λ frequency was less than 95 per cent, showing that suicide cannot stop infection in well-mixed cultures if the suicidal strain is not sufficiently common, because T4rII multiplication (burst size 100–300) within E. coli HK97 outweighs suicide-based phage eradication.
Figure 2.
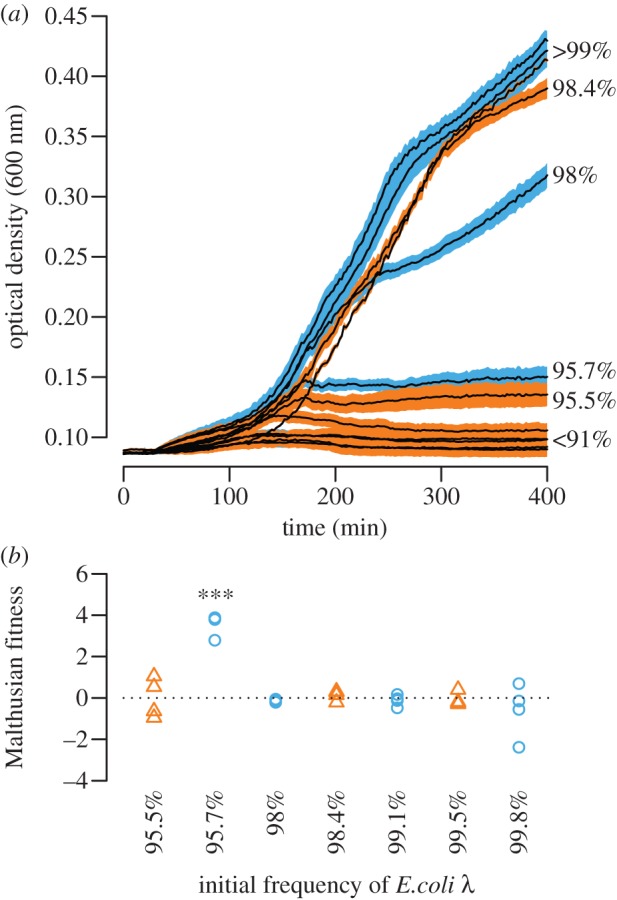
(a) In co-cultures where Escherichia coli λ was very common (>95%), suicide could eradicate T4rII (MOI = 0.02) infections. In one case (95.7%), co-cultures entered a growth-arrest phase during which E. coli λ significantly increased in frequency suggesting that suicide could be delayed or circumvented for unknown reasons. Co-cultures in which E. coli λ was less common (<95%) went extinct, showing that suicide cannot stop infection in well-mixed populations with a susceptible strain. Growth curves are means of four replicates, shaded areas represent standard errors, and colours indicate the type of marker plasmid used for E. coli λ (blue circles, pGFP; orange triangles, pDsRed). (b) In those cases where suicide could eradicate T4rII, E. coli HK97 successfully hitchhiked along with E. coli λ, but never experienced significant fitness benefits (t-tests, from left to right: t6 = 0, p = 1.00; t6 =−13.38, p < 0.0001; t6 = 1.43, p = 0.20; t6 =−1.04, p = 0.34; t6 = 0.73, p = 0.49; t6 = 0.56, p = 0.59; t6 = 1.39, p = 0.21). This indicates that E. coli λ suicide bears negligible costs.
(c). Relatedness and the evolution of host suicide
The earlier-mentioned analyses in well-mixed cultures (where relatedness r ≈ 0) show that owing to its cost-free nature, altruistic host suicide can be maintained even with r = 0 (i.e. non-suicidal strains cannot invade). On the other hand, the data also show that altruistic host suicide cannot evolve with r = 0 because populations go extinct before invasion of the Abi trait is possible. Thus, we predict marginal relatedness (r < 0) to be required for successful invasion. Our fitness assays in structured environments confirmed this hypothesis.
In a highly structured environment (0.4% agarose, high relatedness), we found that T4rII infections could always be stopped, even at very high MOIs normally sufficient to eradicate E. coli λ in liquid culture. Furthermore, E. coli λ significantly outcompeted E. coli HK97 under all conditions (figure 3a), whereby the relative fitness of E. coli λ significantly increased with phage concentration (ANOVA: F1,72 = 24.66, p < 0.0001) but was independent of the initial frequency of E. coli λ (F1,72 = 1.51, p = 0.22). These findings are consistent with the view that phage–bacteria interaction occurred at a local scale, where T4rII managed to drive local E. coli HK97 patches to extinction. By contrast, T4rII was eradicated from E. coli λ patches owing to suicide, leading to large fitness benefits for surviving E. coli λ, even when initially rare.
