Abstract
Aim
We hypothesize that moderate cardiac-selective overexpression of the angiotensin type 2 receptor (AT2R) would protect the myocardium from ischemic injury after a myocardial infarction (MI) induced by coronary artery ligation.
METHODS AND RESULTS
For the in vitro studies, Ad-G-AT2R-EGFP was used to overexpress AT2R in rat neonatal cardiac myocytes (RNCM). Expression of AT2R, measured by real-time PCR and immunostaining demonstrated efficient transduction of AT2R in a dose-dependent pattern. AT2R constitutively induced apoptosis in RNCM in dose-dependent patterns. For the in vivo studies, 4×1010 vector genome (vg) of rAAV9-CBA-AT2R was injected into the left ventricle chamber of the heart in 5-day-old Sprague-Dawley rats. At six weeks of age, hearts were harvested and expression of AT2R determined by real time PCR and western blotting. Expression was increased one fold over controls and no apoptosis was detected. Two subsequent in vivo studies were performed. In a prevention study 4×1010 vg of rAAV9-CBA-AT2R was injected into the left ventricle chamber of the heart in 5-day-old Sprague-Dawley rats and MI was induced at six week of age. For a post treatment study 4×1010 vg of rAAV9-CBA-AT2R was administrated to the peri-infarcted myocardium area immediately after MI in six week old animals. For both in vivo studies, cardiac functions were assessed using echocardiography and hemodynamic measurements four weeks after coronary artery ligation. In the in vivo studies the MI rats showed significant decreases in fractional shortening and dP/dt with an increased left ventricular end diastolic pressure and a ventricular hypertrophy. For the prevention study, the moderate cardiac-selective overexpression of AT2R attenuated the above MI-induced impairments and also caused a decrease in ventricular wall thinning. In the post treatment study, the overexpression of AT2R partially reversed the MIinduced cardiac dysfunction. MI also induced the up-regulation of AT1R, ACE, and Collagen I mRNA expression, all of which were attenuated by the overexpression of AT2R.
CONCLUSION
Moderate cardiac-selective overexpression of AT2R protects heart function from ischemic injury, which may be mediated, at least in part, through modulation of components of the cardiac RAS and collagen levels in the myocardium.
Keywords: Myocardial infarction, Angiotension type 2 receptor, Apoptosis, Gene Therapy
Introduction
Myocardial infarction (MI) occurs when the blood supply to a part of the heart is interrupted. The resulting ischemia causes irreversible damage to the heart tissue (Williams and Benjamin., 2000). The damage after myocardial infarction results in left ventricular (LV) remodeling, characterized by molecular, cellular and interstitial changes. LV remodeling is manifested as adverse alterations in the size, shape and function of the ventricle, often leading to left ventricular dysfunction, dilated cardiomyopathy and heart failure (Tiyyagura and Pinney., 2006) (Pfeffer and Braunwald., 1990). Evidence shows that the adverse alterations in the heart after MI are associated with a marked increase in cardiovascular morbidity and mortality (Anavekar and Solomon., 2005).
It is well established that components of the renin angiotensin system (RAS) contributes to the progression of myocardial infarction and heart failure. It is well documented that angiotensin II (AngII) plays a critical role in the development of LV remodeling after MI (Anavekar and Solomon., 2005). Ang II has two major receptor subtypes, type 1 (AT1R) and type 2 receptors (AT2R), both of which are expressed in the heart (Ozono, et al., 2000) and play a crucial role in cardiovascular physiology and disease. AT1R signaling contributes to the cardiac remodeling following a MI by mediating vasoconstriction, cardiomyocyte hypertrophy, fibroblast proliferation, and interstitial collagen deposition (Matsubara, 1998) (Weber and Brilla., 1991). AT2R is generally thought to exert an opposing effect to AT1R in the cardiovascular system. Several experimental findings have demonstrated that the AT2R is upregulated under pathological conditions like heart failure (Tsutsumi, et al., 1998) (Regitz-Zagrosek, et al., 1995). It has also been reported that direct stimulation of AT2R improves systolic and diastolic function post-MI (Kaschina, et al., 2008). Pharmacological inhibitors of the RAS have demonstrated significant protection against MI and heart failure in both animals models and in patients (Gerc and Buksa., 2010). Use of angiotensin receptor blockers (ARBs) or ACE inhibitors also has been shown to significantly increase AT2R density in the left ventricle of high-salt diet mice (Le Corvoisier, et al., 2010). It is also suggested that part of protective effects of ARBs are possibly mediated through AT2R (Matsubara, 1998), since unbounded AngII can stimulate AT2R.
We have recently demonstrated that overexpression of Angiotensin-(1–7) can attenuate ischemic induced cardiac pathophysiology (Qi, et al., 2011). Using a coronary ligation rodent model we induced cardiac damage and cardiac remodeling that was significantly attenuated by overexpressing Ang-(1–7) in the myocardium. We also demonstrated that Ang-(1–7) was protective against hypoxia-induced cell death in cardiac myocyte cell culture (Qi, et al., 2011). In these overexpression studies we observed an increase expression of AT2R in the protected cardiomoycte, suggesting that the increase in AT2R may be part of the cardioprotective mechanism. Zisman et al (Zisman, et al., 2003) has previously demonstrated a direct correlation between AT2R expression and Ang-(1–7)-forming activity in the ventricles of patients with pulmonary hypertension. AT2R have also been reported to interact functionally with Ang-(1–7) through the Mas receptor (Castro, et al., 2005) (Gurzu, et al., 2005). We therefore hypothesized that Ang-(1–7) may mediate, at least in part, its cardioprotective effects via an upregulation of the AT2R. Studies by others also suggest that AT2R overexpression in the myocardium may be beneficial. Transgenic mice that overexpress AT2R exhibit improved baseline LV systolic function as well as preservation of systolic function after MI when compared to wild type mice (Yang, et al., 2002). Consistent with this finding, deletion of AT2R in knockout mice cause an enhanced deterioration of heart failure and an increased mortality after MI (Oishi, et al., 2003). However, there are opposing studies that do not support a cardioprotective effect for AT2R overexpression. In contrast, chronic over-expression of AT2R has been reported to depress myocardial contractility in transgenic mice (Nakayama, et al., 2005), and a high level of ventricular-specific expression of AT2R was reported to cause heart failure and dilated cardiomyopathy in transgenic mice compared to a lower level of overexpression of AT2R and in wild type control mice (Yan, et al., 2003).
We have recently demonstrated that utilization of a recombinant adeno associated virus serotype 9 vector (rAAV9) administered in vivo resulted in cardio-selective transduction (Qi, et al., 2009). Therefore in the present study we utilized this viral vector to determine whether a moderate cardiac selective overexpression of AT2R can prevent cardiac dysfunction in a rat MI model, and to evaluate potential cardioprotective mechanism(s) modulated by cardiac overexpression of AT2R.
Materials and Methods
Characterization of Ad-CMV-GFP and Ad-G-AT2R-EGFP for in vitro studies
To evaluate whether different levels of myocardial AT2R overexpression could modulate myocyte function, in vitro studies were conducted. For these in vitro studies, adenoviral vector containing enhanced green fluorescent protein (EGFP) gene controlled by a cytomegalovirus promoter (Ad-CMV-EGFP) and adenoviral vector containing genomic AT2R (G-AT2R) DNA and EGFP gene controlled by cytomegalovirus promoters (Ad-G-AT2R-EGFP) were produced and characterized as detailed previously (Li, et al., 2007). Rat neonatal cardiac myocyte (RNCM) were isolated following the previously reported protocol (Qi, et al., 2009). 4× 106 RNCM were plated into six-well Nunc tissue culture plates. On the following day, RNCM were transduced with Ad-G-AT2R-EGFP or the control vector Ad-CMV-EGFP. Changes in cell morphology were observed using an Olympus BX41 fluorescence microscope. Four different doses of adeno viral vectors (0.5 ifu/cell, 5 ifu/cell, 50 ifu/cell, and 100 ifu/cell) were used. Transduced RNCM were used at different time point after viral transduction depending on the specific protocol. DeadEnd Colorimetric terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling (TUNEL) System was used according to the manufacturer's instructions to measure the extent of apoptosis for in vitro studies (G7130, Promega, WI, USA).
