Abstract
The Themba Lethu Clinical Cohort was established in 2004 to allow large patient-level analyses from a single HIV treatment site to evaluate National Treatment Guidelines, answer questions of national and international policy relevance and to combine an economic and epidemiologic focus on HIV research. The current objectives of the Themba Lethu Clinical Cohort analyses are to: (i) provide cohort-level information on the outcomes of HIV treatment; (ii) evaluate aspects of HIV care and treatment that have policy relevance; (iii) evaluate the cost and cost-effectiveness of different approaches to HIV care and treatment; and (iv) provide a platform for studies on improving HIV care and treatment. Since 2004, Themba Lethu Clinic has enrolled approximately 30 000 HIV-positive patients into its HIV care and treatment programme, over 21 000 of whom have received anti-retroviral therapy since being enrolled. Patients on treatment are typically seen at least every 3 months with laboratory monitoring every 6 months to 1 year. The data collected include demographics, clinical visit data, laboratory data, medication history and clinical diagnoses. Requests for collaborations on analyses can be submitted to our data centre.
How did the study come about?
Since its beginning in 2004, the roll-out of South Africa’s national anti-retroviral treatment (ART) programme has led to a dramatic increase in the number of patients accessing life-saving treatment. The public health approach adopted by the national programme1,2 has led to more than 1 million patients on treatment in 2010,3 the largest HIV treatment programme in the world.4
This massive scale-up of care and treatment services has presented numerous operational, logistical and clinical challenges. The 2010 revisions to South Africa’s National Treatment Guidelines illustrate the challenges the programme has been grappling with given limited resources.5 These include what CD4 threshold to use for treatment initiation,6–8 what ART regimens to use for first- and second-line treatment, what cadre of care provider to use to initiate and maintain patients on therapy and when to initiate patients who are pregnant or co-infected with tuberculosis (TB).
The Themba Lethu Clinic in Johannesburg was started in 2004 as a public sector HIV treatment roll-out site run by the South African Department of Health9 as part of its development of accredited Comprehensive Care, Management and Treatment (CCMT) sites. Whereas Themba Lethu Clinic is a government clinic, it also receives support from Right to Care, a South African NGO supporting ART roll-out throughout South Africa with funding through the United States Agency for International Development (USAID) from the President’s Emergency Plan for AIDS Relief (PEPFAR) programme. Since 2004, Themba Lethu Clinic has enrolled approximately 30 000 patients into its HIV care and treatment programme, of whom over 21 000 have received ART since then. The size of the Themba Lethu Clinical Cohort makes it an excellent location to evaluate aspects of the government roll-out and explore ways to improve access to and delivery of treatment.
Since its inception, Themba Lethu Clinic has used a rich patient-level electronic data collection system to keep electronic patient medical records. These records include routinely captured demographic, laboratory, medication and clinical diagnosis fields (Table 1). To analyse this detailed data set, Right to Care has fostered a productive relationship with the Health Economics and Epidemiology Research Office (HE2RO) in South Africa, a collaboration between the University of the Witwatersrand and Boston University. These groups developed the Themba Lethu Clinical Cohort to allow large patient-level analyses from a single HIV treatment site to evaluate National Treatment Guidelines, answer questions of national and international policy relevance and to combine an economic and epidemiologic focus on HIV research.9–32 As part of this strategy, they have also initiated collaborations with the University of North Carolina at Chapel Hill, Duke University, the Wistar Institute and the University of Bern.
Table 1.
Data fields collected routinely on those enrolled on care at Themba Lethu Clinical Cohort in Johannesburg, South Africa
| Data fields | Variable list |
|---|---|
| Demographics | Name, national ID number, contact details, gender, date of birth, employment status, alcohol use, smoking history, ethnicity and education level |
| Clinical visit data | Date of visit (scheduled and actual), TB screening, urine analysis, vital signs, height, weight, description and duration of new symptoms and systems-based clinical examination (e.g. cardiology, neurology, and respiratory) |
| Laboratory results | ART initiation and monitoring bloods, including CD4 count, HIV viral load, full blood counts, liver function tests, renal function tests, TB microscopy and culture results, lactate levels and glucose and lipid profiles |
| Medication history | Date of start and stop of ART and non-ART medications, reasons for treatment discontinuation and self-reported treatment adherence |
| Clinical diagnoses | Pregnancy, opportunistic infections including TB, hepatitis, PCP, AIDS-related malignancies including Kaposi sarcoma, ART toxicities including peripheral neuropathy, anaemia, hyperlactataemia/lactic acidosis and lipoatrophy |
What does the study cover and how has this changed?