Figure 3.
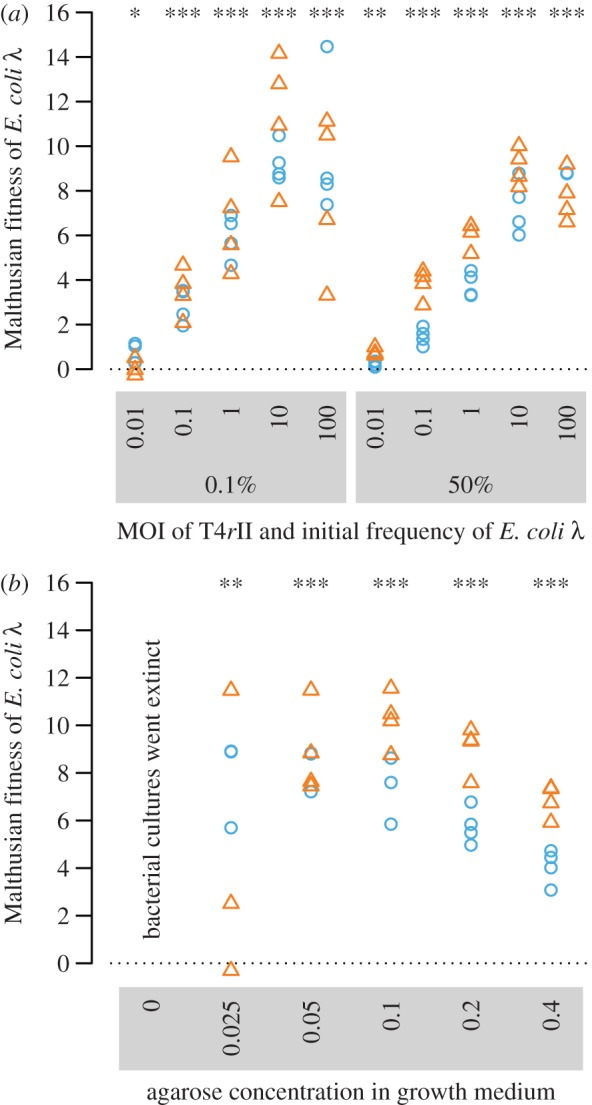
(a) On solid agarose (0.4%, high relatedness) Escherichia coli λ significantly outcompeted E. coli HK97 at all phage concentrations, even when initially rare (t-tests, from left to right: t7 = 3.5, p = 0.0097; t7 = 9.6, p < 0.0001; t7 = 10.7, p < 0.0001; t7 = 12.8, p < 0.0001; t7 = 7.6, p = 0.0001; t7 = 4.6, p = 0.0024; t7 = 5.5, p = 0.0009; t7 = 10.6, p < 0.0001; t7 = 17.1, p < 0.0001; t7 = 25.1, p < 0.0001). (b) Escherichia coli λ also significantly outcompeted E. coli HK97 (in a 1 : 1 mix) when the degree of population structure was reduced to a minimum, showing that only marginal relatedness is required for host suicide to be selectively favoured (t-tests, from left to right: t7 = 4.8, p = 0.0020; t7 = 18.2, p < 0.0001; t7 = 14.2, p < 0.0001; t7 = 10.9, p < 0.0001; t7 = 9.5, p < 0.0001). Colours indicate the type of marker plasmid used for E. coli λ (blue circles, pGFP; orange triangles, pDsRed).
Crucially, we observed that E. coli λ also experienced large fitness benefits when moving from solid (0.4% agarose, reflecting high relatedness) to almost liquid (in 0.025% agarose-shaken medium, reflecting relatedness close to zero) conditions (figure 3b). This shows that even marginal population structure suffices for E. coli λ to significantly outcompete E. coli HK97. Moreover, our data show that fitness benefits for E. coli λ significantly peaked at intermediate agarose concentrations (quadratic regression: F2,37 = 5.59, p = 0.008, figure 3). This pattern indicates that in extremely structured environments not only bacteria but also phage mobility is greatly limited. Consequently, susceptible E. coli HK97 performs better in these environments because of a reduced risk of encountering phages.
