Summary
Axon degeneration is observed at the early stages of many neurodegenerative conditions and this often leads to subsequent neuronal loss. We previously showed that inactivating the c-Jun N-terminal kinase (JNK) pathway leads to axon degeneration in Drosophila mushroom body (MB) neurons. To understand this process, we screened candidate suppressor genes and found that the Wallerian degeneration slow (WldS) protein blocked JNK axonal degeneration. Although the nicotinamide mononucleotide adenylyltransferase (Nmnat1) portion of WldS is required, we found that its nicotinamide adenine dinucleotide (NAD+) enzyme activity and the WldS N-terminus (N70) are dispensable, unlike axotomy models of neurodegeneration. We suggest that WldS-Nmnat protects against axonal degeneration through chaperone activity. Furthermore, ectopically expressed heat shock proteins (Hsp26 and Hsp70) also protected against JNK and Nmnat degeneration phenotypes. These results suggest that molecular chaperones are key in JNK- and Nmnat-regulated axonal protective functions.
Key words: Axon degeneration, Nmnat, JNK, Heat shock proteins, Drosophila
Introduction
Axonal loss is detected at the early stages of many neurodegenerative pathologies (Coleman, 2005; Luo and O'Leary, 2005; Saxena and Caroni, 2007; Wang et al., 2012) and some studies show blocking axonal degeneration can reduce progressive degenerative phenotypes, such as in motorneuron loss (Ferri et al., 2003; Pun et al., 2006). Understanding the basis of axon degeneration and how it can be prevented could provide rational bases to treat common or unique neurodegenerative conditions.
It is important to note selective degeneration of axons, dendrites and synapses also occurs naturally during development and plasticity (Luo and O'Leary, 2005). Such pruning events share common features with neurodegenerative events. However, neurons are not lost during developmental pruning. Whether blocking all known neurodegenerative mechanisms is equally effective in preventing axon degeneration, under natural or pathological conditions, is still unclear. Nonetheless, present studies reveal cell death, axonal and dendritic degenerative phenotypes are distinct, despite some mechanistic overlaps (Sagot et al., 1995; Finn et al., 2000; Coleman, 2005; Luo and O'Leary, 2005; Hoopfer et al., 2006; Kuo et al., 2006; Williams et al., 2006; Beirowski et al., 2008; Nikolaev et al., 2009; Schoenmann et al., 2010; Vohra et al., 2010).
Cell-protective mechanisms that control molecular chaperones, autophagy, the ubiquitin-proteosome system (UPS), oxidative stress and mitochondrial functions are also key. These are not only cell-essential but their manipulations can alleviate neurodegenerative pathologies (Watts et al., 2003; Zhai et al., 2003; Lin and Beal, 2006; Voisine et al., 2010; Wong and Cuervo, 2010; Bingol and Sheng, 2011). These can act by detecting and responding to potentially degenerative stimuli, by promoting neuronal integrity and initiating repair upon nerve damage.
The JNK pathway is a central regulator of diverse neuropathologies. Aberrant JNK signalling is implicated in Alzheimer's (Morishima et al., 2001; Okazawa and Estus, 2002), Parkinson's (Peng and Andersen, 2003), and Huntington's (Morfini et al., 2009; Perrin et al., 2009) diseases, where its activation leads to neuronal cell death. JNK also induces neurodegeneration in response to stress stimuli, such as toxins and excitotoxicity (Yang et al., 1997; Kuan et al., 2003; Brecht et al., 2005; Waetzig et al., 2006), growth factor deprivation (Xia et al., 1995; Eilers et al., 1998; Maroney et al., 1998; Ghosh et al., 2011) and acute physical injury (Miller et al., 2009). Axon fragmentation is evident in many of these cases.
Despite its central pro-degenerative and pro-apoptotic activities, JNKs are also neuroprotective (Brecht et al., 2005) and involved in neuronal patterning during axonal outgrowth (Oliva et al., 2006), dendritogenesis (Rosso et al., 2005) and in synaptic plasticity (Sanyal et al., 2002) and transmission (Thomas et al., 2008). We, and others, previously showed JNK signals have conserved functions in maintaining axonal stability and we showed that sustained JNK activities throughout development are essential, prior to the onset of degenerative events (Chang et al., 2003; Rallis et al., 2010). Physical injury paradigms in C. elegans and Drosophila show JNK is also required post-injury during axonal regeneration (Ayaz et al., 2008; Nix et al., 2011). This may also be conserved in mammals (Herdegen et al., 1998; Raivich et al., 2004; Barnat et al., 2010). Therefore, determining how JNK promotes axonal stability and regeneration (while avoiding its pro-degenerative effects) can be useful in defining the strategies required to prevent neurodegenerative pathologies and promote repair programs upon nerve damage.
Here we show that inactivating the JNK gene (basket; bsk) in Drosophila neurons induces an age-dependent, Wallerian-like axon degeneration phenotype. This is not due to aberrant developmental pruning and cannot be suppressed by neuroprotective molecules linked to apoptosis, autophagy, the Ubiquitin-Proteosome (UPS) pathway, mitochondrial function or translational repression. Instead, we find JNK axonal degeneration (in this study defined as those caused by JNK inactivation) is linked to the axonal-protective effects of WldS.
WldS was discovered from the molecular cloning of spontaneously generated slow Wallerian degeneration (WldS) mutant mice that showed a strong capacity to promote axonal survival following acute physical lesion (Lunn et al., 1989; Coleman and Freeman, 2010). The WldS protein has neuroprotective effects across different species and in different neurodegeneration models (Coleman and Freeman, 2010; Feng et al., 2010; Vohra et al., 2010; Barrientos et al., 2011; Ali et al., 2012; Bhattacharya et al., 2012; Fang et al., 2012). The WldS gene product results from the fusion of first 70 residues of the UBE4B gene (N70), that is involved in polyubiquitination, with the entire nicotinamide mononucleotide adenylyltransferase protein sequence (Nmnat1) that is involved in nicotinamide adenine dinucleotide (NAD+) biosynthesis (Conforti et al., 2000; Mack et al., 2001). Different portions of WldS can confer neuroprotective function (Coleman and Freeman, 2010). However, WldS function remains unclear. For example, despite its predominant nuclear localisation, it is axonal localisation that appears to be key to neuroprotection, even though WldS and different Nmnat isoforms have subtle and distinct subcellular locations (Berger et al., 2005; Conforti et al., 2007; Beirowski et al., 2009; Babetto et al., 2010; Sasaki and Milbrandt, 2010; Yahata et al., 2009) (supplementary material Fig. S2). Also, while in many neurodegenerative paradigms the Nmnat enzyme activity is essential, it is unclear how the NAD+ pathway contributes to axonal protection (Araki et al., 2004; Wang et al., 2005; Kaneko et al., 2006; Conforti et al., 2007; Avery et al., 2009; Sasaki et al., 2009; Coleman and Freeman, 2010). Furthermore, some studies suggest Nmnat neuroprotective functions are enzyme-independent (Zhai et al., 2006; Zhai et al., 2008; Wen et al., 2011). To date, the relationship between WldS function(s) and axon-neuronal damage and repair also remains unclear, although recent data suggest WldS-Nmnat regulation of mitochondrial motility and calcium buffering functions may underlie key neuroprotective responses to physical injury in Drosophila and mouse axons (Avery et al., 2012). A further report suggests Drosophila Nmnat (dNmnat or nmnat) also controls axonal mitochondria levels and their availability is key to neuroprotection following acute injury (Fang et al., 2012). Previous data suggest WldS-Nmnat localisation within mitochondria may also be the underlying basis of axonal neuroprotection (Yahata et al., 2009).
When tested ectopically, many Nmnat isoforms and homologs show axonal-protective effects even though some appear to be weaker, possibly due to labile effects (Sasaki et al., 2009; Gilley and Coleman, 2010). However, apart from Drosophila Nmnat (Wen et al., 2011; Fang et al., 2012), currently only mouse Nmnat2 has an endogenous role in promoting axonal stability (Gilley and Coleman, 2010; Hicks et al., 2012). It is important to note, beyond their neuronal roles, Nmnats also have obligate roles in NAD+ metabolism and multiple cellular processes across species (Zhai et al., 2009; Lin et al., 2010). Very recent reports show Nmnat1 mutations cause Leber congenital amaurosis (LCA), highlighting its importance in retinal degenerative diseases in humans (Chiang et al., 2012; Falk et al., 2012; Koenekoop et al., 2012; Perrault et al., 2012).
Here we show that the WldS protein protects against axon degeneration triggered by JNK inactivation. Contrary to previous models, while the Nmnat1 region is sufficient, we find that its enzyme activity is dispensable for WldS neuroprotection. The results suggest that Nmnat and JNK axonal-protective functions occur through molecular chaperones.
