Abstract
Background Age at menarche is an important determinant of hormonal-related neoplasia and other chronic diseases. Spatial and temporal variations in age at menarche have been observed in industrialised countries and several environmental factors were reported to have an influence.
Method We examined geographical variations in self-reported age at menarche and explored the effects of both latitude and ultraviolet radiation (UVR) dose on the onset of menarche in 88 278 women from the French E3N cohort (aged 40–65 years at inclusion).
Results The mean age at menarche was 12.8 years. After adjustment for potential confounders (birth cohort, prematurity, birth weight and length, father’s income index, body silhouette in childhood, food deprivation during World War II, population of birthplace, number of siblings, breastfeeding exposure and indoor exposure to passive smoking during childhood), latitude and UVR dose (annual or spring/summer) in county of birth were significantly associated with age at menarche (Ptrend < 0.0001). Women born at lower latitudes or in regions with higher annual or spring/summer UVR dose had a 3- to 4-month earlier menarche than women born at higher latitudes or in regions with lower UVR. On a continuous scale, a 1° increment in latitude resulted in a 0.04-year older age at menarche [95% confidence interval (CI): 0.03, 0.05], whereas a 1-kJ/m2 increment in annual UVR dose resulted in a 0.42-year younger age at menarche (95% CI: −0.55, −0.29).
Conclusion These data further suggest that light exposure in childhood may influence sexual maturation in women.
Keywords: Age at menarche, ultraviolet radiation, latitude, cohort study
Introduction
Age at menarche is an important determinant of hormone-related neoplasia and many other chronic diseases.1–5 Spatial and temporal variations in age at menarche have been observed in industrialised countries,6,7 and several environmental factors were reported to have an influence, including health status, diet and socio-economic conditions.8 A north-south gradient in menarcheal age has been described across several countries of the northern hemisphere9,10 but the mechanisms that mediate the association are unclear. Since northern latitude is inversely correlated with dose of ultraviolet radiation (UVR), a role of UVR may be speculated. However, to our knowledge, no study has explored the association between UVR exposure and timing of menarche.
In this report, we examined geographical variations in age at menarche and explored the associations between both latitude and UVR dose, and age at menarche in the French Etude Epidémiologique auprès de femmes de l’Education Nationale (E3N) cohort.
Method
Study cohort
E3N is an ongoing prospective cohort study investigating major chronic diseases in women. Participants were 98 995 women born in 1925–1950 and affiliated with a French national health insurance plan covering mostly teachers, and who volunteered to join the study (20% of the invited members of the health insurance plan). Women were living in France when they were enrolled in 1990 and returned an informed consent along with a self-administered questionnaire on their lifestyle and medical history. Follow-up questionnaires were sent approximately every 24–36 months thereafter (until now, nine follow-up questionnaires have been sent to the participants with an average response rate of ∼80% at each questionnaire, and only 517 women failed to answer any follow-up questionnaire).
Age at menarche: data collection
Self-reported age at menarche was recorded in the first two questionnaires, where women answered the question: ‘At what age did you have your first menstrual period?’, with an age ranking from 8 to 19 years (one-year categories), and three additional categories for ≤7 or ≥20 years, and for women who never menstruated. Among participants with available age at menarche in both questionnaires (n = 79 283), the correlation coefficient between responses was 0.92 (P < 0.0001), and the Kappa coefficient was 0.68 (P for symmetry < 0.0001), which represent substantial agreement between the two measures.11 When ages at menarche were discordant between the two questionnaires, the mean between the two values was used (except if this difference was >1 year, where the value was considered missing).
Potential confounders: data collection
Data on county of birth, education, body silhouette at age 8 years and at menarche, level of suffering from World War II (WWII) food deprivation, and physical activity at ages 8–15 years were collected at baseline. The baseline questionnaire also collected data on skin complexion and skin sensitivity to sun exposure, which was defined as the skin burning and tanning responses when exposed to the sun for the first time in summer. The second questionnaire provided data on fathers’ professional categories and passive smoking during childhood. Birth-related data, such as prematurity and birth weight and length, were collected in the seventh questionnaire. A detailed description of these data and their relation to age at menarche in this sample has been reported elsewhere.12
Assessment of childhood UVR exposure
Since geographical area of residence during childhood was not available, we used county of birth as a proxy variable. We hypothesised that most women remained in the same area between birth and menarche, since French women born in 1925–1950 were not likely to move before marriage.13 In a sensitivity analysis, we restricted the data set to a sub-sample of women with identical county of birth and of residence at baseline (n = 34 914). County of birth was linked to a database containing mean daily erythemal doses (UVR in kJ/m2/day) and latitude in French metropolitan counties, which we obtained from the Joint Research Centre of the European Commission.14 The database covers the period from 1 January 1984 to 31 August 2003, with UVR maps covering Europe with a spatial resolution of 0.05°. UVR doses were estimated with a mapping algorithm, using satellite data, as previously described.15
Briefly, UVR doses were obtained by interpolation in a look-up table built using the UVspec code16 of the libRadtran radiative transfer model package, entries being solar zenith angle, total column ozone amount, cloud liquid water thickness, near-surface horizontal visibility, surface elevation and Earth surface UV albedo. Both satellite (Meteosat, GOME, TOMS) and non-satellite (synoptic observations, meteorological model results, digital elevation model) data were exploited to assign values to the influencing factors. Daily UVR doses were constructed by numerical integration of the dose rate estimated at half-hourly intervals from, and including, the local solar noon (for each 0.05° x 0.05° pixel). The erythemal UV doses were produced by weighing the UV spectrum with the CIE87 action spectrum. The quality of the satellite-derived estimates has been assessed at several sites in Europe with usually good relative difference between the satellite estimates and the measured ground erythemal daily doses and small bias (<3%).17
On the basis of these estimations, we computed the annual and spring/summer mean UVR dose for each French county and assigned it to the corresponding county of birth.