Characterization of rAAV9-GFP and rAAV9-AT2R for in vivo studies
For the in vivo studies, a recombinant adeno-associated virus serotype 9 (rAAV9) containing either EGFP or AT2R driven by a ubiquitous chicken-β-actin promoter (rAAV9-EGFP and rAAV9-AT2R) was produced by the Viral Vector Core at University of Florida. The viral vector production, harvest, purification, and testing were performed as detailed previously (Qi, et al., 2009). We chose a dose of 4×1010 vector genome (vg) to first evaluate if this level of overexpression would result in a significant increase in AT2R without inducing any apoptosis. 5-day-old SD rats were lightly anesthetized with 2% isoflurane (Pittman-Moore, Washington Crossing, NJ, USA). rAAV9- AT2R (4×1010 vg) was injected into the left ventricle chamber of 5-day-old SD rats, and rAAV9 results in a prolonged trangene expression for at least two months post-viral administration as described previously (Qi, et al., 2009). Rats were closely monitored for any discomfort until they fully recovered from anesthesia and returned to their mothers. 6 weeks after viral administration (at the same time that coronary ligation was performed in the in vivo prevention study, see below) the heart was harvested to determine the expression of AT2R and detection of apoptosis using TUNEL assay.
Rat myocardial infarction model
Six-week-old rats were separated into six groups; 1) control, 2) rAAV9-AT2R, 3) rAAV9-GFP, 4) MI, 5) MI+AT2R(PT), and 6) MI+AT2R(P) (N=4–8 animals per group). Myocardial infarction was induced by ligating the left anterior descending coronary artery. Rats were anesthetized with isoflurane (5% in oxygen for five minutes), after which rats were intubated with an 18-gauge intravenous catheter and mechanically ventilated with this isoflurane-oxygen mixture (2.5% in oxygen) using a Harvard ventilator (model 683, Harvard Apparatus, Holliston, Mass). After the chest was cleaned, rats were underwent a left thoracotomy. The thorax was entered via the left fourth and fifth intercostal space, and the pericardium gently torn to expose the heart. The heart was exposed, and ligated at the proximal left anterior descending coronary artery 2–3 mm from its origin between the pulmonary artery conus and the left atrium with a 7–0 polypropylene suture. Successful cessation of blood flow was indicated by elevation of ST segment on electrocardiogram and cyanosis of anterior LV wall. Fluid and air were evacuated from the thorax before closing the site. All of the animals received buprenorphine hydrochloride (Buprenex, 0.03 mg/kg, Reckitt and Colman Pharmaceuticals) and were closely monitored for signs of discomfort. Sham rats underwent the mock surgery. In the present study, the operation-related mortality was approximately 10%.
In vivo studies with moderate AT2R overexpression using rAAV9
Two sets of in vivo studies were performed to overexpress AT2R in the rat myocardium: a prevention study (P) where AT2R overexpression occurred prior to the cardiac insult and a study where the viral vector was administered at the time of coronary artery ligation (PT). In this set of experiments the overexpression of AT2R would occur after the insult as transduction should take place 12–24 hours after injection of the viral vector. All of the animal protocols were approved by the institutional animal care and use committee (IACUC) and conducted according to National Institutes of Health guidelines.
For the prevention study(P), five-day–old male Sprague-Dawley rats under isoflurane anesthesia received a single intra-ventricular injection of 4×1010 vector genome(vg) of rAAV9-GFP or rAAV9-AT2R viral vectors in 30 µL 1× DPBS, as previously described (Qi, et al., 2009). This method of gene transfer by rAAV vector provides a high animal survival rate and has been established to produce efficient, cardiac-selective and long-term transduction in the heart. After viral administration, animals were returned to their mothers until weaning. At 6 weeks of age, rats were subjected to either coronary artery ligation surgery or mock surgery.
For the post treatment study (PT), rAAV9-AT2R (4×1010 vg) in 100 µL 1× DPBS was injected into 3–5 multiple sites of healthy myocardium tissue on the periphery of the infarct area immediately after coronary artery ligation surgery, in six week old Sprague-Dawley rats under isoflurane anesthesia, using a 0.5 ml insulin syringe with a 28 gauge needle.
Echocardiography
Cardiac function was evaluated using a Hewlett Packard Sonos Model 5500 with a 12-Hz transducer at four weeks after coronary artery ligation surgery. Rats were anesthetized with 2.5% isofluorane in oxygen during the assessment. Images were obtained from the parasternal short axis. All measurements were based on the average of three consecutive cardiac cycles. Left ventricle internal diameter at end diastole (LVIDd) and end systole (LVIDs) were obtained. The fractional shortening was calculated according to the formulae: fractional shortening (FS) = [(LVIDd-LVIDs)/LVIDd] × 100
Hemodynamic Measurements
Rats were anesthetized with ketamine/xylazine/acepromazine rodent cocktail (100 mg/20 mg/10mg/kg, i.m.). The rats were placed in supine position and the body temperature was maintained at 37 °C by a heated pad throughout the experiment. Left ventricular function was measured using a pressure catheter (SPR-320, Millar Instruments, Houston, TX, USA), which was inserted into the right carotid artery and advanced into the ascending aorta. After 10 min stabilization, arterial blood pressure was recorded for 10 min. Then, the catheter was advanced into the left ventricle and stabilized for 5 min. The signals were continuously recorded for 10 min by the Powerlab Chart 4 software system (ADInstruments Inc., Colorado Springs, CO, USA). The parameters for cardiac functions include a peak systolic pressure of left ventricle (LVSP), a maximal positive and negative rate of rise in left ventricular pressure (dP/dtmax and dP/dtmin), heart rate (HR), and left ventricular end diastolic pressure (LVEDP).
Histological Analysis
Following the hemodynamic measurement, the hearts were harvested. The ventricles were separated from the atria, rinsed in 1× PBS, weighed, and cut into three thick sections made perpendicular to the long axis. The basal and apex section was snap frozen in liquid nitrogen and stored at −80°C for further analysis. The middle section was used to measure cardiac remodeling. Ventricular hypertrophy was determined by measuring wet weights of rat heart ventricles normalized to tibia length. Cardiac hypertrophy can be more accurately quantified by relative heart weight to tibia length than to body weight, as tibia length is a reliable reference to normalize heart weight in conditions in which body weight changes (Yin, et al., 1982). Cross sections of the ventricles were then fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 4 µm. Ventricular sections were stained with Picro-Sirius Red to measure ventricular wall thickness and degree of fibrosis. Left ventricular (LV) wall thickness and degree of fibrosis were examined using ImageJ program. Quantification of left ventricular wall thickness was carried out by an individual who was blinded to the treatments.
Western Blot
A piece of the left ventricle of rat heart from the peri-infarcted area was homogenized in radioimmuno-precipitation assay buffer (RIPA buffer). Equal amounts of protein (30µg total protein/well) were separated on 12% SDS-polyacrylamide gels and transferred electrophorectically onto a nitrocellulose membrane (Bio-Rad Laboratories, USA). The membranes were blocked with 5% non-fat milk solution in Tris-buffered saline (TBS) with 0.1% Tween 20 (TBS-T) for 2 h and incubated with anti-angiotensin II type 2 receptor (extracellular) antibody (AAR-012, 1:400, Alomone Labs, Israel) overnight at 4 °C. Mouse monoclonal anti-GAPDH antibody (1:10000, G8795, Sigma Aldrich, MO, USA) was used as a loading control. After overnight incubation with the primary antibody, the membranes were washed three times for 30 minutes in TBS-T, and then incubated with secondary antibody conjugated with horseradish peroxidase (anti-rabbit IgG 1:5000 or anti-mouse IgG 1:5000) (NA934V, NA931V, GE HealthCare, UK) for 1 hour. Finally, the membranes were subjected to a chemiluminescence detection system (NEL104001EA, Perkinelmer, USA); and exposed to a photographic film. AT2R and GAPDH levels were quantified by densitometric analysis of X-ray film of western blots using a GS-800 densitometer with Quantity One software (Bio-Rad).
