Abstract
Honeybees, like other insects, accumulate electric charge in flight, and when their body parts are moved or rubbed together. We report that bees emit constant and modulated electric fields when flying, landing, walking and during the waggle dance. The electric fields emitted by dancing bees consist of low- and high-frequency components. Both components induce passive antennal movements in stationary bees according to Coulomb's law. Bees learn both the constant and the modulated electric field components in the context of appetitive proboscis extension response conditioning. Using this paradigm, we identify mechanoreceptors in both joints of the antennae as sensors. Other mechanoreceptors on the bee body are potentially involved but are less sensitive. Using laser vibrometry, we show that the electrically charged flagellum is moved by constant and modulated electric fields and more strongly so if sound and electric fields interact. Recordings from axons of the Johnston organ document its sensitivity to electric field stimuli. Our analyses identify electric fields emanating from the surface charge of bees as stimuli for mechanoreceptors, and as biologically relevant stimuli, which may play a role in social communication.
Keywords: electric surface charge, mechanoreceptors as sensors for electric fields, Johnston organ, learning of electric field stimuli
1. Introduction
Ever since Exner's [1] discovery in 1895 that the feathers of birds and the hairs of mammals store the electric charge created by friction with other materials and by air, the phenomenon of surface charge in animals and humans has been known. Heuschmann [2] added to this the observation that insect cuticle also accumulates electric charge by friction. In insects, the wax layer of the exocuticle causes a high electrical resistance between the inner and outer parts of the body, and along the body surface leading to charge accumulation when insects walk or fly [3–5]. It is now clear that surface charge in insects is a biological phenomenon reflecting their movements and the movements of their body parts [6]. It is also clear that insects respond to electric fields, e.g. by avoidance [7] or by modifying their locomotion [8]. However, it is less clear whether electric fields serve a biological function and whether they are sensed by the insect itself.
Naturally occurring surface charge is thought to play a role in pollination [9–11] reviewed by [12], since flowers were found to be usually negatively charged, whereas the arriving insects appear to carry a positive surface charge. The electric charge that accumulates on the cuticle of flying insects discharges only partially upon landing because of the high contact resistance between legs and ground. Warnke [13] in his early studies assumed a current flow between the bees' body parts and from the cuticle to the ground to explain the effects of natural surface charge, but this may not be necessary for sensing movements, because it could be that the cuticular structures of mechanoreceptors are charged as well; and therefore, they may be moved by the coulomb forces in an electric field. In fact, electric fields have been used as well-defined stimuli in physiological studies of mechanoreceptors in Drosophila, where electrostatically induced movements of the antennal arista caused by coulomb forces were used to probe the transduction mechanism in antennal mechanoreceptor cells [14–17]. In these latter studies, voltages of less than or equal to 4 V delivered at a distance of 0.3 mm from the arista resulted in effective forces of less than or equal to 100 pN and displacements of the arista of up to ca 2 µm [14]. Given that flying insects accumulate surface charges of more than 100 V, electric fields emanating from the insect body may constitute biologically relevant signals. In such a situation, no current needs to flow from or to the insect body. However, an essential requirement is that the cuticular structures of the mechanoreceptors are also charged. As one can learn from any physics textbook, electric charge on the surface of an insulator is fixed, thus it will not move along the surface and it is rather the insulator (e.g. the cuticular structure of a mechanoreceptor) that will be moved in the gradient of a constant electric field or by a modulated electric field.
Here, we ask what kind and under which conditions honeybees produce electric fields, how they sense them, whether they respond to them and whether they learn them. We deal with constant and modulated electric fields emanating from stationary or moving cuticle surfaces. Thus, depending on its own movement, the body of a bee is surrounded by both static and changing electric fields. Since wings and other body parts can also rub against each other during other body movements (e.g. during wing fanning as observed in singing drosophilid flies [18]); bees and insects in general may refresh their electric charge and emanate modulated electric fields when walking.
To assess whether and how electric fields are produced and received in honeybees, we (i) measured the static and modulated electric fields of flying, landing and dancing bees, (ii) monitored the movement of the antennal flagellum, and (iii) associated the activity of the mechanosensory neurons of Johnston's organ (JO). Then we asked whether bees perceive electric fields by exposing them to such fields under different test conditions. By training the bees to constant and modulated electric fields, we examined whether the JOs of the antennae are potentially involved in sensing the electric field. We found that bees emanate modulated electric fields when moving their wings and body during the waggle dance. When bees walking stationary on a treadmill were stimulated with the modulation patterns of these electric fields in the absence of any mechanical stimulation they responded selectively and more strongly to the natural field patterns. Bees were also able to associate constant and modulated electric fields with reward. Ablation experiments show that mechanoreceptors of the antennae are most probably involved in sensing electric fields.
2. Material and methods
(a). Measurement of the electric fields of arriving and dancing bees
The basics of physical conditions for the coupling between charged insulators and their effective coulomb forces are described in the electronic supplementary material, figure S1a,b. We also describe the calibration of the electrometer for measuring the electric charge of the wing beat in the electronic supplementary material, figure S2. The electric charge carried by bees arriving at the hive entrance was measured with an electrometer array consisting of eight electrodes (see the electronic supplementary material, figure S3a). The electrodes picked up the electric field at a distance of 2–3 cm, both during the last 1–2 cm of flight and after landing. In all our charge measuring devices described below, we made sure that there was no conductive material close by or between the animal and the detector potentially influencing the signals. Similarly, the dialectic material was selected for low permittivity.
