Summary
Prior to ovulation, mammalian oocytes complete their first meiotic division and arrest at metaphase II. During this marked asymmetric cell division, the meiotic spindle moves dramatically from the center of the oocyte to the cortex to facilitate segregation of half of its chromosomal content into the diminutive first polar body. Recent investigations have documented crucial roles for filamentous actin (F-actin) in meiotic spindle translocation. However, the identity of the upstream regulators responsible for these carefully orchestrated movements has remained elusive. Utilizing fluorescently tagged probes and time-lapse confocal microscopy, we document that phosphatidylinositol 3,4,5-trisphosphate [PtdIns(3,4,5)P3] is constitutively synthesized with spatial and temporal dynamics similar to that of F-actin and Formin 2 (Fmn2). Blockage of PtdIns(3,4,5)P3 synthesis by LY294002, a specific inhibitor of phosphoinositide 3-kinase (PI3K), disrupts cytoplasmic F-actin organization and meiotic spindle migration to the cortex. F-actin nucleator Fmn2 and Rho GTPase Cdc42 play roles in mediating the effect of PtdIns(3,4,5)P3 on F-actin assembly. Moreover, the spatial and temporal dynamics of PtdIns(3,4,5)P3 is impaired by depletion of MATER or Filia, two oocyte proteins encoded by maternal effect genes. Thus, PtdIns(3,4,5)P3 is synthesized during meiotic maturation and acts upstream of Cdc42 and Fmn2, but downstream of MATER/Filia proteins to regulate the F-actin organization and spindle translocation to the cortex during mouse oocyte meiosis.
Key words: PtdIns(3,4,5)P3; Oocyte meiosis; Spindle translocation; Filamentous actin; Cdc42
Introduction
During the first meiotic division, mammalian oocytes undergo cell division to extrude half of their chromosomes into a small polar body. The dramatic asymmetry of this division enables eggs to preserve a substantial cytoplasmic store of maternal factors necessary for early development preceding activation of the embryonic genome. To ensure the success of asymmetric cell division, oocytes must relocate their meiosis I spindles from the cell center to the cortex (Verlhac et al., 2000). Recent studies provide strong evidence that the F-actin meshwork plays important roles in mediating this translocation (Azoury et al., 2008; Leader et al., 2002; Li et al., 2008a; Schuh and Ellenberg, 2008). During meiotic maturation, the nuclear membrane (germinal vesicle) of mouse oocytes breaks down and F-actin initially forms a symmetric meshwork surrounding chromosomes. Gradually the meshwork cloud becomes asymmetric as it moves behind the chromosome during the time in which the spindle approaches the cortex of the oocyte (Li et al., 2008a). The actin motor protein myosin 2 generates the pulling forces required for spindle translocation (Schuh and Ellenberg, 2008).
Although it is clear that F-actin assembly plays critical role in meiotic spindle migration, upstream regulation required for the dynamic assembly of F-actin is relatively less understood. Three types of actin nucleators are implicated in regulating F-actin assembly during meiosis I spindle translocation. The branched actin nucleator Arp2/3 complex and its upstream activators Wave2 and JMY are required for spindle migration and asymmetric division (Sun et al., 2011a; Sun et al., 2011b; Sun et al., 2011c). Formins regulate the assembly of unbranched actin filaments (Kovar and Pollard, 2004). Formin 2 (Fmn2), a member of formin proteins containing FH1 and FH2 domains (Leader and Leder, 2000), is necessary for dynamic F-actin assembly and meiotic spindle migration (Dumont et al., 2007; Leader et al., 2002; Li et al., 2008a). Spire proteins are a novel class of actin nucleator which drives the nucleation of straight actin microfilaments (Bosch et al., 2007; Quinlan et al., 2005). Spire 1 and 2 cooperate with Fmn2 to regulate F-actin assembly as well as meiotic spindle migration (Pfender et al., 2011). In addition, cytoskeletal regulator Cdc42 and Mos/MEK1/2/MAPK pathway also play roles in meiotic spindle migration in mouse oocytes (Na and Zernicka-Goetz, 2006; Verlhac et al., 2000; Yu et al., 2007). More recently, a cis-Golgi protein GM 130 was found to regulate asymmetric division in mouse oocytes (Sun and Kim, 2011; Zhang et al., 2011).
Phosphatidylinositol 3,4,5-trisphosphate [PtdIns(3,4,5)P3], a major in vivo product of phosphoinositide 3-kinase (PI3K) (Auger et al., 1989), is an important regulator of actin polymerization. In a broad range of cell types, PtdIns(3,4,5)P3 functions as an instructive signal for actin polymerization. An increase in PtdIns(3,4,5)P3 level alone is sufficient to induce F-actin assembly and the spatial and temporal dynamics of PtdIns(3,4,5)P3 parallel that of actin polymerization (Insall and Weiner, 2001). PI3K is constitutively active during mouse preimplantation development and its enzymatic product PtdIns(3,4,5)P3 is required for embryo survival (Halet et al., 2008). PI3K and PtdIns(3,4,5)P3 also play roles in follicle growth (Reddy et al., 2009; Reddy et al., 2008). However, whether PtdIns(3,4,5)P3 is synthesized and regulates F-actin assembly required for meiotic spindle migration and asymmetric division of mouse oocyte remains elusive. Utilizing fluorescently tagged probes and time-lapse confocal microscopy, we herein provide evidence that the constitutive synthesis of PtdIns(3,4,5)P3 during meiotic maturation plays critical roles in regulating F-actin assembly and meiotic spindle translocation to cortex. In addition, we propose that Fmn2 and Rho GTPase Cdc42 function as mediators for coupling PtdIns(3,4,5)P3 synthesis to F-actin organization, and that maternal proteins MATER and Filia act as upstream regulators of PtdIns(3,4,5)P3 synthesis during meiosis in mouse oocytes.
