Abstract
It is currently estimated that about 20%–30% of adults and 10%–40% of children diagnosed with epilepsy suffer from uncontrolled or poorly controlled seizures, despite optimal medical management. In addition to its huge economic costs, treatment-refractory epilepsy has a widespread impact on patients’ health-related quality of life. The present paper focuses on the concepts of refractory and difficult-to-treat seizures and their pharmacological management. Evidence on efficacy and tolerability of rational pharmacotherapy with antiepileptic drug combinations and of non-pharmacological treatment options such as epilepsy surgery, neurostimulation, metabolic treatment and herbal remedies is reviewed. The importance of early identification of the underlying etiology of the specific epilepsy syndrome is emphasized, to inform early prognosis and therapeutic strategies.
Keywords: refractory epilepsy, intractable seizures, treatment, pharmacotherapy, antiepileptic drugs
Introduction
Epilepsy is generally deemed intractable or refractory to treatment if the use of two or more appropriately chosen antiepileptic drugs (AEDs) fails to adequately control seizures. The AEDs in question must be at the maximum tolerated doses, at a sufficient therapeutic level and not be discontinued due to side effects. A less commonly used definition of intractability requires monthly seizures for a period of 18 months.1 Despite optimal medical management with appropriate AEDs, 20%–30% of adults and 10%–40% of children diagnosed with epilepsy continue to suffer from seizures.2–8 Furthermore, after the failure in efficacy of two AEDs, the chance of achieving seizure freedom by introducing subsequent drug regimens has been shown to be less than 10%.2,8–12
Intractable epilepsy is a significant problem for the quality of life (QOL) of the individual patients. Persistent seizures heavily impact on the patients’ behavior, cognitive ability and psychosocial well-being. It has been shown that this condition can lead to poor academic achievements, increased probability of unemployment, low self-esteem, anxiety and depression, social isolation and ultimately to decreased quality of life.13,14 In addition, the unremitting seizures can pose a significant financial burden, with patients suffering from poor seizure control reported to represent approximately 25% of epilepsy cases but accounting for greater than 85% of epilepsy associated costs, mainly due to repeated hospital admissions and extensive investigations.15 Further to the financial burden, patients with refractory seizures have higher mortality rate than patients whose seizures are well-controlled, who have mortality rates comparable to the general population.16 This is likely to be due to differences in the underlying etiology rather than the effect of seizures “per se”. Patients with refractory seizures have higher rates of structural abnormalities or inborn errors of metabolism (IEM). In addition, sudden unexpected death in epilepsy (SUDEP) is forty times more common in patients who continue to suffer from seizures compared to patients who are seizure free.17
The number of new AEDs has significantly increased in the last 20 years, effectively doubling the number of anticonvulsants available for physicians to prescribe. Whilst the efficacy of new molecules in controlling seizures has not dramatically increased, safety and tolerability profiles are significantly better than for the older AEDs. Many of the new drugs have novel mechanisms of action and exhibit fewer drug-interactions.
When considering the most appropriate choice of treatment, it is essential to be confident about the diagnosis and underlying etiology. This is of particular importance in children with refractory epilepsy, a large proportion of whom present specific age-dependent epilepsy syndromes. Accurate identification of the epilepsy syndrome is the foundation for the choice of an appropriate treatment and increases the likelihood of seizure remission.18 In some instances, identifying etiologies can radically alter the potential treatment options, for example metabolic treatments such as the ketogenic diet for epilepsies resulting from IEM.19 Surgery may be the most appropriate treatment option in some cases, especially where strong evidence exists for its efficacy, such as for the anterior temporal lobectomy in drug-resistant temporal lobe epilepsy.20 However, the most appropriate timing for surgery remains debated. When surgery is not a suitable treatment option, neurostimulation therapies such as vagus nerve stimulation (VNS) may be considered.
This review will outline the evidence for the use of different treatment options for patients with intractable seizures. Whilst the focus will be on the pharmacological options, we will also review the extent and quality of evidence supporting the efficacy of surgical, metabolic, neurostimulatory and herbal interventions in the context of the associated side-effects, safety and patient preferences. When appropriate, the costs of the interventions will also be discussed.
Pharmacological Options
Before considering any changes to pharmacological treatment in a patient with refractory seizures, confirmation that the events are epileptic in origin is essential. It is estimated that up to 30% of patients seen in specialist epilepsy clinics present with non-epileptic attacks alone or in combination with epileptic seizures.21 In these patients, the prescription of AEDs is often inappropriate and can lead to significant side effects, in addition to the financial burden to the health services. In many patients with refractory seizures, video-electroencephalography (video-EEG) monitoring is essential to characterize the events and reach diagnostic conclusions with a high level of confidence.
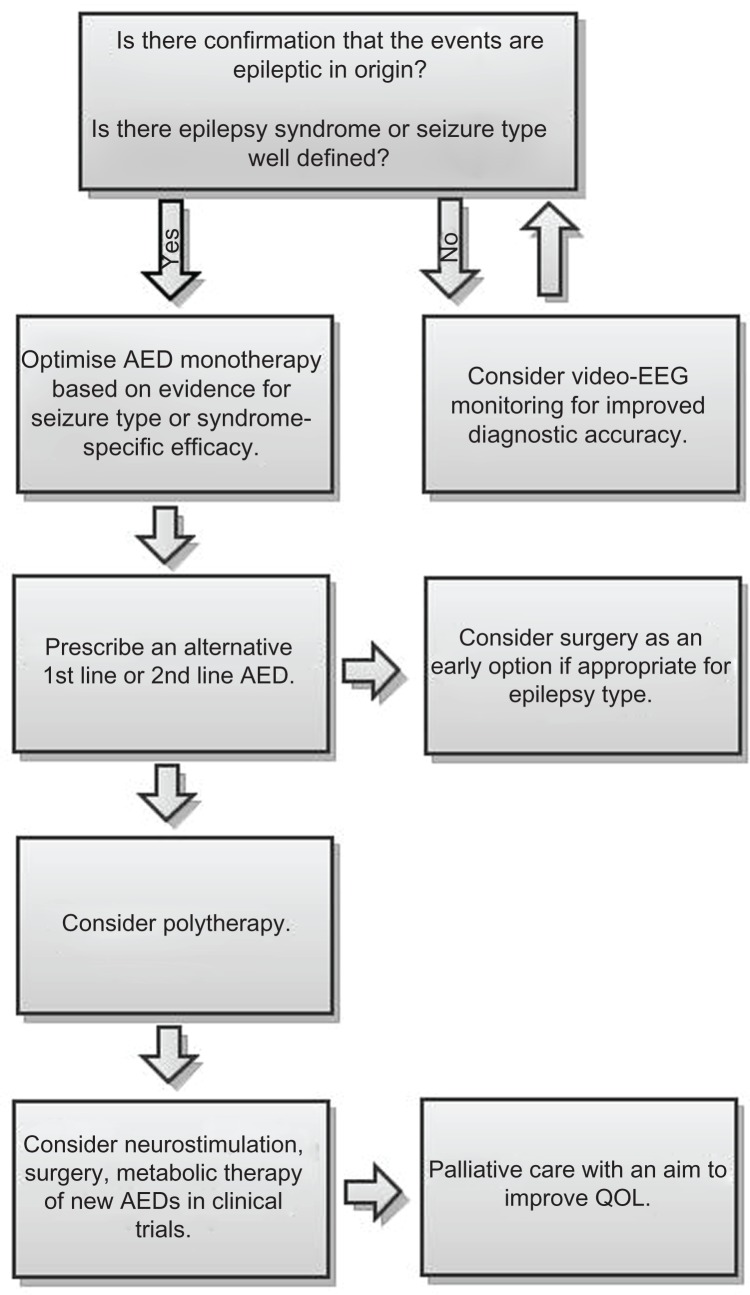
A better understanding of the underlying etiology can inform treatment decisions and lead to optimal seizure control over a shorter time period. This is also the case for patients who suffer from a specific epilepsy syndrome, for whom the importance of correct classification at an early stage should also be emphasized. The correct identification and classification of seizures and epilepsy types allows evidence-based choice of syndrome-specific or etiology-specific treatment intervention, as is the case for Valproate treatment as a first line agent in juvenile myoclonic epilepsy.18 This stepwise approach to correct diagnosis and subsequent treatments options is outlined in Figure 1.
