Abstract
Group-living animals often do not maintain territories, but instead have highly overlapping ranges, even though in principle these are economically defendable. We investigate whether this absence of range defence reflects a collective action problem, since a territory can be considered a public good. In a comparative analysis comprising 135 primate species, we find a positive association between range overlap and group size, controlling for economic defendability and phylogenetic non-independence. We subsequently demonstrate that groups with multiple adults of both sexes suffer levels of range overlap twice as high as groups with only a single adult representative of either sex, consistent with the presence of a collective action problem. Finally, we reveal that this collective action problem can be overcome through philopatry of the larger sex. These results suggest that a social complication of group living is a stronger determinant of between-group relations among social animals than ecological factors, but also that collective defence is still achieved where the dominant sex is philopatric and effective defence is critical to reproductive success and survival. In addition, our findings support the idea that human-like warfare, defined as escalated collective territorial conflict, has an evolutionary basis reflected by cases of convergent evolution among non-human primates.
Keywords: between-group conflict, within-group cooperation, home range defence, human warfare, public good, social dilemma
1. Introduction
In many animal species an individual will defend the area it occupies against intrusions by (usually same-sex) conspecifics, at least during some stages of its life cycle. The basic economic approach (or cost-benefit analysis: [1]), which estimates the so-called defendability of an area, has successfully been applied to solitary and pair-forming species many years ago, and has led to an understanding of both the conditions under which territoriality is expected, as well as the approximate sizes of sustainable territories [2]. In the absence of territoriality, high overlap between home ranges should be observed, with animals either avoiding contact or meeting at resource patches. In this scenario, the outcome of an encounter is dependent on the relative fighting abilities of the participants [3].
If this rationale is applied to species living in stable mixed-sex groups, however, the fit between economic defendability and range defence is far from perfect [4,5]. Moreover, in some taxa, conflict between-groups over space often appears absent where it would be expected (rodents: [6]; primates: [7–9]), or may only become apparent at unusually high population densities [10,11]. This poor fit to theories developed to account for range defence by individuals can possibly be ascribed to two inherent complications of group living.
First, in his revival of sexual selection theory, Trivers [12] argued that the fitness of males tends to be limited by access to mates while that of females is restrained by access to resources and safety. Thus, males are expected to engage in range defence to acquire or defend sexual access to mates, whereas females should be most involved when access to food, water or shelter is concerned [13]. As a result, in different situations one or the other sex should participate most actively [8,14,15]. However, where one sex can systematically dominate the other owing to sexual dimorphism in body size or weaponry, participation by the dominant sex may suppress participation by the subordinate sex. If this is the case, the interests of individuals of the smaller sex are not expressed by the observed range defence behaviours. Conversely, for some species in which males tend to be the larger sex and dominate females, it has been suggested that males may defend female interests (i.e. engage in resource defence) if this enhances their mates’ reproductive output (the ‘hired-guns’-hypothesis: [16]). These interactions between male and female interests may explain confusing observations on one sex being involved in some situations and the other in others, while both are involved in yet other situations [17].
The second complication of group living, and the main focus of this study, is that in many animal societies, the maintenance of home ranges or territories requires collective defence against conspecifics (ants: [18,19]; birds: [20,21]; carnivores: [22,23]; primates: [24,25]). This need for cooperation at the group level renders the defence of a common range vulnerable to a collective action problem (CAP; [26]), which can prevent it from being expressed behaviourally, even if economic conditions are propitious for all concerned. The reason for this is that, if collective action creates a public good that is more or less equally shared among group members (e.g. a territory), natural selection favours the emergence of free-riders or ‘laggards’ [27], who reap the benefits without incurring the costs of producing them [28,29]. As a result, an individual's selfish incentive will be to defect and collective action breaks down, which can result in a sub-optimal outcome to all (akin to a tragedy of the commons: [30,31]).
In primates, van Schaik [32] could indeed show that the number of males in a group affected range defence more (and negatively so) than economic defendability of the home range in both langurs and sifakas, two taxa in which range defence is predominantly a male affair. Nunn & Deaner [33] found evidence for free-riding by females in the collective defence of ranging areas in ring-tailed lemurs (Lemur catta), a species in which females are the most active sex in this context. Likewise, Crofoot & Gilby [34] demonstrated location-dependent defection by both male and female white-faced capuchins (Cebus capucinus) in collective range defence behaviours during simulated home range intrusions. The presence of CAPs then, may explain why primate home ranges are often not defended to the extent we would expect them to be.
To test the generality of the CAP in between-group relations, we compiled information from the literature on a large number of primate species. Primates are among the most intensively studied social animals, and exhibit remarkable interspecific variation in both general socio-ecology and the extent to which home ranges are defended. In addition, variation in range defence has been documented at the inter-individual and inter-populational level, yet to our knowledge, only one previous study has examined this across species. Using phylogenetic comparative methods, Nunn [35] could show that primate males were less likely to produce loud between-group vocalizations as the benefits produced were more public, i.e. more equally shared. However, the focus in his study was exclusively on male between-group competition over mates (a relatively monopolizable resource), whereas the present study targets between-group conflict over space (a much more public resource).
Here, we first aim to establish whether evidence for a CAP can be found in the context of home range defence across the primate taxon. As a proxy for the effectiveness of collective defence, we take the proportion of home range overlap and assess to what extent the observed interspecific variation can be accounted for by key social and ecological variables, while controlling for phylogenetic relatedness among species. Based on previous work on primate home ranges and territoriality [4,36–38], we consider group size and economic defendability of the home range, as well as the species’ activity period, substrate use, habitat type and diet, as potential explanatory variables. Because an absence of range defence is known to lead to higher overlap [39], under the CAP-hypothesis we would expect home range overlap to increase with group size, regardless of the influence of any additional variables, as collective defence in larger groups breaks down owing to increasing levels of free-riding and diminishing individual returns [26].