The Themba Lethu Clinical Cohort was initially developed for monitoring and evaluating the treatment roll-out. Prior to 2007, records were kept on paper and then entered into an electronic patient record system. Since then, live data capturing into an electronic medical record at the time of the patient encounter has been used. Since the clinical database was being developed at a time when anti-retroviral (ARV) roll-out was occurring at a rapid pace throughout resource-limited settings, it quickly became a valuable source of data to evaluate the rapid scale-up. Examples of early use of Themba Lethu Clinical Cohort data were studies of patient loss to follow-up33 and hepatitis co-infection.10
The current objectives of the Themba Lethu Clinical Cohort analyses are to: (i) provide cohort-level information on the outcomes of HIV treatment; (ii)evaluate aspects of HIV care and treatment that have policy relevance; (iii) evaluate the cost and cost-effectiveness of different approaches to HIV care and treatment; and (iv) provide a platform for studies on improving HIV care and treatment.
The large size of the data set has led to important contributions in the fields of health economics and epidemiology, including contributing to cost models used to determine the budget needed by the South African government for HIV treatment from 2011/12 to 2016/17.34 The data have also led to analyses of resistance profiles in patients failing first-line ART22 and to one of the largest studies24 included in a recent meta-analysis35 on the effect of TB on mortality in HIV-positive people. Themba Lethu Clinic also contributes data annually to the South African International Epidemiologic Databases to Evaluate AIDS (IeDEA-SA) network that pools data from multiple treatment sites throughout South Africa.36–44 In addition, the clinic data have been used as a tool to train pre-doctoral and doctoral students.
Where is the study area?
The Themba Lethu Clinic is located in the city of Johannesburg in the Gauteng Province in north central South Africa (Figure 1). Gauteng Province has the fifth largest number of infected patients with an estimated prevalence of 15.2%,45 but has the second largest number of patients on ART in South Africa with over 207 000 estimated to be receiving treatment at the province’s CCMT sites at the end of March 2010.3 The clinic is located in an ambulatory care wing at the Helen Joseph Hospital, a large urban secondary-level public sector teaching hospital. The clinic currently operates according to the 2010 South African National ART Guidelines,46 although until April 2010 it operated under the 2004 Guidelines.1 Despite the large number of patients, 400–500 seen per day, Themba Lethu Clinic functions with a modest clinical staff. There are six to eight full-time doctors, nine nurses, three pharmacists and a team of five administrative and eight data entry staff.
Figure 1.
Map of Johannesburg South Africa: location of the Themba Lethu Clinic
Themba Lethu Clinic provides HIV testing services at the clinic to about 12 000 people per year, but the majority of patients who enrol in care test elsewhere. Themba Lethu Clinic provides both pre-ART and ART care and operates a TB focal point where TB can be diagnosed and the necessary treatment initiated. TB patients are then referred to satellite clinics to continue TB treatment until completion. The clinic is supported by a team of Infectious Disease and HIV specialists from the Helen Joseph Hospital and the Clinical HIV Research Unit (CHRU), an HIV clinical trials research group. The clinic has also been used as a teaching facility for community service doctors and registrars.
Until April 2010, patients were initiated onto ART with a CD4 count <200 cells/mm3 or with a WHO Stage IV condition. Pregnant women could be initiated with higher CD4 counts. In April 2010, national guidelines changed to allow initiation at a CD4 count <350 cells/mm3 for pregnant women and those with TB. In September 2011, guidelines were again changed to allow initiation of all patients with a CD4 count <350 cells/mm3. Whereas the clinic has some flexibility with which drug regimens to use, before April 2010, patients were largely initiated onto stavudine–lamivudine–efavirenz. Tenofovir was substituted for stavudine after April 2010. Details of other regimens used are given in Table 2.
Table 2.
Public sector-recommended ART regimens according to the 2004 and 2010 South African National Treatment Guidelines
| Regimen | 2004 Guidelines | 2010 Guidelines |
|---|---|---|
| Drugs | Drugs | |
| First-line | d4T/3TC/EFV | TDF/3TC or FTC/EFV |
| Alternative first-line | d4T/3TC/NVP | TDF/3TC or FTC/NVP |
| Second-line | AZT/ddI/LPVr | AZT/3TC/LPVr |
3TC: lamivudine, AZT: zidovudine, ddI: didanosine, d4T: stavudine, FTC: emtricitabine, EFV: efavirenz, LPVr: lopinavir–ritonavir, NVP: nevirapine and TDF: tenofovir.
Since 2009, Themba Lethu Clinic has been ‘down-referring’ stable patients (i.e. those on ART for at least 11 months, with an undetectable viral load in the previous 10 months, stable weight, a CD4 count >200 cells/mm3, <5% weight loss over the last three visits and no opportunistic infections) to one of two primary health clinics for monitoring and treatment, Crosby and Rex Clinics. At these primary health clinics, space is allotted and nurses are dedicated to managing down-referred patients. The down-referral sites are linked to the clinic in real time through the electronic patient management system. Patients who do not remain stable and respond to treatment at the down-referral site are ‘up-referred’ back to Themba Lethu Clinic for care and continued treatment until they are eligible to be down-referred again.15,28
Who is in the sample?
