Abstract
Background Only a limited number of studies have investigated the correlation between haematocrit (HCT) and mortality in the general population, and few of those studies have had data on a wide range of low and high levels of HCT. We investigated the association between baseline HCT and mortality in a prospective cohort study of 49 983 adult subjects in Iran with a broad spectrum of HCT values.
Methods Data on socio-demographic and life-style factors, past medical history, and levels of HCT were collected at enrollment. During a mean follow-up of 5 years (follow-up success rate ∼99%), 2262 deaths were reported. Cox proportional hazards regression models were used to estimate hazard ratios and corresponding 95% confidence intervals.
Results There was a U-shaped relationship between categories of HCT and mortality in both sexes: both low and high levels of HCT were associated with increased overall mortality and mortality from cardiovascular disease. The U-shaped relationship persisted after several sensitivity analyses were done, including analyses restricted to non-smokers and non-users of opium; analyses excluding deaths from accidents and other external causes as well as deaths of persons with self-reported ischemic heart disease at the baseline interview for the study; and analyses excluding the first 2 years of follow-up. Self-reported past medical history and lack of data about lipids and other cellular blood components were the major limitations of the study.
Conclusions Low and high levels of HCT are associated with increased mortality in the general population. The findings in the present study can be of particular importance for low- and middle-income countries in which a substantial proportion of the population lives with suboptimal levels of HCT.
Keywords: Anaemia, cancer, cardiovascular disease, erythrocytosis, haematocrit, mortality
Introduction
An ample body of evidence, including that provided by randomized trials and observational studies, exists for the association of haematocrit (HCT) or haemoglobin (Hb) levels with all-cause mortality, disease-specific mortality, or quality-of-life measures in specific populations such as the elderly1–3 and patients with end-stage renal disease,4,5 heart failure,6,7 cancer,8,9 human immunodeficiency virus (HIV) infection/acquired immune deficiency syndrome (AIDS),9,10 and inflammatory states such as rheumatoid arthritis11,12 or irritable bowel disease.13 Studies of HCT in the general population, however, have been largely focused on the association of high HCT with cardiovascular disease (CVD).14 Most of these studies have been conducted in Europe and North America, where the proportion of individuals with a suboptimal HCT is small, and have therefore been limited by insufficient data for individuals with a low HCT. Thus, for example, the lowest category of HCT analysed by Kunnas and colleagues in a study of Finnish men was that below 44%.15 Few population-based studies have investigated the correlation between HCT and mortality from causes other than CVD.16
In this paper we report the association between HCT and mortality from all causes combined and specifically from CVD and from cancer in men and women enrolled in the Golestan Cohort Study (GCS), a large prospective cohort study of more than 50 000 adult subjects from Golestan Province in northeast Iran. The study covers a wide range of values of HCT including both high and low levels.
Golestan Province consists of deserts and mountainous and forested areas, with a climate that is humid and moderate. Its population has a socioeconomic status ranging from low to intermediate.17 Most of the men and women in the province are farmers and housewives, respectively.18 The rate of ever having smoked cigarettes is moderate among men (∼ 38%) and low among women (∼ 2%).19 The chewing of nass (a tobacco product that is mixed with lime and ash) is a relatively common habit practiced chiefly among men (∼ 16% of men), particularly in rural areas.19 Average body mass index (BMI) in this area is high, particularly among women.20 Studies have suggested a low intake of vitamins in rural area of the province and among women.21 The four main causes of death in the study population have been (in order): cardiovascular diseases, cancer, cerebrovascular events, and external causes.22
Methods
The GCS is a prospective population-based cohort study for which enrollment was conducted between January 2004 and June 2008 and included 50 045 participants from eastern parts of Golestan Province and ranging in age from 40–75 years.19 Our primary goal was to recruiting 50 000 healthy individuals (40–75 years old), with equal numbers of men and women, 20% from urban areas and 80% of Turkmen ethnicity. However, the actual numbers were slightly different at the end of recruitment. We report our primary goal here because after publication of one of our earlier articles from this cohort, some readers thought that the difference in proportion of subgroups (e.g. 20% urban and 80% rural) was excursively because of difference in participation rates. Eligibility criteria for the study were permanent residence in the study area and a negative history of upper gastrointestinal cancer. A total of 16 599 urban inhabitants within the specified age range for the study were selected randomly from Gonbad City, the main urban area in eastern Golestan, by systematic clustering based on the number of residents in a household. In rural areas, all residents of all villages in the study catchment area who were within the specified age range were invited to participate. In rural areas, recruitment took advantage of the network of primary health-care centers known as health houses, which are present in each group of villages. With participation rates of approximately 70% for women and 50% for men, the GCS enrolled a total of 10 032 urban participants. In rural areas, 40 013 participants were enrolled from 326 villages, with participation rates of 84% for women and 70% for men. All participants received a personal GCS identification card at the time of enrollment, which allows them to utilize a specialized gastrointestinal clinic established by the study group in the study area, and provides free services for GCS participants if they experience any gastrointestinal symptoms.
