Summary
Cyclic AMP (cAMP) is a ubiquitous second messenger that regulates a variety of essential processes in diverse cell types, functioning via cAMP-dependent effectors such as protein kinase A (PKA) and/or exchange proteins directly activated by cAMP (EPAC). In an intact tissue it is difficult to separate the contribution of each cAMP effector in a particular cell type using genetic or pharmacological approaches alone. We, therefore, utilized optogenetics to overcome the difficulties associated with examining a multicellular tissue. The transgenic photoactive adenylyl cyclase bPAC can be activated to rapidly and reversibly generate cAMP pulses in a cell-type-specific manner. This optogenetic approach to cAMP manipulation was validated in vivo using GAL4-driven UAS–bPAC in a simple epithelium, the Drosophila renal (Malpighian) tubules. As bPAC was expressed under the control of cell-type-specific promoters, each cAMP signal could be directed to either the stellate or principal cells, the two major cell types of the Drosophila renal tubule. By combining the bPAC transgene with genetic and pharmacological manipulation of either PKA or EPAC it was possible to investigate the functional impact of PKA and EPAC independently of each other. The results of this investigation suggest that both PKA and EPAC are involved in cAMP sensing, but are engaged in very different downstream physiological functions in each cell type: PKA is necessary for basal secretion in principal cells only, and for stimulated fluid secretion in stellate cells only. By contrast, EPAC is important in stimulated fluid secretion in both cell types. We propose that such optogenetic control of cellular cAMP levels can be applied to other systems, for example the heart or the central nervous system, to investigate the physiological impact of cAMP-dependent signaling pathways with unprecedented precision.
Key words: Photoactive adenylyl cyclase, PAC, Optogenetics, cAMP signaling pathway, PKA, EPAC, Renal fluid secretion, Drosophila Malpighian tubules
Introduction
The second messenger cAMP controls a variety of processes in diverse cell types, including the relay of ligand-mediated receptor activation into an appropriate cellular response. This requires activation of one or more alternative cAMP effectors such as protein kinase A (PKA), cAMP-gated ion channels (CNGs), phosphodiesterases (PDEs) and/or exchange proteins directly activated by cAMP (EPACs) (Kandel, 2001; Kaupp and Seifert, 2002; Gloerich and Bos, 2010). While many cellular responses have traditionally been attributed to activation of PKA and CNGs, contemporary models increasingly emphasize both a contribution of EPAC (a guanine nucleotide exchange factor for Ras-like small GTPases), and strong interplay between these pathways (Bos, 2003; Bos et al., 2003; Bos, 2005; Stokman et al., 2011). However, it is difficult to disentangle these complex interactions and unambiguously identify the cAMP-dependent signaling pathways responsible for a particular physiological process in vivo. There are two reasons for this: firstly, the pharmacological agents used for selective activation of alternative cAMP sensors (Chepurny et al., 2010) do not provide cell-type specificity; and secondly, genetic approaches based on cell-type-specific promoters lack the fine temporal control over transgene activity – typically seconds – needed for physiological study. In the present study, we overcame these limitations with a genetically encoded bacterial photoactive adenylyl cyclase (bPAC) from the filamentous bacterium Beggiatoa sp., which rapidly elevated cellular cAMP levels when stimulated by blue light (Schröder-Lang et al., 2007; Ryu et al., 2010; Stierl et al., 2011). As PACs can be expressed under the control of cell-type-specific promoters, this optogenetic approach allowed both temporal and spatial control of cAMP signaling. Parallel manipulation of either PKA or EPAC using standard genetic or pharmacological techniques allowed further resolution of these two signaling pathways.
The validity of this approach was explored in excised renal (Malpighian) tubules of Drosophila melanogaster, as this tissue has various advantages for physiological studies (see Fig. 1A for a schematic overview). Firstly, the renal tubule is a highly transparent simple epithelium, allowing easy penetration of the blue light required for bPAC activation. Secondly, the main segment of the tubule is composed of only two cell types; the principal cells and the stellate cells. The GAL4/UAS system can be used to target transgene expression specifically to either subset of cells within the tissue (Sözen et al., 1997; Kerr et al., 2004). Thirdly, the contribution of each cell type to fluid secretion is well established: the principal cells actively transport potassium from the basolateral to apical surface of the tubule via a defined array of ion transporters, including a basolateral Na+-K+-ATPase (Torrie et al., 2004) and inward-rectifier K+ channels (Evans et al., 2005); and apically, a plasma membrane H+ V-ATPase and an alkali-metal/proton exchanger (Day et al., 2008; Dow, 2009; Beyenbach et al., 2010). The role of the stellate cells is to regulate water flux and anion shunt conductance, via chloride channels (O'Donnell et al., 1998), aquaporins (Kaufmann et al., 2005), or regulation of paracellular transport routes through the modification of tight junctions (Beyenbach et al., 2010). Fourthly, renal fluid secretion is under neuroendocrine control, and has been studied extensively in Drosophila and other insects (Davies, 2000; Coast and Garside, 2005; Dow, 2007; Coast, 2009). Activation of specific receptors triggers a diuretic or anti-diuretic response via a cascade of second messengers, including Ca2+ and the cyclic nucleotides cAMP and cyclic GMP (cGMP) (Dow and Davies, 2003). However, although the cognate second messengers for each ligand are known, the downstream mechanisms that control fluid transport are still being investigated.
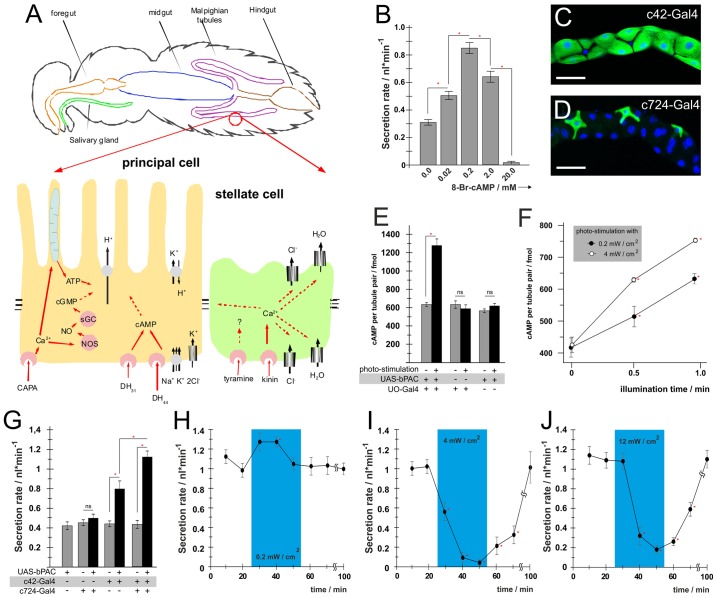
Fig. 1.
The impact of cAMP signals on Drosophila renal fluid secretion defined by pharmacology and optogenetics. (A) Drosophila renal (Malpighian) tubules are adjacent to the gastrointestinal system and are devoted to water balance and ionic homeostasis, orthologous to the mammalian kidney. Two cell types, principal and stellate cells, provide distinct functions to the process of secretion: principal cells accomplish the net active transport of potassium from the basolateral to apical surface via various classes of ion transporters and thereby provide an electrochemical gradient that energizes the process of fluid secretion, whereas stellate cells regulate conductance of anions and water. Established (solid lines) and assumed (dotted lines) contributors to regulation of fluid secretion within the Malpighian tubule are shown in the lower diagram (for review see Beyenbach et al., 2010). (B) Application of the cell-permeable cAMP derivative 8-Br-cAMP stimulated fluid secretion in isolated tubules when applied in micromolar concentrations, whereas millimolar amounts resulted in inhibition. (C,D) Cell-type-specific expression of the UAS-GFP reporter construct illustrating specificity of Gal4 lines used for selective manipulation of either principal (C) or stellate cells (D). Nuclei were stained with DAPI. Scale bars: 30 µm. (E) Photostimulation with 2 mW/cm2 for 15 min in the presence of the PDE inhibitor IBMX resulted in cAMP increase when bPAC was expressed in the principal cells, but not in genetic controls bearing either the Gal4 or the UAS element alone. n = 3. (F) When bPAC was expressed in the principal cells, the increase in cytosolic cAMP correlated with the duration and intensity of photostimulation. n = 3 (G) Expression of the photoactive bPAC transgene stimulates secretion in the dark because of background activity when expressed in the principal cells, but not the stellate cells. (H–J) The bPAC transgene stimulates fluid secretion when simultaneously expressed in principal and stellate cells under control of c42– and c724–Gal4 drivers, respectively. Illumination with various intensities [0.2 (H), 4.0 (I) or 12.0 (J) mW/cm2] of blue light (indicated by blue shading) resulted in dynamic and reversible modulation of fluid secretion. Different kinetics of cAMP action of either the principal or stellate cells is probably responsible for delay of inhibition. All data in B and G–J are means ± s.e.m., n>20 tubules from two independent experiments. Asterisks indicate significant differences (P<0.05).
