Abstract
Burst fractures in acute spinal traumas are a difficult problem to solve. Different approaches and techniques have been utilized, but with high incidence of morbidity and mortality, besides unsatisfactory clinical and radiological results. Mini-open approaches recently emerged and have been shown to be safe and effective in the treatment of several spinal conditions. Here we report a case of acute lumbar burst fracture at L2 treated by minimally invasive true lateral approach posteriorly instrumented with percutaneous pedicle screws. The minimum disruptive access in addition to a rigid construction allowed a lumbar corpectomy without the morbidity of standard open approaches, lowering surgery costs and accelerating the patient recovery with successfully clinical and radiological results.
1. Introduction
The treatment of burst fractures in acute spinal traumas represents a complex decision-making process [1]. Some considerations must be analyzed in order to decide which way to proceed. Posterior-only approaches are widely utilized, but failure to maintain the sagittal plane correction has been observed [2]. However, when the main goal is decompression of the spinal canal and stabilization of the segment, the anterior approach should be the technique of choice [3].
This technique provides direct decompression of the neural structures, providing an appropriate anterior support and load sharing with the use of a vertebral body replacement device [4]. However, excessive blood loss, damage of the abdominal wall, permanent injuries in diaphragm, and incisional pain are related to the anterior approach [5].
Recently, mini-open approaches to the thoracolumbar spine have been shown to be safe and effective in the treatment of several spinal conditions including vertebral fractures, with minimum blood loss, muscle splitting, and pain [6]. Here we report a mini-open true lateral transpsoas approach for lumbar corpectomy supplemented with percutaneous pedicle screws in the treatment of an acute lumbar burst fracture.
2. Case Report
HK, 55-year-old male, was involved in a fall from 3 meters height. The patient complained of immediate back pain with some irradiation numbness and weakness to the right leg. He was initially admitted in a countryside hospital and further transferred to São Paulo, SP, Brazil, to be followed up by our group.
Initial physical examinations showed tenderness on palpation of the back at the level of L2 spinal process. The patient had anterior tight numbness and weakness in knee extension, with motor strength grade 4, an ASIA motor score of 98 (normal = 100), sensitive score of 110 (normal = 112), and D in Frankel scale. Moreover, the patient was conscious and has no additional injuries.
Initial anteroposterior and lateral X-rays evidenced a burst fracture of L2 vertebra with an increased distance between the pedicles, a loss of 32% of the vertebral heights, and increased local kyphosis in 11.7°, besides a 4.4 mm retrolisthesis. Cross-sectional CT scan showed an intracanal fragment that filled around 80% of the canal area, in addition to a transverse process fracture on both sides.
The authors classified this fracture as a Type C burst fracture according to Denis classification [7] and A3 according to AO/Magerl classification (Burst fracture) [8] (Figure 1).
Figure 1.
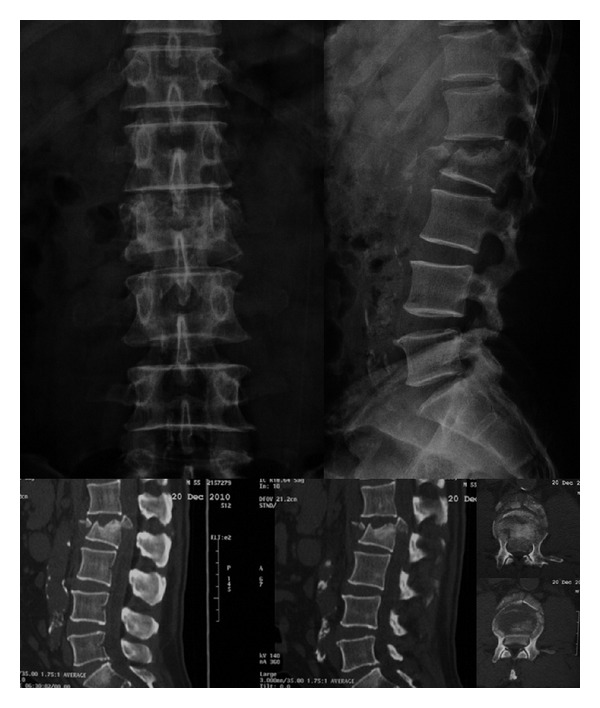
X-ray and CT scan showing a burst fracture on L2 vertebra with a fragment inside the spinal canal.
