Abstract
Bone accumulations faithfully record historical ecological data on animal communities, and owing to millennial-scale bone survival on high-latitude landscapes, have exceptional potential for extending records on arctic ecosystems. For the Porcupine Caribou Herd, maintaining access to calving grounds on the Arctic National Wildlife Refuge (ANWR, Alaska) is a central management concern. However, variability in calving ground geography over the 30+ years of monitoring suggests establishing the impacts of climate change and potential petroleum development on future calving success could benefit from extended temporal perspectives. Using accumulations of female antlers (shed within days of calving) and neonatal skeletons, we test if caribou calving grounds develop measureable and characteristic bone accumulations and if skeletal data may be helpful in establishing a fuller, historically integrated understanding of landscape and habitat needs. Bone surveys of an important ANWR calving area reveal abundant shed antlers (reaching 103 km–2) and high proportional abundance of newborn skeletal individuals (up to 60% neonate). Openly vegetated riparian terraces, which compose less than 10 per cent of ANWR calving grounds, yield significantly higher antler concentrations than more abundant habitats traditionally viewed as primary calving terrain. Differences between habitats appear robust to potential differences in bone visibility. The distribution of antler weathering stages mirrors known multi-decadal calving histories and highlights portions of the antler accumulation that probably significantly extends records of calving activity. Death assemblages offer historically integrated ecological data valuable for the management and conservation of faunas across polar latitudes.
Keywords: conservation palaeobiology, Arctic National Wildlife Refuge, Porcupine Caribou Herd, ecological baselines, historical ecology, taphonomy
1. Introduction
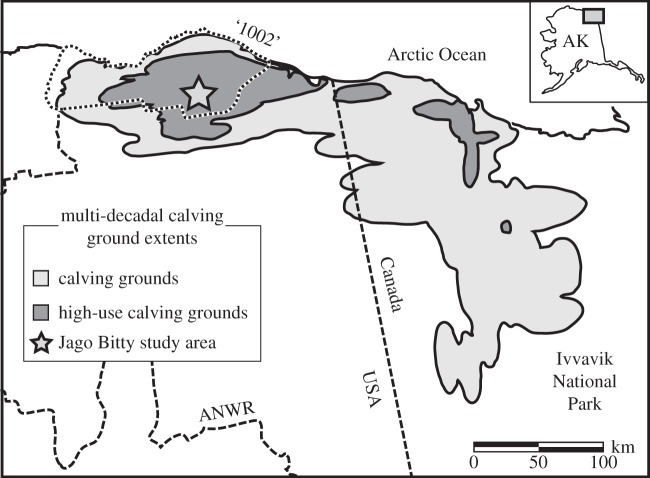
As animal communities around the globe continue to face significant ecological perturbations owing to climate change, habitat loss and over-exploitation [1–3], a pervasive challenge to their conservation and management is the limited duration over which populations have been studied [1,3,4]. This predicament is exemplified in the Arctic National Wildlife Refuge (ANWR: Alaska, USA) where the ecological effects of climate change are expected to be particularly severe, and continued interest in petroleum exploration could directly impact the Coastal Plain calving grounds of the Porcupine Caribou Herd (PCH, Rangifer tarandus; figure 1) [5,6]. Compounding issues, non-continuous aerial surveys between 1972 and 2010 have shown the location of PCH calving grounds to vary strongly across annual-to-decadal timescales [5,7]. This variability observed across a limited survey duration suggests a broader observational window could be beneficial for establishing the ramifications of possible petroleum development, the overall climatic influences on calving ground choice, and the diversity of areas needed to maintain successful calving in the decades and centuries to come. Overall, management of the PCH and other herds could benefit from baseline data prior to the initiation of secular warming trends and other anthropogenic disturbances (i.e. before the initiation of modern surveys). Natural accumulations of bones and antlers (death assemblages) survive on the surfaces of temperate and arctic landscapes from centuries [8,9] to millennia [10,11], and may significantly expand the timescales over which arctic calving grounds are studied. Death assemblages in temperate and tropical settings are known to provide high-quality ecological and biogeographic data on source species and communities [8,12–15], but bone accumulations of arctic ecosystems have not been studied in detail. Here, we employ bone surveys of an important PCH calving ground on the Jago River (ANWR, Alaska; figure 1) to provide a first test of the fidelity with which arctic death assemblages capture ecological data. Specifically, we test if calving grounds develop measureable and characteristic bone accumulations and record patterns of past use. As part of this work, we also establish a quantitative morphological framework for identifying the gender of caribou antlers on the ANWR landscape using measurements of the antler–skull attachment surface of known-gender museum specimens (see the electronic supplementary material, text 1.0). These first steps will help determine if more extensive bone surveys can provide unique and valuable historically integrated data on caribou calving activity across the ANWR Coastal Plain.
Figure 1.
Map showing extent of calving grounds for the Porcupine Caribou Herd in both ANWR (AK) and Ivvavik National Park (Yukon, Canada). General calving areas and more heavily used calving areas over the last 30 years are shown. ‘1002’ area is the zone under consideration for petroleum exploration. Star indicates the Jago Bitty study area. (Map adapted from [5].)
Unique aspects of caribou biology and ecology make bone assemblages ideally suited to identify areas used for calving and early rearing. Caribou are the only cervid for which females, like males, annually grow and shed antlers. Male caribou (and non-pregnant females) generally shed their antlers following breeding, while pregnant females keep their antlers until casting them within days of calving [16,17]. On the calving grounds of the PCH, tens of thousands of female caribou bear young within a few weeks in concentrated areas [5] after migrating hundreds of kilometres from breeding grounds [18]. Furthermore, calf mortality rates can be high (more than one-third of births [19]), with the majority of deaths (up to nearly three-quarters) occurring within 48 h of birth [19], and prior to large movements away from calving sites [5,19]. Thus, there are opportunities for the development of two geographically faithful skeletal accumulations: concentrations of shed female antlers and newborn carcasses.
(a). Additional tests of ecological fidelity: comparing the Arctic National Wildlife Refuge and Yellowstone death assemblages
Beyond testing the Jago Bitty death assemblage for characteristics indicative of caribou calving activity, we further test the quality of ecological data contained in such death assemblages by comparing arctic caribou antler and neonatal bone accumulations (ANWR) to those of temperate elk in Yellowstone National Park, WY (USA). In Yellowstone, newborn skeletal remains and shed male elk antlers faithfully record decadal-scale patterns of landscape use, including elk calving areas and the relative importance of male elk wintering grounds [15]. However, differences between Yellowstone and ANWR in ungulate landscape use, migration patterns, population density and environment-driven bone weathering rates should produce characteristic dissimilarities between their death assemblages.