Statistical analyses
All analyses were performed using SAS (Version 9.3, SAS Institute, Cary, NC, USA). All statistical tests and corresponding P-values were two-sided. Means and 95% confidence intervals (CIs) in UVR and latitude categories were estimated using multilevel models with two levels (individual and county of birth), using the SAS PROC MIXED. Latitude and UVR were only available at the county level whereas all other covariates were available at the individual level. Confounders were selected based on their association with age at menarche and/or UVR dose in our cohort and included birth cohort (1925–1930; 1931–1935; 1936–1940; 1941–1945; 1946–1950), father’s income index (quartiles), population of birthplace (<1000; 1000–4999; 5000–99 999; ≥100 000 inhabitants), number of siblings (0; 1; 2; ≥3), WWII food deprivation (none or little; moderate; much; menarche <1940; not born; missing), birth weight and length (low; medium; high; missing), body silhouette at 8 years and at menarche (lean: 1; medium: 2; large: 3–8; missing), indoor exposure to passive smoking during childhood (never; rarely; a few hours per week; a few hours per day; several hours per day; missing) and extra school physical activity during childhood (none; 1–4 hours/week; ≥5 hours/week; missing). As results from multivariable models were very similar to those of univariate models, only results from multivariable models were presented. Since phenotypic characteristics may influence UVR-mediated effects, we further adjusted our analyses for skin sensitivity to sun exposure (none, moderate, high), and skin complexion (very fair, fair, dark, very dark). For all adjustment factors, multiple imputation of missing values was performed. The data were imputed five times using a fully conditional specification (FCS) method (SAS procedure PROC MI). Variables used for imputation were age at menarche, UVR dose, birth cohort, father’s income index, population of birthplace, number of siblings, WWII food deprivation, birth weight and length, body silhouette at 8 years and at menarche, indoor exposure to passive smoking during childhood and extra school physical activity during childhood. The pooled estimate was obtained by averaging the estimates from the five imputed datasets and the confidence intervals took into account within- and between-imputation variances. Results obtained with multiple imputation were compared with those from the complete case analysis (n = 30 489). Since the results obtained with the two methods were almost identical, we decided to present only those arising from multiple imputations.
We tested for potential interactions by entering interaction terms between UVR dose or latitude and each individual covariate into the multivariable model; however, none of the interaction terms was statistically significant (Wald test) and therefore interaction terms were not included in the final model. Tests for linear trend were performed by creating an ordinal score across categories of each variable and by adding this score in the model.
Results
Of the 98 995 cohort participants, we excluded 2477 women (2.50%) with missing or imprecise (≤7 or ≥20) menarcheal age, and 25 (0.03%) who reported primary amenorrhoea. Since UVR data were only available in metropolitan France, we restricted our analysis to this geographical area, thus excluding another 7530 (7.61%) women born in foreign countries or the French overseas territories. We also excluded 655 (0.66%) subjects with missing data on county of birth and 30 (0.03%) who were deported during WWII before menarche, thus leaving a final sample of 88 278 women for analysis.