At baseline, and after obtaining written informed consent from prospective participants, trained nurses and physicians collected data on their demographic characteristics and several life-style factors in face-to-face interviews and through the use of a structured questionnaire. Additionally, members of the study staff measured anthropometric indices and blood pressure (BP). To identify deceased persons within the cohort and to ascertain the causes of their death, all participants in the GCS are actively followed through annual telephone calls and by local health workers in their communities, and through a review of monthly provincial death registration reports. When the death of a GCS participant is reported, a validated verbal autopsy questionnaire23 is completed through an interview of the closest relative of the deceased, and copies of all available and relevant medical documents for deceased cohort members are collected from hospitals and pathology laboratories in Golestan and neighbouring provinces. All collected documents are reviewed by at least two expert physicians to determine the cause of death. Further details on the design of GCS and ascertainment of causes of death are provided elsewhere.19,23 The success rate of the follow-up in the GCS has been ∼99%. The high telephone coverage; a low immigration rate, particularly of persons in the age range of the study participants; the ability to contact local health workers in rural areas and free services provided in the GCS for the management of gastrointestinal diseases may be among the factors that contributed to high success rate of the follow-up.
Individuals examined for enrollment in the GCS were considered tobacco users if they had smoked cigarettes, a water pipe (hookah), or a pipe, or had used nass at least once a week for a period of 6 months or more. Cigarette smokers who smoked during the year before the enrollment interview were classified as current cigarette smokers and those who smoked ≥20 cigarettes/day during the year before the interview were considered current heavy smokers. Opium users were defined as persons who had consumed opium at least once a week for at least 6 months. The self-reported use of tobacco and opium is a reliable and valid indicator of exposure to these substances in this population.24 Body mass index (BMI) was calculated by dividing body weight (kg) by the squared value of height (m). Participants were considered hypertensive if they used anti-hypertensive medication or fulfilled the criteria of having a systolic blood pressure ≥140 mm Hg or a diastolic blood pressure above ≥90 mm Hg.25 Data on chronic conditions, such as diabetes and ischemic heart disease (IHD), were obtained using pre-specified questions and defined as self-reported diagnoses made by a physician.
Values of HCT were determined in blood samples obtained at the time of baseline interviews for the GCS. For the determination of HCT, venous blood was well and immediately mixed with ethylene diamine tetra-acetic acid (EDTA). Anticoagulated blood was drawn into microhaematocrit tubes, which were filled to approximately 75% of capacity and centrifuged for 10 minutes. The values of HCT were determined with an HCT reader. To eliminate the effect of outliers, value of HCT of <15% and ≥70% were excluded from the study analyses. Values of HCT were categorized in units of 5%, except for the lowest and highest categories, which included HCTs of 15%–29% and 55%–69%, respectively, because of the small number of participants with these values of HCT. According to the World Health Organization’s (WHO) definition for individuals ≥15 years of age, the threshold values of hemoglobin (Hb) below which individuals are considered anemic are 13 g/dl (∼HCT 39%) for men and 12 g/dl (∼HCT 36%) for non-pregnant women.26 The practical definition of erythrocytosis (an abnormally high HCT) consistent with the WHO threshold for white persons (Caucasians) is an Hb ≥18.5 g/dl (∼HCT 55%) in men or ≥16.5 g/dl (∼HCT 50%) in women for Caucasians.27 The ‘normal’ range of HCT values may differ across populations28; among the commonly used ranges are 40–50% for men and 36–44% for women.29 In the analyses done for the GCS, men with HCTs of 35–39% and women with HCTs of 40–44% were assigned as reference groups.
The GCS was approved by the institutional review boards of the Digestive Disease Research Center of Tehran University of Medical Sciences, the US National Cancer Institute (NCI), the World Health Organization International Agency for Research on Cancer (IARC), and the Mount Sinai School of Medicine.
Statistical analysis
In the analysis of the study data, numbers and percentages were calculated and presented for categorical variables, and means and standard deviations (SD) for continuous variables. Multivariate linear-regression models were used to investigate correlates of HCT levels in men and women separately. In these models, HCT was included as a continuous variable, and adjusted mean HCT values (predicted by the models) for categorical variables and regression coefficients for continuous variables were calculated, along with corresponding P values. Values of P for trend were obtained from the same models by assigning consecutive numbers to categories within each categorical variable.
Cox proportional-hazards regression models were used to estimate hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) for the association between categories of HCT and mortality, separately for men and women. The HRs (95% CIs) were adjusted for age; ethnicity (Turkmen, other); place of residence (Gonbad City, rural areas); education level; cigarette smoking (both never/current/former status and cumulative use); water-pipe smoking; chewing of nass; opium use; BMI; hypertension; and self-reported diabetes, IHD, and cancer (excluding upper gastrointestinal cancers, because these cancers were among the exclusion criteria in the study). Sensitivity analyses were done by excluding ever-smokers of cigarettes or hookahs and opium users (because tobacco smoking and opium use have been shown to be associated with a higher mortality in this population22; cases of death during the first 2 years of follow-up; cases of death from external causes (e.g., suicide, burns, and vehicle or other accidents); and persons with self-reported IHD at the baseline interview. As additional procedures we repeated our analyses after considering cardiovascular and cancer mortality as outcomes representing failure of interest. When there was only a single case of death in a category, the category was combined with the next category; when there was no next category, the category in which the death occurred was combined with the previous category.
We used likelihood ratio tests to compare and test for goodness of fit of adjusted linear vs. nonlinear models. We compared two models, one with HCT alone as a continuous variable and the other with linear plus squared and cubed terms of HCT. The latter model showed a better fit, with P < 0.0001 for both women and men, and we therefore used fractional polynomial terms in the multivariate Cox proportional-hazards models to investigate the non-linear association between HCT and mortality. The variables included in these models were the same as those in the Cox proportional-hazards models described above, but HCT was used as a continuous rather than as a categorical variable. The powers for fractional polynomials were chosen from the set of −2, −1, −1/2, 0, 1/2, 1, 2, and 3, in which the power of 0 refers to the natural logarithm, based on the best fit to the data.30 All statistical analyses were done with STATA statistical software version 11 (Stata, College Station, TX, USA). All reported values of P are two-sided, and P < 0.05 was considered statistically significant.