Second, based on findings from our first analysis, we investigate a number of additional social variables for their influence on home range overlap in social primates. Naively, the CAP-hypothesis would predict home range overlap to increase with both adult male and female numbers as CAPs can occur in either sex. However, because these numbers are likely to be correlated with each other as well as total group size, other factors may prove more informative. Therefore, we also look for differences owing to the social structure of a species’ modal grouping pattern (i.e. single-male single-female (sM–sF), single-male multi-female (sM–mF), multi-male single-female (mM–sF) or multi-male multi-female (mM–mF)). Moreover, because of the possibility that range defence predominantly reflects the interests of the larger, dominant sex, the CAP-hypothesis predicts that (regardless of the identity of this sex) home range overlap among species in which multiple individuals of the larger sex reside within a group, is higher than among species in which only one individual of the larger sex is present in a group.
Third, we explore the broad variation observed in home range overlap among mM–mF taxa, which have the highest levels of overlap, and ask whether there is evidence for mechanisms that may help avoid the expression of a CAP. Several evolutionary pathways have been identified to explain both the evolution and maintenance of cooperation in animal societies [40–42] and among these, shared genes (kin-selection: [43]) and shared benefits (including by-product benefits: [44], and multi-level selection: [45]) are thought to be particularly relevant to maintain collective defence of a common home range [46]. We, therefore, investigate whether philopatry of the larger sex, which increases familiarity as well as genetic relatedness amongst its members, is associated with lower home range overlap.
2. Material and methods
(a). Data collection
An extensive review was undertaken on the primatological literature published between 1970 and 2012 using Thomson Reuters Web of Knowledge (http://apps.webofknowledge.com) and Google Advanced Scholar (http://scholar.google.com). In addition, various books, edited volumes and postgraduate theses were consulted. Studies included in the review were restricted to free-ranging and non-provisioned populations of group-living species, and minimally had to report figures on home range overlap and total group size from the same study group(s) to be included. Also of key interest were home range size and mean day journey length that were used to calculate economic defendability of the home ranges of focal group(s). In the primatological literature, economic defendability has been quantified by two different, but related indices: the Mitani–Rodman D-index and the Lowen–Dunbar M-index ([37,38]; for a detailed account of both, see our note to the electronic supplementary material, table S4). We independently ran all analyses using either index, but here present findings based on the empirically more established D-index (results obtained using the M-index can be found in the electronic supplementary material, tables S4.1–S4.3). We emphasize that the choice of index did not affect our results or conclusions in any way.
To this list of variables, species-specific characteristics such as activity period (diurnal or nocturnal/cathemeral), substrate use (arboreal or -at least to some extent- terrestrial), habitat type (open/wooded) and diet (the proportion of leaves in the diet) were added from reviews in Smuts et al. [47], Nunn & van Schaik [48] and Campbell et al. [49]. Additional references were located through the All the World's Primates database [50]. Where available, social and life-history information was collated on the number of adult males and females, the social structure of the study group(s) (i.e. sM–sF, sM–mF, mM–sF or mM–mF), male and female dispersal patterns (dispersal/philopatry), and sexual dimorphism (male biased/female biased). Values for species represented by multiple populations were obtained by either taking averages or medians (for continuous and categorical variables, respectively).
(b). Statistical analyses
To control for the potentially confounding influence of phylogenetic relatedness among species, phylogenetic generalized least-squared analyses (PGLS) were conducted, using the ‘Caper’ [51] package in R v. 2.15.2 [52]. Maximum-likelihood estimation was used in all models to most appropriately incorporate the magnitude of the phylogenetic signal λ (i.e. the trait similarity between species owing to a shared common ancestry, assuming a Brownian model of evolution; [53,54]).
A Bayesian consensus tree incorporating an initial 124 species was obtained from the 10K Trees Project (v. 3; [55]) and extended with the most recent phylogenies for Presbytis spp. and the Saguinus nigricollis group [56,57]. Simias concolor and Presbytis siamensis were added following van Woerden et al. [58] so that the complete phylogenetic tree contained 135 species (see the electronic supplementary material, figure S1). Where required, data were transformed prior to analyses using the appropriate procedure (arcsine square-root for proportional data, natural log for continuous variables and (non-zero) count data; [59]). Statistical analyses were conducted in three consecutive steps.
First, a multivariate model was constructed to express the proportion of home range overlap as a function of key socio-ecological variables. As is common with comparative datasets, however, not all variables were available for each species. This implied that nested models were based on samples differing in both the number and composition of species, rendering the use of standard model selection procedures [60] uninformative. Instead, findings of the full model were corroborated by a follow-up model that only retained the significant predictor variables identified by both multivariate and univariate models. This approach allowed us to estimate effect sizes as accurately as possible by maximizing sample size. Second, given the outcome of the first analysis, additional social variables (number of males and females, social structure of a group, identity and number of individuals (one/multiple) of the larger sex) were investigated for possible relationships with home range overlap, always controlling for the confounding effect of home range defendability. Third, for species living in mM–mF groups (which showed the highest levels of home range overlap) a model was constructed to assess whether the dispersal pattern of the dominant sex (proxied by whether sexual dimorphism was male or female biased) affected home range overlap, again controlling for the variation already accounted for by the economic defendability of home ranges.