Results and Discussion
Constitutive synthesis of PtdIns(3,4,5)P3 in the vicinity of chromosomes during meiotic maturation of mouse oocytes
A pleckstrin homology (PH) domain tagged with GFP provides an excellent monitor of inositide species within live cells (Balla and Varnai, 2009). We tagged the PH domain from Akt with GFP at the C-terminus (PH-Akt–GFP) to monitor the synthesis and localization of PtdIns(3,4,5)P3 as previously reported (Srinivasan et al., 2003; Wang et al., 2002). In vitro transcribed mRNAs encoding PH-Akt–GFP were injected into germinal vesicle-intact (GV) oocytes and time lapse images were acquired during oocyte in vitro maturation. As shown (Fig. 1A and supplementary material Movie 1), a green fluorescent ring was visible surrounding the nucleus in the GV oocyte. Upon germinal vesicle breakdown (GVBD), GFP formed symmetric cloud around chromosomes. Thereafter, the symmetric spindle-like structure assumed an asymmetric distribution as the chromosomes migrated to the cortex of the oocyte. In agreement with the localization of PtdIns(3,4,5)P3, the regulatory subunit of class I PI3K (p85) was detected in the vicinity of chromosomes after GVBD and formed spindle-like structures at metaphase (Fig. 1B, arrowhead).
Fig. 1.
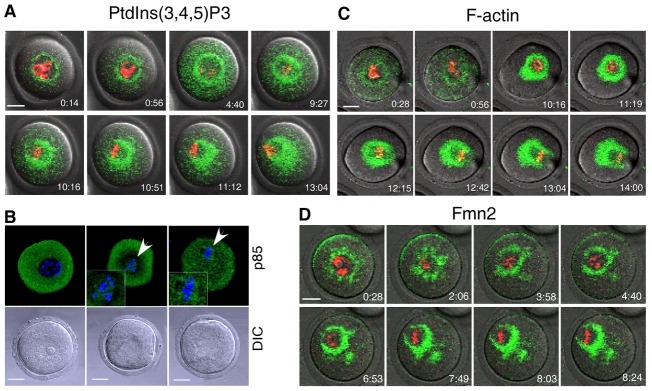
Constitutive synthesis of PtdIns(3,4,5)P3 during meiotic maturation of mouse oocytes. GV oocytes were injected with mRNAs encoding PH-Akt–GFP, EGFP–Lifeact and EGFP–Fmn2 and imaged by time-lapse confocal microscopy during meiotic maturation to document the localization of (A) PtdIns(3,4,5)P3, (C) F-actin and (D) Fmn2. (B) Fixed GV oocytes were stained with an antibody against p85 to document the presence of PI3K in the vicinity of chromosomes (arrowheads). DNA (red or blue) was labeled with Hoechst 33258. Images in A, C and D were taken from individual sections of confocal images at the indicated time points (hr:min). Scale bars: 20 µm.
The spatial-temporal distribution pattern of PtdIns(3,4,5)P3 is similar to that of F-actin and Fmn2 in mouse oocytes (Li et al., 2008a). To verify these earlier observations, we used EGFP–Lifeact and EGFP–Fmn2 in time-lapse microscopy (Leader et al., 2002; Riedl et al., 2008) to monitor F-actin and Fmn2, respectively. As shown (Fig. 1C,D), the dynamic distribution of F-actin and Fmn2 was consistent with previous reports (Li et al., 2008a) and correlated well with that of PtdIns(3,4,5)P3. These results suggested a potential role of PtdIns(3,4,5)P3 in regulating F-actin organization and meiotic spindle translocation.
PtdIns(3,4,5)P3 is required for F-actin organization and meiotic spindle translocation
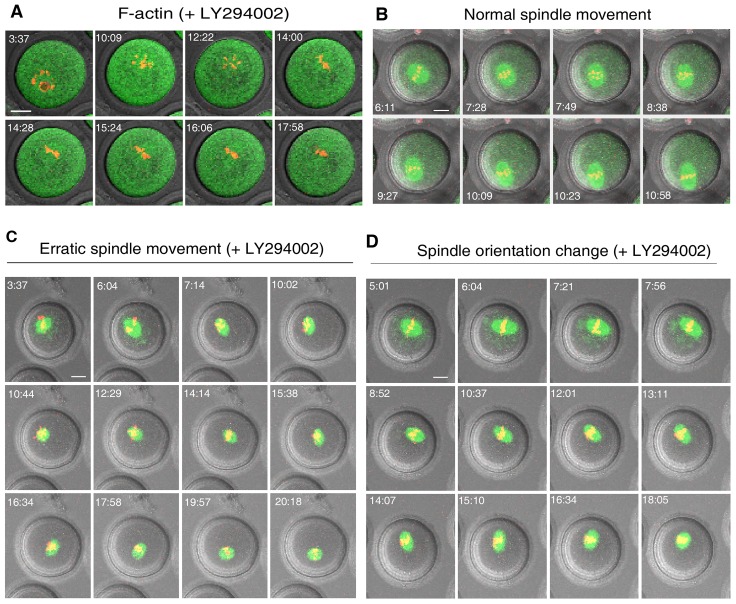
LY294002 is a potent and specific PI3K inhibitor at concentrations of ≤30 µM (Backer, 2000; Halet et al., 2008; O'Neill, 2008). We cultured the oocytes in the presence of 20 µM LY294002 which could efficiently block the synthesis of PtdIns(3,4,5)P3 (data not shown), and F-actin organization was investigated, as described above, by time-lapse microscopy. In control oocytes treated with vehicle (DMSO) alone, F-actin was normally organized during oocyte maturation (Fig. 1C). In sharp contrast, treatment with LY294002 abolished the dynamic assembly of F-actin in all oocytes examined (35/35, Fig. 2A) as indicated by diffusion of green fluorescence throughout the cytoplasm. Similarly, Fmn2 lost its polarized distribution in the presence of LY294002 during in vitro maturation (supplementary material Fig. S1). However, the cortical F-actin cap formed when the chromosomes approach the oocyte cortex was not impaired (supplementary material Fig. S2, arrow). Thus, the dynamic cytoplasmic F-actin assembly was under the control of PtdIns(3,4,5)P3 signaling, while cortical organization of F-actin cap was regulated by distinct mechanism as suggested by others (Deng et al., 2007).