Results
Of the 50 045 cohort participants, 62 (0.1%) were excluded from the GCS because their HCT levels were out of the selected range (15%–69%) or were missing. The characteristics of the remaining 49 983 participants, and the distribution of HCT values measured at baseline, are summarized in Table 1. A total of 7.9% of the women in the study had an HCT < 35% and 12.1% had an HCT ≥ 45%. The proportions of men with an HCT < 40% or ≥ 50% were 15.1% and 7.3%, respectively.
Table 1.
Characteristics of baseline cohort of study populationa
| Characteristic | Women | Men | Both sexes |
|---|---|---|---|
| Total | 28 776 (100) | 21 207 (100) | 49 983 (100) |
| Age (years) | 51.3 (8.6) | 53.2 (9.4) | 52.1 (9.0) |
| Ethnicity | |||
| Non-Turkmen | 7595 (26.4) | 5175 (24.4) | 12 770 (25.5) |
| Turkmen | 21 181 (73.6) | 16 032 (75.6) | 37 213 (74.5) |
| Place of residence | |||
| Rural | 22 692 (78.9) | 17 287 (81.5) | 39 979 (80.0) |
| Urban | 6084 (21.1) | 3920 (18.5) | 10 004 (20.0) |
| Education level | |||
| No school | 24 673 (85.7) | 10 406 (49.1) | 35 079 (70.2) |
| Primary school | 2909 (10.1) | 5543 (26.1) | 8452 (16.9) |
| Middle school | 455 (1.6) | 1785 (8.4) | 2240 (4.5) |
| High school/higher | 739 (2.6) | 3473 (16.4) | 4212 (8.4) |
| Cigarette smoking | |||
| Never smoker | 28 338 (98.5) | 13 057 (61.6) | 41 395 (82.8) |
| Former smoker | 133 (0.4) | 3572 (16.8) | 3705 (7.4) |
| Current light smoker | 249 (0.9) | 2724 (12.8) | 2973 (6.0) |
| Current heavy smoker | 56 (0.2) | 1854 (8.7) | 1910 (3.8) |
| Cumulative use of cigarettes | |||
| 1–5 pack-years | 293 (1.0) | 2471 (11.7) | 2764 (5.5) |
| 5.1–10 pack-years | 60 (0.2) | 1200 (5.6) | 1260 (2.5) |
| 10.1–20 pack-years | 46 (0.2) | 1751 (8.3) | 1797 (3.6) |
| >20 pack-years | 39 (0.1) | 2713 (12.8) | 2752 (5.5) |
| Water-pipe smoking | |||
| Never | 28 418 (98.8) | 21 010 (99.1) | 49 428 (98.9) |
| Ever | 345 (1.2) | 187 (0.9) | 532 (1.1) |
| Nass chewing | |||
| Never | 28 477 (99.0) | 17 727 (83.6) | 46 204 (92.4) |
| Ever | 299 (1.0) | 3476 (16.4) | 3775 (7.6) |
| Opium use | |||
| Never | 26 421 (91.8) | 15 066 (71.1) | 41 487 (83.0) |
| Ever | 2352 (8.2) | 6139 (28.9) | 8491 (17.0) |
| Body mass index (kg/m2) | 27.8 (5.8) | 25.1 (4.6) | 26.7 (5.5) |
| Hypertension | |||
| No | 15 408 (53.8) | 13 187 (62.5) | 28 595 (57.5) |
| Yes | 13 251 (46.2) | 7917 (37.5) | 21 168 (42.5) |
| Self-reported diabetes | |||
| No | 26 424 (91.8) | 20 107 (94.8) | 46 531 (93.1) |
| Yes | 2349 (8.2) | 1098 (5.2) | 3447 (6.9) |
| Self-reported IHD | |||
| No | 26 991 (93.8) | 19 945 (94.1) | 46 936 (93.9) |
| Yes | 1785 (6.2) | 1262 (5.9) | 3047 (6.1) |
| Self-reported cancerb | |||
| No | 28 683 (99.7) | 21 163 (94.1) | 49 847 (97.3) |
| Yes | 93 (0.3) | 44 (0.2) | 137 (0.3) |
| Haematocrit (%) | |||
| Continuous variable | 40.0 (4.1) | 43.6 (4.3) | 41.5 (4.5) |
| Categories | |||
| 15–29 | 313 (1.1) | 89 (0.4) | 402 (0.8) |
| 30–34 | 1955 (6.8) | 415 (2.0) | 2370 (4.7) |
| 35–39 | 10 040 (34.9) | 2701 (12.7) | 12 741 (25.5) |
| 40–44 | 12 987 (45.1) | 8931 (42.1) | 21 918 (43.9) |
| 45–49 | 3286 (11.4) | 7509 (35.4) | 10 795 (21.6) |
| 50–54 | 168 (0.6) | 1451 (6.8) | 1619 (3.2) |
| 55–69 | 27 (0.1) | 111 (0.5) | 138 (0.3) |
aIHD, ischaemic heart disease. Figures are numbers (column percentages) except for age, body mass index, and haematocrit (continuous variable), for which mean (SD) is reported. Numbers may not add to column totals because of missing data.
bExcluding upper gastrointestinal cancer, which was among the exclusion criteria for this study.