3. Results
Information on home range overlap was collated from well over 200 study populations, representing a total of 135 social primate species. A highly significant PGLS model (λML = 0.663, F7,109 = 5.34, p < 0.0001; table 1) showed that home range overlap across the primate taxon was determined by group size (t = 4.27, p < 0.0001) and economic defendability of the home range (t = −2.02, p < 0.05): groups containing more animals experienced more overlap, whereas economically more defendable ranges were found to overlap less. None of the other variables in the model explained a significant amount of variation (see also electronic supplementary material, figure S2 for Bonferroni-corrected univariate analyses). To maximize the number of species against which to assess this finding, a follow-up analysis considered only the two significant predictor variables identified by the full model. This not only corroborated the initial result (λML = 0.728, F3,123 = 16.19, p < 0.0001; electronic supplementary material, table S1), but also revealed that the unique proportion of the total variance accounted for by group size (ΔR2 = 0.142, t = 4.67, p < 0.0001) was much higher than that explained by economic defendability (ΔR2 = 0.032, t =−2.44, p < 0.05). Home range overlap in social primates is thus strongly affected by social factors on top of the expected influence of the economic defendability of a ranging area, which is highly suggestive of the presence of a CAP in the context of collective range defence.
Table 1.
Socio-ecological determinants of home range overlap in 116 species of non-human primate for which information was available on all variables. (Parameter estimates (B) and significance values as obtained from a PGLS analysis. λML = 0.663; R2Adj = 0.185, F7,109 = 5.34 and p < 0.0001. Italics denote p-values <0.05.)
| variable | B | s.e. | t-value | p-value |
|---|---|---|---|---|
| intercept | 0.187 | 0.30 | 0.63 | 0.5333 |
| group size | 0.226 | 0.05 | 4.27 | <0.0001 |
| defendability | −0.110 | 0.05 | −2.02 | 0.0463 |
| activity period | −0.068 | 0.19 | −0.36 | 0.7226 |
| substrate use | −0.069 | 0.12 | −0.59 | 0.5502 |
| habitat type | −0.011 | 0.16 | −0.07 | 0.9450 |
| diet | −0.072 | 0.13 | −0.55 | 0.5867 |
We next looked in more detail into the social conditions under which a CAP can emerge. While controlling for differences in economic defendability, home range overlap was found to increase with both the number of adult males (t = 3.40, p =< 0.001) and females (t = 4.58, p < 0.0001) in a group (see the electronic supplementary material, table S2). The interpretation of this finding, that CAPs can emerge in both sexes, however, was confounded by strong collinearities between the number of males and females in a group (nspecies = 130, rPearson = 0.906, p < 0.0001) and total group size (males: nspecies = 132, rPearson = 0.880, p < 0.0001; females: nspecies = 130, rPearson = 0.952, p < 0.0001). A more informative result, was obtained by comparing species with a sM–sF social structure (in which a CAP among same-sex individuals is not possible) to all other species (PGLS: λML = 0.754, F5,121 = 6.47, p < 0.0001; table 2a and figure 1). The difference in home range overlap between species living in sM–sF (mean ± s.e. = 22.44 ± 3.76%) and mM–mF (mean ± s.e. = 55.70 ± 4.23%) groups reached statistical significance (t = 2.82, p < 0.01), whereas differences between sM–sF species on the one hand, and sM–mF (mean ± s.e. = 33.76 ± 4.49%) or mM–sF (mean ± s.e. = 20.41 ± 7.01%) on the other, did not (t = 0.50, p = 0.62 and t =−0.72, p = 0.47, respectively). Thus, merely having multiple same-sex individuals in a group is not a sufficient (albeit necessary) condition for a CAP to emerge. We, therefore, asked next whether the relative dominance of the sex represented by multiple individuals affected home range overlap. The model constructed to answer this question (PGLS: λML = 0.756, F4,108 = 5.27, p < 0.001; table 2b) revealed that, regardless of which sex is larger (t = 0.70, p = 0.48; figure 2a), having multiple individuals of this sex reside within a group is indeed associated with increased levels of home range overlap (t = 2.86, p < 0.01; figure 2b).
Table 2.
The effect of social structure (a), and the sex and number of individuals of the larger sex in the group (b) on home range overlap in 126 and 112 species of non-human primate, respectively. (Parameter estimates (B) and significance values as obtained from PGLS analyses. Italics denote p-values <0.05.)
| variable | B | s.e. | t-value | p-value |
|---|---|---|---|---|
| (a) λML = 0.754; R2Adj = 0.149, F5,121 = 6.47, p < 0.0001 | ||||
| intercept | 0.502 | 0.26 | 1.96 | 0.0528 |
| social structure: | ||||
| sM–sF | — | — | — | — |
| sM–mF | 0.053 | 0.11 | 0.50 | 0.6191 |
| mM–sF | −0.099 | 0.14 | −0.72 | 0.4741 |
| mM–mF | 0.279 | 0.10 | 2.82 | 0.0056 |
| controlling for: | ||||
| defendability | −0.151 | 0.05 | −2.89 | 0.0046 |
| (b) λML = 0.756; R2Adj = 0.103, F4,108 = 5.27, p < 0.001 | ||||
| intercept | 0.469 | 0.27 | 1.74 | 0.0855 |
| IDlarger sex: | ||||
| male | — | — | — | — |
| female | 0.065 | 0.09 | 0.70 | 0.4837 |
| nlarger sex: | ||||
| one | — | — | — | — |
| multiple | 0.194 | 0.07 | 2.86 | 0.0050 |
| controlling for: | ||||
| defendability | −0.133 | 0.06 | −2.34 | 0.0212 |
Figure 1.