Results
JNK inactivation causes age-dependent axonal degeneration in Drosophila MB neurons
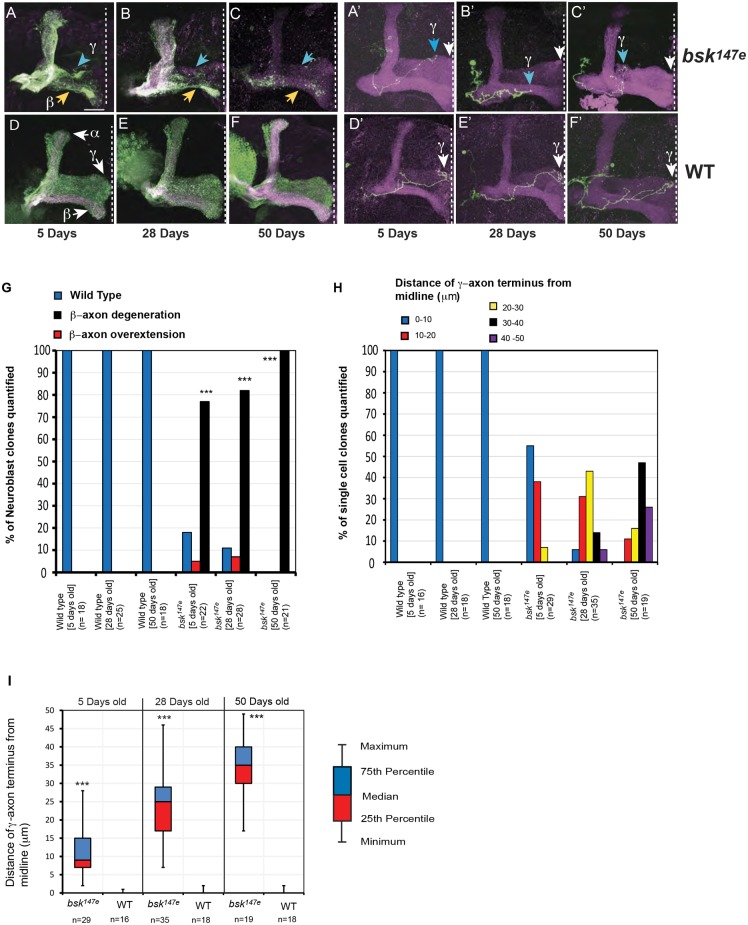
We previously showed that Drosophila JNK mutant mushroom body (MB) neurons have axon degeneration phenotypes (Rallis et al., 2010). These are not a consequence of axon growth defects. Also, a small fraction of axons have overextension phenotypes, which is distinct from axon degeneration. This study focused only on the axon degeneration phenotype in the β-lobe in neuroblast clones and in γ-single clones. To examine how this occurs, we generated JNK mutant clones (using the bsk147E null allele) using MARCM (Lee and Luo, 1999) and analyzed this phenotype at different adult stages. At 5 days post-eclosion, although many bsk axons did show some degenerative phenotypes, most MB axons were still visible (Fig. 1A,G). This degenerative phenotype becomes more severe at 28 days and 50 days post-eclosion (Fig. 1B,C,G). By generating single-cell clones, we could measure the extent to which each axon reaches the wild-type termination point. We found a greater terminal loss in aged axons (Fig. 1A′–C′,H,I), suggesting that a ‘dying back’ (Wallerian-like) degeneration is progressing with age.
Fig. 1.
Drosophila JNK loss results in age-dependent axon degeneration. (A–C) Adult MB JNK (bsk147e) neuroblast clones exhibit axon degeneration with axon thinning and terminal fragmentation in β- (yellow arrows) and γ-axons (blue arrows). This phenotype worsens with age, as seen between 5- (A), 28- (B) and 50-day-old (C) flies (post-eclosion). (D–F) Aged-matched wild-type (WT) controls show no degeneration. Arrows label the α-, β- and γ-MB subsets with axon defects, as shown. (A′–C′) Adult bsk γ-single neuron clones show age-dependent axonal loss from the distal terminal ends (white arrows on the right). Note the increasing distance away from the midline (dashed line) as axon degeneration proceeds in aged animals. (D′–F′) Aged-matched wild-type axons do not display any terminal loss. All clonal generated neurons were labeled with CD8-GFP (green). FasII immunostaining (magenta) labels all γ and α/β subset of MB axons. As FasII labels both mutant and wild-type axons, this provides a useful marker to compare the MB axon terminal zones between mutant and wild-type axons. Confocal images are z-stacks of serial sections taken at 1 µm intervals. Scale bar: 20 µm. (G) Quantification of β-axon degeneration in neuroblast clones at indicated stages. The neurodegenerative phenotypes are extremely significant (***P<0.0001; Fisher's exact test) in bsk147e compared with wild-type (WT) age-matched neuroblast clones; n indicates the number of clones analyzed. (H) Quantification of the γ-axon terminal loss in single cell clones by measuring the distance from the γ-axon terminals to the midline. (I) A box-and-whisker plot representation (by measuring the distance of a single cell axon terminus from the midline) showing that dying-back degeneration increases with age. The P-values (***P<0.001; Mann-Whitney U-test) were highly significant between age-matched bsk147e and wild-type axon terminals.
Axon pruning and myosin-II based retraction are not involved in JNK axonal degeneration
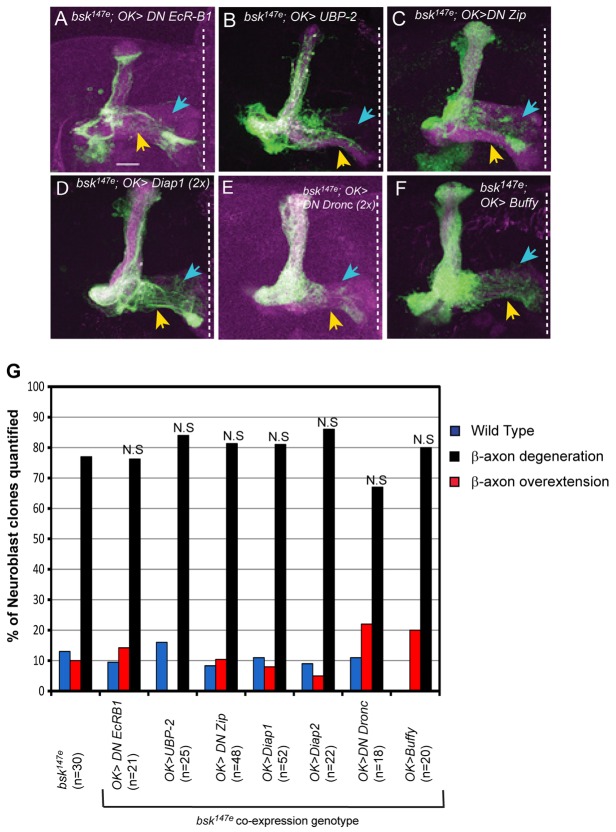
To determine whether developmental regulated axon pruning is involved in bsk axonal degeneration, we expressed a dominant-negative (DN) form of the ecdysone receptor EcR-B1 (Cherbas et al., 2003) in bsk-null MB neuroblast clones. During Drosophila metamorphosis, the steroid hormone ecdysone initiates local axonal degeneration in MB γ-neurons through a nuclear receptor complex composed of ultraspiracle (USP) and EcR-B1 (Lee et al., 2000). With EcR-B1 DN, even though pruning was blocked (not shown; Hoopfer et al., 2006), it failed to suppress the bsk degeneration phenotype (Fig. 2A,G). The UPS pathway is also required in axon pruning and ectopic expression of the ubiquitin protease UBP-2 blocks MB axon pruning (not shown; Watts et al., 2003). However, UBP-2 does not block the bsk phenotype (Fig. 2B,G). These results suggest the bsk degeneration phenotype is not due to ectopic axon pruning. Given the ‘dying-back’ phenotype, we tested whether myosin II based retraction is involved. Myosin II activity causes axonal retraction in vitro (Wylie and Chantler, 2003; Gallo, 2004; Gallo, 2006) and in vivo in MB neurons (Billuart et al., 2001) by generating actin-based contractile forces. However, dominant-negative Zipper expression, which encodes Drosophila non-muscle Myosin II (Dawes-Hoang et al., 2005), did not suppress the axon degeneration phenotype (Fig. 2C,G), suggesting that myosin-II is not the key effector in the bsk phenotype.
Fig. 2.
JNK axon degeneration is not caused by aberrant axonal pruning or myosin-based retraction or affected by apoptotic inhibitors. (A–C) Overexpressing a dominant-negative (DN) form of EcR-B1 (A) or the ubiquitin protease UBP-2 (B) in bsk147e MB neuroblast clones fails to suppress the JNK axon degeneration phenotype in either γ- or β-neurons (blue and yellow arrows, respectively). MB neuroblast clones were analyzed at 14 days post-eclosion. (C) Dominant-negative Zipper (DN Zip) expression also fails to rescue the bsk degeneration phenotype. (D–F) Overexpression of anti-apoptotic regulators including Diap1 (D), a dominant-negative (DN) form of Dronc (E) or Buffy (F) does not suppress JNK degeneration phenotype. Green, CD8-GFP (neurons); magenta, FasII (axons). Scale bar: 20 µm. (G) Quantification of β-axon degeneration in JNK-null genotypes in the presence of the indicated transgenes. n indicates the number of MB neuroblast clones analyzed. No significant differences were found between age-matched bsk147e mutant and bsk147e UAS coexpression genotypes (P>0.05; using Fisher's exact test).
JNK axonal degeneration is not due to ectopic caspase activities
Previous Drosophila studies show developmental pruning in dendrites requires non-apoptotic, caspase activity (Kuo et al., 2006; Williams et al., 2006; Schoenmann et al., 2010; Tao and Rolls, 2011). Caspase activity is also triggered in response to Alzheimer's disease-causing forms of the Beta-amyloid precursor protein (APP) resulting in axonal fragmentation and cell death (Nikolaev et al., 2009). Various caspase inhibitors were tested. Drosophila IAP1 (DIAP1) is an E3 ubiquitin ligase that promotes the ubiquitination of caspases, thereby preventing caspase activation (Muro et al., 2002). DIAP1 expression also blocks dendrite pruning (Schoenmann et al., 2010; Tao and Rolls, 2011) in Drosophila mechanosensory neurons and neurodegeneration in MB neurons overexpressing mutant ataxin-3 (Ghosh and Feany, 2004). However, using two copies of UAS-DIAP1 (2X), DIAP1 overexpression did not alter the bsk degeneration phenotype (Fig. 2D,G). We also tested a related protein, DIAP2. Like DIAP1, DIAP2 also suppresses naturally occurring cell death as well as by cell death activators reaper (Rpr) or head involution defective (Hid) (Hay et al., 1995). Interestingly, DIAP2 does show some differences in caspase-inhibitory preferences (Ribeiro et al., 2007). Nonetheless, DIAP2 overexpression did not rescue the bsk phenotype (data not shown; Fig. 2G). We also tested a dominant-negative (DN) form of Dronc, an initiator caspase, which blocks cell death induced by cell death activators (Hawkins et al., 2000; Meier et al., 2000). Dronc also controls dendritic pruning in Drosophila neurons (Kuo et al., 2006). However, expressing two copies of DN Dronc (2X) transgenes failed to suppress the bsk degeneration (Fig. 2E,G). Bcl-2 family proteins are known inhibitors of caspase-dependent events and required for mitochondria integrity and function (Vander Heiden et al., 1997). Buffy, the sole Drosophila anti-apoptotic Bcl-2 homologue, can suppress cell death phenotypes associated with caspase activity (Quinn et al., 2003) and mitochondrial dysfunction in the Drosophila PINK1 model of an early onset form of Parkinson's disease (PD) (Park et al., 2006). Nonetheless, ectopic Buffy did not alter the bsk phenotype (Fig. 2F,G). These results suggest caspase activities or Bcl-2 functions are unrelated to the JNK axon degeneration phenotype.