Fig. 2.
Blockage of PtdIns(3,4,5)P3 synthesis disrupted dynamic F-actin assembly and spindle translocation to the cortex. GV oocytes were matured in vitro and imaged by time-lapse confocal microscopy as described in Fig. 1. After treatment with LY294002 (20 µM) F-actin (A) diffused throughout the cytoplasm and the normal translocation of the spindle from the center to cortex (B) became erratic (C) or occurred with frequent changes in spindle orientation without net displacement (D). F-actin in normal controls was as in Fig. 1C. Scale bars: 20 µm.
We then investigated the roles of PtdIns(3,4,5)P3 in meiotic spindle translocation. After 16 hours of in vitro maturation, oocyte progression to GVBD or metaphase II was evaluated morphologically, and the 1st meiotic spindle position was examined by immunofluorescent staining. In control oocytes undergoing GVBD (n = 104, two repeats), 72.1% (75/104) extruded the first polar body, 22.1% (23/104) contained a meiosis I spindle at their periphery, and 5.8% (6/104) exhibited a 1st meiotic spindles near or at their center. In sharp contrast, treatment with LY294002 significantly decreased the proportions of oocytes reaching metaphase II (13.5%, 14/104) or with a meiosis I spindle at their cortex (43.3%, 45/104). Instead, many more oocytes contained central 1st meiotic spindles (39.4%, 41/104), and four oocytes divided into two-cell like embryos (3.8%, 4/104). To exclude the off-target effects of LY294002 on other protein kinases including DNA protein kinase (DNA-PK) and mTOR, PI3K activity was blocked by wortmannin, another selective and irreversible PI3K inhibitor at nanomolar concentrations (Powis et al., 1994; Ptasznik et al., 1997). Owing to the unstable nature of wortmannin in culture medium (with half-life between 8 and 13 minutes) (Holleran et al., 2003), 50 nM wortmannin was replenished every 2 hours during the 9 hours of in vitro maturation (at 0 hours, 2 hours, 4 hours and 6 hours of culture), as suggested by other studies (Cleveland and Weber, 2010; Franch et al., 2002; Shpetner et al., 1996). Consistently, treatment of wortmannin impaired migration of the meiosis I spindle to the cortex. After 9 hours of maturation, 4.5% (4/89, two repeats) of meiosis I spindles were located in the center of control oocytes undergoing GVBD, whereas the proportion of centrally-located spindles increased to 42.1% (80/190, two repeats) in the wortmannin-treated group. Increasing the concentration of wortmannin to 100 nM did not significantly change the percentage of central spindles (45.6%, 130/285, two repeats). Taken together, these observations confirmed the selective inhibition of LY294002 or wortmannin on PI3K activity. We thereafter utilized 20 µM LY294002 in the following experiments.
To gain a more detailed understanding of abnormal spindle behavior without PtdIns(3,4,5)P3 synthesis, spindles were visualized by GFP-tagged EB1, a microtubule plus-end binding protein that enabled us to monitor the spindle. The spindle movement during the course of oocyte maturation was filmed by time-lapse microscopy. In the control group, 32 out of 37 spindles (86.5%, three repeats) displayed normal behavior, i.e. direct, unwavering movement to the nearest cortex (Fig. 2B, supplementary material Movie 2). The remaining spindles (13.5%, 5/37) remained stationary without obvious change in orientation throughout the experimental time period which could reflect senescence. In distinct contrast, treatment with LY294002 significantly reduced the proportion of spindles undergoing normal translocation (16.3%, 7/43, three repeats). Most spindles (83.7%, 36/43) displayed abnormal behaviors which could be classified into one of three categories: (1) spindles that wandered in the cytoplasm with unpredicted directions (erratic spindle movement, 58.1%, 25/43) (Fig. 2C, supplementary material Movie 3); (2) spindles that changed orientation frequently without net displacement (orientation change, 11.6%, 5/43) (Fig. 2D); (3) spindles that remained stationary without change in orientation (14.0%, 6/43). This latter phenotype occurred at a similar frequency to that observed in the control group and therefore appeared independent of drug treatment. Taken together, these data suggested that PtdIns(3,4,5)P3 was required for F-actin organization and meiotic spindle translocation to the cortex of mouse oocytes.
Apart from F-actin, the actin motor protein myosin 2 is also required for spindle migration by generating pulling forces (Schuh and Ellenberg, 2008). In this study, LY294002 treatment did not impair the activation and localization of myosin 2 as documented by imaging its phosphorylated light chain p-MLC2 (supplementary material Fig. S3). Thus, myosin 2 was not responsible for the failure of spindle migration to the cortex in the absence of PtdIns(3,4,5)P3 synthesis.
Rho GTPase Cdc42 mediates the role of PtdIns(3,4,5)P3 in F-actin organization
Next we sought to investigate how PtdIns(3,4,5)P3 regulates F-actin organization. Previous studies had proposed Rho GTPases as potential candidates to couple PtdIns(3,4,5)P3 signaling and F-actin organization in many cell types (Hall, 1998; Insall and Weiner, 2001). In addition, interruption of Cdc42 activity leads to the failure of meiotic spindle translocation in mouse oocytes, although its influence on F-actin assembly has not been assessed (Na and Zernicka-Goetz, 2006). Based on the above knowledge, we speculated that Cdc42 might couple PtdIns(3,4,5)P3 to F-actin assembly during meiotic maturation of mouse oocytes.