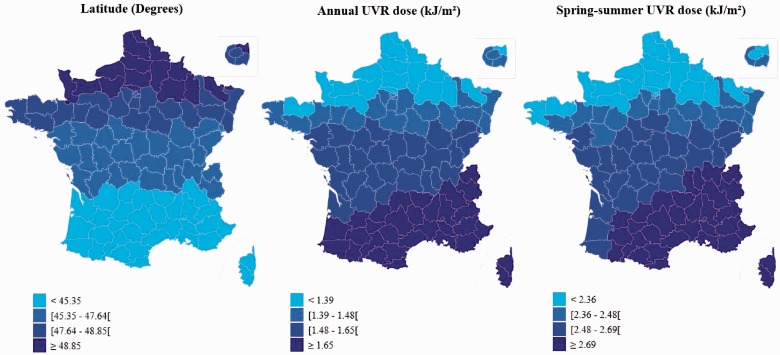
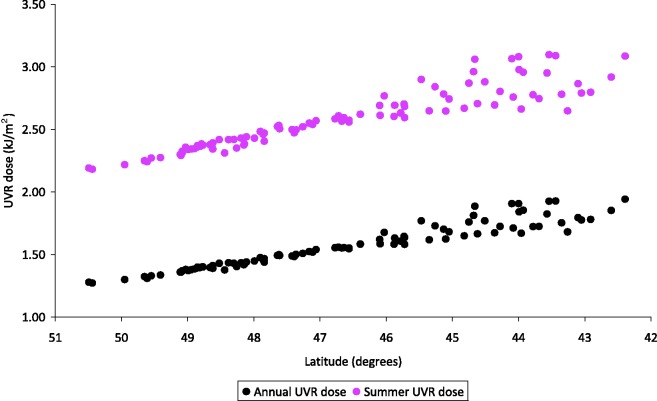
Among them, mean age at menarche was 12.8 years (SD = 1.42) and median age was 13.0 years. Menarche occurred before age 12 in 4.7% of women. Figure 1 presents the geographical distribution of quartiles of annual and spring/summer UVR dose and latitude in our study population. Lower latitude corresponds to higher doses of UVR (Figure 2, correlation coefficient = −0.97), with small differences in the classification since altitude is also taken into account in the UVR estimation. Table 1 presents the mean latitude and UVR dose according to the participants’ characteristics. Lower latitude and higher annual UVR dose in county of birth were observed in women in the lowest quartile of father’s income index, from a rural birthplace (<1000 inhabitants), who did not suffer from WWII food deprivation, with lower birth weight and length or leaner silhouette at age 8 years or at menarche, not exposed to passive smoking during childhood and who reported more extra school physical activity but less walking activity during childhood. Women who were breastfed were also on average exposed to higher residential UVR doses.
Figure 1.
Geographical repartition of quartiles of UV dose and latitude in France (mean data by county)
Figure 2.
Latitude by UVR dose in French counties
Table 1.
Mean (SD) latitude and UVR dose in county of birth according to characteristics of the study population, E3N cohort 1990–1992 (n = 88 278)
| Variable | Missing data | Latitude | Annual UVR dose [kJ/m2] | Spring/Summer UVR dose [kJ/m2] |
|---|---|---|---|---|
| Birth cohort | 0% | |||
| 1925–1930 | 47.28 (2.15) | 1.51 (0.17) | 2.51 (0.23) | |
| 1931–1935 | 47.12 (2.20) | 1.52 (0.18) | 2.53 (0.24) | |
| 1936–1940 | 47.04 (2.22) | 1.53 (0.18) | 2.54 (0.25) | |
| 1941–1945 | 46.90 (2.22) | 1.54 (0.18) | 2.56 (0.25) | |
| 1946–1950 | 47.27 (2.17) | 1.52 (0.18) | 2.52 (0.24) | |
| Ptrend | 0.29 | 0.49 | 0.25 | |
| Father’s income indexa | 27.9% | |||
| Quartile 1 | 46.84 (2.20) | 1.55 (0.18) | 2.56 (0.24) | |
| Quartile 2 | 47.41 (2.17) | 1.50 (0.18) | 2.51 (0.24) | |
| Quartile 3 | 47.01 (2.17) | 1.53 (0.18) | 2.54 (0.24) | |
| Quartile 4 | 47.29 (2.15) | 1.51 (0.18) | 2.52 (0.24) | |
| Ptrend | <0.0001 | <0.0001 | <0.0001 | |
| Population of birthplace | 0% | |||
| < 1000 | 46.80 (2.17) | 1.54 (0.17) | 2.55 (0.22) | |
| 1000–4999 | 49.92 (2.13) | 1.54 (0.17) | 2.55 (0.23) | |
| 5000–99 999 | 47.30 (2.23) | 1.51 (0.18) | 2.52 (0.25) | |
| ≥ 100 000 | 47.19 (2.18) | 1.53 (0.19) | 2.54 (0.26) | |
| Ptrend | <0.0001 | <0.0001 | <0.0001 | |
| Birth | 29.0% | |||
| Singleton | 47.10 (2.18) | 1.53 (0.18) | 2.54 (0.24) | |
| Multiple | 47.14 (2.18) | 1.52 (0.18) | 2.53 (0.24) | |
| Number of siblings | 34.6% | |||
| 0 | 47.13 (2.20) | 1.53 (0.18) | 2.53 (0.24) | |
| 1 | 47.02 (2.21) | 1.53 (0.18) | 2.54 (0.24) | |
| 2 | 47.09 (2.19) | 1.53 (0.18) | 2.54 (0.24) | |
| ≥3 | 47.21 (2.13) | 1.52 (0.17) | 2.52 (0.24) | |
| Ptrend | <0.0001 | <0.0001 | <0.0001 | |