A total of 955 women and 1307 men died during a mean follow-up of 5 years. For both men and women there was a U-shaped relationship between HCT and overall mortality (Table 2), although the association between a high HCT and mortality was stronger in women than in men. As compared with respective unadjusted risk estimates, the adjusted estimates were slightly higher for women but lower for men. The main covariates responsible for these differences were age (the main effect in cases of a low HCT) and hookah smoking (the main effect in cases of a high HCT) in women, and age (for both a low and a high HCT), hypertension (for a high HCT), and cigarette smoking status (for a high HCT) in men. The results for mortality related to CVD and for mortality related to cancer were slightly stronger in both men and women as compared to the results for overall mortality.
Table 2.
Hazard ratios for mortality according to haematocrit, by sex
| Women |
Men |
|||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | |||
| Haematocrit (%) | N | HR (95% CI) | HR (95% CI)a | N | HR (95% CI) | HR (95% CI)a |
| Overall mortality | ||||||
| 15–29 | 15 | 1.90 (1.13–3.20) | 2.12 (1.26–3.58) | 18 | 3.89 (2.42–6.22) | 2.12 (1.30–3.45) |
| 30–34 | 61 | 1.28 (0.96–1.68) | 1.34 (1.01–1.77) | 49 | 2.35 (1.74–3.16) | 1.64 (1.22–2.21) |
| 35–39 | 258 | 1.00 (Referent) | 1.00 (Referent) | 205 | 1.47 (1.25-1.74) | 1.18 (1.00–1.39) |
| 40–44 | 438 | 1.18 (1.01–1.38) | 1.16 (0.99–1.36) | 506 | 1.00 (Referent) | 1.00 (Referent) |
| 45–49 | 167 | 1.73 (1.42–2.10) | 1.58 (1.29–1.93) | 417 | 0.88 (0.77–1.00) | 1.00 (0.88–1.15) |
| 50–54 | 13 | 2.74 (1.57–4.79) | 2.05 (1.17–3.60) | 100 | 1.08 (0.87–1.35) | 1.29 (1.04–1.61) |
| 55–69 | 3 | 4.83 (1.55–15.07) | 5.61 (1.79–17.55) | 12 | 1.53 (0.82–2.85) | 1.25 (0.64–2.42) |
| CVD mortality | ||||||
| 15–29 | 7 | 2.07 (0.97–4.45) | 2.60 (1.21–5.60) | 12 | 5.58 (3.12–9.96) | 3.08 (1.71–5.55) |
| 30–34 | 28 | 1.36 (0.90–2.05) | 1.48 (0.98–2.25) | 16 | 1.67 (1.01–2.78) | 1.21 (0.73–2.01) |
| 35–39 | 111 | 1.00 (Referent) | 1.00 (Referent) | 93 | 1.43 (1.12–1.82) | 1.19 (0.94–1.52) |
| 40–44 | 238 | 1.49 (1.19–1.87) | 1.42 (1.13–1.79) | 236 | 1.00 (Referent) | 1.00 (Referent) |
| 45–49 | 92 | 2.23 (1.69–2.94) | 1.95 (1.47–2.52) | 230 | 1.05 (0.88–1.27) | 1.12 (0.93–1.36) |
| 50–54 | 9 | 4.35 (2.28–8.31) | 2.87 (1.49–5.52) | 63 | 1.49 (1.12–1.97) | 1.61 (1.21–2.15) |
| 55–69 | 1 | 7 | 1.99 (0.88–4.47) | 1.70 (0.75–3.85) | ||
| Cancer mortality | ||||||
| 15–29 | 6 | 3.13 (1.35–7.24) | 2.92 (1.26–6.81) | 3 | 2.94 (0.94–9.28) | 1.96 (0.62–6.18) |
| 30–34 | 17 | 1.45 (0.85–2.48) | 1.45 (0.84–2.53) | 15 | 3.37 (1.96–5.77) | 2. 21 (1.27–3.82) |
| 35–39 | 63 | 1.00 (Referent) | 1.00 (Referent) | 54 | 1.80 (1.30–2.49) | 1.37 (0.98–1.91) |
| 40–44 | 90 | 1.02 (0.74–1.42) | 1.08 (0.77–1.50) | 112 | 1.00 (Referent) | 1.00 (Referent) |
| 45–49 | 23 | 1.02 (0.63–1.64) | 1.05 (0.64–1.71) | 63 | 0.61 (0.44–0.83) | 0.74 (0.54–1.03) |
| 50–54 | 1 | 2.33 (0.73–7.44) | 2.57 (0.80–8.27) | 18 | 0.84 (0.51–1.41) | 1.14 (0.68–1.93) |
| 55–69 | 2 | 2 | 1.37 (0.34–5.55) | 1.21 (0.30–4.98) | ||
CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio; N, number of deaths.
aHazard ratos (95% CIs) are adjusted for age; ethnicity; place of residence; education level; cigarette smoking (both current/former status and cumulative use); water-pipe smoking; nass chewing; opium use; body mass index; and history of hypertension, self-reported diabetes, ischemic heart disease, and cancer (variables as shown in Table 1).
