Abstract
Tarsiers are small nocturnal primates with a long history of fuelling debate on the origin and evolution of anthropoid primates. Recently, the discovery of M and L opsin genes in two sister species, Tarsius bancanus (Bornean tarsier) and Tarsius syrichta (Philippine tarsier), respectively, was interpreted as evidence of an ancestral long-to-middle (L/M) opsin polymorphism, which, in turn, suggested a diurnal or cathemeral (arrhythmic) activity pattern. This view is compatible with the hypothesis that stem tarsiers were diurnal; however, a reversion to nocturnality during the Middle Eocene, as evidenced by hyper-enlarged orbits, predates the divergence of T. bancanus and T. syrichta in the Late Miocene. Taken together, these findings suggest that some nocturnal tarsiers possessed high-acuity trichromatic vision, a concept that challenges prevailing views on the adaptive origins of the anthropoid visual system. It is, therefore, important to explore the plausibility and antiquity of trichromatic vision in the genus Tarsius. Here, we show that Sulawesi tarsiers (Tarsius tarsier), a phylogenetic out-group of Philippine and Bornean tarsiers, have an L opsin gene that is more similar to the L opsin gene of T. syrichta than to the M opsin gene of T. bancanus in non-synonymous nucleotide sequence. This result suggests that an L/M opsin polymorphism is the ancestral character state of crown tarsiers and raises the possibility that many hallmarks of the anthropoid visual system evolved under dim (mesopic) light conditions. This interpretation challenges the persistent nocturnal–diurnal dichotomy that has long informed debate on the origin of anthropoid primates.
Keywords: Tarsius, trichromatic colour vision, opsin polymorphism, activity pattern, nocturnality
1. Introduction
Tarsiers are an enduring source of fascination [1], both because of their many unique features and because they occupy a central position in the primate phylogenetic tree. The genus Tarsius—represented today by a handful of species within insular Southeast Asia—belongs to a relict lineage with an origin approximately 56 Ma [2]. Accordingly, tarsiers are not only allocated to their own family (Tarsiidae) and superfamily (Tarsioidea), but also to their own infraorder (Tarsiiformes). Debate over the phyletic position of tarsiiformes is longstanding [3], but recent phylogenies have unequivocally united tarsiers with anthropoid primates (monkeys, apes and humans) in the semiorder Haplorhini [4,5]. Thus, the functional and behavioural ecology of tarsiers has the potential to inform hypotheses focused on the origin of anthropoid primates [6].
The prevailing view of anthropoid origins emphasizes a fundamental shift from nocturnal to diurnal foraging behaviours, perhaps at a tarsier-like body size of 100 g [6]. Evidence for this view stems in part from derived attributes of the anthropoid visual system. Traits such as highly convergent orbits, high concentrations of retinal cones and ganglion cells, and extreme cortical magnification of foveal regions of the retina are all associated with enhanced visual acuity under diurnal conditions. Yet, the strength of this evidence is weakened in part by living tarsiers, which, though nocturnal, share many of these same traits. For example, tarsiers possess a fovea, a macula lutea and a central retina with relatively high densities of cones ([7–10]; figure 1a). These features have long provoked an important question: are these character traits convergent in tarsiers and anthropoids, and, therefore, unrelated to diurnality, or are they synapomorphic retentions from a diurnal stem haplorhine?
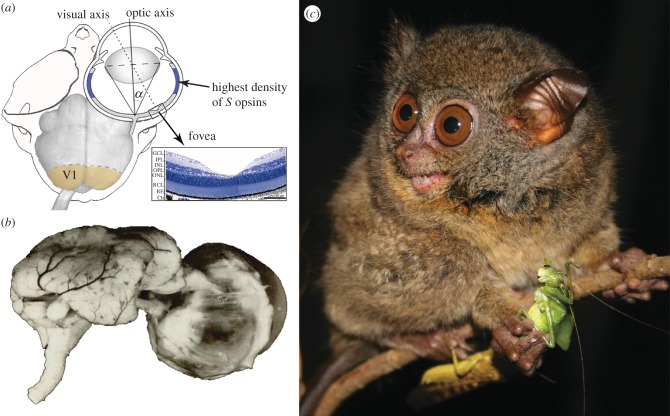
Figure 1.
(a) The skull, eye and brain of Tarsius bancanus. At 21%, tarsiers have proportionally more primary visual cortex (V1) than any other primate [9]; this degree of cortical magnification is associated with foveal acuity and colour vision. The foveal pit of Tarsius spectrum (=T. tarsier) is shown in histological cross section. A curious feature of the retina is the high density of S opsins in the periphery (shown in blue). Redrawn from Hendrickson et al. [7], Ross [8] and Collins et al. [9]. (b) Heinrich Sprankel's preparation of the eye and brain of T. bancanus [11] illustrates the comparable volume of the two structures [12]; thus, the eyes of T. bancanus are enormous, both in absolute size and in proportion to the size of the 120–134 g animal. Polyak [13] concluded that the eye size relative to body size of tarsiers is unmatched by any mammal. (c) A Sulawesi tarsier (Tarsius lariang) on the verge of consuming an orthopteran insect (photograph by Stefan Merker, reproduced with permission).