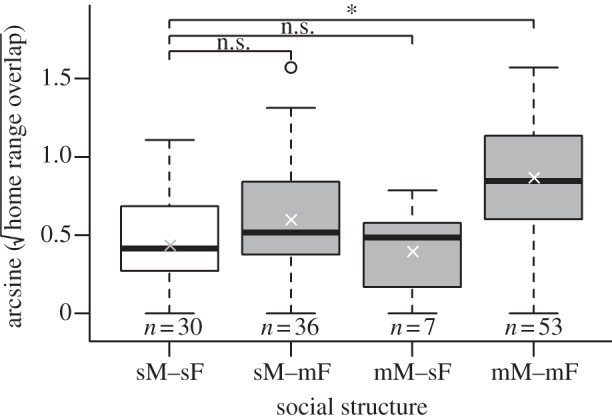
PGLS comparisons of home range overlap between the different social structures of non-human social primates. Taking species with sM–sF groups as reference group, only mM–mF species exhibited significantly increased levels of home range overlap, consistent with the presence of a CAP in range defence. This and all other analyses controlled for differences in the economic defendability of home ranges. Medians (thick lines), means (crosses) and extreme values (open circles), along with the number of species (n) are displayed here, as in all figures.
Figure 2.

(a) The effect of identity and (b) number of individuals of the larger sex in a group on home range overlap across the primate taxon. A PGLS model revealed that the number of individuals of the larger sex, but not their identity (male/female), affects whether a collective action problem (CAP) arises.
Finally, we were interested in whether the dispersal pattern of the dominant sex (assumed to typically be the larger sex) could affect home range overlap among species that suffer from CAPs in the collective defence of ranging areas (i.e. those living in groups with a mM–mF social structure; see the electronic supplementary material, figure S3). A significant model (PGLS: λML = 0.000, F3,46 = 5.09, p < 0.005; table 3 and figure 3) showed home range overlap among mM–mF species with habitual dispersal by the larger sex (mean ± s.e. = 61.47 ± 4.49%) to be higher than that of species in which the larger sex remained philopatric (mean ± s.e. = 30.58 ± 7.73%). Note that maximum-likelihood estimation of the phylogenetic signal in this analyses (λML) indicated complete independence among species (pλ = 0 = 1.00, whereas pλ = 1 < 0.0001), reflecting the repeated and independent evolution of male philopatry and female dispersal (the derived dispersal patterns amongst mammals: [61]) within the primate lineage. Moreover, a post hoc analysis confirmed that average home range overlap among species living in mM–mF groups characterized by philopatry of the larger sex, was not significantly different from that of sM–sF species, whereas that of mM–mF species in which the larger sex disperses, was (PGLS: λML = 0.000, F4,75 = 14.36, p < 0.0001; electronic supplementary material, table S3).
Table 3.
The effect on home range overlap in 49 species with mM–mF groups of the dispersal pattern of the larger sex. (Parameter estimates (B) and significance values as obtained from a PGLS analysis. λML = 0; R2Adj = 0.146, F3,46 = 5.09, p < 0.005. Italics denote p-values <0.05.)
| variable | B | s.e. | t-value | p-value |
|---|---|---|---|---|
| intercept | 0.946 | 0.06 | 16.24 | <0.0001 |
| dispersal pattern: | ||||
| dispersal | — | — | — | — |
| philopatry | −0.387 | 0.12 | −3.10 | 0.0033 |
| controlling for: | ||||
| defendability | −0.057 | 0.08 | −0.74 | 0.4650 |
Figure 3.

The effect of the dispersal pattern of the larger sex on home range overlap in species with mM–mF groups. A PGLS analysis found home range overlap to be significantly lower in species in which the larger sex is philopatric. A follow-up analysis moreover revealed that levels of home range overlap in species in which the larger sex remains philopatric, were not different from baseline (see the electronic supplementary material, table S3), strongly suggesting the CAP was effectively overcome.
4. Discussion
In this study, we explored the proposition that the mismatch between predictions from classic ecological theory on animal territoriality [1,2] and observations on range defence in many group-living species can be attributed to an inherent complication of sociality: the need for cooperation at the group level. In social primates, we found that social factors (group size) affected the effectiveness of collective range defence as proxied by home range overlap more than the economic defendability of a ranging area. More specifically, a series of phylogenetic comparative analyses indicated that, while accounting for economic defendability, co-residence of multiple individuals of the larger sex within a group was associated with increased home range overlap. We interpret our findings as strong evidence for the presence of a CAP [26] in the context of range defence across the primate lineage. In addition, we identify a life-history trait that has effectively enabled certain species to overcome this CAP. Below, we first recapitulate our main results before proposing that the ‘territorial tragedy of the commons’ we describe (i.e. the reduction in size of the defended area of exclusive use, owing to a CAP), may explain many of the discrepancies between current ecological theory and empirical data on the economics of territoriality in group-living animals.
In accordance with Olson's central thesis on the logic of collective action in human groups [26], our first analysis confirmed that, also in non-human primates, larger groups are less effective than smaller groups in securing a common good (here, a collectively defended area of exclusive use, or territory). As in humans and other social animals [27,28], this observed breakdown of collective action at the group level in non-human primates can be attributed to increasing levels of free-riding and diminishing returns to participating individuals in larger groups [35].