Exclusion of the first 2 years of follow-up reduced the strength of the association between mortality and a low HCT in both men and women (Table 3); in men, the association of mortality with a high HCT was no longer apparent, whereas in women it was still present but more weakly so. An analysis restricted to never-users of cigarettes, a hookah, or opium resulted in a stronger association between overall mortality and an increased HCT in men. Excluding external causes of death and participants with self-reported IHD at the baseline interview had relatively little effect on the results for HCT and mortality.
Table 3.
Sensitivity analyses for association of haematocrit and mortality, by sex
| Women |
Men |
|||
|---|---|---|---|---|
| Haematocrit (%) | N | HR (95% CI) | N | HR (95% CI) |
| Excluding first 2 years of follow-up | ||||
| 15–29 | 6 | 1.47 (0.65–3.32) | 9 | 1.73 (0.89–3.37) |
| 30–34 | 32 | 1.18 (0.81–1.73) | 24 | 1.31 (0.86–1.99) |
| 35–39 | 160 | 1.00 (Referent) | 104 | 0.94 (0.76–1.18) |
| 40–44 | 292 | 1.19 (0.98–1.44) | 337 | 1.00 (Referent) |
| 45–49 | 120 | 1.72 (1.35–2.19) | 265 | 0.93 (0.79–1.10) |
| 50–54 | 8 | 1.92 (0.98–3.78) | 61 | 1.16 (0.88–1.53) |
| 55–69 | 1 | 5 | 0.83 (0.31–2.24) | |
| Mortality in never-users of cigarettes, hookahs, or opium | ||||
| 15–29 | 12 | 2.38 (1.32–4.26) | 2 | 0.74 (0.18–3.00) |
| 30–34 | 49 | 1.52 (1.11–2.09) | 17 | 2.25 (1.36–3.70) |
| 35–39 | 191 | 1.00 (referent) | 78 | 1.40 (1.08–1.82) |
| 40–44 | 350 | 1.28 (1.07–1.53) | 195 | 1.00 (Referent) |
| 45–49 | 127 | 1.75 (1.39–2.20) | 172 | 0.94 (0.76–1.16) |
| 50–54 | 10 | 2.28 (1.21–4.34) | 53 | 1.47 (1.08–2.01) |
| 55–69 | 2 | 4.59 (1. 13–18.60) | 5 | 1.98 (0.63–6.18) |
| Excluding external causes of death | ||||
| 15–29 | 14 | 2.11 (1.23–3.62) | 18 | 2.21 (1.36–3.61) |
| 30–34 | 60 | 1.40 (1.05-1.86) | 48 | 1.69 (1.25–2.28) |
| 35–39 | 242 | 1.00 (Referent) | 197 | 1.20 (1.01–1.42) |
| 40–44 | 417 | 1.18 (1.00–1.38) | 474 | 1.00 (Referent) |
| 45–49 | 163 | 1.64 (1.34–2.02) | 378 | 0.98 (0.85–1.13) |
| 50–54 | 13 | 2.18 (1.24–3.82) | 92 | 1.28 (1.02-1.61) |
| 55–69 | 3 | 5.96 (1.90–18.68) | 12 | 1.31 (0.68–2.55) |
| Excluding individuals with self-reported IHD at baseline | ||||
| 15–29 | 11 | 1.89 (1.03–3.47) | 11 | 1.88 (1.00–3.53) |
| 30–34 | 49 | 1.28 (0.93–1.75) | 46 | 1.95 (1.43–2.66) |
| 35–39 | 216 | 1.00 (Referent) | 179 | 1.25 (1.05–1.50) |
| 40–44 | 373 | 1.20 (1.01–1.42) | 415 | 1.00 (Referent) |
| 45–49 | 137 | 1.58 (1.27–1.97) | 349 | 1.05 (0.90–1.22) |
| 50–54 | 11 | 2.43 (1.32–4.47) | 81 | 1.32 (1.03–1.69) |
| 55–69 | 3 | 10.46 (3.34–32.77) | 10 | 1.14 (0.54–2.43) |
CI, confidence interval; HR, hazard ratio; IHD, ischaemic heart disease; N, number of deaths.
Hazard ratios (95% CIs) are adjusted for age; ethnicity; place of residence; education level; cigarette smoking (both current/former status and cumulative use); water-pipe smoking; nass chewing; opium use; body mass index; and history of hypertension, self-reported diabetes, ischemic heart disease, and cancer (variables as shown in Table 1).
Hazard ratios (95% CIs) in never-users of cigarettes, a hookah, or opium were not adjusted for the variables of cigarette, hookah, and opium use.