| In utero exposure to maternal smoking | 20.7% | |||
| No | 47.1 (2.20) | 1.53 (0.18) | 2.54 (0.24) | |
| Yes | 47.2 (2.14) | 1.52 (0.18) | 2.53 (0.24) | |
| Breastfeeding exposure | 28.0% | |||
| Never | 47.30 (2.16) | 1.51 (0.18) | 2.51 (0.24) | |
| Ever | 47.00 (2.21) | 1.54 (0.18) | 2.55 (0.25) | |
| Suffering from WWII deprivationa | 2.8% | |||
| None or little | 46.95 (2.20) | 1.54 (0.18) | 2.55 (0.24) | |
| Moderate | 47.20 (2.20) | 1.52 (0.18) | 2.53 (0.25) | |
| Much | 47.27 (2.26) | 1.52 (0.19) | 2.53 (0.26) | |
| Ptrend | <0.0001 | <0.0001 | 0.75 | |
| Premature birth | 20.0% | |||
| No | 47.10 (2.20) | 1.53 (0.18) | 2.54 (0.24) | |
| Yes | 47.11 (2.17) | 1.53 (0.18) | 2.54 (0.24) | |
| Birth weight (kg) in women born at full term | 5.4% | |||
| <2.5 | 46.97 (2.16) | 1.53 (0.17) | 2.55 (0.23) | |
| 2.5–4 | 47.10 (2.20) | 1.53 (0.18) | 2.54 (0.24) | |
| >4 | 47.15 (2.25) | 1.52 (0.18) | 2.53 (0.25) | |
| Ptrend | 0.0006 | 0.004 | 0.002 | |
| Birth height (cm) in women born at full term | 12.7% | |||
| <48 | 47.00 (2.20) | 1.53 (0.18) | 2.54 (0.24) | |
| 48–52 | 47.10 (2.20) | 1.53 (0.18) | 2.54 (0.24) | |
| >52 | 47.14 (2.26) | 1.52 (0.19) | 2.53 (0.25) | |
| Ptrend | 0.0001 | 0.008 | 0.02 | |
| Body silhouette at age 8 years | 5.8% | |||
| 1 | 47.04 (2.23) | 1.53 (0.18) | 2.54 (0.25) | |
| 2 | 47.18 (2.17) | 1.52 (0.18) | 2.53 (0.24) | |
| ≥3 | 47.20 (2.16) | 1.52 (0.18) | 2.52 (0.24) | |
| Ptrend | <0.0001 | <0.0001 | <0.0001 | |
| Body silhouette at menarche | 4.3% | |||
| ≤2 | 47.04 (2.25) | 1.53 (0.18) | 2.54 (0.25) | |
| 3 | 47.11 (2.21) | 1.53 (0.18) | 2.54 (0.24) | |
| ≥4 | 47.15 (2.17) | 1.52 (0.18) | 2.53 (0.24) | |
| Ptrend | <0.0001 | <0.0001 | <0.0001 | |
| Passive smoking during childhood | 18.0% | |||
| Both parents smoked | 47.30 (2.15) | 1.52 (0.18) | 2.52 (0.25) | |
| One parent smoked | 47.20 (2.21) | 1.52 (0.18) | 2.53 (0.24) | |
| Parents did not smoke | 46.98 (2.17) | 1.54 (0.18) | 2.55 (0.24) | |
| Ptrend | <0.0001 | <0.0001 | <0.0001 | |
| Frequency of exposure to passive smoking during childhood | 19.0% | |||
| Never | 46.97 (2.18) | 1.54 (0.18) | 2.55 (0.24) | |
| Rarely | 47.09 (2.19) | 1.53 (0.18) | 2.54 (0.24) | |
| A few hours per week | 47.27 (2.18) | 1.51 (0.18) | 2.52 (0.24) | |
| A few hours per day | 47.22 (2.21) | 1.52 (0.18) | 2.53 (0.25) | |
| Several hours per day | 47.39 (2.14) | 1.51 (0.18) | 2.51 (0.24) | |
| Ptrend | <0.0001 | <0.0001 | <0.0001 | |
| Extra school physical activity at 8-15 years old | 13.4% | |||
| No extra school physical activity | 47.10 (2.18) | 1.53 (0.18) | 2.54 (0.24) | |
| 1–4 h/week | 47.11 (2.17) | 1.53 (0.18) | 2.54 (0.24) | |
| ≥ 5 h/week | 47.00 (2.21) | 1.54 (0.18) | 2.55 (0.25) | |
| Ptrend | 0.10 | 0.001 | 0.0003 | |
| Walking activity between 8 and 15 years old | 19.5% | |||
| ≤ 2 h/week | 47.04 (2.18) | 1.53 (0.18) | 2.54 (0.24) | |
| 3–4 h/week | 47.06 (2.15) | 1.53 (0.18) | 2.54 (0.24) | |
| ≥ 5 h/week | 47.18 (2.17) | 1.52 (0.18) | 2.53 (0.24) | |
| Ptrend | <0.0001 | <0.0001 | <0.0001 |
aAmong women born ≤ 1945.