Our second set of analyses subsequently established that both males and females can fall prey to this CAP. Our findings, however, cautioned that merely having multiple same-sex individuals (agents with similar selfish interests) within a group did not necessarily result in a territorial tragedy: neither species with sM–mF nor mM–sF groups showed elevated levels of home range overlap from species in which CAPs cannot occur (figure 1). We hypothesized that this could be attributed to the inherent dissimilarities in the competitive abilities of the sexes owing to sexual dimorphism [36,62], and reasoned that where one sex is larger than the other, this can subdue the expression of range defence behaviour by the smaller sex, along with any potential CAP therein. In line with this suggestion, the presence of multiple individuals of the larger sex (male or female) in a group was associated with increased home range overlap (figure 2). From this we inferred that, collective range defence by both males and females is prone to a breakdown in cooperation to the detriment of all, owing to a CAP, unless prevented by interference by the other, larger sex. In this respect, males in species living in sM–mF social groups can be said to provide a double service to their females: not only can they defend access to resources more efficiently because of their larger body size or more elaborate weaponry (the ‘hired-guns’-hypothesis: [16]), but also because there is no risk of a CAP emerging.
As the magnitude of the observed territorial tragedy in species living in mM–mF groups was very large (home range overlap was more than twice as high as in species with sM–sF groups: mean ± s.e. = 55.70 ± 4.23% and 22.44 ± 3.76%, respectively), we subsequently asked whether in some of these species mechanisms have evolved through which the breakdown of cooperation in range defence can be avoided. We found that, of all species in which multiple individuals of the larger sex co-reside in the modal social unit, those in which the larger sex remains philopatric did not suffer from significantly increased home range overlap, whereas species in which the larger sex disperses, did.
A striking observation here was that in eight out of 10 species in which the CAP is overcome through philopatry of the larger sex (three Ateles spp., Brachyteles arachnoides, Lagothrix lagotricha and three Pan (sub-)spp.), males are the cooperating sex (the remaining species (in which females are the dominant sex) being Eulemur macaco flavifrons and L. catta). As males compete for a non-shareable resource (fertilizations), one would not expect them to cooperate. However, in all eight cases, the species live in a social system with high fission–fusion dynamics in which individual males cannot possibly protect local females and offspring on their own. In the absence of consistent female gregariousness, therefore, male cooperative defence of a communal range may be the evolutionarily stable strategy [63], with the potential costs of within-community cooperation readily offset by the benefits accrued through between-community competition (trait-group or multi-level selection: [42,45]). In three of these four genera (Ateles: [64], Brachyteles: [65], Pan: [66]) free-riding may furthermore be held in check by the fact that lone males are vulnerable to lethal attacks by raiding parties of neighbouring communities, with the fourth genus (Lagothrix) remaining relatively poorly studied in the wild. As a result, cooperation becomes all but mutualistic [40], especially when additional indirect fitness benefits accrue owing to philopatry of the cooperating sex. Maximum-likelihood estimates of the phylogenetic signal in our final analyses (λML = 0.000, p = 1.00), indeed suggest that philopatry of the larger sex, which in most primate species are males, has evolved independently in these taxa.
Our results clearly show that the CAP affects the territory economics of many social primates (approx. 30% of species in our sample), resulting in a sub-optimal defence of a common range or territory. As a priori there is no compelling reason to assume that social primates are unique amongst animals living in stable mixed-sex groups, we conclude that CAPs are likely to be a major evolutionary and ecological force in the social dynamics of many group-living taxa, including their territorial behaviour. Extrapolating this to our own species, we observe that, either through shared ancestry with Pan or independently, male bonding and philopatry in societies with high fission–fusion tendencies also characterize modal group dynamics in Homo sapiens, with coalitionary lethal raids into the territories of neighbouring groups occurring in even the most ancient of human societies [67]. Overcoming the CAP in territory economics, through similar evolutionary pathways as other highly social primates, may thus very well have brought ‘war before civilization’ [67] to our species.
Acknowledgements
We thank Janneke van Woerden, Karin Isler and Natasha Arora for instructive discussions on phylogenetic comparative methods, and help in locating the most appropriate and recent primate phylogenies. We also acknowledge Ralph Schwarz for constructing a preliminary database. This study would never have been possible without the efforts and hard work of many field primatologists over the last four decades, and we thank all authors of the original studies that (unwittingly) contributed data to this study. This research was financially supported by the Swiss National Science Foundation (Sinergia grant CRSI33_133040 to C.P.v.S.) and the University of Zurich's Forschungskredit (postdoctoral grant to E.P.W.).