The consensus view—that stem haplorhines were diurnal and that this heritage is partly obscured by a reversion to nocturnality in tarsiers [3]—helps account for one of the most striking aspects of the tarsier visual system: their outsized eyes (figure 1b; [6]). It has long been suggested that the extreme ocular hypertrophy of tarsiers is related to the absence of a tapetum lucidum (the structure that results in the phenomenon of ‘eye shine’; [14]). Most nocturnal mammals, including lorises and lemurs, possess a tapetum lucidum because it improves visual sensitivity at low light levels. Accordingly, the enlarged eyes of tarsiers are often interpreted as a compensatory trait that functions to improve visual sensitivity, and thus further evidence of a visual system that is secondarily adapted to nocturnality [14–16].
(a). Tarsiers and trichromatic colour vision
Many primates exhibit a colour vision polymorphism that results from allelic variation of the single-locus long-to-middle (L/M) wavelength opsin gene on the X chromosome. Females that are heterozygous for the gene have trichromatic vision, whereas all other individuals possess dichromatic vision. The discovery in 1999 of discrete M and L opsin genes in two sister species, Tarsius bancanus (Bornean tarsier) and Tarsius syrichta (Philippine tarsier), respectively, is provocative because it suggests that alleles of the L/M opsin gene existed in the ancestral population [17]. The heterozygous females of this population probably possessed foveate trichromatic colour vision [17], and it follows that their activity pattern resembled those of living primates with a similar combination of traits. Thus, the last common ancestor of T. bancanus and T. syrichta is hypothesized to have had a diurnal or cathemeral (arrhythmic) activity pattern [17]. This hypothesis is compatible with the view that stem tarsiers were diurnal; however, the evolution of hyper-enlarged orbits during the Middle Eocene [18] and Middle Miocene [19] predates the divergence of T. bancanus and T. syrichta in the Late Miocene [20,21]. Extraordinarily large eyes are strongly suggestive of activity under dark (scotopic) light levels. Taken together, the implication of these findings—that some nocturnal tarsiers possessed high-acuity trichromatic vision—is profound, as it challenges prevailing views on the origin of the anthropoid visual system. It is, therefore, important to explore the plausibility and antiquity of trichromatic colour vision in the genus Tarsius. We do so here by drawing attention to a more basal species from Sulawesi, Tarsius tarsier (figure 1c).
As demonstrated for New World (platyrrhine) monkeys [22], the simultaneous presence of L/M opsin alleles in an ancestral population, and thus the potential for trichromatic colour vision, can be inferred if the coding regions of the genes cluster together irrespective of phylogenetic distance, while intron and synonymous-site trees reflect species relationships. Therefore, if a site tree reconstructed using non-synonymous nucleotide sequences results in T. tarsier clustering more closely with either T. syrichta (in the case of the L opsin gene) or T. bancanus (in the case of the M opsin gene), then this result can be interpreted as evidence of an ancestral polymorphism and fixation, versus independent derivation, of the L/M opsin gene in the various lineages. We test this prediction here by comparing the L/M opsin genes of extant tarsiers.
2. Material and methods
(a). Study subjects and data collection
Genomic DNA was extracted from the buccal cells (Gentra Puregene Buccal Cell Kit, Qiagen) of T. tarsier (one male, two females; n = 5 X chromosomes) housed at the Ueno Zoo, Tokyo. The precise alpha taxonomy of each individual is uncertain because individual provenances in Sulawesi are unknown; however, long tail tufts are an unambiguous character trait of the T. tarsier complex [23,24] (see the electronic supplementary material, figure S1). We also collected buccal DNA from a female Philippine tarsier at the Ueno Zoo (T. syrichta; n = 2 X chromosomes). This sampling protocol conformed to the guidelines of the Ueno Zoo and was approved by the Institutional Animal Care and Use Committee of Dartmouth College (approval no. 11-11-02AT). The genomic DNA of a male Bornean tarsier (T. bancanus; n = 1 X chromosome) was extracted from a fibroblast cell line [25].
For each tarsier, we conducted PCR amplification and sequencing of a continuous section (approx. 4 kb) of the L/M opsin gene containing exon 3, intron 3, exon 4, intron 4 and exon 5. This gene section contains three nucleotide sites that are critical for spectral tuning of the photopigment, encoding residue 180 in exon 3 and residues 277 and 285 in exon 5 (the three-sites rule [26–28]). In contrast to platyrrhine monkeys, polymorphisms are reported for strepsirrhine primates and tarsiers at site 285 only [17,29]; however, variation at any of the three sites can shift photopigment sensitivity and we, therefore, examined each. PCR primers were designed to amplify overlapping segments in this region based on conservation of nucleotide sequences among previously reported L/M opsin genes of other primates (see the electronic supplementary material, figure S2).