Here, we systematically probed the impact of light-induced cAMP signals on fluid secretion, using mutants, cell-specific transgenics and optogenetics; and uncovered a complex framework of PKA and EPAC signals with functionally distinct roles in the principal and stellate cells of the Drosophila renal tubules.
Results
Cyclic AMP exerts a bimodal control on fluid secretion
The cell-permeable cAMP analogue, 8-Br-cAMP, was applied to excised tubules and the resultant impact on fluid secretion measured (Fig. 1B). While micromolar concentrations of 8-Br-cAMP increased fluid secretion, millimolar concentrations decreased secretion to below the resting basal level. The observed impact of cAMP on fluid secretion is similar to that described for cGMP (O'Donnell et al., 1996), and invites further investigation into the molecular effectors of this bimodal relationship. However, as 8-Br-cAMP is likely to penetrate both the principal and stellate cells, it does not allow us to elucidate cell-type-specific responses. To overcome this limitation we utilized the photoactive adenylyl cyclase bPAC, an optogenetic transgene that generates cAMP upon stimulation with blue light (Ryu et al., 2010; Stierl et al., 2011). Importantly using the GAL4/UAS binary expression system in Drosophila (Brand and Perrimon, 1993), bPAC can be driven specifically in either the principal or stellate cells of the Malpighian tubules by crossing UAS-bPAC flies to the appropriate GAL4 drivers, i.e. c42 (Rosay et al., 1997) or Uro (Terhzaz et al., 2010) for principal cells, and c724 (Sözen et al., 1997) for stellates, allowing the impact of increased cAMP in each cell type to be investigated. Driving expression using c42–Gal4 results in transgene expression in the principal cells of the tubules (Fig. 1C), while c724–Gal4 reliably drives expression in the stellate cells (Fig. 1D). Previous publications have demonstrated that the intracellular level of cAMP correlates with the intensity and duration of activating blue light in both prokaryotic (Ryu et al., 2010) and eukaryotic model systems containing bPAC (Stierl et al., 2011). Similarly, there is a significant increase in cellular cAMP upon light stimulation of Drosophila renal tubules expressing bPAC in the principal cells (Fig. 1E). The increase in cellular cAMP correlates with the time period and intensity of the illumination, indicating both temporal and cell-type-specific control of activation (Fig. 1F).
Although bPAC is activated by blue light illumination, there is low-level residual cyclase activity in the dark (Schröder-Lang et al., 2007; Weissenberger et al., 2010; Ryu et al., 2011; Stierl et al., 2011). When expressed in the principal cells we observed a 1.7-fold increase in cellular cAMP levels attributable to bPAC when tissues were maintained in the dark, and PDE activity was inhibited by 3-isobutyl-1-methylxanthine (IBMX; data not shown). Accordingly, we assessed the impact of basal bPAC activity on tubule function without blue light stimulation (Fig. 1G). While bPAC did not affect fluid secretion when expressed in the stellate cells, expression in the principal cells increased secretion from 0.5 to 0.8 nl/min. When simultaneously expressed in both cell types secretion was further increased to 1.1 nl/min. This background activity must thus be considered when analyzing experiments performed using the c42–Gal4 driver. We addressed this issue by quantifying fluid secretion before, during and after a 30-minute stimulation of the tubules with various intensities of blue light (Fig. 1H–J). When bPAC was expressed in either cell type the basal rate of fluid secretion was elevated as expected, but it was further increased upon illumination with low intensity blue light (0.2 mW/cm2). In contrast, intermediate (4.0 mW/cm2) or high (12.0 mW/cm2) intensities inhibited fluid secretion completely (Fig. 1I,J). This data demonstrates a bimodal secretion response to bPAC-derived cAMP, similar to that observed with 8-Br-cAMP. Although there is residual dark activity of the bPAC transgene, it is easily distinguished from any light-induced changes.
Inhibition of secretion at high cAMP concentration is caused by action on the principal cells
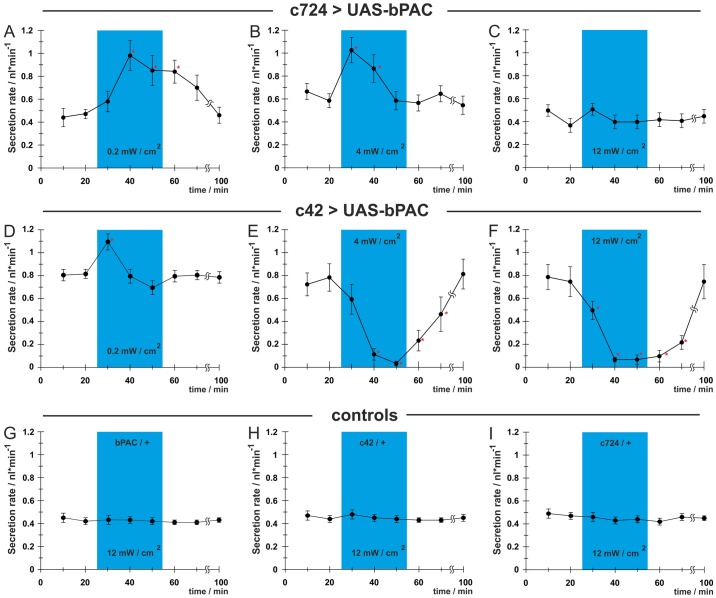
To elucidate the effect of cAMP stimulation on the principal and stellate cells, UAS–bPAC was driven in either the principal or stellate cells by c42– or c724–Gal4, respectively. The rate of fluid secretion was recorded under illumination with low, intermediate or high intensity blue light, i.e. 0.2, 4.0 or 12.0 mW/cm2, respectively (Fig. 2). When bPAC was expressed in the stellate cells we observed a stimulatory stellate cAMP signal that increased fluid secretion after activation with low to intermediate intensities of blue light (Fig. 2A,B). In contrast, high intensity stimulations did not increase fluid secretion above the basal rate (Fig. 2C). As high cAMP levels in the stellate cells do not decrease secretion, another mechanism must be invoked for the total inhibition of fluid secretion observed with high concentrations of 8-Br-cAMP, or with ubiquitously driven bPAC stimulated with high light intensities. It seems likely therefore, that this effect originates from the principal cells. Interestingly, in principal cells when bPAC was activated by low light a stimulatory principal cAMP signal was observed (Fig. 2D), but when bPAC was activated by intermediate to high light intensities, an inhibitory principal cAMP signal that disrupted fluid secretion was observed (Fig. 2E,F). All of these effects were reversible within a few minutes without blue light. Genetic controls bearing either of the Gal4 drivers or bPAC alone did not show any significant modulation of fluid secretion when maximally illuminated (Fig. 2G–I).
Fig. 2.
Optogenetic control of cellular cAMP signals reveals cell-specific responses. When bPAC was expressed in stellate cells under the control of the c724–Gal4 driver, light activation with low/intermediate intensities resulted in stimulation of fluid secretion (A,B), whereas high intensities did not affect fluid secretion (C). When expressed in principal cells under the control of c42–Gal4, bPAC stimulated fluid secretion at a light intensity of 0.2 mW/cm2 (D), but higher light intensities resulted in inhibition (E,F). Genetic controls bearing one of the transgenes alone showed no change in fluid secretion when illuminated at 12.0 mW/cm2 (G–I). All data are means ± s.e.m., n>20 from two independent experiments. Asterisks indicate significant differences (P<0.05).
