Abstract
Objective. The randomized controlled trials (RCTs) on Guanxinning injection (GXN) in treating angina pectoris were published only in Chinese and have not been systematically reviewed. This study aims to provide a PRISMA-compliant and internationally accessible systematic review to evaluate the efficacy of GXN in treating angina pectoris. Methods. The RCTs were included according to prespecified eligibility criteria. Meta-analysis was performed to evaluate the symptomatic (SYMPTOMS) and electrocardiographic (ECG) improvements after treatment. Odds ratios (ORs) were used to measure effect sizes. Subgroup analysis, sensitivity analysis, and metaregression were conducted to evaluate the robustness of the results. Results. Sixty-five RCTs published between 2002 and 2012 with 6064 participants were included. Overall ORs comparing GXN with other drugs were 3.32 (95% CI: [2.72, 4.04]) in SYMPTOMS and 2.59 (95% CI: [2.14, 3.15]) in ECG. Subgroup analysis, sensitivity analysis, and metaregression found no statistically significant dependence of overall ORs upon specific study characteristics. Conclusion. This meta-analysis of eligible RCTs provides evidence that GXN is effective in treating angina pectoris. This evidence warrants further RCTs of higher quality, longer follow-up periods, larger sample sizes, and multicentres/multicountries for more extensive subgroup, sensitivity, and metaregression analyses.
1. Introduction
Ischemic heart disease (IHD) is a major cause of death and global healthcare burden [1]. Angina pectoris, a symptom of IHD, is a severe chest pain due to ischemia of the heart muscle, during obstruction or spasm of the coronary arteries [2]. In the United States, IHD accounts for 26.6% of all deaths in 2005, with an age-adjusted male-to-female mortality ratio of 1.5 [3]. The morbidity and mortality of angina in middle-aged and elderly people were ranked the top among all common diseases in China [4]. Three categories of conventional Western medicine including nitrates (e.g., isosorbide mononitrate), beta-receptor blockers (e.g., atenolol), and calcium channel blockers (e.g., amlodipine) are commonly used in treating angina [3].
Guanxinning injection (GXN, also known as Danshen Chuanxiong Injection) comprises extracts from two well-known traditional Chinese medicines Danshen (Salvia miltiorrhiza) and Chuanxiong (Ligustrazine, Ligustium Wallichii Franch) [5]. Danshen and its active compounds tanshinones and isotanshinones have bioactivities against myocardial ischemia, inflammation, and angiotensin-converting enzyme [6]. Chuanxiong and its active compounds tetramethylpyrazine and ferulic acid can dilate coronary arteries, increase myocardial oxygen, and decrease platelet aggregation and thrombosis [7].
GXN was tested to be more effective than nitrates [8], beta-receptor blockers [9], and calcium channel blockers [10] in treating angina. Since the launch of GXN (2002) and prior to this study, there has been only one systematic review, which is not compliant with PRISMA [11] and includes only nine randomized controlled trials (RCTs) published in Chinese between 2002 and 2010 [12]. The methods and results of quality assessment of the included RCTs were not clearly reported in the systematic review. Sensitivity and subgroup analyses were missing. Hence, this study aims to provide an internationally accessible, comprehensive, and timely systematic review and meta-analysis in compliance with PRISMA to assess the efficacy of GXN as a monotherapy and combined therapy with conventional Western or Chinese medicines in treating angina pectoris.
2. Methods
The procedures of this systematic review and meta-analysis were conducted in accordance with the PRISMA guideline [11], including the search and selection of studies, data extraction from the studies, and meta-analysis (overall, subgroup, sensitivity, publication bias, and metaregression analysis).
2.1. Search Strategies
RCTs published on the efficacy of GXN in treating angina pectoris were retrieved from major bibliographical databases including Medline, PubMed, Cochrane Library, ScienceDirect, Embase, China National Knowledge Infrastructure (CNKI), WanFang Data, China Master Theses Full-text Database (CMTD), and China Doctor Dissertations Full-text Database (CDMD) between the inception dates of databases and 2012 (last search on 18 March 2012). A simple search strategy, that is, searching for the keywords “Guanxinning” or “danshen chuanxiong” or “danshenchuanxiong,” was used to search all fields. For instance, the search in WanFang Data using the keyword “Guanxinning” found 196 records and “danshen chuanxiong” found 17 records and “danshenchuanxiong” found none. Exact search strategies and query syntax for specific databases were customized according to the same strategy.
2.2. Study Selection
Inclusion criteria for each study were (a) the participants were suffering from and being treated for angina pectoris; (b) the study was claimed as an RCT; (c) the study compared the efficacy of GXN with conventional (Western and Chinese medicine) drugs. Exclusion criteria were (a) the study was a duplicated or redundant publication and (b) the study did not include symptomatic improvement as a major outcome.
Two reviewers (Y. Jia and F. Pan) independently searched the databases and selected studies according to the inclusion and exclusion criteria. Disagreements between reviewers were resolved by consensus after discussion. Figure 1 shows a flow diagram of study selection.
Figure 1.
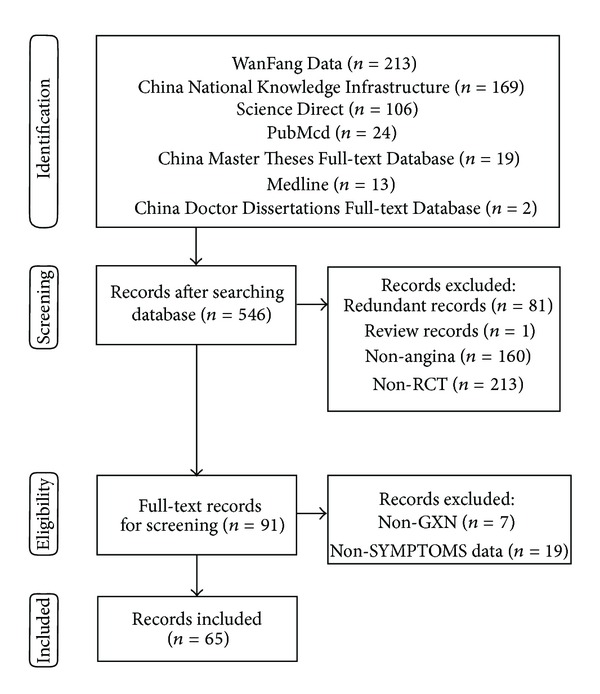
Process of searching and screening studies.
2.3. Data Extraction
Two reviewers (Y. Jia and F. Pan) independently extracted data items, including (a) years of publication; (b) numbers of authors; (c) follow-up periods; (d) baseline characteristics of participants between groups; (e) sample sizes; (f) outcome measures; (g) dosages and follow-up periods; (h) type of angina; (i) frequencies of adverse events (AE); and (j) the type of angina.
2.4. Quality Assessment of Included Studies
Two reviewers (Y. Jia and F. Pan) independently assessed the quality of the included studies according to the Jadad scale [13], its refined version the M scale [14], and the Cochrane Collaboration's tool for assessing risk of bias [15]. The Jadad scale focused on three criteria including “randomization,” “blinding,” and “dropouts” for assessing the quality of RCT. The M scale added two criteria “baseline comparison of participants” and “adverse event report” on top of the Jadad scale. The Cochrane Collaboration's tool for assessing risk of bias includes “random sequence generation,” “allocation concealment,” “blinding of participants and personnel,” “blinding of outcome assessment (patient-reported outcomes),” “blinding of outcome assessment (SYMPTOMS),” “incomplete outcome data addressed,” “reporting bias,” and “other sources of bias.”
2.5. Criteria for Symptomatic and ECG Improvements
Effective symptomatic improvements should achieve at least 50% (basic) or 80% (significant) reduction in frequency of feeling angina chest pain [16]. Effective ECG improvements should achieve (a) at least 0.05 mV lowering at ST segment in ECG (basic) or (b) nearly normal (significant) ECG during an exercise test according to the International Society and Federation of Cardiology/World Health Organization [16].
2.6. Meta-Analysis
Effect sizes were represented by odds ratios (ORs) [17] and their 95% confidence intervals (CI) [18]. Overall meta-analysis and subgroup analysis employed the random-effects model for conservative generalizability. Heterogeneity among studies was assessed by Chi-squared (χ 2) and I-squared (I 2) tests [19].
2.7. Subgroup and Sensitivity Analyses
Subgroup analysis was conducted to evaluate the overall effects in the subgroups according to years of publication (≤2008 or >2008), numbers of authors (1 or >1), follow-up periods (≤14 days or >14 days), sample sizes (<mean sample size or ≥mean sample size), quality scores of the studies (<mean or ≥mean), different type of angina, and different daily dosage of GXN. The overall effects were also analyzed in subgroups of GXN for monotherapy and adjunctive therapy. Sensitivity analysis was carried out according to different criteria outcomes (basic or significant) in SYMPTOMS and ECG and excluding studies with maximum GXN dosage to assess their influence on the overall effect sizes. The Mann-Whitney-Wilcoxon test was used to compare two subgroups. The Kruskal-Wallis test and the Bonferroni correction were used to compare multiple subgroups. Kendall correlation between ORs of symptoms and ECG was performed.
2.8. Metaregression and Risk of Bias across Studies
Funnel plots [20], Begg's test [21], and Egger's test [22] were employed to assess publication bias. Trim-and-fill method [23] was conducted to identify and correct the funnel plot asymmetry arising from publication bias. Metaregression [24] was conducted to find the possible relationship between the overall effects and the factors such as sample sizes, follow-up periods, M scores, and years of publication.
2.9. Adverse Events
Information about adverse events (AEs) of RCTs, including nonreported adverse events and types and frequency of adverse events reported, was tabulated and analyzed by basic statistics.
2.10. Statistical Analysis
All data analyses, including meta-analysis, forest plot generation, funnel plot generation, metaregression, Kendall correlation, Mann-Whitney-Wilcoxon test, Kruskal-Wallis test, Begg's test, and Egger's test, were performed using statistical software R [25] and its “metafor” package for meta-analysis. P values lower than 0.05 were considered statistically significant.