PCRs were carried out in volumes of 50 μl containing 1.5 units of Ex Taq polymerase hot start version (Takara, Tokyo, Japan) with 1× Ex Taq Buffer, 0.2 mM of dNTP, 1.5 mM MgCl2, 1 μM of the forward and reverse primers, and ca 1 ng of the tarsier genomic DNA. Cycles were set at 94°C for 5 min followed by 40 cycles at 94°C for 30 s, 62°C for 30 s and 72°C for 30–120 s. Pure water was used as the template for the negative control in every reaction. The amplified DNA fragments were purified using UltraClean 15 DNA Purification Kit (MO BIO Laboratories, Solana Beach, CA, USA). Both strands of the purified DNA samples were directly sequenced using Applied Biosystems model 3130 automatic sequencer with Big Dye Terminator v. 3.1 Cycle Sequencing kit (Applied Biosystems Japan, Tokyo) and the PCR primers and other primers designed inside the amplified region.
The PCR products of the L/M opsin gene from female samples were subjected to DNA cloning to distinguish the X-chromosomal allelic sequences. The amplified DNA fragments were cloned into pGEM-T vectors (Promega, Madison, WI, USA) and were sequenced as described above. We confirmed their nucleotide sequences in duplicate experiments.
Nucleotide sequences were aligned using Clustal W and alignment was refined visually. We used Mega5 [30] to conduct phylogenetic analyses of aligned nucleotide sequences. The number of nucleotide differences was corrected for multiple substitutions by the Juke–Cantor formula [31]. The number of nucleotide substitutions per synonymous site (dS) and per non-synonymous site (dN) for each pair of sequences was estimated using the Nei–Gojobori model [32]. In our calculations, gap sites were removed in a pairwise fashion. Phylogenetic trees were constructed following the neighbour-joining method [33] and their reliabilities were evaluated via bootstrap analyses with 1000 replications.
3. Results
Distinct sequence types were found and a sequence from each species—T. tarsier L no. 1; T. syrichta L no. 1 and T. bancanus M (see the electronic supplementary material, figure S3)—was deposited in the DDBJ/EMBL/GenBank Database under accession nos AB675925, AB675926 and AB675927, respectively. The four sequence types of T. tarsier and both sequence types of T. syrichta were identical in amino acid composition at the three critical tuning sites, having Ala, Tyr and Thr at residues 180, 277 and 285 (designated Ala/Tyr/Thr), respectively. This amino acid composition is in agreement with a previous report on T. syrichta [17], which inferred an L opsin with a peak spectral absorbance (λmax) of 553 nm based on the three-sites rule [27,28]. The amino acid composition of T. bancanus (Ala/Tyr/Ala) also agrees with a previous report [17], which inferred an M opsin with a λmax of 538 nm [27,28].
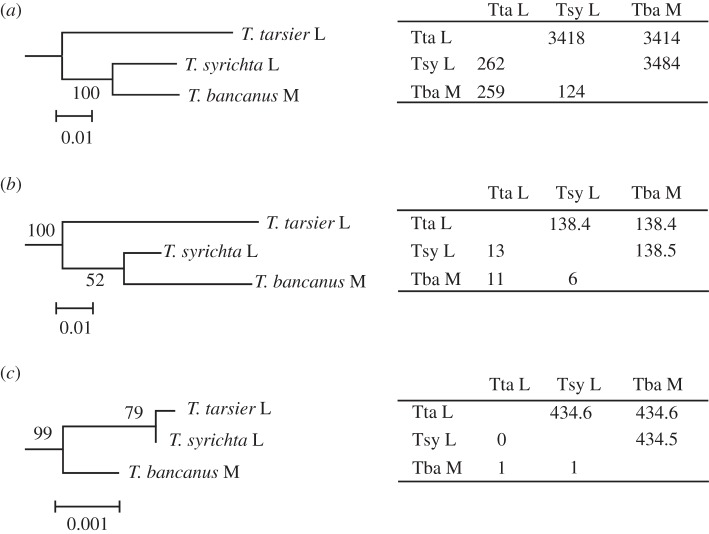
We reconstructed phylogenic trees of the tarsier L/M opsin genes based on introns 3 and 4 (figure 2a) and the synonymous (figure 2b) and non-synonymous sites (figure 2c) of the exons 3, 4 and 5. The intron and synonymous trees agreed well with the established phyletic relationships of tarsiers. The L opsin gene of T. tarsier was cast as the most basal lineage, whereas the M opsin gene of T. bancanus and the L opsin gene of T syrichta were closely related. We used the first sequence listed for each species (see the electronic supplementary material, figure S3) when constructing the tree, but the choice of sequences did not affect the tree topology and reliability (data not presented). Intraspecific differences in the four sequence types of T. tarsier ranged from 0.1 to 1.0 per cent of the intron sites and 0 to 1.5 per cent of the synonymous exon sites. The two sequences of T. syrichta differed in 0.4 per cent of the intron sites and were identical in the synonymous sites. In contrast to the phylogenies based on the intron and synonymous sites, the non-synonymous-site phylogeny grouped the L opsin genes of T. tarsier and T. syrichta with each other, to the exclusion of the M opsin gene of T. bancanus (figure 2c).
Figure 2.
