Abstract
To assess ecological consequences of bushmeat hunting in African lowland rainforests, we compared paired sites, with high and low hunting pressure, in three areas of southeastern Nigeria. In hunted sites, populations of important seed dispersers—both small and large primates (including the Cross River gorilla, Gorilla gorilla diehli)—were drastically reduced. Large rodents were more abundant in hunted sites, even though they are hunted. Hunted and protected sites had similar mature tree communities dominated by primate-dispersed species. In protected sites, seedling communities were similar in composition to the mature trees, but in hunted sites species with other dispersal modes dominated among seedlings. Seedlings emerging 1 year after clearing of all vegetation in experimental plots showed a similar pattern to the standing seedlings. This study thus verifies the transforming effects of bushmeat hunting on plant communities of tropical forests and is one of the first studies to do so for the African continent.
Keywords: bushmeat hunting, seedling community, seed dispersal
1. Introduction
Habitat destruction is regarded as a major threat to primate populations and global biodiversity [1,2]. Over recent decades, it has become clear that defaunation of otherwise undisturbed tropical forests is an additional and severe threat [3–5]. Losses of populations of large frugivores by hunting have occurred in Asia and South America as well as in Africa [2,4,6–8]. Two factors are primarily responsible for this phenomenon. First, growing human populations in the tropics, rural and urban, increase the demand for meat [6,9]. Secondly, improved infrastructure, partly as a consequence of logging in remote forest areas and for the transportation of agricultural products to urban markets, facilitates the transportation of hunted animals from the forest to urban consumers [10,11]. Large frugivores may decline even in structurally intact forest reserves as a result of illegal hunting, and in extreme cases, the decline may lead to a forest empty of large vertebrates [12]. In several cases, the loss of large frugivores has been followed by an increase in small seed predators, which are less sensitive to hunting [5,13]. There is strong evidence showing that where large vertebrate frugivores are reduced by illegal hunting and other anthropogenic disturbances, their seed dispersal services are also disrupted [14–16].
Large primates are among the largest frugivores in African forests and play a significant ecological role through primary seed dispersal [17,18]. They are particularly important dispersers of large-seeded plants and may be the sole dispersers of some tropical plant species [15,19,20]. This means that seed dispersal and subsequent recruitment of many plant species may be severely disrupted without the large primates. Although it is still not clear if their dispersal role will be fully, or even partially, compensated by other frugivores in the event of total extirpation, present evidence suggests that it is unlikely [5,21]. It is therefore likely that the more effective a frugivore is at dispersing seeds, the greater the consequence of its decline on tropical plant community regeneration, demography and forest structure.
The Janzen–Connell model [22,23] provides a framework to make predictions for some consequences of altering seed dispersal. Specifically, it predicts that provided seed dispersal is not restricted, recruitment will be highest at some distance away from the parent tree. This is because the higher density of fruits and seeds near the tree attracts predators and pathogens, causing disproportionate mortality of conspecific propagules. Without effective dispersal agents, most animal-dispersed plants will experience depressed recruitment. Recent studies corroborate that recruitment is influenced by density-dependent processes (seed predation beneath parent trees and sibling competition among clustered seedlings beneath parent trees) and that the decline in effective seed dispersers may affect the recruitment of plants [14,24]. Dispersal of large-seeded species by mammals reduces dispersal limitation [25–27] and is therefore critical for the successful recruitment of these species. In defaunated forests, we would expect increased recruitment of species with other dispersal modes as a consequence of the absence of the mammal dispersed species. However, both seed predators and smaller seed dispersers may increase in the absence of the large frugivores [5] and the eventual outcome in terms of plant recruitment can be difficult to accurately predict.
Recent studies from the Neotropics have demonstrated compositional changes in the forest understory associated with the decline in populations of large dispersers [5,13,28–31]. At present, such evidence is rare in African forests (but see [20,32,33]). Given the widespread occurrence of the bushmeat harvest and habitat loss and their effects on tropical forest biodiversity [4], quantifying the impact of the decline of large frugivores on community-wide regeneration in structurally intact forests is important. In addition, with a few exceptions from the Amazon [5,31], previous studies have not been sufficiently replicated or designed as mensurative experiments. At least some replication is needed for making generalizations of the results possible, and as true experiments of the effects of hunting would not be feasible, nor desirable, well-controlled mensurative experiments are the best alternative.
Here, we present a combined field survey and manipulative experiment from a set of paired, replicated field sites in Nigerian rainforests, that are either relatively well protected from hunting, or not. The overall aim is to quantify the effects of hunting on the abundance of mammals and plant seedlings using a robust study design.
We hypothesize that primates, especially the large species, will be severely affected by hunting. Other species may or may not benefit from hunting depending on relative importance and severity of hunting pressure experienced versus their potentially increased food availability in the absence of primates
If hunting leads to decline in primate numbers and primate-dispersed plant species are severely dispersal limited, we predict lower densities of seedlings of such species in hunted sites relative to intact sites, at least away from fruiting trees. Under fruiting trees, fruits will be aggregated in high densities owing to lack of seed dispersal, which may either result in high seed mortality by host-specific natural enemies, or in some cases may result in dense aggregations of conspecific seedlings [27,34]. However, this scenario is not explored in this study. Plants with other dispersal modes should benefit from hunting, as less space and other resources are taken up by primate-dispersed species, which may often be the superior competitors [35]. We thus predict that reduced dispersal of primate-dispersed species should lead to increased abundance of species with other dispersal modes, among standing seedlings in the forest. Without competition for space or resources between seedlings, recruitment should be more closely related to the seed rain of the different groups of species. To minimize the effect of competition among seedlings, we cleared experimental plots of all vegetation and studied the recruitment 1 year later. In these cleared plots, we predict lower abundance of primate-dispersed seedlings in the hunted sites, owing to reduced dispersal. However, the dispersal of seedlings of other dispersal modes should be unaffected by hunting; and therefore, we would expect to find comparable densities in the cleared plots in hunted as in protected sites.