The Themba Lethu Clinical Cohort consists of all patients ever enrolled for HIV care (pre-ART and ART) since April 2004 and by October 1, 2011, this included 8217 pre-ART patients (never initiated ART) and 21 101 ART patients (Table 3). Of these, there are currently 4570 patients actively in pre-ART care and 12 398 receiving ART (40% of those ever enrolled). The 12 398 ART patients have been observed for 53 530 person-years, for a median [interquartile range (IQR)] of 1.9 (0.6–4.1) person-years per person and a range of 0–7.7 years. Patients had a median (IQR) of 19 (5–44) visits in a total of 724 257 visits over the total observation time. As with most HIV treatment programmes in sub-Saharan Africa, the majority of the cohort (64%) are female, are predominately of Black or of African ethnicity (93%) with a median age of 36 years (IQR: 30.8–42.6 years).
Table 3.
Characteristics of patients in the Themba Lethu Clinical HIV Cohort in Johannesburg, South Africa
| Characteristics | Exposure | Pre-ART (n = 8217), n (%) | ART (n = 21 101), n (%) |
|---|---|---|---|
| Gender | Female | 5222 (63.6) | 13 428 (63.6) |
| Male | 2995 (36.4) | 7671 (36.4) | |
| Missing | 0 (0) | 2 (0) | |
| Nationality | South African | 7608 (92.6) | 19 195 (91.0) |
| Non-South African | 609 (7.4) | 1904 (9.0) | |
| Missing | 0 (0) | 2 (0) | |
| Education level | No formal education | 1151 (14.0) | 634 (3.0) |
| Primary school | 648 (7.9) | 2946 (14.0) | |
| Secondary school | 2445 (29.8) | 113 20 (53.7) | |
| Tertiary education | 160 (2.0) | 744 (3.5) | |
| Missing | 3813 (46.3) | 5357 (25.8) | |
| Employment status | Unemployed | 4434 (54.0) | 11 121 (52.7) |
| Employed | 3783 (46.0) | 9978 (47.3) | |
| Missing | 0 (0) | 2 (0) | |
| Characteristics at HAART initiation only | |||
| Age | Median (IQR) | 36 (30.8–42.6) | |
| BMI (kg/m2) | <18.5 | 3140 (14.9) | |
| 18.5–24.9 | 9025 (42.8) | ||
| 25–29.9 | 2804 (13.3) | ||
| ≥30 | 1302 (6.2) | ||
| Missing | 4830 (22.9) | ||
| Median (IQR) | 21.7 (19.1–25) | ||
| CD4 count category (cells/mm3) | <50 | 5224 (24.8) | |
| 50–100 | 3231 (15.3) | ||
| 100–200 | 5725 (27.1) | ||
| 200–350 | 2359 (11.2) | ||
| >350 | 705 (3.3) | ||
| Missing | 3857 (18.3) | ||
| Median (IQR) | 103 (39–178) | ||
| HIV viral load (copies/ml) | ≤100 000 | 3197 (7.2) | |
| >100 000 | 1527 (15.2) | ||
| Missing | 16 377 (77.6) | ||
| Haemoglobin | Median (IQR) | 11.6 (10.0–13.1) | |
| TB | Yes | 2519 (11.9) | |
| No | 8582 (88.1) | ||
| Missing | (0) | ||
| Current status | Alive | 13 067 (62.0) | |
| Deceased | 1701 (8.1) | ||
| Lost to follow-up | 4576 (21.7) | ||
| Transferred out | 1757 (8.3) | ||
| Missing | 0 (0) |
Patients who enrol in ART care are largely from the Johannesburg area. However, the clinic does serve a diverse set of nationalities since it has provided free care since not long after opening and because it is seen as a large, anonymous treatment site. Whereas the vast majority of those enrolled in care are South African (91%), the clinic also sees patients originating from Zimbabwe (4%), Malawi (1%), Lesotho (0.3%) and Zambia (0.2%). Whereas the clinic population is diverse, the population is poor with only 47% reporting having formal employment at ART initiation.
Patients on ART at Themba Lethu have generally been immunosuppressed at treatment initiation. The median CD4 cell count at initiation was 103 cells/mm3 (IQR 39–178 cells/mm3), body mass index (BMI) was 21.7 kg/m2 (IQR 19.1–25.0 kg/m2) and haemoglobin was 11.6 g/dl (IQR 10.0–13.1 g/dl). Viral load is not routinely collected at baseline, but for the 22% with a viral load at treatment initiation, 32.3% had a viral load above 100 000. In addition, 12% of the patients had TB at the time of initiating ART. As expected, based on the treatment guidelines, the majority of patients prior to 2010 initiated either stavudine–lamivudine–efavirenz (75%) or stavudine–lamivudine–nevirapine (8%), whereas after April of 2010, the majority of the patients (70%) initiated tenofovir–lamivudine–efavirenz. Between 2004 and 2007, ∼11% of the women initiating ART at Themba Lethu Clinic were pregnant. Since 2007, women initiating ART during pregnancy have been transferred to nearby facilities, mainly Rahima Moosa Mother and Child Hospital, for care and treatment during the duration of their pregnancy.
What has been measured?