Figure 1.
Stepwise approach for evaluation and treatment of a patient with refractory seizures.
Patients with refractory seizures have, by definition, tried at least two appropriate AEDs and their seizures have failed to be adequately controlled. At this point the physician faces the decision to either add a further AED to the current treatment or to pursue sequential monotherapy. If sequential monotherapy is chosen, the drugs that are failing to control seizures are slowly tapered off and replaced with different AEDs which can potentially improve seizure control. Sequential monotherapy was the main treatment paradigm from the 1970s through to the 1990s, when sodium channel blocking AEDs were the only treatment option. Combining drugs with the same mechanism of action lead to a slightly better seizure control, but at the expense of a marked increase in adverse drug interactions and pharmacodynamic amplification of side effects.22 This practice changed in the 1990s, when new AEDs were introduced with novel mechanisms of action. These drugs exhibited fewer pharmacokinetic interactions, exhibited fewer side effects when combined and raised the possibility of synergistic drug combinations. Clinical practice over the past 20 years has been characterized by “rational polytherapy”: numerous drug combinations have been considered and a wealth of evidence on which combinations are most effective and where drug interactions are most likely to occur has accumulated.23
When contemplating polypharmacy for a patient with epilepsy, it is important to consider the concept of “total drug load” in addition to the number of drugs being prescribed. Although general agreement on the definition has not been reached, drug load is usually defined as the total amount of drug exposure for a treatment regimen, and has been quantified as the prescribed daily dose of AED(s) divided by the defined daily dose for the AED(s) as outlined by the World Health Organization. Drug load has been demonstrated to correlate with the number of adverse events better than the number of AEDs being prescribed.24
It has been argued that for the use of multiple AEDs to be rational, the combination of two drugs must provide supra-additive therapeutic efficacy, whereby the combined efficacy of the two drugs is greater than when the drugs are given alone. Alternatively, combining AEDs may be more advantageous than prescribing monotherapy at standard doses if they demonstrate simple additive efficacy but less than additive side effects. However, pharmacokinetic drug interactions often contraindicate specific drug combinations.
Some of the traditional AEDs, including Phenobarbital, Phenytoin and Carbamazepine are metabolized in the liver and are potent inducers of the cytochrome P450 (CYP450) system. These drugs act by blocking voltage-dependent sodium channels preventing the repetitive and uncontrolled neuronal discharges seen in epilepsy. Phenobarbital, in addition, enhances GABA mediated inhibition, which further acts to prevent the repetitive neuronal firing.25 In the developed world, Phenobarbital is now prescribed rarely due to its sedative side effect profile. However due to its relatively low cost it is regularly prescribed in developing countries. All of the drugs described above induce hepatic enzymes and decrease the serum concentration of additional AEDs that may be prescribed. As a consequence of this effect, each drug may impede the other, by preventing effective serum concentrations being reached.22 It is therefore advised that the combination of an enzyme-inducing AED with another AED which is metabolized in the liver requires particular caution. Interactions with other medications should also be considered when prescribing hepatic enzyme inducing drugs, particularly in patients taking exogenous contraceptive hormones, immunosuppressant therapy, a number of corticosteroids and some antineoplastic chemotherapeutic agents, all of which can be the subject of enzyme-induction. Physicians should also be aware of the additional adverse effects that enzyme-inducing drugs can produce, including secondary hyperparathyroidism, reduced serum estosterone concentrations, menstrual irregularities and elevated cholesterol levels, which may impact on a patient’s cardiovascular disease risk. Consequently, it has been suggested that the older, enzyme-inducing AEDs should not be considered as first-line treatment options for patients with refractory seizures.26 Among older AEDs, Valproate is characterized by a number of mechanisms of action, including sodium channel blockade and potentiation of GABA mediated inhibition. It is also metabolized by the liver, but has no enzyme-inducer properties.
The newer AEDs, including Gabapentin, Levetiracetam, Pregabalin, Vigabatrin, Lamotrigine, Oxcarbazepine, Topiramate and Zonisamide, are characterized by a more favorable tolerability profile, as their hepatic enzyme induction properties are minimal or absent. Many of these drugs have novel or unknown mechanisms of action and demonstrate similar levels of efficacy and fewer side effects, less adverse pharmacokinetic drug interactions and are tolerated better by patients.25 When compared to Carbamazepine in double-blind randomized control trials for treatment of partial onset seizures, Gabapentin, Lamotrigine and Oxcarbazepine were found to have an similar efficacy but better levels of tolerability.27–29
Until recently, direct comparison studies in treatment-refractory epilepsy populations have been sparse. A recent systematic review and meta-analysis of studies on patients with refractory partial epilepsy suggested that Topiramate and Levetiracetam have a greater efficacy in controlling seizure frequency than the other new AEDs.30 The same study found that Gabapentin is likely to be less effective than other new AEDs. In terms of tolerability, Oxcarbazepine and Topiramate were associated with significantly more withdrawals and therefore more poorly tolerated than the other new AEDs. Gabapentin and Levetiracetam, on the other hand, were associated with significantly fewer withdrawals and were therefore better tolerated. It should be noted that these conclusions were based on indirect comparisons and should be treated with caution.
Although the newer AEDs exhibit fewer side effects than traditional AEDs, the potential for significant cognitive and behavioral side effects should be considered when prescribing. Drugs that have a mechanism of action involving the potentiation of GABA mediated inhibition such as Vigabatrin, Tiagabine, Topiramate and Gabapentin have been associated with sedation and negative effects on mood. Some patients who are prescribed these AEDs are at risk of developing depression as a side effect, especially patients with a past psychiatric history.31 Topiramate has been associated with unfavorable cognitive effects, including impaired concentration, psychomotor slowing, language regression, comprehension problems and memory deficits.32 Other new AEDs which act by inhibiting the glutamatergic excitatory neurotransmission have been associated with an ‘activating’ effect, with improved mood but potentially anxiogenic effects. Taking advantage of these properties, Lamotrigine may be considered in patients with concomitant depression, apathy and hypersomnia.31 In addition, psychotic symptoms have been reported as adverse events following AED administration, especially with Topiramate and Levetiracetam, although the extent that the medication plays in producing these symptoms remains uncertain.33 It is recommended that baseline mood and cognitive ability is taken into account when deciding which AED to prescribe in refractory patients, in order to minimize any impact that prescribing may have on mood and cognition. This will improve AED tolerability and ensure the minimum detrimental impact on QOL.