The patient was brought to OR on day 1 after injury and the lateral transpsoas approach (XLIF) was chosen in order to perform the corpectomy and fusion due to its minimal disruption of the soft tissue, direct visualization of the vertebral body, neural elements and spinal canal, and the possibility to insert an expandable vertebral body replacement device in a minimal invasive fashion.
The patient was placed and taped in a true lateral position in a radiolucent table. A mark was made on the skin based on fluoroscopy that identified the fracture level. Then, a 7 cm incision was made and the retroperitoneal space was dissected, as previously described [9].
To cross the psoas muscle in a safe position, neuromonitoring was obtained using a free-run electromyography (EMG) with somatosensory and motor evoked potentials (SSEP & MEP, NeuroVision M5, NuVasive, Inc., San Diego, CA, USA). Real-time neuromonitoring was used during passage through the psoas muscle, retractor expansion, and instrument implantation. Sequential tube dilation was used to distract the psoas muscle until docking the expandable retractor (MaXcess, NuVasive, Inc.) over the disc space. Initially, inferior and superior discectomies in the upper and lower levels of the fracture and coagulation of segmental vessels were performed. The corpectomy was then conducted working within the space defined by the retractor on lateral projection from the upper to the lower disc space, the retropulsed fragments were mobilized, and the spinal canal was decompressed using standard instruments and techniques.
Vertebral body replacement was performed using a wide-footprint expandable Ti cage (XCore, NuVasive, Inc.). Autograft was used inside and outside the cage from the vertebral body itself. The cage was expanded reducing partially the local kyphosis using the vertebral endplates of L1 and L3 as points of fixation. Due to the wide footprint, the cage rests on the ring apophysis, enhancing biomechanical support. After closing the operative wound in a standard fashion, the patient was positioned in ventral decubitus and a supplemental internal fixation was done utilizing posterior percutaneous pedicle screw fixation (MIP, MDT, Inc.) one level above and one below fracture (Figure 2).
Figure 2.
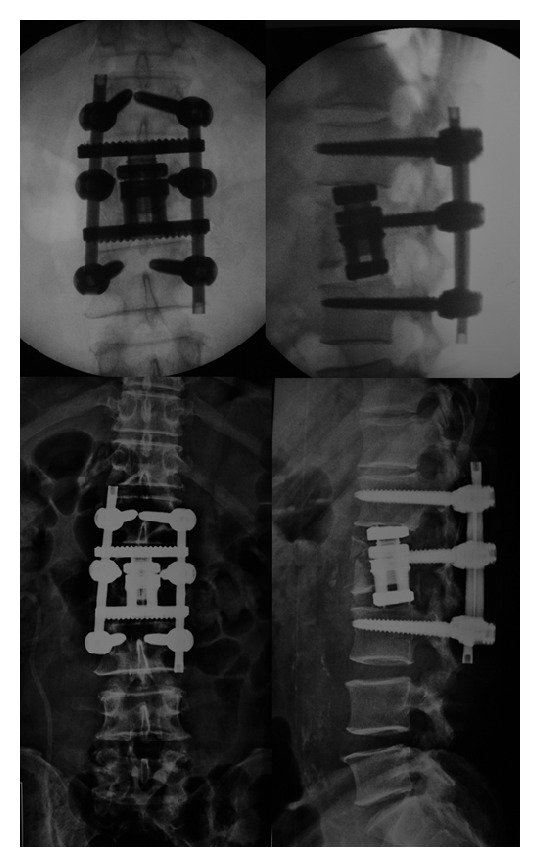
Intraoperative fluoroscopy and immediate postoperative X-ray showing implant position and fracture reduction.
The overall duration of the procedure was 300 minutes, with intraoperative blood loss of 350 mL. Patient did not need to stay in the intensive care unit, no blood transfusion was required, and the total length of hospital stay was only one day. Standing position and ambulation were also performed on the first postoperative day, before hospital discharge.