2. Material and methods
(a). Study sites
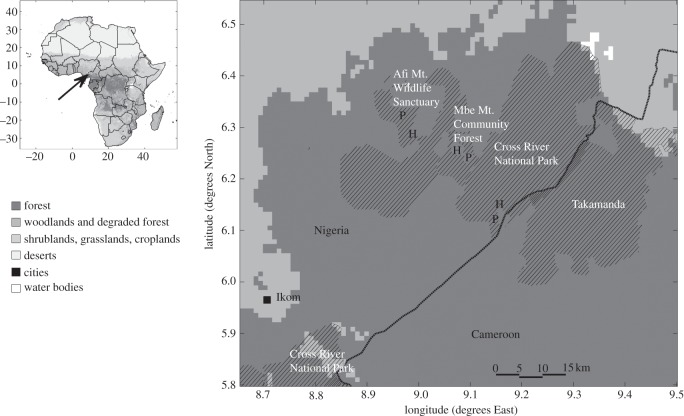
Our study area is situated in Cross River state, southeastern Nigeria (approx. 6°15′ N, 9°0′ E; figure 1). This is a large area classified as closed evergreen lowland forest according to the Global Land Cover 2000-map of Africa [37] with several protected areas [36]. Not all the area outside reserves that is classified as closed forest by the land cover map is structurally intact forest, but intact forests still extend far outside the protected areas (E. O. Effiom and O. Olsson 2009–2012, personal observations). We established pairs of study sites in three different but adjacent protected areas: (i) Mbe Mountain Community Forest (MMCF, total area 80 km2); (ii) Afi Mountain Wildlife Sanctuary (AMWS, total area 100 km2); and (iii) Okwangwo division of the Cross River National Park (CRNP, total area 920 km2). One of the sites in each pair was relatively well protected from hunting (hereafter, the protected site) and the other was poorly protected (hereafter, the hunted site). There is very little documented information about the history of hunting in these areas. Some hunting at low levels might have occurred for a long time, but human populations in the region are increasing rapidly [38,39]. According to the information we have been able to obtain from locals and officials (at Cross River Forestry Commission and Wildlife Conservation Society), the sites we use have had the same hunting status for the last 10–20 years. To date, all sites are structurally intact forest, undisturbed by logging [8,40]. The distances between the different pairs of sites are 10–20 km. Each site within a pair was at least 4–5 km across and at least 4–5 km from the other site in the pair. As the sites are situated within larger continuous forest areas they should be considered as samples of those areas, rather than distinct entities. The two sites in a pair were chosen relatively close to be as edaphically and floristically similar as possible to each other. They are still far enough apart that habitat selection and/or population processes of mammals could generate different population densities with little movement between sites, and seed dispersal between sites would be negligible.
Figure 1.
Map of the study area. The arrow on the small inset map at the top left shows the location of the study region in Africa (black square with white edges). The main map shows the study region in more detail. Shadings represent different land covers, and most of the study region is covered by forest. Reserved areas as given by the World Database on Protected Areas [36] are shown by diagonal hatch. Boundaries given for CRNP appear uncertain as they extend into Cameroon. Locations of the study sites are given by the letters P and H, where P is a site where animals are well protected from hunting, and H where hunting occurs.
Study sites are naturally structured moist tropical lowland rainforest [8,40]. The study area is characterized by two distinct seasons, one dry season (November to March) and one wet season (April to October) with a peak of rainfall from June to August. Rainfall averages 3000 mm yr−1, and humidity is high (rarely less than 90%) with daily temperatures ranging from 15°C to 33°C, with minor seasonal differences.
(b). Wildlife censuses
To estimate mammal abundance, we performed diurnal standardized line transect censuses [41], during the rainy seasons in May and June 2009 and 2010. The mammals in these forests do not have any strong seasonal migration or movement patterns, and we assume that our estimates from May and June represent the whole year well. Four transects, each of 1 km, were made in all sites. Diurnal counts on transects were conducted during both mornings (06.00 to 11.00) and afternoons (14.00 to 17.00), i.e. the transects were walked up during the morning hours and down again later during afternoon hours. Although the survey was conducted during the rainy season, the survey was not disturbed by the rains, as rains came late in the evenings after fieldwork. Animals seen or heard were recorded by a team of three observers. The data used for subsequent analyses were the number of observed groups (of one or more individuals). This choice was based on the fact that the number of individuals was highly over-dispersed, which could lead to inflated type I errors. Using the number of groups is conservative, but might increase type II error risk. Given the high topographic heterogeneity of the study sites, we used recce walks [42]. The recce walk method relaxes the rules of a line transect by allowing deviations from transects for ease of movement [42]. Like the line transect method, recce walks also allow for the quantification of observations per kilometre, thus providing estimates of wildlife abundance [42]. Observers moved at a pace of 1 km h−1.
Mammals recorded were classified into four ‘guilds’: (i) Large primates (gorillas, Gorilla gorilla diehli; chimpanzee, Pan troglodytes ellioti; and drill, Mandrillus leucophaeus); (ii) Other monkeys (putty-nosed monkey, Cercopithecus nictitans; Mona monkey, Cercopithecus mona and red-eared monkey, Cercopithecus erythrotis); (iii) Large rodents (squirrels, Anomalurus spp.; brush-tailed porcupine, Atherurus africanus) and (iv) Ungulates (blue duiker, Philantomba monticola; other duikers Cephalophus spp.; red river hog, Potamochoerus porcus).
For several reasons, detectability probably varies strongly between the guilds which means that we cannot use our estimates to compare abundances between guilds. However, as the forest structure was similar between sites, the estimates are useful for comparisons between sites for each of the guilds.
Elephants were not included in our analysis as only a single feeding sign was recorded in one of the sites. Bats are considered much less important as dispersers in Africa, as they disperse few plants when compared with the Neotropics [43]. Therefore, we did not perform any censuses of bats.
(c). Plant censuses
Mature trees were surveyed to assess the densities of trees with different dispersal modes. We identified and counted mature trees (greater than or equal to 10 cm at dbh) within a 10 m distance on each side of the same transects used for the mammal counts. Tree censuses were only initiated after the mammal counts were completed in order to avoid disturbance.
Seedling counts were conducted to enable a community-wide quantification of the regeneration cohort. All plants (including herbs) with true leaves and less than or equal to 1 m tall in plots of 25 m2 (5 × 5 m) were identified and recorded in May through June 2009 (in CRNP and AMWS) and 2010 (in MMCF). Two plots were placed along each transect, i.e. a total of 48 plots. All plots were randomly located in fully shaded understory, but with the constraint that no plot was established directly beneath adult primate-dispersed trees, to avoid possible aggregations of seedlings of a single species. One of the plots at each transect was randomly selected to be cleared and the other to be left intact. In the 24 experimental plots, all vegetation was cleared after the initial seedling identification and recording. In these experimental plots, all emergent seedlings were identified and counted 1 year after the plots were cleared (i.e. in 2010 and 2011, respectively). In cases where seedlings could not be identified in the field, leaf samples from the same species, outside the plots, were taken to the Cross River State Forestry Commission, Nigeria and the Forestry department of the University of Calabar, Nigeria for identification.
