Abstract
The survival of patients with HIV infection has improved dramatically over the past 20 years, largely owing to a significant reduction in opportunistic infections and AIDs-defining malignancies, such as lymphoma and Kaposi sarcoma. However, with improved survival, patients with HIV are experiencing morbidity and mortality from other (non-AIDs-defining) complications, such as solid organ malignancies. Of these, the leading cause of mortality in the HIV-infected population is lung cancer, accounting for nearly 30% of all cancer deaths and 10% of all non-HIV-related deaths. Importantly, the average age of onset of lung cancer in the HIV-infected population is 25 to 30 years earlier than that in the general population and at lower exposure to cigarette smoke. This article provides an overview of the epidemiology of lung cancer in the HIV-infected population and discusses some of the important risk factors and pathways that may enhance the risk of lung cancer in this population.
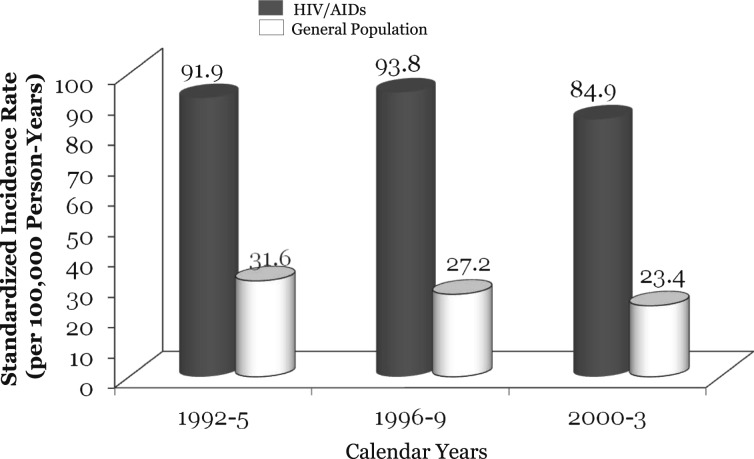
The introduction and widespread use of combination antiretroviral therapy (cART) in the mid-1990s has dramatically improved the health outcomes of individuals with HIV infection and AIDS.1 However, the longer life expectancy now observed in these individuals has led to the development of diseases with a longer latency period, such as solid organ malignancies. Although Kaposi sarcoma and non-Hodgkin’s lymphoma, the two most frequent AIDS-defining cancers (ADCs), have decreased substantially since 1996, non-AIDs-defining cancers (NADCs) have rapidly escalated in individuals with HIV.2‐6 Shiels et al7 estimated that the risk of ADCs has decreased by threefold, whereas NADCs have increased by threefold from 1991 through 1995 to 2001 through 2005.7 NADCs now account for 50% of all cancers among individuals with HIV.2 Of these, the most prevalent is lung cancer.2‐4,8,9 The risk of lung cancer is now nearly three times higher in HIV- than non-HIV-infected populations (Fig 1). Despite this statistic, there remains limited recognition among clinicians of the growing importance of lung cancer in individuals with HIV infection. This article provides an overview of the risk of lung cancer in individuals with HIV infection before and after the introduction of cART and explores the salient risk factors for lung cancer in this population.
Figure 1.
Incidence of lung cancer in patients with HIV infection. Incidence rates in the general population derived from the Surveillance, Epidemiology, and End Results program of the National Cancer Institute. Data from Patel et al.5
Incidence of Lung Cancer: Pre- and Post-cART Era
Several studies have examined the risk of lung cancer in the HIV-infected population (Table 1). Approximately one-half of these studies used a case-control design, whereas the other half used a longitudinal cohort approach. Of note, the average age at lung cancer diagnosis in this population was between 38 and 57 years. In contrast, the average age at lung cancer diagnosis in the general population is approximately 70 years. On a discouraging note, most of the cases were discovered in stages III or IV, and the median survival of these patients was measured in months from the time of diagnosis (Table 1).
Table 1.
—The Clinical Characteristics of Individuals With HIV Infection and Lung Cancer
| Study | Study Period | Sample Size | No. With Lung Cancer | Average Age, y | Male Sex, % | Smoker, % | IVDU, % | cART, % | CD4 Count, cells/μL | Histology (% of Total) | Stage (% of Total) | Survival, mo |
| Karp et al10 | 1983-1991 | 205 | 7 | 38 | 86 | 100 | 100 | 0 | N/A | Adeno (100) | IV (100) | 1 |
| Sridhar et al11 | 1986-1991 | 1,336 | 19 | 47 | 100 | N/A | 21 | 0 | 121 | Adeno (42) | IV (58) | 3 |
| Alshafie et al2 | 1990-1994 | 127 | 11 | 50 | 82 | 90 | 82 | N/A | 329 | Adeno (45) | IV (45) | 3 |
| Vyzula and Remick12 | 1988-1995 | N/A | 16 | 45 | 94 | N/A | 63 | 0 | 184 | Adeno (50) | N/A | 5.4 |
| Parker et al13 | 1990-1995 | 26,181 | 36 | 49 | 97 | N/A | N/A | 0 | N/A | Adeno (33) | IIIB/IV (89) | N/A |
| Tirelli et al14 | 1986-1998 | 138 | 36 | 38 | 89 | N/A | 69 | 8 | 150 | Adeno (42) | IV (55) | 5 |
| Bower et al15 | 1986-2001 | 8,400 | 11 | 45 | 91 | N/A | N/A | 55 | 160 | Adeno (45) | IV (54) | 2 |
| Spano et al16 | 1993-2002 | N/A | 22 | 45 | 86 | N/A | 23 | N/A | 364 | Squamous(50) | III/IV (75) | 7 |
| Powles et al17 | 1996-2002 | 36 | 9 | 45 | N/A | N/A | N/A | N/A | 160 | Adeno(66) | IV (66) | 4 |
| Engels et al18 | 1989-2003 | 5,238 | 33 | 46 | 67 | 69 | 57.5 | 57.1 | > 200 | Adeno(48) | N/A | N/A |
| Hakimian et al19 | 1996-2003 | N/A | 34 | 44 | 68 | N/A | 86 | 60 | > 200 | NSCLC (88) | IV (53) | 8.2 |
| Brock et al20 | 1986-2004 | 5,065 | 92 | 46 | 67 | 89 | 58 | 62 | 305 | Adeno (48) | IV (69) | 6.3 |
| Lavolé et al21 | 1996-2007 | 5,170 | 49 | 46 | 86 | N/A | 35 | 73 | 350 | Adeno (67) | III/IV (84) | 8.1 |