The patient was evaluated throughout 24 months, showing improvement in clinical and radiological conditions. The 12-month X-rays show improvement in sagittal and coronal alignments (Figure 3), while solid fusion was achieved 24 months after surgery (Figure 4), maintaining favorable clinical and radiological statuses.
Figure 3.
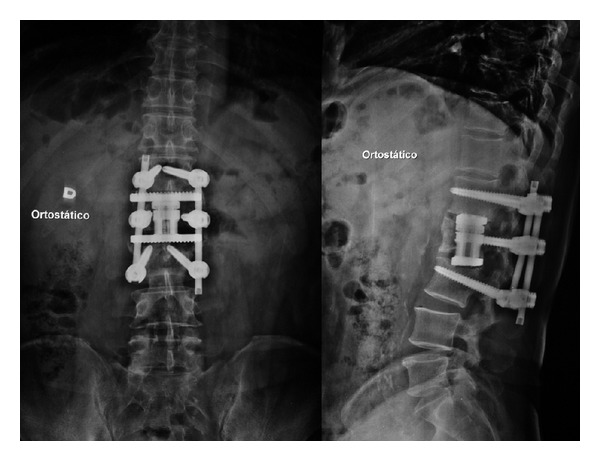
AP and lateral X-rays showing good coronal and sagittal alignments 12 months after surgery.
Figure 4.
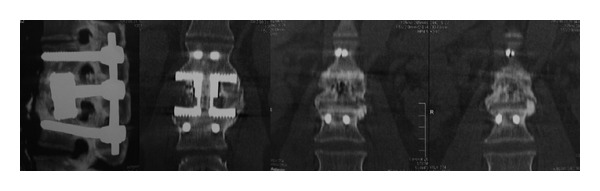
CT scan showing solid fusion 24 months after the procedure. The device design promotes the ossification inside and outside of the prosthesis.
3. Discussion
Thoracolumbar fractures are very common in spine practice, and there are several therapeutic options for its treatment [10–13]. When surgery is needed, its goals are to decompress the spinal canal and neural elements to facilitate neurologic recovery, restore and maintain the vertebral body height for coronal and sagittal alignments, generate a rigid construction to allow early ambulation and rehab, and prevent deformity progression and unbalance of the spine to avoid neurological deficit, while limiting the number of instrumented segments fused [14–17].
The anterior approach to the burst fracture is indicated for cases with severe canal compromise and kyphotic deformity [18, 19]. However, the operative risk is relatively high and includes excessive blood loss, permanent diaphragm failure, abdominal wall injuries, pulmonary complications, and prolonged incisional pain with high infection rates [20, 21]. Mini-open anterior approaches proved to be a less invasive but still open alternative to access the thoracolumbar spine, with the 3-dimensional view of the structures that facilitates the surgical procedure and corpectomy cage insertion [6].
Regarding perioperative factors, Lu et al. [20] found a mean operative time of 445 minutes in patients with anterior-only corpectomy, with a mean EBL of 1506 mL. When adding a posterior approach, mean operative time was 729 minutes with a mean blood loss of 3154 mL. Using a lateral approach to the spine with percutaneous pedicle screw supplementation (one level above and one below), we were able to perform a 1-level corpectomy in 300 minutes with only 350 mL of blood loss, with minimal soft tissue dissection and muscle splitting, which enabled a decreased length of hospital stay and faster return of normal daily living.
One of the major difficulties in anterior approaches is to reduce the kyphotic fractures, being long fixation using pedicle screws (2 above and 2 below fracture) the most adequate to this indication [19, 22]. Using an expandable vertebral body replacement device, we were able to restore the sagittal alignment of the spine without the need of extent posterior manipulation, saving motion segments. Differently from nonexpandable corpectomy devices [23], particularly with cylindrical cages that rest inside the border of the apophyseal ring [24], the lateral approach also permits the insertion of a wider footprint device that reaches apophyseal ring bilaterally, enhancing biomechanical stability and preventing subsidence, kyphosis progression, and restenosis [25–27].