Since the clinic was founded, data collection has been a central focus of clinic management. Themba Lethu Clinic uses an electronic patient management system called TherapyEdge-HIV™. This system can be used as both an electronic patient medical record and an interactive tool that provides up-to-date information on HIV patient care, including real-time alerts for better care and treatment.
When the clinic first opened, data were collected on paper registers in a form that could easily be entered into the electronic database by a team of data capturers. TherapyEdge-HIV™ went live at the clinic in mid-2007, so that clinicians could enter data in real time at the point of clinical encounter with the patient. Themba Lethu Clinic continues to maintain data capturers who are used to verify and clean data that are found to be missing, out of range or logically impossible. In addition, monitoring and evaluation teams, academic researchers and a TherapyEdge-HIVTM operations team identify and forward data validation queries to a dedicated data cleaning team for checking and correction where possible.
At Themba Lethu Clinic, pre-ART care includes wellness and adherence counselling. Follow-up of patients occurs generally on a schedule of visits every 3–6 months, depending on the patient’s CD4 count, and visits include monitoring CD4 cell count and WHO Stage to determine when the patient becomes eligible for ART.
From 2004 to 2010, prior to a patient initiating ART, various laboratory investigations, including full blood counts, haemoglobin and liver function tests were conducted to determine the appropriate ART regimen. In April 2010, when tenofovir was substituted for stavudine, measurement of creatinine clearance was also required prior to initiation of treatment to determine patient’s renal function. In accordance with South African National Guidelines, viral loads are not generally taken prior to ART initiation. Whereas the schedule varies depending on the regimen, patients are typically seen for medical follow-up visits at months 1, 3 and 6 and 12-monthly thereafter. Patients come for ARV pickups monthly for the first 6–12 months on treatment and every 2 months thereafter once stable. Patients have their first monitoring of viral load to assess suppression at 4 months as well as a CD4 count. Monitoring tests were done every 6 months after that until the 2010 guideline changes. They are now done at 6 and 12 months and yearly thereafter. Other parameters routinely measured include full blood counts, haemoglobin, liver function tests and creatinine clearance. Tests such as lipid profiles, lactate levels and glucose are measured as clinically indicated. Specimens are processed and analysed at the National Health Laboratory Service (NHLS), which has a branch at Helen Joseph Hospital.
At all visits, the dates the visit was scheduled and when it was actually completed are recorded as well as all clinical characteristics and laboratory results. More recently (2010), the database was upgraded to include the ability to integrate and download all laboratory results electronically from the NHLS, ensuring high quality, complete data. Finally, conditions reported at the clinical encounter (e.g. peripheral neuropathy and TB) are recorded, though with less completeness than the laboratory data.
The clinic employs several strategies to deal with patients lost to follow-up. Dedicated loss to follow-up counsellors make up to three attempts to contact lost patients and return them to care or determine their vital status. Mortality is ascertained routinely through family or hospital report, active tracing and linkage with the South African National Vital Registration Infrastructure Initiative. Over half (52%) of the patients in the clinic have a legitimate national ID number recorded and have been linked to the death registry. During our first match in 2008, the number of deaths more than doubled (4.2–10.9%).11
What is attrition like?
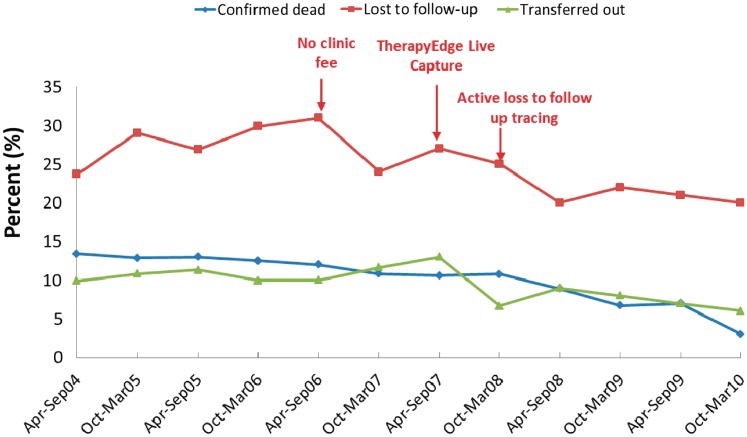
Since this is a clinical cohort, we consider attrition to be an important outcome that we measure and track as well as strive to reduce. There are three types of attrition for ART patients in our cohort (Figure 2). Firstly, patients can become lost to follow-up. We define loss to follow-up (for research purposes) as having occurred as soon as a patient is 3 months late for their last scheduled visit. Since April 2004, 22% of all the patients who initiated ART have become lost, typical for clinical cohorts in this region.47,48 Secondly, patients can leave the cohort through formal transfer to another facility. Of all patients initiated onto ART, 9% have transferred to another facility. Finally, attrition can happen through death, which has occurred in 9% of all patients, the majority within the first 6 months after ART initiation. This leaves over 60% of all patients who initiated ART remaining alive and in care. Figure 2 shows attrition from the cohort over time in relation to events at the clinic, including the initiation of live capturing of patient data, the removal of clinic fees and initiation of active tracing of lost patients.