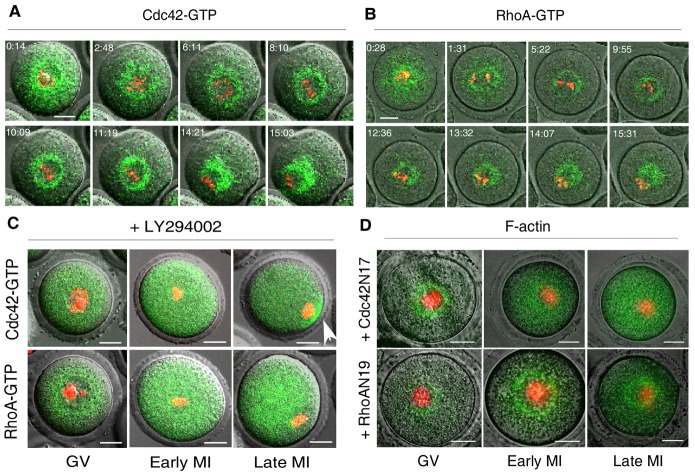
To test this hypothesis, we first examined the activation status of Cdc42 and RhoA, the latter does not play a role in spindle translocation and herein served as negative control (Na and Zernicka-Goetz, 2006; Schuh and Ellenberg, 2008). Rho GTPases cycle between inactive (GDP-bound) and active (GTP-bound) conformations and their activation can be traced by specific binding domains. We used GFP-tagged Cdc42 binding domain (CBD) of the Wiscott–Aldrich syndrome protein (WASP) (WASP-CBD–GFP) (Nalbant et al., 2004) and Rho binding domain (RBD) of the Rhotekin protein (Rhotekin-RBD–GFP) (Li et al., 2002; Ren et al., 1999) to visualize active Cdc42 and RhoA, respectively. The dynamics of GFP distribution was monitored by time-lapse microscopy throughout the course of oocyte maturation. Cdc42 and RhoA were activated in the cytoplasm with dynamic patterns similar to that of PtdIns(3,4,5)P3, F-actin or Fmn2 (Fig. 3A,B, supplementary material Movie 4). We then went on to investigate the influence of PtdIns(3,4,5)P3 on Rho GTPases activation. As indicated by diffusion of the green fluorescence (Fig. 3C), treatment with LY294002 (20 µM) abolished the normal distribution of active Cdc42 and RhoA in the cytoplasm (Fig. 3A,B). This result demonstrated that PtdIns(3,4,5)P3 was required for cytoplasmic activation of Cdc42 and RhoA. Interestingly, a cortical cap of active Cdc42 was formed even after treatment with LY294002 (Fig. 3C, arrow). This was in agreement with our observation of the continued presence of the cortical F-actin cap (supplementary material Fig. S2, arrow) as well as with previous report on cortical activation of Rac1 (Halet and Carroll, 2007). Thus, distinct molecular bases were involved in the regulation of cytoplasmic and cortical polarity.
Fig. 3.
Cdc42 mediated the effect of PtdIns(3,4,5)P3 on cytoplasmic F-actin assembly. (A,B) GV oocytes were matured in vitro and imaged as described in Fig. 1 to document activation of (A) Cdc42 (Cdc42–GTP) and (B) RhoA (RhoA–GTP) using fluorescently tagged WASP-CBD–GFP and Rhotekin-RBD–GFP, respectively. (C) In contrast to normal controls (A,B), treatment with LY294002 (20 µM) led to diffusion of Cdc42–GTP (upper panels) and RhoA–GTP (lower panels) in the cytoplasm. Note that the cortical cap of active Cdc42 (arrow) was not affected by LY294002. (D) F-actin organization was perturbed by ectopic expression of dominant-negative mutant Cdc42N17 (upper panels), but not RhoAN19(lower panels). In C and D, oocytes were examined at the indicated stages of meiosis by confocal microscopy. GV, germinal vesicle stage; MI, metaphase I stage. Scale bars: 20 µm.
Finally, we determined if Cdc42 could influence the organization of F-actin assembly during meiotic maturation. To this end, we interrupted Rho GTPase activity with dominant-negative mutants. The mRNAs of RhoAN19 or Cdc42N17 at equivalent concentration were co-injected with EGFP–Lifeact into GV oocytes. F-actin assembly was examined by confocal microscopy at different stages of maturation. In agreement with previous reports (Na and Zernicka-Goetz, 2006; Schuh and Ellenberg, 2008), F-actin assembly was not impaired by ectopic expression of RhoAN19. However, expression of Cdc42N17 perturbed F-actin organization as reflected by the even distribution of GFP in the cytoplasm (Fig. 3D). Taken together, these data supported the conclusion that Cdc42 mediated the regulatory role of PtdIns(3,4,5)P3 in F-actin assembly. Here we did not examine the Rho GTPase Rac1. A previous study had reported its essential roles in regulating meiotic spindle stability and anchoring to cortex (Halet and Carroll, 2007). Whether Rac1 displays cytoplasmic activation under the control of PtdIns(3,4,5)P3 and plays roles in F-actin assembly as well as spindle translocation deserves further exploration.
Fmn2, a well documented regulator of F-actin assembly and meiotic spindle translocation (Leader et al., 2002; Li et al., 2008a), was also influenced by PtdIns(3,4,5)P3 (supplementary material Fig. S1). Formin proteins can be classified into two groups, one contains Rho GTPase binding (RGB) domain and the other does not. Binding of active Rho GTPase to the RGB region plays a regulatory role on the activity of the former group. Fmn2 does not contain the RGB domain, and its function on actin nucleation is not modulated by Rho GTPase (Higgs, 2005). Therefore, Cdc42 and Fmn2 might be two irrelevant downstream effectors of PtdIns(3,4,5)P3 in regulating F-actin assembly.