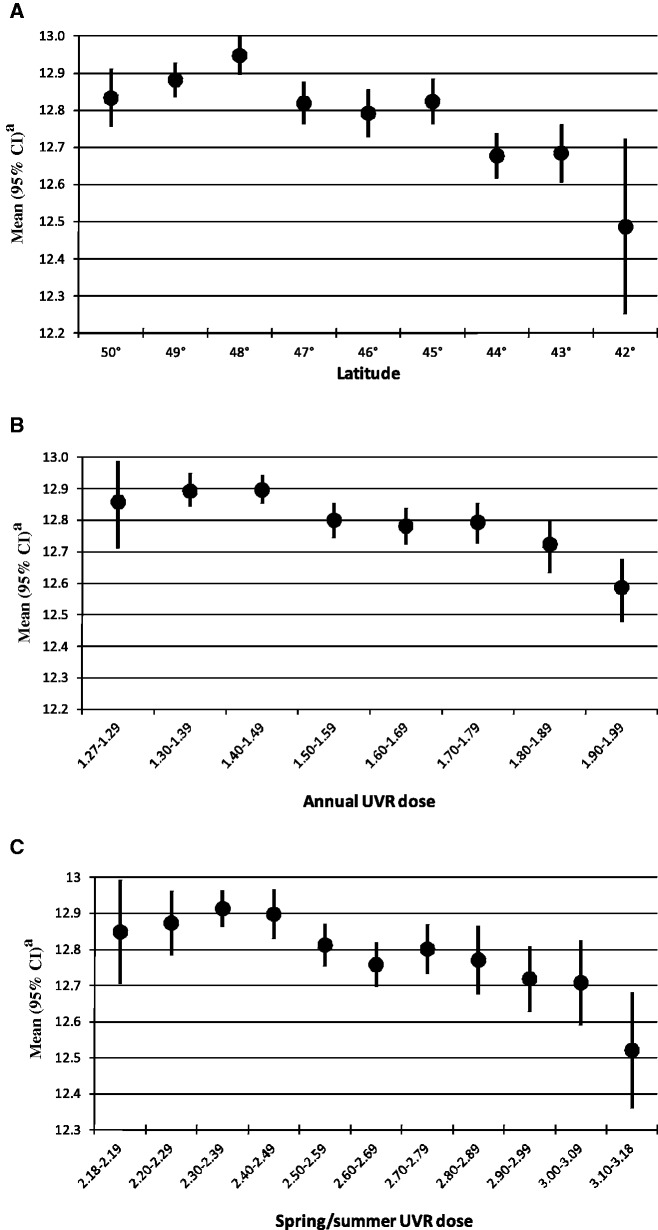
After adjustment for potential confounders, latitude and UVR dose (annual or spring/summer) in county of birth were significantly associated with age at menarche (Ptrend < 0.0001, Figure 3). Women born at the lowest latitudes had an average age at menarche 3–4 months earlier than those born at the highest latitudes. In contrast, women born in counties with the highest annual or spring/summer UVR dose were 3–4 months younger at menarche on average than those born in counties with the lowest UVR doses. A slightly older age at menarche(∼12.9 years) was observed among women born at latitude 48°, an annual UVR dose of 1.40–1.49 kJ/m2 or a spring/summer UVR dose of 2.30–2.49 kJ/m2. On a continuous scale, an increment of 1° in latitude resulted in a 0.04–year older age at menarche (95% CI: 0.03, 0.05) whereas an increment of 1 kJ/m2 in annual UVR dose resulted in a 0.42-year younger age at menarche (95% CI: −0.55, −0.29). Further adjustment for skin complexion or sensitivity to sun exposure did not alter the estimates for either UVR dose or latitude. In addition, estimates and trends were almost identical when restricting the analyses to the sub-sample of women who reported living in the same county at birth and at baseline (data not shown).
Figure 3.
Effects of latitude and UVR dose in county of birth on age at menarche, E3N cohort 1990–1992 (n = 88 278). (A) Latitude (Ptrend < 0.0001) (B) Average annual UVR dose [kJ/m2] (Ptrend < 0.0001) (C) Spring/summer average UVR dose [kJ/m2] (Ptrend < 0.0001)
aAdjusted for birth cohort, premature birth, birth weight, birth height, father’s income index, body silhouette at age 8 years, body silhouette at menarche, suffering from WWII deprivation, size of place of birth, number of siblings, breastfeeding exposure and frequency of indoor exposure to passive smoking during childhood
We found no evidence for a significant interaction between latitude or UVR dose (annual or spring/summer) and any of the individual covariates considered in the model. In addition, there was no evidence for an interaction with skin complexion, and results were very similar among women with fair or dark skin (data not shown).
Discussion
To our knowledge, this large cross-sectional analysis of a prospective study among women is the first to report an inverse association between age at menarche and UVR dose in county of birth. Women born in regions with the lowest category of latitude or the highest category of annual or spring/summer UVR dose had their menarche 3–4 months earlier than women born in region with lower latitudes or higher UVR doses. In comparison, we have previously shown that girls with larger body silhouettes and with more than three siblings had their menarche about 5 months earlier compared with those who were leaner or with no siblings.12
Main strengths of our study include its large sample size and the use of reliable data on UVR dose and latitude in French counties. Solar irradiance was documented by satellite UVR dose calculations that take into account both the atmospheric ozone content and cloud conditions, the two main modulators of UVR doses.14 Thus, use of UVR in addition to latitude may have reduced possible exposure misclassification with regard to sunlight exposure.
Use of average UVR dose estimated over the period 1984–2003 may account for an important limitation of our study, as UVR dose calculations between 1935 and 1960 were not available and were probably different due to pollution and climate changes. However, we found significant inverse correlations between latitude and UVR in county of birth, and we observed a similar inverse trend of age at menarche as related to latitude.