References
- 1.Brown JL. 1964. The evolution of diversity in avian territorial systems. Wilson Bull. 76, 160–169 [Google Scholar]
- 2.Davies NB, Houston AI. 1984. Territory economics. In Behavioural ecology: an evolutionary approach (eds Krebs JR, Davies NB.), pp. 148–169, 2nd edn Oxford, UK: Blackwell Scientific Publications [Google Scholar]
- 3.Milinski M, Parker GA. 1991. Competition for resources. In Behavioural ecology: an evolutionary approach (eds Krebs JR, Davies NB.), pp. 137–168, 3rd edn Oxford, UK: Blackwell Scientific Publications [Google Scholar]
- 4.Grant JWA, Chapman CA, Richardson KS. 1992. Defended versus undefended home range size of carnivores, ungulates and primates. Behav. Ecol. Sociobiol. 31, 149–161 10.1007/bf00168642 (doi:10.1007/bf00168642) [DOI] [Google Scholar]
- 5.Campbell RD, Rosell F, Nolet BA, Dijkstra VAA. 2005. Territory and group sizes in Eurasian beavers (Castor fiber): echoes of settlement and reproduction? Behav. Ecol. Sociobiol. 58, 597–607 10.1007/s00265-005-0942-6 (doi:10.1007/s00265-005-0942-6) [DOI] [Google Scholar]
- 6.Schradin C. 2004. Territorial defense in a group-living solitary forager: who, where, against whom? Behav. Ecol. Sociobiol. 55, 439–446 10.1007/s00265-003-0733-x (doi:10.1007/s00265-003-0733-x) [DOI] [Google Scholar]
- 7.Waser PM, Homewood K. 1979. Cost-benefit approaches to territoriality: a test with forest primates. Behav. Ecol. Sociobiol. 6, 115–119 10.1007/BF00292557 (doi:10.1007/BF00292557) [DOI] [Google Scholar]
- 8.van Schaik CP, Assink PR, Salafsky N. 1992. Territorial behavior in Southeast Asian langurs: resource defense or mate defense? Am. J. Primatol. 26, 233–242 10.1002/ajp.1350260402 (doi:10.1002/ajp.1350260402) [DOI] [PubMed] [Google Scholar]
- 9.Cowlishaw G. 1995. Behavioural patterns in baboon group encounters: the role of resource competition and male reproductive strategies. Behaviour 132, 75–86 10.1163/156853995X00298 (doi:10.1163/156853995X00298) [DOI] [Google Scholar]
- 10.Jolly A, Rasamimanana R, Kinnaird MF, O'Brien TG, Crowley HM, Harcourt CS, Gardner S, Davidson JM. 1993. Territoriality in Lemur catta groups during the birth season at Berenty, Madagascar. In Lemur social sytems and their ecological basis (eds Kappeler PM, Ganzhorn JU.), pp. 85–110 London, UK: Plenum Press [Google Scholar]
- 11.Sugiura H, Saito C, Sato S, Agetsuma N, Takahashi H, Tanaka T, Furuichi T, Takahata Y. 2000. Variation in intergroup encounters in two populations of Japanese macaques. Int. J. Primatol. 21, 519–535 10.1023/a:1005448120967 (doi:10.1023/a:1005448120967) [DOI] [Google Scholar]
- 12.Trivers RL. 1972. Parental investment and sexual selection. In Sexual selection and the descent of man (ed. Campbell P.), pp. 136–179 Chicago, IL: Aldine [Google Scholar]
- 13.Emlen ST, Oring LW. 1977. Ecology, sexual selection, and evolution of mating systems. Science 197, 215–223 10.1126/science.327542 (doi:10.1126/science.327542) [DOI] [PubMed] [Google Scholar]
- 14.Grinnell J. 2002. Modes of cooperation during territorial defense by African lions. Hum. Nat. 13, 85–104 10.1007/s12110-002-1015-4 (doi:10.1007/s12110-002-1015-4) [DOI] [PubMed] [Google Scholar]
- 15.Boydston EE, Morelli TL, Holekamp KE. 2001. Sex differences in territorial behavior exhibited by the spotted hyena (Hyaenidae, Crocuta crocuta). Ethology 107, 369–385 10.1046/j.1439-0310.2001.00672.x (doi:10.1046/j.1439-0310.2001.00672.x) [DOI] [Google Scholar]
- 16.Wrangham R, Rubenstein DI. 1986. Social evolution in birds and mammals. In Ecological aspects of social evolution: birds and mammals (eds Rubenstein DI, Wrangham R.), pp. 452–470 Princeton, NJ: Princeton University Press [Google Scholar]
- 17.Cheney DL. 1981. Inter-group encounters among free-ranging vervet monkeys. Folia Primatol. 35, 124–146 10.1159/000155970 (doi:10.1159/000155970) [DOI] [PubMed] [Google Scholar]
- 18.Adams ES. 1990. Boundary disputes in the territorial ant Azteca trigona: effects of asymmetries in colony size. Anim. Behav. 39, 321–328 10.1016/s0003-3472(05)80877-2 (doi:10.1016/s0003-3472(05)80877-2) [DOI] [Google Scholar]
- 19.Tanner CJ. 2006. Numerical assessment affects aggression and competitive ability: a team-fighting strategy for the ant Formica xerophila. Proc. R. Soc. B 273, 2737–2742 10.1098/rspb.2006.3626 (doi:10.1098/rspb.2006.3626) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woolfenden GE, Fitzpatrick JW. 1977. Dominance in the Florida scrub jay. Condor 79, 1–12 10.2307/1367524 (doi:10.2307/1367524) [DOI] [Google Scholar]
- 21.Carlson A. 1986. Group territoriality in the rattling cisticola, Cisticola chiniana. Oikos 47, 181–189 10.2307/3566044 (doi:10.2307/3566044) [DOI] [Google Scholar]