The sensor for measuring the electric fields of dancing bees consisted of a transparent flat sensor consisting of two round Plexiglas plates at a distance of 0.5 mm filled with a low concentration (0.1 molar) of an electrolyte (KCl). The sensor was located directly above the respective animal in an open observation hive and also allowed video recording of the dance communication process (figure 1). The conducting fluid between the two transparent plates made contact via a ring-like Ag wire (diameter 0.5 mm) to the input of an AC amplifier mimicking the average diameter of the dance rounds. The location of the sensor for measuring the electric fields of dancing bees was chosen according to the area used by the dancing bee. Such geometry of the flat electrode was necessary for detecting the complex spatial–temporal movements of the body parts of the dancing bee. The concentration of KCl was kept low (0.1 M) to avoid strong electric field reflections. The amount of electric field reflection was measured by determining the strength of the electric field in front and behind the sensor and was found to be less than 10 per cent for this low KCl concentration. The low-noise AC amplifier (AD624, Analog Devices, bandwidth 1 Hz–10 kHz) was connected to an AD converter (CED1401, Cambridge Instruments). The hive was set up in a Faraday cage. As figure 1 shows, the input voltage divider based on the capacitor between animal and sensor and the input resistor was calibrated with a sine wave generator at the wing beat frequency. Further details of the calibration of the transmission of power are given in the electronic supplementary material, figures S1 and S2. The level of power emitted by a flying bee was found to be at least 1.78 µW at the input resistor Ri (100 MΩ). The spatial coverage of the measuring device equalled 10 per cent of the full sphere. Note that the total amount of emitted power from the animal is higher because the electrode receives only a fraction of the power emanating from the animal.
Figure 1.
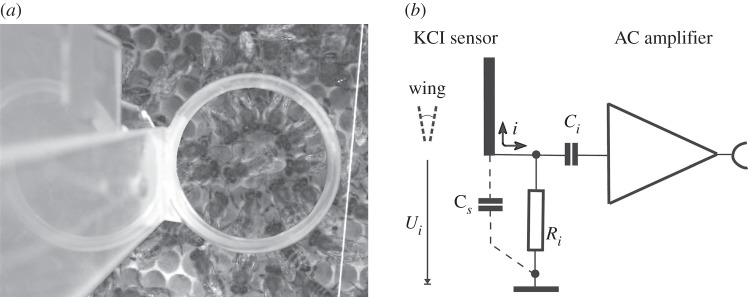
Measuring the electric field of a dancing bee. (a) Measuring arrangement for the electric field. The transparent KCl electrode allows us to view and video record the targeted animal. The dancing bee is seen in the middle surrounded by 12 attending bees. Note that the attending bees are positioned around the dancing bee. A silver wire inside the flat sheet of KCl solution is connected to the input of the AC amplifier. (b) The bee's body with moving wings and the KCl electrode (vertical bar) function as a coupling input capacitor. The voltage Ui produced by the wing beat of the bee depends mainly on the distance between the flat KCL electrode and the animal (see the electronic supplementary material, figures S1 and S2). The capacitor Cs is minimized to avoid input signal loss. The low-noise instrumentation amplifier has a gain of 100× to shift signal levels above noise from the set-up. The input resistor Ri is set to 100 MΩ to avoid static load from the KCL electrode. The input capacitor Ci is 10 nF. (See the electronic supplementary material for the calibration of this recording device.)
(b). Vibrations of the antennal flagellum
To measure sound and electric field-induced vibrations of the antennal flagellum, bees were affixed ventrum down on top of a Teflon rod with beeswax (see the electronic supplementary material, figure S4). Prior to taking the measurements, the head, wings, legs and the base of the antenna were stabilized with wax to minimize movement. All measurements were carried out at room temperature (20°C–23°C) on a vibration isolation table (Technical Manufacturing Corporation (Peabody, USA)). The experimental set-up has already been published [19]. In short, stimulus-induced vibrations were measured in non-loading condition at the tip of the antennal flagellum using a PSV-400 scanning laser Doppler vibrometer with a close-up unit (7 cm focal length) and a DD-5000 displacement decoder (Polytec, Waldbronn). The rod holding the bee was placed at focal length (7 cm) from the laser Doppler vibrometer, coaxially to the direction of mechanical actuation. The position of the laser spot (ca 50 μm diameter) was controlled online during the measurements using a coaxial video system of the vibrometer (see the electronic supplementary material, figure S4). Flagellar displacement amplitudes were determined as Fourier amplitudes (Hanning window, 1 Hz frequency resolution) at the frequency of stimulation (for details, see [19]).
(c). Neural responses of the Johnston organ
In order to measure the neural responses of the mechanoreceptors in the Johnston organ, an antenna was cut at its base close to the head capsule and the scapus was pushed over the tip of a sharpened 200 µm tungsten wire acting as ground electrode. The cut end was quickly sealed and glued to the wire with UHU Bastelkleber (without solvent, UHU GmbH, Brühl, Germany) to prevent it from drying out and rotating around the wire. Another sharpened tungsten wire was used as an active electrode and its tip was gently inserted into the soft cuticle between scapus and pedicel. The signals were amplified (100×) using the head stage of a preamplifier (npi electronic, Tamm, Germany). The high pass of the filter was set to 30 Hz and the low pass to 1 kHz. Hum noise (50 Hz) was eliminated by an additional filter (Hum Bug; Digitimer, Hertfordshire, UK). Neural activity was sampled at a rate of 20 kHz through an analogue-to-digital converter (1401 micro MKII; Cambridge Electronic Design, Cambridge, UK) and initial data analysis was performed by Spike2 software (Cambridge Electronic Design), including signal storage. The flagellum was stimulated by a Styrofoam ball that was friction-charged to about 100 V by rubbing it with a cotton tissue or with an electrode (an exposed 3 mm wire of a shielded coax cable) at a distance of 3 mm to the flagellum. Styrofoam was used because its electric charge could be measured by mounting it on a moving stick driven by a loudspeaker or Piezo stepper at a defined distance in front of a calibrated electrode connected to the electrometer described earlier. The detector described in figure 1 was calibrated as explained in the electronic supplementary material, figures S1 and S2. Using a micromanipulator, the Styrofoam ball was slowly moved towards the flagellum (final distance of 3 mm) thus avoiding any mechanical stimulation. For modulated electric field stimulation, a shielded cable with an exposed end (3 mm) was placed at a distance of 3 mm from the flagellum. The voltages varied between 20 and 100 Vpp (Vpp is voltage peak-to-peak) produced by a function generator.