RNA Isolation and Realtime Reverse Transcription-PCR
A piece of ventricular tissue collected from the peri-infarct area of LV free wall of the rat heart was homogenized and total RNA was isolated using RNAqueous- 4 polymerase chain reaction (PCR) kit (Ambion, Foster City, CA, USA) according to the manufacturer's instructions. 200ng RNA was reverse transcribed with iScript cDNA Synthesis kit (Bio-Rad, Hercules, CA, USA). The AT1R, AT2R, Collagen I, Collagen III, ACE2, ACE, Mas, and GAPDH were analyzed by realtime RT-PCR using Taqman probe (Applied Biosystems). Realtime RT-PCR was run using ABI Prism 7000 sequence detection system. All cDNA samples were assayed in triplicate. Data were normalized to GAPDH mRNA.
Statistical Analysis
For in vitro experiments, viral transduction was done in triplicate wells and repeated thrice. Data are expressed as mean ± SD and analyzed using one or two-way ANOVA as appropriate followed by a post hoc test (Bonferroni) to compare individual means. Values of P<0.05 were considered statistically significant.
Results
Overexpression of AT2R in RNCM constitutively induced cell death in a dose-dependent pattern
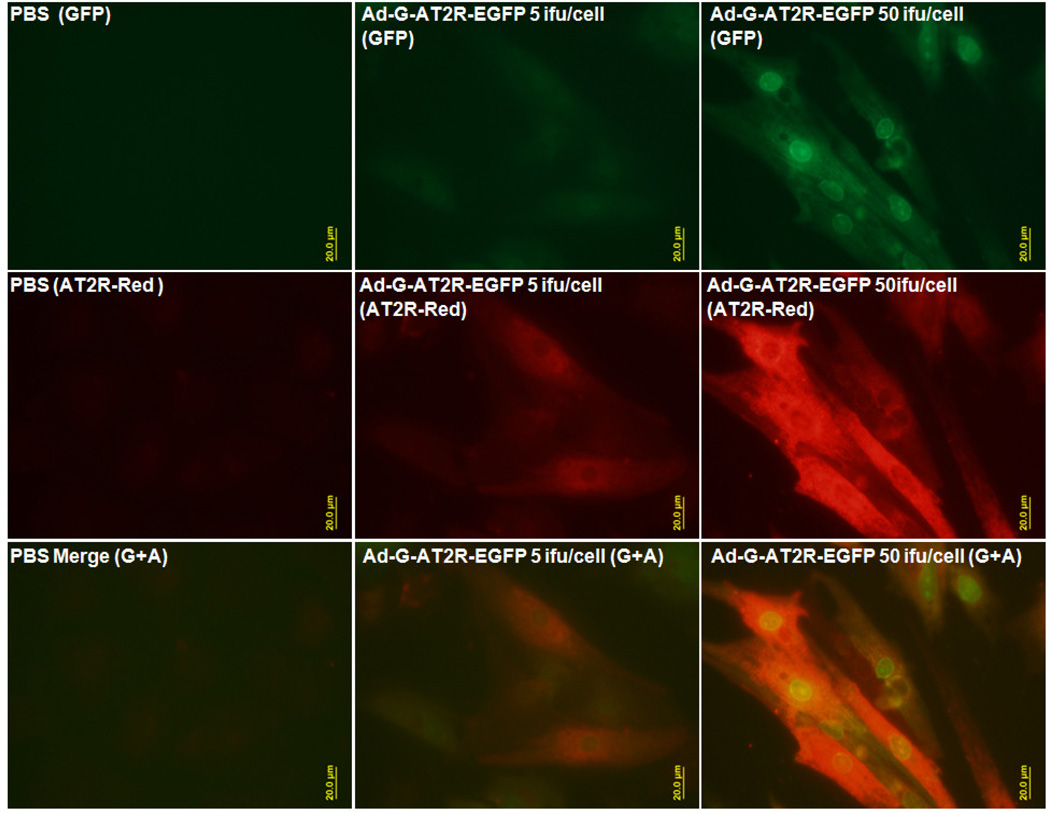
The endogenous level of AT2R in RNCM determined by both immunostaining and realtime RT-PCR (Figure 1- left column and Figure 2d) was detected at very low level. Ad-G-AT2R-EGFP efficiently transduced RNCM and produced a high level of AT2R immune-reactivity and EGFP when compared with PBS control (Figure 1-middle and right columns, Figure2a, 2c, and 2d).
Figure 1.
Basal and adeno viral vector mediated expression of AT2R in RNCM. PBS (left) was used as negative control. RNCM were transduced with either 5 ifu/cell (middle) or 50 ifu/cell (right) of Ad-G-AT2R-EGFP for 48 h. Viral transduction was confirmed by detection of EGFP fluorescence and AT2R immunoreactivity using an anti-AT2R antibody. Representative fluorescence micrographs from Ad-G-AT2R-EGFP transduced cells showing GFP (green fluorescence), AT2R immunostaining (red fluorescence), and merge (GFP+AT2R). Scale bars, 20 µm.
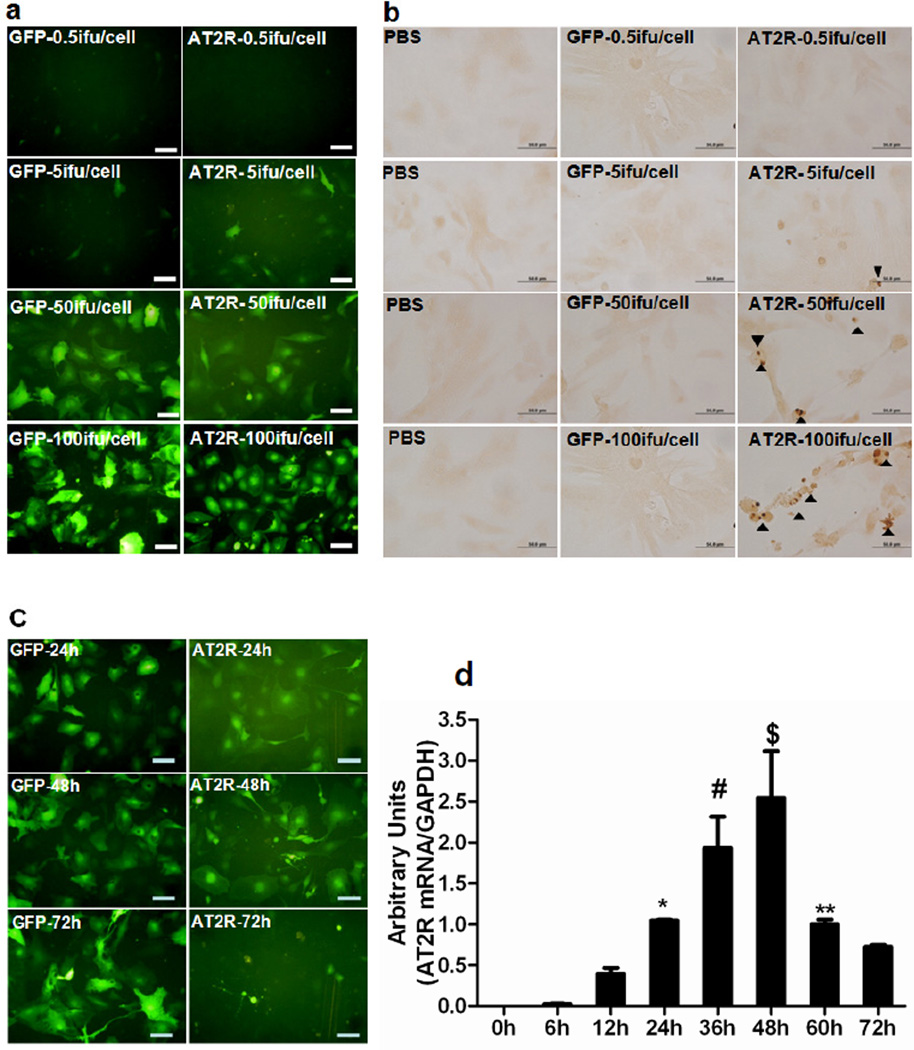
Figure 2.