Figure 2.
Trends in total attrition from the Themba Lethu Clinical Cohort in Johannesburg, South Africa
What has been found?
Analyses conducted using the Themba Lethu Clinic data follow several themes. Examples of these include:
Treatment outcomes: we have demonstrated good clinical, virologic and immunologic outcomes among patients initiating first-9 and second-line therapy in our cohort, with roughly 80% of the patients on second-line therapy alive and in care 1 year later.12 The database has also been used as a platform to explore the association between baseline renal function and renal toxicity and mortality among patients initiated onto tenofovir-based regimens.26 Recently, we showed that stable patients down-referred from doctor-managed ART clinics to nurse-managed primary health clinics were less likely to die [hazard ratio (HR) 0.2; 95% confidence interval (95% CI): 0.04–0.8], become lost to follow-up (HR 0.3; 95% CI: 0.2–0.6) or experience viral rebound (RR 0.6; 95% CI 0.4–0.9) than stable patients who remained at the site where they initiated treatment.28
Response to co-infections: we have shown that TB at time of ART initiation does not increase all-cause mortality during follow-up,24 but may increase short-term risks of drug toxicities, especially stavudine toxicities.25 We have explored outcomes in patients co-infected with Kaposi’s sarcoma herpes virus (KSHV), finding a detectable KSHV viral load rather than KSHV seropositivity to be associated with markers of advanced HIV disease at ART initiation.49
Attrition for HIV care: data from Themba Lethu Clinic have been used for several analyses of attrition in ART programmes. By matching with the National Vital Registration System, we showed that 37% of the patients lost from ART care died within 3 years of being lost. Themba Lethu data also showed that missing two or more medical visits was associated with at least a 2-fold increased risk of mortality compared with missing no visits (HR 2.1; 95% CI: 1.0–4.3).27
Pregnancy and HIV treatment: we have shown that there is a high rate of pregnancy among women (especially young women) after ART initiation, and that incident pregnancy after ART initiation may increase risks of virologic failure.23
Cost and cost-effectiveness: Themba Lethu has been the subject of costing analyses as well as a source of inputs for larger costing models. In the former category, cost and outcomes for Themba Lethu patients were compared with other sites and showed that in comparison with these sites, Themba Lethu had the lowest cost for producing a patient who was in care and responding.17,20 In the latter category, data from Themba Lethu were used to model the probabilities of patient movement between CD4 categories as well as rates of survival and attrition over time in order to model the overall cost of HIV care in South Africa as well as the cost implications of changes to the 2010 National Treatment Guidelines.34
What future analyses are planned?
We plan to continue to use Themba Lethu Clinical Cohort data to ask questions that will help guide not only the South African National treatment programme but also other programmes in resource-limited settings. As an example, current plans include evaluating the impact of changes in the 2010 National ART Guidelines, including changes allowing the initiation of pregnant women and TB patients at higher CD4 counts (>350 cells/mm3), changes related to nurse initiated and managed HIV treatment and changes in first-line ART regimens.
What are the main strengths and weaknesses of the study?
The Themba Lethu Clinical Cohort has several strengths that make it a valuable research asset. Perhaps the biggest strength is the size of the cohort. The Themba Lethu Clinical Cohort is one of the largest single site ART cohorts in all of South Africa and globally. As such, questions can be answered in a single cohort that typically require pooled data sets like the IeDEA networks to answer. Whereas such collaborations are invaluable, the differing care protocols make them best suited to answering questions about the variation across sites. The Themba Lethu Clinical Cohort is able to answer questions of critical importance among patients following a common treatment protocol.
The second strength is the depth of the data. Due to the history of using a standardized, electronic data capture system, data on ART regimens, visit dates, outcomes, drug side effects, laboratory investigations and demographics are of high quality. The employment of dedicated data cleaners helps maintain the quality over time and the integration of the data collection system electronically with the National Health Laboratory System, linking with the South African National Vital Registration Infrastructure Initiative, and plans to link with the national TB and cancer registries allow for accurate capture of clinical data.
The third strength is the dynamic nature of the cohort. Since we continue to enrol patients, we can evaluate changes to both the clinic and national policies as they are implemented. The prospective nature also means we have and can continue to improve data collection systems and quality over time. New data collection modules can be added for substudies of interest and fields that are repeatedly not collected can be removed.
The Themba Lethu Clinical Cohort also has weaknesses. Since it is a clinical cohort, missing data occur when patients miss visits and when the clinician seeing the patient does not enter information about the patient encounter. Some conditions, including those requiring inpatient workup and diagnosis (such as malignancies) or those where treatment is accessed at other facilities (TB and chronic conditions such as hypertension or diabetes), are commonly under-reported in the data set and need to be interpreted and analysed with caution. In addition, as a result of data not being captured, confounding could be a common problem in analyses. The data collection system was designed to capture critical information for patient care, but variables one would often want to adjust for (e.g. socio-economic status, adherence and parity) are either not collected and need to be evaluated in substudies or are poorly recorded.