3. Results
3.1. Study Selection
Figure 1 depicts the process of study selection. The search of bibliographical databases found 401 records, including 196 records from WanFang Data, 162 records from CNKI, 19 records from CMTD, 11 records from ScienceDirect, 6 records from Medline, 5 records from PubMed, and 2 records from CDMD. According to prespecified selection criteria as described in Methods, 65 studies [26–90] were included for further quality assessment and meta-analysis.
3.2. Study Characteristics
Table 1 lists the main characteristics of the included studies. All included studies were published in the Chinese language between 2004 and 2011 with a total of 6064 participants. The mean sample size was 93.3 (median: 88.0; 95% CI: [56.5, 130.1]). The follow-up periods were between 1 and 30 days. GXN was compared with the conventional treatments in the included RCTs. Drugs in control group mainly included nitrates, beta-receptor blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors, and some conventional Chinese medicinal products for treating heart disease. Fifty-nine out of 65 RCTs employed GXN plus the conventional treatments in the treatment group while the conventional treatments were employed in control group. Dosage details were listed in Supplementary Table 1 in the Supplementary Material available online at http://dx.doi.org/10.1155/2013/282707. For outcome measures, all 65 included studies reported symptomatic (SYMPTOMS) changes while 38 studies also reported ECG changes.
Table 1.
Characteristics of the included studies.
| Study | Number of authors | Trial date report | Sample size |
Followup (day) |
Baseline comparison | AE | Outcomes measure |
Treatment group dosage | Angina |
|---|---|---|---|---|---|---|---|---|---|
| Chen 2009 | 1 | 1 | 100 | 15 | 1 | 0 | SYM, ECG | GXN 20 mL/d + CG | Angina |
| Chen et al. 2011 | 3 | 1 | 100 | 10 | 0 | 0 | SYM | GXN 20 mL/d + CG | Angina |
| Chen 2006 | 1 | 1 | 62 | 14 | 1 | 1 | SYM, ECG | GXN 20 mL/d + CG | Angina |
| Cheng and Zang 2010 | 2 | 1 | 43 | 14 | 1 | 0 | SYM | GXN 30 mL/d + CG | Unstable |
| Cheng et al. 2011 | 3 | 1 | 76 | 14 | 1 | 1 | SYM, ECG | GXN 30 mL/d + CG | Angina |
| Dong XP 2009 | 1 | 0 | 100 | 1 | 0 | 0.5 | SYM | GXN 20 mL/d | Angina |
| Fu and Meng 2011 | 2 | 0 | 47 | 10 | 1 | 0 | SYM, ECG | GXN 20 mL/d + CG | Angina |
| Fu et al. 2010 | 4 | 1 | 56 | 14 | 1 | 0 | SYM, ECG | GXN 200 ml + CG + shenmaiyin 40 ml | Angina |
| Gao et al. 2005 | 3 | 1 | 60 | 14 | 1 | 1 | SYM, ECG | GXN 20 mL/d | Angina |
| Gong et al. 2009 | 3 | 1 | 85 | 14 | 1 | 1 | SYM, ECG | GXN 20 mL/d + xueshuantong 20 ml | Stable |
| He and Meng 2007 | 1 | 1 | 49 | 15 | 1 | 0 | SYM | GXN 20 mL/d + CG | Unstable |
| He 2009 | 1 | 1 | 120 | 28 | 1 | 1 | SYM, ECG | GXN 30 mL/d + atorvastatin 10 mg | Unstable |
| Hou and Gao 2009 | 2 | 1 | 128 | 14 | 1 | 1 | SYM, ECG | GXN 20 mL/d + CG | Stable |
| Huang et al. 2011 | 4 | 0 | 120 | 7 | 1 | 1 | SYM | GXN 20 mL/d + CG + xueshuantong 400 mg | Angina |
| Jiang et al. 2010 | 3 | 1 | 116 | 10 | 1 | 0 | SYM, ECG | GXN 20 mL/d + CG | Unstable |
| Jiang 2009 | 1 | 1 | 68 | 20 | 1 | 1 | SYM, ECG | GXN 20 mL/d | Angina |
| Jiang et al. 2010 | 5 | 0 | 56 | 7 | 0 | 1 | SYM | GXN 30 mL/d | Angina |
| Kong 2009 | 1 | 0 | 100 | 14 | 1 | 1 | SYM | GXN 30 mL/d + CG | Unstable |
| Lan et al. 2006 | 3 | 1 | 64 | 14 | 1 | 1 | SYM | GXN 20 mL/d | Angina |
| Li and Jia 2011 | 2 | 1 | 200 | 14 | 1 | 0 | SYM, ECG | GXN 30 mL/d + CG | Angina |
| Li and Lei 2005 | 2 | 1 | 156 | 14 | 0 | 1 | SYM, ECG | GXN 20 mL/d + CG | Angina |
| Li et al. 2009 | 5 | 1 | 168 | 14 | 1 | 0 | SYM | GXN 20 mL/d + CG | Angina |
| Li and Ran 2009 | 2 | 1 | 160 | 10 | 1 | 1 | SYM | GXN 20 mL/d + CG | Angina |
| Li 2004 | 1 | 0 | 83 | 7 | 1 | 0 | SYM, ECG | GXN 20 mL/d + CG | Unstable |
| Liang and Feng 2010 | 2 | 0 | 120 | 14 | 1 | 0 | SYM, ECG | GXN 20 mL/d + CG | Unstable |
| Liu 2004 | 1 | 1 | 104 | 10 | 1 | 1 | SYM | GXN 20 mL/d + CG | Unstable |
| Liu and Li 2007 | 2 | 1 | 88 | 12 | 0 | 1 | SYM | GXN 20 mL/d + CG | Unstable |
| Liu 2005 | 1 | 1 | 80 | 30 | 1 | 0 | SYM, ECG | GXN 20 mL/d + CG | Unstable |
| Liu 2011 | 1 | 1 | 152 | 28 | 1 | 0 | SYM, ECG | GXN 20 mL/d + CG | Angina |
| Lu et al. 2006 | 3 | 1 | 68 | 30 | 1 | 0 | SYM | GXN 30 mL/d + CG | Angina |
| Ma and Peng 2008 | 2 | 1 | 120 | 14 | 1 | 0 | SYM | GXN 30 mL/d + CG | Unstable |
| Nie and Chen 2007 | 2 | 1 | 60 | 14 | 1 | 0 | SYM, ECG | GXN 20 mL/d + CG | Angina |
| Qiao and Wu 2004 | 2 | 0 | 81 | 28 | 1 | 1 | SYM, ECG | GXN 20 mL/d + CG | Stable |
| Song 2010 | 1 | 1 | 82 | 7 | 0 | 0.5 | SYM | GXN 20 mL/d + CG + diltiazem 90 mg/d | Unstable |
| Su 2009 | 1 | 1 | 90 | 15 | 0 | 1 | SYM, ECG | GXN 6 mL/d + CG | Angina |
| Sun 2010 | 1 | 1 | 90 | 14 | 1 | 0 | SYM, ECG | GXN 30 mL/d + CG | Unstable |
| Sun et al. 2006 | 5 | 1 | 98 | 15 | 0 | 0 | SYM | GXN 20 mL/d + CG | Angina |
| Tian and Wu 2006 | 2 | 1 | 62 | 14 | 1 | 1 | SYM | GXN 30 mL/d + CG | Unstable |
| Wan and Xu 2009 | 2 | 0 | 120 | 14 | 1 | 1 | SYM, ECG | GXN 30 mL/d + CG | Unstable |
| Wang 2007 | 1 | 1 | 100 | 14 | 1 | 1 | SYM, ECG | GXN 30 mL/d + CG | Angina |
| Wang 2011 | 1 | 1 | 85 | 14 | 1 | 1 | SYM, ECG | GXN 20 mL/d + CG | Unstable |
| Wang 2011 | 2 | 1 | 112 | 14 | 1 | 1 | SYM, ECG | GXN 20 mL/d + CG | Unstable |
| Wang and Ji 2008 | 2 | 1 | 60 | 14 | 0 | 0 | SYM, ECG | GXN 20 mL/d + CG | Unstable |
| Wang and Sun 2007 | 2 | 1 | 92 | 10 | 0 | 0 | SYM | GXN 20 mL/d + CG | Unstable |
| Wang 2005 | 2 | 1 | 60 | 15 | 1 | 0 | SYM | GXN 20 mL/d + CG | Unstable |
| Wang 2010 | 1 | 0 | 80 | 14 | 1 | 1 | SYM, ECG | GXN 20 mL/d + CG | Stable |
| Wang 2005 | 1 | 0 | 76 | 15 | 0 | 0 | SYM | GXN 20 mL/d + CG + shenmai 30 mL/d + tongxinluo 9 pills/d | Unstable |
| Wang 2005 | 1 | 1 | 60 | 14 | 1 | 1 | SYM | GXN 20 mL/d + CG | Unstable |
| Wang et al. 2011 | 4 | 1 | 60 | 14 | 1 | 1 | SYM | GXN 20 mL/d + CG | Unstable |
| Wu et al. 2008 | 3 | 0 | 108 | 14 | 1 | 0 | SYM | GXN 20 mL/d + CG | Angina |
| Wu et al. 2011 | 4 | 1 | 144 | 7 | 1 | 0 | SYM | GXN 20 mL/d + CG + shenmai 50 mL/d | Unstable |
| Xia 2011 | 1 | 1 | 90 | 14 | 1 | 0 | SYM, ECG | GXN 30 mL/d + CG | Unstable |
| Yang and Ma 2008 | 2 | 1 | 90 | 14 | 1 | 0 | SYM, ECG | GXN 30 mL/d + CG | Unstable |
| Ye et al. 2008 | 3 | 0 | 76 | 15 | 0 | 1 | SYM | GXN 20 mL/d + CG | Unstable |
| Yu and Wang 2009 | 2 | 1 | 75 | 15 | 1 | 0 | SYM, ECG | GXN 20 mL/d + CG | Angina |
| Yuan 2005 | 1 | 0 | 104 | 14 | 1 | 0 | SYM, ECG | GXN 20 mL/d + CG | Angina |
| Zhang 2005 | 1 | 1 | 60 | 14 | 1 | 0 | SYM, ECG | GXN 10 mL/d | Angina |
| Zhang 2010 | 1 | 1 | 240 | 15 | 1 | 1 | SYM, ECG | GXN 20 mL/d + CG | Unstable |
| Zhang 2004 | 1 | 1 | 102 | 14 | 1 | 1 | SYM, ECG | GXN 10 mL/d + CG + ginkgo leaf injection 10 mL/d | Angina |
| Zhang 2004 | 1 | 1 | 42 | 7 | 0 | 1 | SYM | GXN 20 mL/d + CG | Angina |
| Zhao et al. 2010 | 6 | 1 | 100 | 14 | 1 | 1 | SYM, ECG | GXN 10 mL/d + CG + xueshuangtong 120 mg | Unstable |
| Zhao and An 2008 | 2 | 1 | 90 | 28 | 1 | 1 | SYM, ECG | GXN 20 mL/d + CG + simvastatin 10–20 mg/d | Unstable |
| Zhao 2010 | 1 | 1 | 86 | 14 | 1 | 0 | SYM, ECG | GXN 30 mL/d + CG | Angina |
| Zhong et al. 2007 | 8 | 1 | 60 | 10 | 0 | 0 | SYM | GXN 20 mL/d + CG | Angina |
| Zhu 2005 | 1 | 1 | 80 | 15 | 0 | 0.5 | SYM, ECG | GXN 20 mL/d + CG | Unstable |
GXN is Guanxinning injection; LMWH is low molecular weight heparin; and shenmai is Shenmai injection. CG is interventions of control group; SYM is SYMPTOMS; ECG is electrocardiogram; and AE is adverse event. The column of “Trial date report” shows that study did (1) or did not (0) report the trial date. The column of “Baseline comparison” shows that the study did (1) or did not (0) report the baseline comparison between the treatment and control groups.