Another limitation is that, by exploring the link between age at menarche and UVR dose in county of birth, we made the strong hypothesis that women remained in the same geographical area between birth and menarche. In addition, no data were available in the E3N cohort on behavioural sun exposure during childhood. However, results from our sensitivity analysis excluding women who moved to different French counties between birth and cohort baseline led to almost identical results. It is also possible that women living in the north of France at the beginning of WWII moved to the south of France, where people were less exposed to restrictions. Such migrations would have attenuated the effects observed for latitude and UVR and resulted in lower estimates in older women (i.e. born before WWII).
Another limitation is the fact that age at menarche was retrospectively collected from women aged 40–65 years at baseline, and therefore long after its occurrence. However, an independent validation study showed that women around the menopause have an accurate recall of their age at menarche and body size during childhood (correlation between original and recalled = 0.8 for age and 0.6 for body size) and concluded that retrospective data are valid exposure measures that can be used in epidemiological studies.18 Therefore, although there is a possibility for recall bias in our study, we believe that it is unlikely to be of great magnitude, and since this bias is likely non-differential between early and late menarche, it would most likely lead to an underestimation of our results.
Participants in the E3N cohort were mainly female teachers with high levels of education and socio-economic status. Although this constitutes a selected population as compared with the general French population, there is no biological reason that our results on the association between UVR doses in region of birth and age at menarche do not apply to all women. However, we cannot completely rule out a spurious association due to unknown mediators or residual confounders. Although a minimal difference was observed in our point estimates before and after adjustment for several recognized potential determinants of menarcheal age, residual confounding may still be present. Indeed, this minimal difference could also indicate that the confounders used did not measure the concepts of interest well or did not include key confounders. In particular, although better nutrition could be patterned geographically at the time the study subjects were children and related to earlier menarche, it may not have been appropriately captured by the adjusting variables.
It should also be noted that the association between age at menarche and UVR dose or latitude was not strictly linear since a small non-significant older age at menarche was observed among women born at latitude 48° or annual UVR dose of 1.40–1.49 kJ/m2. Since these areas include the Paris region, these associations might be explained by the confounding effect of factors related to this large urban area, such as differences in lifestyle, pollution or mobility.
An association between latitude and menarcheal age has been previously described19 and, to our knowledge, only one ecological study suggested sunlight-mediated endocrine effects on the control of pubescence.10 Several mechanisms can be proposed to explain our findings. First, higher photoperiod (i.e. day length)—and therefore exposure to light—associated with latitude could at least partly explain our observed associations with menarcheal age. Studies on seasonality of menarche indicated summer peaks in menarche occurrence which might be due in part to higher photoperiod.20–25 An inverse association between artificial light exposure and age at menarche has also been suggested.26 The hypothesis of a possible effect of light on the activation of the hypothalamus to trigger puberty mainly derives from previous observations in birds27 and rodents.28 Photoperiodic information in mammals is coded into a biochemical signal to the hypothalamic reproductive system via melatonin,29,30 a pineal hormone with circadian production rhythm. UVR has also been associated with melatonin production.31,32 However, the well-described decline of melatonin at the same time as pubertal development may be the consequence of maturation of the neuroendocrine–gonadal axis, rather than the manifestation of a negative regulatory role of melatonin.33,34
New insights on endocrine control of puberty may suggest a role of kisspeptin in its onset.35 Observed photoperiodic variations in kisspeptin combined with the evidence that pinealectomy alters KISS-1 expression36 suggest responsiveness of the kisspeptin system to melatonin, although the presence of functional melatonin receptors on kisspeptin neuronal cells is still debated.29,37,38 Alternatively, a role of vitamin D deficiency in women less exposed to sunlight might be speculated, but studies on this topic have been conflicting.39,40,41
Next to these potential biological pathways, some differences in diet, temperature or genetic factors across French regions may also account for our observations. Regarding dietary differences, previous studies on diet and nutrient intake in relation to age at menarche40,41 suggested that menarcheal onset was rather related to weight changes due to variations in dietary habits than to the diet composition itself. In the present study, body sizes at and before menarche have been taken into account in the analyses, as well as food deprivation during WWII. Our results might also be explained by temperature differences between southern and northern France, although no clear association has been found between temperature and menarcheal age.19 On the other hand, studies have shown that girls living at high altitude had a delayed menarche.19 This observation is in apparent contradiction with a negative association of UVR with menarcheal age since UVR doses correlate positively with altitude. However, the cohort did not include enough women living at high altitudes to bias our estimates.
Conclusion
In conclusion, we demonstrated an inverse dose-effect relationship between latitude and UVR dose in county of birth in relation to age at menarche in women participating in the E3N cohort who were born in metropolitan France. These data further suggest that light exposure in childhood may influence sexual maturation. Future research should involve a wider span of latitude and accurate data on living areas and sun exposure before menarche to refine this association.