Phylogenetic trees of the tarsier L/M opsin gene using (a) introns 3 and 4, (b) the synonymous sites of exons 3, 4 and 5, and (c) the non-synonymous sites of exons 3, 4 and 5. For each species by species comparison, the length of the nucleotide sequence (bp) used and the number of nucleotides differing between each pair are presented, above and below the diagonal, respectively, in the tables adjacent to each phylogeny. The bootstrap probability is given at each node. We determined the phylogenetic root for tree (a) using the common marmoset L/M opsin gene (Ensembl Marmoset release 70, Genome assembly C_jacchus3.2.1 (GCA 000004665.1) (http://www.ensembl.org/Callithrix_jacchus/Info/Index), X:140703132–140704540 for intron 3 and X:140704707–140706490 for intron 4) and for trees (b) and (c) using three spectral alleles of the common marmoset L/M opsin gene (GenBank AB046546–AB046548) as the out-group for each tree. Scale bars indicate the number of nucleotide substitutions per site.
4. Discussion
We have replicated an earlier report of M opsin genes in Bornean tarsiers (T. bancanus) and L opsin genes in Philippine tarsiers (T. syrichta) [17]. In addition, we have shown that Sulawesi tarsiers (T. tarsier) possess an L opsin gene. In accordance with the species phylogeny, the L opsin gene of T. tarsier fell outside the T. bancanus–T. syrichta cluster in the intron and the synonymous-site trees. However, in the non-synonymous-site tree, the L opsin gene of T. tarsier clustered with the L opsin gene of T. syrichta. This result suggests that alleles of the L/M opsin gene were present in the last common ancestor of crown tarsiers and that polymorphic trichromatic vision persisted until the divergence of T. syrichta and T. bancanus in the Late Miocene [20,21]. This pattern is consistent with homogenization of the alleles in the introns and synonymous sites by recombination, and natural selection (balancing selection) against homogenization of the non-synonymous sites to maintain the spectral polymorphism of the alleles.
An alternate interpretation of our results is that the last common ancestor of crown tarsiers was uniformly dichromatic and similar to all living tarsiers studied to date. If so, based on the intron and synonymous-site trees, the M opsin gene of T. bancanus is most plausibly a derivation of the L opsin gene of T. syrichta by a nucleotide substitution at codon 285. Yet, the L/M opsin genes of each species are otherwise identical at non-synonymous sites throughout exons 3–5 (i.e. in amino acid sequence). It is highly improbable that the only substitution present among the approximately 435 possible non-synonymous sites, and among the three possible directions of nucleotide change, occurred at a spectral tuning site by chance. Given the strict conservation of the amino acid sequence, the chance of a non-synonymous mutation being selectively neutral is doubtful because the fixation probability of a neutral mutation is generally low. Thus, if the M opsin gene is derived from the L opsin gene and not fixed via random drift alone, strong positive selection should have favoured the M opsin gene in Bornean tarsiers. This raises the question of why a shift from deutan to protan dichromacy, with an intermittent stage of polymorphic vision including trichromacy, was adaptive for tarsiers inhabiting the rainforests of Borneo versus those of Sulawesi or the Philippines.
(a). Implications for anthropoid origins
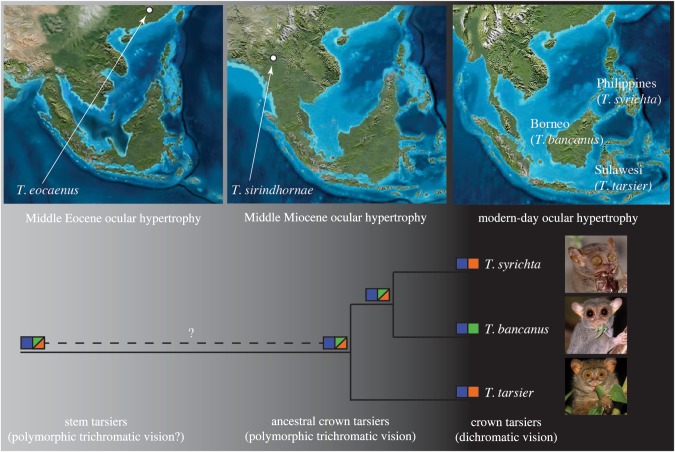
Our results indicate that the last common ancestor of crown tarsiers possessed polymorphic trichromatic vision. Such a finding would normally suggest a diurnal activity pattern because high-acuity trichromatic vision is strongly associated with bright (photopic) conditions; however, this Middle Miocene population of tarsiers [21,34] postdates or is coincident with the existence of hyper-enlarged orbits in the genus ([18,19]; figure 3). The evolution of extraordinary eye size is functionally incompatible with diurnality and inconsistent with cathemerality, an activity pattern that favours intermediate ocular morphologies [35–38]. This paradoxical tandem of polymorphic trichromatic vision and enlarged eyes is, therefore, puzzling, and it raises the possibility that dim (mesopic) light rather than a particular diel pattern was an important factor during tarsier evolution. Indeed, recent findings suggest that full moonlight and twilight are sufficient for cone-mediated colour vision yet dim enough to favour enlarged eyes for greater sensitivity [39].
Figure 3.