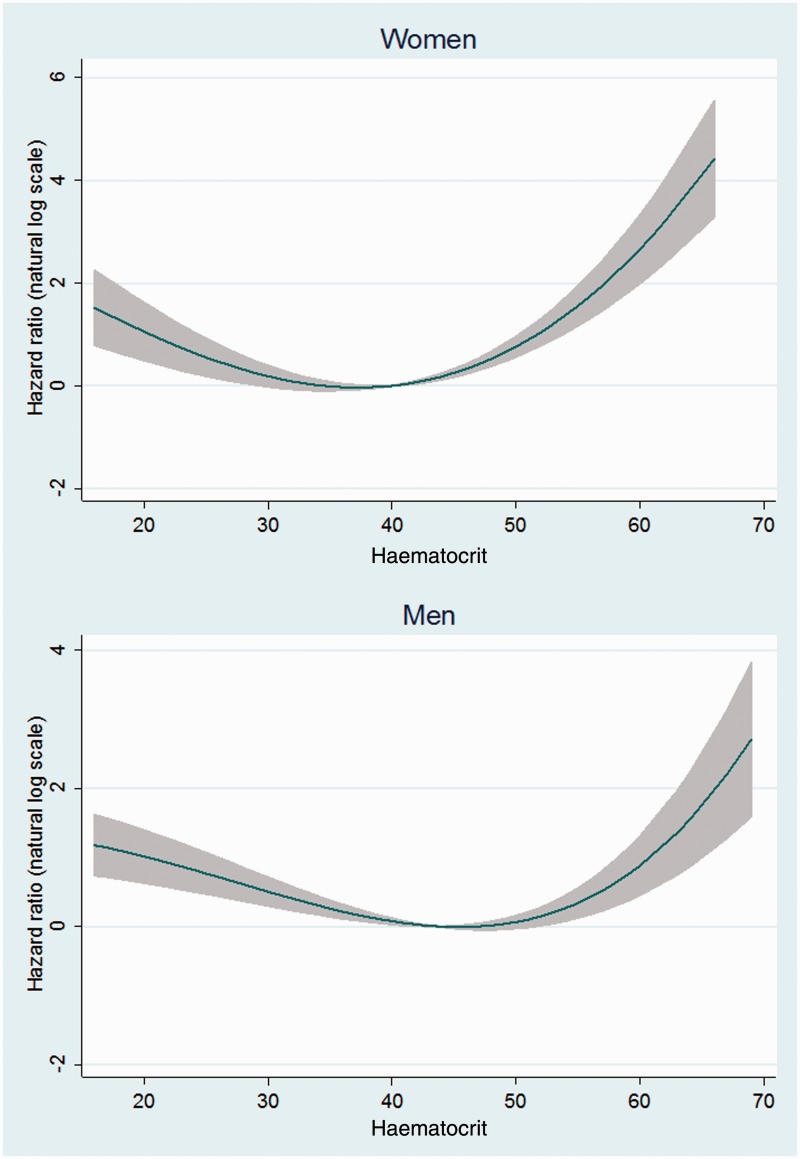
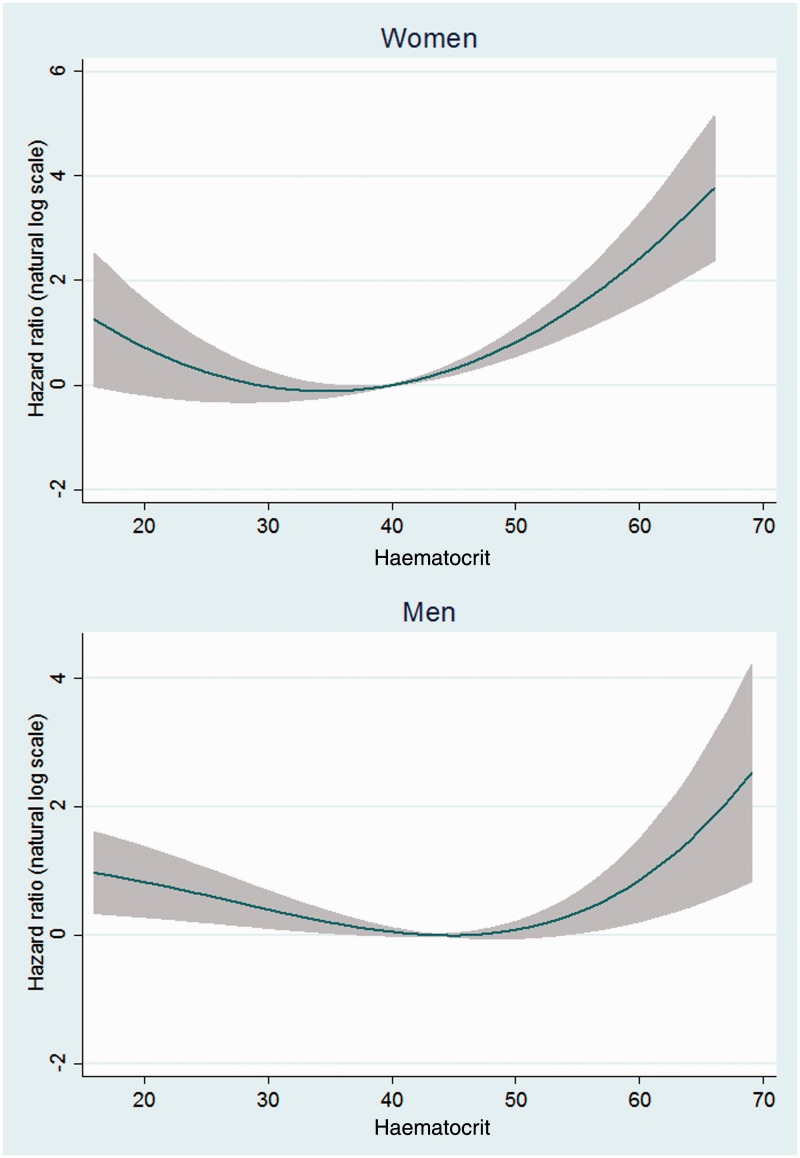
The results of fractional polynomial analyses using HCT as a continuous variable also showed a U-shaped association between HCT and mortality in both the overall analyses (Figure 1) and after exclusion of the first 2 years of follow-up (Figure 2) for men and women.
Figure 1.
Non-linear risk estimates for the association between haematocrit and mortality.