(d). Behavioural analysis
Behavioural analyses were performed under two conditions. (i) Animals performing stationary walking on a Styrofoam ball (diameter 8 cm) floating on an air stream were stimulated with electric and acoustic signals (see the electronic supplementary material, figure S5 and video S1). Electric field stimulation was performed via an exposed (3 mm) wire placed at a distance of 3 mm from the animals' antennae simulating a ratio of more than 4 : 1 surface area between wing and flagellum (see the electronic supplementary material on capacitive coupling and figure S1). The wire was connected to a shielded cable reaching voltages between 20 and 80 Vpp. Acoustic signals were broadcast via a loudspeaker (Ekulit, Piezo-AL-60P12) driven by an audio amplifier (Pioneer, aux input gain 10). The acoustic signal ranged from a sound particle velocity of 0.05–50 mm s−1. The movement of the flagellae induced by the respective stimulation was recorded by a web camera and analysed frame–by-frame offline.
(ii) For training bees to electric fields, foragers were collected at the hive entrance, cooled individually on ice and fixed to a plastic tube. After feeding to satiation with 1.25 molar sugar solution, they were stored overnight in a humid chamber. The bees were conditioned either to a static electric field or to a modulated electric field following the procedure developed for olfactory stimuli [20]. In the first case, a static electric field was produced by friction-charging a piece of Styrofoam to about 100 V (charge measurement see above) at a distance of 3 mm from the antennae. The bees were differentially conditioned by forward pairing of this electric field (as the conditioned stimulus, CS+) for 3 s, and then feeding sucrose solution for 3 s (1 s overlap with electric field stimulation). Ten minutes later a similar piece of uncharged Styrofoam was presented without sucrose reward (specifically unpaired stimulus, CS−). Three CS+ and three CS− trials were given to each bee at trial intervals of 10 min. A conditioned response (CR) was recorded when the animal extended its proboscis during the first 2 s of CS+ or CS− presentation. Two hundred animals were tested. In the second set of experiments, the CS+ was a modulated electric field (160 Vpp, 60 Hz produced by a function generator) emitted from a probe 3 mm above the bee's antenna. Fifty-three animals were tested. The training trials were always performed with the shortest distance to the stimulating source, and the distance varied in extinction trials. Four distances were tested (1.0, 0.5, 0.75 and 0.6 cm tests in this order, n = 36). To test whether learning and responding to the electric field stimulation required charged antennae, we washed the antennae with water to eliminate any surface charge. To examine whether the antennae were involved in receiving the electric field, we either covered the flagellae and/or the scapus with wax, fixed the scapus to the head or pedicel to the flagellum with a drop of wax, or removed the antennae by cutting them at the base of the scapus. Wax was applied because of its high electrical resistance. The wax cover was uncharged when applied. A control of the effect of wax on the charge of the antennae consisted in conditioning animals which managed to free the respective joints of the antennae by the muscles in the antennae without removing the wax layer.
Statistical analysis. We used the Statistica (Statsoft, Inc. Tulsa, USA) package to evaluate the data. Data presented in figures 5–7 were analysed by ANOVA or ANOVA for repeated measurements. Further details are given in the legends to the figures.
Figure 5.
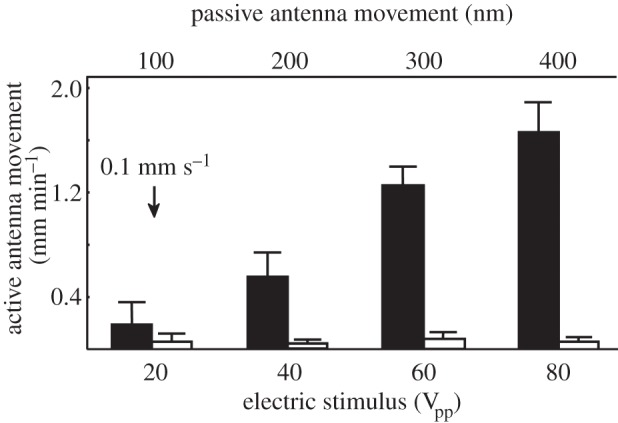
Flagellar displacement of resting animals to electric field stimuli of four different strengths (black bars). As control the same animals were exposed to similar manipulations, but electric field was switched off in every second trial (open bars). The ordinate at the base gives the flagellar displacement in millimetres relative to the respective resting position. Four electric field stimulus strengths of the dance pattern (figure 2) were tested (20, 40, 60 and 80 Vpp, at a distance of 3 mm to the antennae) in six animals in random order. The average amplitude of flagellar displacement as estimated by laser vibrometry is given on the top scale for each stimulus strength. The significantly larger flagellar displacement for the two higher stimuli indicates an active component leading to amplitudes of 1.5 mm that correspond to an angular movement of 50°. The arrow marks the flagellar responses to acoustic stimuli induced by the same dance pattern at 0.1 mm s−1 (at a distance of 10 mm). Responses for 40, 60 and 80 Vpp are significantly different from all control groups (between groups: d.f. = 1, d.f.error = 76, F = 185.82, p < 0.01 ANOVA repeated measures, between stimuli: d.f. = 3, d.f.error = 228, F = 25.25, p < 0.01 ANOVA repeated measures), and the responses for 20 Vpp (post hoc comparison C20 versus R20 n.s.).
Figure 7.