(a), RNCM were transduced with four different dose of Ad-CMV-EGFP or Ad-G-AT2R-EGFP (0.5 ifu/cell, 5 ifu/cell, 50 ifu/cell, and 100 ifu/cell) for 48 h. PBS was used as negative control and Ad-CMV-EGFP was used as viral control. (b), Apoptosis was detected using the DeadEnd Colorimetric TUNEL System kit. The brown-colored nuclei as indicated by arrow head represent TUNEL-positive (apoptotic) cells. (c), Representative fluorescence micrographs from RNCM transduced with 50 ifu/cell of Ad-CMV-EGFP or Ad-G-AT2R-EGFP. (d), AT2R mRNA level was quantified by realtime RT-PCR. (P<0.05, * 24h vs 0h and 6h; # 36h vs 0h, 6h, 12h, and 72h; $ 48h vs 0h,6h,12h,24h,60h, and 72h;** 60h vs72h.). Scale bars, 50 µm.
RNCM were transduced with four different doses of Ad-CMV-EGFP or Ad-AT2R-EGFP (0.5 ifu/cell, 5 ifu/cell, 50 ifu/cell, and 100 ifu/cell) for 48h (Figure 2a). There was an AT2R dose dependently induced apoptosis observed in cultured RNCM, which was confirmed by the finding that incubating RNCM with Ad-G-AT2R-EGFP for 48h produced a significant increase in TUNEL labeling compared to the RNCM transduced with the same dose of Ad-CMV-EGFP (Figure 2b). RNCM were then transduced with 50 ifu/cell of either Ad-G-AT2R-EGFP or Ad-CMV-EGFP, and monitored over the time after viral transduction. AT2R level peaked at 48h (Figure 2c and d). After 48h, AT2R level decreased over the time as the RNCM expressing AT2R died over this time (Figure 2d).
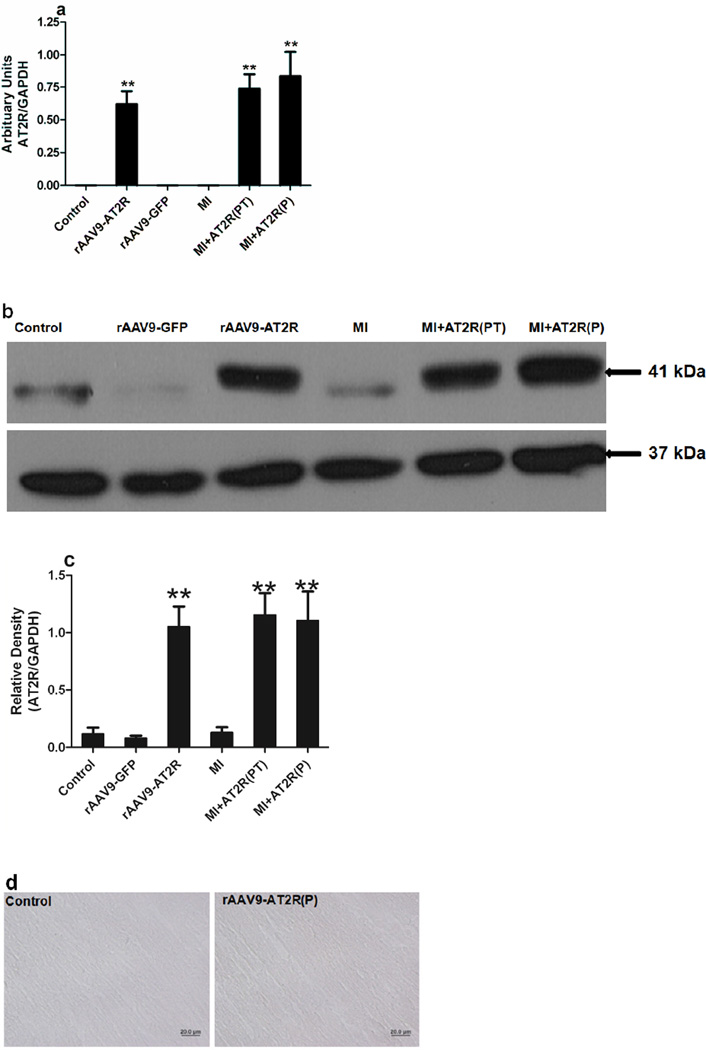
rAAV9 Mediated Cardiac-Selective Overexpression of AT2R in Rat Hearts
rAAV9-AT2R was used to mediate overexpression of AT2R in the rat hearts for two in vivo studies. AT2R level was determined by realtime RT-PCR (Figure 3a) and western blotting (Figure 3b and c). The endogenous level of AT2R in the rat heart was low and rAAV9-AT2R significantly increased AT2R expression in the rat hearts. AT2R mRNA levels in the rat left ventricles was a moderate one, around one fold higher than the controls, for both of the in vivo studies (Figure 3). The level of expression was similar to that observed in RNCM transduced with Ad-G-AT2R-EGFP (50ifu/cell) at 24h (Figure 2d). At this time point, the level of AT2R did not induce apoptosis in vitro. Likewise the in vivo AT2R overexpression mediated by rAAV9-AT2R in the rat hearts did not cause apoptosis, which was confirmed by staining the rat hearts with TUNEL assay kit (Figure 3d) as rats with an increased level of AT2R in the myocardium did not show any positive staining compared to the control group.
Figure 3.
(a) Semi-quantitative Real-Time RT-PCR detection of AT2R mRNA level presented in heart tissues. (b) AT2R in the rat ventricles was detected by western blotting. (c) The relative density of AT2R was determined following densitometric measurements of the protein bands and normalization against the GAPDH signals. (d) Apoptosis was determined in the rAAV9-AT2R transduced rats by DeadEnd Colorimetric TUNEL System kit at 6 weeks after vial administration. Scale bars, 20 µm. (** p<0.05, rAAV9-AT2R, MI+AT2R(PT), and MI+AT2R(P), vs control, MI and rAAV9-GFP)
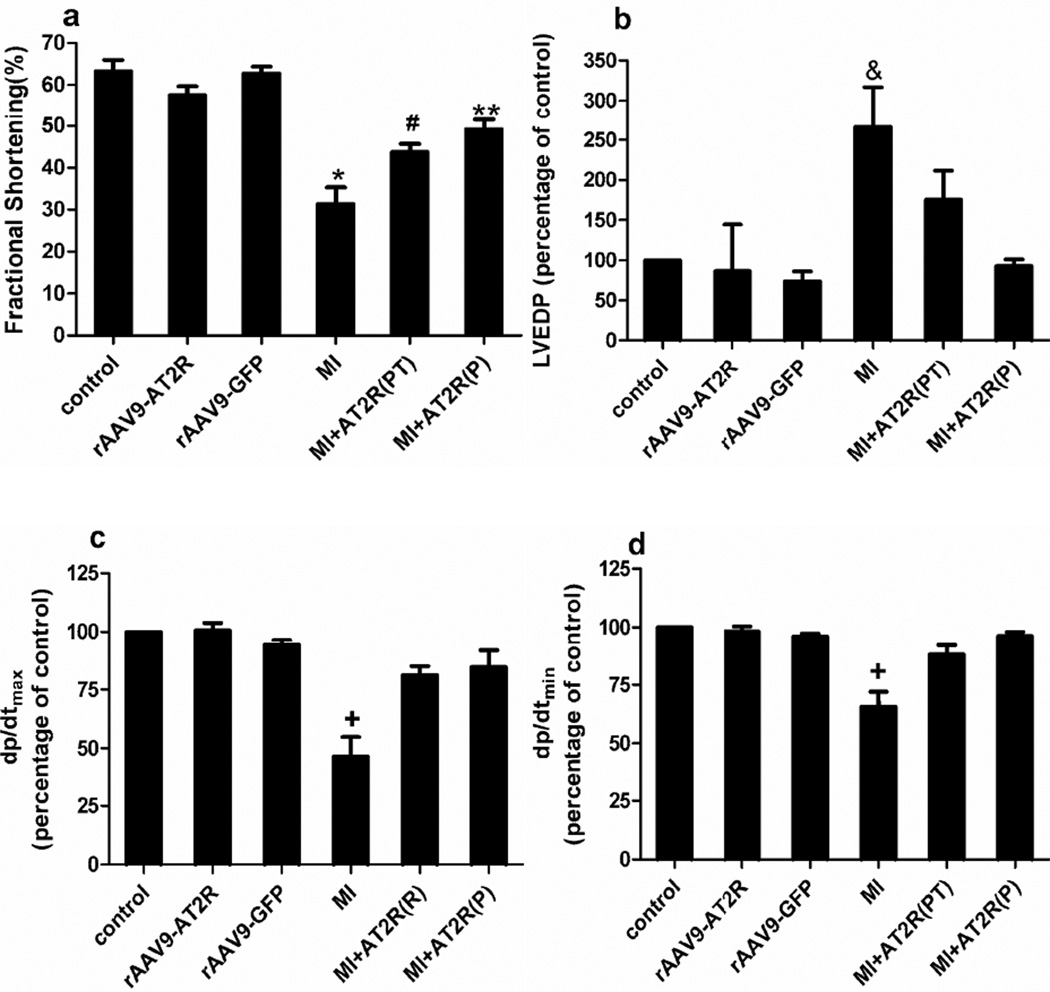
Effects of AT2R Overexpression on Cardiac Function Post-MI
For the in vivo studies, echocardiography and hemodynamic analysis were performed at 4 weeks after myocardial infarction. Myocardial infarction resulted in decreases in fractional shortening, maximum dP/dt, and minimum dP/dt; and an increase in left ventricular end diastolic pressure (Figure 4). In the prevention study, cardiac-selective overexpression of AT2R effectively prevented MI-induced cardiac dysfunction (Figure 4a and b). In the post-treatment study, AT2R overexpression only partially protected the cardiac functions (Figure 4a). Heart rate and left ventricular systolic blood pressure were not significantly different among groups (data not shown).