3.3. Quality Assessment of Included Studies
Table 2 shows the results of quality assessment according to the Jadad scales, M scales, and the Cochrane Collaboration's tool. According to the Jadad scale (with a possible range between 0 and 5 points), 63 studies of all included studies scored 2 with two items “randomization” and “dropouts,” one study [34] scored 3, and one study [47] scored 4. According to the M scale, six studies scored 2, three studies scored 2.5, 30 studies scored 3, 24 studies scored 4, and 2 studies scored 5. Fifty included studies reported baseline comparison of participants in experiment and control groups. Thirty-one studies did not report adverse events. Three studies reported types of adverse events. Thirty-one studies reported types and numbers of adverse events. The assessment results of the Cochrane Collaboration's tool showed (1) low risk of bias in random sequence generation for selection bias, blinding of outcome assessment (SYMPTOMS) for detection bias, and incomplete outcome data addressed for attrition bias, (2) high risk of bias in allocation concealment for selection bias, blinding of participants and personnel for performance bias, blinding of outcome assessment (patient-reported outcomes) for detection bias, and reporting bias for selecting reporting, and (3) unclear risk of bias in other sources of bias for other bias.
Table 2.
Quality assessment of included studies.
| Study | C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | Comparable | Random | Blind | Dropout | AE | Jadad | M |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chen 2009 | Low | High | High | High | Low | Low | High | High | 1 | 1 | 0 | 1 | 0 | 2 | 3 |
| Chen et al. 2011 | Low | Unclear | High | High | Low | Low | High | High | 0 | 1 | 0 | 1 | 0 | 2 | 2 |
| Chen 2006 | Low | High | High | High | Low | Low | Low | Low | 1 | 1 | 0 | 1 | 1 | 2 | 4 |
| Cheng and Zeng 2010 | Low | High | High | High | Low | Low | High | High | 1 | 1 | 0 | 1 | 0 | 2 | 3 |
| Cheng et al. 2011 | Low | High | High | High | Low | Low | Low | Low | 1 | 1 | 0 | 1 | 1 | 2 | 4 |
| Dong 2009 | Low | High | High | High | Low | Low | Unclear | High | 0 | 1 | 0 | 1 | 0.5 | 2 | 2.5 |
| Fu and Meng 2011 | Low | High | High | High | Low | Low | High | High | 1 | 1 | 0 | 1 | 0 | 2 | 3 |
| Fu et al. 2010 | Low | High | High | High | Low | Low | High | High | 1 | 1 | 0 | 1 | 0 | 2 | 3 |
| Gao et al. 2005 | Low | Low | High | High | Low | Low | Low | Low | 1 | 1 | 1 | 1 | 1 | 3 | 5 |
| Gong et al. 2009 | Low | High | High | High | Low | Low | Low | Low | 1 | 1 | 0 | 1 | 1 | 2 | 4 |
| He 2007 | Low | High | High | High | Low | Low | High | High | 1 | 1 | 0 | 1 | 0 | 2 | 3 |
| He 2009 | Low | High | High | High | Low | Low | Low | Low | 1 | 1 | 0 | 1 | 1 | 2 | 4 |
| Hou and Gao 2009 | Low | High | High | High | Low | Low | Low | Low | 1 | 1 | 0 | 1 | 1 | 2 | 4 |
| Huang et al. 2011 | Low | High | High | High | Low | Low | Low | Low | 1 | 1 | 0 | 1 | 1 | 2 | 4 |
| Jiang et al. 2010 | Low | High | High | High | Low | Low | High | High | 1 | 1 | 0 | 1 | 0 | 2 | 3 |
| Jiang 2009 | Low | High | High | High | Low | Low | Low | Low | 1 | 1 | 0 | 1 | 1 | 2 | 4 |
| Jiang et al. 2010 | Low | High | High | High | Low | Low | Low | Low | 0 | 1 | 0 | 1 | 1 | 2 | 3 |
| Kong 2009 | Low | High | High | High | Low | Low | Unclear | Low | 1 | 1 | 0 | 1 | 1 | 2 | 4 |
| Lan et al. 2006 | Low | High | High | High | Low | Low | Low | Low | 1 | 1 | 0 | 1 | 1 | 2 | 4 |
| Li and Jia 2011 | Low | High | High | High | Low | Low | High | High | 1 | 1 | 0 | 1 | 0 | 2 | 3 |
| Li and Lei 2005 | Low | High | High | High | Low | Low | Low | Low | 0 | 1 | 0 | 1 | 1 | 2 | 3 |
| Li et al. 2009 | Low | Low | Low | Low | Low | Low | High | High | 1 | 1 | 2 | 1 | 0 | 4 | 5 |
| Li and Ran 2009 | Low | High | High | High | Low | Low | Low | Low | 1 | 1 | 0 | 1 | 1 | 2 | 4 |
| Li 2004 | Low | High | High | High | Low | Low | High | Low | 1 | 1 | 0 | 1 | 0 | 2 | 3 |
| Liang and Feng 2010 | Low | High | High | High | Low | Low | High | High | 1 | 1 | 0 | 1 | 0 | 2 | 3 |
| Liu 2004 | Low | High | High | High | Low | Low | Low | Low | 1 | 1 | 0 | 1 | 1 | 2 | 4 |
| Liu and Li 2007 | Low | High | High | High | Low | Low | High | High | 0 | 1 | 0 | 1 | 1 | 2 | 3 |
| Liu 2005 | Low | High | High | High | Low | Low | High | High | 1 | 1 | 0 | 1 | 0 | 2 | 3 |
| Liu 2011 | Low | High | High | High | Low | Low | High | High | 1 | 1 | 0 | 1 | 0 | 2 | 3 |
| Lu et al. 2006 | Low | High | High | High | Low | Low | High | High | 1 | 1 | 0 | 1 | 0 | 2 | 3 |
| Ma and Peng 2008 | Low | High | High | High | Low | Low | Low | Low | 1 | 1 | 0 | 1 | 0 | 2 | 3 |
| Nie and Chen 2007 | Low | High | High | High | Low | Low | High | High | 1 | 1 | 0 | 1 | 0 | 2 | 3 |
| Qiao and Wu 2004 | Low | High | High | High | Low | Low | Low | Low | 1 | 1 | 0 | 1 | 1 | 2 | 4 |
| Song 2010 | Low | High | High | High | Low | Low | Low | Unclear | 0 | 1 | 0 | 1 | 0.5 | 2 | 2.5 |
| Su 2009 | Low | Unclear | High | High | Low | Low | Low | Low | 0 | 1 | 0 | 1 | 1 | 2 | 3 |
| Sun 2010 | Low | High | High | High | Low | Low | High | High | 1 | 1 | 0 | 1 | 0 | 2 | 3 |
| Sun et al. 2006 | Low | High | High | High | Low | Low | High | Unclear | 0 | 1 | 0 | 1 | 0 | 2 | 2 |
| Tian and Wu 2006 | Low | High | High | High | Low | Low | Low | Low | 1 | 1 | 0 | 1 | 1 | 2 | 4 |
| Wan and Xu 2009 | Low | High | High | High | Low | Low | Low | Low | 1 | 1 | 0 | 1 | 1 | 2 | 4 |
| Wang 2007 | Low | High | High | High | Low | Low | Low | High | 1 | 1 | 0 | 1 | 1 | 2 | 4 |
| Wang 2011 | Low | High | High | High | Low | Low | Low | Low | 1 | 1 | 0 | 1 | 1 | 2 | 4 |
| Wang 2011 | Low | High | High | High | Low | Low | Low | Low | 1 | 1 | 0 | 1 | 1 | 2 | 4 |
| Wang and Ji 2008 | Low | High | High | High | Low | Low | High | High | 0 | 1 | 0 | 1 | 0 | 2 | 2 |
| Wang and Sun 2007 | Low | High | High | High | Low | Low | High | Unclear | 0 | 1 | 0 | 1 | 0 | 2 | 2 |
| Wang 2005 | Low | High | High | High | Low | Low | High | High | 1 | 1 | 0 | 1 | 0 | 2 | 3 |
| Wang 2010 | Low | High | High | High | Low | Low | Low | Low | 1 | 1 | 0 | 1 | 1 | 2 | 4 |
| Wang 2005 | Low | High | High | High | Low | Low | High | Unclear | 0 | 1 | 0 | 1 | 0 | 2 | 2 |
| Wang 2005 | Low | Low | Unclear | High | Low | Low | Low | Low | 1 | 1 | 0 | 1 | 1 | 2 | 4 |
| Wang et al. 2011 | Low | High | High | High | Low | Low | Low | Low | 1 | 1 | 0 | 1 | 1 | 2 | 4 |
| Wu et al. 2008 | Low | High | High | High | Low | Low | High | Unclear | 1 | 1 | 0 | 1 | 0 | 2 | 3 |