Key differences in the ecological and environmental settings between ANWR and Yellowstone lead to a series of hypotheses concerning antler concentrations and neonatal representation in their skeletal records. Antlers in Yellowstone are shed by male elk in late-winter after congregating in bachelor groups [20], though such aggregations include fewer individuals at lower concentrations than on ANWR calving areas [5,21]. In addition, while bones can persist for centuries in temperate settings [8], they can survive on arctic landscapes for millennia [10,11], significantly increasing the maximum number of generations represented in a bone assemblage. Interestingly, the predator communities of ANWR and Yellowstone are highly similar (both include bears, wolf, fox, wolverine and eagles); so their death assemblages are unlikely to experience highly disparate patterns of biologically mediated bone loss (as might be observed in ecosystems with hyaenas or other highly efficient and ravenous bone consumers [22]). Taken together, we predict that antler concentrations will be higher in ANWR compared with Yellowstone. In addition, we expect the ANWR death assemblage to have higher proportional abundance of neonatal skeletal remains. High neonatal mortality is known from both regions [19,23], but the density of adult (and sub-adult) remains is expected to be lower in ANWR owing to differences in seasonal landscape use. Specifically, while Yellowstone elk are present throughout the year [20] and are commonly found in areas used for calving, the PCH only visits calving grounds for a few weeks annually [5,24], limiting opportunities for adult mortality and, thus, proportionately enriching ANWR's neonatal signal.
2. Material and methods
(a). Determining antler size variation by gender
To determine the discriminatory power with which shed antler size reveals gender identity (even when gnawed or otherwise fragmented), we measured the major- and minor-axes of known-gender, fully developed cranial pedicle surfaces (i.e. antler attachment surfaces). All specimens were from adult mainland North American caribou (Rangifer tarandus, R. t. grantii, R. t. groenlandicus, R. t. caribou). All accessible specimens meeting these criteria were measured at the University of Alaska Museum, the Field Museum of Natural History and the American Museum of Natural History; totalling 35 males and 24 females. Crania with and without antlers were measured. Crania with attached antlers were measured at the antler attachment suture or, when antler attachments were heavily ossified, at the pedicle indentation proximal to the antler bur (see [25]). Pedicle surfaces are roughly ellipsoid, allowing us to estimate surface area as π × semi-major axis × semi-minor axis. Semi-axes were obtained by dividing caliper measurements of axis dimensions (centimetres) in half. For specimens with both left and right pedicles, we used the smaller of the males and the larger of the females, which offered conservative comparisons between genders. We compared geometric distinctiveness of adult male and female pedicle surfaces based on overlap in size-frequency distributions. Genders of unknown (ANWR) antlers were calculated probabilistically based on their pedicle surface areas and the proportional overlap in normal distributions fit to male and female museum specimens. To obtain confidence intervals (CIs) for gender identification and to accommodate differences in sample sizes between available male and female museum specimens, the normal distribution for male antlers was repeatedly fit after subsampling the male data to match available female sample size (randomly sampling n = 24 museum males 10 000 times without replacement). By changing the resulting overlap between female and male distributions, this iterative subsampling produced variability in gender assignment for ANWR antlers and allowed us to calculate the mean probability and 95% CI for gender of each antler and the whole assemblage (see the electronic supplementary material, text 2.0).
(b). Sampling the Arctic National Wildlife Refuge bone assemblage
To sample the ANWR bone assemblage, four sample plots on the Jago Bitty calving ground (figure 1) were examined. Sample plots were 1 km long and 100 m wide (50 m on either side of a midline) and spaced a minimum of 0.5 km from each other to reduce the probability that skeletal remains of single individuals would be sampled in multiple plots [8,12]. Two plots sampled tussock tundra-dominated habitats (tussock tundra, moist/wet sedge: T01–01 and T01–03), and two sampled more openly vegetated riparian terrace habitats (Dryas terrace, riparian shrub: T01–02 and T01–04). Habitat designations followed previous definitions [26] and were identified by J. Jorgenson (US Fish and Wildlife Service). To test for variability among antler concentrations within a small geographical area, all plots were within a 2 km radius. To collect data on the death assemblage, JHM flagged a midline and two observers walked perpendicular to that midline (on opposite sides), flagging all observed antlers, skeletal material, bone fragments, raptor regurgitate and carnivore faeces. JHM took standardized data on all bone occurrences, including location (GPS coordinates), species identity, bone completeness, damage, ontogenetic age of skeletal material (neonate, sub-adult and adult), bone weathering stage (WS), and the lengths of the major- and minor-axes of antler-cranial attachment surfaces. Bone WSs categorize the deterioration state of a subaerially exposed bone from fresh (WS 0) to disintegrating (WS 5) and are a proxy for postmortem duration [8,27]. Only antlers with a rounded pedicle surface were counted as ‘shed.’ For each sample plot, concentrations of shed female antlers were calculated as: no. shed female antlers/plot area (km2) (see the electronic supplementary material, text 3.0). To examine the proportional abundance of neonatal skeletons, caribou skeletal material from each sample plot was first reduced to the minimum number of individuals (MNI) using left- and right-sided skeletal material (i.e. excluding antlers), ontogenetic age, and bone WS ([8,27], electronic supplementary material, text 4.0). Geographical proximity among skeletal materials was also used when assigning bones to each constituent MNI. Neonate concentrations were then calculated as the proportion of neonatal MNI to the total caribou MNI (neonate MNI + sub-adult MNI + adult MNI). These metrics were also used for comparisons to the Yellowstone death assemblage (sampled using 40 sample plots and the same methodology [8,28]). Bone data from ANWR were compared with the entire Yellowstone death assemblage and to sample-size standardized distributions of median Yellowstone values (medians calculated from n = 4 Yellowstone sample plots randomly drawn 10 000 times without replacement). All analyses were conducted in the open source statistical platform R, v. 2.9.2 [29].