Figure 4.
Effects of cardiac-selective AT2R overexpression on cardiac functions. (a), Fractional shortening is analyzed using echocardiography. (P<0.05, * : MI vs control, rAAV9-AT2R,rAAV9-GFP, and MI+AT2R(P); #: MI+AT2R(PT) vs control, rAAV9-AT2R, and rAAV9-GFP;**: MI+AT2R(P) vs control, rAAV9-AT2R, and MI). (b), Hemodynamic analysis: % of left ventricle end-diastolic pressure (LVEDP) changes.(P<0.05, &: MI vs control, rAAV9-AT2R, rAAV9-GFP, and MI+AT2R(P). Control LVEDP was 4.0 ± 1.5 mmHg. (c) and (d), % of +dP/dt and –dP/dt (maximal and minimal peak rate of left ventricular pressure). (P<0.05, +: MI vs all other groups). Control value for dP/dtmax and dP/dtmin were in the 8000 and 5000mmHg ranges, respectively.
Effects of AT2R Overexpression on Post-MI Ventricular Remodeling
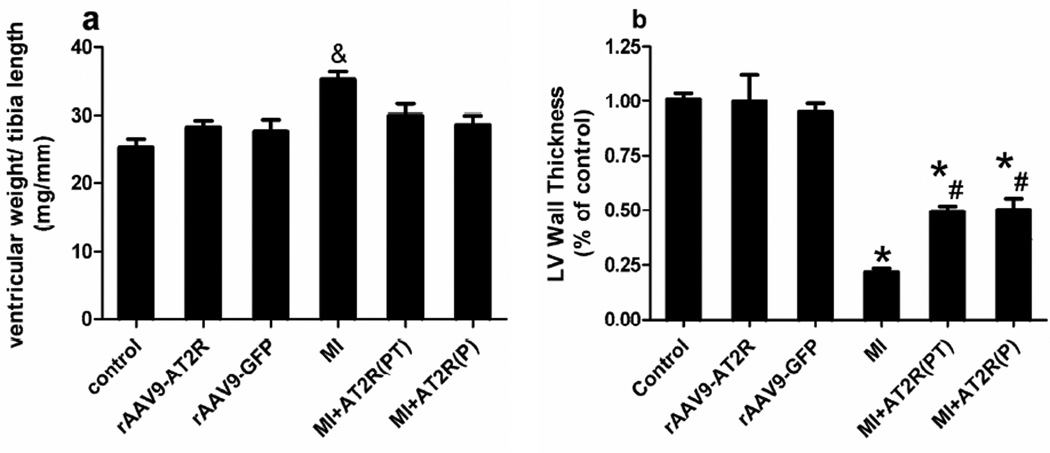
Moderate cardiac-selective overexpression of AT2R attenuated MI-induced cardiac hypertrophy evaluated by ventricular weight to tibia length ratio in both in vivo studies (Figure 5a). MI induced significantly decrease in the left ventricular wall thickness, which was attenuated by AT2R overexpression (Figure 5b).
Figure 5.
Effects of moderate cardiac-selective overexpression of AT2R on ventricular remodeling. (a), Cardiac hypertrophy was evaluated by the ratio of ventricular weight (g) to tibia length (cm) (P<0.05, &: MI vs all other groups). (b), Left ventricular wall thickness was quantified by the percentage of control. (P<0.05, * MI, MI+AT2R(PT), and MI+ AT2R(P) vs control, rAAV9-GFP, and rAAV9-AT2; # MI+rAAV9-AT2R(PT) and MI+rAAV9-AT2R(P) vs MI).
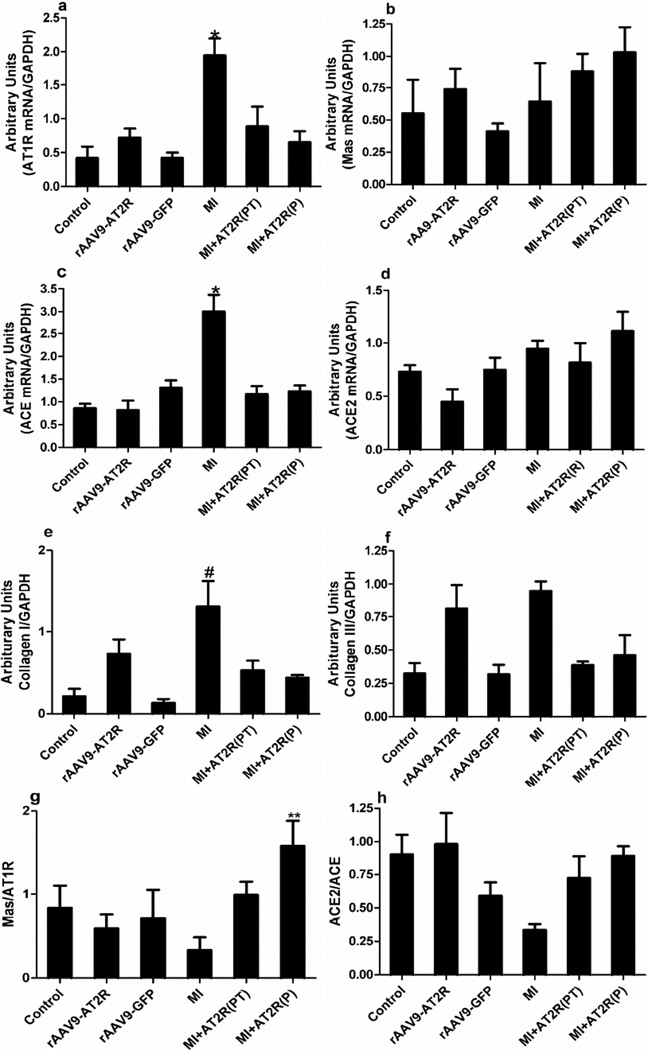
AT2R-mediated Protective Mechanism Post-MI
Genes involved in the RAS and in cardiac remodeling were measured in an attempt to determine possible cardioprotective mechanism(s) of AT2R overexpression. As summarized in figure 6, there was a significant increase in levels of some components of the ACE-AngII-AT1R axis, e.g. upregulation of AT1R (a) and ACE(c) in the MI group. Additionally, Collagen I was upregulated in the MI group. Overexpression of AT2R was able to attenuate these changes in both prevention and post-treatment studies. There was no effect of any of the treatments on mRNA of the Mas receptor (b). AT2R overexpression significantly increased the Mas/AT1 receptor ratio (g) in the prevention study and tended to prevent the suggested decrease in the ACE2/ACE ratio observed in the MI group (h).
Figure 6.