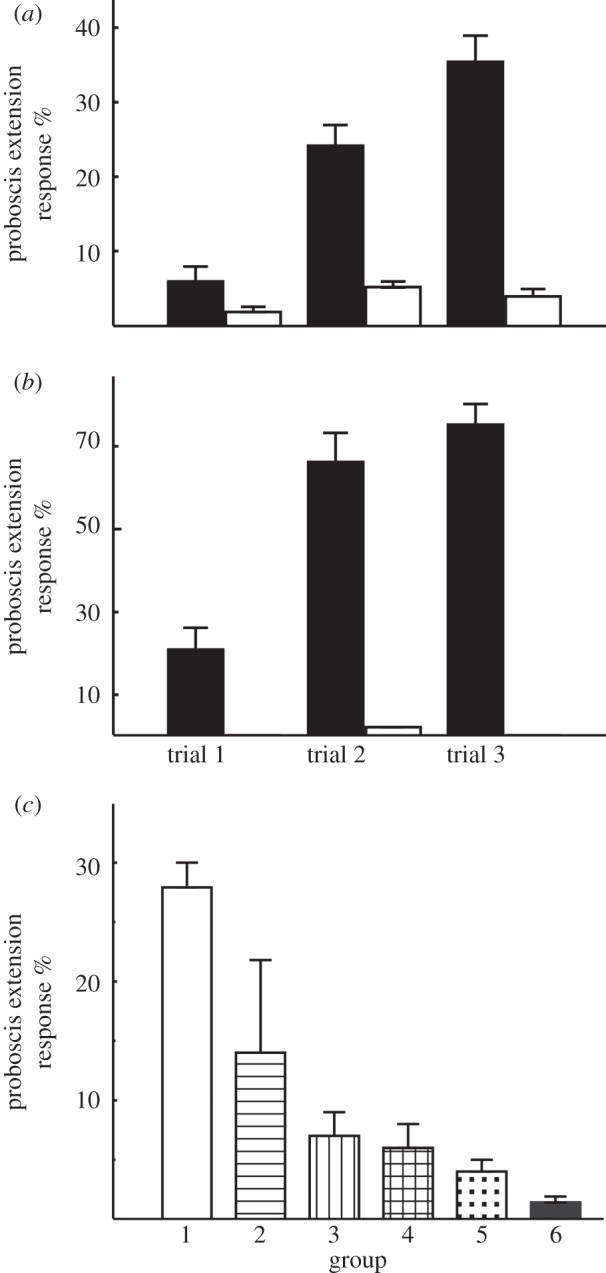
Response acquisition during classical reward conditioning of constant or modulated electric fields. (a) Conditioning of constant electric fields emitted from a charged piece of Styrofoam. The response to CS+ (charged Styrofoam) increased with the number of training trials (closed bars, abscissa) but did not change for the CS− (open bars, uncharged Styrofoam). All response values to the CS+ are significantly different to those to CS− (between groups: d.f. = 1, d.f.error = 96, F = 133.67, p < 0.01 ANOVA repeated measures, between stimuli: d.f. = 2, d.f.error = 192, F = 7.713, p < 0.02 ANOVA repeated measures). (b) Classical reward conditioning of a modulated electric field. The response to CS+ increased with the number of training trials (closed bars, abscissa) but did not change for the CS− (open bars, source without voltage). The response values for all three trials to the CS+ are significantly different to those to CS− (between groups: d.f. = 1, d.f.error = 52, F = 33.67, p < 0.01 ANOVA repeated measures, between stimuli: d.f. = 2, d.f.error = 104, F = 5.143, p < 0.001 ANOVA repeated measures). (c) The effect of manipulation of the antennae on the CR to a constant electric field as provided by charged Styrofoam. Group 1: no preparation of the antennae (control group). Group 2: the flagellae were coated with wax. Group 3: the scapus was fixed to the head. Group 4: the antennae were fully covered with wax, but joints between flagellum and pedicel (JO) were free of wax. Group 5: antennae were fully covered with wax. Group 6: antennae were removed. Group 1 is significantly different from all other groups: d.f. = 5, d.f.error = 101, F = 74.5, p < 0.01 ANOVA repeated measures).
Electronic supplementary material. http://www.honeybee.neurobiologie.fu-berlin.de/Reception and Learning of Electric Fields ESM.html.
3. Results and discussion
(a). Bees as a source of electric fields
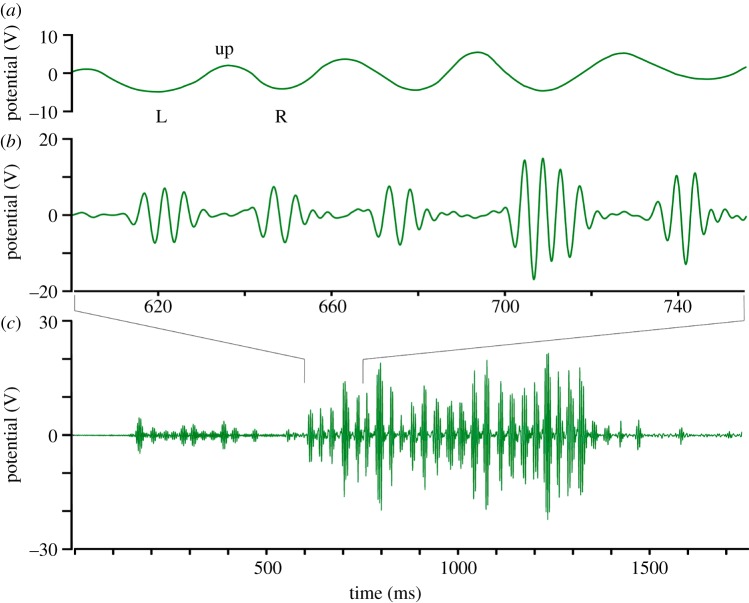
Electric charge of bees arriving at the hive entrance results in a voltage of 0–450 V as measured by a calibrated electrometer array at a distance of 2–3 cm (see the electronic supplementary material, figure S3b). The body charge dissipates very little on landing (usually less than 5%) and stays high also within the hive because the relative humidity in the hive is usually lower than outside (U. Greggers 2009, 2010, personal observations: less than 60% even at greater than 90% outside), and fanning behaviour with additional dielectric charging as well as movement inside the hive leads to an overall accumulation of charge for all bees. Dancing bees not only emit airborne signals (e.g. jet streams) by vibrating the thorax and the wings [21,22] but also electric fields. We measured the electric fields emanating from 40 dancing bees in 300 waggle runs and analysed the middle section of each straight run (450 ms) by comparing high-speed video recordings with the time course of the electric fields. The furthest side-wise extension of the abdomen together with the extreme position during the rolling behaviour was located in the high-speed video stream, and the corresponding reading of the electric signal was determined. These analyses allowed us to relate the modulation of the electric field to the movements of the abdomen and the wing beats. A typical example of body movement and electric field during the waggle run is shown in figure 2.
Figure 2.