3. Results
(a). Antler pedicle surface areas of adult male and female caribou
Museum sampling shows that pedicle attachment area can differentiate adult male and female caribou. While there is some overlap between genders (see figure 2b and the electronic supplementary material, appendix A), the difference between means is highly significant (museum males versus museum females: t57 = 11.021, 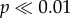 ). Ninety-five percent CIs calculated from the iterative subsample standardization of museum male antlers are small (error bars figure 2a; electronic supplementary material, text 5.0 and figure S1), further demonstrating strong and consistent differences between males and females (mean male antler distribution shown in figure 2b; see the electronic supplementary material, figure S2.0 for all 10 000 subsampled male distributions).
). Ninety-five percent CIs calculated from the iterative subsample standardization of museum male antlers are small (error bars figure 2a; electronic supplementary material, text 5.0 and figure S1), further demonstrating strong and consistent differences between males and females (mean male antler distribution shown in figure 2b; see the electronic supplementary material, figure S2.0 for all 10 000 subsampled male distributions).
Figure 2.
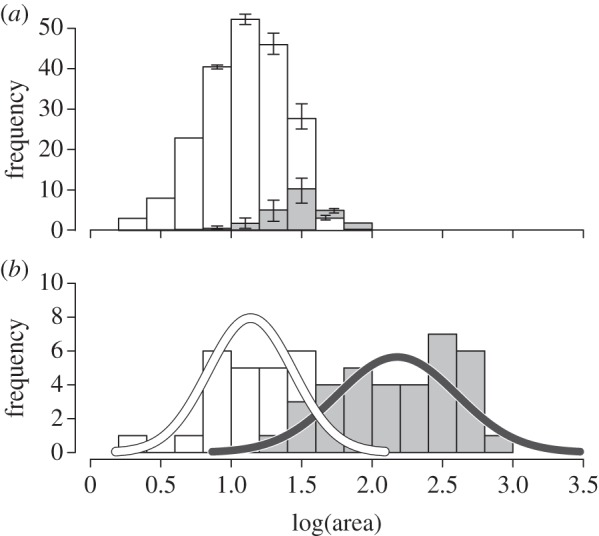
Log surface area for caribou antler pedicle surfaces from (a) the ANWR bone assemblage and (b) museum collections. Female antlers are white, males are grey. Error bars are 95% CIs of gender assignment probabilities. Adult male and female antlers are distinguishable. The Jago Bitty calving ground is dominated by female antlers: 89% (CI: 86–93%).
(b). The Arctic National Wildlife Refuge death assemblage
Data from bone surveys show that caribou skeletal material and shed antlers are common on the Jago Bitty (ANWR) calving ground, with a mean concentration of 842 ± 388 (s.e.) caribou bones (including antlers) per square kilometre (excluding bones from rodents, carnivores, birds and caribou teeth; table 1). Total shed antler concentrations on the Jago calving grounds averaged 573 ± 281 km−2 (table 1; electronic supplementary material, appendix B), and of the 232 antlers, 228 could be assigned to gender based on basal antler measurements (damage prevented some antlers from being measured; table 2). ANWR antlers are dominated by adult female sheds (89%, CI: 86–93%; figure 2a and table 2). Field observations note the absence of any large male antlers, which is confirmed quantitatively by the lack of ANWR antlers in the largest two-thirds (65%) of the log-based range of known adult male antler surface areas (figure 2a). Furthermore, nearly the entire size-frequency distribution of shed antlers from ANWR falls within the range of adult females sampled from museum collections (figure 2). The entire sample of ANWR antlers and museum females share the same mean and standard deviation (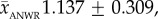
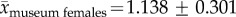 ), and like museum females, are significantly different from museum male antlers (ANWR versus museum males: t40 = 13.963,
), and like museum females, are significantly different from museum male antlers (ANWR versus museum males: t40 = 13.963, 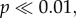
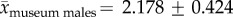 ). Together, this suggests the percentage of shed female antlers on the Jago Bitty calving ground may be higher than the 86–93% calculated here.
). Together, this suggests the percentage of shed female antlers on the Jago Bitty calving ground may be higher than the 86–93% calculated here.
Table 1.
Results from sample plots of the Jago Bitty bone assemblage. (‘Caribou bones and antlers’ provide counts of all skeletal material, including antlers attached to crania and shed antlers (including those gnawed and buried). ‘Shed antlers’ provides counts of all encountered shed antlers (and concentrations per square kilometre). ‘Buried antlers’ are counts (and proportional abundances) of all shed antlers buried more than 50% in soil or vegetation. ‘Gnawed antlers’ provides counts (and proportional abundances) of shed antlers with evidence of gnawing damage by carnivores or ungulates. ‘Rodent occurrences’ are counts of the locations where one or a collection of rodent bones were found within a few metres of each other, providing a measure of the frequency with which millimetre-sized bones were observed. Skeletal remains are readily available on the Jago Bitty calving ground.)
| terrain | sample plot | habitat | area (km2) | caribou bones and antlers (per km2) | shed antlers (per km2) | buried antlers (prop.) | gnawed antlers (prop.) | rodent occurrences |
|---|---|---|---|---|---|---|---|---|
| tussock tundra | T01–01 | tussock/hummocky tundra | 0.0996 | 4 (40) | 3 (30) | 1 (33%) | 2 (67%) | 8 |
| T01–03 | moist/wet sedge | 0.0984 | 33 (335) | 15 (152) | 5 (33%) | 4 (27%) | 7 | |
| riparian terrace | T01–02 | barren gravel/riparian shrub | 0.102 | 169 (1657) | 100 (980) | 13 (13%) | 59 (59%) | 12 |
| T01–04 | Dryas terrace | 0.101 | 135 (1337) | 114 (1129) | 7 (6%) | 71 (62%) | 3 | |
| pooled sum | 0.401 | 341 (850) | 232 (579) | 26 (11%) | 136 (59%) | 30 | ||
Table 2.