It was also noteworthy that, even when activating light levels were used, secretion decreased under sustained illumination (see Fig. 2A,B,D). This might reflect long-term adaptations of the bPAC transgene, or alternatively might reveal the activation of antagonizing mechanisms, such as inhibition of PKA, or activation of cyclic nucleotide-specific phosphodiesterases (PDEs).
Taken together, these results establish that the manipulation of cAMP levels can result in distinct downstream effects in both the principal and stellate cells. To further investigate this we examined the roles of the cAMP-dependent signaling molecules protein kinase A (PKA) and the exchange protein directly activated by cAMP (EPAC) in control of renal fluid secretion.
Control of secretion requires both PKA and EPAC
Classically, many cAMP-dependent cellular responses have initially been attributed to activation of PKA. However, the contribution of EPACs, and crosstalk between both cAMP-dependent pathways, is increasingly being recognized. In order to distinguish their role in the control of fluid secretion, we took advantage of appropriate PKA and EPAC mutant fly lines. In Drosophila the most abundant catalytic subunit of PKA is encoded by the DC0 gene and PKA activity is markedly reduced in flies which are heterozygous for the DC0581 or DC0B10 allele; homozygotes are not viable (Kalderon and Rubin, 1988). Heterozygous DC0 mutants exhibited strongly reduced resting secretion rates, confirming that PKA activity is necessary for maintenance of basal fluid secretion (Fig. 3A). In contrast, null epac mutants exhibited basal secretion rates indistinguishable from those of wild-type Canton-S (Fig. 3B), but failed to respond to stimulation by the cell-permeable EPAC-specific agonist 2 mM 8-pCPT-2′-O-Me-cAMP (Enserink et al., 2002). Together, these results support several major conclusions: firstly, maintaining a basal rate of renal fluid secretion is EPAC independent but PKA dependent, and secondly, stimulation of fluid secretion requires an EPAC-dependent component. Although these manipulations are not cell specific, the findings are nonetheless remarkable, as they demonstrate that basal and stimulated fluid secretion are under separate control. Generation of null EPAC mutants is outlined in Fig. 3C,E.
Fig. 3.
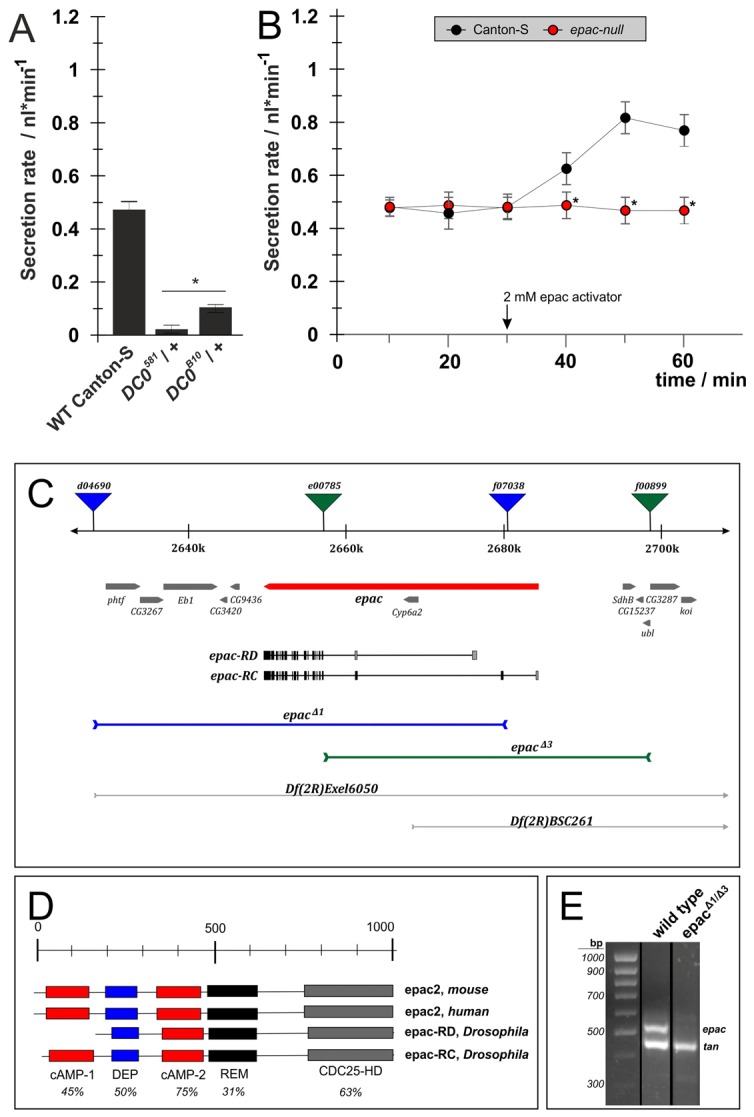
The cAMP effectors PKA and EPAC have separable effects on fluid secretion. For manipulation of PKA we used different alleles of the PKA catalytic subunit DC0 isolated by Kalderon and Rubin (Kalderon and Rubin, 1988). For manipulation of the non-canonical cAMP target EPAC we generated a null EPAC mutant by targeted deletion. (A) Basal fluid secretion rates in DC0581 or DC0B10 heterozygotes were strongly reduced compared with wild-type Canton-S controls. (B) In epac null tubules, basal secretion was indistinguishable from wild-type Canton-S controls. However, they failed to respond to application of 2 mM 8-pCPT-2′-O-Me-cAMP, a cell-permeable, EPAC-specific agonist. All data are means ± s.e.m. (n>20) from two independent experiments. Significant (P<0.05) differences are denoted by asterisks (Student's t-test, two-tailed). (C) Deletions at the unique Drosophila epac locus were generated by remobilization of FRT-containing P-elements: epacΔ1 was generated by combining d04690 and f07038; epacΔ3 by combining e00785 and f00899. (D) Homology comparison of epac proteins from human, mouse and Drosophila indicates high homology. (E) QRT-PCR on cDNA generated from either epacΔ1/epacΔ3 or wild-type Canton-S flies. Specific primers were used to amplify products from either Drosophila epac or tan, which served as an internal control.
Mapping distinct functions for PKA signals in fluid secretion
Having formally established roles for PKA and EPAC in the control of basal and stimulated fluid secretion, we employed a variety of genetic techniques to manipulate PKA or EPAC signaling in a cell-type-specific manner, in an effort to understand their cell-specific actions.
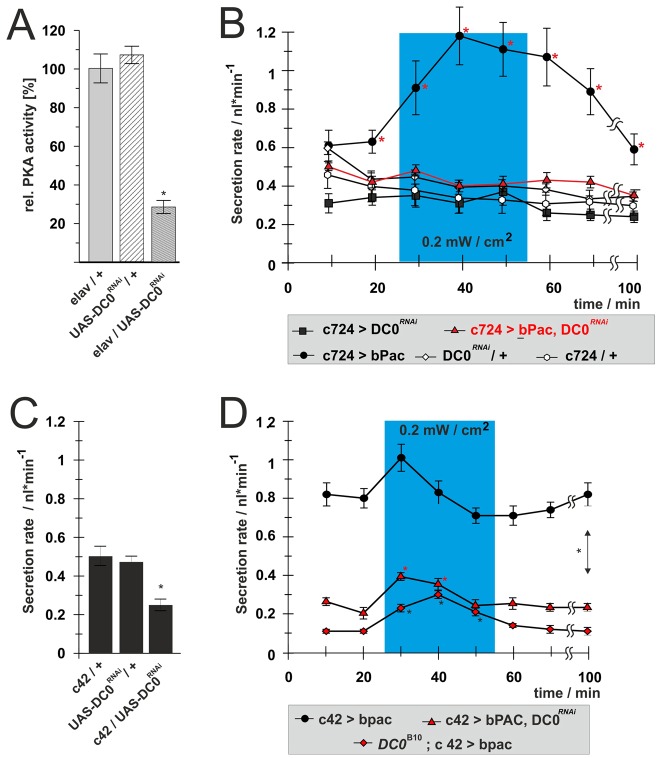
The GAL4/UAS system allows targeted gene knockdown by RNA interference, and expression of UAS–DC0RNAi reduces PKA transcript and protein levels by at least 70% (Iijima-Ando et al., 2009) (Fig. 4A). When UAS–DC0RNAi was driven in stellate cells in combination with bPAC, the light-dependent stimulation of secretion was abolished (Fig. 4B). However, PKA knockdown did not affect basal fluid secretion rates, as no significant differences were observed when compared to genetic controls. Therefore PKA is necessary to activate the stimulatory stellate cell cAMP signal, but not for maintenance of the basal secretion rate, implying that basal rates might be controlled by the principal cells.