- 22.Mosser A, Packer C. 2009. Group territoriality and the benefits of sociality in the African lion, Panthera leo. Anim. Behav. 78, 359–370 10.1016/j.anbehav.2009.04.024 (doi:10.1016/j.anbehav.2009.04.024) [DOI] [Google Scholar]
- 23.Furrer RD, Kyabulima S, Willems EP, Cant MA, Manser MB. 2011. Location and group size influence decisions in simulated intergroup encounters in banded mongooses. Behav. Ecol. 22, 493–500 10.1093/beheco/arr010 (doi:10.1093/beheco/arr010) [DOI] [Google Scholar]
- 24.Cheney DL. 1987. Interactions and relationships between groups. In Primate societies (eds Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT.), pp. 267–281 Chicago, IL: University of Chicago Press [Google Scholar]
- 25.Kitchen DM, Cheney DL, Seyfarth RM. 2004. Factors mediating inter-group encounters in savannah baboons (Papio cynocephalus ursinus). Behaviour 141, 197–218 10.1163/156853904322890816 (doi:10.1163/156853904322890816) [DOI] [Google Scholar]
- 26.Olson M. 1965. The logic of collective action: public goods and the theory of groups. Cambridge, MA: Harvard University Press [Google Scholar]
- 27.Heinsohn R, Packer C. 1995. Complex cooperative strategies in group-territorial African lions. Science 269, 1260–1262 10.1126/science.7652573 (doi:10.1126/science.7652573) [DOI] [PubMed] [Google Scholar]
- 28.Nunn CL, Lewis RJ. 2001. Cooperation and collective action in animal behaviour. In Economics in nature (eds Noë R, van Hooff JARAM, Hammerstein P.), pp. 42–66 Cambridge, UK: Cambridge University Press [Google Scholar]
- 29.Kitchen DM, Beehner JC. 2006. Factors affecting individual participation in group-level aggression among non-human primates. Behaviour 144, 1551–1581 10.1163/156853907782512074 (doi:10.1163/156853907782512074) [DOI] [Google Scholar]
- 30.Hardin G. 1968. The tragedy of the commons. Science 162, 1243–1248 10.1126/science.162.3859.1243 (doi:10.1126/science.162.3859.1243) [DOI] [PubMed] [Google Scholar]
- 31.Rankin DJ, Bargum K, Kokko H. 2007. The tragedy of the commons in evolutionary biology. Trends Ecol. Evol. 22, 643–651 10.1016/j.tree.2007.07.009 (doi:10.1016/j.tree.2007.07.009) [DOI] [PubMed] [Google Scholar]
- 32.van Schaik CP. 1996. Social evolution in primates: the role of ecological factors and male behaviour. In Evolution of social behaviour patterns in primates and man (eds Runciman WG, Maynard Smith J, Dunbar RIM.), pp. 9–32 Oxford, UK: Oxford University Press [Google Scholar]
- 33.Nunn CL, Deaner RO. 2004. Patterns of participation and free riding in territorial conflicts among ringtailed lemurs (Lemur catta). Behav. Ecol. Sociobiol. 57, 50–61 10.1007/s00265-004-0830-5 (doi:10.1007/s00265-004-0830-5) [DOI] [Google Scholar]
- 34.Crofoot MC, Gilby IC. 2012. Cheating monkeys undermine group strength in enemy territory. Proc. Natl Acad. Sci. USA 109, 501–505 10.1073/pnas.1115937109 (doi:10.1073/pnas.1115937109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nunn CL. 2000. Collective benefits, free-riders, and male extra-group conflict. In Primate males (ed. Kappeler PM.), pp. 192–204 Cambridge, UK: Cambridge University Press [Google Scholar]
- 36.Clutton-Brock TH, Harvey PH. 1977. Primate ecology and social organization. J. Zool. 183, 1–39 10.1111/j.1469-7998.1977.tb04171.x (doi:10.1111/j.1469-7998.1977.tb04171.x) [DOI] [Google Scholar]
- 37.Mitani JC, Rodman PS. 1979. Territoriality: the relation of ranging pattern and home range size to defendability, with an analysis of territoriality among primate species. Behav. Ecol. Sociobiol. 5, 241–251 10.1007/BF00293673 (doi:10.1007/BF00293673) [DOI] [Google Scholar]
- 38.Lowen C, Dunbar RIM. 1994. Territory size and defendability in primates. Behav. Ecol. Sociobiol. 35, 347–354 10.1007/BF00184423 (doi:10.1007/BF00184423) [DOI] [Google Scholar]
- 39.Waser PM. 1976. Cercocebus albigena: site attachment, avoidance, and intergroup spacing. Am. Nat. 110, 911–935 10.1086/283117 (doi:10.1086/283117) [DOI] [Google Scholar]
- 40.Clutton-Brock T. 2002. Breeding together: kin selection and mutualism in cooperative vertebrates. Science 296, 69–72 10.1126/science.296.5565.69 (doi:10.1126/science.296.5565.69) [DOI] [PubMed] [Google Scholar]
- 41.Sachs Joel L, Mueller Ulrich G, Wilcox Thomas P, Bull James J. 2004. The evolution of cooperation. Q. Rev. Biol. 79, 135–160 10.1086/383541 (doi:10.1086/383541) [DOI] [PubMed] [Google Scholar]
- 42.Dugatkin LA. 1997. The evolution of cooperation. Bioscience 47, 355–362 10.2307/1313150 (doi:10.2307/1313150) [DOI] [Google Scholar]
- 43.Hamilton WD. 1964. Genetical evolution of social behaviour I, II. J. Theor. Biol. 7, 1–52 10.1016/0022-5193(64)90038-4 (doi:10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- 44.Connor RC. 1995. Altruism among non-relatives: alternatives to the ‘Prisoner's Dilemma’. Trends Ecol. Evol. 10, 84–86 10.1016/s0169-5347(00)88988-0 (doi:10.1016/s0169-5347(00)88988-0) [DOI] [PubMed] [Google Scholar]