WldS is a long-term suppressor of JNK axonal degeneration
Other candidate genes were tested but none of these suppressed the bsk phenotype (supplementary material Fig. S1). For example, we also test various regulators (TOR, HDAC6, 4EBP and Parkin) associated with autophagy, protein translational inhibition and mitochondrial quality control that were previously linked to neurodegenerative phenotypes.
TOR inhibition has been demonstrated to promote neuroprotection by inducing autophagy and reducing levels of translational activity (Ravikumar et al., 2002). However, TOR overexpression, which also causes a TOR loss-of-function effect in Drosophila (Hennig and Neufeld, 2002), did not modify the bsk axonal degeneration phenotype (supplementary material Fig. S1A,E). We also examined the effect of HDAC6 overexpression in bsk MB neuroblast clones. HDAC6 has been shown to act at an intersection between the UPS, the principle non-lysosomal degradative pathway of ubiquitinated proteins, and autophagy, a lysosomal degradative pathway. HDAC6 expression rescues neurodegeneration in an autophagy-dependent manner when the UPS system is impaired in a Drosophila model of spinobulbar muscular atrophy (Pandey et al., 2007). Furthermore, HDAC6 has recently been found to regulate the autophagosome-lysosome fusion step during autophagy (Lee et al., 2010). However, overexpressed Drosophila HDAC6 failed to rescue the bsk axonal degeneration phenotype (supplementary material Fig. S1B,E). This potentially suggests that the bsk axonal degeneration phenotype might not be a consequence of UPS or autophagy dysfunction.
TOR can also confer neuroprotection via protein translational repression in Drosophila models of familial Parkinson's disease (PD) (Tain et al., 2009; Liu and Lu, 2010). In these models, PD neurodegeneration is associated with mitochondrial dysfunction, aberrant protein synthesis and degradation and oxidative stress (Abou-Sleiman et al., 2006; Farrer, 2006). Ectopic expression of the TOR effector pathway, the 4EBP translation inhibitor is sufficient to suppress neurodegeneration and other pathological phenotypes that occur in PD gene mutants, parkin, Pink1 and in LRRK2 transgenic animals (Imai et al., 2008; Tain et al., 2009). Nonetheless, overexpression of 4EBP (Drosophila Thor) in a bsk-null genetic background failed to rescue the axonal degeneration phenotype (supplementary material Fig. S1C,E), suggesting that JNK inactivation results in a distinct form of degeneration from that observed in the fly PD models.
In PD models, the E3 ubiquitin ligase Parkin acts as part of a mitochondrial quality control system through its recruitment to dysfunctional mitochondria, where it ubiquitinates outer mitochondrial membrane proteins and promote autophagy of defective mitochondria (Narendra et al., 2010; Narendra and Youle, 2011). Additionally, Parkin also mediates Beclin-dependent mitophagy in a mouse Alzheimer’s model and in the autophagic clearance of ubiquitinated Aβ in vivo (Khandelwal et al., 2011). Nonetheless, Parkin overexpression had no effect on the bsk phenotype (supplementary material Fig. S1D,E), suggesting that the JNK inactivation phenotype is unrelated to defective mitophagy. WldS is a promising candidate given its wide-ranging neuroprotective effects (Coleman and Freeman, 2010). However, WldS does not suppress all forms of axon degeneration (Coleman, 2005), such as developmental pruning of Drosophila MB axons or the reorganisation of mouse retinotectal axons (Hoopfer et al., 2006). We tested two different WldS transgenic lines (1 and 2), both of which fully suppressed the bsk axonal phenotype (Fig. 3). Furthermore, WldS maintained axonal integrity in aged bsk clones (Fig. 3A,C,D) and the single-axon analyses showed it blocked the dying-back phenotype in aged clones (Fig. 3B,E,F,Q). Therefore, the WldS protective effect was sustained, and not transient, as previously reported in other contexts (Mack et al., 2001; Beirowski et al., 2008; Beirowski et al., 2010).
Fig. 3.
WldS confers sustained protection against JNK axon degeneration that is Nmnat enzyme-independent. (A,B) Graphs quantifying JNK axon degeneration in neuroblast (A) or single cell clones (B) expressing WldS in 5- and 28-day-old adults. P-values were highly significant comparing age-matched bsk147e neuroblast clones with those in the presence of WldS::Myc. (C–F) WldS expression blocks JNK axon degeneration and terminal loss phenotypes in bsk neuroblast (C,D) and single cell clones (E,F), respectively. (G) Quantification of axon degeneration in 28-day-old bsk neuroblast clones expressing mNmnat1, mNmnat2, mNmnat3 or WldS-dead. Fisher's exact test between age-matched bsk147e neuroblast clones and those in the presence of the WldS::Myc lines (#1 and #2), WldS-dead, Nmnat1::Myc and Nmnat3::Myc were highly significant, but not significant for Nmnat2::Myc. (H–K) Representative images of genotypes. Yellow and blue arrows indicate axonal degeneration in β- and γ-axons, respectively. (L) Quantification of the terminal loss in 28-day-old JNK single-cell clones expressing WldS, WldS-dead, mNmnat1, mNmnat2 and mNmnat3. (M–P) Representative images of clones. Blue arrow indicates where axon terminal loss has occurred. Green, CD8-GFP; magenta, FasII. Scale bar: 20 µm. (Q) Box-and-whisker representation of the axon degeneration phenotype, measuring the distance of single axon terminus from the midline. Significant differences were found between 5- and 28-day-old age-matched bsk147e single γ-axons compared with bsk clones with WldS::Myc. (R) Similar plots with other Nmnat lines, including WldS-dead, Nmnat1::Myc and Nmnat3::Myc, also showed significant differences compared with bsk147e mutants alone. By contrast, bsk147e clones expressing Nmnat2::Myc exhibited no significant difference. **P<0.01; ***P<0.001; N.S., P>0.05.
WldS neuroprotection requires Nmnat1 but is independent of enzyme activity
Different portions of WldS are thought to confer neuroprotective function (Coleman and Freeman, 2010). When the mouse Nmnat1 gene (mNmnat1) was expressed, this robustly rescued the bsk degeneration phenotype, surpassing the WldS effect (Fig. 3G,H,L,R). Single axon studies show many of these neurons had wild-type termination points (Fig. 3L,M,R). This suggests that the mNmnat1 region is fully sufficient and that the WldS N70 region is dispensable for neuroprotection here.
Other Nmnat isoforms were subsequently tested. While mNmnat2 failed to suppress the bsk phenotype (P>0.05; Fig. 3G,I,L,N,R), mNmnat3 expression provided some rescue (Fig. 3G,J,L,O,R), although not to the same extent as with WldS or mNmnat1. Taken together, these data suggest that Nmnat1 and, to some extent, mNmnat3 provide the greatest axonal protective functions in bsk-dependent degeneration. This closely parallels other reports that show Nmnats 1 and 3 are potent suppressors of axonal degeneration induced by physical injury (Avery et al., 2009; Coleman and Freeman, 2010; Avery et al., 2012; Fang et al., 2012). As all WldS and Nmnat transgenes used here are myc tagged, we could verify their expression by immunohistochemistry. Their expression levels were broadly similar in MB neurons (supplementary material Fig. S2). This suggests the differences in Nmnat neuroprotective actions are unlikely to be due to differences in expression levels.
We tested whether Nmnat enzyme activity is important by expressing WldS-dead, an enzyme-inactive form of mNmnat1 (Avery et al., 2009). WldS-dead also suppressed bsk axon degeneration to a degree similar to WldS and mNmnat1 (Fig. 3G,K,L,P,R). This suggests while the mNmnat1 portion confers axonal protection, its enzyme activity is dispensable. This is in contrast to Drosophila and mouse axotomy models, where its enzyme activity is essential (Araki et al., 2004; Avery et al., 2009; Conforti et al., 2009; Yahata et al., 2009).
Drosophila Nmnat inactivation results in axon degeneration and neuronal loss
To test the endogenous function of Nmnat, we made mutant clones of Drosophila Nmnat (Zhai et al., 2006). nmnat1 loss-of-function clones showed an axonal phenotype similar to bsk and mutants of the upstream JNK regulators, hep and Mkk4, where the β-lobe was lost (Fig. 4A-C, respectively, quantified in Fig. 4E) (Rallis et al., 2010). Interestingly, in nmnat1 clones, earlier-born neurons (γ and α′β′) were not visible, suggesting that apart from axonal maintenance it is also required for neuronal viability. Some neuronal cell loss was also evident in nmnat1 αβ neurons.
Fig. 4.

Nmnat inactivation results in axon degeneration. (A–D) Images of 28-day-old nmnat1 (A), bsk147e (B), hep/Mkk4 double mutant (C) and wild-type (D) MB α/β neuroblast clones. These loss-of-function mutations all result in β-axon loss (yellow arrows). (E) Quantification from these genotypes indicates highly significant differences compared with wild-type clones. (F,G) Hsp26 or Hsp70 expression blocks axonal degeneration. (H) Quantification of axon degeneration and overextension in neuroblasts expressing Hsp26 or Hsp70. Scale bars: 20 µm. Green, CD8-GFP; Magenta, FasII. ***P<0.001.
Evidence of molecular chaperone involvement in JNK and Nmnat degeneration
One previous report showed that Drosophila Nmnat has a non-enzyme function that involves molecular chaperone activity (Zhai et al., 2008). Drosophila Nmnat was recruited together with the molecular chaperone, Heat shock protein (Hsp) Hsp70 to polyglutamine expanded spinocerebellar ataxin-1 (SCA-1) containing aggregates. Non-enzyme Nmnat functions were involved in regulating protein folding and blocking SCA-1 neurotoxicity. Very recent results show non-enzyme Nmnat also functions to clear tau oligomers in vivo (Ali et al., 2012). We tested the effect of Heat shock proteins (Hsps) on the bsk phenotypes in two ways. In bsk-null neuroblast clones, we found that, like WldS and Nmnats1 and 3, ectopic Hsp70 or Hsp26 also blocked the bsk axon degeneration (Fig. 4F–H).