The ocular hypertrophy of Tarsius eocaenus [18] and Tarsius sirindhornae [19] predates or is coeval with the inferred L/M cone opsin polymorphism in the last common ancestor of crown tarsiers. Squares depict discrete opsins and their spectral sensitivities. The combination of hyper-enlarged eyes and trichromatic vision during the Middle Miocene suggests an activity pattern that includes dim (mesopic) light levels (indicated in grey). By contrast, contemporary tarsiers are active mainly under dark (scotopic) light levels (indicated in black). Palaeogeographic maps depict Sundaland during the Late Eocene (35 Ma) and Early Miocene (20 Ma) (Ron Blakeley and Colorado Plateau Geosystems, Inc.). The photographs of T. syrichta (Daniel Heuclin), T. bancanus (David Haring) and a member of the T. tarsier complex (=T. lariang; Stefan Merker) are reproduced with permission.
Our findings call attention to a light environment that is under-appreciated in the literature on tarsier visual adaptations and, by extension, the origin of anthropoid primates [40]. Accordingly, some speculation is warranted on why early tarsiers were plausibly active under mesopic light levels. Evidence suggests that Sundaland was exceedingly rainy during the Eocene and Miocene [41,42]; and rain is expected to dampen the foraging efficiency of a sit-and-wait ambush predator by immobilizing invertebrate prey [43] or masking prey movement amid broadband noise [44]. Accordingly, we suggest that rain-induced sensory deprivation led tarsiers to expand their foraging activities under mesopic light levels, conditions that favoured high-acuity stereoscopic vision for detecting motionless prey [45]. Perhaps compellingly, polymorphic trichromatic vision is associated with the consumption of green foods at low light levels [46]; and, it could be advantageous for discriminating green generalist orthopteran insects [47], an important food item for many tarsiers ([48,49]; figure 1c). In addition, tarsiers are themselves vulnerable to predation, especially from twilight-active felids and viverrids [50–53]; trichromatic vision is likely to be advantageous for detecting these russet-coloured predators [54]. Mesopic light, then, could have exerted a strong selective tension on the visual system of tarsiers, favouring an evolutionary compromise between sensitivity and high-acuity polymorphic trichromatic vision. Testing the plausibility of this hypothesis will hinge on several factors, which we detail in the supporting information.
A potential problem with our hypothesis is that extant tarsiers remain active under twilight and bright moonlight [55,56], conditions that appear to be mesopic [39]. Thus, it is unclear why tarsiers were independently released from the selective advantages of trichromatic vision during the past 5 Myr. Although the possibility of allelic fixation owing to stochastic processes such as genetic bottlenecks cannot be ruled out, the parallel loss of polymorphic trichromatic vision in at least three species could be associated with diminished rainfall across insular southeast Asia during the Pleistocene [41]. Reduced rainfall is expected to favour a greater commitment to nocturnality among tarsiers and a reversion to audition as the primary sensory modality. Our suggestion that the selective advantages of visually mediated foraging are relaxed under drier conditions is consistent with field observations in Sulawesi. During the dry season, tarsiers preferentially attend to the rustling of invertebrate prey in leaf litter [48], a broadband acoustic cue [57] to which at least one species is exceedingly sensitive [1].
The tarsiid niche is an exemplary model of long-term stability [58], and we suggest that tarsiers achieved dietary stasis by vacillating between scotopic and mesopic light levels, conditions that would alternately stress their unique auditory and visual systems. Furthermore, we suggest that the canon of haplorhine visual adaptations—convergent orbits, high concentrations of retinal cones and ganglion cells, and extreme cortical magnification of foveal regions of the retina—are perhaps better interpreted as mesopic traits that were preadaptive to a diurnal activity pattern. Although speculative, a shared mesopic origin for the last common ancestor of crown tarsiers and stem anthropoids has the advantage of parsimony over the prevailing hypothesis: that the attributes of the anthropoid visual system evolved when stem haplorhines invaded a diurnal niche, a view that entails a full diurnal–nocturnal reversal during tarsier evolution. The present findings challenge this persistent nocturnal–diurnal dichotomy and shed new (dim) light on the evolution of haplorhine and anthropoid primates.
Acknowledgements
The sampling protocol conformed to the guidelines of the Ueno Zoo and was approved by the Institutional Animal Care and Use Committee of Dartmouth College (approval no. 11-11.02AT).
We are grateful to the administration and staff of the Ueno Zoo for permission to conduct this study and we thank Mackenzie Bergstrom for assistance with sample collection. Financial support was received from the David and Lucile Packard Foundation (Fellowship no. 2007-31754 to N.J.D.), the Japan Society for the Promotion of Science (Grants-in-Aid for Scientific Research A nos. 19207018 and 22247036 to S.K.), and the National Science and Engineering Research Council of Canada (Postdoctoral Fellowship to A.D.M.).