Results of gender differentiation for shed antlers in the ANWR bone assemblage and demographic breakdown of caribou MNI. (‘Female antlers’ is the cumulative mean probability and 95% CI of gender assignment for each sample plot. The mean proportion of antlers that are female is also provided. ‘Female sheds per kilometre’ provides the mean concentration of female antlers in each sample plot. ‘Neonatal MNI’ provides counts for each sample plot; the number of neonates with carnivore gnawing is provided parenthetically. Most neonatal specimens are composed of one or more bones from multiple body regions (see the electronic supplementary material, table S1).)
| terrain | sample plot | measured shed antlers | female antlers [95% CI] (proportion) | female sheds per km2 | neonatal MNI (no. gnawed) | adult and sub-adult MNI | proportion neonatal MNI |
|---|---|---|---|---|---|---|---|
| tussock tundra | T01–01 | 3 | 3 [3, 3] (100%) | 30 | 0 (0) | 1 | 0.0 |
| T01–03 | 15 | 14 [13, 15] (93%) | 142 | 1 (1) | 1 | 0.50 | |
| riparian terrace | T01–02 | 98 | 88 [84, 92] (90%) | 863 | 3 (3) | 2 | 0.60 |
| T01–04 | 112 | 99 [95, 104] (88%) | 980 | 1 (0) | 2 | 0.33 | |
| pooled sum | 228 | 203 [195, 214] (89%) | 509 | 5 (4) | 6 | 0.45 |
Concentrations of shed female antlers differ among habitats, with higher densities recovered from more openly vegetated riparian terraces (T01–02 and T01–04) than tussock tundra-dominated habitats (T01–01 and T01–03; table 1, χ12 = 692.371, 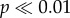 ). While these differences are in the same direction as an observational and/or burial bias against more highly vegetated areas, such sampling biases do not adequately explain the magnitude of difference in antler concentrations. For example, while burial rates owing to trampling and vegetation overgrowth may be higher in tussock tundra habitats, which have between three and nearly six times the proportion of ‘buried’ antlers (defined as buried 50% or more by soil or tundra), openly vegetated riparian terrace habitats yielded 7–38 times the number of total antlers (see table 1 and the electronic supplementary material, appendix B). Higher total antler concentrations on riparian terrace compared with tussock tundra-dominated habitats also persist when analyses are limited to the first two or three WSs, which includes only the most recent antler input, and is least likely to be buried or otherwise obscured from sampling (WS 0 + 1, χ21 = 6.224, p < 0.05; WS 0 + 1 + 2, χ21 = 49.131,
). While these differences are in the same direction as an observational and/or burial bias against more highly vegetated areas, such sampling biases do not adequately explain the magnitude of difference in antler concentrations. For example, while burial rates owing to trampling and vegetation overgrowth may be higher in tussock tundra habitats, which have between three and nearly six times the proportion of ‘buried’ antlers (defined as buried 50% or more by soil or tundra), openly vegetated riparian terrace habitats yielded 7–38 times the number of total antlers (see table 1 and the electronic supplementary material, appendix B). Higher total antler concentrations on riparian terrace compared with tussock tundra-dominated habitats also persist when analyses are limited to the first two or three WSs, which includes only the most recent antler input, and is least likely to be buried or otherwise obscured from sampling (WS 0 + 1, χ21 = 6.224, p < 0.05; WS 0 + 1 + 2, χ21 = 49.131, 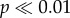 ). Similarly, equal ‘occurrences’ (small accumulations of bones within a few square metres) of rodents were found in tussock tundra as in more openly vegetated riparian terraces (table 1). Visibility of the ground surface within single sample plots was generally consistent, and our ability to detect bones with millimetre-scale dimensions in all sample plots suggests our capacity to find shed caribou antlers is generally high, even if they are somewhat obscured by vegetation. Taken together, this suggests differences in antler detection among examined habitats are probably not causal for observed discrepancies in bone accumulations, and that observed discrepancies are biologically meaningful.
). Similarly, equal ‘occurrences’ (small accumulations of bones within a few square metres) of rodents were found in tussock tundra as in more openly vegetated riparian terraces (table 1). Visibility of the ground surface within single sample plots was generally consistent, and our ability to detect bones with millimetre-scale dimensions in all sample plots suggests our capacity to find shed caribou antlers is generally high, even if they are somewhat obscured by vegetation. Taken together, this suggests differences in antler detection among examined habitats are probably not causal for observed discrepancies in bone accumulations, and that observed discrepancies are biologically meaningful.
Neonatal specimens were observed in three of the four (75%) ANWR sample plots and account for up to 60 per cent of the recovered skeletal individuals (MNI; table 2). While 80 per cent (four of five) of neonatal MNI included skeletal elements with tooth punctures and/or scrapes from carnivorous mammals (table 2), four of five MNI were also represented by paired appendages and/or skeletal elements from multiple body regions (e.g. limbs ± crania or vertebrae; electronic supplementary material, table S1), suggesting only moderate postmortem skeletal dispersal.
(c). Comparisons between Arctic National Wildlife Refuge and Yellowstone
The ANWR antler assemblage exhibits generally higher concentrations of antlers than Yellowstone (ANWRfemale versus Yellowstone: Mann–Whitney U = 116, p = 0.073, one-tailed; ANWRall antlers versus Yellowstone: Mann–Whitney U = 119, p = 0.058, one-tailed), with abundances reaching nearly an order of magnitude higher on the Jago Bitty calving ground than in many Yellowstone regions (figure 3a). Interestingly, the Yellowstone plots closest to the maximum ANWR female antler concentration are from localities that are heavily used by bull elk during the late-winter period of maximum antler shedding [15]. Sample-size standardizing the Yellowstone plots to those of ANWR accentuates differences between the two localities (figure 3a, ‘Yellowstone sample stand.’).
Figure 3.
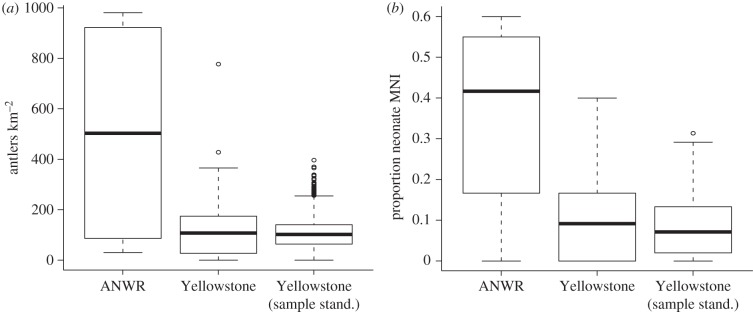
Bone survey results from the Jago Bitty calving ground, including (a) concentrations of female antlers (as estimated in figure 2) and (b) proportion of neonatal caribou. Concentrations of ANWR antlers and neonatal individuals (n = 4 sample plots) are higher than those recovered from Yellowstone (n = 40 sample plots) [15]. ‘Yellowstone (sample stand.)’ are the distributions of median values generated from a sample-size standardization of the Yellowstone data to match the sampling intensity of ANWR. Similar to ANWR, concentrations of shed antlers and neonatal remains in Yellowstone faithfully document regions used as late-wintering areas (where elk shed their antlers) and spring calving areas [15].