| Wu et al. 2011 | Low | High | High | High | Low | Low | High | High | 1 | 1 | 0 | 1 | 0 | 2 | 3 |
| Xia 2011 | Low | Low | Unclear | High | Low | Low | High | Unclear | 1 | 1 | 0 | 1 | 0 | 2 | 3 |
| Yang and Ma 2008 | Low | Low | Unclear | High | Low | Low | High | High | 1 | 1 | 0 | 1 | 0 | 2 | 3 |
| Ye et al. 2008 | Low | High | High | High | Low | Low | Low | Low | 0 | 1 | 0 | 1 | 1 | 2 | 3 |
| Yu and Wang 2009 | Low | High | High | High | Low | Low | High | High | 1 | 1 | 0 | 1 | 0 | 2 | 3 |
| Yuan 2005 | Low | High | High | High | Low | Low | High | High | 1 | 1 | 0 | 1 | 0 | 2 | 3 |
| Zhang 2005 | Low | High | High | High | Low | Low | High | High | 1 | 1 | 0 | 1 | 0 | 2 | 3 |
| Zhang 2010 | Low | High | High | High | Low | Low | Low | Low | 1 | 1 | 0 | 1 | 1 | 2 | 4 |
| Zhang 2004 | Low | High | High | High | Low | Low | Low | Low | 1 | 1 | 0 | 1 | 1 | 2 | 4 |
| Zhang 2004 | Low | High | High | High | Low | Low | Low | High | 0 | 1 | 0 | 1 | 1 | 2 | 3 |
| Zhao et al. 2010 | Low | High | High | High | Low | Low | Low | Low | 1 | 1 | 0 | 1 | 1 | 2 | 4 |
| Zhao and An 2008 | Low | High | High | High | Low | Low | Low | Low | 1 | 1 | 0 | 1 | 1 | 2 | 4 |
| Zhao 2010 | Low | High | High | High | Low | Low | High | Unclear | 1 | 1 | 0 | 1 | 0 | 2 | 3 |
| Zhong et al. 2007 | Low | High | High | High | Low | Low | High | High | 0 | 1 | 0 | 1 | 0 | 2 | 2 |
| Zhu 2005 | Low | High | High | High | Low | Low | Unclear | Unclear | 0 | 1 | 0 | 1 | 0.5 | 2 | 2.5 |
C1 is random sequence generation for selection bias; C2 is allocation concealment for selection bias; C3 is blinding of participants and personnel for performance bias; C4 is blinding of outcome assessment (patient-reported outcomes) for detection bias; C5 is blinding of outcome assessment (SYMPTOMS) for detection bias; C6 is incomplete outcome data addressed for attrition bias; C7 is reporting bias for selecting reporting; C8 is other sources of bias for other bias; Comparable is participants in treat group and control group comparable; Random is study described as randomized; Blind is study described as blinding; Dropout is withdrawals and dropouts of participants; AE is the adverse effects; Low is low risk of bias; High is high risk of bias; Unclear is unclear risk of bias.
3.4. Overall Effects of Included Studies
As shown in Figure 2 and Table 3, the overall OR of SYMPTOMS was 3.32 (95% CI: [2.72, 4.04], Z = 11.93, P < 0.0001) with significant heterogeneity (tau = 0.23, I 2 = 37%, P = 0.0030) among the 65 studies with SYMPTOMS outcome. Figure 3 and Table 4 show that the overall OR of ECG was 2.59 (95% CI: [2.14, 3.15], Z = 9.68, P < 0.0001) with nonsignificant heterogeneity (tau = 0.11, I 2 = 32%, P = 0.0539) among the 38 studies with ECG outcome. Both ORs (SYMPTOMS and ECG) indicated that GXN was more effective than the drugs in control group in treating angina pectoris. The Kendall correlation between SYMPTOMS and ECG in ORs was statistically significant (tau = 0.2644; P = 0.0200).
Figure 2.

Forest plot of outcome measure SYMPTOMS.
Table 3.
Subgroups and sensitivity analysis on SYMPTOMS outcomes.
| Group | Number of RCTs |
Number of participants |
OR | Wilcoxon test |
95% CI | Z | P (eff) | I 2 | χ 2 | P (het) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| M score | ≤3 | 40 | 3625 | 3.21 | W = 546 | 2.36, 4.35 | 7.46 | <0.0001 | 54% | 0.50 | <0.0001 |
| >3 | 25 | 2439 | 3.51 | P = 0.5395 | 2.78, 4.43 | 10.50 | <0.0001 | 0% | 0 | 0.9858 | |
| Sample size | <93 | 39 | 2772 | 3.22 | W = 445.5 | 2.59, 4.01 | 10.51 | <0.0001 | 0% | 0 | 0.6150 |
| ≥93 | 26 | 3292 | 3.37 | P = 0.4140 | 2.39, 4.76 | 6.89 | <0.0001 | 60% | 0.47 | <0.0001 | |
| Number of authors | 1 | 27 | 2485 | 3.18 | W = 1189 | 2.39, 4.24 | 7.92 | <0.0001 | 28% | 0.16 | 0.1253 |
| >1 | 38 | 3579 | 3.40 | P = 0.7558 | 2.60, 4.46 | 8.87 | <0.0001 | 44% | 0.30 | 0.0031 | |
| Publication year | ≤2008 | 31 | 2495 | 3.80 | W = 441.5 | 3.01, 4.81 | 11.19 | <0.0001 | 1% | 0.01 | 0.2929 |
| >2008 | 34 | 3569 | 2.94 | P = 0.2642 | 2.20, 3.93 | 7.32 | <0.0001 | 48% | 0.34 | 0.0016 | |
| Trial date report | Reported | 51 | 4793 | 3.19 | W = 2112.5 | 2.57, 3.95 | 10.52 | <0.0001 | 36% | 0.21 | 0.0189 |
| Not reported | 14 | 1271 | 3.84 | P = 1 | 2.33, 6.33 | 5.28 | <0.0001 | 47% | 0.40 | 0.0254 | |
| Baseline comparison |
Reported | 50 | 4808 | 3.56 | W = 2112.5 | 2.84, 4.45 | 11.10 | <0.0001 | 40% | 0.25 | 0.0057 |
| Not reported | 15 | 1256 | 2.53 | P = 1 | 1.75, 3.68 | 4.89 | <0.0001 | 14% | 0.08 | 0.1545 | |
| Adverse events |
Reported | 31 | 2947 | 3.20 | W = 1006 | 2.58, 3.97 | 10.59 | <0.0001 | 0% | 0 | 0.4304 |
| Not reported | 34 | 3117 | 3.48 | P = 0.4678 | 2.53, 4.78 | 7.68 | <0.0001 | 51% | 0.44 | 0.0003 | |
| Follow-up period (day) |
≤14 | 48 | 4461 | 3.38 | W = 440 | 2.75, 4.16 | 11.51 | <0.0001 | 28% | 0.14 | 0.1321 |
| >14 | 17 | 1603 | 3.05 | P = 0.6382 | 1.81, 5.16 | 4.18 | <0.0001 | 61% | 0.71 | 0.0005 | |
| GXN daily | 6–200 mL | 65 | 6064 | 3.32 | W = 2059 | 2.72, 4.04 | 11.93 | <0.0001 | 37% | 0.23 | 0.0030 |
| Dosage (mL) | 6–30 mL | 64 | 6008 | 3.34 | P = 0.9231 | 2.73, 4.07 | 11.83 | <0.0001 | 38% | 0.24 | 0.0025 |
| GXN daily | <20 | 4 | 352 | 3.42 | χ 2 = 0.4290 | 1.48, 7.91 | 2.88 | 0.0040 | 38% | 0.28 | 0.1717 |
| Dosage (mL) | 20 | 45 | 4235 | 3.16 | df = 2 | 2.45, 4.07 | 8.85 | <0.0001 | 46% | 0.33 | 0.0004 |
| >20 | 16 | 1477 | 3.87 | P = 0.8069 | 2.84, 5.29 | 8.51 | <0.0001 | 0% | 0 | 0.8315 | |
| Types of angina | Stable | 4 | 374 | 3.42 | χ 2 = 0.9900 | 1.89, 6.21 | 4.05 | <0.0001 | 0% | 0 | 0.7151 |
| Unstable | 31 | 2892 | 3.07 | df = 2 | 2.26, 4.16 | 7.18 | <0.0001 | 47% | 0.34 | 0.0013 | |
| Angina | 30 | 2798 | 3.61 | P = 0.6096 | 2.72, 4.81 | 8.81 | <0.0001 | 32% | 0.19 | 0.1179 | |
| Improvement | >50% | 65 | 6064 | 3.32 | W = 02.5 | 2.72, 4.04 | 11.93 | <0.0001 | 37% | 0.23 | 0.0030 |
| >80% | 63 | 5856 | 1.75 | P < 0.0001 | 1.54, 1.98 | 8.65 | <0.0001 | 25% | 0.06 | 0.0557 | |
| GXN | 1 | 6 | 408 | 3.19 | χ 2 = 0.4891 | 1.86, 5.49 | 4.21 | <0.0001 | 0% | 0 | 0.8454 |
| GXN + CG | 2 | 49 | 4681 | 3.43 | df = 2 | 2.81, 4.19 | 12.07 | <0.0001 | 21% | 0.11 | 0.1177 |
| GXN + CG + additional | 3 | 10 | 975 | 3.07 | P = 0.7830 | 1.47, 6.41 | 2.99 | 0.00228 | 72% | 0.98 | <0.0001 |
CI is confidence interval; Z and P (eff) are statistical terms for evaluating overall effect; I 2, χ 2, and P (het) are statistical terms for assessing heterogeneity among studies.