References
- 1.Ramsier MA, Cunningham AJ, Moritz GL, Finneran JJ, Williams CV, Ong PS, Gursky-Doyen SL, Dominy NJ. 2012. Primate communication in the pure ultrasound. Biol. Lett. 8, 508–511 10.1098/rsbl.2011.1149 (doi:10.1098/rsbl.2011.1149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsui A, Rakotondraparany F, Munechika I, Hasegawa M, Horai S. 2009. Molecular phylogeny and evolution of prosimians based on complete sequences of mitochondrial DNAs. Gene 441, 53–66 10.1016/j.gene.2008.08.024 (doi:10.1016/j.gene.2008.08.024) [DOI] [PubMed] [Google Scholar]
- 3.Williams BA, Kay RF, Kirk EC. 2010. New perspectives on anthropoid origins. Proc. Natl Acad. Sci. USA 107, 4797–4804 10.1073/pnas.0908320107 (doi:10.1073/pnas.0908320107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jameson NM, Hou Z-C, Sterner KN, Weckle A, Goodman M, Steiper ME, Wildman DE. 2011. Genomic data reject the hypothesis of a prosimian primate clade. J. Hum. Evol. 61, 295–305 10.1016/j.jhevol.2011.04.004 (doi:10.1016/j.jhevol.2011.04.004) [DOI] [PubMed] [Google Scholar]
- 5.Perelman P, et al. 2011. A molecular phylogeny of living primates. PLoS Genet. 7, e1001342. 10.1371/journal.pgen.1001342 (doi:10.1371/journal.pgen.1001342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross CF. 2000. Into the light: the origin of Anthropoidea. Annu. Rev. Anthropol. 29, 147–194 10.1146/annurev.anthro.29.1.147 (doi:10.1146/annurev.anthro.29.1.147) [DOI] [Google Scholar]
- 7.Hendrickson A, Djajadi HR, Nakamura L, Possin DE, Sajuthi D. 2000. Nocturnal tarsier retina has both short and long/medium-wavelength cones in an unusual topography. J. Comp. Neurol. 424, 718–730 (doi:10.1002/1096-9861(20000904)424:4<718::aid-cne12>3.0.co;2-z) [DOI] [PubMed] [Google Scholar]
- 8.Ross CF. 2004. The tarsier fovea: functionless vestige or nocturnal adaptation? In Anthropoid origins: new visions (eds Ross CF, Kay RF.), pp. 477–537 New York, NY: Kluwer Academic [Google Scholar]
- 9.Collins CE, Hendrickson A, Kaas JH. 2005. Overview of the visual system of Tarsius. Anat. Rec. A 287, 1013–1025 10.1002/ar.a.20263 (doi:10.1002/ar.a.20263) [DOI] [PubMed] [Google Scholar]
- 10.Wong P, Collins CE, Kaas JH. 2010. Overview of sensory systems of Tarsius. Int. J. Primatol. 31, 1002–1031 10.1007/s10764-009-9388-4 (doi:10.1007/s10764-009-9388-4) [DOI] [Google Scholar]
- 11.Sprankel H. 1965. Untersuchungen an Tarsius I. Morphologie des schwanzes nebst ethologischen bemerkungen. Folia Primatol. 3, 153–188 10.1159/000155027 (doi:10.1159/000155027) [DOI] [PubMed] [Google Scholar]
- 12.Castenholz A. 1984. The eye of Tarsius. In Biology of tarsiers (ed. Niemitz C.), pp. 303–318 Stuttgart, Germany: Gustav Fischer Verlag [Google Scholar]
- 13.Polyak S. 1957. The vertebrate visual system. Chicago, IL: University of Chicago Press [Google Scholar]
- 14.Cartmill M. 1980. Morphology, function, and evolution of the anthropoid postorbital septum. In Evolutionary biology of the New World monkeys and continental drift (eds Ciochon RL, Chiarelli AB.), pp. 243–274 New York, NY: Plenum Press [Google Scholar]
- 15.Martin RD. 1990. Primate origins and evolution: a phylogenetic reconstruction. Princeton, NJ: Princeton University Press [Google Scholar]
- 16.Martin RD, Ross CF. 2005. The evolutionary and ecological context of primate vision. In The primate visual system: a comparative approach (ed. Kremers J.), pp. 1–36 Chichester, UK: John Wiley & Sons [Google Scholar]
- 17.Tan Y, Li W-H. 1999. Trichromatic vision in prosimians. Nature 402, 36. 10.1038/46947 (doi:10.1038/46947) [DOI] [PubMed] [Google Scholar]
- 18.Rossie JB, Ni X, Beard KC. 2006. Cranial remains of an Eocene tarsier. Proc. Natl Acad. Sci. USA 103, 4381–4385 10.1073/pnas.0509424103 (doi:10.1073/pnas.0509424103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaimanee Y, Lebrun R, Yamee C, Jaeger J-J. 2011. A new Middle Miocene tarsier from Thailand and the reconstruction of its orbital morphology using a geometric–morphometric method. Proc. R. Soc. B 278, 1956–1963 10.1098/rspb.2010.2062 (doi:10.1098/rspb.2010.2062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meireles CM, Czelusniak J, Page SL, Wildman DE, Goodman M. 2003. Phylogenetic position of tarsiers within the order Primates: evidence from γ-globin DNA sequences. In Tarsiers: past, present, and future (eds Wright PC, Simons EL, Gursky S.), pp. 145–160 New Brunswick, Canada: Rutgers University Press [Google Scholar]