As shown previously (Rallis et al., 2010), compared to wild-type axons, bsk axons showed more abnormal protrusions and swellings along the axons and terminals (Fig. 5A,B,B′, respectively; quantified in Fig. 5F). When Hsp70, WldS, Nmnat and Nmnat enzyme-inactive forms were expressed in these clones, these were reduced suggesting that this phenotype is also linked to Hsps and non-enzyme Nmnat activities (Fig. 5C–E; quantified in Fig. 5F).
Fig. 5.
Enzyme-inactive Nmnats and Hsps can block JNK and Nmnat inactivation phenotypes. (A–B′) Images of 28-day-old wild-type (A) and bsk147e single cell γ-neuron axon terminals (B,B′). Enlarged and supernumerous axonal protrusions (red arrowheads) are evident in bsk mutant axons along the axon shaft (B) and terminals (magnified image in B′). (C–E) Magnified images of 28-day-old bsk clones expressing Hsp70 (C), WldS (D) or WldS-dead (E). These large protrusions were reduced by Hsp70, WldS or WldS-dead expression. Green, CD8-GFP. Scale bars: 20 mm (A,B), 10 µm (B′–E). (F) Quantification of enlarged protrusions (>1 µm in diameter) within 20 µm of axonal terminals, scored from the above genotypes, including the effects of Nmnats 1 and Nmnats 3. n indicates number of single cell clones analyzed. (G,H) Nmnat RNAi transgene in MB neurons resulted in axonal loss, visible in newly eclosed adults (1 day old) (G). In aged adults (7 days old), MB neurons were no longer visible (H), suggesting that Nmnat is an obligate cell maintenance factor. Only the labeled antennal lobes (AL), ventral to the MB lobes, were visibly labeled. The midline is indicated by a dashed white line. (I,J) Nmnat RNAi axonal and neuronal loss phenotype was blocked with mNmnat1(H24A) and Hsp70 expression at the 7-day-old adult stage (I and J, respectively). (K) The quantified neuronal loss phenotype shows that Nmnats and enzyme inactive forms of mNmnat1(H24A) and WldS-Dead are more potent at blocking Nmnat RNAi neuronal loss than Hsp26 and Hsp70. (L) Using the GAL80ts system to control Nmnat RNAi expression, flies were raised at 18°C (off-state) or at 29°C (on-state). By transferring flies to 29°C, RNAi transgene expression was induced either throughout the life cycle (all stages, beginning at embryogenesis) or at defined pupal (0 hours after pupal formation; APF) or adult stages (1 day old). As controls, sibling genotypes that contained no GAL80ts or Nmnat RNAi transgenes (but raised throughout at 29°C) were also analyzed for phenotypes. All flies were dissected and analyzed at the 7-day-old stage. Note that the cell number for the no GAL80ts control suggests that, even at the restrictive temperature of 29°C, the presence of GAL80ts does reduce the level of UAS-GAL4-mediated expression.
To further test the neuroprotective activity of Hsps, we turned to Nmnat RNAi assays (Fig. 5G–L). When Nmnat RNAi was expressed in MB neurons, this resulted in a β-axon loss phenotype similar to nmnat1 loss-of-function clones above. Some neuronal loss was visible in newly eclosed adults (1-day-old adults). However, almost all neurons were lost in 7-day-old adults (Fig. 5G,H, respectively; quantified in Fig. 5K), suggesting that Nmnat is an obligate maintenance factor, consistent with previous reports (Zhai et al., 2006). We found the Nmnat RNAi axon and neuronal cell loss was rescued by enzyme-inactive forms of mNmnat1 (H24A) and WldS-dead (Fig. 5I; quantified in Fig. 5K; not shown). Furthermore, Hsp26 and Hsp70 expression also partially suppressed the Nmnat RNAi phenotype (Fig. 5J,K; not shown). Together, these results suggest non-enzyme Nmnat and chaperone activities are linked to JNK axonal functions.
Using the GAL80ts system (McGuire et al., 2003) to control JNK temporal expression, we previously showed that JNK activity is required throughout development, even though the axon degeneration phenotype occurs mainly at adult stages (Rallis et al., 2010; this study). To determine Nmnat's temporal requirements, we coupled Nmnat RNAi to GAL80ts control and induced the loss-of-function phenotype at various stages of development (Fig. 5L). We found that RNAi throughout the development and adult phase caused the strongest neuronal loss phenotype. RNAi induction at pupal or adult stages also caused neuronal loss, albeit at a weaker levels. These results suggest Nmnat is required throughout development as well as adult stages. Even though the Nmnat RNAi phenotype is more severe in adults, as in bsk mutants, unlike bsk, Nmnat's genetic requirements extend beyond the developmental stages and are essential at adult stages. This suggests Drosophila Nmnat may have additional roles at adult stages that may be independent of JNK activity.
Discussion
Here we reveal a ‘Wallerian-like’ degeneration occurs in JNK mutant axons, which progressively worsens with age. This ‘dying-back’ degeneration is prevalent in many neurodegenerative conditions and closely linked to WldS-Nmnat activities (Finn et al., 2000; Sajadi et al., 2004; Mi et al., 2005; Hasbani and O'Malley, 2006; Kaneko et al., 2006; Howell et al., 2007; Beirowski et al., 2008; Wang et al., 2012). However, unlike the prevalent model of Nmnat function, our studies show non-enzyme Nmnat and chaperone functions are key interactors with the JNK pathway in controlling axonal stability.
Many experiments have addressed how WldS confers neuroprotection (Coleman and Freeman, 2010). Previous axotomy models using primary neuronal cultures, mouse and Drosophila models show both the N70 and the Nmnat enzyme activity are essential (Conforti et al., 2007; Watanabe et al., 2007; Avery et al., 2009; Yahata et al., 2009). Here, we found that only the Nmnat portion is required to protect against JNK axonal degeneration. Furthermore, its enzyme activity is dispensable. Interestingly, this non-enzyme requirement was also reflected in the Nmnat RNAi rescue assays. Therefore, one possibility is that the N70 and the Nmnat enzyme activity are differentially axon-protective; essential for transected axons but not for uncut (but degenerating) axons, such as in JNK and Nmnat loss-of-function or SCA-1 paradigms, where its non-enzyme chaperone activity has a greater role (this study; Zhai et al., 2006; Zhai et al., 2008). This also extends to a recent Tau neurotoxicity model in Drosophila where non-enzyme Nmnat also has a neuroprotective effect (Ali et al., 2012). Another recent report suggest Nmnat also has a role in dendritic maintenance that is enzyme-independent (Wen et al., 2011). All these studies put together suggest a growing involvement of non-enzyme Nmnat function in various neuropathological conditions that is intimately linked to neuronal maintenance and stability.
Neuroprotective effects of molecular chaperones in neurodegenerative disease models are well-documented (Cummings et al., 1998; Cummings et al., 2001; Auluck et al., 2002; Magrané et al., 2004; Gifondorwa et al., 2007; Fonte et al., 2008; Voisine et al., 2010). Recent studies show that increasing Hsp activity restores peripheral injured nerves to functional recovery by promoting axonal growth (Ma et al., 2011). Upon nerve damage, Hsps are upregulated and present in rat dorsal root ganglion axons (Willis et al., 2005). The Hsps tested here appear to be less neuroprotective than Nmnat1. One possibility is that chaperones require Nmnat to provide greater recruitment potential to sites of nerve damage. Alternatively, acting together, they provide stronger neuroprotection.
Two questions emerge from these results. First, how does Nmnat and chaperone activities interact with JNK signalling? One possibility is that Nmnats are JNK kinases substrates. While Nmnats are phosphorylation targets (Schweiger et al., 2001; Berger et al., 2007), we have yet to test if JNK is directly involved. Another possibility is that JNK signals via the AP-1 transcription program to modulate Nmnat expression. Using immunostaining protocols, no significant changes to Nmnat protein levels were found when either Bsk or AP-1 was altered (using gain- or loss-of-function paradigms) in MB neurons (unpublished observations). However, the interactions may also occur through extensive regulators. Given that the JNK pathway is highly tuned to stress responses and nerve damage (Brecht et al., 2005; Leyssen et al., 2005; Ayaz et al., 2008; Nix et al., 2011), JNK signals may act to indirectly coordinate a mechanism by which Nmnat and molecular chaperones function together to prevent axon degeneration and maintain neural integrity at sites of damage. Recent axonal injury paradigms suggest Nmnat responses reside in the mitochondria (Avery et al., 2012; Fang et al., 2012; Yahata et al., 2009). It would be interesting to determine whether non-enzyme Nmnat axonal-protective function similarly occur in mitochondria and whether this also applies to other neurodegenerative paradigms, such as those provoked by genetic mutations and toxicological stress.
Secondly, what are the protein substrates regulated by Hsps, Nmnat and JNK signals? Identifying these substrates and how they may be regulated may be key to determining how axonal stability and degeneration is controlled during neural development, maintenance and in neuropathological situations.
Materials and Methods
Drosophila strains
The bsk147e, hepR39 and nmnat1 flies are null strains (Glise et al., 1995; Sluss et al., 1996; Zhai et al., 2006). The Mkk4e01458 is a loss-of-function allele (Thibault et al., 2004; Rallis et al., 2010). Additional strains used: UAS-EcRB1 DN (Cherbas et al., 2003); UAS-ZipDN::YFP (Dawes-Hoang et al., 2005); UAS-UBP2 (DiAntonio et al., 2001); UAS-Diap1, UAS-Diap2 and UAS-Dronc DN (ΔNdronc.C>A) (Meier et al., 2000; Ribeiro et al., 2007); UAS-Buffy (Quinn et al., 2003); UAS-mTOR (Hennig and Neufeld, 2002); UAS-HDAC6 (Pandey et al., 2007); UAS-Thor (4E-BP) (Miron et al., 2001); UAS-Parkin (Greene et al., 2003); UAS-dNmnat RNAi (Bloomington Drosophila Stock Center; 29402); UAS-Hsp70 (Warrick et al., 1999); UAS-Hsp26 (Wang et al., 2004); Myc tagged UAS-WldS, Nmnat, and their variant strains (Avery et al., 2009). WldS and Nmnat enzyme-inactive variants correspond to the H24A mutation that disrupts the ATP binding site, reducing the enzyme activity to ∼0.3% of wild-type levels (Sasaki et al., 2009).