Can I get hold of the data?
Analyses of the data are approved for analysis by the Human Research Ethics Committee (Medical) of the University of the Witwatersrand. All participating institutions who conduct analyses on the data seek their own ethics approval, often using de-identified data. All investigators working with Themba Lethu Clinical Cohort sign a data-use agreement. Themba Lethu staff has a long history of collaborating with partner institutions to ask questions of importance and will continue to do so. Whereas the data are not given out to outside researchers, requests for analyses that are relevant to the research missions of the participating institutions can be considered. Requests should be made in writing to Lynne McNamara (lmcnamara@witshealth.co.za) with a specific study question and the types of data that would be needed.
Where can I find out more?
Information about the Themba Lethu Clinical Cohort can be found at http://www.righttocare.org/tlc/tlc.pdf or by contacting the Right to Care offices.
Funding
USAID (674-A-00-08-00007-00) through Right to Care; National Institute of Allergy and Infectious Diseases (K01AI083097 to M.P.F.); National Institute of Child Health and Development (4 R00-HD-06-3961-02 to D.W.); National Institute of Allergy and Infectious Diseases (5 T32 AI 07001-32); the South Africa Mission of the US Agency for International Development (674-A-00-09-00018-00 to L.L.).
Acknowledgements
The authors wish to thank Babatyi Malope-Kgokong for her efforts and input in developing the database. Annelies Van Rie and Charles van der Horst helped in mentoring some of those who developed the database and coding. We thank Melinda Wilson and Clint Cavanaugh of USAID for support of the cohort development. We thank the clinical staff who saw the patients and the data capturers who helped collect the data. Most importantly, we thank the patients who contributed to this cohort. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the article. The content is solely the responsibility of the authors and does not necessarily represent the official views of WHO, USAID, the National Institute of Allergy and Infectious Diseases, the National Institutes of Health or other parties.
Conflict of interest: None declared.
KEY MESSAGES.
The following are the main findings from the Themba Lethu Clinical Cohort to date:
HIV-infected patients initiating first- and second-line therapy have good clinical, virologic and immunologic outcomes, with roughly 80% of the patients on second-line therapy alive and in care 1 year later.
Stable patients down-referred from doctor-managed ART clinics to nurse-managed primary health clinics were less likely to die, become lost to follow-up or experience viral rebound than stable patients remaining at the site where they initiated treatment.
TB at the time of ART initiation does not increase all-cause mortality during follow-up, but may increase short-term risks of drug toxicities, especially stavudine toxicities.
Data from Themba Lethu were used to model the overall cost of HIV care in South Africa as well as the cost implications of changes to the 2010 National Treatment Guidelines.
References
- 1.South Africa National Ministry of Health. National Antiretroviral Treatment Guidelines. 1st edn. Pretoria: Ministry of Health, 2004. [Google Scholar]
- 2.World Health Organization. Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a Public Health Approacy, 2010 Revision. Geneva: WHO; 2010. [PubMed] [Google Scholar]
- 3.Department of Health Republic of South Africa. National Strategic Plan for HIV and AIDS/CCMT Monthly Statistics. Pretoria: South African Department of Health; April 2010. [Google Scholar]
- 4. WHO, UNICEF. Towards Universal Access: Scaling Up Priority HIV/AIDS Interventions in the Health Sector. Geneva: World Health Organization, 2010. [Google Scholar]
- 5. National Department of Health, Republic of South Africa. Clinical Guidelines for the Management of HIV & AIDS in Adults and Adolescents. Pretoria: South African Department of Health, 2010. [Google Scholar]
- 6.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–26. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Severe P, Juste MA, Ambroise A, et al. Early versus standard antiretroviral therapy for HIV-infected adults in Haiti. N Engl J Med. 2010;363:257–65. doi: 10.1056/NEJMoa0910370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox MP, Sanne IM, Conradie F, et al. Initiating patients on antiretroviral therapy at CD4 cell counts above 200 cells/μl is associated with improved treatment outcomes in South Africa. AIDS. 2010;24:2041–50. doi: 10.1097/QAD.0b013e32833c703e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanne IM, Westreich D, Macphail AP, Rubel D, Majuba P, Van Rie A. Long term outcomes of antiretroviral therapy in a large HIV/AIDS care clinic in urban South Africa: a prospective cohort study. J Int AIDS Soc. 2009;12:38. doi: 10.1186/1758-2652-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Firnhaber C, Reyneke A, Schulze D, et al. The prevalence of hepatitis B co-infection in a South African urban government HIV clinic. S Afr Med J. 2008;98:541–44. [PMC free article] [PubMed] [Google Scholar]