In addition to the above evidence, clinical studies on polytherapy are informative when considering using a combination of AEDs. One study on 47 patients with cognitive impairment showed that seizures were controlled more effectively by a combination of Phenobarbital and Phenytoin or Phenobarbital and Carbamazepine, than by Phenytoin and Carbamazepine combination.34 This suggested that a combination of two sodium-channel blocking AEDs does not improve seizure control significantly. Other studies indicate that a combination of Carbamazepine and Valproate or Carbamazepine and Vigabatrin is more effective in seizure control than a combination of Carbamazepine and Phenytoin, although these studies were not controlled for drug concentration.35,36 The AED combination for which there is the most convincing evidence is Lamotrigine and Valproate. A large study aiming to assess the efficacy of Lamotrigine monotherapy also evaluated the effect on seizure control whilst taking Lamotrigine as add-on to the first AED (Carbamazepine, Phenytoin or Valproate). This study showed that patients who were taking Lamotrigine and Valproate experienced an 83% reduction in seizures, whilst those taking the Lamotrigine and Carbamazepine or Lamotrigine and Phenytoin combinations experienced only a 43% and 34% reduction in seizures, respectively.37 These findings should be interpreted with some caution, however, as different doses of Lamotrigine were used in each combination.
Non-Pharmacological Options
Surgery
For resective surgery to be a viable option, the patient with treatment-refractory epilepsy should ideally have a single epileptogenic focus in a non-eloquent cortical region (ie, not involved in key language, memory or motor processes). It should be noted that exceptional cases do exist, where larger resections such as hemispherectomies are acceptable if the seizures are severe and the benefit gained from such surgery can be justified.38 Major surgery such as corpus callosotomy can be considered as last treatment option in palliative care for patients with intractable seizures. This invasive intervention is usually aimed at preventing secondary generalization in patients who are regularly experiencing loss of consciousness and a high frequency of seizure-related injuries.
Most epileptologists consider surgical management as second line therapy for patients with intractable seizures.39 This is especially true for patients with drug-resistant temporal lobe epilepsy (TLE), for whom temporal lobectomy can represent an effective treatment option. A randomized control trial found that 58% of patients randomly allocated to surgery for temporal lobe resection did not present with any seizures which impaired consciousness after one year, compared to 8% in the treatment arm receiving the optimum medication regimen (P < 0.001).20 A multi-center study which followed 339 patients with epilepsy for 2 years post-operatively showed that temporal lobe resection is the most effective type of resective surgery in this patient population: 68% of the patients who received temporal lobectomies experienced seizure remission for 2 years, compared to 50% of the patients who underwent extratemporal resections.40 Another non-randomized controlled study which followed up patients with refractory seizures, assigned either to surgical treatment or continued drug therapy, found that 44.6% of 242 patients with pharmacoresistant TLE who received surgery were seizure-free for 12 months, compared to 4.3% of those who received AEDs only (P < 0.001).41 It should be emphasized, however, that not all patients with TLE have the same probability of achieving seizure freedom through temporal lobe resection. A meta-analysis of 83 studies including 7,343 patients undergoing epilepsy surgery showed that the proportion of patients experiencing seizure freedom for at least 5 years post-operatively was highest in those who underwent temporal lobe resection (66%), even though a substantial proportion of patients undergoing occipital, parietal or frontal lobe resections demonstrated post-operative seizure freedom (46%, 46% and 27% respectively).42 It should be noted that post-operative seizure freedom for the first few years following surgery might not persist long-term. Isolated relapses can still occur many years after the procedure, with a probability of seizure relapse of 4% per year for the 5 years following surgery.43 Overall, patients with structural abnormalities apparent on pre-operative imaging studies, such as hippocampal sclerosis or foreign tissue lesions are more likely to experience post-operative seizure freedom compared to patients with no obvious lesion.44–46
When considering surgery for refractory epilepsy, there are a number of risks that need to be taken into account alongside the potential benefits. The surgery itself can cause a substantial period of disability, with patients typically having to take 4–8 weeks off work or school.47 The most common medical complications are deep vein thrombosis, infection and transient endocrine abnormalities. Psychiatric complications are reported post-resection in 25% of the patients, including transient dysphoria, depression and occasionally mania.48 Although less commonly reported, permanent neurological and neuropsychological complications can result from resective surgery. These differ depending on the site of resection, and tend to be more common and disabling if the site is close to areas of the cortex involved in key cognitive functions. Furthermore, the procedure has the possibility to create new lesions which may become epileptogenic and trigger subsequent seizures.47 On the other hand, patients are often withdrawn from AEDs following surgery, and therefore relieved from many of the adverse effects of these medications.
Surgical procedures are generally considered after the failure of two appropriate drug regimens, although the number of unsuccessful trials with AEDs, alone or in combination, before considering surgery remains a matter of debate.47,49 When contemplating surgery, it is important to assess the likelihood of seizure remission using further drug regimens compared to surgical resection, whilst considering the risks associated with such procedures. Although current evidence suggests that temporal lobe surgery is the most effective treatment option after the failure of two AEDs, the same cannot be said for other surgical procedures, such as frontal lobe resection, whereby the overall benefit from surgery may not be greater than trying alternative drug regimens. In these cases, the risks for each treatment option should be weighed up on a case-by-case basis until more definitive data is available to guide therapeutic decisions.
Neurostimulation
Patients with intractable epilepsy who are not suitable for epilepsy surgery or those who continue to experience seizures post-operatively may benefit from neurostimulation. This treatment option for patients with intractable epilepsy involves electrical stimulation of the nervous system using surgically implanted devices, or non-invasive stimulation as is the case for repetitive transcranial magnetic stimulation (rTMS). Vagal nerve stimulation (VNS) therapy is the only neurostimulation method to have received approval by the USA Food and Drug Administration, whilst both VNS and deep brain stimulation (DBS) are approved for use in patients with refractory epilepsy in the UK.