Plant species were classified according to dispersal mode into three different groups: (i) dispersed primarily by large and medium primates, (ii) dispersed primarily by other animals and (iii) dispersed primarily by wind or ballistic ejection and those dispersed by vegetative means (hereafter, ‘abiotic’). Dispersal mode was primarily determined from literature sources. A complete list of all species, their dispersal modes and references for these are provided in the electronic supplementary material, S1. Where we have been unable to locate literature to determine dispersal, we based our classification on knowledge by local people or on the type of fruit (e.g. fleshy and edible for humans, or winged nuts, etc.). When such information was unavailable or ambiguous, we left the dispersal mode of the species as unknown, and removed the species from the dataset before analysis. Some species had more than one dispersal mode. In those cases, we identified which of those was the primary, and used that in our main analyses. However, we also ran models in which we used the secondary dispersal modes of those species, to evaluate the effect of this ambiguity.
(d). Data analysis
The datasets are available at datadryad.org (doi:10.5061/dryad.g3n23) [44]. We analysed data using generalized linear mixed effects models (GLMM) with Poisson or quasi-Poisson distributed error terms, using glmer from package lme4 [45] in R v. 2.11.1 [46]. To represent the field design, we used a random structure with random intercept terms for area, sites and transect. For the trees and seedlings data, we additionally used random intercept terms for sections or plots, respectively. For mammals, the fixed factors considered were hunting and guild, and for plants it was hunting and dispersal mode. All models with these factors, alone, in combination, with their interaction, as well as with just the intercept were considered. To select best model structure for the fixed and random part of the models, we followed Bolker et al. [47], and based our selection on AICC (i.e. corrected for small sample sizes, or quasi-AICC when data were over-dispersed). From the best models, we calculated estimated marginal means and 95% CIs for the means, based on 5000 simulations, using package arm in R [48].
To test if competition from primate-dispersed seedlings determined the number of abiotically dispersed seedlings recruiting, we ran Poisson regressions separately in hunted and protected sites using glm in R. That is, we treated each individual study plot as an independent observation, without accounting for study area. We chose this approach to maximize power of the test, as a significance would indicate that our assumption for the clearing experiment was false. Although we use an information theoretic approach for the other analyses, we here chose to explicitly test the null hypothesis that our assumption of no relation is true.
3. Results
Mammal communities differed between hunted and protected sites (figure 2). The best model for the number of observed groups showed that there was a difference depending on hunting, a difference between guilds and an interaction between these two factors. The AICC-weight of this model was w = 0.99999, and alternative models had w ≤ 10−5 (ΔAICC > 20 in all cases). Complete fit statistics of all models analysed are provided in the electronic supplementary material, S2. Number of primate groups, both large and smaller, were reduced to less than half in the hunted sites, whereas the rodents were much more abundant in the hunted sites (figure 2). For all these three guilds, there was a significant effect of hunting as the mean values in one type of site did not overlap with CI of the other. For ungulates, there was no clear pattern. The effect size of hunting corresponded to 3.16 times more groups of large primates in protected forests (unstandardized log-scale effect size of hunting EH =−1.15, with s.e. ± 0.33), 2.05 times as many groups of smaller primates in protected sites (EH =−0.72 ± 0.19), 14.0 times as many rodents in hunted sites (EH = +2.64 ± 0.73) and 2.00 times as many ungulates in hunted sites (EH = +0.69 ± 0.39). As stated above, the latter effect is not different from zero. The interaction in this analysis is largely a consequence of the different effects of hunting on the rodents and ungulates on the one hand, when compared with the primates on the other. To investigate if the large and smaller primates react differently to hunting, we ran a new analysis, on only the two primate guilds. The best model did not include the interaction but had an AICC-weight of only w = 0.68, and the second best, with the interaction had w = 0.26 (ΔAICC = 1.94), which indicates that these two models fit the data similarly. The log likelihood of both models is very similar (−19.9 and −19.6, respectively, electronic supplementary material, S2) and this means that the interaction guild × hunting is a pretending variable, which does not truly improve model fit [49]. The proportional change of both guilds in response to hunting was very similar. The number of observed groups of both large and smaller primates was less than half (on average 44%) in the hunted sites.
Figure 2.
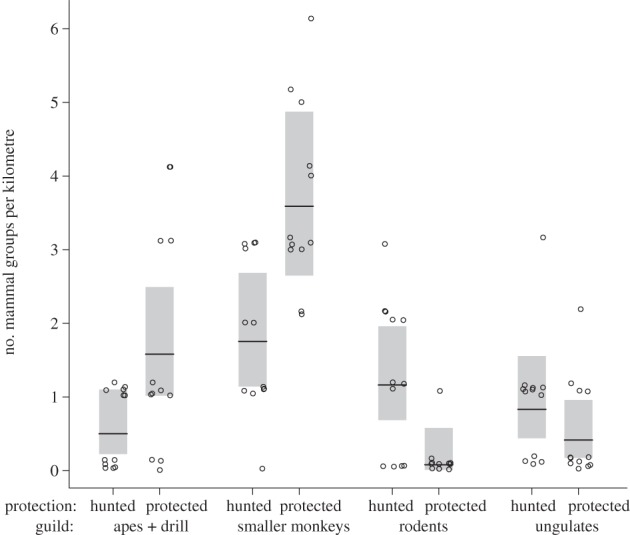
Number of observed groups of mammals along 1 km transects. Data are shown separately for four ‘guilds’ of mammals: large primates, smaller primates, rodents and ungulates. Species belonging to the different groups are listed in §2. Circles indicate the individual data points, i.e. number of observed groups per transect, and different sites are separated horizontally. Dots are jittered for visibility. The horizontal lines show the estimated marginal means from the best Poission-GLMM model. The shaded box represents the 95% CI of the mean.
Mature trees of different dispersal modes were similarly represented among all sites, and regardless of hunting intensity (figure 3a). Most individual trees were primate-dispersed. Approximately, half as many were abiotically dispersed, and even fewer were dispersed by other animals than primates. The best model (w = 0.72) had dispersal mode as its only fixed factor. The second best model (ΔAICC = 2.08, w = 0.25) also included hunting, but the quantitative effect was very weak: 3 per cent lower density of trees in protected sites. As ΔAICC only differs by approximately 2 and log likelihood of the two models is similar (−459.4 in both cases, electronic supplementary material, S2) hunting is only a pretending variable. A model also including the interaction between dispersal mode and hunting provided a poorer fit to the data (ΔAICC = 6.2, w = 0.03), and models with only hunting or the intercept as fixed factors had provided very poor fit (ΔAICC > 130, w ≈ 0).