4. Discussion
We showed that E. coli λ suicide upon T4rII infection was highly beneficial as it saved the clonal population from extinction, but entailed no detectable cost for cells committing suicide. This resulted in suicidal behaviour being favoured even when relatedness between committers and survivors approached zero. No detectable costs of suicide could arise owing to a combination of low metabolic maintenance costs of the Rex system, and the fact that infected bacteria are moribund anyway. Because infection causes the rapid death of the host, an infected individual has little or no opportunity to reproduce until its death, such that the commitment of suicide might simply hasten an individual's inevitable doom. Our findings demonstrate that altruistic host suicide can clearly be delimited from other forms of suicidal behaviours in microbes, such as cell lysis to release toxins [25] or nutrients [26], cell sacrifices to overcome immune systems [27] or to form fruiting body stalks [28,29]. In contrast to the altruistic suicide described in our study, these other behaviours require substantial relatedness among interacting individuals because suicidal cells lose direct fitness benefits.
The absence of detectable costs for E. coli λ suicide further raises the question, whether this behaviour represents a true altruistic act, which per some definitions implies c > 0 [30,31]. There are two ways to deal with this issue. First, it could be argued that, although not detectable with our methods, there are certainly some costs (c > 0) involved in Rex maintenance, such that E. coli λ suicide indeed represents an altruistic act. For instance, marginal costs of carrying Rex might be masked in our experiment because the two prophages (HK97 and λ) differ in a number of minor aspects other than Rex. Second, c = 0 could be true, in that case E. coli λ suicide could be considered as a novel type of cooperative behaviour, which is neither altruistic (c > 0) nor mutually beneficial (c < 0) [31]. However, we do not think that the proclamation of a novel type of cooperative behaviour is useful, and rather endorse the view that the definition of altruism should be expanded to c ≥ 0 (in line with Foster [32]).
Our results further reveal that under a narrow range of conditions (probably depending on MOI) the Rex system rather induces dormancy than suicide (figure 2a, E. coli λ frequency of approx. 95%). This phenomenon was first described by Slavcey & Hayes [23,24], who showed that a fraction of dormant cells are able to resume growth after approximately 2 days. The proximate basis of this dormancy is unknown. Furthermore, it is also unclear whether this form of dormancy is adaptive, providing direct fitness benefits, or whether it represents a by-product of the Rex system, which has primarily evolved to induce suicide. The latter explanation seems more probable, because the mechanism of cell death is clearly established [8,13] and is triggered across most conditions examined here.
Our fitness-based (ultimate) analyses reveal that bacterial suicide is adaptive and represents a highly efficient host-defence mechanism. Our findings are in close agreement with the work of Fukuyo et al. [33], who used an engineered E. coli–phage system to demonstrate for the first time that host suicide is adaptive in structured habitats. Our study and the work of Fukuyo et al. [33] contrast with previous studies, where evolutionary conclusions were mainly based on proximate (mechanistic) aspects of cell death [8,10,11]. This is problematic because mechanistic approaches elucidating how things work can provide only limited answers on why a given behaviour has evolved [31].
More generally, evolutionary conclusions based on proximate findings resulted in much controversy on whether mechanisms resulting in microbial cell death have indeed evolved for the purpose of committing suicide or whether they represent by-products of selection at other traits [34–36]. While this controversy can never distinctly be resolved for past events, it seems that in the Rex system, rexB may have originally been favoured because it protects E. coli from the killing mediated by addiction modules [37]. In combination with the sensor RexA, which is triggered by several unrelated phages [38], the system may have subsequently been coopted into an adaptive suicidal host-defence mechanism. Co-option, which is a common phenomenon in evolution [32], might have also been involved in shaping some of the other Abi systems described in bacteria [9–11]. Moreover, it might be that not all Abi systems involve active suicide (i.e. a cell expressing a trait with the purpose to kill itself). It might rather be that certain mechanisms have evolved to block phage progeny production within the host, which consequently lead to bacterial death. Such traits would however spread under the same conditions as the Rex system examined in our study.