MARCM clones, GAL80ts experiments and immunohistochemical analyses were similarly performed as previously (Rallis et al., 2010). All UAS expression lines were verified either by immunofluorescence staining (using available antibodies), or by RT-PCR, using primer pairs that detect only UAS-derived mRNA transcripts (5′-CAAGCGCAGCTGAACAAGCTAAACAATCTG-3′ and gene-specific primers) (data not shown; (Wang et al., 2004). Overexpression of EcRB1 DN, UBP2 or ZipDN::YFP causes phenotypes which are consistent with previously reported defects in axon pruning [EcRB1 DN and UBP2 (Watts et al., 2003; Hoopfer et al., 2006)] or in cytokinesis and axon overgrowth [ZipDN::YFP (Billuart et al., 2001); data not shown].
Supplementary Material
Acknowledgments
We thank Andrea Brand, Marc Freeman, Shigeo Hayashi, Pascal Meier, Leonie Quinn, John-Paul Taylor, Horng-Dar Wang, Alex Whitworth, the VDRC and Bloomington Drosophila Stock Centers who provided additional fly strains. We also thank Takahiro Chihara and members of the Lu laboratory for discussions and help. J.N. thanks Greg Jefferis for continued support.
Footnotes
Funding
Funded by the Wellcome Trust [grant number 078045 to J.N.]; and the National Institutes of Health (NIH) [grant numbers R01AR054926 and R01MH080378 to B.L.]. Deposited in PMC for release after 6 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.117259/-/DC1
References
- Abou–Sleiman P. M., Muqit M. M., Wood N. W. (2006). Expanding insights of mitochondrial dysfunction in Parkinson's disease. Nat. Rev. Neurosci. 7, 207–219 10.1038/nrn1868 [DOI] [PubMed] [Google Scholar]
- Ali Y. O., Ruan K., Zhai R. G. (2012). NMNAT suppresses tau-induced neurodegeneration by promoting clearance of hyperphosphorylated tau oligomers in a Drosophila model of tauopathy. Hum. Mol. Genet. 21, 237–250 10.1093/hmg/ddr449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki T., Sasaki Y., Milbrandt J. (2004). Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science 305, 1010–1013 10.1126/science.1098014 [DOI] [PubMed] [Google Scholar]
- Auluck P. K., Chan H. Y., Trojanowski J. Q., Lee V. M., Bonini N. M. (2002). Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson's disease. Science 295, 865–868 10.1126/science.1067389 [DOI] [PubMed] [Google Scholar]
- Avery M. A., Sheehan A. E., Kerr K. S., Wang J., Freeman M. R. (2009). Wld S requires Nmnat1 enzymatic activity and N16-VCP interactions to suppress Wallerian degeneration. J. Cell Biol. 184, 501–513 10.1083/jcb.200808042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery M. A., Rooney T. M., Pandya J. D., Wishart T. M., Gillingwater T. H., Geddes J. W., Sullivan P. G., Freeman M. R. (2012). WldS prevents axon degeneration through increased mitochondrial flux and enhanced mitochondrial Ca2+ buffering. Curr. Biol. 22, 596–600 10.1016/j.cub.2012.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaz D., Leyssen M., Koch M., Yan J., Srahna M., Sheeba V., Fogle K. J., Holmes T. C., Hassan B. A. (2008). Axonal injury and regeneration in the adult brain of Drosophila. J. Neurosci. 28, 6010–6021 10.1523/JNEUROSCI.0101-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babetto E., Beirowski B., Janeckova L., Brown R., Gilley J., Thomson D., Ribchester R. R., Coleman M. P. (2010). Targeting NMNAT1 to axons and synapses transforms its neuroprotective potency in vivo. J. Neurosci. 30, 13291–13304 10.1523/JNEUROSCI.1189-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnat M., Enslen H., Propst F., Davis R. J., Soares S., Nothias F. (2010). Distinct roles of c-Jun N-terminal kinase isoforms in neurite initiation and elongation during axonal regeneration. J. Neurosci. 30, 7804–7816 10.1523/JNEUROSCI.0372-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos S. A., Martinez N. W., Yoo S., Jara J. S., Zamorano S., Hetz C., Twiss J. L., Alvarez J., Court F. A. (2011). Axonal degeneration is mediated by the mitochondrial permeability transition pore. J. Neurosci. 31, 966–978 10.1523/JNEUROSCI.4065-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beirowski B., Babetto E., Coleman M. P., Martin K. R. (2008). The WldS gene delays axonal but not somatic degeneration in a rat glaucoma model. Eur. J. Neurosci. 28, 1166–1179 10.1111/j.1460-9568.2008.06426.x [DOI] [PubMed] [Google Scholar]
- Beirowski B., Babetto E., Gilley J., Mazzola F., Conforti L., Janeckova L., Magni G., Ribchester R. R., Coleman M. P. (2009). Non-nuclear Wld(S) determines its neuroprotective efficacy for axons and synapses in vivo. J. Neurosci. 29, 653–668 10.1523/JNEUROSCI.3814-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beirowski B., Morreale G., Conforti L., Mazzola F., Di Stefano M., Wilbrey A., Babetto E., Janeckova L., Magni G., Coleman M. P. (2010). WldS can delay Wallerian degeneration in mice when interaction with valosin-containing protein is weakened. Neuroscience 166, 201–211 10.1016/j.neuroscience.2009.12.024 [DOI] [PubMed] [Google Scholar]
- Berger F., Lau C., Dahlmann M., Ziegler M. (2005). Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J. Biol. Chem. 280, 36334–36341 10.1074/jbc.M508660200 [DOI] [PubMed] [Google Scholar]
- Berger F., Lau C., Ziegler M. (2007). Regulation of poly(ADP-ribose) polymerase 1 activity by the phosphorylation state of the nuclear NAD biosynthetic enzyme NMN adenylyl transferase 1. Proc. Natl. Acad. Sci. USA 104, 3765–3770 10.1073/pnas.0609211104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya M. R., Gerdts J., Naylor S. A., Royse E. X., Ebstein S. Y., Sasaki Y., Milbrandt J., DiAntonio A. (2012). A model of toxic neuropathy in Drosophila reveals a role for MORN4 in promoting axonal degeneration. J. Neurosci. 32, 5054–5061 10.1523/JNEUROSCI.4951-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billuart P., Winter C. G., Maresh A., Zhao X., Luo L. (2001). Regulating axon branch stability: the role of p190 RhoGAP in repressing a retraction signaling pathway. Cell 107, 195–207 10.1016/S0092-8674(01)00522-0 [DOI] [PubMed] [Google Scholar]
- Bingol B., Sheng M. (2011). Deconstruction for reconstruction: the role of proteolysis in neural plasticity and disease. Neuron 69, 22–32 10.1016/j.neuron.2010.11.006 [DOI] [PubMed] [Google Scholar]
- Brecht S., Kirchhof R., Chromik A., Willesen M., Nicolaus T., Raivich G., Wessig J., Waetzig V., Goetz M., Claussen M.et al. (2005). Specific pathophysiological functions of JNK isoforms in the brain. Eur. J. Neurosci. 21, 363–377 10.1111/j.1460-9568.2005.03857.x [DOI] [PubMed] [Google Scholar]
- Chang L., Jones Y., Ellisman M. H., Goldstein L. S., Karin M. (2003). JNK1 is required for maintenance of neuronal microtubules and controls phosphorylation of microtubule-associated proteins. Dev. Cell 4, 521–533 10.1016/S1534-5807(03)00094-7 [DOI] [PubMed] [Google Scholar]
- Cherbas L., Hu X., Zhimulev I., Belyaeva E., Cherbas P. (2003). EcR isoforms in Drosophila: testing tissue-specific requirements by targeted blockade and rescue. Development 130, 271–284 10.1242/dev.00205 [DOI] [PubMed] [Google Scholar]
- Chiang P. W., Wang J., Chen Y., Fu Q., Zhong J., Chen Y., Yi X., Wu R., Gan H., Shi Y.et al. (2012). Exome sequencing identifies NMNAT1 mutations as a cause of Leber congenital amaurosis. Nat. Genet. 44, 972–974 10.1038/ng.2370 [DOI] [PubMed] [Google Scholar]
- Coleman M. (2005). Axon degeneration mechanisms: commonality amid diversity. Nat. Rev. Neurosci. 6, 889–898 10.1038/nrn1788 [DOI] [PubMed] [Google Scholar]
- Coleman M. P., Freeman M. R. (2010). Wallerian degeneration, wld(s), and nmnat. Annu. Rev. Neurosci. 33, 245–267 10.1146/annurev-neuro-060909-153248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti L., Tarlton A., Mack T. G., Mi W., Buckmaster E. A., Wagner D., Perry V. H., Coleman M. P. (2000). A Ufd2/D4Cole1e chimeric protein and overexpression of Rbp7 in the slow Wallerian degeneration (WldS) mouse. Proc. Natl. Acad. Sci. USA 97, 11377–11382 10.1073/pnas.97.21.11377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti L., Fang G., Beirowski B., Wang M. S., Sorci L., Asress S., Adalbert R., Silva A., Bridge K., Huang X. P.et al. (2007). NAD(+) and axon degeneration revisited: Nmnat1 cannot substitute for Wld(S) to delay Wallerian degeneration. Cell Death Differ. 14, 116–127 10.1038/sj.cdd.4401944 [DOI] [PubMed] [Google Scholar]
- Conforti L., Wilbrey A., Morreale G., Janeckova L., Beirowski B., Adalbert R., Mazzola F., Di Stefano M., Hartley R., Babetto E.et al. (2009). Wld S protein requires Nmnat activity and a short N-terminal sequence to protect axons in mice. J. Cell Biol. 184, 491–500 10.1083/jcb.200807175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings C. J., Mancini M. A., Antalffy B., DeFranco D. B., Orr H. T., Zoghbi H. Y. (1998). Chaperone suppression of aggregation and altered subcellular proteasome localization imply protein misfolding in SCA1. Nat. Genet. 19, 148–154 10.1038/502 [DOI] [PubMed] [Google Scholar]
- Cummings C. J., Sun Y., Opal P., Antalffy B., Mestril R., Orr H. T., Dillmann W. H., Zoghbi H. Y. (2001). Over-expression of inducible HSP70 chaperone suppresses neuropathology and improves motor function in SCA1 mice. Hum. Mol. Genet. 10, 1511–1518 10.1093/hmg/10.14.1511 [DOI] [PubMed] [Google Scholar]