Effects of cardiac-selective overexpression of AT2R on mRNA levels of the RAS, collagen I, and collagen III. Realtime RT- PCR was used to measure (a) AT1 receptor, (b) Mas receptor, (c) ACE, (d) ACE2, (e) Collagen I, and (f) Collagen III levels in the left ventricles of hearts. AT2R increased the ratio of heart (g) Mas/AT1R and (h) ACE2/ACE mRNA levels. Data are expressed as mean ± SD. (P<0.05, * MI versus all other groups, # MI vs control, rAAV9-GFP, and MI+AT2R(P); ** MI+AT2R(P) vs all other groups.)
Discussion
The present study examined the in vitro effects of different levels of AT2R on the viability of RNCM and the in vivo effects of moderate AT2R overexpression on the cardiac function and ventricular remodeling in a rat myocardial infarction model. In vitro experiments demonstrated that AT2R induced apoptosis in a dose-dependent pattern. Apoptosis was observed only when the level of AT2R reached certain threshold whereas only a moderate increase in the level of AT2R did not cause cell death. For the in vivo prevention studies, this moderate cardiac-selective overexpression of AT2R prevented left ventricular dysfunction after myocardial infarction as shown by preserving the fractional shortening, dP/dt, and left ventricular end diastolic pressure, and attenuating the ventricular hypertrophy and wall thinning. For the in vivo post treatment studies, moderate cardiac-selective overexpression of AT2R provided partial protection against the ischemic injury.
The level of AT2R appears to modulate the effects of AT2R on the different cells, whether it is anti-proliferation, anti-hypertrophy, or even the opposite effect-hypertrophy. AT2R has been shown to inhibit cells growth and proliferation in coronary endothelial cells (Stoll, et al., 1995), cardiac fibroblasts (van Kesteren, et al., 1997), neonatal cardiomyocytes (van Kesteren, et al., 1997), adult ventricular cardiomyocytes (Booz and Baker., 1996), and vascular smooth muscle cells (Yamada, et al., 1998). Increased level of AT2R constitutively induces apoptosis in prostate cancer cell lines (Li, et al., 2009) and vascular smooth muscle cells (Nakajima, et al., 1995). On the contrary, increased level of the AT2R resulted in ligand-independent, constitutive cardiomyocyte hypertrophy (D'Amore, Black and Thomas., 2005). Yan (Yan, et al., 2003) suggested that high level of AT2R caused LV dysfunction and myocyte apoptosis compared to low level of AT2R and wild-type control. In the present study, high level of AT2R constitutively induced apoptosis in RNCM, which is consistent with Yan’s report (Yan, et al., 2003). Moderate and low level of AT2R did not result in apoptosis on cardiac myocytes or the intact rat hearts, and therefore we targeted this level of expression in the MI studies.
The level of AT2R in the adult is relatively low and limited to certain organs; however, the adult organism retains its ability to re-express AT2R without any age limit. AT2R is re-expressed and/or upregulated under pathophysiological conditions such as in patients with ischemic heart disease, heart failure, and dilated cardiomyopathy (Nio, et al., 1995) (Wharton, et al., 1998) (Haywood, et al., 1997) and stroke (Zhu, et al., 2000). It suggests that AT2R is upregulated in the tissues following injury or insult to somehow reduce and/or repair the injured tissue. It is conceivable that the endogenous upregulation of AT2R may not be adequate to exert any significant beneficial effects, or may only be transient in nature and thus not able to restore normal function. There are several reports suggesting that AT2R protects cardiac functions, or mediates part of the protective effects of AT1R antagonists (Yang, et al., 2002) (Matsubara, 1998) (Oishi, et al., 2003) (Oishi, et al., 2006). Yet, other investigators have reported that transgenic overexpression of AT2R in cardiomyocytes in vivo results in enhanced hypertrophy and dilated cardiomyopathy (Yan, et al., 2003). Many of these latter studies are performed in either AT2R knockout animals or transgenic animals that overexpress AT2R. In these cases the AT2R has already been increased or knocked out at birth and before myocardial infarction. Since the AT2R has been implicated in the developmental processes (Yu, et al., 2010), this early overexpression or knockout may lead to yet to be determined compensatory changes that could cloud the interpretation of the findings. The current study used recombinant adeno associated viral vector serotype 9 (rAAV9) to deliver AT2R into the heart. Our group (Qi, et al., 2009) has reported that administration of rAAV9 into rat heart mediates efficient and cardiac-selective expression of transgene in the rats. rAAV9 mediated overexpression of AT2R may avoid the compensatory changes, control the level of AT2R, and may be a better tool to investigate the roles of AT2R in cardiac functions after ischemic injury.
It is intriguing to investigate whether the AT2R mediated cardioprotective effects are mediated through preventing the adverse remodeling process or can be beneficial if the overexpression occurs post injury. Though we did not assess the time course for incorporating the transgene for the ‘post-treatment’ study, previous reports suggest that transgene expression can occur as early as one day after the direct intra-cardiac injection (Su, et al., 2006), and as long as one year after the intra-cardiac injection by a trans-diaphragmatic approach (Woo, et al., 2005). Collectively the results suggested that an overexpression of AT2R in the heart, shortly after birth or immediately after the cardiac ischemic insult, does provide beneficial effects on cardiac function and structure. AT2R more efficiently protected the cardiac functions when AT2R was overexpressed before the ischemic injury than after, although we cannot say that the degree of overexpression was necessarily the same even though we used a similar viral titer. Additionally the timing of the overexpression of the transgene in relationship to the cardiac insult may be important in providing optimal cardioprotective effects of AT2R. Also we were not able to determine if in the ‘prevention’ study whether overexpression of AT2R would actually protect the cells in the immediate area of infarct, to reduce the original extent of the primary injury and thus resulting in a greater beneficial effect than overexpressing tissue adjacent to the injury after the insult.
Angiotensin receptor blockers (ARBs) reduce cardiovascular mortality and morbidity in patients with heart failure after MI (Jugdutt and Menon., 2004). Selectively blockade of AT1R with ARBs results in an elevation of the levels of circulating AngII which can then stimulate the unopposed AT2R. Thus, it is hypothesized that the beneficial effects of ARBs may be mediated, at least in part, through AT2R activation. There are several literature reports that suggest the concept that AT2R exerts its protective effects on the heart post MI through interacting with ACE2-Ang-(1–7)-Mas axis. Administration of ARBs is associated with an upregulation of cardiac ACE2 and Ang-(1–7) levels (Ishiyama, et al., 2004) (Trask, et al., 2007). Both ACE2 and Ang-(1–7) have been reported to provide cardioprotective effects against heart failure and cardiac hypertrophy. Cardiac overexpression of ACE2 or Ang-(1–7) mediated by Lenti-viral vector preserved cardiac functions and attenuated left ventricular wall thinning following myocardial infarction(Der Sarkissian, et al., 2008) (Qi, et al., 2011). Whereas, chronic Ang-(1–7) treatment not only attenuated the development of heart failure in the MI model (Ishiyama, et al., 2004) but also prevented cardiac hypertrophy and fibrosis in rats (Iwata, et al., 2005) (Wang, et al., 2005) (Grobe, et al., 2007). It has also been demonstrated that ACE2 activity or Ang-(1–7) forming activity directly correlated with AT2R density (Zisman, et al., 2003). In addition, ARB (irbesartan) or ACEi (ramipril) treatment significantly increased AT2R density in the left ventricle of high-salt diet mice, and the ACEi induced change was more pronounced than that of the ARB (Le Corvoisier, et al., 2010). Taken together, it is hypothesized that AT2R exerts its protective effects on the heart post MI through interacting with ACE2-Ang-(1–7)-Mas axis. In the current study, overexpression of AT2R attenuated the MI-induced upregulation of AT1R, ACE, collagen I, and collagen III in both the prevention and post treatment studies. Also, in the prevention study, overexpression of AT2R also significantly increased the Mas/AT1R ratio. These results would support the hypothesis that AT2R may exerts its protective effects on the heart following MI possibly through antagonizing ACE-AngII-AT1R and stimulating the ACE2-Ang-(1–7)-mas axis. It has also been suggested that Ang-(1–7) may act through both AT2R and Mas receptors, as both AT2R antagonist (PD123319) and Mas receptor antagonist (A779) abrogated the Ang (1–7)–evoked vasoprotection and atheroprotection and the reciprocal changes in eNOS and superoxide in a model of atherosclerosis (Tesanovic, et al., 2010). Thus, it is conceivable that Ang-(1–7) may be the ligand for the AT2R. Further experimentation would be required to test this hypothesis.