The pattern of electric fields produced by a dancing bee at two temporal resolutions. The electric field pattern was recorded with the transparent sensor (figure 1). The high-frequency components of the full electric field pattern produced during the straight waggle phase lasting for 1700 ms are shown in (c). (a,b) Give an extended section of the middle part of the pattern as indicated. (a) The low-frequency components (less than 70 Hz) of electric field patterns (not included in (c)), and (b) the corresponding high-frequency components (greater than 70 Hz included in (c)). The time scales for (a,b) are the same. Note that all three panels have the same ordinate (electric potential in V). The trace in (c) relates to 12 left-right wagglings of the body. Two groups of typically three to five wing electric field beats (in (b)) can be seen for each outmost body position (left L and right R) of each waggle. As the abdomen is swinging to the sides (in (a)), it is in addition lowered in the outmost position compared with the middle position (up). This combination of movements results in different effective distances to the recording electrode, leading to a modulation of the electric field signal. (Online version in colour.)
Waggling of the body consisted of up-down and side-to-side movements at a basic frequency of 16.5 Hz. Since the body was at a higher position (closer to the recording electrode, figure 2, up) when straight and at a lower position (further away from the electrode, figure 2, L) when moved to the side as judged by video observations from the side, waggling resulted in a rhythmic rolling and side-to-side movement. The side-to-side movements were synchronized with buzzing of the wings, leading to an average of three to five (range 1–8) electric pulses. These pulses originated from the wing vibrations at 230 Hz (s.e.±5 Hz, n = 300) leading to a voltage of typically 200 V (s.e.±60 V, n = 300) as measured at a distance of 6–9 mm. The interval between episodes of wing buzzing was 29.09 ms (s.e.±1.9 ms, n = 300), and the interval between left/low and right/low was 30.26 ms (s.e.±2.14 ms). A comparison with the recordings of the sound signals [22] indicates similar values (typically five wing beat episodes at 36 Hz with 250 Hz wing beat frequency).
(b). Electric fields induce vibration of the antennal flagellum
Dancing bees produce air flows and electric fields, both of which induce vibrations of the antennal flagellum. Such flagellar vibrations were observed when we oscillated an isolated wing placed 2 mm from the bee's head with an oscillation frequency of 40 Hz producing both air flow and electric field. In order to compare antennal vibrations with and without charge on the test antenna and/or on the stimulating wing, they were discharged by water. During the first few seconds, the vibrating wing recharged allowing us to measure the effect on a charged or discharged antenna either in combination or separately. Laser Doppler vibrometric measurements revealed that the flagellum did vibrate in response to air flow alone, but the vibration amplitude was increased by almost one order of magnitude. Under all our test conditions, the wings had an electric charge due to the nature of their movements (figure 3a). An exception is the wing discharged with water in figure 3a.
Figure 3.
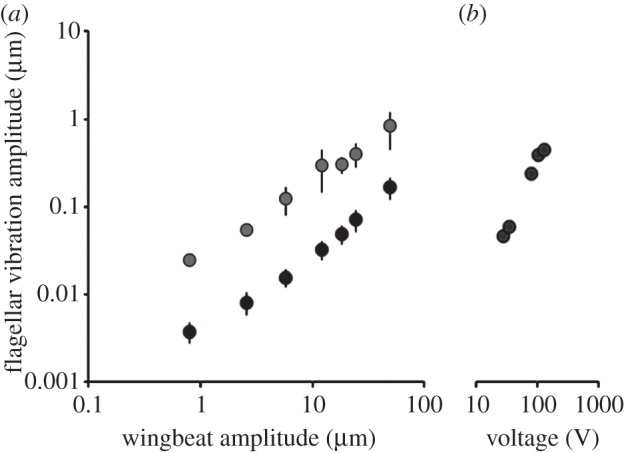
(a) Vibration amplitude of the antennal flagellum as a function of the oscillation amplitude of an isolated wing placed at a distance of 2 mm from the bee's head (oscillation frequency: 40 Hz, n = 3 flagella, means±1 s.d. See also electronic supplementary material, figure S4). Vibration amplitudes were measured as Fourier amplitudes at the frequency of oscillation. Black symbols: wing charged by contact to a piece of charged Styrofoam, grey symbols: wing uncharged so that the flagellum is only driven by air flow. (b) Flagellar vibration amplitude as a function of the voltage amplitude of a 40 Hz sinusoid fed to an electrostatic probe placed at a distance of 2 mm from the bee's head. Statistics: uncharged versus charged bodies differ significantly (d.f. = 1, d.f.error = 6, F = 8.15, p < 0.029, ANOVA for repeated measures).
Electric fields sufficed to induce flagellar vibrations as confirmed by applying electrostatic force via an electrostatic probe (figure 3b). Hence, electric fields at frequencies as produced during the waggle dance induce vibrations of the antennal flagellum whose amplitudes exceed those caused by air flows of the beating wing.
(c). Constant electric fields induce neural activity in Johnston's organ
Bees monitor vibrations of their antennal flagellum with JO, a mechanosensory chordotonal organ in their second antennal segment. JO was found to respond electrically to flagellar displacements above some 10–100 nm [23], which is well in the range of displacement amplitudes induced by oscillations of a charged wing (figure 3a). To directly test whether electric fields elicit neural JO responses, we recorded compound action potentials from the axons of the JO during stimulation with acoustic, modulated and constant electric fields (figure 4). A sharp onset of such stimuli induced a phasic–tonic response. This is not seen in figure 4, because here we show the effects of stimulating with a charged probe which was moved slowly in order to avoid any mechanical stimulation. Close examination of the flagellum revealed small movements of the flagellum relative to the scapus when the charged probe was slowly approached. Stimulation with modulated electric fields transmitted from an electric probe induced also in-phase discharges of the JO, and these disappeared when the flagellum was not charged (not shown).
Figure 4.
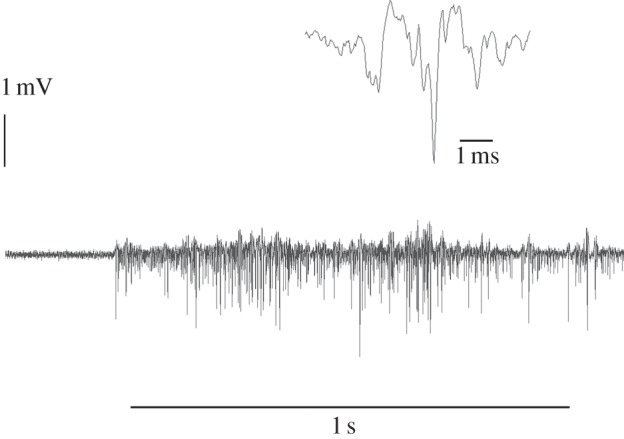
Compound action potentials recorded from the axons of the Johnston organ in the pedicel of the honeybee antenna. Two traces with different temporal resolutions are shown. The flagellum was stimulated with a charged Styrofoam ball moved slowly close (2 mm) to the flagellum for the time period marked by the black bar. No mechanical movement stimulus was involved.