Note. Risk estimates are adjusted for age, ethnicity, place of residence, education level, cigarette smoking (both current/former status and cumulative use), water-pipe smoking, nass chewing, opium use, body mass index, hypertension, and self-reported diabetes, ischemic heart disease, and cancer (variables as shown in Table 1)
Figure 2.
Non-linear risk estimates for the association between haematocrit and mortality after excluding the first two years of follow-up.
Note. Risk estimates are adjusted for age, ethnicity, place of residence, education level, cigarette smoking (both current/former status and cumulative use), water-pipe smoking, nass chewing, opium use, body mass index, hypertension, and self-reported diabetes, ischemic heart disease, and cancer (variables as shown in Table 1)
The correlates of HCT level are shown in Table 4. The strongest predictor of HCT was smoking, and particularly current heavy smoking, with the mean HCT of current heavy smokers being 1.54 units higher than that of never-smokers. There were smaller but statistically significant differences among the levels of other predictors of HCT. Whereas older age was positively correlated with higher HCT in women, there was an inverse association in men. Men and women of Turkmen ethnicity and participants residing in urban areas had higher HCTs than those of other ethnicities and those residing in rural areas, respectively. Education showed an inverse association with HCT for both men and women. Ever-smoking of a hookah, BMI, and hypertension were associated with a higher HCT in both sexes. Self-reported diabetes was associated with a higher HCT in women only. The results of a polynomial logistic regression analysis of correlates of HCT as a categorical variable were consistent with those of the linear regression models reported above (the method used in the logistic regression models is shown in the footnote to Supplementary Table 1, available as Supplementary data at IJE online).
Table 4.
Associations between haematocrit and selected variables in the baseline cohort
| Women |
Men |
|||||
|---|---|---|---|---|---|---|
| Characteristic | Mean HCT (SD)a | Adjusted Mean (regression coefficient)b | P-valueb | Mean HCT (SD)a | Adjusted Mean (regression coefficient)b | P-valueb |
| Total | 39.98 (4.1) | 39.97 | – | 43.56 (4.3) | 43.56 | – |
| Age (years), continuous | – | [0.011] | <0.001 | – | (–0.061) | <0.001 |
| Ethnicity | ||||||
| Non-Turkmen | 39.86 (4.1) | 39.80 | Referent | 43.46 (4.0) | 43.25 | Referent |
| Turkmen | 40.02 (4.1) | 40.03 | <0.001 | 43.59 (4.3) | 43.66 | <0.001 |
| Place of residence | ||||||
| Rural | 39.83 (4.1) | 39.83 | Referent | 43.26 (4.3) | 43.28 | Referent |
| Urban | 40.53 (4.1) | 40.50 | <0.001 | 44.87 (4.2) | 44.77 | <0.001 |
| Education level | ||||||
| No school | 40.00 (4.1) | 40.02 | Referent | 43.26 (4.4) | 43.86 | Referent |
| Primary school | 39.80 (4.1) | 39.65 | <0.001 | 43.59 (4.4) | 43.35 | <0.001 |
| Middle school | 39.62 (4.3) | 39.29 | <0.001 | 43.78 (4.2) | 43.17 | <0.001 |
| High school/higher | 40.18 (3.9) | 39.91 | 0.46 | 44.31 (4.0) | 43.17 | <0.001 |
| P for trend | 0.001 | <0.001 | ||||
| Cigarette smoking | ||||||
| Status | ||||||
| Never smoker | 39.96 (4.1) | 39.96 | Referent | 43.66 (4.2) | 43.42 | Referent |
| Former smoker | 40.32 (3.6) | 40.16 | 0.57 | 42.81 (4.6) | 43.28 | 0.09 |
| Current light smoker | 40.90 (4.4) | 41.06 | <0.001 | 43.74 (4.4) | 43.94 | <0.001 |
| Current heavy smoker | 41.50 (4.2) | 41.36 | 0.01 | 44.02 (4.6) | 44.46 | <0.001 |
| P for trend | <0.001 | <0.001 | ||||
| Cumulative use | ||||||
| None | 39.96 (4.1) | 39.96 | Referent | 43.66 (4.2) | 43.42 | Referent |
| 1–5 pack-years | 40.69 (4.0) | 40.78 | 0.001 | 43.23 (4.3) | 43.40 | 0.79 |
| 5.1–10 pack-years | 40.58 (4.7) | 40.43 | 0.37 | 43.25 (4.5) | 43.56 | 0.27 |
| 10.1–20 pack-years | 41.76 (3.4) | 41.66 | 0.005 | 43.51 (4.4) | 43.83 | <0.001 |
| > 20 pack-years | 40.85 (5.0) | 40.84 | 0.17 | 43.54 (4.8) | 44.16 | <0.001 |
| P for trend | <0.001 | <0.001 | ||||
| Water-pipe smoking | ||||||
| Never | 39.97 (4.1) | 39.96 | Referent | 43.56 (4.3) | 43.55 | Referent |
| Ever | 40.58 (4.3) | 40.81 | <0.001 | 44.02 (4.9) | 44.46 | 0.003 |
| Nass chewing | ||||||
| Never | 39.98 (4.1) | 39.97 | Referent | 43.84 (4.3) | 43.65 | Referent |
| Ever | 39.33 (3.8) | 39.74 | 0.32 | 42.13 (4.3) | 43.09 | <0.001 |
| Opium use | ||||||
| Never | 39.96 (4.0) | 39.93 | Referent | 43.97 (4.2) | 43.82 | Referent |
| Ever | 40.23 (4.3) | 40.47 | <0.001 | 42.55 (4.5) | 42.90 | <0.001 |
| BMI (kg/m2), continuous | – | [0.104] | <0.001 | – | (0.183) | <0.001 |
| Hypertension | ||||||
| No | 39.61 (4.0) | 39.75 | Referent | 43.42 (4.3) | 43.45 | Referent |
| Yes | 40.40 (4.1) | 40.23 | <0.001 | 43.78 (4.5) | 43.73 | <0.001 |
| Self-reported diabetes | ||||||
| No | 39.91 (4.1) | 39.94 | Referent | 43.54 (4.3) | 43.56 | Referent |
| Yes | 40.71 (4.1) | 40.33 | <0.001 | 43.83 (4.6) | 43.52 | 0.78 |
| Self-reported IHD | ||||||
| No | 39.95 (4.1) | 39.97 | Referent | 43.57 (4.3) | 43.56 | Referent |
| Yes | 40.37 (4.2) | 39.94 | 0.76 | 43.34 (4.9) | 43.48 | 0.51 |
| Self-reported cancer | ||||||
| No | 39.98 (4.1) | 39.97 | Referent | 43.56 (4.3) | 43.56 | Referent |
| Yes | 39.52 (4.8) | 39.39 | 0.17 | 43.41 (6.3) | 43.52 | 0.95 |
BMI, body mass index; HCT, haematocrit; IHD, ischaemic heart disease.