The RAS plays a role in modulating the balance between collagen production and degradation. AngII has been shown to induce the accumulation of cardiac collagen through increased collagen synthesis and decrease collagen degradation by matrix metalloproteinases (MMPs) (Booz, Dostal and Baker., 1999)(Booz, Dostal and Baker., 1999). mRNA for both ACE and AT1R are elevated in hearts of the untreated MI animals, suggesting a role of this arm of the RAS in the pathogenesis of heart failure. This arm of the RAS was basically normalized with AT2R treatment. AT1R and AT2R modulate opposite effects on the collagen degradation. AT1R activation inhibits collagen degradation by inhibiting MMP-2 and MMP-9 activity (Stacy, et al., 2007). On the contrary, AT2R promotes collagen degradation by increasing the activity of MMP-2 and MMP-9 (Stacy, et al., 2007) and decreased the level of tissue inhibitor of metalloproteinases (TIMPs) (Jiang, et al., 2007). The results from the current study findings support these actions of the angiotensin receptors on collagen. Moderate cardiac-selective overexpression of AT2R attenuated the MI-induced increase in the level of collagen I.
In summary, our data demonstrate that moderate cardiac-selective overexpression of AT2R protects cardiac function and attenuates cardiac remodeling post myocardial infarction. These beneficial effects involve restoration of the RAS balance and prevention of the upregulation of fibrotic factors (Collagen I and Collagen III). Collectively, these results suggest that targeting of the AT2R may provide a novel therapeutic strategy in the treatment of myocardial infarction and its associated complications.
References
- Anavekar NS, Solomon SD. Angiotensin II receptor blockade and ventricular remodelling. J.Renin Angiotensin Aldosterone Syst. 2005;6:43–48. doi: 10.3317/jraas.2005.006. [DOI] [PubMed] [Google Scholar]
- Booz GW, Baker KM. Role of type 1 and type 2 angiotensin receptors in angiotensin II-induced cardiomyocyte hypertrophy. Hypertension. 1996;28:635–640. doi: 10.1161/01.hyp.28.4.635. [DOI] [PubMed] [Google Scholar]
- Booz GW, Dostal DE, Baker KM. Paracrine actions of cardiac fibroblasts on cardiomyocytes: implications for the cardiac renin-angiotensin system. Am.J.Cardiol. 1999;83:44H–47H. doi: 10.1016/s0002-9149(99)00257-x. [DOI] [PubMed] [Google Scholar]
- Castro CH, Santos RA, Ferreira AJ, Bader M, Alenina N, Almeida AP. Evidence for a functional interaction of the angiotensin-(1–7) receptor Mas with AT1 and AT2 receptors in the mouse heart. Hypertension. 2005;46:937–942. doi: 10.1161/01.HYP.0000175813.04375.8a. [DOI] [PubMed] [Google Scholar]
- D'Amore A, Black MJ, Thomas WG. The angiotensin II type 2 receptor causes constitutive growth of cardiomyocytes and does not antagonize angiotensin II type 1 receptormediated hypertrophy. Hypertension. 2005;46:1347–1354. doi: 10.1161/01.HYP.0000193504.51489.cf. [DOI] [PubMed] [Google Scholar]
- Der Sarkissian S, Grobe JL, Yuan L, et al. Cardiac overexpression of angiotensin converting enzyme 2 protects the heart from ischemia-induced pathophysiology. Hypertension. 2008;51:712–718. doi: 10.1161/HYPERTENSIONAHA.107.100693. [DOI] [PubMed] [Google Scholar]
- Gerc V, Buksa M. Advantages of renin-angiotensin system blockade in the treatment of cardiovascular diseases. Med.Arh. 2010;64:295–299. [PubMed] [Google Scholar]
- Grobe JL, Der Sarkissian S, Stewart JM, Meszaros JG, Raizada MK, Katovich MJ. ACE2 overexpression inhibits hypoxia-induced collagen production by cardiac fibroblasts. Clin.Sci.(Lond) 2007;113:357–364. doi: 10.1042/CS20070160. [DOI] [PubMed] [Google Scholar]
- Gurzu B, Costuleanu M, Slatineanu SM, Ciobanu A, Petrescu G. Are multiple angiotensin receptor types involved in angiotensin (1–7) actions on isolated rat portal vein. J.Renin Angiotensin Aldosterone Syst. 2005;6:90–95. doi: 10.3317/jraas.2005.015. [DOI] [PubMed] [Google Scholar]
- Haywood GA, Gullestad L, Katsuya T, et al. AT1 and AT2 angiotensin receptor gene expression in human heart failure. Circulation. 1997;95:1201–1206. doi: 10.1161/01.cir.95.5.1201. [DOI] [PubMed] [Google Scholar]
- Ishiyama Y, Gallagher PE, Averill DB, Tallant EA, Brosnihan KB, Ferrario CM. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43:970–976. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- Iwata M, Cowling RT, Gurantz D, et al. Angiotensin-(1–7) binds to specific receptors on cardiac fibroblasts to initiate antifibrotic and antitrophic effects. Am.J.Physiol.Heart Circ.Physiol. 2005;289:H2356–H2363. doi: 10.1152/ajpheart.00317.2005. [DOI] [PubMed] [Google Scholar]
- Jiang XY, Gao GD, Du XJ, Zhou J, Wang XF, Lin YX. The signalling of AT2 and the influence on the collagen metabolism of AT2 receptor in adult rat cardiac fibroblasts. Acta Cardiol. 2007;62:429–438. doi: 10.2143/AC.62.5.2023404. [DOI] [PubMed] [Google Scholar]
- Jugdutt BI, Menon V. AT2 receptor and apoptosis during AT1 receptor blockade in reperfused myocardial infarction in the rat. Mol.Cell.Biochem. 2004;262:203–214. doi: 10.1023/b:mcbi.0000038236.59905.8b. [DOI] [PubMed] [Google Scholar]
- Kaschina E, Grzesiak A, Li J, et al. Angiotensin II type 2 receptor stimulation: a novel option of therapeutic interference with the renin-angiotensin system in myocardial infarction? Circulation. 2008;118:2523–2532. doi: 10.1161/CIRCULATIONAHA.108.784868. [DOI] [PubMed] [Google Scholar]
- Le Corvoisier P, Adamy C, Sambin L, et al. The cardiac renin-angiotensin system is responsible for high-salt diet-induced left ventricular hypertrophy in mice. Eur.J.Heart Fail. 2010;12:1171–1178. doi: 10.1093/eurjhf/hfq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Gao Y, Grobe JL, Raizada MK, Katovich MJ, Sumners C. Potentiation of the antihypertensive action of losartan by peripheral overexpression of the ANG II type 2 receptor. Am.J.Physiol.Heart Circ.Physiol. 2007;292:H727–H735. doi: 10.1152/ajpheart.00938.2006. [DOI] [PubMed] [Google Scholar]
- Li H, Qi Y, Li C, et al. Angiotensin type 2 receptor-mediated apoptosis of human prostate cancer cells. Mol.Cancer.Ther. 2009;8:3255–3265. doi: 10.1158/1535-7163.MCT-09-0237. [DOI] [PubMed] [Google Scholar]
- Matsubara H. Pathophysiological role of angiotensin II type 2 receptor in cardiovascular and renal diseases. Circ.Res. 1998;83:1182–1191. doi: 10.1161/01.res.83.12.1182. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Hutchinson HG, Fujinaga M, et al. The angiotensin II type 2 (AT2) receptor antagonizes the growth effects of the AT1 receptor: gain-of-function study using gene transfer. Proc.Natl.Acad.Sci.U.S.A. 1995;92:10663–10667. doi: 10.1073/pnas.92.23.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M, Yan X, Price RL, et al. Chronic ventricular myocyte-specific overexpression of angiotensin II type 2 receptor results in intrinsic myocyte contractile dysfunction. Am.J.Physiol.Heart Circ.Physiol. 2005;288:H317–H327. doi: 10.1152/ajpheart.00957.2003. [DOI] [PubMed] [Google Scholar]