(d). Electric fields of different temporal structures evoke behavioural responses in resting bees
Next we asked whether bees respond behaviourally to modulated electric field signals. We monitored the behaviour of bees walking on a Styrofoam ball floating on air. After several hours of walking on the ball the animals rested, as witnessed by a loss in muscle tonus and associated downward movements of their antennae. Upon electric field stimulation with 20–80 Vpp and 700 ms duration, the bees transiently re-lifted their antennae to the normal position characteristic for the awake state. After stimulation, the antennae reassumed their resting position within some 50 s (figure 5a and electronic supplementary material, figure S5 and video S1).
Also acoustic stimuli were applied to resting animals under similar conditions. The acoustic signal produced sound particle velocities ranging from 0.05 to 50 mm s−1 at 230 Hz. A threshold for antennal responses in live animals was found at a sound particle velocity of 0.1 mm s−1. Note that the passive movement of the flagellum at threshold (see upper scale in figure 5) was several times larger than the neural response threshold (ca 10–100 nm) measured by Tsujiuchi et al. [23].
(e). Stationary walking animals respond to biologically relevant electric field patterns
If an electrode emitted greater than 100 Vpp at a distance of 3 mm, the animals were aroused and started walking with actively moving antennae for a period of minutes. We used this response to test the efficiency of biologically relevant patterns of electric fields with different frequencies and temporal structures as provided by an electrode and compared the responses to control animals, which were treated equally but not stimulated (figure 6).
Figure 6.
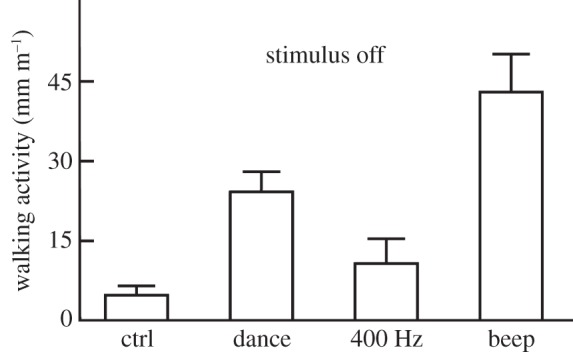
Electric field induced walking activity in animals in response to biologically relevant electric field patterns. The electric field strength was 40 Vpp at a distance of 3 mm. The ordinate gives the average walking activity induced by the stimuli as expressed in walking speed (mm min–1) for five animals. Each bee was tested for 10 h during which the animal was exposed to multiple tests consisting of 10 stimulations followed by 10 min of no stimulation (a total of 60 tests per bee; see electronic supplementary material, figure S5 and video S1). Four different stimuli were used, those recorded from a dancing bee (dance, dominant frequency 230 Hz), those recorded from bees following a dancing bee and producing a stop signal (beep, dominant frequency 400 Hz), and a sine wave of 400 Hz with the same intensity as that of the stop signal. The controls did not receive any stimuli during the same sample periods in which the electric field source was switched off. Significant differences were found for group comparisons with the control group and the dance and beep group (between groups: d.f. = 1, d.f.error = 3, F = 8.213, p < 0.02 ANOVA repeated measures, between stimuli: d.f. = 3, d.f.error = 15, F = 7.713, p < 0.02 ANOVA repeated measures) but not with the 400 Hz group (post hoc comparison 400 Hz group versus control n.s.).
Walking responses outlasted the stimulation period. Therefore, we analysed walking activity during the intervals between repetitive stimulations. The most effective electric signals were the two biologically relevant electric signals, the dance signal and the bees' stop signal, also called a beep [24,25]. The stop signal consisted of short (130 ms) modulated pulses with a dominant frequency of 400 Hz repeated at intervals of 1 s. A non-modulated signal with a constant frequency of 400 Hz was significantly less effective than both biologically relevant signals. These results indicate that biologically relevant electric field signals (beep and dance signals) induce long-lasting activity in bees walking on a floating ball, suggesting that the bees both receive and may even discriminate electric fields with different modulation patterns.
(f). Bees learn to associate constant and modulated electric field with reward
We conditioned the proboscis extension response (PER) to electric fields as the conditioned stimulus (CS+) by forward pairing it with the reward (i.e. the unconditioned stimulus sucrose solution) and by exposing it to a control stimulus without reward (CS−) [20]. Both constant and modulated electric fields were used as CS+. The constant electric field was produced by rubbing pieces of Styrofoam against each other (see above). The CS− was a similar uncharged piece of Styrofoam. Modulated electric field emanated from a voltage source providing effective stimulus strength of 160 Vpp (60 Hz). The CS− came from the same inactivated voltage source. Bees learned to respond with PER to both CS+ electric field stimuli (figure 7a,b).
In both cases, the CRs increased with the number of forward pairings between the respective electric field as the CS+ and sucrose reward, whereas the response to the unrewarded stimulus (CS−, uncharged Styrofoam or inactivated current source) remained unaltered. We also tested whether bees stored the learned electric field stimuli in their memory and found a significantly higher level of conditioned PER 24 h later (n = 64, d.f. = 1, F = 24.7, p < 0.01, ANOVA repeated measures). These results demonstrate the bees' sensitivity to constant and modulated electric field and document that bees are able to associate these cues with reward.
(g). Mechanoreceptors on the antennae are required for responses to the learned electric field stimulus
We used the CR to test whether sensory receptors of the antennae are required for detecting electric field. The flagellae of the antennae carry a large number of olfactory receptors in pore plates, contact chemoreceptors in hair cells and mechanoreceptors at the base of short hairs. Additional mechanoreceptors are located between the joints of the flagellum and the pedicel (JO), and between scapus and head. Bees were conditioned with fully functioning antennae. Then the flagellae were covered with wax so that the joints between the flagellum and the scapus or between the scapus and the head were fixed. In one group, the antennae were removed after training (figure 7c). We used wax because we wanted to ensure that the electric charge on the antenna is altered as little as possible when interfering with the movability and surface of the antenna.