- 21.Shekelle M, Meier R, Wahyu I, Wirdateti W, Ting N. 2010. Molecular phylogenetics and chronometrics of Tarsiidae based on 12S mtDNA haplotypes: evidence for Miocene origins of crown tarsiers and numerous species within the Sulawesian clade. Int. J. Primatol. 31, 1083–1106 10.1007/s10764-010-9457-8 (doi:10.1007/s10764-010-9457-8) [DOI] [Google Scholar]
- 22.Boissinot S, Tan Y, Shyue S-K, Schneider H, Sampaio I, Neiswanger K, Hewett-Emmett D, Li W-H. 1998. Origins and antiquity of X-linked triallelic color vision systems in New World monkeys. Proc. Natl Acad. Sci. USA 95, 13 749–13 754 10.1073/pnas.95.23.13749 (doi:10.1073/pnas.95.23.13749) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill WCO. 1953. Caudal cutaneous specializations in Tarsius. Proc. Zool. Soc. Lond. 123 17–26 10.1111/j.1096-3642.1953.tb00150.x (doi:10.1111/j.1096-3642.1953.tb00150.x) [DOI] [Google Scholar]
- 24.Shekelle M, Groves C, Gursky S, Neri-Arboleda I, Nietsch A. 2008. A method for multivariate analysis and classification of tarsier tail tufts. In Primates of the oriental night (eds Shekelle M, Maryanto I, Groves C, Schulze H, Fitch-Snyder H.), pp. 71–84 Jakarta, Indonesia: LIPI Press [Google Scholar]
- 25.Kawamura S, Kubotera N. 2004. Ancestral loss of short wave-sensitive cone visual pigment in lorisiform prosimians, contrasting with its strict conservation in other prosimians. J. Mol. Evol. 58, 314–321 10.1007/s00239-003-2553-z (doi:10.1007/s00239-003-2553-z) [DOI] [PubMed] [Google Scholar]
- 26.Neitz M, Neitz J, Jacobs GH. 1991. Spectral tuning of pigments underlying red-green color vision. Science 252, 971–974 10.1126/science.1903559 (doi:10.1126/science.1903559) [DOI] [PubMed] [Google Scholar]
- 27.Yokoyama S, Radlwimmer FB. 2001. The molecular genetics and evolution of red and green color vision in vertebrates. Genetics 158, 1697–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hiramatsu C, Radlwimmer FB, Yokoyama S, Kawamura S. 2004. Mutagenesis and reconstitution of middle-to-long-wave-sensitive visual pigments of New World monkeys for testing the tuning effect of residues at sites 229 and 233. Vision Res. 44, 2225–2231 10.1016/j.visres.2004.04.008 (doi:10.1016/j.visres.2004.04.008) [DOI] [PubMed] [Google Scholar]
- 29.Veilleux CC, Bolnick DA. 2009. Opsin gene polymorphism predicts trichromacy in a cathemeral lemur. Am. J. Primatol. 71, 86–90 10.1002/ajp.20621 (doi:10.1002/ajp.20621) [DOI] [PubMed] [Google Scholar]
- 30.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739 10.1093/molbev/msr121 (doi:10.1093/molbev/msr121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nei M, Kumar S. 2000. Molecular evolution and phylogenetics. New York, NY: Oxford University Press [Google Scholar]
- 32.Nei M, Gojobori T. 1986. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol. Biol. Evol. 3, 418–426 [DOI] [PubMed] [Google Scholar]
- 33.Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4, 406–425 [DOI] [PubMed] [Google Scholar]
- 34.Merker S, Driller C, Perwitasari-Farajallah D, Pamungkas J, Zischler H. 2009. Elucidating geological and biological processes underlying the diversification of Sulawesi tarsiers. Proc. Natl Acad. Sci. USA 106, 8459–8464 10.1073/pnas.0900319106 (doi:10.1073/pnas.0900319106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirk EC. 2004. Comparative morphology of the eye in primates. Anat. Rec. A 281A, 1095–1103 10.1002/ar.a.20115 (doi:10.1002/ar.a.20115) [DOI] [PubMed] [Google Scholar]
- 36.Kirk EC, Kay RF. 2004. The evolution of high visual acuity in the Anthropoidea. In Anthropoid origins: new visions (eds Ross CF, Kay RF.), pp. 539–602 New York, NY: Kluwer Academic [Google Scholar]
- 37.Kirk EC. 2006. Effects of activity pattern on eye size and orbital aperture size in primates. J. Hum. Evol. 51, 159–170 10.1016/j.jhevol.2006.02.004 (doi:10.1016/j.jhevol.2006.02.004) [DOI] [PubMed] [Google Scholar]
- 38.Ross CF, Kirk EC. 2007. Evolution of eye size and shape in primates. J. Hum. Evol. 52, 294–313 10.1016/j.jhevol.2006.09.006 (doi:10.1016/j.jhevol.2006.09.006) [DOI] [PubMed] [Google Scholar]
- 39.Melin AD, Moritz GL, Fosbury RAE, Kawamura S, Dominy NJ. 2012. Why aye-ayes see blue. Am. J. Primatol. 74, 185–192 10.1002/ajp.21996 (doi:10.1002/ajp.21996) [DOI] [PubMed] [Google Scholar]