- Dawes–Hoang R. E., Parmar K. M., Christiansen A. E., Phelps C. B., Brand A. H., Wieschaus E. F. (2005). folded gastrulation, cell shape change and the control of myosin localization. Development 132, 4165–4178 10.1242/dev.01938 [DOI] [PubMed] [Google Scholar]
- DiAntonio A., Haghighi A. P., Portman S. L., Lee J. D., Amaranto A. M., Goodman C. S. (2001). Ubiquitination-dependent mechanisms regulate synaptic growth and function. Nature 412, 449–452 10.1038/35086595 [DOI] [PubMed] [Google Scholar]
- Eilers A., Whitfield J., Babij C., Rubin L. L., Ham J. (1998). Role of the Jun kinase pathway in the regulation of c-Jun expression and apoptosis in sympathetic neurons. J. Neurosci. 18, 1713–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk M. J., Zhang Q., Nakamaru–Ogiso E., Kannabiran C., Fonseca–Kelly Z., Chakarova C., Audo I., Mackay D. S., Zeitz C., Borman A. D.et al. (2012). NMNAT1 mutations cause Leber congenital amaurosis. Nat. Genet. 44, 1040–1045 10.1038/ng.2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y., Soares L., Teng X., Geary M., Bonini N. M. (2012). A novel Drosophila model of nerve injury reveals an essential role of Nmnat in maintaining axonal integrity. Curr. Biol. 22, 590–595 10.1016/j.cub.2012.01.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer M. J. (2006). Genetics of Parkinson disease: paradigm shifts and future prospects. Nat. Rev. Genet. 7, 306–318 10.1038/nrg1831 [DOI] [PubMed] [Google Scholar]
- Feng Y., Yan T., Zheng J., Ge X., Mu Y., Zhang Y., Wu D., Du J. L., Zhai Q. (2010). Overexpression of Wld(S) or Nmnat2 in mauthner cells by single-cell electroporation delays axon degeneration in live zebrafish. J. Neurosci. Res. 88, 3319–3327 10.1002/jnr.22498 [DOI] [PubMed] [Google Scholar]
- Ferri A., Sanes J. R., Coleman M. P., Cunningham J. M., Kato A. C. (2003). Inhibiting axon degeneration and synapse loss attenuates apoptosis and disease progression in a mouse model of motoneuron disease. Curr. Biol. 13, 669–673 10.1016/S0960-9822(03)00206-9 [DOI] [PubMed] [Google Scholar]
- Finn J. T., Weil M., Archer F., Siman R., Srinivasan A., Raff M. C. (2000). Evidence that Wallerian degeneration and localized axon degeneration induced by local neurotrophin deprivation do not involve caspases. J. Neurosci. 20, 1333–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonte V., Kipp D. R., Yerg J., 3rd, Merin D., Forrestal M., Wagner E., Roberts C. M., Link C. D. (2008). Suppression of in vivo beta-amyloid peptide toxicity by overexpression of the HSP-16.2 small chaperone protein. J. Biol. Chem. 283, 784–791 10.1074/jbc.M703339200 [DOI] [PubMed] [Google Scholar]
- Gallo G. (2004). Myosin II activity is required for severing-induced axon retraction in vitro. Exp. Neurol. 189, 112–121 10.1016/j.expneurol.2004.05.019 [DOI] [PubMed] [Google Scholar]
- Gallo G. (2006). RhoA-kinase coordinates F-actin organization and myosin II activity during semaphorin-3A-induced axon retraction. J. Cell Sci. 119, 3413–3423 10.1242/jcs.03084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Feany M. B. (2004). Comparison of pathways controlling toxicity in the eye and brain in Drosophila models of human neurodegenerative diseases. Hum. Mol. Genet. 13, 2011–2018 10.1093/hmg/ddh214 [DOI] [PubMed] [Google Scholar]
- Ghosh A. S., Wang B., Pozniak C. D., Chen M., Watts R. J., Lewcock J. W. (2011). DLK induces developmental neuronal degeneration via selective regulation of proapoptotic JNK activity. J. Cell Biol. 194, 751–764 10.1083/jcb.201103153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifondorwa D. J., Robinson M. B., Hayes C. D., Taylor A. R., Prevette D. M., Oppenheim R. W., Caress J., Milligan C. E. (2007). Exogenous delivery of heat shock protein 70 increases lifespan in a mouse model of amyotrophic lateral sclerosis. J. Neurosci. 27, 13173–13180 10.1523/JNEUROSCI.4057-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilley J., Coleman M. P. (2010). Endogenous Nmnat2 is an essential survival factor for maintenance of healthy axons. PLoS Biol. 8, e1000300 10.1371/journal.pbio.1000300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glise B., Bourbon H., Noselli S. (1995). hemipterous encodes a novel Drosophila MAP kinase kinase, required for epithelial cell sheet movement. Cell 83, 451–461 10.1016/0092-8674(95)90123-X [DOI] [PubMed] [Google Scholar]
- Greene J. C., Whitworth A. J., Kuo I., Andrews L. A., Feany M. B., Pallanck L. J. (2003). Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc. Natl. Acad. Sci. USA 100, 4078–4083 10.1073/pnas.0737556100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbani D. M., O'Malley K. L. (2006). Wld(S) mice are protected against the Parkinsonian mimetic MPTP. Exp. Neurol. 202, 93–99 10.1016/j.expneurol.2006.05.017 [DOI] [PubMed] [Google Scholar]
- Hawkins C. J., Yoo S. J., Peterson E. P., Wang S. L., Vernooy S. Y., Hay B. A. (2000). The Drosophila caspase DRONC cleaves following glutamate or aspartate and is regulated by DIAP1, HID, and GRIM. J. Biol. Chem. 275, 27084–27093 [DOI] [PubMed] [Google Scholar]
- Hay B. A., Wassarman D. A., Rubin G. M. (1995). Drosophila homologs of baculovirus inhibitor of apoptosis proteins function to block cell death. Cell 83, 1253–1262 10.1016/0092-8674(95)90150-7 [DOI] [PubMed] [Google Scholar]
- Hennig K. M., Neufeld T. P. (2002). Inhibition of cellular growth and proliferation by dTOR overexpression in Drosophila. Genesis 34, 107–110 10.1002/gene.10139 [DOI] [PubMed] [Google Scholar]
- Herdegen T., Claret F. X., Kallunki T., Martin–Villalba A., Winter C., Hunter T., Karin M. (1998). Lasting N-terminal phosphorylation of c-Jun and activation of c-Jun N-terminal kinases after neuronal injury. J. Neurosci. 18, 5124–5135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks A. N., Lorenzetti D., Gilley J., Lu B., Andersson K. E., Miligan C., Overbeek P. A., Oppenheim R., Bishop C. E. (2012). Nicotinamide mononucleotide adenylyltransferase 2 (Nmnat2) regulates axon integrity in the mouse embryo. PLoS ONE 7, e47869 10.1371/journal.pone.0047869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoopfer E. D., McLaughlin T., Watts R. J., Schuldiner O., O'Leary D. D., Luo L. (2006). Wlds protection distinguishes axon degeneration following injury from naturally occurring developmental pruning. Neuron 50, 883–895 10.1016/j.neuron.2006.05.013 [DOI] [PubMed] [Google Scholar]
- Howell G. R., Libby R. T., Jakobs T. C., Smith R. S., Phalan F. C., Barter J. W., Barbay J. M., Marchant J. K., Mahesh N., Porciatti V.et al. (2007). Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J. Cell Biol. 179, 1523–1537 10.1083/jcb.200706181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y., Gehrke S., Wang H. Q., Takahashi R., Hasegawa K., Oota E., Lu B. (2008). Phosphorylation of 4E-BP by LRRK2 affects the maintenance of dopaminergic neurons in Drosophila. EMBO J. 27, 2432–2443 10.1038/emboj.2008.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S., Wang J., Kaneko M., Yiu G., Hurrell J. M., Chitnis T., Khoury S. J., He Z. (2006). Protecting axonal degeneration by increasing nicotinamide adenine dinucleotide levels in experimental autoimmune encephalomyelitis models. J. Neurosci. 26, 9794–9804 10.1523/JNEUROSCI.2116-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandelwal P. J., Herman A. M., Hoe H. S., Rebeck G. W., Moussa C. E. (2011). Parkin mediates beclin-dependent autophagic clearance of defective mitochondria and ubiquitinated Abeta in AD models. Hum. Mol. Genet. 20, 2091–2102 10.1093/hmg/ddr091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenekoop R. K., Wang H., Majewski J., Wang X., Lopez I., Ren H., Chen Y., Li Y., Fishman G. A., Genead M.et al. ; Finding of Rare Disease Genes (FORGE) Canada Consortium (2012). Mutations in NMNAT1 cause Leber congenital amaurosis and identify a new disease pathway for retinal degeneration. Nat. Genet. 44, 1035–1039 10.1038/ng.2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuan C. Y., Whitmarsh A. J., Yang D. D., Liao G., Schloemer A. J., Dong C., Bao J., Banasiak K. J., Haddad G. G., Flavell R. A.et al. (2003). A critical role of neural-specific JNK3 for ischemic apoptosis. Proc. Natl. Acad. Sci. USA 100, 15184–15189 10.1073/pnas.2336254100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. T., Zhu S., Younger S., Jan L. Y., Jan Y. N. (2006). Identification of E2/E3 ubiquitinating enzymes and caspase activity regulating Drosophila sensory neuron dendrite pruning. Neuron 51, 283–290 10.1016/j.neuron.2006.07.014 [DOI] [PubMed] [Google Scholar]
- Lee T., Luo L. (1999). Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22, 451–461 10.1016/S0896-6273(00)80701-1 [DOI] [PubMed] [Google Scholar]
- Lee T., Marticke S., Sung C., Robinow S., Luo L. (2000). Cell-autonomous requirement of the USP/EcR-B ecdysone receptor for mushroom body neuronal remodeling in Drosophila. Neuron 28, 807–818 10.1016/S0896-6273(00)00155-0 [DOI] [PubMed] [Google Scholar]