The antennae were clearly necessary for sensing the learned electric field stimulus. Ablating the antennae eliminated learned electric field responses (figure 7c, group 6). Immobilizing both joints (flagellum–pedicel and scapus–head; figure 7c, group 5) strongly reduced learned electric field responses. Leaving the flagellum–pedicel joint free but otherwise covering the antennae fully with wax (figure 7c, group 4) led to an almost equal reduction of learned electric field responses. Even if only the scapus was fixed to the head (figure 7c, group 3), the learned electric field responses were significantly lower than in the untreated control group. If, however, both joints were left untouched but the flagella and scapus were coated with wax (figure 7c, group 2) response reduction was still significantly lower than in the untreated control group albeit less marked. Thus, interfering with the movement of either or both of the joints reduced the electric field sensitivity most strongly. We thus conclude that mechanosensory organs associated with both joints of the antennae are most probably responsible for the ability of bees to sense electric field. However, we cannot exclude that other mechanoreceptors might be involved in electric field reception. The PER conditioning paradigm is known to work particularly effectively with conditioned stimuli that are received via the antennae (odours, humidity, mechanosensory and thermosensory stimuli, [26]). Other mechanosensory receptors may be involved in electric field sensing in other contexts, e.g. wing movement, surface probing. Future experiments need to address this question to gain a better understanding of the electro-mechanical sense in insects.
4. Conclusion
It has long been recognized that insects become electrostatically charged when flying or walking, or when their body parts are rubbed together, but little is known about whether and how emanating fields of such charge are sensed and potentially used as meaningful signals. Electric fields can act on mechanoreceptors of their cuticular appendages if these appendages are charged, and indeed such fields have been used to stimulate insect mechanosensory organs [15,27]. Electric fields induce responses in insects [7,8,28]. A biologically relevant property of body surface charge in pollinating insects was traced to the opposite polarity of their body surface charge to that of flowers, potentially facilitating pollen collection and possibly leaving a cue after electrical neutralization of the flowers' electric field [9–12]. It has also be noted that charged pollen powder sprayed over flowers leads to fertilization of these flowers [29], and biopesticides may be more effective if electrically charged [30]. Taken together these observations indicate that electric surface charge is a frequently found property of plants and animals and may play a role in pollination. However, the question of whether honeybees themselves receive electric fields, respond to them, learn them and possibly exploit them in their natural habitat, including social context has not been considered so far.
Our findings show that electric fields produced by the movement of the bee's wings even at very low amplitudes, as for example in dancing bees, induce movements of the antennal flagellum 10 times more strongly than the mechanical stimulus of the wing alone. Similar flagellar movements can be achieved by applying modulated electric fields at strengths well within the range of those of the bee's body surface charge. The response of bees differs for different modulation patterns indicating that naturally occurring electric stimuli are received. By training bees to static and modulated electric fields, we have confirmed that bees perceive electric fields. These training experiments point to mechanoreceptors in the two joints of the antennae, but other receptors on the bee body may also be involved in electrosensing via mechanoreceptors. Collectively, these results establish electric fields as a behaviourally relevant stimulus component in honeybees potentially used as a cue in social communication. Humidity in the hive would not cause a problem because of the high temperature inside the hive, and the fanning behaviour leads to relatively low humidity and continuous recharging owing to the friction between the body parts. Furthermore, fanning and friction between different bees will also keep surface charge high.
To develop a full picture of this potential information channel in social communication additional studies will be needed that monitor and manipulate electric fields of dancing bees and interfere selectively with the reception of electric fields by dance followers.
Acknowledgements
We thank Peter Knoll and Gisela Manz for technical assistance; Friederike Hagen and Jürgen Rybak for supporting research. We are grateful to Anne Carney for help with the English manuscript. The work was supported by the Dr Klaus Tschira Stiftung and Gemeinnützige Hertie Stiftung.
References
- 1.Exner S. 1895. Über die elektrischen Eigenschaften der Haare und Federn. Pflügers Archiv. 61, 1–98 10.1007/BF01661733 (doi:10.1007/BF01661733) [DOI] [Google Scholar]
- 2.Heuschmann O. 1929. Über die elektrischen Eigenschaften der Insekten Haare. Z. vergl. Physiol. 10, 594–664 [Google Scholar]
- 3.Warnke U. 1976. Effects of electric charges on honeybees. Bee World 57, 50–56 [Google Scholar]
- 4.McGonigle DF, Jackson CW. 2002. Effect of surface material on electrostatic charging of houseflies (Musca domestica L). Pest Manag. Sci. 58, 374–380 10.1002/ps.463 (doi:10.1002/ps.463) [DOI] [PubMed] [Google Scholar]