In VNS therapy, intermittent electrical stimulation is delivered to the left vagus nerve, which has ascending fibers with widespread connections to the limbic, autonomic and reticular brain regions. The reduced thalamic blood flow that is observed in VNS has been proposed to underlie the mechanism for seizure reduction.50
In addition to a number of retrospective studies, efficacy for this treatment option has been demonstrated in two randomized, placebo-controlled, double-blind trials, which reported a median seizure reduction of 24.5%–28.0% in the group receiving high level VNS compared to just 6.1%–15.0% in patients receiving low level VNS (P = 0.01 and P = 0.04, respectively).51,52 The risks associated with implantation of the VNS device are relatively low, with a 3%–5% chance of infection.53–55 Vocal cord dysfunction, throat discomfort, change in voice quality and sleep apnea can present post-operatively, and such consequences should be discussed with the patient when considering this treatment. An additional factor that should be considered when planning VNS therapy is that patients are usually unable to undergo magnetic resonance imaging (MRI) after implantation, since the changing magnetic fields may induce electrical currents in the device.56
DBS involves electrical stimulation of specific subcortical nuclei, which have widespread neural connections. The anterior nucleus of the thalamus is often the target of DBS due to its widespread projections to the limbic system. In a randomized controlled trial of 109 patients with refractory epilepsy who had DBS electrodes implanted in the anterior nucleus of the thalamus there was a 29% greater reduction in seizures for 54 patients who had the stimulator switched on compared to 55 patients who had their stimulation turned off after a blinded period of 3 months (P = 0.002).57 Infection, hemorrhage and stimulation-induced seizures were amongst the most frequently reported complications. In the same study, impaired memory and higher levels of depression were observed in the group receiving the active treatment. DBS has been recently licensed in the UK, and NICE guidelines suggest that this method should be used in highly selected cases, due to the lack of efficacy data and the associated risks.58 The centromedian nucleus is also a potential location for DBS, since it is part of the reticulo-thalamo-cortical system which is involved in mediating cortical excitability and wakefulness. One double-blind crossover trial reported a 30% reduction in generalized tonic-tonic seizures when the device was turned on compared to when it was off, although this change was not significant, possibly due to the small sample size of 7 patients.59 Further evidence for centromedian nucleus stimulation efficacy can be found in a longitudinal study which reported good efficacy for generalized tonic-clonic seizures, atypical absences and tonic seizures, however caution should be taken in interpreting these findings because of the lack of control groups.60
Unlike other methods of neurostimulation, responsive neurostimulation (RNS) does not deliver electrical stimulation at specific frequencies throughout the day. Instead, the RNS device is composed of a combined recorder and stimulator device, which detects clinically relevant epileptiform discharges and delivers an appropriate electrical stimuli in response. A recent multicenter double-blind randomized controlled trial in adults with intractable partial seizures demonstrated a reduction in seizure frequency of 37.9% in the treatment arm compared to 17.3% in the control group (P = 0.012).61
The fourth and only non-invasive neurostimulation technique, low frequency rTMS, is thought to suppress cortical excitability either via inducing long term depression or alternatively by enhancing GABAergic inhibition.62 Although evidence for its efficacy is limited, a number of small-scale studies suggest antiepileptic properties (see Fregni and Pascual-Leone for a comprehensive review).63
Metabolic treatment
Over the past century, the efficacy of dietary therapies for intractable seizures has increasingly been recognized. Currently there are four main types of metabolic therapy in use for the reduction of seizures. Three of these have a high fat content and low carbohydrate content—the classic ketogenic diet, the medium chain triglyceride (MCT) diet and the modified Atkins diet (MAD)—and the fourth controls the carbohydrate quality, the low glycemic index treatment (LGIT). These treatments are usually considered in patients with intractable epilepsy who have failed to respond to several AEDs and are not suitable candidates for surgery.
The classic ketogenic diet is a high fat and low carbohydrate diet that uses long chain fatty acids (LCFAs) as its main source of fat. Typically the patient must consume 3–4 grams of fat for every 1 gram of carbohydrate plus protein. As a result, between 86% and 90% of the calories from this diet are provided from lipids.64 The MCT diet provides more ketones per calorie consumed than in the classic ketogenic diet, which uses mainly LCFAs. In MCT, more carbohydrates and proteins can be consumed for every gram of fat. The MAD recommends increased consumption of foods high in fat whilst restricting the amount of carbohydrates. Unlike the classic ketogenic and MCT diets, there is no limit on the amount of protein consumed or the total number of calories per day.65 The typical MAD uses a ratio of 1 gram of fat for every gram of carbohydrate plus protein, and consequently is less restrictive than the other two high fat diets. The LGIT restricts carbohydrate intake to food items with a glycemic index of less than 50 at 40–60 grams per day, in order to prevent large fluctuations in blood glucose concentrations, which are thought to exacerbate seizures.66
There is a large body of evidence demonstrating the efficacy of the metabolism-based therapies for refractory seizures, with the majority focusing on the classic ketogenic diet. Two meta-analyses of observational studies showed that 15.6%–15.8% of patients on the diet can achieve seizure freedom whilst 33.0%–55.8% of the patients can have a >50% reduction in number of seizures.67 One of the only randomized controlled trials to investigate the efficacy of the classic ketogenic diet followed children with intractable or focal-onset epilepsy who were randomized to a ketogenic diet or continuation of a normal diet for a 3-month period. Of the patients who received the ketogenic diet, 38% had a seizure reduction of >50%. This was significantly greater than the control group, where only 6% had >50% seizure reduction (P < 0.001).68 The results of a follow-up study comparing the effectiveness of the LCFA versus the MCT diet suggest there is no increased benefit using either of the two diets for seizure reduction.69
Despite the lack of prospective randomized control trials, the MAD has also demonstrated substantial efficacy in controlling seizures. Of the patients who have been studied on the MAD, 45% have demonstrated 50%–90% seizure reduction and 28% have >90% seizure reduction. Similar results have been reported in two retrospective studies of the LGIT, with 38%–73% of patients achieving >50% seizure reduction.70,71 The best candidates for the MAD tend to be adults and adolescents, for whom compliance on the LCFA and MCT diets is an issue.64 This is because the food consumed on the MAD is similar to that being consumed by peers. The same can be said about the LGIT, which in addition can be considered for patients who find the other 3 high-fat diets unpalatable.
There is a body of evidence suggesting that metabolism-based therapy can be considered earlier in the treatment plan for patients with specific epilepsy syndromes. One notable example is West syndrome (infantile spasms), where the ketogenic diet is successfully used as a first line agent.72 The same diet is also thought to be especially effective in unspecified symptomatic generalized epilepsy, multifocal epilepsy, juvenile absence epilepsy, continuous spike and slow wave of sleep, myoclonic-astatic epilepsy (MAE or Doose syndrome), severe myoclonic epilepsy of infancy (Dravet syndrome), and seizures in patients with Tuberous Sclerosis Complex and Landau-Kleffner syndrome.68,73,74 Ketogenic diets are also recommended for patients suffering from glucose transporter defects and pyruvate dehydrogenase deficiency, who are unable to utilize glucose for brain metabolism.75,76
The most common side effects of dietary treatments are constipation, acidosis, temporary hypercholesterolemia, kidney stones and hunger.64 Growth restriction has also been observed in children.77 The ketogenic diet is contraindicated in patients who are unable to use ketone bodies as an energy source or those who require high levels of glucose. Such conditions include pyruvate decarboxylase deficiency, primary carnitine deficiency, fatty acid oxidation abnormalities, the porphyrias and some mitochondrial disorders.78
Generally, as with AEDs, it is suggested that after adhering to dietary therapy for a period of 2 years, the treatment should slowly be tapered off. Many patients who become seizure free whilst adhering to the diets remain seizure free off the diet. This has been demonstrated in one study, which reported the risk of seizures recurring when stopping the diet at only 20%.79 It has been hypothesized that the dietary therapies have a long-term, disease modifying effects, although the observed phenomenon could just be explained by the natural history of the seizure disorder.64
In addition to showing high levels of efficacy, similar to many AEDs, the dietary interventions are generally quite tolerable, can have associated positive effects such as weight loss,80 and demonstrate effectiveness in a relatively short time period. Whilst AEDs may take several weeks to be titrated up to a therapeutic dose and VNS may take months or years to show an optimal effect,81 the effects of a ketogenic diet can be seen within a few days. Furthermore, the diets are cost effective compared to many AEDs.64 In practice, a median of 5 AEDs are prescribed before considering a metabolic treatment such as the ketogenic diet.82 Furthermore, 60% of child neurologists only use a ketogenic diet as a last resort treatment.83 It is therefore suggested that the metabolic treatments should be considered earlier in the course of treatment, especially in patients with refractory seizures who are not suitable for or willing to undergo resective surgery.
