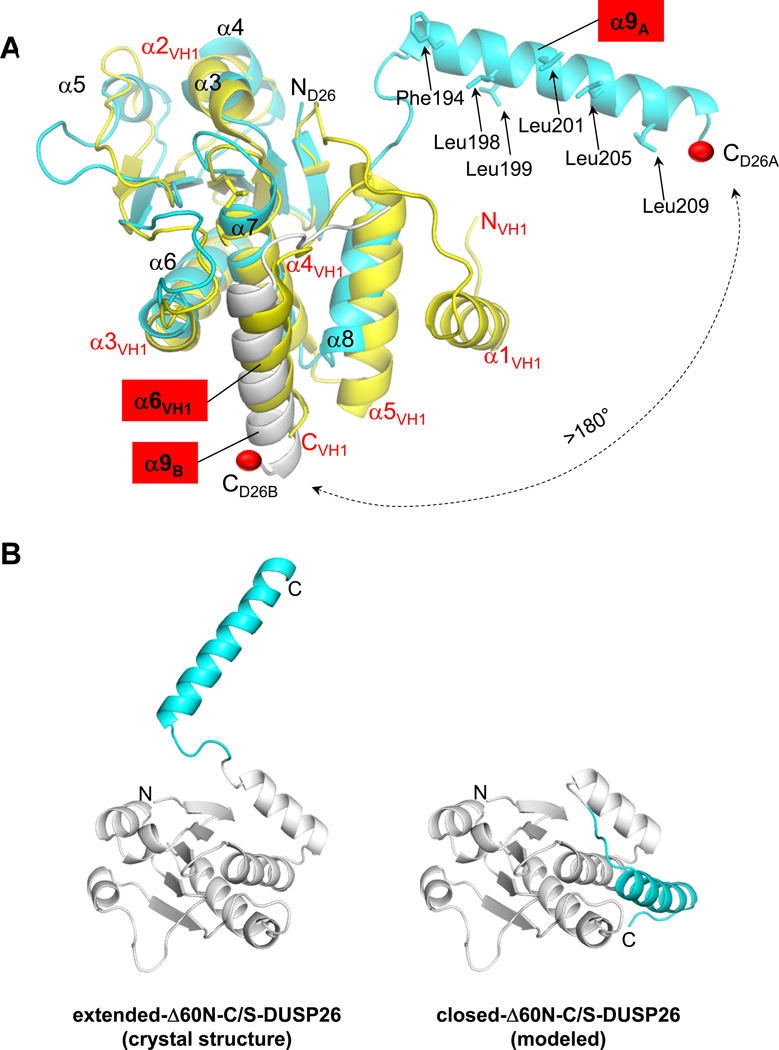
Figure 6. Flexibility of DUSP26 C-terminal helix α9.
(A) Superimposition of ΔN60-C/S-DUSP26 with VH1 (colored in cyan and yellow, respectively). For clarity, only α-helices are labeled; helix α9, and its counterpart in VH1, helix α6VH1 are highlighted in red. Hydrophobic residues protruding on the surface of helix α9 are shown as sticks and their position indicated by arrows. Helix α9 of protomer B (α9B) is shown as a gray ribbon. A red ball indicates the position of DUSP26 α9A and α9B C-termini; a dashed arrow illustrates the putative trajectory of the conformational change helix α9 would undergo from the position observed crystallographically to that adopted by helix α6VH1 in VH1 (or helix α9B in protomer B). (B) Ribbon diagram of ΔN60-C/S-DUSP26 protomer observed crystallographically, with helix α9 in an extended conformation (‘extended-ΔN60-C/S-DUSP26’), and of a model of ΔN60-C/S-DUSP26 with helix α9 packed against the DSP-core like in VH1 (‘closed-ΔN60-C/S-DUSP26’). In both diagrams, the DSP-core is colored in gray and helix α9 is in cyan.