Figure 3.
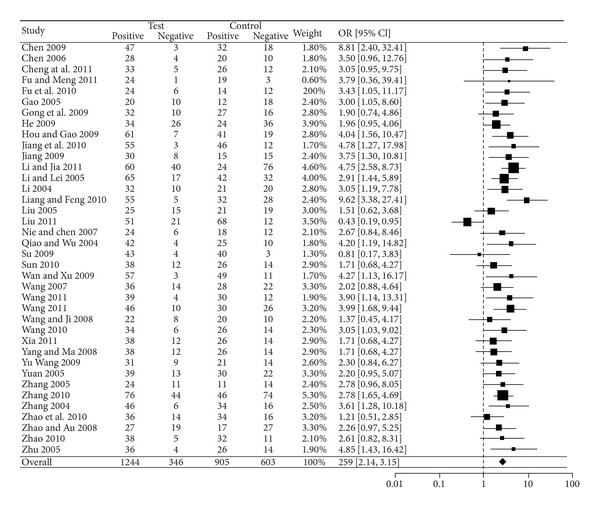
Forest plot of outcome measure ECG.
Table 4.
Subgroups and sensitivity analysis on ECG outcomes.
| Group | Number of RCTs |
Number of participants |
OR | Wilcoxon test |
95% CI | Z | P (eff) | I 2 | χ 2 | P (het) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| M score | ≤3 | 21 | 1995 | 2.47 | W = 149 | 1.79, 3.41 | 5.53 | <0.0001 | 52% | 0.28 | 0.0025 |
| >3 | 17 | 1709 | 2.71 | P = 0.3945 | 2.17, 3.39 | 8.77 | <0.0001 | 0% | 0 | 0.9136 | |
| Sample size | <93 | 23 | 1734 | 2.42 | W = 127 | 1.93, 3.02 | 7.72 | <0.0001 | 0% | 0 | 0.9776 |
| ≥93 | 15 | 1970 | 2.86 | P = 0.1789 | 1.94, 4.21 | 5.33 | <0.0001 | 67% | 0.37 | 0.0002 | |
| Number of authors | 1 | 19 | 1872 | 2.30 | W = 140 | 1.72, 3.08 | 5.65 | <0.0001 | 41% | 0.16 | 0.0358 |
| >1 | 19 | 1832 | 2.98 | P = 0.2428 | 2.34, 3.80 | 8.81 | <0.0001 | 13% | 0.04 | 0.4435 | |
| Publication year | ≤2008 | 15 | 1268 | 2.49 | W = 200 | 1.94, 3.20 | 7.17 | <0.0001 | 0% | 0 | 0.9538 |
| >2008 | 23 | 2436 | 2.68 | P = 0.4200 | 1.99, 3.61 | 6.47 | <0.0001 | 52% | 0.26 | 0.0025 | |
| Trial date report | Reported | 31 | 3069 | 2.43 | W = 57 | 1.97, 3.00 | 8.29 | <0.0001 | 34% | 0.12 | 0.0511 |
| Not reported | 7 | 635 | 3.67 | P = 0.0548 | 2.36, 5.70 | 5.79 | <0.0001 | 8% | 0.03 | 0.5363 | |
| Baseline comparison |
Reported | 34 | 3318 | 2.64 | W = 85 | 2.14, 3.24 | 9.20 | <0.0001 | 35% | 0.12 | 0.0513 |
| Not reported | 4 | 386 | 2.29 | P = 0.4325 | 1.25, 4.19 | 2.68 | 0.0074 | 23% | 0.09 | 0.2200 | |
| Adverse events |
Reported | 19 | 1955 | 2.67 | W = 192 | 2.16, 3.30 | 9.14 | <0.0001 | 0% | 0 | 0.8792 |
| Not reported | 19 | 1749 | 2.54 | P = 0.7480 | 1.80, 3.59 | 5.28 | <0.0001 | 54% | 0.31 | 0.0020 | |
| Follow-up period (day) |
≤14 | 27 | 2528 | 2.83 | W = 129 | 2.34, 3.42 | 10.67 | <0.0001 | 2% | 0 | 0.7120 |
| >14 | 11 | 1176 | 2.21 | P = 0.5407 | 1.37, 3.57 | 3.27 | <0.0001 | 65% | 0.39 | 0.0024 | |
| GXN daily | 6–200 mL | 38 | 3704 | 2.59 | W = 707.5 | 2.14, 3.15 | 9.68 | <0.0001 | 32% | 0.11 | 0.0539 |
| dosage (mL) | 6–30 mL | 37 | 3648 | 2.58 | P = 0.9662 | 2.12, 3.14 | 9.45 | <0.0001 | 33% | 0.1175 | 0.0448 |
| GXN daily | <20 | 4 | 352 | 1.89 | χ 2 = 3.4288, | 1.00, 3.55 | 1.96 | 0.0497 | 27% | 0.1148 | 0.2425 |
| dosage (mL) | 20 | 24 | 2324 | 2.80 | df = 2 | 2.14, 3.66 | 7.51 | <0.0001 | 43% | 0.1820 | 0.0246 |
| >20 | 10 | 1028 | 2.53 | P = 0.1801 | 1.85, 3.46 | 5.81 | <0.0001 | 14% | 0.0365 | 0.5413 | |
| Types of angina | Stable | 4 | 374 | 3.03 | χ 2 = 0.7010 | 1.80, 5.09 | 4.18 | <0.0001 | 0% | 0 | 0.6688 |
| Unstable | 16 | 1676 | 2.48 | df = 2 | 1.95, 3.15 | 7.42 | <0.0001 | 10% | 0.02 | 0.2332 | |
| Angina | 18 | 1654 | 2.60 | P = 0.7043 | 1.87, 3.61 | 5.68 | <0.0001 | 46% | 0.22 | 0.0191 | |
| Improvement | >50% | 38 | 3704 | 2.59 | W = 1050 | 2.14, 3.15 | 9.68 | <0.0001 | 32% | 0.11 | 0.0539 |
| >80% | 38 | 3704 | 1.84 | P < 0.0001 | 1.59, 2.14 | 8.06 | <0.0001 | 0% | 0 | 0.8367 | |
| GXN | 1 | 3 | 188 | 3.15 | χ 2 = 1.6604 | 1.71, 5.81 | 3.68 | 0.0002 | 0% | 0 | 0.9202 |
| GXN + CG | 2 | 29 | 2963 | 2.68 | df = 2 | 2.10, 3.41 | 7.98 | <0.0001 | 42% | 0.17 | 0.0157 |
| GXN + CG + additional | 3 | 6 | 553 | 2.09 | P = 0.4360 | 1.45, 3.01 | 3.94 | <0.0001 | 0% | 0 | 0.6382 |
CI is confidence interval; Z and P (eff) are statistical terms for evaluating overall effect; I 2, χ 2, and P (het) are statistical terms for assessing heterogeneity among studies.
3.5. Subgroup Analysis
ORs of the subgroups in both SYMPTOMS (Table 3) and ECG (Table 4) were compared based on the study characteristics including M scores (≤3 or >3), sample sizes (<93 or ≥93), number of authors (1 or >1), years of publication (before or after January 1, 2008), reports of trial dates (yes or no), baseline comparison of participants (yes or no), reports of adverse events (yes or no), follow-up periods (≤14 days or >14 days), GXN daily dosages (<20 mL, 20 mL, >20 mL), different angina types, and different treatments including GXN monotherapy versus control treatment, GXN + control versus control, and GXN mixed treatment + control versus control. There was no statistically significant difference between ORs of these subgroups.
3.6. Sensitivity Analysis
When the improvement criteria were raised to the significant level from the basic level, the overall results remained effective (i.e., OR > 1) and statistically significant. The OR of overall SYMPTOMS decreased from 3.32 to 1.75 (95% CI: [1.54, 1.98], Z = 8.65, P < 0.0001). The OR of overall ECG decreased from 2.59 to 1.84 (95% CI: [1.59, 2.14], Z = 8.06, P < 0.0001). There was a statistically significant correlation between the changes in ORs of SYMPTOMS and ECG outcomes (tau = 0.2971, P = 0.0089). When study [33] with maximum GXN dosage was excluded, there was no statistically significant difference between ORs of groups in both SYMPTOMS and ECG data.
3.7. Metaregression
Table 5 shows the results of metaregression between log OR and study characteristics. There seemed to be no statistically significant relationship between GXN's efficacy and study characteristics, except that follow-up periods made a significant difference (P = 0.0093) on the log OR with ECG data.
Table 5.
Metaregression analysis of the relationship between outcomes and the study characteristics.
| log OR | Number of RCTs | Number of participants | Factor | Coefficient | z | P |
|---|---|---|---|---|---|---|
| SYMPTOMS | 65 | 6064 | M score | 0.0663 | 0.4378 | 0.6615 |
| Sample size | −0.0013 | −0.4955 | 0.6203 | |||
| Number of authors | −0.0466 | −0.6283 | 0.5298 | |||
| Publication year | −0.0838 | −1.9158 | 0.0554 | |||
| Trial date report | −0.1931 | −0.7634 | 0.4453 | |||
| Baseline comparison | 0.3299 | 1.3376 | 0.1810 | |||
| Adverse events | −0.0965 | −0.4646 | 0.6422 | |||
| Follow-up period | 0.0116 | 0.6126 | 0.5401 | |||
|
| ||||||
| ECG | 38 | 3704 | M score | 0.1191 | 0.7160 | 0.4740 |
| Sample size | 0.0006 | 0.2938 | 0.7689 | |||
| Number of authors | −0.0100 | −0.1071 | 0.9147 | |||
| Publication year | −0.0180 | −0.4296 | 0.6675 | |||
| Trial date report | −0.4255 | −1.5606 | 0.1186 | |||
| Baseline comparison | 0.1520 | 0.4458 | 0.6558 | |||
| Adverse events | 0.1066 | 0.5300 | 0.5961 | |||
| Follow-up period | −0.0423 | −2.6000 | 0.0093 | |||
3.8. Risk of Bias Across Studies
Visual assessment of funnel plots (Figure 4) found obvious asymmetry, indicating that there were publication biases in the results of both SYMPTOMS and ECG. Egger's test (SYMPTOMS: t = 2.0555, P = 0.0440; ECG: t = 0.9358, P = 0.3556) and Begg's test (SYMPTOMS: z = 0.1898, P = 0.0257; ECG: z = 0.2571, P = 0.0236) detected statistically significant publication biases. Trim-and-fill method found that there were 24 missing studies for SYMPTOMS and 13 missing studies for ECG on the left side of the corresponding funnel plots.