- 11.Fox MP, Brennan A, Maskew M, MacPhail P, Sanne I. Using vital registration data to update mortality among patients lost to follow-up from ART programmes: evidence from the Themba Lethu Clinic, South Africa. Trop Med Int Health. 2010;15:405–13. doi: 10.1111/j.1365-3156.2010.02473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox MP, Ive P, Long L, Maskew M, Sanne I. High rates of survival, immune reconstitution, and virologic suppression on second-line antiretroviral therapy in South Africa. J Acquir Immune Defic Syndr. 2010;53:500–06. doi: 10.1097/QAI.0b013e3181bcdac1. [DOI] [PubMed] [Google Scholar]
- 13.Larson BA, Brennan A, McNamara L, et al. Lost opportunities to complete CD4+ lymphocyte testing among patients who tested positive for HIV in South Africa. Bull World Health Organ. 2010;88:675–80. doi: 10.2471/BLT.09.068981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larson BA, Brennan A, McNamara L, et al. Early loss to follow up after enrolment in pre-ART care at a large public clinic in Johannesburg, South Africa. Trop Med Int Health. 2010;15:43–47. doi: 10.1111/j.1365-3156.2010.02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long L, Brennan A, Fox M, et al. Treatment outcomes and cost-effectiveness of shifting management of stable ART patients to nurses in South Africa: an observational cohort. PLoS Med. 2011;8:1–10. doi: 10.1371/journal.pmed.1001055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long L, Fox M, Sanne I, Rosen S. The high cost of second-line antiretroviral therapy for HIV/AIDS in South Africa. AIDS. 2010;24:915–19. doi: 10.1097/QAD.0b013e3283360976. [DOI] [PubMed] [Google Scholar]
- 17.Rosen S, Ketlhapile M, Sanne I, DeSilva MB. Characteristics of patients accessing care and treatment for HIV/AIDS at public and nongovernmental sites in South Africa. J Int Assoc Physicians AIDS Care. 2008;7:200–07. doi: 10.1177/1545109708320684. [DOI] [PubMed] [Google Scholar]
- 18.Rosen S, Ketlhapile M, Sanne I, Desilva MB. Differences in normal activities, job performance and symptom prevalence between patients not yet on antiretroviral therapy and patients initiating therapy in South Africa. AIDS. 2008;22:S131–39. doi: 10.1097/01.aids.0000327634.92844.91. [DOI] [PubMed] [Google Scholar]
- 19.Rosen S, Long L, Fox M, Sanne I. Cost and cost-effectiveness of switching from stavudine to tenofovir in first-line antiretroviral regimens in South Africa. J Acquir Immune Defic Syndr. 2008;48:334. doi: 10.1097/QAI.0b013e31817ae5ef. [DOI] [PubMed] [Google Scholar]
- 20.Rosen S, Long L, Sanne I. The outcomes and outpatient costs of different models of antiretroviral treatment delivery in South Africa. Trop Med Int Health. 2008;13:1005–15. doi: 10.1111/j.1365-3156.2008.02114.x. [DOI] [PubMed] [Google Scholar]
- 21.Rosen S, Long L, Sanne I, Stevens WS, Fox MP. The net cost of incorporating resistance testing into HIV/AIDS treatment in South Africa: a Markov model with primary data. J Int AIDS Soc. 2011;14:24. doi: 10.1186/1758-2652-14-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallis CL, Mellors JW, Venter WD, Sanne I, Stevens W. Varied patterns of HIV-1 drug resistance on failing first-line antiretroviral therapy in South Africa. J Acquir Immune Defic Syndr. 2010;53:480–84. doi: 10.1097/QAI.0b013e3181bc478b. [DOI] [PubMed] [Google Scholar]
- 23.Westreich D, Cole SR, Nagar S, Maskew M, van der Horst C, Sanne I. Pregnancy and virologic response to antiretroviral therapy in South Africa. PLoS One. 2011;6:e22778. doi: 10.1371/journal.pone.0022778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westreich D, MacPhail P, Van Rie A, et al. Effect of pulmonary tuberculosis on mortality in patients receiving HAART. AIDS. 2009;23:707–15. doi: 10.1097/QAD.0b013e328325d115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Westreich DJ, Sanne I, Maskew M, et al. Tuberculosis treatment and risk of stavudine substitution in first-line antiretroviral therapy. Clin Infect Dis. 2009;48:1617–23. doi: 10.1086/598977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brennan A, Evans D, Maskew M, et al. Renal dysfunction and the risk of nephrotoxicity and mortality. AIDS. 2011;25:1603–09. doi: 10.1097/QAD.0b013e32834957da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brennan AT, Maskew M, Sanne I, Fox MP. The importance of clinic attendance in the first six months on antiretroviral treatment: a retrospective analysis at a large public sector HIV clinic in South Africa. J Int AIDS Soc. 2010;13:49. doi: 10.1186/1758-2652-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brennan A, Long L, Maskew M, et al. Outcomes of stable HIV-positive patients down-referred from doctor-managed ART clinics to nurse-managed primary health clinics for monitoring and treatment. AIDS. 2011;25:2027–36. doi: 10.1097/QAD.0b013e32834b6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maskew M, Brennan AT, Macphail AP, Sanne IM, Fox MP. Poorer ART outcomes with increasing age at a large public sector HIV clinic in Johannesburg, South Africa. J Int Assoc Physicians AIDS Care. 2011;11:57–65. doi: 10.1177/1545109711421641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malope BI, MacPhail P, Mbisa G, et al. No evidence of sexual transmission of Kaposi's sarcoma herpes virus in a heterosexual South African population. AIDS. 2008;22:519–26. doi: 10.1097/QAD.0b013e3282f46582. [DOI] [PubMed] [Google Scholar]