Herbal remedies
Herbal treatments are generally viewed as alternative and complementary forms of medicine by both patients and physicians. There is, however, a small body of evidence demonstrating the effectiveness of some herbs at controlling seizures. Out of all the herbal treatments, cannabinoids have the largest body of evidence supporting their use as anticonvulsants. One randomized, double-blind controlled trial reported 50% of patients with secondary generalized epilepsy receiving Cannabidiol becoming virtually seizure-free for the duration of the study. However, only 15 patients were included and to our knowledge no follow-up study has been performed.84 The use of Cannabis for epilepsy has been legalized in Canada and licensed in 14 states within the United States, although it is prohibited in most European countries.85Huperzine A. has been shown to be effective in animal models of epilepsy, and a clinical trial to assess its effectiveness has been planned.86,87 There is also laboratory data supporting the efficacy of kava (Piper methysticum) and mistletoe (Viscum sp), but no clinical evidence exists to support their use. A number of other herbal treatments that are commonly used to treat seizures have either been shown to have no effect on the severity of seizures, or in fact demonstrate proconvulsant properties and exacerbate the condition. In addition, much evidence is anecdotal, or when clinical studies have been performed, these often display poor methodology and do not have sufficient levels of statistical power to make firm conclusions from. Further, good quality evidence in the form of controlled trials is required to justify the use of herbal treatments in a clinical setting (see Pearl et al for a comprehensive review).88
Conclusions
Patients with treatment refractory seizures continue to represent a large proportion of patients diagnosed with epilepsy. The fact that recurrent seizures and associated side effects from AEDs significantly impact on patient’s QOL means that treatment decisions should be made in light of the best evidence for improved seizure control, enhanced QOL and the greatest tolerability profile. Confirmation that the events are epileptogenic in origin and early identification of an underlying etiology or specific epilepsy syndrome is essential to guide appropriate treatment for which syndrome or etiology specific efficacy exists. This may be in the form of monotherapy, polytherapy or alternative treatment options including surgery, neurostimulation, metabolic treatments and herbal remedies. Despite the fact that decisions to alter a treatment regimen may be influenced by local availability and funding, physicians should balance the evidence for efficacy in seizure reduction with the associated risks, side effects and patient preference.
Footnotes
Author Contributions
Wrote the first draft of the manuscript: JWM. Contributed to the writing of the manuscript: JWM, AEC. Agree with manuscript results and conclusions: JWM, AEC, SS. Jointly developed the structure and arguments for the paper: JWM, AEC, SS. Made critical revisions and approved final version: JWM, AEC, SS. All authors reviewed and approved of the final manuscript.
Competing Interests
AEC received fees for Board Membership and other manuscript preparation from Eisai Pharmaceuticals and Lectureship grants from Eisai Pharmaceuticals and Janssen-Cilag; SS received Unrestricted Teaching Grants from Eisai Pharmaceuticals and UCB Pharma.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest. Provenance: the authors were invited to submit this paper.
References
- 1.Berg AT, Kelly MM. Defining intractability: Comparisons among published definitions. Epilepsia. 2006;47(2):431–6. doi: 10.1111/j.1528-1167.2006.00440.x. [DOI] [PubMed] [Google Scholar]
- 2.Dlugos DJ, Sammel MD, Strom BL, Farrar JT. Response to first drug trial predicts outcome in childhood temporal lobe epilepsy. Neurology. 2001;57(12):2259–64. doi: 10.1212/wnl.57.12.2259. [DOI] [PubMed] [Google Scholar]
- 3.Berg AT, Shinnar S, Levy SR, Testa FM, Smith-Rapaport S, Beckerman B. Early development of intractable epilepsy in children—A prospective study. Neurology. 2001;56(11):1445–52. doi: 10.1212/wnl.56.11.1445. [DOI] [PubMed] [Google Scholar]
- 4.Camfield C, Camfield P, Gordon K, Smith B, Dooley J. Outcome of childhood epilepsy—a population-based study with a simple predictive scoring system for those treated with medication. J Pediatr. 1993;122(6):861–8. doi: 10.1016/s0022-3476(09)90008-7. [DOI] [PubMed] [Google Scholar]
- 5.Go C, Snead OC. Pharmacologically intractable epilepsy in children: diagnosis and preoperative evaluation. Neurosurg Focus. 2008;25(3) doi: 10.3171/FOC/2008/25/9/E2. [DOI] [PubMed] [Google Scholar]
- 6.Sillanpaa M, Jalava M, Kaleva O, Shinnar S. Long-term prognosis of seizures with onset in childhood. New Engl J Med. 1998;338(24):1715–22. doi: 10.1056/NEJM199806113382402. [DOI] [PubMed] [Google Scholar]
- 7.Beghi E. Efficacy and tolerability of the new antiepileptic drugs: comparison of two recent guidelines. Lancet Neurol. 2004;3(10):618–21. doi: 10.1016/S1474-4422(04)00882-8. [DOI] [PubMed] [Google Scholar]
- 8.Kwan P, Brodie MJ. Early identification of refractory epilepsy. The New England Journal of Medicine. 2000;342(5):314–9. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 9.Mohanraj R, Brodie MJ. Diagnosing refractory epilepsy: response to sequential treatment schedules. Eur J Neurol. 2006;13(3):277–82. doi: 10.1111/j.1468-1331.2006.01215.x. [DOI] [PubMed] [Google Scholar]
- 10.Brodie MJ, Kwan P. Staged approach to epilepsy management. Neurology. 2002;58(8):S2–8. doi: 10.1212/wnl.58.8_suppl_5.s2. [DOI] [PubMed] [Google Scholar]
- 11.Arts WFM, Brouwer OF, Peters ACB, et al. Course and prognosis of childhood epilepsy: 5-year follow-up of the Dutch study of epilepsy in childhood. Brain. 2004;127:1774–84. doi: 10.1093/brain/awh200. [DOI] [PubMed] [Google Scholar]