| Bertolaccini et al22 | 2003-2007 | N/A | 26 | 39 | 85 | N/A | 58 | 85 | 143 | NSCLC (81) | IV (33) | 23 |
| Pakkala et al23 | 1995-2008 | N/A | 80 | 52 | 80 | N/A | 25 | 55 | 304 | Adeno (38) | IV (49) | 6.1 |
| D’Jaen et al24 | 1996-2008 | 36,569 | 75 | 50 | 83 | 76 | 30 | 80 | 340 | Adeno (46) | IIIB/IV (77) | 9 |
| Engsig et al25 | 1995-2009 | 5,053 | 29 | 57 | 93 | 71 | 10 | 69 | 299 | Squamous (28) | N/A | 2 |
| Ruiz26 | 2002-2009 | 2,060 | 16 | 49 | 69 | N/A | N/A | 100 | 211 | Adeno (67) | IIIB/IV (85) | N/A |
| Clifford et al27 | 1985-2010 | 405 | 68 | 50 | 79 | 73 | 37 | 74 | N/A | Adeno (32) | N/A | N/A |
Adeno = adenocarcinoma; cART = combination antiretroviral therapy; IVDU = IV drug use; N/A = not available; NSCLC = non-small cell lung cancer.
The use of cART significantly reduces the risk of ADCs, such as Karposi sarcoma and non-Hodgkin’s lymphoma.28 The effect of cART on lung cancer risk has been less clear. In the pre-cART era, some studies demonstrated a significantly elevated risk of lung cancer in patients with HIV infection (Table 2); others, however, failed to show this relationship. In the post-cART era, there has been less heterogeneity in results, with most published studies showing that HIV infection is a significant independent risk factor for lung cancer (Table 2). The reason for the increased incidence of lung cancer in the cART era is not entirely clear. One possible explanation is competing risk of death. With the reduction in AIDS-related causes of morbidity and mortality, common causes of death in the community, such as ischemic heart disease and cancer, may become predominant.3,7,33 However, as stated previously, the observed rates of lung cancer in the HIV population adjusted for age are several fold higher than those in the general population, suggesting other reasons for the rise in lung cancer rates in these patients. Another plausible explanation is the high rate of smoking in patients with HIV infection. However, although smoking plays an important role, statistical adjustments for smoking do not appear to materially alter the increased risk of lung cancer in patients with HIV infection compared with the general population.3
Table 2.
—The Relationship Between Lung Cancer and HIV Infection
| Study | Study Duration | Location | SIR | 95% CI |
| Grulich et al29 | 1980-1993 | Australia | 3.8 | 1.39-8.29 |
| Cooksley et al30 | 1975-1994 | Texas | 0.7 | 0.4-1.1 |
| Gallagher et al31 | 1981-1994 | New York | 3.3 | 2.86-3.75 |
| Parker et al13 | 1990-1995 | Texas | 6.5 | 4.5-8.9 |
| Frisch et al8 | 1978-1996 | 11 US areas | 4.5 | 4.2-4.8 |
| 1978-1996 | … | 2.8 | 2.4-3.1 | |
| Grulich et al29 | 1985-1999 | Australia | 1.44 | 0.84-2.30 |
| Herida et al32 | 1996-1999 (men) | France | 2.12 | 1.67-2.65 |
| 1992-1995 (men) | … | 1.13 | 0.71-1.72 | |
| 1996-1999 (women) | … | 6.59 | 3.40-11.52 | |
| 1992-1995 (women) | … | 1.08 | 0.01-5.98 | |
| Hessol et al33 | 1990-2000 | San Francisco, CA | 2.6 | 2.1-3.2 |
| Engels et al3 | 1980-1989 | 11 US regions | 2.5 | 1.9-3.3 |
| 1990-1995 | … | 3.3 | 2.9-3.8 | |
| 1996-2002 | … | 2.6 | 2.1-3.1 | |
| Bower et al15 | 1986-1996 | England | 0.8 | 0.2-1.4 |
| 1997-2002 | … | 6.7 | 3.5-9.9 | |
| Engels et al34 | 1991-2002 | Multiple US areas | 2.6 | 2.1-3.1 |
| Clifford et al35 | 1985-2003 | Switzerland | 3.2 | 1.7-5.4 |
| Patel et al5 | 1992-1995 | 13 US areas | SRR = 3.5 | 2.5-4.9 |
| 1996-1999 | … | SRR = 3.8 | 2.8-5.0 | |
| 2000-2003 | … | SRR = 3.6 | 2.8-4.6 | |
| Dal Maso et al36 | 1986-1996 | Italy | 2.1 | 1.2-3.3 |
| 1997-2004 | … | 4.1 | 2.9-5.5 | |
| Bedimo et al37 | 1997-2004 | United States | IRR = 2.0 | 1.8-2.2 |
| Long et al4 | 1996-2005 | Baltimore, MD | 5.5 | 3.7-8.0 |
| Guiguet et al28 | 1998-2006 (CD4 count > 500) | France | RR = 1.0 | … |
| 1998-2006 (CD4 count 350-499) | … | RR = 2.2 | 1.3-3.6 | |
| Powles et al6 | 1983-1995 | Europe | 0 | 0.00-1.52 |
| 1996-2001 | … | 3.1 | 1.34-6.11 | |
| 2002-2007 | … | 2.37 | 1.14-4.36 | |
| Silverberg et al38 | 1996-2007 | California | RR = 1.9 | 1.4-2.5 |
| Shiels et al39 | 1996-2007 | Multiple US areas | 3 | 2.8-3.2 |
| Engsig et al25 | 1995-2009 | Denmark | IRR = 2.38 | 1.61-3.53 |
| Grulich et al29, a | 1978-2003 | Meta-analysis | 2.72 | 1.91-3.87 |
| Shiels et al9, a | 1981-2005 | Meta-analysis | 2.6 | 2.1-3.1 |
| Chaturvedi et al40, b | 1980-2002 (−60 to +60 mo AIDS onset) | 11 US regions | 3.8 | 3.6-4.1 |
| 1980-2002 (−6 to +3 mo AIDS onset) | … | 10.5 | 9.7-11.4 | |
| Kirk et al41, b | 1988-2003 | Baltimore, MD | HR = 3.6 (risk of death) | 1.6-7.9 |
| Engels et al18, b | 1989-2003 | Baltimore, MD | SIR = 2.5 | 1.6-3.5 |
| Shiels et al42, b | 1988-2007 | Baltimore, MD | HR = 2.3 (risk of lung cancer) | 1.1-5.1 |
| … | HR = 3.8 (risk of death) | 0.92-15 |
HR = hazard ratio; IRR = incidence rate ratio; RR = relative risk; SIR = standardized incidence rate; SRR = standardized rate ratio.