- Lee J. Y., Koga H., Kawaguchi Y., Tang W., Wong E., Gao Y. S., Pandey U. B., Kaushik S., Tresse E., Lu J.et al. (2010). HDAC6 controls autophagosome maturation essential for ubiquitin-selective quality-control autophagy. EMBO J. 29, 969–980 10.1038/emboj.2009.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyssen M., Ayaz D., Hébert S. S., Reeve S., De Strooper B., Hassan B. A. (2005). Amyloid precursor protein promotes post-developmental neurite arborization in the Drosophila brain. EMBO J. 24, 2944–2955 10.1038/sj.emboj.7600757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M. T., Beal M. F. (2006). Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443, 787–795 10.1038/nature05292 [DOI] [PubMed] [Google Scholar]
- Lin H., Kwan A. L., Dutcher S. K. (2010). Synthesizing and salvaging NAD: lessons learned from Chlamydomonas reinhardtii. PLoS Genet. 6, e1001105 10.1371/journal.pgen.1001105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Lu B. (2010). Reduction of protein translation and activation of autophagy protect against PINK1 pathogenesis in Drosophila melanogaster. PLoS Genet. 6, e1001237 10.1371/journal.pgen.1001237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn E. R., Perry V. H., Brown M. C., Rosen H., Gordon S. (1989). Absence of Wallerian Degeneration does not Hinder Regeneration in Peripheral Nerve. Eur. J. Neurosci. 1, 27–33 10.1111/j.1460-9568.1989.tb00771.x [DOI] [PubMed] [Google Scholar]
- Luo L., O'Leary D. D. (2005). Axon retraction and degeneration in development and disease. Annu. Rev. Neurosci. 28, 127–156 10.1146/annurev.neuro.28.061604.135632 [DOI] [PubMed] [Google Scholar]
- Ma C. H., Omura T., Cobos E. J., Latrémolière A., Ghasemlou N., Brenner G. J., van Veen E., Barrett L., Sawada T., Gao F.et al. (2011). Accelerating axonal growth promotes motor recovery after peripheral nerve injury in mice. J. Clin. Invest. 121, 4332–4347 10.1172/JCI58675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack T. G., Reiner M., Beirowski B., Mi W., Emanuelli M., Wagner D., Thomson D., Gillingwater T., Court F., Conforti L.et al. (2001). Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat. Neurosci. 4, 1199–1206 10.1038/nn770 [DOI] [PubMed] [Google Scholar]
- Magrané J., Smith R. C., Walsh K., Querfurth H. W. (2004). Heat shock protein 70 participates in the neuroprotective response to intracellularly expressed beta-amyloid in neurons. J. Neurosci. 24, 1700–1706 10.1523/JNEUROSCI.4330-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroney A. C., Glicksman M. A., Basma A. N., Walton K. M., Knight E., Jr, Murphy C. A., Bartlett B. A., Finn J. P., Angeles T., Matsuda Y.et al. (1998). Motoneuron apoptosis is blocked by CEP-1347 (KT 7515), a novel inhibitor of the JNK signaling pathway. J. Neurosci. 18, 104–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire S. E., Le P. T., Osborn A. J., Matsumoto K., Davis R. L. (2003). Spatiotemporal rescue of memory dysfunction in Drosophila. Science 302, 1765–1768 10.1126/science.1089035 [DOI] [PubMed] [Google Scholar]
- Meier P., Silke J., Leevers S. J., Evan G. I. (2000). The Drosophila caspase DRONC is regulated by DIAP1. EMBO J. 19, 598–611 10.1093/emboj/19.4.598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi W., Beirowski B., Gillingwater T. H., Adalbert R., Wagner D., Grumme D., Osaka H., Conforti L., Arnhold S., Addicks K.et al. (2005). The slow Wallerian degeneration gene, WldS, inhibits axonal spheroid pathology in gracile axonal dystrophy mice. Brain 128, 405–416 10.1093/brain/awh368 [DOI] [PubMed] [Google Scholar]
- Miller B. R., Press C., Daniels R. W., Sasaki Y., Milbrandt J., DiAntonio A. (2009). A dual leucine kinase-dependent axon self-destruction program promotes Wallerian degeneration. Nat. Neurosci. 12, 387–389 10.1038/nn.2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron M., Verdú J., Lachance P. E., Birnbaum M. J., Lasko P. F., Sonenberg N. (2001). The translational inhibitor 4E-BP is an effector of PI(3)K/Akt signalling and cell growth in Drosophila. Nat. Cell Biol. 3, 596–601 10.1038/35078571 [DOI] [PubMed] [Google Scholar]
- Morfini G. A., You Y. M., Pollema S. L., Kaminska A., Liu K., Yoshioka K., Björkblom B., Coffey E. T., Bagnato C., Han D.et al. (2009). Pathogenic huntingtin inhibits fast axonal transport by activating JNK3 and phosphorylating kinesin. Nat. Neurosci. 12, 864–871 10.1038/nn.2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima Y., Gotoh Y., Zieg J., Barrett T., Takano H., Flavell R., Davis R. J., Shirasaki Y., Greenberg M. E. (2001). Beta-amyloid induces neuronal apoptosis via a mechanism that involves the c-Jun N-terminal kinase pathway and the induction of Fas ligand. J. Neurosci. 21, 7551–7560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muro I., Hay B. A., Clem R. J. (2002). The Drosophila DIAP1 protein is required to prevent accumulation of a continuously generated, processed form of the apical caspase DRONC. J. Biol. Chem. 277, 49644–49650 10.1074/jbc.M203464200 [DOI] [PubMed] [Google Scholar]
- Narendra D. P., Youle R. J. (2011). Targeting mitochondrial dysfunction: role for PINK1 and Parkin in mitochondrial quality control. Antioxid. Redox Signal. 14, 1929–1938 10.1089/ars.2010.3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra D. P., Jin S. M., Tanaka A., Suen D. F., Gautier C. A., Shen J., Cookson M. R., Youle R. J. (2010). PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 8, e1000298 10.1371/journal.pbio.1000298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaev A., McLaughlin T., O'Leary D. D., Tessier–Lavigne M. (2009). APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature 457, 981–989 10.1038/nature07767 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nix P., Hisamoto N., Matsumoto K., Bastiani M. (2011). Axon regeneration requires coordinate activation of p38 and JNK MAPK pathways. Proc. Natl. Acad. Sci. USA 108, 10738–10743 10.1073/pnas.1104830108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazawa H., Estus S. (2002). The JNK/c-Jun cascade and Alzheimer's disease. Am. J. Alzheimers Dis. Other Demen. 17, 79–88 10.1177/153331750201700209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva A. A., Jr, Atkins C. M., Copenagle L., Banker G. A. (2006). Activated c-Jun N-terminal kinase is required for axon formation. J. Neurosci. 26, 9462–9470 10.1523/JNEUROSCI.2625-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey U. B., Nie Z., Batlevi Y., McCray B. A., Ritson G. P., Nedelsky N. B., Schwartz S. L., DiProspero N. A., Knight M. A., Schuldiner O.et al. (2007). HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature 447, 859–863 10.1038/nature05853 [DOI] [PubMed] [Google Scholar]
- Park J., Lee S. B., Lee S., Kim Y., Song S., Kim S., Bae E., Kim J., Shong M., Kim J. M.et al. (2006). Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature 441, 1157–1161 10.1038/nature04788 [DOI] [PubMed] [Google Scholar]
- Peng J., Andersen J. K. (2003). The role of c-Jun N-terminal kinase (JNK) in Parkinson's disease. IUBMB Life 55, 267–271 10.1080/1521654031000121666 [DOI] [PubMed] [Google Scholar]
- Perrault I., Hanein S., Zanlonghi X., Serre V., Nicouleau M., Defoort–Delhemmes S., Delphin N., Fares–Taie L., Gerber S., Xerri O.et al. (2012). Mutations in NMNAT1 cause Leber congenital amaurosis with early-onset severe macular and optic atrophy. Nat. Genet. 44, 975–977 10.1038/ng.2357 [DOI] [PubMed] [Google Scholar]
- Perrin V., Dufour N., Raoul C., Hassig R., Brouillet E., Aebischer P., Luthi–Carter R., Déglon N. (2009). Implication of the JNK pathway in a rat model of Huntington's disease. Exp. Neurol. 215, 191–200 10.1016/j.expneurol.2008.10.008 [DOI] [PubMed] [Google Scholar]
- Pun S., Santos A. F., Saxena S., Xu L., Caroni P. (2006). Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nat. Neurosci. 9, 408–419 10.1038/nn1653 [DOI] [PubMed] [Google Scholar]
- Quinn L., Coombe M., Mills K., Daish T., Colussi P., Kumar S., Richardson H. (2003). Buffy, a Drosophila Bcl-2 protein, has anti-apoptotic and cell cycle inhibitory functions. EMBO J. 22, 3568–3579 10.1093/emboj/cdg355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raivich G., Bohatschek M., Da Costa C., Iwata O., Galiano M., Hristova M., Nateri A. S., Makwana M., Riera–Sans L., Wolfer D. P.et al. (2004). The AP-1 transcription factor c-Jun is required for efficient axonal regeneration. Neuron 43, 57–67 10.1016/j.neuron.2004.06.005 [DOI] [PubMed] [Google Scholar]
- Rallis A., Moore C., Ng J. (2010). Signal strength and signal duration define two distinct aspects of JNK-regulated axon stability. Dev. Biol. 339, 65–77 10.1016/j.ydbio.2009.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar B., Duden R., Rubinsztein D. C. (2002). Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Hum. Mol. Genet. 11, 1107–1117 10.1093/hmg/11.9.1107 [DOI] [PubMed] [Google Scholar]
- Ribeiro P. S., Kuranaga E., Tenev T., Leulier F., Miura M., Meier P. (2007). DIAP2 functions as a mechanism-based regulator of drICE that contributes to the caspase activity threshold in living cells. J. Cell Biol. 179, 1467–1480 10.1083/jcb.200706027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso S. B., Sussman D., Wynshaw–Boris A., Salinas P. C. (2005). Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat. Neurosci. 8, 34–42 10.1038/nn1374 [DOI] [PubMed] [Google Scholar]