Absence of MATER/Filia protein in oocytes impairs PtdIns(3,4,5)P3 synthesis and normal spindle translocation
We have shown the essential roles of PtdIns(3,4,5)P3 in F-actin organization and meiotic spindle translocation in mouse oocytes. Deciphering the upstream factors responsible for the polarized PtdIns(3,4,5)P3 synthesis would provide further insight into the regulation of meiosis in mammals. Mater (official name Nlrp5) is one of the first maternal effect genes described in mice. It is located in the subcortex of mature oocytes and persists to the blastocyst stage of development, but its function(s) remains incompletely understood (Li et al., 2008b; Ohsugi et al., 2008; Tong et al., 2000). In an independent experiment to characterize oocytes from Mater−/− females, we observed a striking defect in meiotic spindle translocation (i.e. spindles displayed erratic movement with many turns) and unstable anchoring to cortex during in vitro maturation (Fig. 4A, supplementary material Movie 5). Their wild-type counterparts cultured in the same droplets displayed normal spindle migration (supplementary material Fig. S4). This prompted us to examine the possible correlation between PtdIns(3,4,5)P3 and MATER protein. Although Mater−/− mice have genetic background different than those used above (C57BL6×SV129 versus B6D2[C57BL6×DBA/2] F1), the PtdIns(3,4,5)P3 dynamics in oocytes from the wild-type counterparts (supplementary material Fig. S5) was similar to that described in B6D2[C57BL6×DBA/2] F1 mice (Fig. 1A). This suggested that the constitutive synthesis of PtdIns(3,4,5)P3 and its roles during oocyte maturation might be irrelevant to genetic background. In contrast to the wild-type control (supplementary material Fig. S5), Mater−/− oocytes displayed abnormal PtdIns(3,4,5)P3 synthesis as evidenced by the even distribution of GFP throughout the cytoplasm (Fig. 4B). Concordantly, we failed to detect normal Fmn2 (supplementary material Fig. S6) and Cdc42 activation (supplementary material Fig. S7) in Mater−/− oocytes during meiotic maturation. The symmetric F-actin assembly surrounding the chromosomes observed in normal controls (supplementary material Fig. S8) was not detected in all Mater−/− oocytes at the early stage of meiosis. However, asymmetric F-actin cloud could be observed behind the chromosomes in most of the mutant oocytes when chromosomes approached close to the cortex (Fig. 4C). In mouse oocytes, MATER binds to another maternal protein Filia (official name, Khdc3) (Ohsugi et al., 2008)), which is also important for early embryogenesis (Zheng and Dean, 2009). Similar defects in spindle translocation (supplementary material Fig. S9A) as well as PtdIns(3,4,5)P3 synthesis (supplementary material Fig. S9B) were observed in Filia−/− oocytes when compared to the wild-type counterparts (supplementary material Figs S4, S5). These results together suggested that the maternal protein complex MATER/Filia was involved in regulating PtdIns(3,4,5)P3 dynamics during meiosis.
Fig. 4.
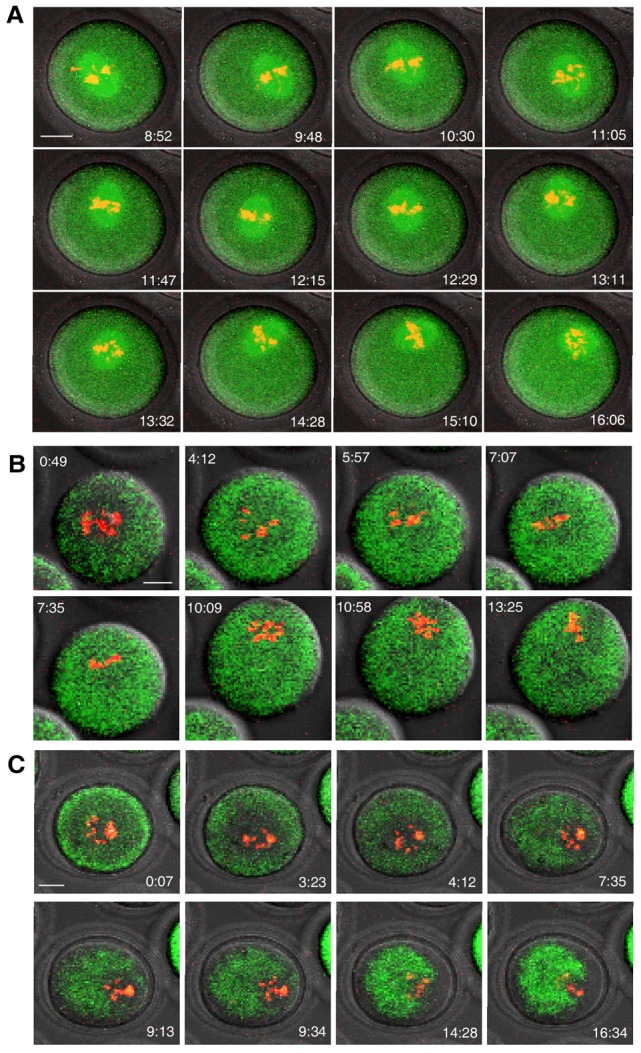
Absence of MATER protein impaired PtdIns(3,4,5)P3 synthesis and spindle translocation. GV oocytes from Mater−/− females and the wild-type littermates were matured in the same droplet of medium. The zona pellucida was removed from mutant or normal oocytes. (A,B) Time-lapse confocal microscopy was performed as described in Fig. 1 to document that meiotic spindles move erratically in mutant oocytes (A) compared with normal oocytes (supplementary material Fig. S4) , and that (B) PtdIns(3,4,5)P3 was diffusely present in oocytes lacking MATER but not in normal control oocytes (supplementary material Fig. S5). (C) Symmetric F-actin assembly was lost in Mater−/− oocytes but not in normal control oocytes (supplementary material Fig. S8). However, the asymmetric F-actin cloud was visible when chromosomes approached to the cortex. Scale bars: 20 µm.
Due to limitation of our method which is best suited for detecting the polarized distribution of PtdIns(3,4,5)P3, we could not distinguish whether the even distribution of PH-Akt–GFP in Mater−/− or Filia−/− oocytes reflected the lack of PtdIns(3,4,5)P3 synthesis or homogenous diffusion of PtdIns(3,4,5)P3. However, based on the observation that meiotic spindles in Mater−/− or Filia−/− oocytes moved faster than those in LY294002-treated oocytes (supplementary material Movies 3, 5), we speculate that PtdIns(3,4,5)P3 synthesis is not just simply lost in these mutant oocytes. Instead, PtdIns(3,4,5)P3 might lose its polarized distribution in cytoplasm. If so, synthesis and accurate distribution of PtdIns(3,4,5)P3 should be key in ensuring success of asymmetric division during meiosis. MATER or Filia localizes to the cortex of oocytes and it seems unlikely that either directly regulates the cytoplasmic synthesis of PtdIns(3,4,5)P3. Whether some unknown signaling is involved in coupling the cortical events and the polarized PtdIns(3,4,5)P3 synthesis remains to be determined.