- 31.Malope BI, Pfeiffer RM, Mbisa G, et al. Transmission of Kaposi sarcoma-associated herpesvirus between mothers and children in a South African population. J Acquir Immune Defic Syndr. 2007;44:351–55. doi: 10.1097/QAI.0b013e31802f12ea. [DOI] [PubMed] [Google Scholar]
- 32.Malope-Kgokong BI, Macphail P, Mbisa G, et al. Kaposi's sarcoma associated-herpes virus (KSHV) seroprevalence in pregnant women in South Africa. Infect Agent Cancer. 2010;5:14. doi: 10.1186/1750-9378-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maskew M, MacPhail P, Menezes C, Rubel D. Lost to follow up: contributing factors and challenges in South African patients on antiretroviral therapy. S Afr Med J. 2007;97:853–57. [PubMed] [Google Scholar]
- 34.Meyer-Rath G, Brennan A, Long L, et al. 18th International AIDS Conference; 2010 July 18–23. Vienna: 2010. Total cost and potential cost savings of the national antiretroviral treatment (ART) programme in South Africa 2010 to 2017. [Google Scholar]
- 35.Straetemans M, Bierrenbach AL, Nagelkerke N, Glaziou P, van der Werf MJ. The effect of tuberculosis on mortality in HIV positive people: a meta-analysis. PLoS One. 2010;5:e15241. doi: 10.1371/journal.pone.0015241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keiser O, Chi B, Gsponer T, et al. Outcomes of antiretroviral treatment in programmes with and without routine viral load monitoring in Southern Africa. AIDS. 2011;25:1761–69. doi: 10.1097/QAD.0b013e328349822f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brinkhof MW, Dabis F, Myer L, et al. Early loss of HIV-infected patients on potent antiretroviral therapy programmes in lower-income countries. Bull World Health Organ. 2008;86:559–67. doi: 10.2471/BLT.07.044248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brinkhof MW, Egger M, Boulle A, et al. Tuberculosis after initiation of antiretroviral therapy in low-income and high-income countries. Clin Infect Dis. 2007;45:1518–21. doi: 10.1086/522986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cornell M, Grimsrud A, Fairall L, et al. Temporal changes in programme outcomes among adult patients initiating antiretroviral therapy across South Africa, 2002–2007. AIDS. 2010;24:2263–70. doi: 10.1097/QAD.0b013e32833d45c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Egger M, Ekouevi DK, Williams C, et al. Cohort Profile: The international epidemiological databases to evaluate AIDS (IeDEA) in sub-Saharan Africa. Int J Epidemiol. 2011 doi: 10.1093/ije/dyr080. doi: 10.1093/ije/dyr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fenner L, Forster M, Boulle A, et al. Tuberculosis in HIV programmes in lower-income countries: practices and risk factors. Int J Tuberc Lung Dis. 2011;15:620–27. doi: 10.5588/ijtld.10.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keiser O, Anastos K, Schechter M, et al. Antiretroviral therapy in resource-limited settings 1996 to 2006: patient characteristics, treatment regimens and monitoring in sub-Saharan Africa, Asia and Latin America. Trop Med Int Health. 2008;13:870–79. doi: 10.1111/j.1365-3156.2008.02078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keiser O, Tweya H, Boulle A, et al. Switching to second-line antiretroviral therapy in resource-limited settings: comparison of programmes with and without viral load monitoring. AIDS. 2009;23:1867–74. doi: 10.1097/QAD.0b013e32832e05b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keiser O, Tweya H, Braitstein P, et al. Mortality after failure of antiretroviral therapy in sub-Saharan Africa. Trop Med Int Health. 2010;15:251–58. doi: 10.1111/j.1365-3156.2009.02445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shisana O, Rehle T, Simbayi L, et al. South African National HIV Prevalence, Incidence, Behaviour and Communication Survey, 2008: A Turning Tide Among Teenagers? Cape Town: HSRC Press; 2009. [Google Scholar]
- 46.South Africa National Ministry of Health. The South African Antiretroviral Treatment Guidelines. Pretoria: National Department of Health Republic of South Africa; 2010. [Google Scholar]
- 47.Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: systematic review. Trop Med Int Health. 2010;15:1–15. doi: 10.1111/j.1365-3156.2010.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med. 2007;4:e298. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maskew M, Macphail AP, Whitby D, Egger M, Wallis CL, Fox MP. Prevalence and predictors of Kaposi sarcoma herpes virus seropositivity: a cross-sectional analysis of HIV-infected adults initiating ART in Johannesburg, South Africa. Infect Agent Cancer. 2011;6:22. doi: 10.1186/1750-9378-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]