Compared with the Yellowstone bone assemblage, the ANWR calving ground shows significantly higher relative abundances of neonatal MNI (Mann–Whitney U = 128: p = 0.021, one-tailed, figure 3b; electronic supplementary material, appendix C). While neonatal remains in Yellowstone are also faithful to known elk calving grounds [15], even localities with the highest relative abundances of neonatal MNI are below the median concentration of neonates in ANWR. Sample-size standardizing the Yellowstone plots further accentuates these differences (figure 3b, ‘Yellowstone sample stand.’).
4. Discussion
(a). Antler pedicle size and gender differentiation
Antler pedicle surfaces of adult caribou show strong differences between females and males, permitting gender differentiation of adult antlers on landscape surfaces. Though antlers of sub-adult males are known to have some size overlap with adult females [25] (consistent with the small overlap between adult males and females shown here), which could confound gender differentiation, sub-adult males of the PCH are generally not known to migrate to calving areas with pregnant females. Furthermore, if they do, antlers from sub-adult males will have been shed prior to their arrival [30]. Thus, the possible minor inclusion of sub-adult male antlers should not alter overall patterns. Establishing additional morphological characteristics for differentiating sub-adult male and adult female antlers may be more important when studying caribou herds that undergo short spring migrations, where sub-adult males and parturient females may be more likely to drop their antlers in closer proximity.
(b). Bone accumulations indicate patterns of landscape use
The distribution of antlers among sampled habitats suggests that, when available, female caribou at Jago Bitty preferentially visit Dryas terrace and dwarf shrub within days of calving. While we do not know how long the skeletal neonates survived before perishing (many probably died within 48 h: [19]), the prevalence of multiple body regions (see the electronic supplementary material, table S1), suggests that most MNI were not widely dispersed postmortem (table 2). While determining how far calves were from their birthing site at death is non-trivial using skeletal data, the confluence of abundant shed antlers (which have relatively low transport risk from carnivores and ungulates) and only moderately dispersed neonatal remains, suggests skeletal concentrations delineate high-use areas (and possibly some calving sites) during calving and the first days of rearing (i.e. the calving period). Bone concentrations from these surveys are mutually highest in openly vegetated riparian habitats, which, including similar habitats on older river terraces some distance from active river-margins, compose less than 5–10% of the ANWR Coastal Plain and the PCH calving grounds [5,26]. Further study will test the consistency with which bone accumulations of riparian terraces differentiate themselves from those of nearby tussock tundra-dominated habitats (including moist-sedge, wet-sedge and herbaceous tussock tundra settings), which compose roughly 50 to over 70 per cent of PCH calving areas [5,26].
To further illuminate differential habitat use within the calving grounds, we compared observed with expected antler concentrations for the Jago Bitty field area based on available historical survey data on population size, demographics, parturition rates and patterns of geographical use (see figure 4 and the electronic supplementary material, text 6.0). To match the timeframe provided by aerial surveys (1983–2010), we only included antlers in more recent WSs (WS 0 through to 3; bones beyond WS 3 are probably from generations that predate the initiation of aerial surveys [8]). The expected antler concentration (based on uniform use of all habitats) falls near the median of the combined Jago Bitty bone accumulation, with openly vegetated riparian zones exhibiting antler concentrations above expectations and tussock tundra-dominated habitats showing lower concentrations than null expectations. While further data collection will test this finding, this comparison shows: (i) overall observed female antler accumulations are consistent with expectations based on available data on caribou use and demographics (expected antler concentration = 451 antlers km−2, median observed female antler concentration = 503 antlers km−2, electronic supplementary material, text 6.0), and (ii) habitats are used non-randomly during the calving period. Thus, even within a small geographical area (2 km radius), bone surveys suggest strong stratification in habitat use among female caribou. Regardless of precise use (e.g. calving sites, early rearing areas and/or migration corridors), openly vegetated riparian terraces show higher-than-expected use during the calving period. If their use is consistently high across PCH calving grounds, preservation of these areas should be a priority in future decisions regarding management and land-use changes, including natural resource development.
Figure 4.
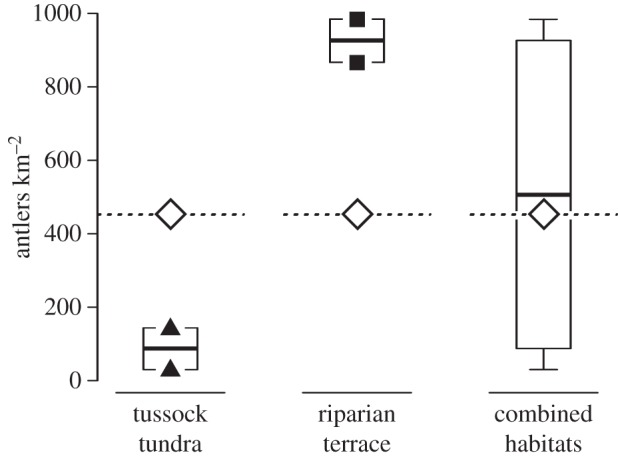
Observed (boxplots) versus expected (diamonds) female antler concentrations at the Jago Bitty calving ground. Expected antler concentration is based on aerial survey data from 1983 to 2010 (see the electronic supplementary material, text 6.0). To approximate the time period represented by aerial surveys, observed antler concentrations only include shed female antlers in WSs 0–3. Expected antler concentration at Jago Bitty is near the median of observed antler concentrations from the bone assemblage (combined habitats). Plots of tussock tundra-dominated habitats have lower-than-expected concentrations of antlers. Riparian terraces have higher-than-expected concentrations of shed female antlers. Data suggest parturient female caribou strongly stratify their habitat use during portions of the calving period in favour of riparian terraces.