Meta-analysis.
Adjusted for smoking.
Risk Factors for Lung Cancer
Table 3 summarizes the proposed mechanisms that link HIV with lung cancer.
Table 3.
—Summary of the Proposed Mechanisms Linking HIV With Lung Cancer
| Theory | Mechanisms | Key References |
| Direct oncogenic effect of HIV | Virus-inducing microsatellite alterations and widespread genomic instability. | Wistuba et al43 |
| Tat, an essential gene for HIV-1 replication, increases expression of protooncogenes and proliferation of the human adenocarcinoma cell line by downregulating tumor suppressor gene p53. | el-Solh et al44 | |
| Downregulation of HIV Tat-interacting protein (TIP30) has been found to promote metastasis of lung cancer. | Baker et al,45 Tong et al46 | |
| HIV-induced immunosuppression | Conflicting evidence, wherein immunosuppression may lead to a reduction in tumor surveillance, thus enabling tumor growth. | Bower et al,15 Engels47 |
| Chronic inflammation | Chronic inflammation has been recognized as a risk factor for lung cancer. | Engels48 |
| Individuals with HIV infection and chronic pneumonia and asthma are at higher risk of lung cancer. | Shebl et al,49 Kirk et al41 | |
| The rate of pneumonia is nearly six times higher in patients with HIV infection and CD4 counts > 500 cells/μL than in control subjects without HIV. | Sogaard et al50 | |
| Cigarette smoking | Smoking is an independent risk factor for lung cancer in individuals with HIV infection. | Guiguet et al28 |
| Smoking is two to three times more prevalent among individuals with HIV infection than in the general population. | Engels et al,18 Giordano and Kramer51 | |
| IV drug use | IV drug users with HIV infection have an increased risk of lung cancer compared with nonusers with HIV. | Serraino et al52 |
Tat = transactivator of transcription.
Smoking
Smoking is an independent risk factor in the development of lung cancer in individuals with HIV infection.28 Sixty percent to 80% of this population in the United States are smokers, and smoking is two to three times more prevalent among those with HIV infection than in the general population.18,51 Elevated smoking rates in this patient population account for some of the excess risk of lung cancer compared with the general population; however, there is accumulating evidence that other factors may be involved.18,41,42
Although previous older studies have been limited by incomplete smoking data, Engels et al18 adjusted for smoking in an analysis involving 5,238 patients with HIV infection living in urban Baltimore, Maryland. The authors found that lung cancer incidence was 2.5 times greater than predicted based on general population rates after statistical adjustments for cigarette smoking.18 In a sensitivity analysis in which they assumed that all patients with HIV infection were smokers, they found that the smoking-adjusted standardized incidence ratio (SIR) for lung cancer was 1.7, suggesting that cigarette smoke could not fully account for the higher risk of lung cancer in patients with HIV infection. A prospective study by Shiels et al42 of 2,495 injection drug users with and without HIV infection reported that HIV infection doubled the risk of lung cancer after controlling for smoking history and other covariates (hazard ratio [HR], 2.3). Two recent studies controlled for smoking and found similar results.14,15
Similar to lung cancer incidence, lung cancer mortality appears to be higher in patients with HIV than in patients without HIV infection, independent of smoking. For instance, Kirk et al41 studied lung cancer mortality in a cohort of 2,086 injection drug users and compared the risk in those with HIV vs those without HIV infection. After adjusting for age, sex, smoking status, and cART use, lung cancer mortality was > 250% higher in those with HIV infection (HR, 3.6).