Figure 4.
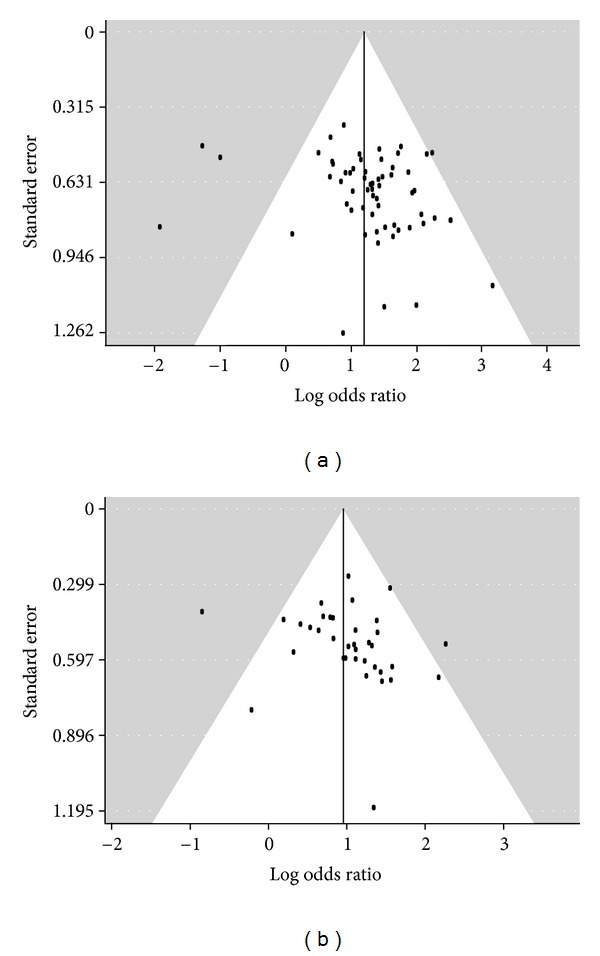
Funnel plots of (a) the included studies with SYMPTOMS data and (b) the included studies with ECG data.
3.9. Adverse Events
As shown in Table 6, the most frequently reported adverse event of GXN was headache. All adverse effects were minor or well tolerated as they did not cause dropouts except in one study [31] where six participants dropped out because of the adverse effects. Headache, epigastria discomfort, and palpitation were noted as the top three adverse effects of drugs in control group. Adverse effects of GXN were less than those of control drugs in the number of types, severity, and frequency.
Table 6.
Adverse events reported in the included studies.
| Treatment group | Control group | |||
|---|---|---|---|---|
| Number of AEs | Number of studies | Number of AEs | Number of studies | |
| Headache | 10 | 4 | 9 | 3 |
| Dizziness | 1 | 1 | 1 | 1 |
| Palpitation | 4 | 2 | 7 | 3 |
| Skin ecchymosis | 8 | 2 | 6 | 1 |
| Serum transaminase elevated | 1 | 1 | NR | NR |
| Nausea | 1 | 1 | 3 | 1 |
| Epigastria discomfort | 4 | 2 | 8 | 3 |
| Abnormal liver function | 1 | 1 | NR | NR |
| Skin allergy | NR | NR | 1 | 1 |
| General weakness | NR | NR | 1 | 1 |
| Cold sweat | NR | NR | 5 | 1 |
| Hypotension | NR | NR | 1 | 1 |
| Skin mucosal bleeding | NR | NR | 1 | 1 |
| No AEs | 0 | 27 | 0 | 24 |
| Total AEs reports | 30 | 9 | 43 | 11 |
| No AE report | 0 | 28 | 0 | 29 |
NR: not reported; AEs: adverse events.
4. Discussion
This study provides the first comprehensive, up-to-date, and PRISMA-compliant systematic review on the efficacy of GXN in treating angina pectoris. Among 65 included RCTs with 6064 participants, overall ORs of SYMPTOMS and ECG were 3.32 (95% CI: [2.72, 4.04]) (P < 0.0001) and 2.59 (95% CI: [2.14, 3.15]) (P < 0.0001), respectively. Subgroup analysis also found statistical significance in the differences between GXN treatment group and control group in testing GXN monotherapy and adjunctive therapy. These results indicated that GXN treatment is effective in treating angina pectoris.
The results of this meta-analysis were robust as shown in subgroup analysis, sensitivity analysis, and metaregression on various parameters including sample sizes, follow-up periods, daily dosages of GXN, types of angina pectoris, and the quality scores of RCTs. Although funnel plots, Begg's test, Egger's test, and trim-and-fill method found publication biases, the overall effects would still favor GXN treatment after enough number of less favorable studies were published to restore the symmetry of funnel plots.
The efficacy of GXN in both monotherapy and adjunctive therapy of angina pectoris exemplifies potential uses of chemical components of GXN as one of the herbal products that have offered great potentials in developing multitarget agents to treat complex diseases [91]. Experimental studies also showed that the aqueous extracts from both Danshen and Chuanxiong significantly reduced the myocardial infarct size in rat myocardial ischemia/reperfusion injury [92]. As seen from the clinical and experimental findings, GXN seems to be a promising resource for identifying new therapeutic agents or new drug targets [93] in treating angina pectoris. Although subgroup analysis and sensitivity analysis did not suggest any significant factors which would influence the efficacy of GXN, clinical heterogeneity may contribute to heterogeneity of this meta-analysis.
The limitations of this study include small sample sizes and short follow-up periods. The mean sample size was 93, which was lower than 124 as required by an alpha of 0.05, the proportions of 0.899 for GXN and 0.742 for control group, and a power of 0.8 [94]. The patients of angina pectoris would need long-term treatment [95], but most available RCTs have short follow-up periods.
Another major limitation of this systematic review is the low quality of included studies although most of included RCT reports achieved the average quality of Chinese RCTs [96, 97], which is still inadequate. Almost all (63 out of 65) studies scored 2 at the Jadad scale, which ranges between 0 and 5. One study [34] reported single blinding and another study [47] reported double blinding. Twenty-four RCTs scored 4 at the M scale and 40 RCTs scored less than 4 at the M scale. There is evidence of the Cochrane Library's tool to show high risks of bias with the aspects of selection bias, performance bias, and detection bias. More than that, less than but almost half of included RCTs (28/65) did not report adverse events, one possible reason of which is high reporting bias for selecting reporting. Safety of GXN intervention cannot be assessed because of incomplete reporting data. Despite the fact that subgroup analysis found no statistically significant differences in ORs of SYMPTOMS and ECG between the RCTs of low and medium M scores, high-quality RCTs would be necessary to further support the efficacy of GXN-based medicines over conventional Western drugs in treating angina pectoris.
Seventy-three out of 6064 participants had AE. The main AEs included headache (19), skin ecchymosis (14), epigastria discomfort (12), and palpitation (11). Headache was the most frequent AE in this paper. The AE mechanisms of GXN are not clear and definite. The functions of dilated blood vessels and coronary artery blood circulation activating are possible reasons that lead to adverse events.
According to this meta-analysis, GXN seems to be effective in treating angina pectoris. As GXN contains the herbal extracts from Salvia miltiorrhiza and Ligustrazine, hence DSS, PAC, PAL, CAA, and SAB as the main active ingredients with potential effects on coronary heart disease, angina pectoris, and cardiovascular diseases [98] by enhancing coronary blood flow, improving the myocardial systolic functions, and protecting myocardial cells [99], further clinical, herbal formulation and pharmacological studies are warranted for further research and development.
5. Conclusion
This meta-analysis of eligible RCTs provides evidence that GXN is effective in treating angina pectoris. This evidence warrants further RCTs of higher quality, longer follow-up periods, larger sample sizes, and multicentres/multicountries for more extensive subgroup, sensitivity, and metaregression analyses.
Supplementary Material
Supplementary Table 1 provides the treatment (drugs and dosages for control and treatment groups) details about the RCTs included in this study.
Conflict of Interests
The authors have no conflict of interests.
Authors' Contributions
S. Leung and M. Lee conceived the meta-analytic assessment of GXN's efficacy. S. Leung, M. Lee, and Y. Jia conducted the study design. G. Cui, X. Huang and M. Lee reviewed the pharmacology and potential clinical applications of GXN. Y. Jia and F. Pan searched the databases for RCTs, retrieved the studies, evaluated the quality of the studies, and extracted the data. Y. Jia and S. Leung analyzed the data and wrote the paper. All authors revised, read, and approved the paper.
Acknowledgments
The work of Y. Jia, F. Pan, and S. Leung was supported by a research Grant from the University of Macau for a study on “Open systematic reviewing of clinical trials” (MYRG190 (Y2-L3)-ICMS11-LSW). The work of G. Cui, X. Huang, M. Lee was supported by a Research Grant from the Science and Technology Development Fund, Macao, for a study on “Mechanistic studies of active compounds from Chinese medicine and their combinations for minimising and treating cardio-cerebrovascular complications” (ref. no. 014/2011/A1).