Results of previous work on calving habitats of the PCH have also shown that riparian zones and dwarf shrub areas are selected by caribou cows in annual calving grounds [5,24], though tussock tundra-dominated habitats are generally considered the dominant calving habitat [5,24], particularly in densely used calving areas (i.e. ‘concentrated calving grounds’, sensu [5]). The Jago Bitty was part of the concentrated calving areas of the 1990s and early 2000s [5,24]. Thus, contrary to our data, there is some expectation that the most notable antler and neonatal bone assemblages should be recovered from regions of tussock tundra. If bone data are picking up calving sites themselves, discrepancies between bone and aerial surveys may largely be manifestations of differences in geographical scale and spatial resolution of the data. Bone surveys, for example, can differentiate and sample finer habitat patches than possible when aerial observations or GPS data are projected onto the coarser resolutions of many vegetation models. An additional contributing factor to differences in results may include the vegetation structure of Jago Bitty itself, where tussock tundra habitats include tightly spaced tussocks reaching 45 cm in height (approx. equal to or greater than neonatal caribou chest-heights [31]). Tussock tundra at Jago Bitty could be a particularly uncomfortable calving area for cows and may be difficult to navigate for newborn calves under predator stress, leading to increased use of flatter, more open areas. Further sampling across ANWR will explore the contribution of tussock habitat structure on abundance of neonatal MNI. If, instead, bone data are largely delineating regions of post-calving importance (not calving sites themselves), the mismatch between aerial and bone surveys speak to changes in habitat use during different stages of the calving period, with bone data providing a unique dataset on landscape use of mother and calf during the critical first few days of life. Overall, while tussock tundra habitats are important to the overall calving process [5,24,32], riparian terraces appear to have previously unrecognized significance during the calving period.
(c). Antler damage and loss
Previous descriptions of modern subarctic landscapes suggest that antlers are readily consumed by rodents, carnivores and ungulates [33,34] (gnaw marks from each are morphologically distinguishable [34]), impacting maximum survival duration of antlers on the landscape. While some gnawing of antlers in ANWR is common (59% of the antlers show damage from carnivores and/or caribou; table 1; electronic supplementary material, appendix B), damage is minimal to moderate and largely concentrated on protein-rich [35] distal antler ends. Importantly, no bones (including antlers) at the Jago Bitty show clear evidence of rodent gnawing (see the electronic supplementary material, text 7.0). This pattern of low damage and the lack of rodent gnawing is a significant departure from previous observations of bones in northern ecosystems [33,34]. On the Coastal Plain of ANWR (and, perhaps, on many arctic landscapes), antler loss rates appear more closely tied to bone weathering (decomposition) and burial (trampling and tundra overgrowth) than bone consumption, enhancing the potential of regional bone accumulations to supply valuable ecological data spanning centennial to millennial timescales.
(d). Antler weathering stage distribution mirrors multi-decadal patterns of caribou landscape use
While a precise WS calibration is not available for arctic bones, knowledge of bone weathering in other settings [8,28] provides preliminary expectations of the time encapsulated by the WS profile of antlers on Jago Bitty and the match between aerial surveys and the antler record. Historical aerial surveys show that the Jago Bitty was an important calving area in the 1980s and 1990s [5], but was not used as often in the early 2000s (calving did occur in 2005, 2006 and 2010 [5,7]). The WS distribution of shed female antlers in the Jago Bitty bone assemblage (figure 5) shows small numbers of antlers in WS 0 and WS 1, and a strong peak in WS 2 and WS 3. While antlers in WS 4 are present, antlers in WS 5 were not observed. In Yellowstone, bones do not generally stay in WS 0 beyond the first year postmortem, and only rarely persist in WS 1 beyond 5 years [28]. It is consistent that ANWR antlers in WSs 0 and 1 (which are probably weathering more slowly than Yellowstone bones [9–11]) record the limited PCH calving history occurring at Jago Bitty over the last decade; with the greater abundance of antlers in WS 1 emphasizing calving during 2005 and 2006. Yellowstone bones can last in WSs 2 and 3 for at least 10 and 30 years, respectively [28], suggesting the observed peak in ANWR antlers probably records calving from the 1990s and 1980s. Yellowstone bones can persist in WSs 4 and 5 for up to two centuries [8], suggesting that ANWR antlers in WS 4 may include (or be dominated by) calving events prior to the initiation of aerial surveys. We also note that overall extended weathering durations in arctic settings [9–11], suggest that some antlers in WS 3 may also predate modern biomonitoring (which may contribute to the high concentration of antlers observed in figure 4). Radiocarbon dating will establish the temporal resolution of WSs and maximum survival durations of antlers and neonatal bones in ANWR. While the neonatal bone record is probably shorter than the antler record [27], the co-recovery of neonatal bones and female-dominated antler concentrations in this recent calving area suggests that calving grounds from more distant historical periods (such as those probably represented by antlers in WS 4 and possibly WS 3 at Jago Bitty) may still be identified from antler remains alone.
Figure 5.
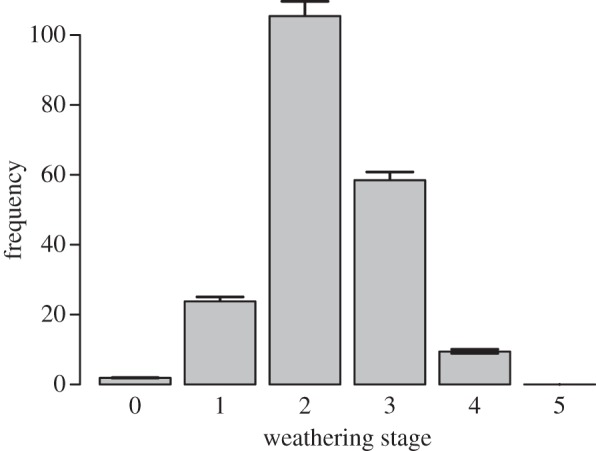
WS frequency distribution of shed female antlers mirrors historical caribou use of the Jago Bitty calving ground over the last few decades (including the few months prior to sampling; the presence of WS 0 antlers). The lack of antlers in WS 5 suggests that Jago Bitty may not have had the same prominence as a calving ground for some historical generations. Error bars are 95% CIs based on probabilistic gender assignment of antlers.
The WS distribution of Jago Bitty antlers also indicates how bone surveys may provide historical data on caribou landscape use that complement and expand traditional survey techniques. The distribution of bone WSs, for example, can faithfully track major changes in population abundance or habitat use of ungulates across decadal timescales [8,13]. Similarly, the lack of WS 5 antlers and bones at Jago Bitty (figure 5) may be an indication that this locality was not widely used as a calving area during some historical periods (WS 5 bones have been observed from other PCH calving grounds; electronic supplementary material, text 8.0). Furthermore, geographically extensive bone surveys (including regions not previously known to host calving) may identify calving grounds that were historically important, but have gone unused in the last few decades. Using skeletal data to test our current understanding of calving ground geography and variability may help us understand the broader patterns and climatic drivers of calving ground choice and, in turn, lead to improved projections of future needs. This expanded dataset will enable a fuller understanding of how caribou have used arctic landscapes prior to and after the initiation of modern climatic and anthropogenic pressures, and help manage these ecologically, culturally, economically and internationally important herds.