Figure 3.
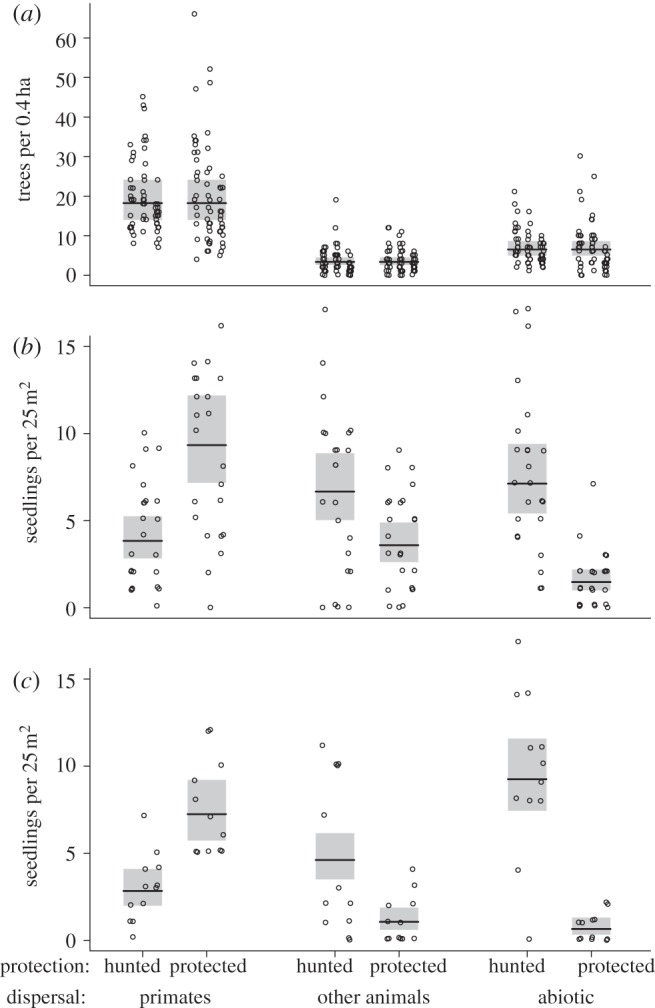
(a) The number of mature trees counted in 20 m wide and 200 m long bands along the transects. Data are shown separately for the three dispersal modes. Each circle represents one 200 m section of a transect, and different sites are separated horizontally. The horizontal lines show the estimated marginal means from the best quasi-Poisson-GLMM model. The shaded box represents the 95% CI of that mean. (b) The number of standing seedlings in 5×5 m plots. Each circle represents one plot. Horizontal line and shaded box as described in (a). (c) The number of seedlings emerging in experimental plots cleared 1 year before.
In intact plots, standing seedlings of different dispersal modes were differently represented in hunted and in protected sites (figure 3b). In protected sites, the representation was similar to that of mature trees (primate dispersed > dispersed by other animals > abiotically dispersed). However, in hunted sites, primate-dispersed species were less abundant than the other dispersal modes (dispersed by other animals ≈ abiotically dispersed > primate dispersed). The best model was the full model, including the interaction between dispersal mode and hunting (w = 0.9999). All other models fit the data poorly (ΔAICC > 19, w < 10−4). Primate-dispersed seedlings were 2.41 times as common in protected sites as in hunted (EH =−0.88 ± 0.38), seedlings dispersed by other animals were 1.86 times more common in hunted sites (EH =+0.62±0.39) and abiotically dispersed seedlings were 4.82 times more common in hunted sites (EH =+1.57±0.46).
In experimentally cleared plots, the representation of emerging seedlings was similar to that of the standing seedlings in intact plots. The effect of hunting was very different between dispersal modes, i.e. the number of seedlings per 25 m2 was significantly higher for species dispersed by other animals and species with abiotic dispersal, in the hunted sites when compared with the protected sites. By contrast, hunted sites had significantly lower densities of primate-dispersed seedlings. The full model with the interaction term provided a superior fit (w > 0.9999) than alternative models (ΔAICC > 48, w < 10−10). Primate-dispersed seedlings were 2.55 times more common in protected sites (EH =−0.93±0.16), seedlings dispersed by other animals were 4.37 times more common in hunted sites (EH = 1.47±0.23) and abiotically dispersed seedlings were 14.2 times more common in hunted sites (EH = 2.65±0.27). There was no indication that the number of abiotically dispersed seedlings in the plots declined with increasing number of primate-dispersed seedlings. This was the case in both hunted (regression coefficient with s.e., β =−0.02±0.041, χ2 = 0.42, d.f. = 1, p = 0.5) and in the protected sites (β = −0.11 ± 0.13, χ2 = 0.79, d.f. = 1, p = 0.4).
Some plant species had ambiguous dispersal modes (see the electronic supplementary material, S3). To account for this, we ran a series of analyses in which we used the secondary, rather than the primary, dispersal mode for those species and also analyses where we formed a new group of those species with mixed dispersal mode. These analyses are fully described in the electronic supplementary material, S3. In none of the cases did this ambiguity change our conclusions, and it was always the same model that came out best.
4. Discussion
The seedling layer in hunted sites was strikingly different from that in protected sites. In protected sites, seedlings dispersed by primates dominated, but in the hunted sites, the other dispersal modes were more common. However, the densities of mature trees were similar between hunted and protected sites, for all dispersal modes. We therefore conclude that the difference of the seedling layer is caused by the paucity of large mammalian dispersers in the hunted sites, possibly in combination with the increase in large rodent seed predators in hunted sites.
It is notable that the surveys of the intact study plots and the clearing experiment showed the same pattern: that the difference in seedling layer regeneration is largely associated with the difference in hunting pressure. Our results strongly suggest that in hunted forests, there are differences in dispersal and/or seed predation that appear to inhibit the regeneration of primate-dispersed species compared with sites protected against hunting. The depletion of primates, as well as the effects on the seedling communities in our study, are similar to those of other studies from Africa and the Neotropics [5,31,32].