Funding
This work was supported by the French National Cancer Institute (Institut National du Cancer - INCa) and the French National Research Agency (Agence Nationale de la Recherche - ANR). The E3N cohort is supported by the French League Against Cancer; the Mutuelle Générale de l’Education Nationale; the Institut Gustave Roussy; and the Institut National de la Santé et de la Recherche Médicale. LD is supported by the Institut de Recherche en Santé Publique. MK is supported (in part) by a Marie Curie International Outgoing Fellowship within the 7th European Community Framework Programme (#PIOF-GA-2011-302078).
Conflict of interest: None declared.
References
- 1.Ma H, Bernstein L, Pike MC, Ursin G. Reproductive factors and breast cancer risk according to joint estrogen and progesterone receptor status: a meta-analysis of epidemiological studies. Breast Cancer Res. 2006;8:R43. doi: 10.1186/bcr1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dossus L, Allen N, Kaaks R, et al. Reproductive risk factors and endometrial cancer: The European prospective investigation into cancer and nutrition. Int J Cancer. 2010;127:442–51. doi: 10.1002/ijc.25050. [DOI] [PubMed] [Google Scholar]
- 3.Ong KK. Early determinants of obesity. Endocr Dev. 2010;19:53–61. doi: 10.1159/000316897. [DOI] [PubMed] [Google Scholar]
- 4.He C, Zhang C, Hunter DJ, et al. Age at menarche and risk of type 2 diabetes: results from 2 large prospective cohort studies. Am J Epidemiol. 2010;171:334–44. doi: 10.1093/aje/kwp372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lakshman R, Forouhi NG, Sharp SJ, et al. Early age at menarche associated with cardiovascular disease and mortality. J Clin Endocrinol Metab. doi: 10.1210/jc.2009-1789. 200994:4953–60. [DOI] [PubMed] [Google Scholar]
- 6.Euling SY, Herman-Giddens ME, Lee PA, et al. Examination of US puberty-timing data from 1940 to 1994 for secular trends: panel findings. Pediatrics. 2008;121(Suppl 3):S172–S191. doi: 10.1542/peds.2007-1813D. [DOI] [PubMed] [Google Scholar]
- 7.Ong KK, Ahmed ML, Dunger DB. Lessons from large population studies on timing and tempo of puberty (secular trends and relation to body size): the European trend. Mol Cell Endocrinol. 2006;254–255:8–12. doi: 10.1016/j.mce.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 8.Karapanou O, Papadimitriou A. Determinants of menarche. Reprod Biol Endocrinol. 2010;8:115. doi: 10.1186/1477-7827-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev. 2003;24:668–93. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- 10.Grivas TB, Vasiliadis E, Mouzakis V, Mihas C, Koufopoulos G. Association between adolescent idiopathic scoliosis prevalence and age at menarche in different geographic latitudes. Scoliosis. 2006;1:9. doi: 10.1186/1748-7161-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landis JR, Koch GG. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics. 1977;33:363–74. [PubMed] [Google Scholar]
- 12.Dossus L, Kvaskoff M, Bijon A, et al. Determinants of age at menarche and time to menstrual cycle regularity in the French E3N cohort. Ann Epidemiol. 2012;22:723–30. doi: 10.1016/j.annepidem.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Courgeau D. Geographical mobility, marriage and reproduction. Population. 1976;31:901–15. [Google Scholar]
- 14.Verdebout J. A European satellite-derived UV climatology available for impact studies. Radiat Prot Dosimetry. 2004;111:407–11. doi: 10.1093/rpd/nch063. [DOI] [PubMed] [Google Scholar]
- 15.Verdebout J. A method to generate surface UV radiation maps over Europe using GOME, Meteosat, and ancillary geophysical data. J Geophys Res. 2000;105:5049–58. [Google Scholar]
- 16.Mayer B, Kylling A. Technical note: The libRadtran software package for radiative transfer calculations – description and examples of use. ACP. 2005;5:1855–77. [Google Scholar]
- 17.Arola AS, Kalliskota PN, den Outer K, et al. Assessment of four methods to estimate surface UV radiation using satellite data, by comparison with ground measurements from four stations in Europe. J Geophys Res. 2002;107:D16. [Google Scholar]
- 18.Must A, Phillips SM, Naumova EN, et al. Recall of early menstrual history and menarcheal body size: after 30 years, how well do women remember? Am J Epidemiol. 2002;155:672–79. doi: 10.1093/aje/155.7.672. [DOI] [PubMed] [Google Scholar]
- 19.Zacharias L, Wurtman RJ. Age at menarche. Genetic and environmental influences. N Engl J Med. 1969;280:868–75. doi: 10.1056/NEJM196904172801606. [DOI] [PubMed] [Google Scholar]
- 20.Albright DL, Voda AM, Smolensky MH, Hsi BP, Decker M. Seasonal characteristics of and age at menarche. Chronobiol Int. 1990;7:251–58. doi: 10.3109/07420529009056983. [DOI] [PubMed] [Google Scholar]