(e). Arctic National Wildlife Refuge and Yellowstone death assemblages reveal meaningful differences between ecosystems
The recovery of expected differences between the ANWR and Yellowstone death assemblages in antler concentrations and neonatal proportional representation offers an encouraging, further endorsement of the ecological data contained in landscape bone accumulations. Fundamental differences in the ecological (e.g. population densities and migratory patterns) and bone weathering settings allow these populations to faithfully discriminate themselves by their skeletal records (particularly their proportional abundances of neonatal MNI). A growing appreciation for the biological data contained in death assemblages has come from repeatedly observing high similarities between biological metrics of living source communities and their death assemblages (i.e. live–dead studies [8,13–15,36]). It is, perhaps, not surprising that bone-based comparisons (dead–dead studies) among different ecosystems and taphonomic settings (the set of biotic and abiotic variables that accumulate and/or remove skeletal material) can also provide meaningful information. While more exhaustive study of landscapes across ANWR and elsewhere is warranted, the Jago Bitty calving ground currently holds the highest recorded proportional abundance of neonatal remains in a modern bone assemblage, further illustrating the biological and taphonomic uniqueness of this area and, probably, the PCH calving grounds more broadly.
5. Conclusions
In this study, we demonstrate that adult female and male caribou antlers can be differentiated using simple morphological measurements. We further provide, to our knowledge, the first documentation that caribou calving grounds can develop measurable accumulations of shed female antlers and bones of neonatal fatalities, and that landscape bone accumulations provide unique data on fine-scale habitat use during the calving interval. More extensive bone surveys will continue to test the ecological fidelity with which calving activity is captured in bone assemblages and compare current geographical patterns of caribou calving (and its variability) against historical states. For the PCH, extended perspectives on the long-term value of regions across the ANWR Coastal Plain may also inform predictive models relating to calving ground choice, which can help inform wildlife management and conservation policy, and contribute to the preservation of successful calving for future decades. Taken together, complementary data from bone and aerial surveys can resolve a more detailed understanding of ecosystems and their recent and historical changes. These insights are valuable across the arctic, including Alaska, Canada, Russia and Scandinavia, for unveiling historical ecological data on landscape use (from single localities to regional perspectives) of caribou and other arctic fauna. Finally, fossil caribou antlers are available in Pleistocene deposits, suggesting that if postmortem transport can be constrained, the biogeography of caribou calving and breeding (from shed male antlers; see [37] for a modern example) may be extended by hundreds of thousands of years or longer.
Acknowledgements
We thank J. Jorgenson, M. Boldenow and W. Elsner for their assistance in the field and D. C. Payer and US Fish and Wildlife for providing logistical support for fieldwork. The University of Alaska Fairbanks provided additional logistical support. We thank J. Jorgenson, E. Wald, D. C. Payer, L. Lawson and three anonymous reviewers for helpful comments on previous drafts of this manuscript. We also thank E. Westwig (American Museum of Natural History), B. Stanley and L. Heaney (Field Museum of Natural History), L. Olson, B. Jacobsen and A. Gunderson (University of Alaska Museum) for access to specimens and their facilities.
References
- 1.National Research Council 2005. The geological record of ecological dynamics: understanding the biotic effects of future environmental change. Washington, DC: National Academies Press [Google Scholar]
- 2.Butchart SHM, et al. 2010. Global biodiversity: indicators of recent declines. Science 328, 1164–1168 10.1126/science.1187512 (doi:10.1126/science.1187512) [DOI] [PubMed] [Google Scholar]
- 3.Saenz-Arroyo A, Roberts CM, Torre J, Carino-Olvera M, Enriquez-Andrade RR. 2005. Rapidly shifting environmental baselines among fishers of the Gulf of California. Proc. R. Soc. B 272, 1957–1962 10.1098/rspb.2005.3175 (doi:10.1098/rspb.2005.3175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGill BJ, Hadly EA, Maurer BA. 2005. Community inertia of Quaternary small mammal assemblages in North America. Proc. Natl Acad. Sci. USA 102, 16 701–16 706 10.1073/pnas.0504225102 (doi:10.1073/pnas.0504225102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffith B, Douglas DC, Walsh NE, Young DD, McCabe TR, Russell DE, White RG, Cameron RD, Whitten KR. 2002. The Porcupine Caribou Herd. In Biological sciences report (eds Douglas DC, Reynolds PE, Rhode EB.), pp. 8–37 Reston, VA: US Geological Survey, Biological Resources Division, Biological Sciences Report USGS/BRD/BSR-2002–0001 [Google Scholar]
- 6.Moritz R, Bitz C, Steig E. 2002. Dynamics of recent climate change in the Arctic. Science 297, 1497–1502 10.1126/science.1076522 (doi:10.1126/science.1076522) [DOI] [PubMed] [Google Scholar]
- 7.Caikoski J. 2010. Porcupine Caribou Herd calving and post-calving surveys, June–July 2010. Alaska Department of Fish and Game, Division of Wildlife Conservation. Memorandum (12-8-10), 6
- 8.Miller JH. 2011. Ghosts of Yellowstone: multi-decadal histories of wildlife populations captured by bones on a modern landscape. PLoS ONE 6, e18057. 10.1371/journal.pone.0018057 (doi:10.1371/journal.pone.0018057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douglas M, Smol J, Savelle J, Blais J. 2004. Prehistoric Inuit whalers affected Arctic freshwater ecosystems. Proc. Natl Acad. Sci. USA 101, 1613–1617 10.1073/pnas.0307570100 (doi:10.1073/pnas.0307570100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meldgaard M. 1986. The Greenland caribou—zoogeography, taxonomy, and population dynamics. Meddelelser om Gronland, Bioscience 20, 1–88 [Google Scholar]