The idea of infected hosts committing suicide in order to prevent disease transmission to healthy relatives has also received considerable attention in higher organisms. While theoretical work convincingly revealed the conditions required for host suicide to evolve [39,40], empirical work has often been criticized [41,42]. In particular, the adaptive significance of host suicide has been challenged because studies involved eusocial Hymenoptera [43–45] or clonal aphids [46], where complex life histories impeded rigorous fitness tests and the exclusion of alternative explanations. Our findings now reveal that, owing to its low cost, altruistic host suicide might easily evolve in any organism, even when relatedness is low. Possible examples include the observation that infected social insect workers leave the colony to die in isolation (i.e. altruistic self-removal [44,45]), a behaviour that could come at negligible costs because infected individuals might be strongly compromised in carrying out worker tasks anyway.
Acknowledgements
We thank John W. Little, Ing-Nang Wang, Benjamin Kerr, Bärbel Stecher, Martin Ackermann and Winfried Boos for providing bacteria and phage strains, the flow cytometry laboratory at the ETH Zürich, Anette Schütz and Olin Silander for help with flow cytometry, and Jim Bull, Tim Cooper, Fred Inglis, Laurent Keller, Stu West and an anonymous reviewer for valuable comments. This work was supported by the Competence Center Environment and Sustainability of the ETH (to D.R.), the Swiss National Science Foundation, and the European Commission (to R.K.).
References
- 1.Hamilton WD. 1964. The genetical evolution of social behaviour. J. Theor. Biol. 7, 1–52 10.1016/0022-5193(64)90038-4 (doi:10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- 2.Abbot P, et al. 2011. Inclusive fitness theory and eusociality. Nature 471, E1–E4 10.1038/nature09831 (doi:10.1038/nature09831) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boomsma JJ. 2007. Kin selection versus sexual selection: why the ends do not meet. Curr. Biol. 17, R673–R683 10.1016/j.cub.2007.06.033 (doi:10.1016/j.cub.2007.06.033) [DOI] [PubMed] [Google Scholar]
- 4.Hughes WOH, Oldroyd BP, Beekman M, Ratnieks FLW. 2008. Ancestral monogamy shows kin selection is key to the evolution of eusociality. Science 320, 1213–1216 10.1126/science.1156108 (doi:10.1126/science.1156108) [DOI] [PubMed] [Google Scholar]
- 5.Cornwallis CK, West SA, Davis KE, Griffin AS. 2010. Promiscuity and the evolutionary transition to complex societies. Nature 466, 969–972 10.1038/nature09335 (doi:10.1038/nature09335) [DOI] [PubMed] [Google Scholar]
- 6.Lukas D, Clutton-Brock T. 2012. Cooperative breeding and monogamy in mammalian societies. Proc. R. Soc. B 279, 2151–2156 10.1098/rspb.2011.2468 (doi:10.1098/rspb.2011.2468) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mollineux IJ. 1991. Host–parasite interactions: recent developments in the genetics of abortive phage infections. New Biol. 3, 230–236 [PubMed] [Google Scholar]
- 8.Parma DH, Snyder M, Sobolevski S, Nawroz M, Brody E, Gold L. 1992. The Rex system of bacteriophage λ: tolerance and altruistic cell death. Genes Dev. 6, 497–510 10.1101/gad.6.3.497 (doi:10.1101/gad.6.3.497) [DOI] [PubMed] [Google Scholar]
- 9.Snyder L. 1995. Phage-exclusion enzymes: a bonanza of biochemical and cell biology reagents? Mol. Microbiol. 15, 415–420 10.1111/j.1365-2958.1995.tb02255.x (doi:10.1111/j.1365-2958.1995.tb02255.x) [DOI] [PubMed] [Google Scholar]
- 10.Chopin M-C, Chopin A, Bidnenko E. 2005. Phage abortive infection in lactococci: variations on a theme. Curr. Opin. Microbiol. 8, 473–479 10.1016/j.mib.2005.06.006 (doi:10.1016/j.mib.2005.06.006) [DOI] [PubMed] [Google Scholar]