- 12.Schiller Y, Najjar Y. Quantifying the response to antiepileptic drugs. Neurology. 2008;70(1):54–65. doi: 10.1212/01.wnl.0000286959.22040.6e. [DOI] [PubMed] [Google Scholar]
- 13.Markand ON, Salanova V, Whelihan E, Emsley CL. Health-related quality of life outcome in medically refractory epilepsy treated with anterior temporal lobectomy. Epilepsia. 2000;41(6):749–59. doi: 10.1111/j.1528-1157.2000.tb00238.x. [DOI] [PubMed] [Google Scholar]
- 14.Sabaz M, Cairns DR, Lawson JA, Bleasel AF, Bye AM. The health-related quality of life of children with refractory epilepsy: a comparison of those with and without intellectual disability. Epilepsia. 2001;42(5):621–8. doi: 10.1046/j.1528-1157.2001.25200.x. [DOI] [PubMed] [Google Scholar]
- 15.Begley CE, Famulari M, Annegers JF, et al. The cost of epilepsy in the United States: An estimate from population-based clinical and survey data. Epilepsia. 2000;41(3):342–51. doi: 10.1111/j.1528-1157.2000.tb00166.x. [DOI] [PubMed] [Google Scholar]
- 16.Sillanpaa M, Shinnar S. Long-term mortality in childhood-onset epilepsy. New Engl J Med. 2010;363(26):2522–9. doi: 10.1056/NEJMoa0911610. [DOI] [PubMed] [Google Scholar]
- 17.Tomson T. Mortality in epilepsy. J Neurol. 2000;247(1):15–21. doi: 10.1007/s004150050004. [DOI] [PubMed] [Google Scholar]
- 18.Hussain S, Sankar R. Pharmacologic treatment of intractable epilepsy in children: a syndrome-based approach. Semin Pediatr Neurol. 2011;18(3):171–8. doi: 10.1016/j.spen.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Rauchenzauner M, Klepper J, Leiendecker B, Luef G, Rostasy K, Ebenbichler C. The ketogenic diet in children with Glut1 deficiency syndrome and epilepsy. J Pediatr. 2008;153(5):716–8. doi: 10.1016/j.jpeds.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Wiebe S, Blume WT, Girvin JP, Eliasziw M. Effectiveness, efficiency of surgery for temporal lobe epilepsy study G. A randomized, controlled trial of surgery for temporal-lobe epilepsy. New Engl J Med. 2001;345(5):311–8. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 21.Sirven JI, Glosser DS. Psychogenic nonepileptic seizures: theoretic and clinical considerations. Neuropsychiatry Neuropsychol Behav Neurol. 1998;11(4):225–35. [PubMed] [Google Scholar]
- 22.French JA, Faught E. Rational polytherapy. Epilepsia. 2009;50(Suppl 8):63–8. doi: 10.1111/j.1528-1167.2009.02238.x. [DOI] [PubMed] [Google Scholar]
- 23.Ferrendelli JA. Relating pharmacology to clinical practice: the pharmacologic basis of rational polypharmacy. Neurology. 1995;45(3 Suppl 2):S12–6. [PubMed] [Google Scholar]
- 24.Deckers CL, Hekster YA, Keyser A, Meinardi H, Renier WO. Reappraisal of polytherapy in epilepsy: a critical review of drug load and adverse effects. Epilepsia. 1997;38(5):570–5. doi: 10.1111/j.1528-1157.1997.tb01142.x. [DOI] [PubMed] [Google Scholar]
- 25.Czapinski P, Blaszczyk B, Czuczwar SJ. Mechanisms of action of antiepileptic drugs. Curr Topics Med Chemistry. 2005;5(1):3–14. doi: 10.2174/1568026053386962. [DOI] [PubMed] [Google Scholar]
- 26.Mintzer S, Mattson RT. Should enzyme-inducing antiepileptic drugs be considered first-line agents? Epilepsia. 2009;50(Suppl 8):42–50. doi: 10.1111/j.1528-1167.2009.02235.x. [DOI] [PubMed] [Google Scholar]
- 27.Brodie MJ, Richens A, Yuen AW. Double-blind comparison of lamotrigine and carbamazepine in newly diagnosed epilepsy. UK Lamotrigine/Carbamazepine Monotherapy Trial Group. Lancet. 1995;345(8948):476–9. doi: 10.1016/s0140-6736(95)90581-2. [DOI] [PubMed] [Google Scholar]
- 28.Houtkooper MA, Lammertsma A, Meyer JW, et al. Oxcarbazepine (GP 47.680): a possible alternative to carbamazepine? Epilepsia. 1987;28(6):693–8. doi: 10.1111/j.1528-1157.1987.tb03702.x. [DOI] [PubMed] [Google Scholar]
- 29.Dam M, Ekberg R, Loyning Y, Waltimo O, Jakobsen K. A double-blind study comparing oxcarbazepine and carbamazepine in patients with newly diagnosed, previously untreated epilepsy. Epilepsy Res. 1989;3(1):70–6. doi: 10.1016/0920-1211(89)90070-3. [DOI] [PubMed] [Google Scholar]
- 30.Costa J, Fareleira F, Ascencao R, Borges M, Sampaio C, Vaz-Carneiro A. Clinical comparability of the new antiepileptic drugs in refractory partial epilepsy: a systematic review and meta-analysis. Epilepsia. 2011;52(7):1280–91. doi: 10.1111/j.1528-1167.2011.03047.x. [DOI] [PubMed] [Google Scholar]
- 31.Cavanna AE, Ali F, Rickards HE, McCorry D. Behavioral and cognitive effects of anti-epileptic drugs. Discov Med. 2010;9(45):138–44. [PubMed] [Google Scholar]
- 32.Eddy CM, Rickards HE, Cavanna AE. The cognitive impact of antiepileptic drugs. Ther Adv Neurol Dis. 2011;4(6):385–407. doi: 10.1177/1756285611417920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piedad JR, Besag H FMC, Cavanna AE. Beneficial and adverse psychotropic effects of anti epileptic drugs in patients with epilepsy. CNSDrugs. 2012;26(4):319–35. doi: 10.2165/11599780-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 34.Cereghino JJ, Brock JT, Van Meter JC, Penry JK, Smith LD, White BG. The efficacy of carbamazepine combinations in epilepsy. Clin Pharmacol Therap. 1975;18(06):733–41. doi: 10.1002/cpt1975186733. [DOI] [PubMed] [Google Scholar]
- 35.Tanganelli P, Regesta G. Vigabatrin vs. carbamazepine monotherapy in newly diagnosed focal epilepsy: a randomized response conditional crossover study. Epilepsy Res. 1996;25(3):257–62. doi: 10.1016/s0920-1211(96)00074-5. [DOI] [PubMed] [Google Scholar]
- 36.Walker JE, Koon P. Carbamazepine versus valproate versus combined therapy for refractory partial complex seizures with secondary generalization. Epilepsia. 1988;29:693–4. [Google Scholar]
- 37.Brodie MJ, Yuen AW. Lamotrigine substitution study: evidence for synergism with sodium valproate? 105 Study Group. Epilepsy Res. 1997;26(3):423–32. doi: 10.1016/s0920-1211(96)01007-8. [DOI] [PubMed] [Google Scholar]