- 21.Boldsen JL. Season of birth and recalled age at menarche. J Biosoc Sci. 1992;24:167–73. doi: 10.1017/s0021932000019702. [DOI] [PubMed] [Google Scholar]
- 22.Brundtland GH, Liestol K. Seasonal variations in menarche in Oslo. Ann Hum Biol. 1982;9:35–43. doi: 10.1080/03014468200005471. [DOI] [PubMed] [Google Scholar]
- 23.Gueresi P. Monthly distribution of menarche in three provinces of north Italy. Ann Hum Biol. 1997;24:157–68. doi: 10.1080/03014469700004892. [DOI] [PubMed] [Google Scholar]
- 24.Wolanski N, Dickinson F, Siniarska A. Seasonal rhythm of menarche as a sensitive index of living conditions. Stud Hum Ecol. 1994;11:171–91. [PubMed] [Google Scholar]
- 25.Matchock RL, Susman EJ, Brown FM. Seasonal rhythms of menarche in the United States: correlates to menarcheal age, birth age, and birth month. Womens Health Issues. 2004;14:184–92. doi: 10.1016/j.whi.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Jafarey NA, Khan MY, Jafarey SN. Role of artificial lighting in decreasing the age of menarche. Lancet. 1971;297:471. doi: 10.1016/s0140-6736(70)90092-9. [DOI] [PubMed] [Google Scholar]
- 27.Lewis PD, Sharp PJ, Wilson PW, Leeson S. Changes in light intensity can influence age at sexual maturity in domestic pullets. Br Poult Sci. 2004;45:123–32. doi: 10.1080/00071660410001668950. [DOI] [PubMed] [Google Scholar]
- 28.Rivest RW, Lang U, Aubert ML, Sizonenko PC. Daily administration of melatonin delays rat vaginal opening and disrupts the first estrous cycles: evidence that these effects are synchronized by the onset of light. Endocrinology. 1985;116:779–87. doi: 10.1210/endo-116-2-779. [DOI] [PubMed] [Google Scholar]
- 29.Greives TJ, Kriegsfeld LJ, Bentley GE, Tsutsui K, Demas GE. Recent advances in reproductive neuroendocrinology: a role for RFamide peptides in seasonal reproduction? Proc Biol Sci. 2008;275:1943–51. doi: 10.1098/rspb.2008.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li XF, Kinsey-Jones JS, Cheng Y, et al. Kisspeptin signalling in the hypothalamic arcuate nucleus regulates GnRH pulse generator frequency in the rat. PLoS One. 2009;4:e8334. doi: 10.1371/journal.pone.0008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fischer TW, Sweatman TW, Semak I, Sayre RM, Wortsman J, Slominski A. Constitutive and UV-induced metabolism of melatonin in keratinocytes and cell-free systems. FASEB J. 2006;20:1564–66. doi: 10.1096/fj.05-5227fje. [DOI] [PubMed] [Google Scholar]
- 32.Claustrat B, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med Rev. 2005;9:11–24. doi: 10.1016/j.smrv.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Terasawa E, Kurian JR, Guerriero KA, Kenealy BP, Hutz ED, Keen KL. Recent discoveries on the control of gonadotrophin-releasing hormone neurones in nonhuman primates. J Neuroendocrinol. 2010;22:630–38. doi: 10.1111/j.1365-2826.2010.02027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macchi MM, Bruce JN. Human pineal physiology and functional significance of melatonin. Front Neuroendocrinol. 2004;25:177–95. doi: 10.1016/j.yfrne.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Roa J, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. New frontiers in kisspeptin/GPR54 physiology as fundamental gatekeepers of reproductive function. Front Neuroendocrinol. 2008;29:48–69. doi: 10.1016/j.yfrne.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Revel FG, Saboureau M, Masson-Pevet M, Pevet P, Mikkelsen JD, Simonneaux V. Kisspeptin mediates the photoperiodic control of reproduction in hamsters. Curr Biol. 2006;16:1730–35. doi: 10.1016/j.cub.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 37.Roseweir AK, Millar RP. The role of kisspeptin in the control of gonadotrophin secretion. Hum Reprod Update. 2009;15:203–12. doi: 10.1093/humupd/dmn058. [DOI] [PubMed] [Google Scholar]
- 38.Li Q, Rao A, Pereira A, Clarke IJ, Smith JT. Kisspeptin cells in the ovine arcuate nucleus express prolactin receptor but not melatonin receptor. J Neuroendocrinol. 2011;23:871–82. doi: 10.1111/j.1365-2826.2011.02195.x. [DOI] [PubMed] [Google Scholar]
- 39.Villamor E, Marin C, Mora-Plazas M, Baylin A. Vitamin D deficiency and age at menarche: a prospective study. Am J Clin Nutr. 2011;94:1020–25. doi: 10.3945/ajcn.111.018168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer F, Moisan J, Marcoux D, Bouchard C. Dietary and physical determinants of menarche. Epidemiology. 1990;1:377–81. doi: 10.1097/00001648-199009000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Maclure M, Travis LB, Willett W, MacMahon B. A prospective cohort study of nutrient intake and age at menarche. Am J Clin Nutr. 1991;54:649–56. doi: 10.1093/ajcn/54.4.649. [DOI] [PubMed] [Google Scholar]