4. Conclusion
The miniopen lateral approach offers the advantages of minimally invasive surgery (MIS) for lumbar corpectomy without the morbidity of standard open approaches, facilitating a wider cage insertion, reducing operative time, blood loss, and adjacent tissue and muscle injuries, lowering surgery costs, and accelerating the patient recovery with the same or better clinical and radiological results of traditional techniques.
Disclosure
The author L. Pimenta is a consultant to and shareowner of NuVasive, Inc., San Diego, CA, USA.
References
- 1.Smith WD, Dakwar E, Le TV, Christian G, Serrano S, Uribe JS. Minimally invasive surgery for traumatic spinal pathologies: a mini-open, lateral approach in the thoracic and lumbar spine. Spine. 2010;35, supplement:S338–S346. doi: 10.1097/BRS.0b013e3182023113. [DOI] [PubMed] [Google Scholar]
- 2.Sasso RC, Cotler HB. Posterior instrumentation and fusion for unstable fractures and fracture- dislocations of the thoracic and lumbar spine: a comparative study of three fixation devices in 70 patients. Spine. 1993;18(4):450–460. [PubMed] [Google Scholar]
- 3.Dai LY. Remodeling of the spinal canal after thoracolumbar burst fractures. Clinical Orthopaedics and Related Research. 2001;(382):119–123. doi: 10.1097/00003086-200101000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Verlaan JJ, Diekerhof CH, Buskens E, et al. Surgical treatment of traumatic fractures of the thoracic and lumbar spine: a systematic review of the literature on techniques, complications, and outcome. Spine. 2004;29(7):803–814. doi: 10.1097/01.brs.0000116990.31984.a9. [DOI] [PubMed] [Google Scholar]
- 5.Dimar JR, Fisher C, Vaccaro AR, et al. Predictors of complications after spinal stabilization of thoracolumbar spine injuries. Journal of Trauma. 2010;69(6):1497–1500. doi: 10.1097/TA.0b013e3181cc853b. [DOI] [PubMed] [Google Scholar]
- 6.Payer M, Sottas C. Mini-open anterior approach for corpectomy in the thoracolumbar spine. Surgical Neurology. 2008;69(1):25–32. doi: 10.1016/j.surneu.2007.01.075. [DOI] [PubMed] [Google Scholar]
- 7.Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine. 1983;8(8):817–831. doi: 10.1097/00007632-198311000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Magerl F, Aebi M, Gertzbein SD, Harms J, Nazarian S. A comprehensive classification of thoracic and lumbar injuries. European Spine Journal. 1994;3(4):184–201. doi: 10.1007/BF02221591. [DOI] [PubMed] [Google Scholar]
- 9.Uribe JS, Dakwar E, Cardona RF, Vale FL. Minimally invasive lateral retropleural thoracolumbar approach: cadaveric feasibility study and report of 4 clinical cases. Neurosurgery. 2011;68(1, supplement):32–39. doi: 10.1227/NEU.0b013e318207b6cb. [DOI] [PubMed] [Google Scholar]
- 10.Gerszten PC, Monaco EA. Complete percutaneous treatment of vertebral body tumors causing spinal canal compromise using a transpedicular cavitation, cement augmentation, and radiosurgical technique. Neurosurgical Focus. 2009;27(6):E9.1–E9.7. doi: 10.3171/2009.9.FOCUS09184. [DOI] [PubMed] [Google Scholar]
- 11.Eleraky MA, Duong HT, Esp E, Kim KD. Expandable versus nonexpandable cages for thoracolumbar burst fracture. World Neurosurgery. 2011;75(1):149–154. doi: 10.1016/j.wneu.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 12.Eck JC. Minimally invasive corpectomy and posterior stabilization for lumbar burst fracture. The Spine Journal. 2011;11(9):904–908. doi: 10.1016/j.spinee.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Gnanenthiran SR, Adie S, Harris IA. Nonoperative versus operative treatment for thoracolumbar burst fractures without neurologic deficit: a meta-analysis. Clinical Orthopaedics and Related Research. 2012;470(2):567–577. doi: 10.1007/s11999-011-2157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang KW. A reduction-fixation system for unstable thoracolumbar burst fractures. Spine. 1992;17(8):879–886. doi: 10.1097/00007632-199208000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs RR, Casey MP. Surgical management of thoracolumbar spinal injuries. General principles and controversial considerations. Clinical Orthopaedics and Related Research. 1984;189:22–35. [PubMed] [Google Scholar]