Materials and Methods
Expression constructs and mRNA synthesis
The PH domain from mouse Akt (Balla and Varnai, 2009), the Cdc42 binding domain from WASP (Nalbant et al., 2004), the RhoA binding domain from rhotekin (Li et al., 2002; Ren et al., 1999), and the EB1 coding region were cloned into pcDNA3.1-CT-GFP (Invitrogen). pCS2-Fmn2-EGFP and pEGFP-N1-Lifeact were kindly provided by Dr Philip Leder (Harvard Medical School) and Dr Roland Wedlich-Soldner (Max Planck Institute of Biochemistry), respectively. The fragment encoding Lifeact–EGFP was cloned into pcDNA3.1(+) (Invitrogen). Dominant-negative mutants of RhoAN19 and Cdc42N17 were generated by in vitro site directed mutagenesis (Quikchange Site-directed Mutagenesis Kit, Stratagene). Coding regions for wild-type RhoA, RhoAN19, wild-type Cdc42 and Cdc42N17 were cloned into pcDNA3.1(+). For in vitro mRNA synthesis, expression constructs were linearized and capped mRNAs were synthesized with T7 or SP6 polymerase (mMessage mMachine Kit, Ambion). Isolated mRNA was dissolved in injection buffer (10 mM Tris-HCl, pH 7.5 and 0.1 mM EDTA) and stored at −80°C.
Collection and culture of immature oocytes
Ovaries were dissected from female mice (B6D2[C57BL6×DBA/2] F1 or C57BL6×SV129, 5–6 weeks of age). Grown follicles were punctured to release cumulus-oocyte complexes (COCs) in M2 medium containing 50 ng/ml of 3-isobutyl-1-methylxanthine (IBMX, Sigma) to prevent resumption of meiosis (Li et al., 2008a). COCs were denuded of cumulus cells. Healthy looking GV oocytes were matured in KSOM medium (Sutton-McDowall et al., 2010). In some experiment, oocytes were treated with 20 µM of PI3K inhibitor LY294002 (Sigma) or wortmannin (50 nM or 100 nM) (Selleckchem). Because wortmannin is very unstable in culture medium with a half-life of between 8 and 13 minutes (Holleran et al., 2003), it was replenished every 2 hours during the treatment period. All experiments were conducted in compliance with the guidelines of the Animal Care and Use Committees of the National Institutes of Health, USA and Kunming Institute of Zoology, the Chinese Academy of Sciences.
Microinjection and time-lapse confocal microscopy
In vitro transcribed mRNAs (500 ng/µl) were injected into the cytoplasm of GV oocytes maintained in M2 medium containing 50 ng/ml IBMX (Sigma). After mRNA injection, oocytes were cultured in KSOM with 50 ng/ml of IBMX for at least 3 hours to allow the translation of injected mRNA. DNA was labeled with 2 ng/ml bisbenzimide (Sigma) for 15 minutes and oocytes were then transferred into 100 µl of KSOM covered with mineral oil in an environmental chamber (37°C, 5% CO2) for time-lapse imaging in which projections were acquired at 7 minutes intervals with a LSM 510 confocal microscope (Carl Zeiss, Thornwood, NY, USA). Because LY294002 is readily absorbed into mineral oil (Halet et al., 2008), oocytes were matured in LY294002-containing medium without mineral oil cover in a small chamber attached on a 35 mm MetTek dish in an environmental chamber in humidified air throughout the observation period. In some cases, oocytes were fixed at different stages of meiosis and imaged by confocal microscopy. All the experiments were repeated more than two times with examined oocyte numbers in each group >30.
Immunofluorescent staining and confocal microscopy
Primary antibodies against p85 (Abcam), p-MLC2 (Cell Signaling Technology), and α-tubulin-FITC (Sigma) were obtained commercially. Secondary antibodies were donkey anti-rabbit conjugated with Alexa Fluor 555 (Invitrogen) and goat anti-rabbit conjugated with Alexa Fluor 488 (Invitrogen). Immunofluorescent staining was performed as described (Zheng and Dean, 2009).
Supplementary Material
Acknowledgments
We thank Dr Philip Leder, Harvard Medical School for providing pCS2-Fmn2–EGFP, and Dr Roland Wedlich-Soldner, Max Planck Institute of Biochemistry for providing pEGFP-N1-Lifeact.