- 38.Kossoff EH, Vining EP, Pillas DJ, et al. Hemispherectomy for intractable unihemispheric epilepsy etiology vs. outcome. Neurology. 2003;61(7):887–90. doi: 10.1212/01.wnl.0000090107.04681.5b. [DOI] [PubMed] [Google Scholar]
- 39.Cross JH, Jayakar P, Nordli D, et al. Proposed criteria for referral and evaluation of children for epilepsy surgery: recommendations of the Subcommission for Pediatric Epilepsy Surgery. Epilepsia. 2006;47(6):952–9. doi: 10.1111/j.1528-1167.2006.00569.x. [DOI] [PubMed] [Google Scholar]
- 40.Spencer SS, Berg AT, Vickrey BG, et al. Predicting long-term seizure outcome after resective epilepsy surgery—The Multicenter Study. Neurology. 2005;65(6):912–8. doi: 10.1212/01.wnl.0000176055.45774.71. [DOI] [PubMed] [Google Scholar]
- 41.Bien CG, Kurthen M, Baron K, et al. Long-term seizure outcome and antiepileptic drug treatment in surgically treated temporal lobe epilepsy patients: A controlled study. Epilepsia. 2001;42(11):1416–21. doi: 10.1046/j.1528-1157.2001.43300.x. [DOI] [PubMed] [Google Scholar]
- 42.Tellez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain. 2005;128:1188–98. doi: 10.1093/brain/awh449. [DOI] [PubMed] [Google Scholar]
- 43.Sperling MR, Nei M, Zangaladze A, et al. Prognosis after late relapse following epilepsy surgery. Epilepsy Res. 2008;78(1):77–81. doi: 10.1016/j.eplepsyres.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 44.Radhakrishnan K, So EL, Silbert PL, et al. Predictors of outcome of anterior temporal lobectomy for intractable epilepsy—A multivariate study. Neurology. 1998;51(2):465–71. doi: 10.1212/wnl.51.2.465. [DOI] [PubMed] [Google Scholar]
- 45.McIntosh AM, Kalnins RM, Mitchell LA, Fabinyi GCA, Briellmann RS, Berkovic SF. Temporal lobectomy: long-term seizure outcome, late recurrence and risks for seizure recurrence. Brain. 2004;127:2018–30. doi: 10.1093/brain/awh221. [DOI] [PubMed] [Google Scholar]
- 46.Tonini C, Beghi E, Berg AT, et al. Predictors of epilepsy surgery outcome: a meta-analysis. Epilepsy Res. 2004;62(1):75–87. doi: 10.1016/j.eplepsyres.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 47.Kwan P, Sperling MR. Refractory seizures: try additional antiepileptic drugs (after two have failed) or go directly to early surgery evaluation? Epilepsia. 2009;50(Suppl 8):57–62. doi: 10.1111/j.1528-1167.2009.02237.x. [DOI] [PubMed] [Google Scholar]
- 48.Kohler CG, Carran MA, Bilker W, O’Connor MJ, Sperling MR. Association of fear auras with mood and anxiety disorders after temporal lobectomy. Epilepsia. 2001;42(5):674–81. doi: 10.1046/j.1528-1157.2001.42600.x. [DOI] [PubMed] [Google Scholar]
- 49.Kwan P, Brodie MJ. Issues of medical intractability for surgical candidacy. In: Wyllie E, editor. The Treatment of Epilepsy: Principles and Practice. 4th ed. Philadephia: Lippincott Williams & Wilkins; 2006. pp. 983–91. [Google Scholar]
- 50.Henry TR, Votaw JR, Pennell PB, et al. Acute blood flow changes and efficacy of vagus nerve stimulation in partial epilepsy. Neurology. 1999;52(6):1166–73. doi: 10.1212/wnl.52.6.1166. [DOI] [PubMed] [Google Scholar]
- 51.George R, Sonnen A, Upton A, et al. A randomized controlled trial of chronic vagus nerve-stimulation for treatment of medically intractable seizures. Neurology. 1995;45(2):224–30. doi: 10.1212/wnl.45.2.224. [DOI] [PubMed] [Google Scholar]
- 52.Handforth A, DeGiorgio CM, Schachter SC, et al. Vagus nerve stimulation therapy for partial-onset seizures—A randomized active-control trial. Neurology. 1998;51(1):48–55. doi: 10.1212/wnl.51.1.48. [DOI] [PubMed] [Google Scholar]
- 53.Air EL, Ghomri YM, Tyagi R, Grande AW, Crone K, Mangano FT. Management of vagal nerve stimulator infections: do they need to be removed? Clinical article. J Neurosurg Pediatr. 2009;3(1):73–8. doi: 10.3171/2008.10.PEDS08294. [DOI] [PubMed] [Google Scholar]
- 54.Kabir SMR, Rajaraman C, Rittey C, Zaki HS, Kemeny AA, McMullan J. Vagus nerve stimulation in children with intractable epilepsy: indications, complications and outcome. Child Nerv Syst. 2009;25(9):1097–100. doi: 10.1007/s00381-009-0849-z. [DOI] [PubMed] [Google Scholar]
- 55.Khurana DS, Reumann M, Hobdell EF, et al. Vagus nerve stimulation in children with refractory epilepsy: unusual complications and relationship to sleep-disordered breathing. Child Nerv Syst. 2007;23(11):1309–12. doi: 10.1007/s00381-007-0404-8. [DOI] [PubMed] [Google Scholar]
- 56.Kotagal P. Neurostimulation: Vagus nerve stimulation and beyond. Semin Pediatr Neurol. 2011;18(3):186–94. doi: 10.1016/j.spen.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 57.Fisher R, Salanova V, Witt T, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51(5):899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- 58.National Institute for Health and Clinical Excellence. Deep brain stimulation for refractory epilepsy IPG416. London: National Institute for Health and Clinical Excellence; 2012. [Google Scholar]
- 59.Fisher RS, Uematsu S, Krauss GL, et al. Placebo-controlled pilot study of centromedian thalamic stimulation in treatment of intractable seizures. Epilepsia. 1992;33(5):841–51. doi: 10.1111/j.1528-1157.1992.tb02192.x. [DOI] [PubMed] [Google Scholar]
- 60.Velasco F, Velasco M, Jimenez F, Velasco AL, Marquez I. Stimulation of the central median thalamic nucleus for epilepsy. Stereot Funct Neurosurg. 2001;77(1–4):228–32. doi: 10.1159/000064611. [DOI] [PubMed] [Google Scholar]
- 61.Morrell MJ. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77(13):1295–304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- 62.Kimiskidis VK. Transcranial magnetic stimulation for drug-resistant epilepsies: rationale and clinical experience. Eur Neurol. 2010;63(4):205–10. doi: 10.1159/000282735. [DOI] [PubMed] [Google Scholar]