- Sagot Y., Dubois–Dauphin M., Tan S. A., de Bilbao F., Aebischer P., Martinou J. C., Kato A. C. (1995). Bcl-2 overexpression prevents motoneuron cell body loss but not axonal degeneration in a mouse model of a neurodegenerative disease. J. Neurosci. 15, 7727–7733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajadi A., Schneider B. L., Aebischer P. (2004). Wlds-mediated protection of dopaminergic fibers in an animal model of Parkinson disease. Curr. Biol. 14, 326–330 [DOI] [PubMed] [Google Scholar]
- Sanyal S., Sandstrom D. J., Hoeffer C. A., Ramaswami M. (2002). AP-1 functions upstream of CREB to control synaptic plasticity in Drosophila. Nature 416, 870–874 10.1038/416870a [DOI] [PubMed] [Google Scholar]
- Sasaki Y., Milbrandt J. (2010). Axonal degeneration is blocked by nicotinamide mononucleotide adenylyltransferase (Nmnat) protein transduction into transected axons. J. Biol. Chem. 285, 41211–41215 10.1074/jbc.C110.193904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y., Vohra B. P., Lund F. E., Milbrandt J. (2009). Nicotinamide mononucleotide adenylyl transferase-mediated axonal protection requires enzymatic activity but not increased levels of neuronal nicotinamide adenine dinucleotide. J. Neurosci. 29, 5525–5535 10.1523/JNEUROSCI.5469-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena S., Caroni P. (2007). Mechanisms of axon degeneration: from development to disease. Prog. Neurobiol. 83, 174–191 10.1016/j.pneurobio.2007.07.007 [DOI] [PubMed] [Google Scholar]
- Schoenmann Z., Assa–Kunik E., Tiomny S., Minis A., Haklai–Topper L., Arama E., Yaron A. (2010). Axonal degeneration is regulated by the apoptotic machinery or a NAD+-sensitive pathway in insects and mammals. J. Neurosci. 30, 6375–6386 10.1523/JNEUROSCI.0922-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweiger M., Hennig K., Lerner F., Niere M., Hirsch–Kauffmann M., Specht T., Weise C., Oei S. L., Ziegler M. (2001). Characterization of recombinant human nicotinamide mononucleotide adenylyl transferase (NMNAT), a nuclear enzyme essential for NAD synthesis. FEBS Lett. 492, 95–100 10.1016/S0014-5793(01)02180-9 [DOI] [PubMed] [Google Scholar]
- Sluss H. K., Han Z., Barrett T., Goberdhan D. C., Wilson C., Davis R. J., Ip Y. T. (1996). A JNK signal transduction pathway that mediates morphogenesis and an immune response in Drosophila. Genes Dev. 10, 2745–2758 10.1101/gad.10.21.2745 [DOI] [PubMed] [Google Scholar]
- Tain L. S., Mortiboys H., Tao R. N., Ziviani E., Bandmann O., Whitworth A. J. (2009). Rapamycin activation of 4E-BP prevents parkinsonian dopaminergic neuron loss. Nat. Neurosci. 12, 1129–1135 10.1038/nn.2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J., Rolls M. M. (2011). Dendrites have a rapid program of injury-induced degeneration that is molecularly distinct from developmental pruning. J. Neurosci. 31, 5398–5405 10.1523/JNEUROSCI.3826-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault S. T., Singer M. A., Miyazaki W. Y., Milash B., Dompe N. A., Singh C. M., Buchholz R., Demsky M., Fawcett R., Francis–Lang H. L.et al. (2004). A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nat. Genet. 36, 283–287 10.1038/ng1314 [DOI] [PubMed] [Google Scholar]
- Thomas G. M., Lin D. T., Nuriya M., Huganir R. L. (2008). Rapid and bi-directional regulation of AMPA receptor phosphorylation and trafficking by JNK. EMBO J. 27, 361–372 10.1038/sj.emboj.7601969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden M. G., Chandel N. S., Williamson E. K., Schumacker P. T., Thompson C. B. (1997). Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell 91, 627–637 10.1016/S0092-8674(00)80450-X [DOI] [PubMed] [Google Scholar]
- Vohra B. P., Sasaki Y., Miller B. R., Chang J., DiAntonio A., Milbrandt J. (2010). Amyloid precursor protein cleavage-dependent and -independent axonal degeneration programs share a common nicotinamide mononucleotide adenylyltransferase 1-sensitive pathway. J. Neurosci. 30, 13729–13738 10.1523/JNEUROSCI.2939-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voisine C., Pedersen J. S., Morimoto R. I. (2010). Chaperone networks: tipping the balance in protein folding diseases. Neurobiol. Dis. 40, 12–20 10.1016/j.nbd.2010.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waetzig V., Zhao Y., Herdegen T. (2006). The bright side of JNKs-Multitalented mediators in neuronal sprouting, brain development and nerve fiber regeneration. Prog. Neurobiol. 80, 84–97 10.1016/j.pneurobio.2006.08.002 [DOI] [PubMed] [Google Scholar]
- Wang H. D., Kazemi–Esfarjani P., Benzer S. (2004). Multiple-stress analysis for isolation of Drosophila longevity genes. Proc. Natl. Acad. Sci. USA 101, 12610–12615 10.1073/pnas.0404648101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zhai Q., Chen Y., Lin E., Gu W., McBurney M. W., He Z. (2005). A local mechanism mediates NAD-dependent protection of axon degeneration. J. Cell Biol. 170, 349–355 10.1083/jcb.200504028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. T., Medress Z. A., Barres B. A. (2012). Axon degeneration: molecular mechanisms of a self-destruction pathway. J. Cell Biol. 196, 7–18 10.1083/jcb.201108111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrick J. M., Chan H. Y., Gray–Board G. L., Chai Y., Paulson H. L., Bonini N. M. (1999). Suppression of polyglutamine-mediated neurodegeneration in Drosophila by the molecular chaperone HSP70. Nat. Genet. 23, 425–428 10.1038/70532 [DOI] [PubMed] [Google Scholar]
- Watanabe M., Tsukiyama T., Hatakeyama S. (2007). Protection of vincristine-induced neuropathy by WldS expression and the independence of the activity of Nmnat1. Neurosci. Lett. 411, 228–232 10.1016/j.neulet.2006.09.068 [DOI] [PubMed] [Google Scholar]
- Watts R. J., Hoopfer E. D., Luo L. (2003). Axon pruning during Drosophila metamorphosis: evidence for local degeneration and requirement of the ubiquitin-proteasome system. Neuron 38, 871–885 10.1016/S0896-6273(03)00295-2 [DOI] [PubMed] [Google Scholar]
- Wen Y., Parrish J. Z., He R., Zhai R. G., Kim M. D. (2011). Nmnat exerts neuroprotective effects in dendrites and axons. Mol. Cell. Neurosci. 48, 1–8 10.1016/j.mcn.2011.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. W., Kondo S., Krzyzanowska A., Hiromi Y., Truman J. W. (2006). Local caspase activity directs engulfment of dendrites during pruning. Nat. Neurosci. 9, 1234–1236 10.1038/nn1774 [DOI] [PubMed] [Google Scholar]
- Willis D., Li K. W., Zheng J. Q., Chang J. H., Smit A. B., Kelly T., Merianda T. T., Sylvester J., van Minnen J., Twiss J. L. (2005). Differential transport and local translation of cytoskeletal, injury-response, and neurodegeneration protein mRNAs in axons. J. Neurosci. 25, 778–791 10.1523/JNEUROSCI.4235-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E., Cuervo A. M. (2010). Autophagy gone awry in neurodegenerative diseases. Nat. Neurosci. 13, 805–811 10.1038/nn.2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie S. R., Chantler P. D. (2003). Myosin IIA drives neurite retraction. Mol. Biol. Cell 14, 4654–4666 10.1091/mbc.E03-03-0187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z., Dickens M., Raingeaud J., Davis R. J., Greenberg M. E. (1995). Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270, 1326–1331 10.1126/science.270.5240.1326 [DOI] [PubMed] [Google Scholar]
- Yahata N., Yuasa S., Araki T. (2009). Nicotinamide mononucleotide adenylyltransferase expression in mitochondrial matrix delays Wallerian degeneration. J. Neurosci. 29, 6276–6284 10.1523/JNEUROSCI.4304-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D. D., Kuan C. Y., Whitmarsh A. J., Rincón M., Zheng T. S., Davis R. J., Rakic P., Flavell R. A. (1997). Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature 389, 865–870 10.1038/39899 [DOI] [PubMed] [Google Scholar]
- Zhai Q., Wang J., Kim A., Liu Q., Watts R., Hoopfer E., Mitchison T., Luo L., He Z. (2003). Involvement of the ubiquitin-proteasome system in the early stages of wallerian degeneration. Neuron 39, 217–225 10.1016/S0896-6273(03)00429-X [DOI] [PubMed] [Google Scholar]
- Zhai R. G., Cao Y., Hiesinger P. R., Zhou Y., Mehta S. Q., Schulze K. L., Verstreken P., Bellen H. J. (2006). Drosophila NMNAT maintains neural integrity independent of its NAD synthesis activity. PLoS Biol. 4, e416 10.1371/journal.pbio.0040416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai R. G., Zhang F., Hiesinger P. R., Cao Y., Haueter C. M., Bellen H. J. (2008). NAD synthase NMNAT acts as a chaperone to protect against neurodegeneration. Nature 452, 887–891 10.1038/nature06721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai R. G., Rizzi M., Garavaglia S. (2009). Nicotinamide/nicotinic acid mononucleotide adenylyltransferase, new insights into an ancient enzyme. Cell. Mol. Life Sci. 66, 2805–2818 10.1007/s00018-009-0047-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.