Footnotes
Funding
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, USA (to J. D.); and the Natural Science Foundation of China [grant number 31071274 to P. Zheng]. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.118042/-/DC1
References
- Auger K. R., Serunian L. A., Soltoff S. P., Libby P., Cantley L. C. (1989). PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell 57, 167–175 10.1016/0092-8674(89)90182-7 [DOI] [PubMed] [Google Scholar]
- Azoury J., Lee K. W., Georget V., Rassinier P., Leader B., Verlhac M. H. (2008). Spindle positioning in mouse oocytes relies on a dynamic meshwork of actin filaments. Curr. Biol. 18, 1514–1519 10.1016/j.cub.2008.08.044 [DOI] [PubMed] [Google Scholar]
- Backer J. M. (2000). Phosphoinositide 3-kinases and the regulation of vesicular trafficking. Mol. Cell Biol. Res. Commun. 3, 193–204 10.1006/mcbr.2000.0202 [DOI] [PubMed] [Google Scholar]
- Balla T., Varnai P. (2009). Visualization of cellular phosphoinositide pools with GFP-fused protein domains. Curr. Protoc. Cell Biol. 24, 24.4.1–24.4.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M., Le K. H., Bugyi B., Correia J. J., Renault L., Carlier M. F. (2007). Analysis of the function of Spire in actin assembly and its synergy with formin and profilin. Mol. Cell 28, 555–568 10.1016/j.molcel.2007.09.018 [DOI] [PubMed] [Google Scholar]
- Cleveland B. M., Weber G. M. (2010). Effects of insulin-like growth factor-I, insulin, and leucine on protein turnover and ubiquitin ligase expression in rainbow trout primary myocytes. Am. J. Physiol. 298, R341–R350 10.1152/ajpregu.00516.2009 [DOI] [PubMed] [Google Scholar]
- Deng M., Suraneni P., Schultz R. M., Li R. (2007). The Ran GTPase mediates chromatin signaling to control cortical polarity during polar body extrusion in mouse oocytes. Dev. Cell 12, 301–308 10.1016/j.devcel.2006.11.008 [DOI] [PubMed] [Google Scholar]
- Dumont J., Million K., Sunderland K., Rassinier P., Lim H., Leader B., Verlhac M. H. (2007). Formin-2 is required for spindle migration and for the late steps of cytokinesis in mouse oocytes. Dev. Biol. 301, 254–265 10.1016/j.ydbio.2006.08.044 [DOI] [PubMed] [Google Scholar]
- Franch H. A., Wang X., Sooparb S., Brown N. S., Du J. (2002). Phosphatidylinositol 3-kinase activity is required for epidermal growth factor to suppress proteolysis. J. Am. Soc. Nephrol. 13, 903–909 [DOI] [PubMed] [Google Scholar]
- Halet G., Carroll J. (2007). Rac activity is polarized and regulates meiotic spindle stability and anchoring in mammalian oocytes. Dev. Cell 12, 309–317 10.1016/j.devcel.2006.12.010 [DOI] [PubMed] [Google Scholar]
- Halet G., Viard P., Carroll J. (2008). Constitutive PtdIns(3,4,5)P3 synthesis promotes the development and survival of early mammalian embryos. Development 135, 425–429 10.1242/dev.014894 [DOI] [PubMed] [Google Scholar]
- Hall A. (1998). Rho GTPases and the actin cytoskeleton. Science 279, 509–514 10.1126/science.279.5350.509 [DOI] [PubMed] [Google Scholar]
- Higgs H. N. (2005). Formin proteins: a domain-based approach. Trends Biochem. Sci. 30, 342–353 10.1016/j.tibs.2005.04.014 [DOI] [PubMed] [Google Scholar]
- Holleran J. L., Egorin M. J., Zuhowski E. G., Parise R. A., Musser S. M., Pan S. S. (2003). Use of high-performance liquid chromatography to characterize the rapid decomposition of wortmannin in tissue culture media. Anal. Biochem. 323, 19–25 10.1016/j.ab.2003.08.030 [DOI] [PubMed] [Google Scholar]
- Insall R. H., Weiner O. D. (2001). PIP3, PIP2, and cell movement – similar messages, different meanings? Dev. Cell 1, 743–747 10.1016/S1534-5807(01)00086-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar D. R., Pollard T. D. (2004). Progressing actin: Formin as a processive elongation machine. Nat. Cell Biol. 6, 1158–1159 10.1038/ncb1204-1158 [DOI] [PubMed] [Google Scholar]
- Leader B., Leder P. (2000). Formin-2, a novel formin homology protein of the cappuccino subfamily, is highly expressed in the developing and adult central nervous system. Mech. Dev. 93, 221–231 10.1016/S0925-4773(00)00276-8 [DOI] [PubMed] [Google Scholar]
- Leader B., Lim H., Carabatsos M. J., Harrington A., Ecsedy J., Pellman D., Maas R., Leder P. (2002). Formin-2, polyploidy, hypofertility and positioning of the meiotic spindle in mouse oocytes. Nat. Cell Biol. 4, 921–928 10.1038/ncb880 [DOI] [PubMed] [Google Scholar]
- Li Z., Aizenman C. D., Cline H. T. (2002). Regulation of rho GTPases by crosstalk and neuronal activity in vivo. Neuron 33, 741–750 10.1016/S0896-6273(02)00621-9 [DOI] [PubMed] [Google Scholar]
- Li H., Guo F., Rubinstein B., Li R. (2008a). Actin-driven chromosomal motility leads to symmetry breaking in mammalian meiotic oocytes. Nat. Cell Biol. 10, 1301–1308 10.1038/ncb1788 [DOI] [PubMed] [Google Scholar]
- Li L., Baibakov B., Dean J. (2008b). A subcortical maternal complex essential for preimplantation mouse embryogenesis. Dev. Cell 15, 416–425 10.1016/j.devcel.2008.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na J., Zernicka–Goetz M. (2006). Asymmetric positioning and organization of the meiotic spindle of mouse oocytes requires CDC42 function. Curr. Biol. 16, 1249–1254 10.1016/j.cub.2006.05.023 [DOI] [PubMed] [Google Scholar]
- Nalbant P., Hodgson L., Kraynov V., Toutchkine A., Hahn K. M. (2004). Activation of endogenous Cdc42 visualized in living cells. Science 305, 1615–1619 10.1126/science.1100367 [DOI] [PubMed] [Google Scholar]
- O'Neill C. (2008). Phosphatidylinositol 3-kinase signaling in mammalian preimplantation embryo development. Reproduction 136, 147–156 10.1530/REP-08-0105 [DOI] [PubMed] [Google Scholar]