- 16.Aebi M, Etter C, Kehl T, Thalgott J. Stabilization of the lower thoracic and lumbar spine with the internal spinal skeletal fixation system. Indications, techniques, and first results of treatment. Spine. 1987;12(6):544–551. doi: 10.1097/00007632-198707000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Dai L-Y, Jiang S-D, Wang X-Y, Jiang L-S. A review of the management of thoracolumbar burst fractures. Surgical Neurology. 2007;67(3):221–231. doi: 10.1016/j.surneu.2006.08.081. [DOI] [PubMed] [Google Scholar]
- 18.Haas N, Blauth M, Tscherne H. Anterior plating in thoracolumbar spine injuries: indication, technique, and results. Spine. 1991;16(3, supplement):S100–S111. doi: 10.1097/00007632-199103001-00015. [DOI] [PubMed] [Google Scholar]
- 19.McDonough PW, Davis R, Tribus C, Zdeblick TA. The management of acute thoracolumbar burst fractures with anterior corpectomy and Z-plate fixation. Spine. 2004;29(17):1901–1908. doi: 10.1097/01.brs.0000137059.03557.1d. [DOI] [PubMed] [Google Scholar]
- 20.Lu D, Lau D, Lee JG, Chou D. The transpedicular approach compared with the anterior approach: an analysis of 80 thoracolumbar corpectomies—clinical article. Journal of Neurosurgery. 2010;12(6):583–591. doi: 10.3171/2010.1.SPINE09292. [DOI] [PubMed] [Google Scholar]
- 21.Faciszewski T, Winter RB, Lonstein JE, Denis F, Johnson L. The surgical and medical perioperative complications of anterior spinal fusion surgery in the thoracic and lumbar spine in adults: a review of 1223 procedures. Spine. 1995;20(14):1592–1599. doi: 10.1097/00007632-199507150-00007. [DOI] [PubMed] [Google Scholar]
- 22.Carl AL, Tromanhauser SG, Roger DJ. Pedicle screw instrumentation for thoracolumbar burst fractures and fracture-dislocations. Spine. 1992;17(8, supplement):S317–S324. doi: 10.1097/00007632-199208001-00018. [DOI] [PubMed] [Google Scholar]
- 23.Arts MP, Peul WC. Vertebral body replacement systems with expandable cages in the treatment of various spinal pathologies: a prospectively followed case series of 60 patients. Neurosurgery. 2008;63(3):537–545. doi: 10.1227/01.NEU.0000325260.00628.DC. [DOI] [PubMed] [Google Scholar]
- 24.Hou Y, Luo Z. A study on the structural properties of the lumbar endplate: histological structure, the effect of bone density, and spinal level. Spine. 2009;34(12):E427–E433. doi: 10.1097/BRS.0b013e3181a2ea0a. [DOI] [PubMed] [Google Scholar]
- 25.Uribe J, Smith W, Pimenta L, et al. Minimally invasive lateral approach for symptomatic thoracic disc herniation: initial multi-center clinical experience. Clinical article. Journal of Neurosurgery. 2012;16(3):264–279. doi: 10.3171/2011.10.SPINE11291. [DOI] [PubMed] [Google Scholar]
- 26.Oliveira L, Marchi L, Coutinho E, Pimenta L. A radiographic assessment of the ability of the extreme lateral interbody fusion procedure to indirectly decompress the neural elements. Spine. 2010;35, supplement:S331–S337. doi: 10.1097/BRS.0b013e3182022db0. [DOI] [PubMed] [Google Scholar]
- 27.Pimenta L, Turner AWL, Dooley ZA, Parikh RD, Peterson MD. Biomechanics of lateral interbody spacers: going wider for going stiffer. The Scientific World Journal. 2012;2012:6 pages. doi: 10.1100/2012/381814. 381814 [DOI] [PMC free article] [PubMed] [Google Scholar]