IV Drug Use
Previous studies have reported lung cancer risk to be higher among injection drug users than other HIV risk groups.8,20,31,32,35,36,40 Serraino et al,52 for instance, reported a sixfold increase in the risk of lung cancer among individuals with HIV infection who were IV drug users (SIR, 6.2) compared with those who were not injection drug users. Importantly, this study also showed that IV drug users (even those without HIV) experienced a higher risk of lung cancer than those who did not use injection drugs.52 However, there are some studies with differing conclusions.18,32,41 Kirk et al,41 for instance, found little evidence for the role of illicit drug use (through either injection or inhalation) in the etiology of lung cancer or lung cancer mortality. One reason for the discordances in the findings is the possible confounding effects of cigarette smoking among IV drug users. It is possible that the high prevalence of smoking among IV drug users (eg, 96% in the Swiss HIV Cohort Study) could have obscured the possible harmful effects of injection drug use on the risk of lung cancer.35
Immunodeficiency and CD4 Count
There are conflicting data in the literature regarding the role of immunosuppression on the risk of lung cancer in patients with HIV infection. To examine the possible role of immunosuppression in carcinogenesis, both Frisch et al8 and Chaturvedi et al40 evaluated the risk of lung cancer in patients with HIV infection before and after the diagnosis of AIDS. Frisch et al8 found that the highest risk of lung cancer occurred at the time of AIDS diagnosis (when immunosuppression was the greatest), with a relative risk exceeding 10; the lowest risk was observed in the distant pre-AIDS period (> 25 months prior to AIDS diagnosis), with the relative risk only 1.2. In the recent pre- and post-AIDS period (defined as 27 months prior to and 27 months following AIDS diagnosis), the relative risk of lung cancer was about 2.7 (P < .001 compared with relative risk observed in the distant pre-AIDS period). Similarly, Chaturvedi et al40 found that patients with HIV infection had an increased risk of lung cancer (SIR, 3.8). Importantly, this risk was inversely related to the patient’s CD4 cell count in peripheral blood. However, these data should be interpreted cautiously because there are concerns that diagnostic bias may have inflated the relative risk of lung cancer during the peak of immunosuppression when patients generally are sick and undergo diagnostic tests, such as thoracic imaging studies, that may lead to lung cancer detection. Nevertheless, these data implicate immunosuppression in the pathogenesis of lung cancer in patients with HIV infection.
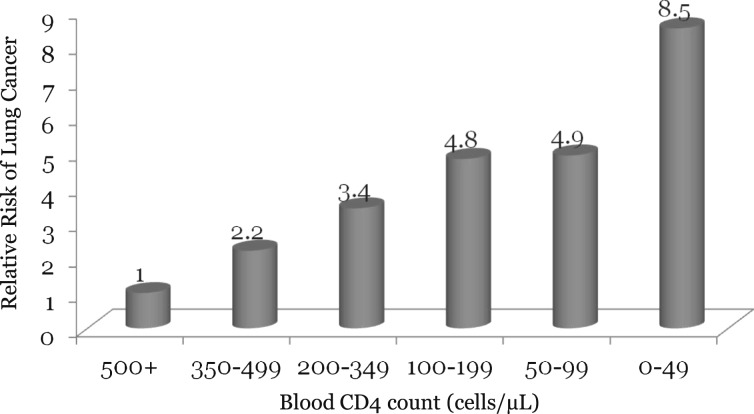
Although the mechanism for this observation is unclear, some have hypothesized that immunosuppression related to the HIV infection promotes uncontrolled tumor growth by reducing adaptive immunity.15,47 Consistent with this theory, a meta-analysis comparing cancer incidence in patients with HIV infection with that among immunosuppressed solid organ transplant recipients demonstrated similar risks between the two groups (SIR, 2.72 vs 2.18, respectively).29 Another study found that the risk of lung cancer doubled when blood CD4 cell count fell from > 500 cells/μL to a range of 350 to 499 cells/μL, and the risk continued to increase with further declines in CD4 counts.28 A negative dose-response relationship between CD4 cell count in the 2 years post-AIDS diagnosis and the risk of lung cancer was noted in the study by Chaturvedi et al40 and by Guiguet et al28 in their large French study (Fig 2).
Figure 2.
The relationship between peripheral blood CD4 counts and the risk of lung cancer in patients with HIV infection. Data from Guiguet et al.28
However, there are some studies that failed to observe a significant association between the risk of lung cancer and CD4 counts.18,27,35,40,41 The Swiss HIV Cohort Study did not show a significant association of CD4 count, HIV viral load, or a history of AIDS-related pulmonary disease with the risk of lung cancer after adjustments for cigarette smoking.27 Some authors have also suggested that CD4 count is an insensitive indicator of immunodeficiency and may not accurately measure immune dysfunction at cancer onset.29 Thus, the role of immunosuppression in the risk of lung cancer remains controversial. Some authors have suggested that cART may have oncogenic potential,37 whereas others have suggested that the increased surveillance of patients with HIV for lung cancer may in part explain the increased prevalence of lung cancer in this population.39,40
Pulmonary Inflammation
Chronic inflammation, whether caused by tobacco smoke, infections, or other diseases, has been recognized as an important risk factor for lung cancer.48 As reported by Engels48 in a review, pulmonary infections by inducing lung inflammation and injury also could play a role in the development of lung cancer. Engels cited epidemiologic studies that demonstrated associations between lung cancer and infectious and inflammatory lung conditions in nonsmokers.
A history of recurrent pneumonia was recently linked to an increase in lung cancer risk in the large HIV/AIDS Cancer Match study.49 Shebl et al49 assessed lung cancer risk over a 10-year period in 322,675 patients receiving a diagnosis of AIDs between 1997 and 2002. Individuals with recurrent pneumonia had a significantly higher risk of lung cancer than those who did not report this history (HR, 1.63; P = .02). This risk was significantly elevated even after 5 to 10 years following the pneumonia event, arguing against reverse causality. However, when the analysis was adjusted for smoking history, the association no longer remained statistically significant. The authors concluded that smoking could account for part of the elevated lung cancer risk among individuals with recurrent pneumonia. Kirk et al41 also demonstrated increased lung cancer risk among patients with preexisting chronic inflammatory lung disease, particularly asthma. Contrary to these findings, Clifford et al27 noted that preexisting pulmonary disease was not observed more frequently among patients with HIV infection and lung cancer than among those with HIV infection but no lung cancer.