Fig. 4.
PKA effect on stimulation or maintenance of fluid secretion is cell-type specific. The impact of PKA on fluid secretion was defined by use of targeted knockdown or systemic mutation of DC0, an abundant PKA catalytic subunit. (A) Targeted knockdown of UAS-DC0-RNAi reduced PKA activity in brain homogenates (Iijima-Ando, 2009). (B) Interfering with PKA activity in the stellate cells by knockdown of DC0 completely abolished stimulation of fluid secretion after photoactivation of bPAC (blue shading). However, basal levels of fluid secretion were not affected by RNA interference. (C) Reducing PKA activity within the principal cells reduced fluid secretion, thus establishing UAS-DC0-RNAi as a potent tool for this cell type. (D) Reducing PKA activity by knockdown in principal cells (c42>UAS-DC0RNAi), or systemically within DC0B10 heterozygous mutants strongly reduced basal fluid secretion rates. However, stimulation of renal function by photoactivation of simultaneously expressed c42>bPAC was unaffected – although starting from a lower level. Overall, upregulation of secretion by 0.2 nl/min could be observed. Significant (P<0.05) differences are denoted by asterisks.
To restrict knockdown of PKA activity to the principal cells we expressed UAS–DC0RNAi under the control of c42–Gal4. Those animals showed significantly reduced levels of basal fluid secretion from the tubules (Fig. 4C), confirming the requirement for PKA activity in the principal cells for the maintenance of basal fluid secretion. Next, we combined bPAC with either targeted knockdown of DC0, or systemic reduction of DC0 expression in DC0B10 heterozygotes (Fig. 4D). Although expressing reduced levels of PKA, both DC0B10 heterozygotes and c42>UAS–DC0RNAi tubules showed significant stimulation of fluid secretion with low light activation of bPAC. These results show that a reduction in PKA activity sufficient to abolish stimulated secretion in stellate cells, drastically reduces resting secretion, but impacts only minimally on stimulated secretion in principal cells, so the major stimulatory signal in principal cells is conveyed independently of PKA.
The manipulation of PKA signaling in specific cell types has revealed multiple functional pathways: stimulatory stellate cAMP signals act via PKA, while maintenance of basal fluid secretion requires PKA activity in the principal cells. Significantly, the principal cell stimulatory cAMP signal requires an effector other than PKA.
Mapping the functional impact of EPAC signals on fluid secretion
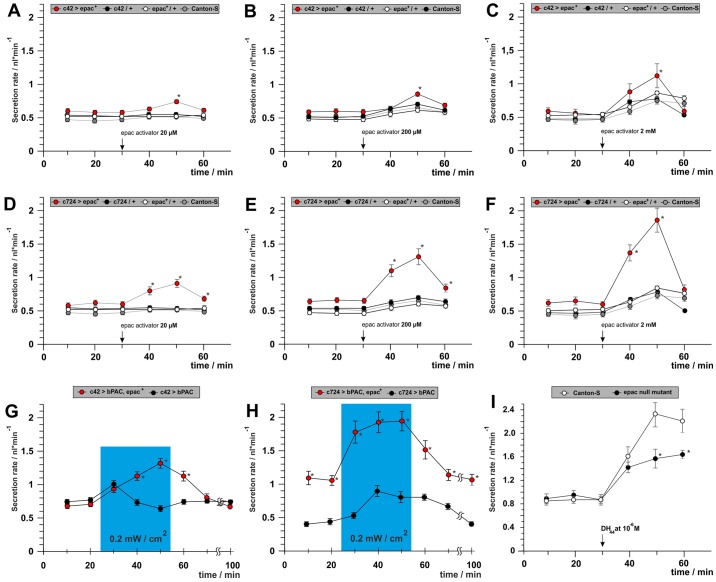
EPAC has already been shown to support stimulation, but not inhibition of fluid secretion. Potentially, this increase in secretion could result from EPAC activity in either the principal or stellate cells, or in both. To further understand the importance of the cellular localization of EPAC in the Drosophila renal tubules (Fig. 5), we firstly assessed the effect of the EPAC agonist 8-pCPT-2′-O-Me-cAMP on fluid secretion when applied to tubules overexpressing EPAC in either the stellate or principal cells (Fig. 5A–F). An increase in fluid secretion in both sets of animals suggested that EPAC signals autonomously in the two cell types. Next, we used cell-type-specific bPAC expression to generate cell-autonomous cAMP signals, and showed that the stimulatory principal cAMP signal potentiates stimulated, but not basal fluid secretion via EPAC (Fig. 5G). In contrast, even in the dark, the stellate cells show an overall upregulation of fluid secretion upon expression of UAS–EPAC and the bPAC transgene (Fig. 5H). Light-induced generation of a stimulatory stellate cAMP signal by photoactivation of bPAC resulted in a rapid, massive and sustained increase in fluid secretion to 2.0 nl/min.
Fig. 5.
EPAC acts in both stellate and principal cells. EPAC signaling can be potentiated by expressing epac+ cDNA in either stellate or principal cells. Expressing epac+ cDNA in either stellate (A–C) or principal cells (D–F) potentiates stimulatory effects of the EPAC-specific agonist 8-pCPT-2′-O-Me-cAMP. (G) Overexpression of epac in principal cells potentiates bPAC-induced stimulation of secretion, but does not affect basal secretion rate. (H) In contrast, epac overexpression in the stellate cells elevates both basal and stimulated fluid secretion. (I) Stimulation of secretion evoked by the DH44 peptide was reduced in epac null tubules. Significant (P<0.05) differences are denoted by asterisks.
It is also vital to verify that EPAC signaling is important under experimental conditions that resemble a natural diuretic stimulus, when cAMP is not artificially increased using bPAC. We used DH44, a diuretic neuropeptide that augments fluid secretion via cAMP signaling in the principal cells (Johnson et al., 2005; Hector et al., 2009) to elicit a physiological response. The stimulatory effects of DH44 were reduced in null epac mutants (Fig. 5I), confirming our earlier conclusion that EPAC signaling is required for maximal augmentation of fluid secretion in the principal cells.
It would be interesting to further investigate the role of EPAC by cell-type-specific photostimulation of bPAC in a null or hypomorphic epac background. Unfortunately, the two known GAL4 drivers for stellate cells, i.e. c710 and c724 (Sözen et al., 1997), both map to tsh which, like epac, is close to the centromere of chromosome 2; and we have so far been unable to obtain recombinants with our epac deletions. In principle, epac RNAi would provide an alternative route; however, of two lines screened (Vienna stocks ID 50372 and 50373), line 50372 was without effect, whereas 50373 showed residual effects even when not driven. Therefore, further work will be required to generate flies in which this experiment can be performed.
Discussion
Here, we pioneered the use of bPAC, a photoactive adenylyl cyclase, as an optogenetic tool to distinguish between the functions of alternative cAMP effectors in the regulation of a physiological process in vivo. To validate bPAC as an in vivo tool we used Drosophila renal tubules to confirm the bPAC transgene could be stimulated with blue light to generate cAMP signals in a cell-type-specific manner. We combined this optogenetic approach with standard techniques that targeted PKA or EPAC to resolve the complex regulatory network of discrete cAMP pathways involved in the control of fluid secretion.
Separate functions for PKA and EPAC within principal cells
Primary urine is generated within the main segment of the Malpighian tubules, where the principal cells establish an electrochemical gradient that provides the driving force for fluid secretion, by actively transporting potassium from the basolateral to the apical surface via a defined array of ion transporters. In parallel, the stellate cells control the anion shunt conductance and water flux of the tubules, via the action of tightly regulated aquaporins and chloride channels.