- Ohsugi M., Zheng P., Baibakov B., Li L., Dean J. (2008). Maternally derived FILIA-MATER complex localizes asymmetrically in cleavage-stage mouse embryos. Development 135, 259–269 10.1242/dev.011445 [DOI] [PubMed] [Google Scholar]
- Pfender S., Kuznetsov V., Pleiser S., Kerkhoff E., Schuh M. (2011). Spire-type actin nucleators cooperate with Formin-2 to drive asymmetric oocyte division. Curr. Biol. 21, 955–960 10.1016/j.cub.2011.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powis G., Bonjouklian R., Berggren M. M., Gallegos A., Abraham R., Ashendel C., Zalkow L., Matter W. F., Dodge J., Grindey G.et al. (1994). Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Res. 54, 2419–2423 [PubMed] [Google Scholar]
- Ptasznik A., Beattie G. M., Mally M. I., Cirulli V., Lopez A., Hayek A. (1997). Phosphatidylinositol 3-kinase is a negative regulator of cellular differentiation. J. Cell Biol. 137, 1127–1136 10.1083/jcb.137.5.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan M. E., Heuser J. E., Kerkhoff E., Mullins R. D. (2005). Drosophila Spire is an actin nucleation factor. Nature 433, 382–388 10.1038/nature03241 [DOI] [PubMed] [Google Scholar]
- Reddy P., Liu L., Adhikari D., Jagarlamudi K., Rajareddy S., Shen Y., Du C., Tang W., Hämäläinen T., Peng S. L.et al. (2008). Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science 319, 611–613 10.1126/science.1152257 [DOI] [PubMed] [Google Scholar]
- Reddy P., Adhikari D., Zheng W., Liang S., Hämäläinen T., Tohonen V., Ogawa W., Noda T., Volarevic S., Huhtaniemi I.et al. (2009). PDK1 signaling in oocytes controls reproductive aging and lifespan by manipulating the survival of primordial follicles. Hum. Mol. Genet. 18, 2813–2824 10.1093/hmg/ddp217 [DOI] [PubMed] [Google Scholar]
- Ren X. D., Kiosses W. B., Schwartz M. A. (1999). Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 18, 578–585 10.1093/emboj/18.3.578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl J., Crevenna A. H., Kessenbrock K., Yu J. H., Neukirchen D., Bista M., Bradke F., Jenne D., Holak T. A., Werb Z.et al. (2008). Lifeact: a versatile marker to visualize F-actin. Nat. Methods 5, 605–607 10.1038/nmeth.1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh M., Ellenberg J. (2008). A new model for asymmetric spindle positioning in mouse oocytes. Curr. Biol. 18, 1986–1992 10.1016/j.cub.2008.11.022 [DOI] [PubMed] [Google Scholar]
- Shpetner H., Joly M., Hartley D., Corvera S. (1996). Potential sites of PI-3 kinase function in the endocytic pathway revealed by the PI-3 kinase inhibitor, wortmannin. J. Cell Biol. 132, 595–605 10.1083/jcb.132.4.595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S., Wang F., Glavas S., Ott A., Hofmann F., Aktories K., Kalman D., Bourne H. R. (2003). Rac and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis. J. Cell Biol. 160, 375–385 10.1083/jcb.200208179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S. C., Kim N. H. (2011). GM130: new insights into oocyte asymmetric division. Cell Cycle 10, 1639–1654 10.4161/cc.10.15.15932 [DOI] [PubMed] [Google Scholar]
- Sun S. C., Sun Q. Y., Kim N. H. (2011a). JMY is required for asymmetric division and cytokinesis in mouse oocytes. Mol. Hum. Reprod. 17, 296–304 10.1093/molehr/gar006 [DOI] [PubMed] [Google Scholar]
- Sun S. C., Wang Z. B., Xu Y. N., Lee S. E., Cui X. S., Kim N. H. (2011b). Arp2/3 complex regulates asymmetric division and cytokinesis in mouse oocytes. PLoS ONE 6, e18392 10.1371/journal.pone.0018392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S. C., Xu Y. N., Li Y. H., Lee S. E., Jin Y. X., Cui X. S., Kim N. H. (2011c). WAVE2 regulates meiotic spindle stability, peripheral positioning and polar body emission in mouse oocytes. Cell Cycle 10, 1853–1860 10.4161/cc.10.11.15796 [DOI] [PubMed] [Google Scholar]
- Sutton–McDowall M. L., Gilchrist R. B., Thompson J. G. (2010). The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction 139, 685–695 10.1530/REP-09-0345 [DOI] [PubMed] [Google Scholar]
- Tong Z. B., Gold L., Pfeifer K. E., Dorward H., Lee E., Bondy C. A., Dean J., Nelson L. M. (2000). Mater, a maternal effect gene required for early embryonic development in mice. Nat. Genet. 26, 267–268 10.1038/81547 [DOI] [PubMed] [Google Scholar]
- Verlhac M. H., Lefebvre C., Guillaud P., Rassinier P., Maro B. (2000). Asymmetric division in mouse oocytes: with or without Mos. Curr. Biol. 10, 1303–1306 10.1016/S0960-9822(00)00753-3 [DOI] [PubMed] [Google Scholar]
- Wang F., Herzmark P., Weiner O. D., Srinivasan S., Servant G., Bourne H. R. (2002). Lipid products of PI(3)Ks maintain persistent cell polarity and directed motility in neutrophils. Nat. Cell Biol. 4, 513–518 10.1038/ncb810 [DOI] [PubMed] [Google Scholar]
- Yu L. Z., Xiong B., Gao W. X., Wang C. M., Zhong Z. S., Huo L. J., Wang Q., Hou Y., Liu K., Liu X. J.et al. (2007). MEK1/2 regulates microtubule organization, spindle pole tethering and asymmetric division during mouse oocyte meiotic maturation. Cell Cycle 6, 330–338 10.4161/cc.6.3.3805 [DOI] [PubMed] [Google Scholar]
- Zhang C. H., Wang Z. B., Quan S., Huang X., Tong J. S., Ma J. Y., Guo L., Wei Y. C., Ouyang Y. C., Hou Y.et al. (2011). GM130, a cis-Golgi protein, regulates meiotic spindle assembly and asymmetric division in mouse oocyte. Cell Cycle 10, 1861–1870 10.4161/cc.10.11.15797 [DOI] [PubMed] [Google Scholar]
- Zheng P., Dean J. (2009). Role of Filia, a maternal effect gene, in maintaining euploidy during cleavage-stage mouse embryogenesis. Proc. Natl. Acad. Sci. USA 106, 7473–7478 10.1073/pnas.0900519106 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.