References
- 1.Gaziano TA. Cardiovascular disease in the developing world and its cost-effective management. Circulation. 2005;112(23):3547–3553. doi: 10.1161/CIRCULATIONAHA.105.591792. [DOI] [PubMed] [Google Scholar]
- 2.Kung HC, Hoyert DL, Xu JQ, Murphy SL. Deaths: final data for 2005. National Vital Statistics Reports. National Center for Health Statistics. 2008;56(10):1–120. [PubMed] [Google Scholar]
- 3.Scottish Intercollegiate Guidelines Networks. Management of Stable Angina. A National Clinical Guideline. no. 96 2007. [Google Scholar]
- 4.Tan SL, Liu CL. Clinical observation of compound danshen dripping pill treating stable angina pectoris. Heilongjiang Medical Journal. 2001;14(2):p. 125. [Google Scholar]
- 5.Chen XF, Lou ZY, Zhang H, et al. Identification of multiple components in Guanxinning injection using hydrophilic interaction liquid chromatography/time-of-flight mass spectrometry and reversed-phase liquid chromatography/time-of-flight mass spectrometry. Rapid Communications in Mass Spectrometry. 2011;25(11):1661–1674. doi: 10.1002/rcm.5003. [DOI] [PubMed] [Google Scholar]
- 6.Cheng TO. Cardiovascular effects of danshen. International Journal of Cardiology. 2007;14:9–22. doi: 10.1016/j.ijcard.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Sun YY, Li SF, Quan C. Solubility of ferulic acid and tetramethylpyrazine in supercritical carbon dioxide. Journal of Chemical and Engineering Data. 2005;50(4):1125–1128. [Google Scholar]
- 8.Chen HP. Efficacy analysis of Guanxinning injection combined with isosorbide mononitrate treating ischemic heart disease. Chinese Journal of Ethnomedicine and Ethnopharmacy. 2009;10:p. 134. [Google Scholar]
- 9.Shen YL, Sang ZL. Efficacy analysis of Guanxinning injection treating ischemic heart disease. World Health Digest Medical Periodieal. 2011;8(47):18–20. [Google Scholar]
- 10.Fu XY, Zhao XH. Clinical analysis of Guanxinning injection treating ischemic heart disease or cerebral infarction. China Modern Medicine. 2009;16(10):p. 194. [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Plos Medicine. 2009;89(9):873–880. [PubMed] [Google Scholar]
- 12.Wang JM. Meta-analysis of Guanxinning injection as adjunctive therapy for unstable Angina pectoris. China Pharmacy. 2011;22(19):1810–1812. [Google Scholar]
- 13.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clinical Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 14.Jia Y, Huang F, Zhang S, Leung SW. Is danshen (Salvia miltiorrhiza) dripping pill more effective than isosorbide dinitrate in treating angina pectoris? A systematic review of randomized controlled trials. International Journal of Cardiology. 2012;157(3):330–340. doi: 10.1016/j.ijcard.2010.12.073. [DOI] [PubMed] [Google Scholar]
- 15.Higgins J, Green S. Cochrane Handbook For Systematic Reviews of Interventions Version 5.1.0. chapter 8 2008. Analyzing data and undertaking meta-analyses. [Google Scholar]
- 16.Nomenclature and criteria for diagnosis of ischemic heart disease. Report of the Joint International Society and Federation of Cardiology/World Health Organization Task Force on standardization of clinical nomenclature. Circulation. 1979;59(3):607–609. doi: 10.1161/01.cir.59.3.607. [DOI] [PubMed] [Google Scholar]
- 17.Lewis T. PROC LOGISTIC: the logistics behind interpreting categorical variable effects. Statistical Data Analysis, pp. 1–7, 2007.
- 18.Breierova L, Choudhari M. An introduction to sensitivity analysis. Massachusetts Institute of Technology. 2001;10:41–107. [Google Scholar]
- 19.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 20.Sterne JAC, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. Journal of Clinical Epidemiology. 2001;54(10):1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 21.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 22.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Increase in studies of publication bias coincided with increasing use of meta-analysis. British Medical Journal. 1997;316:629–634. [Google Scholar]
- 23.Duval SJ, Tweedie RL. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 24.Thompson SG, Higgins JPT. How should meta-regression analyses be undertaken and interpreted? Statistics in Medicine. 2002;21(11):1559–1573. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- 25.R Development Core Team. R: a language and environment for statistical computing. Reference index version 2.12.0. R Foundation for Statistical Computing, Vienna, Austria, 2005, http://www.r-project.org/
- 26.Chen HP. Clinical observation of Guanxinning combined with isosorbide mononitrate in the treatment of Coronary Heart Disease. Chinese Journal of Ethnomedicine and Ethnopharmacy. 2009;10:p. 134. [Google Scholar]
- 27.Chen RJ, Yang X, Yang XS. Clinical observation of Guanxinning combined with potassium magnesium aspartate in the treatment of coronary artery disease. Contemporary Medicine. 2011;17:p. 87. [Google Scholar]
- 28.Chen SG. Clinical observation of Guanxinning injection in the treatment of coronary artery disease. Modern Journal of Integrated Traditional Chinese and Western Medicine. 2006;15:2219–2220. [Google Scholar]
- 29.Cheng HY, Zhang WP. The clinical experience with Guanxinning injection in the treatment of elderly diabetic patients with unstable angina. Journal of Bingtuan Medicine. 2010;25:21–22. [Google Scholar]
- 30.Cheng YS, Tan RB, Zhao DM. Guanxinning injection in the treatment of unstable angina pectoris: a report of 72 cases. China Foreign Medical Treatment. 2011;8:p. 110. [Google Scholar]
- 31.Dong XP. Clinical observation of Guanxinning injection in the treatment of angina pectoris: a report of 60 cases. Chinese Journal of Modern Drug Application. 2009;3:144–145. [Google Scholar]
- 32.Fu YC, Meng LQ. Clinical observation of Guanxinning injection in the treatment of angina pectoris. Journal of New Chinese Medicine. 2011;43:8–9. [Google Scholar]
- 33.Fu YW, Jiao CX, Shi YH, Guo F. Clinical observation of Guanxinning injection combined with Shengmai injection in the treatment of Coronary Heart Disease. Journal of Emergency in Traditional Chinese Medicine. 2010;19:61–62. [Google Scholar]
- 34.Gao GQ, Wang F, Sun HP. Clinical observation of Guanxinning injection in the treatment of angina pectoris. Nei Mongol Journal of Traditional Chinese Medicine. 2005;24:6–7. [Google Scholar]
- 35.Gong CJ, Wang PJ, Huang LM. Clinical observation of Xueshuantong combined with Guanxinning injection in the treatment of angina pectoris. Asia-Pacific Traditional Medicine. 2009;5:52–54. [Google Scholar]
- 36.He HY. Clinical observation of Guanxinning injection in the treatment of unstable angina: a report of 27 cases. China Modern Doctor. 2007;45:43–44. [Google Scholar]
- 37.He YJ. Clinical observation of atorvastatin combined with Guanxinning injection in the treatment of unstable angina. Chinese Journal of Clinical Rational Drug Use. 2009;2:4–5. [Google Scholar]
- 38.Hou GP, Gao XY. Clinical observation of Guanxinning injection and dipyridamole in the treatment of angina pectoris. Journal of Qiqihar Medical College. 2009;30:158–159. [Google Scholar]
- 39.Huang Y, Li B, Li DH, Chen JS. Clinical observation on low molecular weight heparins calcium combined with Guanxinning and Xuesaitong in cerebral infarction. Journal of Clinical Rational Drug Use. 2011;4:7–8. [Google Scholar]
- 40.Jiang S, Xiong ZY, Yong FZ. Clinical observation of Guanxinning injection in the treatment of unstable angina pectoris. China Modern Doctor. 2010;48:125–138. [Google Scholar]
- 41.Jiang SH. Clinical observation of Guanxinning injection in the treatment of unstable angina: a report of 38 cases. China Medical Herald. 2009;6:p. 105. [Google Scholar]
- 42.Jiang SL, Zheng SY, Guo XS, Yi SH, Zou XY. Clinical observation of Guanxinning injection in the treatment of angina pectoris. China Foreign Medical Treatment. 2010;20:p. 123. [Google Scholar]
- 43.Kong LX. Clinical observation of Guanxinning injection combined with ferulic sodium in the treatment of unstable angina pectoris. Clinical Medicine. 2009;29:39–40. [Google Scholar]
- 44.Lan PM, Luo WB, Weng GM. Clinical observation of Guanxinning Injection in the treatment of coronary artery disease and effects on blood lipids. Chinese Medicine Modern Distance Education of China. 2006;4:p. 39. [Google Scholar]
- 45.Li CT, Jia XZ. Clinical observation of Guanxinning injection in the treatment of diabetic patients with angina pectoris: a report of 100 cases. Chinese Journal of Clinical Healthcare. 2011;14:306–307. [Google Scholar]
- 46.Li H, Lei JP. Guanxinning Injection in the treatment of angina pectoris. Journal of Medical Forum. 2005;26:p. 52. [Google Scholar]
- 47.Li H, Cai H, Wang LZ, Yang HY, Ma DM. The improvement of heart function of Guanxinning injection in the patients with angina decubitus. China Medical Herald. 2009;6 [Google Scholar]
- 48.Li L, Ran GX. Clinical observation Guanxinning Injection adjuvant therapy for angina pectoris: a report of 100 cases. Shandong Medical Journal. 2009;49:97–98. [Google Scholar]
- 49.Li XB. Clinical observation of low molecular weight heparin combined with Guanxinning injection in the treatment of unstable angina. Heilongjiang Medical Journal. 2004;28:687–688. [Google Scholar]
- 50.Liang HY, Feng YG. Clinical observation of low molecular weight heparin combined with Guanxinning injection in the treatment of unstable angina. Medical Innovation of China. 2010;7:106–107. [Google Scholar]
- 51.Liu BQ. Clinical observation of low molecular weight heparin and Guanxinning injection in the treatment of unstable angina. Medicine Industry Information. 2004;2:77–124. [Google Scholar]
- 52.Liu L, Li MZ. Clinical observation Guanxinning Injection in the treatment of angina pectoris: a report of 48 cases. Jiangxi Journal of Traditional Chinese Medicine. 2007;38:23–24. [Google Scholar]