Age
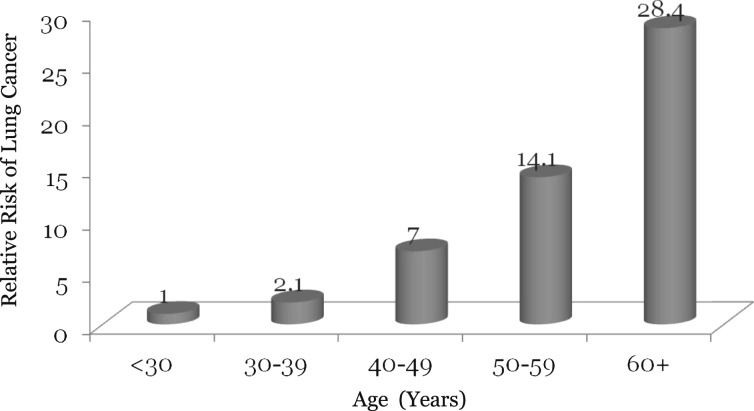
The risk of lung cancer increases with age in the general population. This relationship is exaggerated in patients with HIV infection. In the general population, lung cancer is diagnosed at an average age of 70 years. In patients with HIV infection, however, the average age at lung cancer diagnosis is only 50 years.39 Similar findings have been noted by other groups.18,40 Importantly, Guiguet et al28 found the risk of lung cancer to be increased almost exponentially with age in the HIV-infected population such that by age ≥ 60 years, the risk was 28-fold higher relative to that observed in people aged < 30 (Fig 3). The relationship between age and risk of lung cancer is extremely germane given the increased overall age of contemporaneous patients with HIV infection.4,7 In the United States, the fourfold increase in the AIDS population between 1991 and 2005 has largely been driven by the growth in patients aged ≥ 40 years.7 This represents a substantial growth in the number of people at risk for lung cancer.7 The mechanism for the relationship between age and lung cancer is unclear.
Figure 3.
The relationship between age and the risk of lung cancer in patients with HIV infection. Data from Guiguet et al.28
Sex
Lung cancer incidence appears to be higher among men with HIV than among women with HIV (Table 1). However, a recent meta-analysis reported that the relative risk was higher among women than men when compared with the general population.9 The Women’s Interagency HIV Study also noted a substantially increased risk of lung cancer among both women with HIV and at-risk women without HIV infection compared with population-based expectations.53 The authors suggested that this was perhaps due to higher rates of cigarette smoking among women with HIV infection.
Outcomes
Staging and Prognosis
The histologic subtypes of lung cancer appear to be similar between those with and without HIV. In the western world, adenocarcinomas predominate, accounting for 50% to 75% of all lung cancers, followed by squamous cell and small cell lung cancers.54 Similar to the general population, most lung cancer cases are diagnosed in advanced stages, precluding cure. Less than 15% of the cases are discovered at a local stage, enabling surgical resection for curative intent.39 However, in general, patients with HIV infection who have lung cancer have a worse prognosis than those in the general lung cancer population.1,10‐12,14,20,25,41,42 The median survival is between 3.5 and 6.3 months among patients with HIV infection vs between 9.4 and 10 months among those without HIV infection.11,14,20 Of note, in more recent studies conducted in the cART era, these groups have been shown to have comparable survival times.17,19,21,24 Some researchers have suggested that a more-aggressive form of lung cancer develops in patients with HIV infection because these patients are, on average, 20 years younger than those in the general lung cancer population, whereas others have suggested that immune dysfunction related to HIV infection may be the most important determinant of the prognosis of patients with HIV infection and lung cancer.17
Prognostic Factors
In a multivariate analysis by Lavolé et al,21 three independent prognostic factors for increased survival were identified: stage of disease (I-II), performance status (≤ 1), and use of cART. Specifically, cART exposure was associated with a 60% reduction in the overall risk of death compared with nonexposure and an increased median survival (9 months vs 4.5 months). Similarly, Hessol et al33 reported that at least 6 months of cART use is associated with prolonged survival.
However, the beneficial effects of cART have not been consistently reported in the literature. Some have noted little difference in the median survival of patients with lung cancer between the pre-cART and the post-cART eras.20 In a study of 75 patients with HIV infection and lung cancer, D’Jaen et al24 noted that after controlling for lung cancer stage, cART did not appear to influence the overall median survival of these patients (P = .60). Biggar et al1 also reported little change in survival in patients with AIDS and lung cancer over the past 2 decades, hypothesizing that the stage and histologic subtype of lung cancer are the more prominent contributing factors to survival than HIV infection.
Although some studies have shown a trend for shortened survival with low absolute CD4 counts at lung cancer diagnosis,14,20,23 others have found no correlation between prognosis and CD4 counts.21,41 It has been suggested that the worse prognosis related to decreased CD4 counts may actually reflect the limited treatment options of patients with HIV infection because these patients tend to have multiple comorbid illnesses or demonstrate poor performance status at diagnosis.23
Similar to the general population, advanced stage of lung cancer has been associated with a poor prognosis in patients with HIV infection.20,23,24 There is some suggestion that these patients present with more advanced disease than those without HIV infection.20 Brock et al20 found that 87% of patients with HIV infection and non-small cell lung cancer were given a stage III/IV diagnosis compared with 68% of control patients with undetermined HIV status. However, after adjusting for stage of cancer, HIV infection was not associated with increased mortality from lung cancer. The authors concluded that advanced stage of presentation had a major influence on survival in both the HIV-positive and the HIV-indeterminate groups.