As revealed by our analysis, two distinct cAMP pathways are deployed within the principal cells to sustain fluid secretion: firstly, the basal principal cell PKA pathway, which regulates the rate of basal fluid secretion; and secondly the stimulatory principal cell EPAC pathway, which stimulates fluid secretion above basal levels in a cAMP-dependent manner. Manipulation of EPAC activity altered stimulated secretion but not basal secretion, and manipulation of PKA altered basal secretion but not stimulated secretion. In this respect, the two principal cell secretory control pathways appear to be independent of one another (Fig. 6).
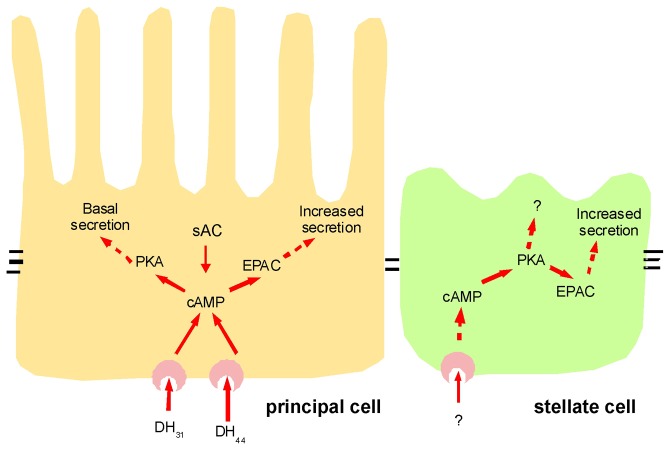
Fig. 6.
Model for camp-dependent regulation of principal and stellate cells. cAMP signals effect numerous functions in the principal cells that are mediated by alternative downstream cAMP sensors. The basal principal component is mediated by PKA and likely to act on apical H+ V-ATPases. Under natural conditions, the appropriate cAMP signal might be generated either by soluble adenylyl cyclases (sAC) or upon activating the G-protein-coupled receptors DH31 or DH44. The stimulating principal component is linked to EPAC as a downstream mediator of instructive cAMP signals.
Is there separate neuroendocrine control of cAMP signaling pathways?
Could these downstream pathways be controlled independently in vivo, through a single second messenger? While imposed cAMP signals feeding into each pathway could be generated by activation of the bPAC transgene with a defined light intensity, in vivo the neuropeptides DH44, related to corticotropin releasing factor (CRF) (Cabrero et al., 2002), and DH31, related to calcitonin/calcitonin gene-related peptide (CGRP), both increase fluid secretion by raising cAMP in the principal cells (Johnson et al., 2004; Johnson et al., 2005). However, there is evidence in other insects that these two neuropeptides might have distinct downstream effects; in the related malarial mosquito Anopheles gambiae, DH31, but not DH44, acts as a natriuretic peptide by increasing basolateral Na+ conductance (Coast et al., 2005). Moreover, DH31 and DH44 have an additive stimulatory effect on fluid secretion, suggesting that they target different transport processes (Coast et al., 2001). Cellular association of specific GPCRs with either PKA or EPAC might well account for the different outputs observed from each GPCR. Another tempting possibility involves a class of soluble adenylyl cyclases (sACs) that are localized near the apical membrane and activated by cellular ionic concentrations rather than GPCRs, as seen in the mammalian kidney (Pastor-Soler et al., 2003; Pastor-Soler et al., 2008; Hallows et al., 2009).
Does cAMP control the plasma membrane V-ATPase?
The apical plasma membrane H+ V-ATPase is the driving force for ion transport in the principal cells, and is therefore an obvious downstream target for stimulatory or inhibitory cAMP signals. (Beyenbach and Wieczorek, 2006). Formation of a functional V-ATPase complex requires PKA-dependent phosphorylation, which prevents the complex from disassembly (Pastor-Soler et al., 2008; Rein et al., 2008; Poulsen et al., 2010). In blowfly salivary gland (another insect epithelium energized by a V-ATPase), cAMP has been shown to promote assembly of the V-ATPase complex (Dames et al., 2006). However, V-ATPase assembly – and thus activation – has also been reported via EPAC signaling within the rat renal collecting duct (Laroche-Joubert et al., 2002). By contrast, intracellular calcium has been shown to activate tubule H+ V-ATPase by directly activating mitochondria, and so increasing the ATP supply (Terhzaz et al., 2006). In this complex field, optogenetic control of cellular cAMP levels in the principal cells will provide a valuable analytical tool to investigate such issues.
How does cAMP inhibit fluid secretion?
A surprising feature of cAMP-dependent fluid secretion is the complete inhibition (below basal) observed with millimolar levels of cell-permeable 8-Br-cAMP, or at very high illumination levels in bPAC-transgenic tubules. Through targeted use of bPAC this effect was localized to the principal cells, and formally established an inhibitory principal cell cAMP signal. It is likely that these manipulations bring intracellular cAMP levels to abnormally high levels that are unlikely to be reached in vivo, where the resting intracellular cAMP concentration is typically in the range 0.1–1.5 µM (Börner et al., 2011); nonetheless, there is a real effect to be explained. At present, we can only speculate on the underlying mechanisms, but it is likely that saturation or desensitization of some component of the signaling pathway is occurring; or that there is cross-talk to, for example calcium signaling via cyclic nucleotide gated calcium channels, which are known to play a role in tubule (MacPherson et al., 2001).
How does cAMP control stellate cells?
In the stellate cells we identified a stimulatory stellate cAMP signal that stimulates fluid secretion via PKA, with moderate illuminations of bPAC. In contrast, high illuminations return fluid secretion to the baseline level, suggesting that dual modulation, i.e. augmentation with low levels and inhibition with high levels of cAMP, is a common theme within the stellate and principal cells. However, further experiments will be required to substantiate this speculation.
Interestingly, the stellate cells are known to be controlled by leucokinin, which acts though calcium, rather than cAMP (Radford et al., 2002), so no extracellular ligand for the stellate cAMP pathway is presently known. Tyramine has also been shown to act on stellate cells, but its second messenger is yet to be established.
Interaction of EPAC and PKA signaling within stellate cells
Selective elevation of cAMP in stellate cells shows that both PKA and EPAC can stimulate fluid secretion. However, these pathways do not act in parallel in the stellate cells; PKA must be upstream of EPAC, because RNAi knockdown of DC0 in stellate cells abolishes the ability of bPAC to stimulate fluid secretion (Fig. 4). In contrast, EPAC is sufficient for secretion when activated in a cAMP-independent manner via the EPAC-specific agonist 8-pCPT-2′-O-Me-cAMP (see Fig. 3). Therefore, cAMP is likely to signal through PKA to EPAC. In turn, EPAC levels are likely to be rate limiting, as stellate-specific overexpression of epac enormously enhanced secretion (Fig. 5D–F).
Benefits and shortcomings of light-induced cAMP signaling in vivo
Here, we have established the use of photoactive adenylyl cyclases (PACs) as a potent tool for investigating organotypic physiological processes in vivo. A unique advantage of this optogenetic transgene is that it acts as a ‘Trojan horse’, allowing cell-type-specific control of cellular cAMP levels with temporal and spatial precision, through simple blue light illumination. It is this feature that has allowed us to deconstruct the complex regulatory network of cAMP pathways involved in fluid secretion control, and to assign function within the Drosophila renal (Malpighian) tubule. We are confident that this experimental approach can easily be adapted to other physiological preparations, for example the central nervous system or the cardiac system, to address similar physiological questions.
Further improvements to bPAC could be achieved; for example it would be beneficial to further reduce the residual dark activity, which must be considered during experimental analysis. Although functional imaging of cAMP has been achieved (Zaccolo, 2009), further development of this complementary technology would be advantageous for studying complex cellular signaling networks. Another feature of light-induced cAMP signals is that, as bPAC is cytoplasmic, the elevation of cAMP is uniform across the cell. In contrast, naturally occurring cAMP is often unevenly distributed on a sub-cellular level, and concentrated in local microdomains (Baillie et al., 2005; Zaccolo, 2006). In addition to the compartmentalization of cAMP, the cAMP sensors PKA and EPAC are also spatially regulated by binding to scaffolding proteins, such as A-kinase anchoring proteins (AKAPs) (Wong and Scott, 2004; Gloerich and Bos, 2010). In future, it should be possible to localize genetically encoded PACs to specific subcellular domains, and embark on a new era of precision optogenetics.