The results can be interpreted in the light of the Janzen–Connell (J–C) model [22,23]. The J–C model is based on two functions. The first is the seed shadow, which indicates that seed density declines sharply with increasing distance from the seed source. The second is the escape curve, which indicates that the probability that a seed will avoid predation or infection increases with distance from the parent tree or, for that matter, any conspecific adult trees. As a result, germination is expected to peak at an intermediate distance from the parent tree where the two curves cross. Near conspecific trees, the chances for a seed to survive until it has germinated are reduced, because it is more easily found by both specialist and generalist seed predators or pathogens [50]. In a forest with primates present, more large seeds are dispersed into safer areas, and as a result more of these seeds can germinate to form seedlings. In a forest with primates, large-seeded species will be better represented among seedlings, whereas in an ‘empty’ forest they will be rarer [12,15,30].
Our mammal surveys showed that in hunted areas primates were decimated, but large rodent seed predators were more common than in protected sites (figure 2). We may then assume that in the hunted sites we have both a truncated seed shadow curve and higher rates of seed predation. Our data concur with the predictions generated by the J–C model: in the protected forests where primates are still relatively abundant, large-seeded and primate-dispersed species are the most common among the seedlings (figure 3b,c), just as they are among mature trees (figure 3a).
The results from the intact seedling plots seem to fit an explanation based on restricted dispersal of large-seeded species [51–53]. Even in faunally intact forests, dispersal is often fecundity limited [27], and few seeds reach safe sites where they can germinate. In the hunted sites, this small fraction of seeds is nearly completely lost for primate-dispersed species. Quantitatively, restricted seed dispersal occurs when dispersers’ activity is insufficient to disperse all seeds away from parents [53,54]. If the species with restricted dispersal are the dominant competitors, less competitive species might win in their absence; a situation described by Hurtt & Pacala [51] as winning by forfeit and recently tested by Terborgh et al. [27].
The results from the cleared plots suggest some additional conclusions. Comparing seedlings in cleared plots in hunted sites, with those in the protected (figure 3c), we find a pattern very similar to that of the comparison of intact plots. However, the cleared plots represent germination and establishment during only 1 year at artificially low densities, whereas the intact plots represent the standing composition at higher and more stable densities. Germination in the intact plots is probably not the same as in the cleared. In the cleared plots, we find that primate-dispersed seedlings are more common in protected sites, whereas seedlings of both of the other dispersal modes are more common in hunted sites. For the primate-dispersed seedlings, as well as for seedlings dispersed by other animals, this may well be explained by dispersal limitation, as in the case of the intact plots. However, the seed rain of abiotically dispersed species should not differ between hunted and protected sites, and the seedling communities in cleared plots are not likely to be structured by competition, as the number of abiotically dispersed seedlings in the cleared plots was unrelated to the number of primate-dispersed seedlings. Therefore, it is surprising that in the cleared plots, abiotically dispersed seedlings have much higher densities in hunted than in protected sites. At this point, we can only speculate about the causes for this, but it is something that future studies should try to shed light on. A possibility is that seed predation is different between hunted and protected sites. In the hunted sites, primate-dispersed seeds are likely aggregated below fruiting trees [27], where they can be more profitably exploited by seed predators, than if they were more evenly dispersed on the forest floor [55,56]. This could cause large seed predators to switch towards primate-dispersed seeds away from abiotically dispersed seeds [57,58], which are often smaller [32]. This would then be a case of apparent competition [59] among seeds of different sizes.
Seed predation can certainly have strong effects. Similar to several other studies [5,13,26], we found that the large rodent seed predators were more common in the hunted sites, despite the fact that these are also hunted. We have no data on the smaller, mostly nocturnal, rodents, which might quantitatively be even more important seed predators [29].
The results indicate that the most common group of tree species, the large seeded and primate dispersed, is on a trajectory to become considerably rarer. At the moment, it does not appear that the remaining dispersing animals (birds or mammals) compensate for the primates lost. That is, there is no indication that primates are redundant in this forest ecosystem. Tree species with abiotic dispersal, and those dispersed by other animals, appear to benefit in hunted forests. However, according to Keay [60], many wind-dispersed species in the area have light-demanding seedlings. They are therefore rarer in a closed forest understory because they become shaded out by large trees. Thus, it is difficult to predict the long-term consequences for the forest community composition and dynamics.
This study, in agreement with several other studies [5,15,32,61], identifies the critical ecosystem function that primates have in tropical forests. They are an important component of biodiversity that provides benefits for other groups of organisms. They might also enhance biodiversity in the areas where they occur. In addition, several of the fruits that primates use, and which rely on primates for dispersal, are also used for human consumption [13,26]. This is a provisioning ecosystem service, which has hitherto not been quantified. Loss of the primates could eventually lead to the reduction or loss of this service.
Plants with different functional traits, such as leaf economics [62], may also have different ecosystem properties [63,64]. Thus, as the loss of primates leads to changed community composition, this may potentially lead to consequences for ecosystem functioning such as net-primary productivity and carbon sequestration [64–66].
We are aware that it is not straightforward to extrapolate from seedlings to future mature tree composition, as e.g. density-dependent mortality may affect which seedlings survive to later stages [53]. Nevertheless, the strongly different representation of dispersal modes among seedlings that we find between hunted and protected sites are highly likely to constrain future outcomes of the forest regeneration processes [53,67].
We find that hunting is severely depressing the regeneration of tree species whose dispersal depend on less common and often more vulnerable specialists [33,68]. Evaluating ecological redundancy among smaller frugivores is critical, as it may offer hope of ameliorating the impact of the loss of large frugivores and may lead to the design of conservation interventions that mitigate changes in forest composition and structure [63,68].
In conclusion, our results support the hypothesis that a forest empty of large seed dispersers is likely to face drastic changes in tree community over the next few tree generations [5,13,30,67]. The predicted future state is a forest with few, if any, large-seeded species dispersed by primates. At that point, such a forest will not only lack primates, but also be unsuitable to them owing to fewer food resources. Hence, this state may be stable and only reversed through large-scale active reintroduction of fruiting trees as well as primates. Our results demonstrate that the decline of large primates is a major threat to tropical biodiversity. In addition, there is a critical need to maintain primate populations for the persistence of many tree species with large, fleshy fruits, which sustain population of many frugivores, including humans.
Acknowledgements
We thank two anonymous reviewers for very valuable comments on previous versions of this manuscript. This study was supported by grant no. SWE-2011-030 from Sida to O.O., grants from Kungliga Fysiografiska Sällskapet to E.E. and by grants from Formas to O.O. and H.G.S. E.E. is employed by the Cross River State Forestry Commission and is a PhD student at Lund University. This is contribution no. 62 from A.P. Leventis Ornithological Research Institute.