Interventions to Reduce the Risk of Lung Cancer Mortality
Table 4 summarizes proposed methods to reduce lung cancer morbidity and mortality in patients with HIV. To curb the growing burden of lung cancer in the HIV population, it is essential that patients who are smokers be counseled aggressively for smoking cessation and treated for tobacco addiction with pharmacologic and nonpharmacologic interventions. Although smoking rates in the HIV-infected population are three to four times higher than those of the general population, successful rates of smoking cessation in the two groups are similar. With comprehensive intervention comprising nicotine replacement therapy, counseling, and follow-up, approximately 20% quit rates can be achieved. It is thus imperative that HIV care providers assess smoking status of their patients at least yearly and foster smoking cessation through education, counseling, and pharmacologic therapies. Smoking cessation reduces the risk of lung cancer mortality by > 50%.59 Because the stage of the tumor is the predominant driver of survival, early detection of lung cancer is desirable. Regrettably, however, > 80% of the cases are discovered in advanced stages, at which point cure is not possible. Plain chest radiograph is not a useful screening test for the detection of early lung cancer. Brock et al,20 for instance, found that in more than one-half of patients later given a diagnosis of lung cancer, chest radiographs did not demonstrate any suspicious lesions, even those that were done within 12 months of the diagnosis. Thus, chest radiographic screening for lung cancer cannot be advocated for patients with HIV infection. Of promise, the National Lung Screening Trial demonstrated a 20% mortality reduction from lung cancer and 7% total mortality reduction with annual thoracic CT screening in heavy former and current smokers.56 For individuals with HIV infection who meet the eligibility criteria for the National Lung Screening Trial (ie, aged 55-74 years, ≥ 30 pack-year smoking history, current or former smokers [quit smoking within 15 years]), annual screening with thoracic CT scans may be considered.60 However, the cost-effectiveness of this intervention is unknown. Thus, there is a pressing need to develop novel (cost-effective) strategies to address the epidemic of lung cancer in patients with HIV infection.
Table 4.
—Proposed Methods to Reduce Lung Cancer Morbidity and Mortality in Patients With HIV Infection
| Intervention | Rationale |
| Smoking cessation | Encourage smoking cessation and refer to smoking cessation programs using the five-step approach.55 |
| Early detection | Have a high clinical suspicion of lung cancer in smokers with HIV. |
| Consider thoracic CT scans in patients at high risk of lung cancer (eg, ≥ 30 pack-y smoking history, family history of lung cancer, detection of emphysema on CT scan or small pulmonary nodules on previous CT scans, COPD).56,57 | |
| cART therapy | Consider initiating cART therapy earlier and maintaining CD4 counts ≥ 500 cells/μL.58 |
| Improve performance status | Encourage proper nutrition and exercise. |
| Monitor for and treat complications of HIV infections. | |
| Follow-up | Schedule regular follow-up and laboratory monitoring to ensure that patients adhere to cART therapy. |
See Table 1 legend for expansion of abbreviation.
Summary
With the widespread use of cART in patients with HIV infection across western nations, there has been a dramatic reduction in the risk of opportunistic infections and hematopoietic malignancies. However, with the continued high rates of cigarette smoking in the HIV-infected population and with aging of these patients, the human and financial burden of lung cancer has become enormous. Because smoking cessation is the most effective way of reducing the risk of lung cancer, there is a pressing need for health-care professionals and clinics involved in the care of patients with HIV infection to develop expertise in tobacco treatment and smoking prevention and implement programs to promote complete smoking abstinence in their patients. There is also an urgent need for more research to understand the mechanisms that drive lung cancer risks in patients with HIV infection and to develop and implement novel tools and therapeutics to reduce the growing human and financial burden of lung cancer in the HIV population.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Hull has received grant support from the National Institute on Drug Abuse (R01-DA-03104301) and honoraria for speaking engagements and consultancy meetings from Bristol-Myers Squibb Company; Gilead; Merck & Co, Inc; Janssen Pharmaceuticals, Inc; Pfizer, Inc; Vertex Pharmaceuticals Incorporated; and ViiV Healthcare. Dr Montaner has received grants from Abbott Laboratories; bioLytical Laboratories; Boehringer Ingelheim GmbH; Bristol-Myers Squibb Company; Gilead; Janssen Pharmaceuticals, Inc; Merck & Co, Inc; and ViiV Healthcare. Dr Sin has received honoraria for speaking engagements from AstraZeneca, Grifols Inc, Takeda Phermaceutical Company Limited, and Merck & Co, Inc and has served on the advisory committee of Merck & Co, Inc; Talecris Plasma Resources, subsidiary of Grifols, Inc; Takeda Phermaceutical Company Limited; and Novartis AG. Drs Winstone, and Man have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Abbreviations
- ADC
AIDS-defining cancer
- cART
combination antiretroviral therapy
- HR
hazard ratio
- NADC
non-AIDS-defining cancer
- SIR
standardized incidence ratio
Footnotes
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Biggar RJ, Engels EA, Ly S, et al. Survival after cancer diagnosis in persons with AIDS. J Acquir Immune Defic Syndr. 2005;39(3):293-299 [DOI] [PubMed] [Google Scholar]
- 2.Alshafie MT, Donaldson B, Oluwole SF. Human immunodeficiency virus and lung cancer. Br J Surg. 1997;84(8):1068-1071 [PubMed] [Google Scholar]