- 53.Liu YL. Clinical observation of Guanxinning injection in the treatment of unstable angina pectoris. Chinese Journal of Integrated Traditional and Western Medicine in Intensive and Critical Care. 2005;12:181–182. [Google Scholar]
- 54.Liu ZH. Qi decoction in the treatment of angina pectoris: a report of 80 cases. Journal of Traditional Chinese Medicine. 2011;26:709–710. [Google Scholar]
- 55.Lu CX, Zhang J, Tang B. Guanxinning injection combined with isosorbide dinitrate treating the aged patients with ischemic heart disease angina pectoris. Journal of Yangtze University (Nature Science Edition) 2006;3(3):p. 230. [Google Scholar]
- 56.Ma XY, Peng LW. Clinical observation of Guanxinning injection in the treatment of unstable angina. Journal of Modern Clinical Medicine. 2008;34:41–42. [Google Scholar]
- 57.Nie YB, Chen HY. Clinical observation of Guanxinning injection in the treatment of angina pectoris. Journal of Huaihai Medicine. 2007;25:p. 544. [Google Scholar]
- 58.Qiao WL, Wu ZR. Guanxinning injection for stable angina pectoris of coronary heart disease. Journal of Henan University of Chinese Medicine. 2004;19:p. 51. [Google Scholar]
- 59.Song GF. Analysis study of Diltiazem combined with Guanxinning in the treatment of unstable angina pectoris. Chronic Pathematology Journal. 2010;12:p. 838. [Google Scholar]
- 60.Su XD. Clinical observation Guanxinning Injection in the treatment of angina pectoris and myocardial ischemia. Proceeding of Clinical Medicine. 2009;18:1813–1814. [Google Scholar]
- 61.Sun SP. Clinical efficacy analysis of Guanxinning combined with Western medicine in the treatment of unstable angina. Jinlin Medical Journal. 2010;31:325–326. [Google Scholar]
- 62.Sun ZH, Dong PF, Sun DW, Zhao LY. Guanxinning injection in the treatment of angina pectoris: a report of 50 cases. Harbin Medical Journal. 2006;26:30–31. [Google Scholar]
- 63.Tian ZQ, Wu L. Clinical observation of Guanxinning combined with Western medicine in the treatment of unstable angina. Chinese Journal of Cardiovascular Rehabilitation Medicine. 2006;15:184–186. [Google Scholar]
- 64.Wan SQ, Xu AY. Clinical observation of Guanxinning injection in the treatment of unstable angina pectoris. Modern Journal of Integrated Traditional Chinese and Western Medicine. 2009;18:2016–2017. [Google Scholar]
- 65.Wang E. Clinical observation of Guanxinning injection in the treatment of angina pectoris. Journal of Emergency in Traditional Chinese Medicine. 2007;16:260–261. [Google Scholar]
- 66.Wang GL. Clinical observation of Guanxinning injection in the treatment of unstable angina pectoris: a report of 43 cases. Chinese Remedies and Clinics. 2011;11:1458–1459. [Google Scholar]
- 67.Wang HT, Yan CM. Clinical observation of Guanxinning injection in the treatment of unstable angina pectoris. Chinese Journal of Clinical Research. 2011;24:792–793. [Google Scholar]
- 68.Wang JJ, Ji XP. Clinical observation of Guanxinning injection in the treatment of unstable angina pectoris. Journal of Liaoning University of TCM. 2008;10:81–82. [Google Scholar]
- 69.Wang LJ, Sun XM. Clinical observation of Guanxinning injection in the treatment of unstable angina pectoris. Journal of Qiqihar Medical College. 2007;28:1350–1351. [Google Scholar]
- 70.Wang Q. Clinical observation of Guanxinning injection in the treatment of angina pectoris. Modern Medicine and Health. 2005;21:1280–1281. [Google Scholar]
- 71.Wang Q. Clinical observation of Guanxinning injection in the treatment of stable angina pectoris: a report of 40 cases. Yunnan Journal of Traditional Chinese Medicine and Materia Medica. 2010;31:p. 47. [Google Scholar]
- 72.Wang RZ. Clinical observation of Guanxinning combined with Western medicine in the treatment of unstable angina: a report of 38 cases. The Journal of Medical Theory and Practice. 2005;18:1156–1157. [Google Scholar]
- 73.Wang Y. Clinical observation of Guanxinning injection treating ischemic heart disease angina pectoris. Modern Medicine Health. 2005;21(10):1280–1281. [Google Scholar]
- 74.Wang ZB, Li HJ, Zhang XY, Li SQ. Clinical observation of Guanxinning injection in the treatment of unstable angina pectoris. Chinese Community Doctors. 2011;13:p. 163. [Google Scholar]
- 75.Wu XF, Wang YF, Liu JP. Clinical observation of guanxinning injection in the treatment of coronary heart disease and angina. China Foreign Medical Treatment. 2008;8:p. 40. [Google Scholar]
- 76.Wu YG, Zhao S, Fan XJ, Zhao WQ. Guanxinning onjection combined with Shenmai injection for unstable angina pectoris: a report of 72 cases. Journal of Anhui TCM College. 2011;30:30–33. [Google Scholar]
- 77.Xia Y. Clinical observation of Guanxinning combined with Western medicine in the treatment of unstable angina: a report of 50 cases. Shaanxi Journal of Traditional Chinese Medicine. 2011;32:1471–1472. [Google Scholar]
- 78.Yang T, Ma ZX. Clinical observation of Guanxinning combined with Western medicine in the treatment of unstable angina. Asia-Pacific Traditional Medicine. 2008;4:22–23. [Google Scholar]
- 79.Ye XW, Luo YS, Gui F. Clinical observation of Low molecular weight heparin combined with Guanxinning injection in the treatment of unstable angina. Zhejiang Journal of Integrated Traditional Chinese and Western Medicine. 2008;18:424–425. [Google Scholar]
- 80.Yu HJ, Wang WF. Clinical observation of Guanxinning injection combined with nitroglycerin in the treatment of angina pectoris: a report of 40 cases. Nei Mongol Journal of Traditional Chinese Medicine. 2009;6:39–40. [Google Scholar]
- 81.Yuan L. Clinical observation of Guanxinning injection in the treatment of angina pectoris. Henan Traditional Chinese Medicine. 2005;25:71–72. [Google Scholar]
- 82.Zhang LX. Guanxinning injection in the treatment of angina pectoris: a report of 35 cases. Zhejiang Journal of Traditional Chinese Medicine. 2005;5:p. 229. [Google Scholar]
- 83.Zhang LX. Analysis study of Guanxinning injection in the treatment of unstable angina pectoris: a report of 120 cases. China Clinical Practical Medicine. 2010;4:133–134. [Google Scholar]
- 84.Zhang Y. Clinical observation of Guanxinning combined with Ginkgo biloba in the treatment of unstable angina. Journal of Changzhi Medical College. 2004;18:179–180. [Google Scholar]
- 85.Zhang ZX. Nitroglycerin combined with Guanxinning injection in the treatment of unstable angina. Zhejiang Journal of Integrated Traditional Chinese and Western Medicine. 2004;14:p. 156. [Google Scholar]
- 86.Zhao FL, Lu XB, Gong CJ, Wang PJ, Huang LM, Guo K. Clinical observation of Guanxinning injection combined with Xueshuantong in the treatment of unstable angina. Journal of Practical Traditional Chinese Medicine. 2010;26:375–377. [Google Scholar]
- 87.Zhao PT, An Y. Clinical observation of Guanxinning Injection combined with simvastatin in the treatment of unstable angina pectoris. Contemporary Medicine. 2008;141:105–106. [Google Scholar]
- 88.Zhao YJ. Clinical observation of Guanxinning in the treatment of angina: a report of 43 cases. Chinese Journal of Integrated Traditional and Western Medicine in Intensive and Critical Care. 2010;8:238–239. [Google Scholar]
- 89.Zhong TH, Li W, Liang YD, et al. Clinical observation of Guanxinning in the treatment of angina: a report of 35 cases. Chinese Medicine Modern Distance Education of China. 2007;5:20–21. [Google Scholar]
- 90.Zhu L. Integrated traditional and Western treatment of unstable angina: a report of 80 cases. Forum on Traditional Chinese Medicine. 2005;20:p. 42. [Google Scholar]
- 91.Wang L, Zhou GB, Liu P, et al. Dissection of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as an effective treatment for promyelocytic leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(12):4826–4831. doi: 10.1073/pnas.0712365105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang DW, Liu JG, Feng JT, et al. Effects of effective components compatibility of aqueous extracts of Salviae Miltiorrhizae and Rhizoma Chuanxiong on rat myocardial ischemia/reperfusion injury. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2010;22(2):109–112. [PubMed] [Google Scholar]
- 93.Li XJ, Zhang HY. Synergy in natural medicines: implications for drug discovery. Trends in Pharmacological Sciences. 2008;29(7):331–332. doi: 10.1016/j.tips.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 94.Wang D, Bakhai A. Clinical Trials: A Practical Guide to Design, Analysis, and Reporting. London, UK: Remedica; 2006. [Google Scholar]
- 95.Gheorghiade M, Bonow RO. Chronic heart failure in the United States: a manifestation of coronary artery disease. Circulation. 1998;97(3):282–289. doi: 10.1161/01.cir.97.3.282. [DOI] [PubMed] [Google Scholar]
- 96.Tang JL, Zhan S, Ernst E. Review of randomized controlled trials of traditional Chinese medicine. British Medical Journal. 1999;319(7203):160–161. doi: 10.1136/bmj.319.7203.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu J, Kjaergard LL, Gluud C. Misuse of randomization: a review of Chinese randomized trials of herbal medicines for chronic hepatitis B. American Journal of Chinese Medicine. 2002;30(1):173–176. doi: 10.1142/S0192415X0200017X. [DOI] [PubMed] [Google Scholar]
- 98.Guo X, Chen X, Li L, et al. LC-MS determination and pharmacokinetic study of six phenolic components in rat plasma after taking traditional Chinese medicinal-preparation: guanxinning lyophilized powder for injection. Journal of Chromatography B. 2008;873(1):51–58. doi: 10.1016/j.jchromb.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 99.Yang SM, Deng GP. The evolvement in clinical use of Perhexiline injection. Modern Hospital. 2007;7(11):68–70. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 provides the treatment (drugs and dosages for control and treatment groups) details about the RCTs included in this study.