References
- 1.Mittermeirer RA, Cheney DL. 1987. Conservation of primates and their habitats. In Primate societies (eds Smuts BB, Cheney DL, Seyfarth R, Wrangham RW, Struhsaker TT.), pp. 477–490 Chicago, IL: University of Chicago Press. [Google Scholar]
- 2.Oates JF. 1996. Habitat alteration, hunting and the conservation of folivorous primates in African forests. Aust. J. Ecol. 21, 1–9 10.1111/j.1442-9993.1996.tb00580.x (doi:10.1111/j.1442-9993.1996.tb00580.x) [DOI] [Google Scholar]
- 3.Peres CA. 1990. Effects of hunting on Western Amazonian primate communities. Biol. Conserv. 54, 47–59 10.1016/0006-3207(90)90041-M (doi:10.1016/0006-3207(90)90041-M) [DOI] [Google Scholar]
- 4.Fa J, Brown D. 2009. Impacts of hunting on mammals in African tropical moist forests: a review and synthesis. Mammal Rev. 39, 231–264 10.1111/j.1365-2907.2009.00149.x (doi:10.1111/j.1365-2907.2009.00149.x) [DOI] [Google Scholar]
- 5.Nuñez-Iturri G, Olsson O, Howe HF. 2008. Hunting reduces recruitment of primate-dispersed trees in Amazonian Peru. Biol. Conserv. 141, 1536–1546 10.1016/j.biocon.2008.03.020 (doi:10.1016/j.biocon.2008.03.020) [DOI] [Google Scholar]
- 6.Milner-Gulland EJ, Bennett EL. 2003. Wild meat: the bigger picture. Trends Ecol. Evol. 18, 351–357 10.1016/S0169-5347(03)00123-X (doi:10.1016/S0169-5347(03)00123-X) [DOI] [Google Scholar]
- 7.Oates JF, Whitesides GH, Davies AG, Waterman PG, Green SM, Dasilva GL, Mole S. 1990. Determinants of variation in tropical forest primate biomass—new evidence from West-Africa. Ecology 71, 328–343 10.2307/1940272 (doi:10.2307/1940272) [DOI] [Google Scholar]
- 8.Bergl R, Warren Y, Nicholas A, Dunn A, Imong I, Sunderland-Groves J, Oates JF. 2012. Remote sensing analysis reveals habitat, dispersal corridors and expanded distribution for the Critically Endangered Cross River gorilla Gorilla gorilla diehli. Oryx 46, 278–289 10.1017/S0030605310001857 (doi:10.1017/S0030605310001857) [DOI] [Google Scholar]
- 9.Fa JE, Currie D, Meeuwig J. 2003. Bushmeat and food security in the Congo Basin: linkages between wildlife and people's future. Environ. Conserv. 30, 71–78 10.1017/S0376892903000067 (doi:10.1017/S0376892903000067) [DOI] [Google Scholar]
- 10.Peres CA, Lake IR. 2003. Extent of nontimber resource extraction in tropical forests: accessibility to game vertebrates by hunters in the Amazon basin. Conserv. Biol. 17, 521–535 10.1046/j.1523-1739.2003.01413.x (doi:10.1046/j.1523-1739.2003.01413.x) [DOI] [Google Scholar]
- 11.Robinson JG, Redford KH, Bennett EL. 1999. Wildlife harvest in logged tropical forests. Science 284, 595–596 10.1126/science.284.5414.595 (doi:10.1126/science.284.5414.595) [DOI] [Google Scholar]
- 12.Redford KH. 1992. The empty forest. Bioscience 42, 412–422 10.2307/1311860 (doi:10.2307/1311860) [DOI] [Google Scholar]
- 13.Wright SJ, Zeballos H, Dominguez I, Gallardo MM, Moreno MC, Ibanez R. 2000. Poachers alter mammal abundance, seed dispersal, and seed predation in a Neotropical forest. Conserv. Biol. 14, 227–239 10.1046/j.1523-1739.2000.98333.x (doi:10.1046/j.1523-1739.2000.98333.x) [DOI] [Google Scholar]
- 14.Wotton DM, Kelly D. 2011. Frugivore loss limits recruitment of large-seeded trees. Proc. R. Soc. B 278, 3345–3354 10.1098/rspb.2011.0185 (doi:10.1098/rspb.2011.0185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapman CA, Onderdonk DA. 1998. Forests without primates: primate/plant codependency. Am. J. Primatol. 45, 127–141 (doi:10.1002/(SICI)1098-2345(1998)45:1<127::AID-AJP9>3.0.CO;2-Y) [DOI] [PubMed] [Google Scholar]
- 16.Terborgh J, Pitman N, Silman M, Schicheter H, Núñez VP. 2002. Maintenance of tree diversity in tropical forests. In Seed dispersal and frugivory: ecology, evolution and conservation (eds Levey DJ, Silva WR, Galetti M.), pp. 1–17 Wallingford, UK: CABI Publishing [Google Scholar]
- 17.Wrangham RW, Chapman CA, Chapman LJ. 1994. Seed dispersal by forest chimpanzees in Uganda. J. Trop. Ecol. 10, 355–368 10.1017/S0266467400008026 (doi:10.1017/S0266467400008026) [DOI] [Google Scholar]
- 18.Poulsen JR, Clark CJ, Smith TB. 2001. Seed dispersal by a diurnal primate community in the Dja Reserve, Cameroon. J. Trop. Ecol. 17, 787–808 10.1017/S0266467401001602 (doi:10.1017/S0266467401001602) [DOI] [Google Scholar]
- 19.Tutin CEG, Williamson EA, Rogers ME, Fernandez M. 1991. A case-study of a plant-animal relationship—Cola-lizae and lowland gorillas in the Lope Reserve, Gabon. J. Trop. Ecol. 7, 181–199 10.1017/S0266467400005320 (doi:10.1017/S0266467400005320) [DOI] [Google Scholar]
- 20.Brodie JF, Helmy OE, Brockelman WY, Maron JL. 2009. Bushmeat poaching reduces the seed dispersal and population growth rate of a mammal-dispersed tree. Ecol. Appl. 19, 854–863 10.1890/08-0955.1 (doi:10.1890/08-0955.1) [DOI] [PubMed] [Google Scholar]
- 21.Peres CA, Dolman PM. 2000. Density compensation in Neotropical primate communities: evidence from 56 hunted and nonhunted Amazonian forests of varying productivity. Oecologia 122, 175–189 10.1007/PL00008845 (doi:10.1007/PL00008845) [DOI] [PubMed] [Google Scholar]
- 22.Janzen DH. 1970. Herbivores and the number of tree species in tropical forests. Am. Nat. 104, 501–528 10.1086/282687 (doi:10.1086/282687) [DOI] [Google Scholar]