- 11.Labrie SJ, Samson JE, Moineau S. 2010. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 8, 317–327 10.1038/nrmicro2315 (doi:10.1038/nrmicro2315) [DOI] [PubMed] [Google Scholar]
- 12.Queller DC, Strassmann JE. 2009. Beyond society: the evolution of organismality. Phil. Trans. R. Soc. B 364, 3143–3155 10.1098/rstb.2009.0095 (doi:10.1098/rstb.2009.0095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ptashne M. 2004. A genetic switch: phage lamda revisited, 3rd edn Cold Spring Harbor, NY: CSH Laboratory Press [Google Scholar]
- 14.Benzer S. 1955. Fine structure of a genetic region in bacteriophage. Proc. Natl Acad. Sci. USA 41, 344–354 10.1073/pnas.41.6.344 (doi:10.1073/pnas.41.6.344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chao L, Levin BR. 1981. Structured habitats and the evolution of anticompetitor toxins in bacteria. Proc. Natl Acad. Sci. USA 78, 6324–6328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kümmerli R, Griffin AS, West SA, Buckling A, Harrison F. 2009. Viscous medium promotes cooperation in the pathogenic bacterium Pseudomonas aeruginosa. Proc. R. Soc. B 276, 3531–3538 10.1098/rspb.2009.0861 (doi:10.1098/rspb.2009.0861) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.West SA, Griffin AS, Gardner A, Diggle SP. 2006. Social evolution theory for microorganisms. Nat. Rev. Microbiol. 4, 597–607 10.1038/nrmicro1461 (doi:10.1038/nrmicro1461) [DOI] [PubMed] [Google Scholar]
- 18.Kümmerli R, van den Berg P, Griffin AS, West SA, Gardner A. 2010. Repression of competition promotes cooperation: experimental evidence from bacteria. J. Evol. Biol. 23, 699–706 10.1111/j.1420-9101.2010.01936.x (doi:10.1111/j.1420-9101.2010.01936.x) [DOI] [PubMed] [Google Scholar]
- 19.R Development Core Team 2010. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 20.Zwietering MH, Jongenburger I, Rombouts FM, van't Ried K. 1990. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 56, 1875–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartl DL, Clark AG. 1997. Principles of population genetics, 3rd edn Sunderland, MA: Sinauer Associates, Inc [Google Scholar]
- 22.Orr HA. 2009. Fitness and its role in evolutionary genetics. Nat. Rev. Genet. 10, 531–539 10.1038/nrg2603 (doi:10.1038/nrg2603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slavcev RA, Hayes S. 2002. Rex-centric mutualism. J. Bacteriol. 184, 857–858 10.1128/JB.184.3.857-858.2002 (doi:10.1128/JB.184.3.857-858.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slavcev RA, Hayes S. 2003. Stationary phase-like properties of the bacteriophage λ Rex exclusion phenotype. Mol. Genet. Genomics 269, 40–48 10.1007/s00438-002-0787-x (doi:10.1007/s00438-002-0787-x) [DOI] [PubMed] [Google Scholar]
- 25.Cascales E, Buchanan SK, Duché D, Kleanthous C, Lloubes R, Postle K, Riley M, Slatin S, Cavard D. 2007. Colocin biology. Microbiol. Mol. Biol. Rev. 71, 158–229 10.1128/MMBR.00036-06 (doi:10.1128/MMBR.00036-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Durand PM, Rashidi A, Michod RE. 2011. How an organsim dies affects the fitness of its neighbors. Am. Nat. 177, 224–232 10.1086/657686 (doi:10.1086/657686) [DOI] [PubMed] [Google Scholar]
- 27.Ackermann M, Stecher B, Freed NE, Songhet P, Hardt W-D, Doebeli M. 2008. Self-destructive cooperation mediated by phenotypic noise. Nature 454, 987–990 10.1038/nature07067 (doi:10.1038/nature07067) [DOI] [PubMed] [Google Scholar]
- 28.Strassmann J, Zhu Y, Queller DC. 2000. Altruism and social cheating in the social amoeba Dictyostelium discoideum. Nature 408, 965–967 10.1038/35050087 (doi:10.1038/35050087) [DOI] [PubMed] [Google Scholar]
- 29.Kraemer SA, Velicer GJ. 2011. Endemic social diversity within natural kin groups of a cooperative bacterium. Proc. Natl Acad. Sci. USA 108, 10 823–10 830 10.1073/pnas.1100307108 (doi:10.1073/pnas.1100307108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lehmann L, Keller L. 2006. The evolution of cooperation and altruism: a general framework and a classification of models. J. Evol. Biol. 19, 1365–1376 10.1111/j.1420-9101.2006.01119.x (doi:10.1111/j.1420-9101.2006.01119.x) [DOI] [PubMed] [Google Scholar]