- Nio Y, Matsubara H, Murasawa S, Kanasaki M, Inada M. Regulation of gene transcription of angiotensin II receptor subtypes in myocardial infarction. J.Clin.Invest. 1995;95:46–54. doi: 10.1172/JCI117675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi Y, Ozono R, Yano Y, et al. Cardioprotective role of AT2 receptor in postinfarction left ventricular remodeling. Hypertension. 2003;41:814–818. doi: 10.1161/01.HYP.0000048340.53100.43. [DOI] [PubMed] [Google Scholar]
- Oishi Y, Ozono R, Yoshizumi M, Akishita M, Horiuchi M, Oshima T. AT2 receptor mediates the cardioprotective effects of AT1 receptor antagonist in post-myocardial infarction remodeling. Life Sci. 2006;80:82–88. doi: 10.1016/j.lfs.2006.08.033. [DOI] [PubMed] [Google Scholar]
- Ozono R, Matsumoto T, Shingu T, et al. Expression and localization of angiotensin subtype receptor proteins in the hypertensive rat heart. Am.J.Physiol.Regul.Integr.Comp.Physiol. 2000;278:R781–R789. doi: 10.1152/ajpregu.2000.278.3.R781. [DOI] [PubMed] [Google Scholar]
- Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- Qi Y, Liu X, Li H, et al. Selective tropism of the recombinant adeno-associated virus 9 serotype for rat cardiac tissue. J.Gene Med. 2009 doi: 10.1002/jgm.1404. [DOI] [PubMed] [Google Scholar]
- Qi Y, Shenoy V, Wong F, et al. Lentiviral mediated overexpression of Angiotensin-(1–7) attenuated ischemia-induced cardiac pathophysiology. Exp.Physiol. 2011 doi: 10.1113/expphysiol.2011.056994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regitz-Zagrosek V, Friedel N, Heymann A, et al. Regulation, chamber localization, and subtype distribution of angiotensin II receptors in human hearts. Circulation. 1995;91:1461–1471. doi: 10.1161/01.cir.91.5.1461. [DOI] [PubMed] [Google Scholar]
- Stacy LB, Yu Q, Horak K, Larson DF. Effect of angiotensin II on primary cardiac fibroblast matrix metalloproteinase activities. Perfusion. 2007;22:51–55. doi: 10.1177/0267659106074793. [DOI] [PubMed] [Google Scholar]
- Stoll M, Steckelings UM, Paul M, Bottari SP, Metzger R, Unger T. The angiotensin AT2-receptor mediates inhibition of cell proliferation in coronary endothelial cells. J.Clin.Invest. 1995;95:651–657. doi: 10.1172/JCI117710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Huang Y, Takagawa J, et al. AAV serotype-1 mediates early onset of gene expression in mouse hearts and results in better therapeutic effect. Gene Ther. 2006;13:1495–1502. doi: 10.1038/sj.gt.3302787. [DOI] [PubMed] [Google Scholar]
- Tesanovic S, Vinh A, Gaspari TA, Casley D, Widdop RE. Vasoprotective and atheroprotective effects of angiotensin (1–7) in apolipoprotein E-deficient mice. Arterioscler.Thromb.Vasc.Biol. 2010;30:1606–1613. doi: 10.1161/ATVBAHA.110.204453. [DOI] [PubMed] [Google Scholar]
- Tiyyagura SR, Pinney SP. Left ventricular remodeling after myocardial infarction: past, present, and future. Mt.Sinai J.Med. 2006;73:840–851. [PubMed] [Google Scholar]
- Trask AJ, Averill DB, Ganten D, Chappell MC, Ferrario CM. Primary role of angiotensin-converting enzyme-2 in cardiac production of angiotensin-(1–7) in transgenic Ren-2 hypertensive rats. Am.J.Physiol.Heart Circ.Physiol. 2007;292:H3019–H3024. doi: 10.1152/ajpheart.01198.2006. [DOI] [PubMed] [Google Scholar]
- Tsutsumi Y, Matsubara H, Ohkubo N, et al. Angiotensin II type 2 receptor is upregulated in human heart with interstitial fibrosis, and cardiac fibroblasts are the major cell type for its expression. Circ.Res. 1998;83:1035–1046. doi: 10.1161/01.res.83.10.1035. [DOI] [PubMed] [Google Scholar]
- van Kesteren CA, van Heugten HA, Lamers JM, Saxena PR, Schalekamp MA, Danser AH. Angiotensin II-mediated growth and antigrowth effects in cultured neonatal rat cardiac myocytes and fibroblasts. J.Mol.Cell.Cardiol. 1997;29:2147–2157. doi: 10.1006/jmcc.1997.0448. [DOI] [PubMed] [Google Scholar]
- Wang LJ, He JG, Ma H, et al. Chronic administration of angiotensin-(1–7) attenuates pressure-overload left ventricular hypertrophy and fibrosis in rats. Di Yi Jun Yi Da Xue Xue Bao. 2005;25:481–487. [PubMed] [Google Scholar]
- Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation. 1991;83:1849–1865. doi: 10.1161/01.cir.83.6.1849. [DOI] [PubMed] [Google Scholar]
- Wharton J, Morgan K, Rutherford RA, et al. Differential distribution of angiotensin AT2 receptors in the normal and failing human heart. J.Pharmacol.Exp.Ther. 1998;284:323–336. [PubMed] [Google Scholar]
- Williams RS, Benjamin IJ. Protective responses in the ischemic myocardium. J.Clin.Invest. 2000;106:813–818. doi: 10.1172/JCI11205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo YJ, Zhang JC, Taylor MD, Cohen JE, Hsu VM, Sweeney HL. One year transgene expression with adeno-associated virus cardiac gene transfer. Int.J.Cardiol. 2005;100:421–426. doi: 10.1016/j.ijcard.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Yamada T, Akishita M, Pollman MJ, Gibbons GH, Dzau VJ, Horiuchi M. Angiotensin II type 2 receptor mediates vascular smooth muscle cell apoptosis and antagonizes angiotensin II type 1 receptor action: an in vitro gene transfer study. Life Sci. 1998;63:PL289–PL295. doi: 10.1016/s0024-3205(98)00448-2. [DOI] [PubMed] [Google Scholar]
- Yan X, Price RL, Nakayama M, et al. Ventricular-specific expression of angiotensin II type 2 receptors causes dilated cardiomyopathy and heart failure in transgenic mice. Am.J.Physiol.Heart Circ.Physiol. 2003;285:H2179–H2187. doi: 10.1152/ajpheart.00361.2003. [DOI] [PubMed] [Google Scholar]
- Yang Z, Bove CM, French BA, et al. Angiotensin II type 2 receptor overexpression preserves left ventricular function after myocardial infarction. Circulation. 2002;106:106–111. doi: 10.1161/01.cir.0000020014.14176.6d. [DOI] [PubMed] [Google Scholar]
- Yin FC, Spurgeon HA, Rakusan K, Weisfeldt ML, Lakatta EG. Use of tibial length to quantify cardiac hypertrophy: application in the aging rat. Am.J.Physiol. 1982;243:H941–H947. doi: 10.1152/ajpheart.1982.243.6.H941. [DOI] [PubMed] [Google Scholar]
- Yu L, Zheng M, Wang W, Rozanski GJ, Zucker IH, Gao L. Developmental changes in AT1 and AT2 receptor-protein expression in rats. J.Renin Angiotensin Aldosterone Syst. 2010 doi: 10.1177/1470320310379065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YZ, Chimon GN, Zhu YC, et al. Expression of angiotensin II AT2 receptor in the acute phase of stroke in rats. Neuroreport. 2000;11:1191–1194. doi: 10.1097/00001756-200004270-00009. [DOI] [PubMed] [Google Scholar]
- Zisman LS, Keller RS, Weaver B, et al. Increased angiotensin-(1–7)-forming activity in failing human heart ventricles: evidence for upregulation of the angiotensin-converting enzyme Homologue ACE2. Circulation. 2003;108:1707–1712. doi: 10.1161/01.CIR.0000094734.67990.99. [DOI] [PubMed] [Google Scholar]