- 3.Engels EA, Pfeiffer RM, Goedert JJ, et al. ; for the HIV/AIDS Cancer Match Study Trends in cancer risk among people with AIDS in the United States 1980-2002. AIDS. 2006;20(12):1645-1654 [DOI] [PubMed] [Google Scholar]
- 4.Long JL, Engels EA, Moore RD, Gebo KA. Incidence and outcomes of malignancy in the HAART era in an urban cohort of HIV-infected individuals. AIDS. 2008;22(4):489-496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel P, Hanson DL, Sullivan PS, et al. ; Adult and Adolescent Spectrum of Disease Project and HIV Outpatient Study Investigators Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992-2003. Ann Intern Med. 2008;148(10):728-736 [DOI] [PubMed] [Google Scholar]
- 6.Powles T, Robinson D, Stebbing J, et al. Highly active antiretroviral therapy and the incidence of non-AIDS-defining cancers in people with HIV infection. J Clin Oncol. 2009;27(6):884-890 [DOI] [PubMed] [Google Scholar]
- 7.Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103(9):753-762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frisch M, Biggar RJ, Engels EA, Goedert JJ; AIDS-Cancer Match Registry Study Group Association of cancer with AIDS-related immunosuppression in adults. JAMA. 2001;285(13):1736-1745 [DOI] [PubMed] [Google Scholar]
- 9.Shiels MS, Cole SR, Kirk GD, Poole C. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr. 2009;52(5):611-622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karp J, Profeta G, Marantz PR, Karpel JP. Lung cancer in patients with immunodeficiency syndrome. Chest. 1993;103(2):410-413 [DOI] [PubMed] [Google Scholar]
- 11.Sridhar KS, Flores MR, Raub WA, Jr, Saldana M. Lung cancer in patients with human immunodeficiency virus infection compared with historic control subjects. Chest. 1992;102(6):1704-1708 [DOI] [PubMed] [Google Scholar]
- 12.Vyzula R, Remick SC. Lung cancer in patients with HIV-infection. Lung Cancer. 1996;15(3):325-339 [DOI] [PubMed] [Google Scholar]
- 13.Parker MS, Leveno DM, Campbell TJ, Worrell JA, Carozza SE. AIDS-related bronchogenic carcinoma: fact or fiction? Chest. 1998;113(1):154-161 [DOI] [PubMed] [Google Scholar]
- 14.Tirelli U, Spina M, Sandri S, et al. ; The Italian Cooperative Group on AIDS and Tumors Lung carcinoma in 36 patients with human immunodeficiency virus infection. Cancer. 2000;88(3):563-569 [DOI] [PubMed] [Google Scholar]
- 15.Bower M, Powles T, Nelson M, et al. HIV-related lung cancer in the era of highly active antiretroviral therapy. AIDS. 2003;17(3):371-375 [DOI] [PubMed] [Google Scholar]
- 16.Spano JP, Massiani MA, Bentata M, et al. Lung cancer in patients with HIV infection and review of the literature. Med Oncol. 2004;21(2):109-115 [DOI] [PubMed] [Google Scholar]
- 17.Powles T, Thirwell C, Newsom-Davis T, et al. Does HIV adversely influence the outcome in advanced non-small-cell lung cancer in the era of HAART? Br J Cancer. 2003;89(3):457-459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engels EA, Brock MV, Chen J, Hooker CM, Gillison M, Moore RD. Elevated incidence of lung cancer among HIV-infected individuals. J Clin Oncol. 2006;24(9):1383-1388 [DOI] [PubMed] [Google Scholar]
- 19.Hakimian R, Fang H, Thomas L, Edelman MJ. Lung cancer in HIV-infected patients in the era of highly active antiretroviral therapy. J Thorac Oncol. 2007;2(4):268-272 [DOI] [PubMed] [Google Scholar]
- 20.Brock MV, Hooker CM, Engels EA, et al. Delayed diagnosis and elevated mortality in an urban population with HIV and lung cancer: implications for patient care. J Acquir Immune Defic Syndr. 2006;43(1):47-55 [DOI] [PubMed] [Google Scholar]
- 21.Lavolé A, Chouaïd C, Baudrin L, et al. Effect of highly active antiretroviral therapy on survival of HIV infected patients with non-small-cell lung cancer. Lung Cancer. 2009;65(3):345-350 [DOI] [PubMed] [Google Scholar]
- 22.Bertolaccini L, Lybéris P, Soncinim S, Di Perri G, Manno E. Clinical characteristic lung cancer in HIV-infected patients. Cancer Ther. 2008;6903-906 [Google Scholar]
- 23.Pakkala S, Chen Z, Rimland D, et al. Human immunodeficiency virus-associated lung cancer in the era of highly active antiretroviral therapy. Cancer. 2012;118(1):164-172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Jaen GA, Pantanowitz L, Bower M, et al. Human immunodeficiency virus-associated primary lung cancer in the era of highly active antiretroviral therapy: a multi-institutional collaboration. Clin Lung Cancer. 2010;11(6):396-404 [DOI] [PubMed] [Google Scholar]
- 25.Engsig FN, Kronborg G, Larsen CS, et al. Lung cancer in HIV patients and their parents: a Danish cohort study. BMC Cancer. 2011;11:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruiz M. Lung cancer in HIV-infected patients: the experience of an urban clinic. J Int Assoc Physicians AIDS Care (Chic). 2010;9(4):214-217 [DOI] [PubMed] [Google Scholar]
- 27.Clifford GM, Lise M, Franceschi S, et al. ; Swiss HIV Cohort Study Lung cancer in the Swiss HIV Cohort Study: role of smoking, immunodeficiency and pulmonary infection. Br J Cancer. 2012;106(3):447-452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guiguet M, Boué F, Cadranel J, Lang JM, Rosenthal E, Costagliola D; Clinical Epidemiology Group of the FHDH-ANRS CO4 cohort Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol. 2009;10(12):1152-1159 [DOI] [PubMed] [Google Scholar]
- 29.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370(9581):59-67 [DOI] [PubMed] [Google Scholar]
- 30.Cooksley CD, Hwang LY, Waller DK, Ford CE. HIV-related malignancies: community-based study using linkage of cancer registry and HIV registry data. Int J STD AIDS. 1999;10(12):795-802 [DOI] [PubMed] [Google Scholar]
- 31.Gallagher B, Wang Z, Schymura MJ, Kahn A, Fordyce EJ. Cancer incidence in New York State acquired immunodeficiency syndrome patients. Am J Epidemiol. 2001;154(6):544-556 [DOI] [PubMed] [Google Scholar]