Materials and Methods
Fly handling and light stimulation of photoactive adenylate cyclase
Flies were raised at 24°C and 60% relative humidity with a 14∶10 light:dark cycle on cornmeal-based food prepared from the Würzburg recipe (Guo et al., 1996). Genetic crosses were performed according to standard procedures. Genetic controls and flies expressing the photoactive adenylyl cyclase bPAC under UAS control (Stierl et al., 2011) were raised and handled in dim red light (λ = 650±20 nm) to avoid uncontrolled activation of bPAC. Photoactivation of bPAC transgenes was performed by use of a custom-built array of 20 light emitting diodes (Luxeon Rebel, royal blue λ = 448±10 nm, Phillips Inc.) mounted underneath a stereo dissecting microscope. Light intensity at the level of the specimen was adjusted by use of a power meter (Laser Check™, Coherent Inc.). Note that we equipped the microscope with a dark red filter to protect experimenter's eyes. Otherwise, the fluid secretion assay was performed as previously described (Dow et al., 1994).
Generation of transgenic flies
To construct the upstream activating sequence (UAS) expression vectors containing wild-type epac cDNA, we obtained a full-length cDNA clone GH01501 containing the epac RD isoform from the Drosophila Genomic Resource Center (DGRC, Bloomington, USA). cDNA was PCR amplified and cloned into the pEntry vector according to the manufacturer's protocol (pENTR/D-TOPO Cloning Kit, Invitrogen Inc.) and further cloned into the pUAST Drosophila transfection vector (Akbari et al., 2009) obtained from DGRC. Generation of transgenic Drosophila by germ-line transformation was performed by BestGene Inc. (Chino Hills, USA).
Measuring cAMP concentrations by enzyme-linked immunosorbent assay
Cytosolic cAMP concentrations were measured in sets of 16 Malpighian tubules isolated from 7-day-old adult male flies. Tubules were dissected in dim red light (λ = 650±20 nm) to avoid uncontrolled activation of bPAC. Photoactivation of bPAC transgenes was performed by use of a custom-built array of 20 light-emitting diodes (Luxeon Rebel, royal blue λ = 448±10 nm, Phillips Inc.) and specimens were immediately frozen in liquid nitrogen. cAMP concentrations were determined using a competitive immunoassay following the manufacturer's procedures (cAMP Biotrak EIA assay kit, GE Healthcare, USA).
Measurement of PKA activity
PKA activity was determined in head homogenate of 7-day-old adult females expressing UAS–DC0-RNAi under control of the neuron-specific elav–Gal4 element. PKA activity was determined using a phosphorylation assay following the manufacturer's procedures (PepTag Non-Radioactive cAMP dependent Protein Kinase Assay System; Promega, USA).
Generation of small deletions covering the Drosophila epac locus
EPACS (exchange proteins activated by cAMP) are cAMP-sensitive signaling molecules that execute a function as guanine nucleotide exchange factors for the small G protein Rap (de Rooij et al., 2000). We used the Drosophila FRT-derived deletion (FDD) system (Parks et al., 2004) to generate a loss-of-function allele for the unique Drosophila epac gene (CG34392). Drosophila epac is located on the right arm of chromosome 2 and codes for 17 exons distributed over 35 kb of genomic DNA (see Fig. 3C). Here, two isoforms, epac-RD and epac-RC, are encoded which differ in the number of camp-binding sites but otherwise have strong homology to mammalian EPACs (see Fig. 3D). FRT-dependent remobilization of the trans-heterozygous P-element combinations d04690/f07038 and e00785/f00899 generated deletions epacΔ1 spanning 52,252 bp, and epacΔ3 spanning 40,493 bp, respectively (see Fig. 3C). Deletions were verified by PCR and subsequently sequenced.
Homozygous epacΔ1 deletion was lethal at the pupal stage, as was the epacΔ1 deletion in trans to Df(2R)EXEL6050, a large deficiency covering the epac locus (data not shown). Mutants homozygous for the epacΔ3 deletion died as first instar larvae, as did flies bearing the epacΔ3 deletion in trans to Df(2R)EXEL6050. Trans combinations of Df(2R)BCS261, another large deficiency that partially covers the proximal part of Drosophila epac, with the epacΔ1 deletion resulted in viable and fertile flies, while with the epacΔ3 deletion animals died at the first instar larval stage (data not shown). These results indicate that lethality is due to deletion of genomic DNA proximal or distal to the epac gene.
Consistently, the combination of epacΔ1 in trans to epacΔ3 gave raise to viable and fertile animals. QRT-PCR confirmed that the trans combination of both deletions abolished epac transcripts (Fig. 3E) thus showing that the epacΔ1/epacΔ3 trans-heterozygote is transcriptionally null.
Genomic DNA isolation and PCR
Genomic DNA for PCR applications was isolated from 30 individual flies by homogenization in 400 µl 100 mM Tris-HCl pH 7.5, 100 mM EDTA, 100 mM NaCl and 0.5% (w/v) SDS. The sample was incubated at 65°C for 30 min. After addition of 800 µl of 1.4 M potassium acetate/4.2 M LiCl, preparations were kept on ice for 15 min. Precipitates were spun down and DNA was precipitated from the supernatant with isopropanol. Standard PCR protocols were employed to determine the deletions generated in the epac gene area.
RNA isolation and RT-PCR
Whole RNA from 30 fly heads was isolated using the ZR Tissue and Insect MicroPrep™ kit (Zymo Research Corp., Orange, CA, USA). 1 µg of RNA was used to generate cDNA from an olgido-dT16-Primer (MWG Biotech AG, Ebersberg, Germany) with the Transcriptor First Strand cDNA Synthesis Kit (Roche Diagnostics, Applied Science, Mannheim, Germany). cDNA-specific primer pairs for the epac- and the tan gene, as an internal control, were designed using the NCBI/Primer BLAST tool. Fragments were amplified with native Taq DNA polymerase (Fermentas GmbH, St. Leon-Rot, Germany) with 35 cycles in a Mastercycler® personal (Eppendorf, Hamburg, Germany).
Acknowledgments
Essential fly stocks and reagents were provided by the Bloomington Stock Center (Indiana, USA), the Drosophila Genomic Resource Center (DGRC) and the Vienna Drosophila RNAi Center (VDRC).
Footnotes
Funding
This research was funded by the National Institutes of Health [grant number 1ROMH086415 to M.S.]; and the Biotechnology and Biological Sciences Research Council [grant numbers BB/J002143/1, BB/H001042/1 to J.A.T.D.]. Deposited in PMC for release after 12 months.