- 11.Sutcliffe AJ, Blake W., Jr 2000. Biological activity on a decaying caribou antler at Cape Herschel, Ellesmere Island, Nunavut, high arctic Canada. Polar Rec. 36, 233–246 10.1017/S0032247400016491 (doi:10.1017/S0032247400016491) [DOI] [Google Scholar]
- 12.Behrensmeyer AK, Western D, Dechant Boaz DE. 1979. New perspectives in vertebrate paleoecology from a recent bone assemblage. Paleobiology 5, 12–21 [Google Scholar]
- 13.Western D, Behrensmeyer AK. 2009. Bone assemblages track animal community structure over 40 years in an African savanna ecosystem. Science 324, 1061–1064 10.1126/science.1171155 (doi:10.1126/science.1171155) [DOI] [PubMed] [Google Scholar]
- 14.Terry RC. 2010. The dead do not lie: using skeletal remains for rapid assessment of historical small-mammal community baselines. Proc. R. Soc. B 277, 1193–1201 10.1098/rspb.2009.1984 (doi:10.1098/rspb.2009.1984) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller JH. 2012. The spatial fidelity of skeletal accumulations: elk wintering and calving grounds revealed by bones on the Yellowstone landscape. Ecology 93, 2474–2482 10.1890/12-0272.1 (doi:10.1890/12-0272.1) [DOI] [PubMed] [Google Scholar]
- 16.Espmark Y. 1971. Antler shedding in relation to parturition in female reindeer. J. Wildl. Manag. 35, 175–177 10.2307/3799887 (doi:10.2307/3799887) [DOI] [Google Scholar]
- 17.Whitten KR. 1995. Antler loss and udder distention in relation to parturition in caribou. J. Wildl. Manag. 59, 273–277 10.2307/3808940 (doi:10.2307/3808940) [DOI] [Google Scholar]
- 18.Fancy SG, Whitten KR, Pank LF, Regelin WL. 1989. Seasonal movements of caribou in arctic Alaska as determined by satellite. Can. J. Zool. 67, 644–650 10.1139/z89-093 (doi:10.1139/z89-093) [DOI] [Google Scholar]
- 19.Whitten KR, Garner GW, Mauer FJ, Harris RB. 1992. Productivity and early calf survival in the Porcupine Caribou Herd. J. Wildl. Manag. 56, 201–212 10.2307/3808814 (doi:10.2307/3808814) [DOI] [Google Scholar]
- 20.Houston DB. 1982. The Northern Yellowstone elk: ecology and management. New York, NY: Macmillan [Google Scholar]
- 21.Northern Yellowstone Cooperative Wildlife Working Group 1988–2007. Late winter elk classification reports (9 annual reports) issued by NPS, Montana Fish, Wildlife, and Parks, USFS, and USGS. Copy on file at the Yellowstone Center for Resources, Yellowstone National Park, WY, USA [Google Scholar]
- 22.Faith JT, Behrensmeyer AK. 2006. Changing patterns of carnivore modification in a landscape bone assemblage, Amboseli Park, Kenya. J. Archaeol. Sci. 33, 1718–1733 10.1016/j.jas.2006.03.004 (doi:10.1016/j.jas.2006.03.004) [DOI] [Google Scholar]
- 23.Barber-Meyer SM, Mech LD, White PJ. 2008. Elk calf survival and mortality following wolf restoration to Yellowstone National Park. Wildl. Monogr. 169, 1–30 10.2193/2008-004 (doi:10.2193/2008-004) [DOI] [Google Scholar]
- 24.Fancy SG, Whitten KR. 1991. Selection of calving sites by Porcupine Herd caribou. Can. J. Zool. 69, 1736–1743 10.1139/z91-242 (doi:10.1139/z91-242) [DOI] [Google Scholar]
- 25.Johnson DR, Nagorsen DW. 1990. Evaluation of cranial and antler characteristics to determine sex of moutain caribou, Rangifer tarandus. Can. Field Nat. 104, 583–584 [Google Scholar]
- 26.Jorgenson JC, Joria PC, Douglas DC. 2002. Vegetation mapping of the Arctic Refuge Coastal Plain. In Arctic Refuge Coastal Plain terrestrial wildlife research summaries (eds Douglas DC, Reynolds PE, Rhode EB.), pp. 4–7 US: Geological Survey, Biological Resources Division, Biological Sciences Report USGS/BRD/BSR-2002–0001 [Google Scholar]
- 27.Behrensmeyer AK. 1978. Taphonomic and ecologic information from bone weathering. Paleobiology 4, 150–162 [Google Scholar]
- 28.Miller J. 2009. The large-mammal death assemblage of Yellowstone National Park: historical ecology, conservation biology, paleoecology. Chicago, IL: The University of Chicago [Google Scholar]
- 29.R Development Core Team 2012. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org [Google Scholar]
- 30.Fuller TK, Keith LB. 1980. Physical characteristics of woodland caribou in Northeastern Alberta. Can. Field Nat. 94, 331–333 [Google Scholar]
- 31.Parker K. 1989. Growth rates and morphological measurements of Porcupine caribou calves. Rangifer 9, 9–13 10.7557/2.9.1.769 (doi:10.7557/2.9.1.769) [DOI] [Google Scholar]
- 32.Thomas DL, Johnson D, Griffith B. 2006. A Bayesian random effects discrete-choice model for resource selection: population-level selection inference. J. Wildl. Manag. 70, 404–412 10.2193/0022-541X(2006)70[404:ABREDM]2.0.CO;2 (doi:10.2193/0022-541X(2006)70[404:ABREDM]2.0.CO;2) [DOI] [Google Scholar]
- 33.McCabe RA. 1957. Observations on the disappearance of shed caribou antlers. J. Mammal 38, 275–277 10.2307/1376336 (doi:10.2307/1376336) [DOI] [Google Scholar]
- 34.Wika M. 1982. Antlers: a mineral source in Rangifer. Acta Zool. 63, 7–10 10.1111/j.1463-6395.1982.tb00752.x (doi:10.1111/j.1463-6395.1982.tb00752.x) [DOI] [Google Scholar]
- 35.Landete-Castillejos T, Estevez J, Martinez A, Ceacero F, Garcia A, Geallego L. 2007. Does chemical composition of antler bone reflect the physiological effort made to grow it? Bone 40, 1095–1102 10.1016/j.bone.2006.11.022 (doi:10.1016/j.bone.2006.11.022) [DOI] [PubMed] [Google Scholar]
- 36.Kidwell SM. 2001. Preservation of species abundance in marine death assemblages. Science 294, 1091–1094 10.1126/science.1064539 (doi:10.1126/science.1064539) [DOI] [PubMed] [Google Scholar]
- 37.Miller F, Barry SJ. 1992. Nonrandom distribution of antlers cast by Peary Caribou bulls, Melville Island, Northwest Territories. Arctic 45, 252–257 [Google Scholar]