- 5.Jackson C, McGonigle D. 2005. Direct monitoring of the electrostatic charge of house-flies (Musca domestica L.) as they walk on a dielectric surface. J. Electrost. 63, 803–805 10.1016/j.elstat.2005.03.075 (doi:10.1016/j.elstat.2005.03.075) [DOI] [Google Scholar]
- 6.Colin ME, Richard D, Chauzy S. 1991. Measurement of electric charges carried by bees: evidence of biological variations. J. Bioelectr. 10, 17–32 [Google Scholar]
- 7.Newland PL, Hunt E, Sharkh SM, Hama N, Takahata M, Jackson CW. 2008. Static electric field detection and behavioural avoidance in cockroaches. J. Exp. Biol. 211, 3682–3690 10.1242/jeb.019901 (doi:10.1242/jeb.019901) [DOI] [PubMed] [Google Scholar]
- 8.Jackson CW, Hunt E, Sharkh S, Newland PL. 2011. Static electric fields modify the locomotory behaviour of cockroaches. J. Exp. Biol. 214, 2020–2026 10.1242/jeb.053470 (doi:10.1242/jeb.053470) [DOI] [PubMed] [Google Scholar]
- 9.Corbet SA, Beament J, Eisikowitch D. 1982. Are electrostatic forces involved in pollen transfer? Plant Cell Environ. 5, 125–129 [Google Scholar]
- 10.Fromm J, Hajirezaei M, Wilke I. 1995. The biochemical response of electrical signaling in the reproductive system of hibiscus plants. Plant Physiol. 109, 375–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaknin Y, Gan-Mor S, Ronen B, Eisikowitch D. 2000. The role of electrostatic forces in pollination. Plant Syst. Evol. 222, 133–142 10.1007/BF00984099 (doi:10.1007/BF00984099) [DOI] [Google Scholar]
- 12.Vaknin Y, Gan-Mor S, Bechar A, Ronen B, Eisikowitch D. 2000. The role of electrostatic forces in pollination. In Pollen and pollination (eds Dafni A, Heese M, Pacini E.), pp. 133–109 Heidelberg, Germany: Springer [Google Scholar]
- 13.Altmann G, Warnke U. 1973. Registrierung von Tierbewegungen mit Hilfe der Körperoberflächenladungen. Experientia 29, 1044. 10.1007/BF01930455 (doi:10.1007/BF01930455) [DOI] [PubMed] [Google Scholar]
- 14.Albert JT, Nadrowski B, Göpfert MC. 2007. Mechanical signatures of transducer gating in the Drosophila ear. Curr. Biol. 17, 1000–1006 10.1016/j.cub.2007.05.004 (doi:10.1016/j.cub.2007.05.004) [DOI] [PubMed] [Google Scholar]
- 15.Göpfert MC, Robert D. 2001. Active auditory mechanics in mosquitoes. Proc. R. Soc. Lond. B 268, 333–339 10.1098/rspb.2000.1376 (doi:10.1098/rspb.2000.1376) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nadrowski B, Albert JT, Gopfert MC. 2008. Transducer-based force generation explains active process in Drosophila hearing. Curr. Biol. 18, 1365–1372 10.1016/j.cub.2008.07.095 (doi:10.1016/j.cub.2008.07.095) [DOI] [PubMed] [Google Scholar]
- 17.Effertz T, Nadrowski B, Piepenbrock D, Albert JT, Gopfert MC. 2012. Direct gating and mechanical integrity of Drosophila auditory transducers require TRPN1. Nat. Neurosci. 15, 1198–1200 10.1038/nn.3175 (doi:10.1038/nn.3175) [DOI] [PubMed] [Google Scholar]
- 18.Nadrowski B, Effertz T, Senthilan PR, Gopfert MC. 2011. Antennal hearing in insects—new findings, new questions. Hear Res. 273, 7–13 10.1016/j.heares.2010.03.092 (doi:10.1016/j.heares.2010.03.092) [DOI] [PubMed] [Google Scholar]
- 19.Albert JT, Nadrowski B, Kamikouchi A, Göpfert MC. 2006. Mechanical tracing of protein function in the Drosophila ear. Nat. Protoc. 10.1038/nprot.2006.364 (10.1038/nprot.2006.364) [DOI] [Google Scholar]
- 20.Bitterman ME, Menzel R, Fietz A, Schäfer S. 1983. Classical conditioning of proboscis extension in honeybees (Apis mellifera). J. Comp. Psychol. 97, 107–119 10.1037/0735-7036.97.2.107 (doi:10.1037/0735-7036.97.2.107) [DOI] [PubMed] [Google Scholar]
- 21.Michelsen A, Kirchner WH, Lindauer M. 1986. Sound and vibrational signals in the dance language of the honeybee, Apis mellifera. Behav. Ecol. Sociobiol. 18, 207–212 10.1007/BF00290824 (doi:10.1007/BF00290824) [DOI] [Google Scholar]
- 22.Michelsen A, Towne WF, Kirchner WH, Kryger P. 1987. The acoustic near field of a dancing honeybee. J. Comp. Physiol. [A] 161, 633–643 10.1007/BF00605005 (doi:10.1007/BF00605005) [DOI] [Google Scholar]
- 23.Tsujiuchi S, Sivan-Loukianova E, Eberl DF, Kitagawa Y, Kadowaki T. 2007. Dynamic range compression in the honey bee auditory system toward waggle dance sounds. PLoS ONE 2, e234. 10.1371/journal.pone.0000234 (doi:10.1371/journal.pone.0000234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nieh JC. 2012. The stop signal in honeybees: reconsidering its message. Behav. Ecol. Sociobiol. 33, 51–56 10.1007/BF00164346 (doi:10.1007/BF00164346) [DOI] [Google Scholar]
- 25.Schlegel T, Visscher PK, Seeley TD. 2012. Beeping and piping: characterization of two mechano-acoustic signals used by honey bees in swarming. Naturwissenschaften 99, 1067–1071 10.1007/s00114-012-0990-5 (doi:10.1007/s00114-012-0990-5) [DOI] [PubMed] [Google Scholar]
- 26.Menzel R, Manz G, Menzel R, Greggers U. 2001. Massed and spaced learning in honeybees: the role of CS, US, the intertrial interval, and the test interval. Learn. Mem. 8, 198–208 10.1101/lm.40001 (doi:10.1101/lm.40001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albert JT, Nadrowski B, Göpfert MC. 2007. Drosophila mechanotransduction—linking proteins and functions. Fly (Austin) 1, 238–241 [DOI] [PubMed] [Google Scholar]
- 28.Hunt EP, Jackson CW, Newland PL. 2005. ‘Electropellancy’ behavior of Periplaneta americana to friction charged dieelectric surfaces. J. Electrostat. 63, 803–808 10.1016/j.elstat.2005.03.075 (doi:10.1016/j.elstat.2005.03.075) [DOI] [Google Scholar]
- 29.Gan-Mor S, Bechar A, Ronen B, Eisikowitch D, Vaknin Y. 2003. Electrostatic pollen applicator development and tests for almond, kiwi, date, and pistachio: an overview. Appl. Eng. Agric. 19, 119–124 [Google Scholar]
- 30.Gan-Mor S, Matthews GA. 2003. Recent developments in sprayers for application of biopesticides: an overview. Biosyst. Eng. 84, 119–125 10.1016/S1537-5110(02)00277-5 (doi:10.1016/S1537-5110(02)00277-5) [DOI] [Google Scholar]