References
- Akbari O. S., Oliver D., Eyer K., Pai C. Y. (2009). An Entry/Gateway cloning system for general expression of genes with molecular tags in Drosophila melanogaster. BMC Cell Biol. 10, 8 10.1186/1471-2121-10-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillie G. S., Scott J. D., Houslay M. D. (2005). Compartmentalisation of phosphodiesterases and protein kinase A: opposites attract. FEBS Lett. 579, 3264–3270 10.1016/j.febslet.2005.03.089 [DOI] [PubMed] [Google Scholar]
- Beyenbach K. W., Wieczorek H. (2006). The V-type H+ATPase: molecular structure and function, physiological roles and regulation. J. Exp. Biol. 209, 577–589 10.1242/jeb.02014 [DOI] [PubMed] [Google Scholar]
- Beyenbach K. W., Skaer H., Dow J. A. (2010). The developmental, molecular, and transport biology of Malpighian tubules. Annu. Rev. Entomol. 55, 351–374 10.1146/annurev-ento-112408-085512 [DOI] [PubMed] [Google Scholar]
- Börner S., Schwede F., Schlipp A., Berisha F., Calebiro D., Lohse M. J., Nikolaev V. O. (2011). FRET measurements of intracellular cAMP concentrations and cAMP analog permeability in intact cells. Nat. Protoc. 6, 427–438 10.1038/nprot.2010.198 [DOI] [PubMed] [Google Scholar]
- Bos J. L. (2003). Epac: a new cAMP target and new avenues in cAMP research. Nat. Rev. Mol. Cell Biol. 4, 733–738 10.1038/nrm1197 [DOI] [PubMed] [Google Scholar]
- Bos J. L. (2005). Linking Rap to cell adhesion. Curr. Opin. Cell Biol. 17, 123–128 10.1016/j.ceb.2005.02.009 [DOI] [PubMed] [Google Scholar]
- Bos J. L., de Bruyn K., Enserink J., Kuiperij B., Rangarajan S., Rehmann H., Riedl J., de Rooij J., van Mansfeld F., Zwartkruis F. (2003). The role of Rap1 in integrin-mediated cell adhesion. Biochem. Soc. Trans. 31, 83–86 10.1042/BST0310083 [DOI] [PubMed] [Google Scholar]
- Brand A. H., Perrimon N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 [DOI] [PubMed] [Google Scholar]
- Cabrero P., Radford J. C., Broderick K. E., Costes L., Veenstra J. A., Spana E. P., Davies S. A., Dow J. A. (2002). The Dh gene of Drosophila melanogaster encodes a diuretic peptide that acts through cyclic AMP. J. Exp. Biol. 205, 3799–3807 [DOI] [PubMed] [Google Scholar]
- Chepurny O. G., Kelley G. G., Dzhura I., Leech C. A., Roe M. W., Dzhura E., Li X., Schwede F., Genieser H. G., Holz G. G. (2010). PKA-dependent potentiation of glucose-stimulated insulin secretion by Epac activator 8-pCPT-2′-O-Me-cAMP-AM in human islets of Langerhans. Am. J. Physiol. 298, E622–E633 10.1152/ajpendo.00630.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coast G. M. (2009). Neuroendocrine control of ionic homeostasis in blood-sucking insects. J. Exp. Biol. 212, 378–386 10.1242/jeb.024109 [DOI] [PubMed] [Google Scholar]
- Coast G. M., Garside C. S. (2005). Neuropeptide control of fluid balance in insects. Ann. N. Y. Acad. Sci. 1040, 1–8 10.1196/annals.1327.001 [DOI] [PubMed] [Google Scholar]
- Coast G. M., Webster S. G., Schegg K. M., Tobe S. S., Schooley D. A. (2001). The Drosophila melanogaster homologue of an insect calcitonin-like diuretic peptide stimulates V-ATPase activity in fruit fly Malpighian tubules. J. Exp. Biol. 204, 1795–1804 [DOI] [PubMed] [Google Scholar]
- Coast G. M., Garside C. S., Webster S. G., Schegg K. M., Schooley D. A. (2005). Mosquito natriuretic peptide identified as a calcitonin-like diuretic hormone in Anopheles gambiae (Giles). J. Exp. Biol. 208, 3281–3291 10.1242/jeb.01760 [DOI] [PubMed] [Google Scholar]
- Dames P., Zimmermann B., Schmidt R., Rein J., Voss M., Schewe B., Walz B., Baumann O. (2006). cAMP regulates plasma membrane vacuolar-type H+-ATPase assembly and activity in blowfly salivary glands. Proc. Natl. Acad. Sci. USA 103, 3926–3931 10.1073/pnas.0600011103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S. A. (2000). Nitric oxide signalling in insects. Insect Biochem. Mol. Biol. 30, 1123–1138 10.1016/S0965-1748(00)00118-1 [DOI] [PubMed] [Google Scholar]
- Day J. P., Wan S., Allan A. K., Kean L., Davies S. A., Gray J. V., Dow J. A. T. (2008). Identification of two partners from the bacterial Kef exchanger family for the apical plasma membrane V-ATPase of Metazoa. J. Cell Sci. 121, 2612–2619 10.1242/jcs.033084 [DOI] [PubMed] [Google Scholar]
- de Rooij J., Rehmann H., van Triest M., Cool R. H., Wittinghofer A., Bos J. L. (2000). Mechanism of regulation of the Epac family of cAMP-dependent RapGEFs. J. Biol. Chem. 275, 20829–20836 10.1074/jbc.M001113200 [DOI] [PubMed] [Google Scholar]
- Dow J. A. (2007). Model organisms and molecular genetics for endocrinology. Gen. Comp. Endocrinol. 153, 3–12 10.1016/j.ygcen.2007.01.023 [DOI] [PubMed] [Google Scholar]
- Dow J. A. (2009). Insights into the Malpighian tubule from functional genomics. J. Exp. Biol. 212, 435–445 10.1242/jeb.024224 [DOI] [PubMed] [Google Scholar]
- Dow J. T., Davies S. A. (2003). Integrative physiology and functional genomics of epithelial function in a genetic model organism. Physiol. Rev. 83, 687–729 [DOI] [PubMed] [Google Scholar]
- Dow J. A., Maddrell S. H., Görtz A., Skaer N. J., Brogan S., Kaiser K. (1994). The malpighian tubules of Drosophila melanogaster: a novel phenotype for studies of fluid secretion and its control. J. Exp. Biol. 197, 421–428 [DOI] [PubMed] [Google Scholar]
- Enserink J. M., Christensen A. E., de Rooij J., van Triest M., Schwede F., Genieser H. G., Døskeland S. O., Blank J. L., Bos J. L. (2002). A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat. Cell Biol. 4, 901–906 10.1038/ncb874 [DOI] [PubMed] [Google Scholar]
- Evans J. M., Allan A. K., Davies S. A., Dow J. A. T. (2005). Sulphonylurea sensitivity and enriched expression implicate inward rectifier K+ channels in Drosophila melanogaster renal function. J. Exp. Biol. 208, 3771–3783 10.1242/jeb.01829 [DOI] [PubMed] [Google Scholar]
- Gloerich M., Bos J. L. (2010). Epac: defining a new mechanism for cAMP action. Annu. Rev. Pharmacol. Toxicol. 50, 355–375 10.1146/annurev.pharmtox.010909.105714 [DOI] [PubMed] [Google Scholar]
- Guo A., Li L., Xia S. Z., Feng C. H., Wolf R., Heisenberg M. (1996). Conditioned visual flight orientation in Drosophila: dependence on age, practice, and diet. Learn. Mem. 3, 49–59 10.1101/lm.3.1.49 [DOI] [PubMed] [Google Scholar]
- Hallows K. R., Alzamora R., Li H., Gong F., Smolak C., Neumann D., Pastor–Soler N. M. (2009). AMP-activated protein kinase inhibits alkaline pH- and PKA-induced apical vacuolar H+-ATPase accumulation in epididymal clear cells. Am. J. Physiol. 296, C672–C681 10.1152/ajpcell.00004.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hector C. E., Bretz C. A., Zhao Y., Johnson E. C. (2009). Functional differences between two CRF-related diuretic hormone receptors in Drosophila. J. Exp. Biol. 212, 3142–3147 10.1242/jeb.033175 [DOI] [PubMed] [Google Scholar]
- Iijima–Ando K., Hearn S. A., Shenton C., Gatt A., Zhao L., Iijima K. (2009). Mitochondrial mislocalization underlies Abeta42-induced neuronal dysfunction in a Drosophila model of Alzheimer's disease. PLoS ONE 4, e8310 10.1371/journal.pone.0008310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. C., Bohn L. M., Taghert P. H. (2004). Drosophila CG8422 encodes a functional diuretic hormone receptor. J. Exp. Biol. 207, 743–748 10.1242/jeb.00818 [DOI] [PubMed] [Google Scholar]
- Johnson E. C., Shafer O. T., Trigg J. S., Park J., Schooley D. A., Dow J. A., Taghert P. H.(2005). A novel diuretic hormone receptor in Drosophila: evidence for conservation of CGRP signaling. J. Exp. Biol. 208, 1239–1246 10.1242/jeb.01529 [DOI] [PubMed] [Google Scholar]