- 32.Herida M, Mary-Krause M, Kaphan R, et al. Incidence of non-AIDS-defining cancers before and during the highly active antiretroviral therapy era in a cohort of human immunodeficiency virus-infected patients. J Clin Oncol. 2003;21(18):3447-3453 [DOI] [PubMed] [Google Scholar]
- 33.Hessol NA, Pipkin S, Schwarcz S, Cress RD, Bacchetti P, Scheer S. The impact of highly active antiretroviral therapy on non-AIDS-defining cancers among adults with AIDS. Am J Epidemiol. 2007;165(10):1143-1153 [DOI] [PubMed] [Google Scholar]
- 34.Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187-194 [DOI] [PubMed] [Google Scholar]
- 35.Clifford GM, Polesel J, Rickenbach M, et al. ; Swiss HIV Cohort Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst. 2005;97(6):425-432 [DOI] [PubMed] [Google Scholar]
- 36.Dal Maso L, Polesel J, Serraino D, et al. ; Cancer and AIDS Registries Linkage (CARL) Study Pattern of cancer risk in persons with AIDS in Italy in the HAART era. Br J Cancer. 2009;100(5):840-847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bedimo RJ, McGinnis KA, Dunlap M, Rodriguez-Barradas MC, Justice AC. Incidence of non-AIDS-defining malignancies in HIV-infected versus noninfected patients in the HAART era: impact of immunosuppression. J Acquir Immune Defic Syndr. 2009;52(2):203-208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silverberg MJ, Chao C, Leyden WA, et al. HIV infection and the risk of cancers with and without a known infectious cause. AIDS. 2009;23(17):2337-2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shiels MS, Pfeiffer RM, Engels EA. Age at cancer diagnosis among persons with AIDS in the United States. Ann Intern Med. 2010;153(7):452-460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaturvedi AK, Pfeiffer RM, Chang L, Goedert JJ, Biggar RJ, Engels EA. Elevated risk of lung cancer among people with AIDS. AIDS. 2007;21(2):207-213 [DOI] [PubMed] [Google Scholar]
- 41.Kirk GD, Merlo C, O’Driscoll P, et al. HIV infection is associated with an increased risk for lung cancer, independent of smoking. Clin Infect Dis. 2007;45(1):103-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shiels MS, Cole SR, Mehta SH, Kirk GD. Lung cancer incidence and mortality among HIV-infected and HIV-uninfected injection drug users. J Acquir Immune Defic Syndr. 2010;55(4):510-515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wistuba II, Behrens C, Milchgrub S, et al. Comparison of molecular changes in lung cancers in HIV-positive and HIV-indeterminate subjects. JAMA. 1998;279(19):1554-1559 [DOI] [PubMed] [Google Scholar]
- 44.el-Solh A, Kumar NM, Nair MP, Schwartz SA, Lwebuga-Mukasa JS. An RGD containing peptide from HIV-1 Tat-(65-80) modulates protooncogene expression in human bronchoalveolar carcinoma cell line, A549. Immunol Invest. 1997;26(3):351-370 [DOI] [PubMed] [Google Scholar]
- 45.Baker ME, Yan L, Pear MR. Three-dimensional model of human TIP30, a coactivator for HIV-1 Tat-activated transcription, and CC3, a protein associated with metastasis suppression. Cell Mol Life Sci. 2000;57(5):851-858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tong X, Li K, Luo Z, et al. Decreased TIP30 expression promotes tumor metastasis in lung cancer. Am J Pathol. 2009;174(5):1931-1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Engels EA. Human immunodeficiency virus infection, aging, and cancer. J Clin Epidemiol. 2001;54(suppl 1):S29-S34 [DOI] [PubMed] [Google Scholar]
- 48.Engels EA. Inflammation in the development of lung cancer: epidemiological evidence. Expert Rev Anticancer Ther. 2008;8(4):605-615 [DOI] [PubMed] [Google Scholar]
- 49.Shebl FM, Engels EA, Goedert JJ, Chaturvedi AK. Pulmonary infections and risk of lung cancer among persons with AIDS. J Acquir Immune Defic Syndr. 2010;55(3):375-379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sogaard OS, Lohse N, Gerstoft J, et al. Hospitalization for pneumonia among individuals with and without HIV infection, 1995-2007: a Danish population-based, nationwide cohort study. Clin Infect Dis. 2008;47(10):1345-1353 [DOI] [PubMed] [Google Scholar]
- 51.Giordano TP, Kramer JR. Does HIV infection independently increase the incidence of lung cancer? Clin Infect Dis. 2005;40(3):490-491 [DOI] [PubMed] [Google Scholar]
- 52.Serraino D, Boschini A, Carrieri P, et al. Cancer risk among men with, or at risk of, HIV infection in southern Europe. AIDS. 2000;14(5):553-559 [DOI] [PubMed] [Google Scholar]
- 53.Levine AM, Seaberg EC, Hessol NA, et al. HIV as a risk factor for lung cancer in women: data from the Women’s Interagency HIV Study. J Clin Oncol. 2010;28(9):1514-1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sigel K, Wisnivesky J, Gordon K, et al. HIV as an independent risk factor for incident lung cancer. AIDS. 2012;26(8):1017-1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.The Tobacco Use and Dependence Clinical Practice Guideline Panel, Staff, and Consortium Representatives A clinical practice guideline for treating tobacco use and dependence: A US Public Health Service report. JAMA. 2000;283(24):3244-3254 [PubMed] [Google Scholar]
- 56.Aberle DR, Adams AM, Berg CD, et al. ; National Lung Screening Trial Research Team Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zurawska JH, Jen R, Lam S, Coxson HO, Leipsic J, Sin DD. What to do when a smoker’s CT scan is “normal”? Implications for lung cancer screening. Chest. 2012;141(5):1147-1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kitahata MM, Gange SJ, Abraham AG, et al. ; NA-ACCORD Investigators Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360(18):1815-1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE; Lung Health Study Research Group The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142(4):233-239 [DOI] [PubMed] [Google Scholar]
- 60.Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012;307(22):2418-2429 [DOI] [PMC free article] [PubMed] [Google Scholar]