- 23.Connell JH. 1971. On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forests trees. In Dynamics of populations (eds den Boer PJ, Gradwell GR.), pp. 298–312 Wageningen, The Netherlands: Centre for Agricultural Publications and Documentation [Google Scholar]
- 24.Chen L, Mi X, Comita LS, Zhang L, Ren H, Ma K. 2010. Community-level consequences of density dependence and habitat association in a subtropical broad-leaved forest. Ecol. Lett. 13, 695–704 10.1111/j.1461-0248.2010.01468.x (doi:10.1111/j.1461-0248.2010.01468.x) [DOI] [PubMed] [Google Scholar]
- 25.Schupp EW, Milleron T, Russo SE. 2002. Dissemination limitation and the origin and maintenance of species-rich tropical forests. In Seed dispersal and frugivory: ecology, evolution and conservation (eds Levey DJ, Silva WR, Galetti M.), pp. 19–33 Wallingford, UK: CABI Publishing [Google Scholar]
- 26.Forget PM, Jansen PA. 2007. Hunting increases dispersal limitation in the tree Carapa procera, a nontimber forest product. Conserv. Biol. 21, 106–113 10.1111/j.1523-1739.2006.00590.x (doi:10.1111/j.1523-1739.2006.00590.x) [DOI] [PubMed] [Google Scholar]
- 27.Terborgh J, Alvarez-Loayza P, Dexter KG, Cornejo F, Carrasco C. 2011. Decomposing dispersal limitation: limits on fecundity or seed distribution? J. Ecol. 99, 935–944 10.1111/j.1365-2745.2011.01836.x (doi:10.1111/j.1365-2745.2011.01836.x) [DOI] [Google Scholar]
- 28.Dirzo R, Miranda A. 1991. Altered patterns of herbivory and diversity in the forest understory: A case study of the possible consequences of contemporary defaunation. In Plant–animal interactions: evolutionary ecology in tropical and temperate regions (eds Price PW, Lewinsohn TM, Fernandes GW, Benson WW.), pp. 273–287 New York, NY: Wiley [Google Scholar]
- 29.Dirzo R, Mendoza E, Ortiz P. 2007. Size-related differential seed predation in a heavily defaunated Neotropical rain forest. Biotropica 39, 355–362 10.1111/j.1744-7429.2007.00274.x (doi:10.1111/j.1744-7429.2007.00274.x) [DOI] [Google Scholar]
- 30.Terborgh J, Nunez-Iturri G, Pitman NC, Valverde FH, Alvarez P, Swamy V, Pringle EG, Paine C. 2008. Tree recruitment in an empty forest. Ecology 89, 1757–1768 10.1890/07-0479.1 (doi:10.1890/07-0479.1) [DOI] [PubMed] [Google Scholar]
- 31.Stevenson PR. 2011. The abundance of large Ateline monkeys is positively associated with the diversity of plants regenerating in Neotropical forests. Biotropica 43, 512–519 10.1111/j.1744-7429.2010.00708.x (doi:10.1111/j.1744-7429.2010.00708.x) [DOI] [Google Scholar]
- 32.Vanthomme H, Belle B, Forget PM. 2010. Bushmeat hunting alters recruitment of large-seeded plant species in Central Africa. Biotropica 42, 672–679 10.1111/j.1744-7429.2010.00630.x (doi:10.1111/j.1744-7429.2010.00630.x) [DOI] [Google Scholar]
- 33.Cordeiro NJ, Howe HF. 2003. Forest fragmentation severs mutualism between seed dispersers and an endemic African tree. Proc. Natl Acad. Sci. USA 100, 14 052–14 056 10.1073/pnas.2331023100 (doi:10.1073/pnas.2331023100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fragoso JMV, Silvius KM, Correa JA. 2003. Long-distance seed dispersal by tapirs increases seed survival and aggregates tropical trees. Ecology 84, 1998–2006 10.1890/01-0621 (doi:10.1890/01-0621) [DOI] [Google Scholar]
- 35.Rees M, Condit R, Crawley M, Pacala SW, Tilman D. 2001. Long-term studies of vegetation dynamics. Science 293, 650–655 10.1126/science.1062586 (doi:10.1126/science.1062586) [DOI] [PubMed] [Google Scholar]
- 36.UNEP-WCMC 2010. Data standards for the world database on protected areas. Cambridge, UK: UNEP-WCMC [Google Scholar]
- 37.Mayaux P, Bartholomé E, Fritz S, Belward A. 2004. A new land-cover map of Africa for the year 2000. J. Biogeogr. 31, 861–877 10.1111/j.1365-2699.2004.01073.x (doi:10.1111/j.1365-2699.2004.01073.x) [DOI] [Google Scholar]
- 38.Bergl R. 2006. Conservation biology of the Cross River gorilla (Gorilla gorilla dielhi). PhD dissertation, The City University of New York, New York, NY, USA. [Google Scholar]
- 39.Imong I, Wood KL, Okeke F. 2009. Great ape and drill census of Afi Mountain Wildlife Sanctuary, Cross River State, Nigeria. Unpublished report of the Wildlife Conservation Society, Pandrillus and Cross River State Forestry Commission
- 40.Sarmiento EE, Oates JF. 2000. The Cross River gorillas: a distinct subspecies, Gorilla gorilla diehli Matschie 1904. Bio. One 3304, 1–55 (doi:10.1206/0003-0082(2000)3304<0001:TCRGAD>2.0.CO;2) [DOI] [Google Scholar]
- 41.Peres CA. 1999. General guidelines for standardizing line transect surveys of tropical forest primates. Neotrop. Primates 7, 11–16 [Google Scholar]
- 42.Kühl HF. 2008. Best practice guidelines for surveys and monitoring of Great ape populations. Gland, Switzerland: IUCN SSC Primate Specialist Group (PSG) [Google Scholar]
- 43.Gautier-Hion A, et al. 1985. Fruit characters as a basis of fruit choice and seed dispersal in a tropical forest vertebrate community. Oecologia 65, 324–337 10.1007/BF00378906 (doi:10.1007/BF00378906) [DOI] [PubMed] [Google Scholar]
- 44.Effiom EO, Nuñez-Iturri G, Smith HG, Ottosson U, Olsson O. Data from: bushmeat hunting changes the regeneration of African rainforests. 10.5061/dryad.g3n23 (doi:10.5061/dryad.g3n23) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bates D, Maechler M. 2010. lme4: linear mixed-effects models using S4 classes. See http://CRAN.R-project.org/package=lme4.