- 63.Fregni F, Pascual-Leone A. Technology insight: noninvasive brain stimulation in neurology-perspectives on the therapeutic potential of rTMS and tDCS. Nature Clinical Practice Neurology. 2007 Jul;3(7):383–93. doi: 10.1038/ncpneuro0530. [DOI] [PubMed] [Google Scholar]
- 64.Kelley SA, Hartman AL. Metabolic treatments for intractable epilepsy. Semin Pediatr Neurol. 2011;18(3):179–85. doi: 10.1016/j.spen.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 65.Kossoff EH, Krauss GL, McGrogan JR, Freeman JM. Efficacy of the Atkins diet as therapy for intractable epilepsy. Neurology. 2003;61(12):1789–91. doi: 10.1212/01.wnl.0000098889.35155.72. [DOI] [PubMed] [Google Scholar]
- 66.Greene AE, Todorova MT, Seyfried TN. Perspectives on the metabolic management of epilepsy through dietary reduction of glucose and elevation of ketone bodies. J Neurochemistry. 2003;86(3):529–37. doi: 10.1046/j.1471-4159.2003.01862.x. [DOI] [PubMed] [Google Scholar]
- 67.Lefevre F, Aronson N. Ketogenic diet for the treatment of refractory epilepsy in children: A systematic review of efficacy. Pediatrics. 2000;105(4):E46. doi: 10.1542/peds.105.4.e46. [DOI] [PubMed] [Google Scholar]
- 68.Neal EG, Chaffe H, Schwartz RH, et al. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008;7(6):500–6. doi: 10.1016/S1474-4422(08)70092-9. [DOI] [PubMed] [Google Scholar]
- 69.Neal EG, Chaffe H, Schwartz RH, et al. A randomized trial of classical and medium-chain triglyceride ketogenic diets in the treatment of childhood epilepsy. Epilepsia. 2009;50(5):1109–17. doi: 10.1111/j.1528-1167.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- 70.Pfeifer HH, Lyczkowski DA, Thiele EA. Low glycemic index treatment: implementation and new insights into efficacy. Epilepsia. 2008;49(Suppl 8):42–5. doi: 10.1111/j.1528-1167.2008.01832.x. [DOI] [PubMed] [Google Scholar]
- 71.Pfeifer HH, Thiele EA. Low-glycemic-index treatment: a liberalized ketogenic diet for treatment of intractable epilepsy. Neurology. 2005;65(11):1810–2. doi: 10.1212/01.wnl.0000187071.24292.9e. [DOI] [PubMed] [Google Scholar]
- 72.Kossoff EH, Hedderick EF, Turner Z, Freeman JM. A case-control evaluation of the ketogenic diet versus ACTH for new-onset infantile spasms. Epilepsia. 2008;49(9):1504–9. doi: 10.1111/j.1528-1167.2008.01606.x. [DOI] [PubMed] [Google Scholar]
- 73.Caraballo RH, Cersosimo RO, Sakr D, Cresta A, Escobal N, Fejerman N. Ketogenic diet in patients with Dravet syndrome. Epilepsia. 2005;46(9):1539–44. doi: 10.1111/j.1528-1167.2005.05705.x. [DOI] [PubMed] [Google Scholar]
- 74.Groomes LB, Pyzik PL, Turner Z, Dorward JL, Goode VH, Kossoff EH. Do patients with absence epilepsy respond to ketogenic diets? J Child Neurol. 2011;26(2):160–5. doi: 10.1177/0883073810376443. [DOI] [PubMed] [Google Scholar]
- 75.Wang D, Pascual JM, Yang H, et al. Glut-1 deficiency syndrome: clinical, genetic, and therapeutic aspects. Ann Neurol. 2005;57(1):111–8. doi: 10.1002/ana.20331. [DOI] [PubMed] [Google Scholar]
- 76.Wexler ID, Hemalatha SG, McConnell J, et al. Outcome of pyruvate dehydrogenase deficiency treated with ketogenic diets. Studies in patients with identical mutations. Neurology. 1997;49(6):1655–61. doi: 10.1212/wnl.49.6.1655. [DOI] [PubMed] [Google Scholar]
- 77.Vining EP. Long-term health consequences of epilepsy diet treatments. Epilepsia. 2008;49(Suppl 8):27–9. doi: 10.1111/j.1528-1167.2008.01828.x. [DOI] [PubMed] [Google Scholar]
- 78.Kossoff EH, Zupec-Kania BA, Amark PE, et al. Optimal clinical management of children receiving the ketogenic diet: recommendations of the International Ketogenic Diet Study Group. Epilepsia. 2009;50(2):304–17. doi: 10.1111/j.1528-1167.2008.01765.x. [DOI] [PubMed] [Google Scholar]
- 79.Martinez CC, Pyzik PL, Kossoff EH. Discontinuing the ketogenic diet in seizure-free children: recurrence and risk factors. Epilepsia. 2007;48(1):187–90. doi: 10.1111/j.1528-1167.2006.00911.x. [DOI] [PubMed] [Google Scholar]
- 80.Smith M, Politzer N, Macgarvie D, McAndrews MP, Del Campo M. Efficacy and tolerability of the modified Atkins diet in adults with pharmacoresistant epilepsy: a prospective observational study. Epilepsia. 2011;52(4):775–80. doi: 10.1111/j.1528-1167.2010.02941.x. [DOI] [PubMed] [Google Scholar]
- 81.Siddiqui F, Herial NA, Ali II. Cumulative effect of vagus nerve stimulators on intractable seizures observed over a period of 3 years. Epilepsy Behav. 2010;18(3):299–302. doi: 10.1016/j.yebeh.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 82.Patel A, Pyzik PL, Turner Z, Rubenstein JE, Kossoff EH. Long-term outcomes of children treated with the ketogenic diet in the past. Epilepsia. 2010;51(7):1277–82. doi: 10.1111/j.1528-1167.2009.02488.x. [DOI] [PubMed] [Google Scholar]
- 83.Mastriani KS, Williams VC, Hulsey TC, Wheless JW, Maria BL. Evidence-based versus reported epilepsy management practices. J Child Neurol. 2008;23(5):507–14. doi: 10.1177/0883073807309785. [DOI] [PubMed] [Google Scholar]
- 84.Cunha JM, Carlini EA, Pereira AE, et al. Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology. 1980;21(3):175–85. doi: 10.1159/000137430. [DOI] [PubMed] [Google Scholar]
- 85.Gloss D, Vickrey B. Cannabinoids for epilepsy (Protocol) Cochrane Database of Systematic Reviews. 2011;(8):CD009270. doi: 10.1002/14651858.CD009270.pub2. [DOI] [PubMed] [Google Scholar]
- 86.Ekstein D, Schachter SC. Natural products in epilepsy—the present situation and perspectives for the future. Pharmaceuticals. 2010;3(5):1426–45. doi: 10.3390/ph3051426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schachter SC. Botanicals and herbs: a traditional approach to treating epilepsy. Neurotherapeutics. 2009;6(2):415–20. doi: 10.1016/j.nurt.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pearl PL, Drillings IM, Conry JA. Herbs in epilepsy: evidence for efficacy, toxicity, and interactions. Semin Pediatr Neurol. 2011;18(3):203–8. doi: 10.1016/j.spen.2011.06.007. [DOI] [PubMed] [Google Scholar]