- Kalderon D., Rubin G. M. (1988). Isolation and characterization of Drosophila cAMP-dependent protein kinase genes. Genes Dev. 2, 1539–1556 10.1101/gad.2.12a.1539 [DOI] [PubMed] [Google Scholar]
- Kandel E. R. (2001). The molecular biology of memory storage: a dialogue between genes and synapses. Science 294, 1030–1038 10.1126/science.1067020 [DOI] [PubMed] [Google Scholar]
- Kaufmann N., Mathai J. C., Hill W. G., Dow J. A. T., Zeidel M. L., Brodsky J. L. (2005). Developmental expression and biophysical characterization of a Drosophila melanogaster aquaporin. Am. J. Physiol. 289, C397–C407 10.1152/ajpcell.00612.2004 [DOI] [PubMed] [Google Scholar]
- Kaupp U. B., Seifert R. (2002). Cyclic nucleotide-gated ion channels. Physiol. Rev. 82, 769–824 [DOI] [PubMed] [Google Scholar]
- Kerr M., Davies S. A., Dow J. A. (2004). Cell-specific manipulation of second messengers; a toolbox for integrative physiology in Drosophila. Curr. Biol. 14, 1468–1474 10.1016/j.cub.2004.08.020 [DOI] [PubMed] [Google Scholar]
- Laroche–Joubert N., Marsy S., Michelet S., Imbert–Teboul M., Doucet A. (2002). Protein kinase A-independent activation of ERK and H,K-ATPase by cAMP in native kidney cells: role of Epac I. J. Biol. Chem. 277, 18598–18604 10.1074/jbc.M201868200 [DOI] [PubMed] [Google Scholar]
- MacPherson M. R., Pollock V. P., Broderick K. E., Kean L., O'Connell F. C., Dow J. A., Davies S. A. (2001). Model organisms: new insights into ion channel and transporter function. L-type calcium channels regulate epithelial fluid transport in Drosophila melanogaster. Am. J. Physiol. 280, C394–C407 [DOI] [PubMed] [Google Scholar]
- O'Donnell M. J., Dow J. A., Huesmann G. R., Tublitz N. J., Maddrell S. H. (1996). Separate control of anion and cation transport in Malpighian tubules of Drosophila melanogaster. J. Exp. Biol. 199, 1163–1175 [DOI] [PubMed] [Google Scholar]
- O'Donnell M. J., Rheault M. R., Davies S. A., Rosay P., Harvey B. J., Maddrell S. H. P., Kaiser K., Dow J. A. T. (1998). Hormonally controlled chloride movement across Drosophila tubules is via ion channels in stellate cells. Am. J. Physiol. 274, R1039–R1049 [DOI] [PubMed] [Google Scholar]
- Parks A. L., Cook K. R., Belvin M., Dompe N. A., Fawcett R., Huppert K., Tan L. R., Winter C. G., Bogart K. P., Deal J. E. (2004). Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat. Genet. 36, 288–292 10.1038/ng1312 [DOI] [PubMed] [Google Scholar]
- Pastor–Soler N., Beaulieu V., Litvin T. N., Da Silva N., Chen Y., Brown D., Buck J., Levin L. R., Breton S. (2003). Bicarbonate-regulated adenylyl cyclase (sAC) is a sensor that regulates pH-dependent V-ATPase recycling. J. Biol. Chem. 278, 49523–49529 10.1074/jbc.M309543200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor–Soler N. M., Hallows K. R., Smolak C., Gong F., Brown D., Breton S. (2008). Alkaline pH- and cAMP-induced V-ATPase membrane accumulation is mediated by protein kinase A in epididymal clear cells. Am. J. Physiol. 294, C488–C494 10.1152/ajpcell.00537.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen H., Morth P., Egebjerg J., Nissen P. (2010). Phosphorylation of the Na+,K+-ATPase and the H+,K+-ATPase. FEBS Lett. 584, 2589–2595 10.1016/j.febslet.2010.04.035 [DOI] [PubMed] [Google Scholar]
- Radford J. C., Davies S. A., Dow J. A. (2002). Systematic G-protein-coupled receptor analysis in Drosophila melanogaster identifies a leucokinin receptor with novel roles. J. Biol. Chem. 277, 38810–38817 10.1074/jbc.M203694200 [DOI] [PubMed] [Google Scholar]
- Rein J., Voss M., Blenau W., Walz B., Baumann O. (2008). Hormone-induced assembly and activation of V-ATPase in blowfly salivary glands is mediated by protein kinase A. Am. J. Physiol. 294, C56–C65 10.1152/ajpcell.00041.2007 [DOI] [PubMed] [Google Scholar]
- Rosay P., Davies S. A., Yu Y., Sözen M. A., Kaiser K., Dow J. A. (1997). Cell-type specific calcium signalling in a Drosophila epithelium. J. Cell Sci. 110, 1683–1692 [DOI] [PubMed] [Google Scholar]
- Ryu M. H., Moskvin O. V., Siltberg–Liberles J., Gomelsky M. (2010). Natural and engineered photoactivated nucleotidyl cyclases for optogenetic applications. J. Biol. Chem. 285, 41501–41508 10.1074/jbc.M110.177600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu J. R., Najand N., Brook W. J. (2011). Tinman is a direct activator of midline in the Drosophila dorsal vessel. Dev. Dyn. 240, 86–96 10.1002/dvdy.22495 [DOI] [PubMed] [Google Scholar]
- Schröder–Lang S., Schwärzel M., Seifert R., Strünker T., Kateriya S., Looser J., Watanabe M., Kaupp U. B., Hegemann P., Nagel G. (2007). Fast manipulation of cellular cAMP level by light in vivo. Nat. Methods 4, 39–42 10.1038/nmeth975 [DOI] [PubMed] [Google Scholar]
- Sözen M. A., Armstrong J. D., Yang M. Y., Kaiser K., Dow J. A. T. (1997). Functional domains are specified to single-cell resolution in a Drosophila epithelium. Proc. Natl. Acad. Sci. USA 94, 5207–5212 10.1073/pnas.94.10.5207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stierl M., Stumpf P., Udwari D., Gueta R., Hagedorn R., Losi A., Gärtner W., Petereit L., Efetova M., Schwarzel M.et al. (2011). Light modulation of cellular cAMP by a small bacterial photoactivated adenylyl cyclase, bPAC, of the soil bacterium Beggiatoa. J. Biol. Chem. 286, 1181–1188 10.1074/jbc.M110.185496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokman G., Qin Y., Genieser H. G., Schwede F., de Heer E., Bos J. L., Bajema I. M., van de Water B., Price L. S. (2011). Epac-Rap signaling reduces cellular stress and ischemia-induced kidney failure. J. Am. Soc. Nephrol. 22, 859–872 10.1681/ASN.2010040423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terhzaz S., Southall T. D., Lilley K. S., Kean L., Allan A. K., Davies S. A., Dow J. A. (2006). Differential gel electrophoresis and transgenic mitochondrial calcium reporters demonstrate spatiotemporal filtering in calcium control of mitochondria. J. Biol. Chem. 281, 18849–18858 10.1074/jbc.M603002200 [DOI] [PubMed] [Google Scholar]
- Terhzaz S., Finlayson A. J., Stirrat L., Yang J., Tricoire H., Woods D. J., Dow J. A., Davies S. A. (2010). Cell-specific inositol 1,4,5 trisphosphate 3-kinase mediates epithelial cell apoptosis in response to oxidative stress in Drosophila. Cell. Signal. 22, 737–748 10.1016/j.cellsig.2009.12.009 [DOI] [PubMed] [Google Scholar]
- Torrie L. S., Radford J. C., Southall T. D., Kean L., Dinsmore A. J., Davies S. A., Dow J. A. T. (2004). Resolution of the insect ouabain paradox. Proc. Natl. Acad. Sci. USA 101, 13689–13693 10.1073/pnas.0403087101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenberger S., Schultheis C., Liewald J. F., Erbguth K., Nagel G., Gottschalk A. (2011). PACα - an optogenetic tool for in vivo manipulation of cellular cAMP levels, neurotransmitter release, and behavior in Caenorhabditis elegans. J. Neurochem. 116, 616–625 [DOI] [PubMed] [Google Scholar]
- Wong W., Scott J. D. (2004). AKAP signalling complexes: focal points in space and time. Nat. Rev. Mol. Cell Biol. 5, 959–970 10.1038/nrm1527 [DOI] [PubMed] [Google Scholar]
- Zaccolo M. (2006). Phosphodiesterases and compartmentalized cAMP signalling in the heart. Eur. J. Cell Biol. 85, 693–697 10.1016/j.ejcb.2006.01.002 [DOI] [PubMed] [Google Scholar]
- Zaccolo M. (2009). cAMP signal transduction in the heart: understanding spatial control for the development of novel therapeutic strategies. Br. J. Pharmacol. 158, 50–60 10.1111/j.1476-5381.2009.00185.x [DOI] [PMC free article] [PubMed] [Google Scholar]