- 40.Ankel-Simons FS, Rasmussen DT. 2008. Diurnality, nocturnality, and the evolution of primate visual systems. Yearb. Phys. Anthropol. 51, 100–117 10.1002/ajpa.20957 (doi:10.1002/ajpa.20957) [DOI] [PubMed] [Google Scholar]
- 41.Morley RJ. 2011. Cretaceous and tertiary climate change and the past distribution of megathermal rainforests. In Tropical rainforest responses to climatic change (eds Bush MB, Flenley JR, Gosling WD.), pp. 1–34 Berlin, Germany: Springer [Google Scholar]
- 42.Clementz MT, Sewall JO. 2011. Latitudinal gradients in greenhouse seawater δ18O: evidence from Eocene sirenian tooth enamel. Science 332, 455–458 10.1126/science.1201182 (doi:10.1126/science.1201182) [DOI] [PubMed] [Google Scholar]
- 43.Niemitz C. 1984. Activity rhythms and use of space in semi-wild Bornean tarsiers, with remarks on wild spectral tarsiers. In Biology of tarsiers (ed. Niemitz C.), pp. 85–115 Stuttgart, Germany: Gustav Fischer Verlag [Google Scholar]
- 44.Brumm H, Slabbekoorn H. 2005. Acoustic communication in noise. Adv. Stud. Behav. 35, 151–209 10.1016/S0065-3454(05)35004-2 (doi:10.1016/S0065-3454(05)35004-2) [DOI] [Google Scholar]
- 45.Heesy CP. 2009. Seeing in stereo: the ecology and evolution of primate binocular vision and stereopsis. Evol. Anthropol. 18, 21–35 10.1002/evan.20195 (doi:10.1002/evan.20195) [DOI] [Google Scholar]
- 46.Yamashita N, Stoner KE, Riba-Hernández P, Dominy NJ, Lucas PW. 2005. Light levels used during feeding by primate species with different color vision phenotypes. Behav. Ecol. Sociobiol. 58, 618–629 10.1007/s00265-005-0936-4 (doi:10.1007/s00265-005-0936-4) [DOI] [Google Scholar]
- 47.Smith AC, Surridge AK, Prescott MJ, Osorio D, Mundy NI, Buchanan-Smith HM. 2012. Effect of colour vision status on insect prey capture efficiency of captive and wild tamarins (Saguinus spp.). Anim. Behav. 83, 479–486 10.1016/j.anbehav.2011.11.023 (doi:10.1016/j.anbehav.2011.11.023) [DOI] [Google Scholar]
- 48.Niemitz C. 1984. Synecological relationships and feeding behaviour of the genus Tarsius. In Biology of tarsiers (ed. Niemitz C.), pp. 59–75 Stuttgart, Germany: Gustav Fischer Verlag [Google Scholar]
- 49.Gursky S. 2000. Effect of seasonality on the behavior of an insectivorous primate, Tarsius spectrum. Int. J. Primatol. 21, 477–495 10.1023/a:1005444020059 (doi:10.1023/a:1005444020059) [DOI] [Google Scholar]
- 50.MacKinnon J, MacKinnon K. 1980. The behavior of wild spectral tarsiers. Int. J. Primatol. 1, 361–379 10.1007/bf02692280 (doi:10.1007/bf02692280) [DOI] [Google Scholar]
- 51.van Schaik CP, Griffiths M. 1996. Activity periods of Indonesian rain forest mammals. Biotropica 28, 105–112 10.2307/2388775 (doi:10.2307/2388775) [DOI] [Google Scholar]
- 52.Gursky S. 2002. Determinants of gregariousness in the spectral tarsier (Prosimian: Tarsius spectrum). J. Zool. 256, 401–410 10.1017/s0952836902000444 (doi:10.1017/s0952836902000444) [DOI] [Google Scholar]
- 53.Řeháková-Petrů M, Peške L, Daněk T. 2012. Predation on a wild Philippine tarsier (Tarsius syrichta). Acta Ethol. 15, 217–220 10.1007/s10211-011-0096-7 (doi:10.1007/s10211-011-0096-7) [DOI] [Google Scholar]
- 54.Caine NG. 2002. Seeing red: consequences of individual differences in color vision in callitrichid primates. In Eat or be eaten: predator sensitive foraging among primates (ed. Miller LE.), pp. 58–73 Cambridge, UK: Cambridge University Press [Google Scholar]
- 55.Crompton RH, Andau PM. 1987. Ranging, activity rhythms, and sociality in free-ranging Tarsius bancanus: a preliminary report. Int. J. Primatol. 8, 43–71 10.1007/bf02737113 (doi:10.1007/bf02737113) [DOI] [Google Scholar]
- 56.Gursky S. 2003. Lunar philia in a nocturnal primate. Int. J. Primatol. 24, 351–367 10.1023/a:1023053301059 (doi:10.1023/a:1023053301059) [DOI] [Google Scholar]
- 57.Goerlitz HR, Greif S, Siemers BM. 2008. Cues for acoustic detection of prey: insect rustling sounds and the influence of walking substrate. J. Exp. Biol. 211, 2799–2806 10.1242/jeb.019596 (doi:10.1242/jeb.019596) [DOI] [PubMed] [Google Scholar]
- 58.Jablonski NG. 2003. The evolution of the tarsiid niche. In Tarsiers: past, present, and future (eds Wright PC, Simons EL, Gursky S.), pp. 35–49 New Brunswick, Canada: Rutgers University Press [Google Scholar]