- 46.R Development Core Team 2010. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 47.Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JSS. 2009. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135 10.1016/j.tree.2008.10.008 (doi:10.1016/j.tree.2008.10.008) [DOI] [PubMed] [Google Scholar]
- 48.Gelman A, Yu-Sung S, Masanao Y, Hill J, Grazia Pittau M, Kerman J, Zheng T. 2010. arm: data analysis using regression and multilevel/hierarchical models. See http://CRAN.R-project.org/package=arm.
- 49.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn New York, NY: Springer [Google Scholar]
- 50.Bagchi R, Swinfield T, Gallery RE, Lewis OT, Gripenberg S, Narayan L, Freckleton RP. 2010. Testing the Janzen–Connell mechanism: pathogens cause overcompensating density dependence in a tropical tree. Ecol. Lett. 13, 1262–1269 10.1111/j.1461-0248.2010.01520.x (doi:10.1111/j.1461-0248.2010.01520.x) [DOI] [PubMed] [Google Scholar]
- 51.Hurtt GC, Pacala SW. 1995. The consequences of recruitment limitation—reconciling chance, history and competitive differences between plants. J. Theor. Biol. 176, 1–12 10.1006/jtbi.1995.0170 (doi:10.1006/jtbi.1995.0170) [DOI] [Google Scholar]
- 52.Jordano P, Godoy JA. 2002. Frugivore-generated seed shadows: a landscape view of demographic and genetic effects. In Seed dispersal and frugivory: ecology, evolution and conservation (eds Levey DJ, Silva WR, Galetti M.), pp. 305–321 New York, NY: CABI Publishing [Google Scholar]
- 53.Howe HF, Miriti MN. 2004. When seed dispersal matters. Bioscience 54, 651–660 10.1641/0006-3568(2004)054[0651:WSDM]2.0.CO;2 (doi:10.1641/0006-3568(2004)054[0651:WSDM]2.0.CO;2) [DOI] [Google Scholar]
- 54.Harms KE, Wright SJ, Calderon O, Hernandez A, Herre EA. 2000. Pervasive density-dependent recruitment enhances seedling diversity in a tropical forest. Nature 404, 493–495 10.1038/35006630 (doi:10.1038/35006630) [DOI] [PubMed] [Google Scholar]
- 55.Olsson O, Brown JS. 2006. The foraging benefits of information and the penalty of ignorance. Oikos 112, 260–273 10.1111/j.0030-1299.2006.13548.x (doi:10.1111/j.0030-1299.2006.13548.x) [DOI] [Google Scholar]
- 56.Olsson O, Molokwu MN. 2007. On the missed opportunity cost, GUD, and estimating environmental quality. Israel J. Ecol. Evol. 53, 263–278 10.1560/IJEE.53.3.263 (doi:10.1560/IJEE.53.3.263) [DOI] [Google Scholar]
- 57.Brown JS, Mitchell WA. 1989. Diet selection on depletable resources. Oikos 54, 33–43 10.2307/3565894 (doi:10.2307/3565894) [DOI] [Google Scholar]
- 58.Garb J, Kotler BP, Brown JS. 2000. Foraging and community consequences of seed size for coexisting Negev Desert granivores. Oikos 88, 291–300 10.1034/j.1600-0706.2000.880207.x (doi:10.1034/j.1600-0706.2000.880207.x) [DOI] [Google Scholar]
- 59.Holt RD. 1977. Predation apparent competition and the structure of prey communities. Theor. Popul. Biol. 12, 197–229 10.1016/0040-5809(77)90042-9 (doi:10.1016/0040-5809(77)90042-9) [DOI] [PubMed] [Google Scholar]
- 60.Keay RWJ. 1957. Wind-dispersed species in a Nigerian forest. J. Ecol. 45, 471–478 10.2307/2256930 (doi:10.2307/2256930) [DOI] [Google Scholar]
- 61.Howe HF. 1984. Implications of seed dispersal by animals for tropical reserve management. Biol. Conserv. 30, 261–281 10.1016/0006-3207(84)90087-9 (doi:10.1016/0006-3207(84)90087-9) [DOI] [Google Scholar]
- 62.Wright IJ, et al. 2004. The worldwide leaf economics spectrum. Nature 428, 821–827 10.1038/nature02403 (doi:10.1038/nature02403) [DOI] [PubMed] [Google Scholar]
- 63.Stoner KE, Vulinec K, Wright SJ, Peres CA. 2007. Hunting and plant community dynamics in tropical forests: a synthesis and future directions. Biotropica 39, 385–392 10.1111/j.1744-7429.2007.00291.x (doi:10.1111/j.1744-7429.2007.00291.x) [DOI] [Google Scholar]
- 64.Lavorel S, Grigulis K. 2012. How fundamental plant functional trait relationships scale-up to trade-offs and synergies in ecosystem services. J. Ecol. 100, 128–140 10.1111/j.1365-2745.2011.01914.x (doi:10.1111/j.1365-2745.2011.01914.x) [DOI] [Google Scholar]
- 65.Lewis SL, et al. 2009. Increasing carbon storage in intact African tropical forests. Nature 457, 1003–1006 10.1038/nature07771 (doi:10.1038/nature07771) [DOI] [PubMed] [Google Scholar]
- 66.Brodie J, Post E, Laurance WF. 2012. Climate change and tropical biodiversity: a new focus. Trends Ecol. Evol. 27, 145–150 10.1016/j.tree.2011.09.008 (doi:10.1016/j.tree.2011.09.008) [DOI] [PubMed] [Google Scholar]
- 67.Comita LS, Aguilar S, Perez R, Lao S, Hubbell SP. 2007. Patterns of woody plant species abundance and diversity in the seedling layer of a tropical forest. J. Veg. Sci. 18, 163–174 10.1658/1100-9233(2007)18[163:POWPSA]2.0.CO;2 (doi:10.1658/1100-9233(2007)18[163:POWPSA]2.0.CO;2) [DOI] [Google Scholar]
- 68.Sethi P, Howe HF. 2009. Recruitment of hornbill-dispersed trees in hunted and logged forests of the Indian eastern Himalaya. Conserv. Biol. 23, 710–718 10.1111/j.1523-1739.2008.01155.x (doi:10.1111/j.1523-1739.2008.01155.x) [DOI] [PubMed] [Google Scholar]