- 31.West SA, Griffin AS, Gardner A. 2007. Social semantics: altruism, cooperation, mutualism, strong reciprocity and group selection. J. Evol. Biol. 20, 415–432 10.1111/j.1420-9101.2006.01258.x (doi:10.1111/j.1420-9101.2006.01258.x) [DOI] [PubMed] [Google Scholar]
- 32.Foster K. 2011. The sociobiology of molecular systems. Nat. Rev. Genet. 12, 193–203 10.1038/nrg2903 (doi:10.1038/nrg2903) [DOI] [PubMed] [Google Scholar]
- 33.Fukuyo M, Sasaki A, Kobayashi I. 2012. Success of a suicidal defense strategy against infection in a structured habitat. Sci. Rep. 2, 238. 10.1038/srep00238 (doi:10.1038/srep00238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gardner A, Kümmerli R. 2008. Social evolution: this microbe will self-destruct. Curr. Biol. 18, R1021–R1023 10.1016/j.cub.2008.09.003 (doi:10.1016/j.cub.2008.09.003) [DOI] [PubMed] [Google Scholar]
- 35.Nedelcu AM, Driscoll WW, Durand PM, Herron MD, Rashidi A. 2011. On the paradigm of altruistic suicide in the unicellular world. Evolution 65, 3–20 10.1111/j.1558-5646.2010.01103.x (doi:10.1111/j.1558-5646.2010.01103.x) [DOI] [PubMed] [Google Scholar]
- 36.Reece SE, Pollitt LC, Colegrave N, Gardner A. 2011. The meaning of death: evolution and ecology of apoptosis in protozoan parasites. PLoS Pathog. 7, e1002320. 10.1371/journal.ppat.1002320 (doi:10.1371/journal.ppat.1002320) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Engelberg-Kulka H, Reches M, Narasimhan S, Schoulaker-Schwarz R, Klemens Y, Aizenman E, Glaser G. 1998. rexB of bacteriophage λ is an anti-cell death gene. Proc. Natl Acad. Sci. USA 95, 15 481–15 486 10.1073/pnas.95.26.15481 (doi:10.1073/pnas.95.26.15481) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Court D, Oppenheim AB. 1983. Phage λ's accessory genes in Lambda II. In Lambda II (eds Hendrix RW, Roberts JW, Stahl FW, Weisberg RA.), pp. 251–277 Cold Spring Harbor, NY: CSH Laboratory Press [Google Scholar]
- 39.Smith Trail DR. 1980. Behavioral interactions between parasites and hosts: host suicide and the evolution of complex life cycles. Am. Nat. 116, 77–92 10.1086/283612 (doi:10.1086/283612) [DOI] [Google Scholar]
- 40.Debarre F, Lion S, van Baalen M, Gandon S. 2012. Evolution of host life-history traits in a spatially structured host-parasite system. Am. Nat. 179, 52–63 10.1086/663199 (doi:10.1086/663199) [DOI] [PubMed] [Google Scholar]
- 41.Tomlinson I, Latta B. 1987. Adaptive and non-adaptive suicide in aphids. Nature 330, 701. 10.1038/330701a0 (doi:10.1038/330701a0)2892138 [DOI] [Google Scholar]
- 42.Poulin R. 1995. ‘Adaptive’ changes in the behaviour of parasitized animals: a critical review. Int. J. Parasitol. 25, 1371–1383 10.1016/0020-7519(95)00100-X (doi:10.1016/0020-7519(95)00100-X) [DOI] [PubMed] [Google Scholar]
- 43.Müller CB, Schmid-Hempel R. 1992. To die for host or parasite? Anim. Behav. 44, 177–179 10.1016/S0003-3472(05)80770-5 (doi:10.1016/S0003-3472(05)80770-5) [DOI] [Google Scholar]
- 44.Heinze J, Walter B. 2010. Moribund ants leave their nests to die in social isolation. Curr. Biol. 20, 249–252 10.1016/j.cub.2009.12.031 (doi:10.1016/j.cub.2009.12.031) [DOI] [PubMed] [Google Scholar]
- 45.Rueppell O, Hayworth MK, Ross NP. 2010. Altruistic self-removal of health-compromised honey bee workers from their hive. J. Evol. Biol. 23, 1538–1546 10.1111/j.1420-9101.2010.02022.x (doi:10.1111/j.1420-9101.2010.02022.x) [DOI] [PubMed] [Google Scholar]
- 46.McAllister MK, Roitberg BD. 1987. Adaptive suicidal behaviour in pea aphids. Nature 328, 797–799 10.1038/328797b0 (doi:10.1038/328797b0)3627227 [DOI] [Google Scholar]