- 45.Wilson David S, Wilson Edward O. 2007. Rethinking the theoretical foundation of sociobiology. Q. Rev. Biol. 82, 327–348 10.1086/522809 (doi:10.1086/522809) [DOI] [PubMed] [Google Scholar]
- 46.Clutton-Brock T. 2009. Cooperation between non-kin in animal societies. Nature 462, 51–57 10.1038/nature08366 (doi:10.1038/nature08366) [DOI] [PubMed] [Google Scholar]
- 47.Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT. 1987. Primate societies. Chicago, IL: University of Chicago Press [Google Scholar]
- 48.Nunn CL, van Schaik CP. 2002. A comparative approach to reconstructing the socioecology of extinct primates. In Reconstructing behavior in the primate fossil record (eds Plavcan JM, Kay RF, Jungers WL, van Schaik CP.), pp. 159–215, 1st edn New York, NY: Kluwer Academic/Plenum Publishers [Google Scholar]
- 49.Campbell CJ, Fuentes A, McKinnon KC, Bearder SK, Stumpf RM. 2011. Primates in perspective, 2nd edn New York, NY: Oxford University Press [Google Scholar]
- 50.Rowe N, Myers M. 2012. All the World's primates. Rhode Island, RI: Primate Conservation Inc; See http://www.alltheworldsprimates.org. [Google Scholar]
- 51.Orme D, Freckleton R, Thomas G, Petzoldt T, Fritz S, Isaac N, Pearse W. 2012. Caper: comparative analyses of phylogenetics and evolution in R, v. 05. See http://cran.r-project.org/package=caper. [Google Scholar]
- 52.R Development Core Team 2012. R: a language and environment for statistical computing, 2.12.1 edn Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 53.Freckleton RP, Harvey PH, Pagel M. 2002. Phylogenetic analysis and comparative data: a test and review of evidence. Am. Nat. 160, 712–726 10.1086/343873 (doi:10.1086/343873) [DOI] [PubMed] [Google Scholar]
- 54.Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401, 877–884 10.1038/44766 (doi:10.1038/44766) [DOI] [PubMed] [Google Scholar]
- 55.Arnold C, Matthews LJ, Nunn CL. 2010. The 10kTrees website: a new online resource for primate phylogeny. Evol. Anthropol. 19, 114–118 10.1002/evan.20251 (doi:10.1002/evan.20251) [DOI] [Google Scholar]
- 56.Meyer D, Rinaldi ID, Ramlee H, Perwitasari-Farajallah D, Hodges JK, Roos C. 2011. Mitochondrial phylogeny of leaf monkeys (genus Presbytis, Eschscholtz, 1821) with implications for taxonomy and conservation. Mol. Phylogenet. Evol. 59, 311–319 10.1016/j.ympev.2011.02.015 (doi:10.1016/j.ympev.2011.02.015) [DOI] [PubMed] [Google Scholar]
- 57.Matauschek C, Roos C, Heymann EW. 2011. Mitochondrial phylogeny of tamarins (Saguinus, Hoffmannsegg 1807) with taxonomic and biogeographic implications for the S. nigricollis species group. Am. J. Phys. Anthropol. 144, 564–574 10.1002/ajpa.21445 (doi:10.1002/ajpa.21445) [DOI] [PubMed] [Google Scholar]
- 58.van Woerden JT, Willems EP, van Schaik CP, Isler K. 2012. Large brains buffer energetic effects of seasonal habitats in catarrhine primates. Evolution 66, 191–199 10.1111/j.1558-5646.2011.01434.x (doi:10.1111/j.1558-5646.2011.01434.x) [DOI] [PubMed] [Google Scholar]
- 59.Sokal RR, Rohlf FJ. 1995. Biometry: the principles and practice of statistics in biological research, 3rd edn New York, NY: W. H. Freeman [Google Scholar]
- 60.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference. New York, NY: Springer [Google Scholar]
- 61.Greenwood PJ. 1980. Mating systems, philopatry and dispersal in birds and mammals. Anim. Behav. 28, 1140–1162 10.1016/s0003-3472(80)80103-5 (doi:10.1016/s0003-3472(80)80103-5) [DOI] [Google Scholar]
- 62.Plavcan JM. 2004. Sexual selection, measures of sexual selection, and sexual dimorphism in primates. In Sexual selection in primates: new and comparative perspectives (eds Kappeler PM, van Schaik CP.), pp. 230–252, 1st edn Cambridge, UK: Cambridge University Press [Google Scholar]
- 63.Maynard Smith J, Price GR. 1973. The logic of animal conflict. Nature 246, 15–18 10.1038/246015a0 (doi:10.1038/246015a0) [DOI] [Google Scholar]
- 64.Aureli F, Schaffner CM, Verpooten J, Slater K, Ramos-Fernandez G. 2006. Raiding parties of male spider monkeys: insights into human warfare? Am. J. Phys. Anthropol. 131, 486–497 10.1002/ajpa.20451 (doi:10.1002/ajpa.20451) [DOI] [PubMed] [Google Scholar]
- 65.Talebi MG, Beltrão-Mendes R, Lee PC. 2009. Intra-community coalitionary lethal attack of an adult male southern muriqui (Brachyteles arachnoides). Am. J. Primatol. 71, 860–867 10.1002/ajp.20713 (doi:10.1002/ajp.20713) [DOI] [PubMed] [Google Scholar]
- 66.Mitani JC, Watts DP, Amsler SJ. 2010. Lethal intergroup aggression leads to territorial expansion in wild chimpanzees. Curr. Biol. 20, R507–R508 10.1016/j.cub.2010.04.021 (doi:10.1016/j.cub.2010.04.021) [DOI] [PubMed] [Google Scholar]
- 67.Keeley LH. 1996. War before civilization: the myth of the peaceful savage. New York, NY: Oxford University Press [Google Scholar]