aMean HCT (SD) is arithmetic mean (standard deviation) of HCT for each category.
bFor continuous variables, adjusted regression coefficients are shown. Adjusted means or regression coefficients and corresponding P-values were calculated with multivariate linear regression models, in which haematocrit (as a continuous variable) was the dependent variable, and adjusted for other variables in the table except for cumulative smoking of cigarettes. Adjusted means and P-values for cumulative smoking of cigarettes were adjusted for the other variables but not for cigarette-smoking status.
Discussion
In the population in northeastern Iran in which the GCS is being conducted, there appears to be a U-shaped relationship between HCT and mortality, with both low and high values of HCT being associated with increased overall and cardiovascular mortality. This relationship was observed for both men and women, after controlling for a self-reported history of IHD, diabetes, and cancer at the baseline of the study. The U-shaped relationship was also maintained after exclusion of the first 2 years of follow-up in the study, done for the purpose of minimizing the effect of conditions prevalent at enrollment in the study. Similar results were seen when HCT was treated as a categorical as when it was treated as a continuous variable. Our results also suggest such a U-shaped association between HCT and cancer mortality, but because the number of deaths from cancer of study participants with high values of HCT was modest, the association in the high HCT direction could not be investigated precisely.
A U-shaped relationship between HCT and mortality in specific population groups, including individuals with heart failure31,32 or IHD,33 and pregnant women,34 has been observed in a number of studies. In the general population, a similar U-shaped relationship between HCT and some health outcomes has also been reported in a few studies done in high-income countries,16,35,36 usually with different patterns of association in men and women. In one such study, restricted to women, in which a high HCT was associated with mortality from IHD, a low HCT was associated with cancer mortality.16 In the Framingham Heart Study, which showed a U-shaped association between HCT and CVD mortality in men and women and between HCT and the incidence of CVD in women,35 the lowest quintiles of HCT in men, of 25–44%, and in women, of 21–39%, also included some ‘normal’ values of HCT based on the WHO definition of this haematological measure.26 In a study does as part of the National Health and Nutrition Examination Survey II, which had only three levels of HCT, there was a U-shaped relationship between HCT and cardiovascular and overall mortality among men in unadjusted analyses, but these associations disappeared after adjustment for risk factors for CVD.36 In women, the same study found a linear relationship of HCT with cardiovascular mortality and a J-shaped relationship of HCT with overall mortality in unadjusted analyses. However, upon stratification for age and after adjustment for risk factors for CVD, a high HCT in younger women was associated with increased cardiovascular mortality whereas a high HCT in older women were associated with a decreased risk of overall mortality.36 A relatively smaller study, of HCT and stroke in rural areas of Japan, which also had only 3 categories of HCT, showed a U-shaped association between HCT and death from stroke in women, with the association for a low HCT being statistically significant.37 In men, there was a non-significantly positive association between HCT and death from stroke.
Our results are based on fine categories, and to our knowledge, this may be the first reported study of the relationship between HCT and mortality from multiple outcomes across a very wide distribution of HCT values for both men and women in the general population. However, despite the U-shaped associations of HCT with mortality in both sexes, the association between high HCT and higher mortality was stronger in women than in men, whereas the association between low HCT and higher mortality was stronger in men. We adjusted our results for several confounding factors that might have dissimilar distributions in women and men. The reasons for these sex differences in the association between HCT and mortality are not clear. Estrogen is associated with an increased risk of vascular thrombosis,38 and menopausal hormone replacement therapy has been associated with an increased risk of CVD.39,40 The thrombotic effect of estrogen might be more pronounced with a high HCT, which is associated with higher blood viscosity. However, the associations between endogenous estrogen levels and CVD have been inconsistent.41–44 Further investigations are needed of sex differences in the association between HCT and mortality.
It is well established that an increased HCT increases the risk of CVD and mortality from CVD35 In a meta-analysis of 16 population-based prospective studies done mainly in Europe and North America, Danesh and colleagues estimated an overall relative risk of 1.16 (95% CI 1.05–1.29) for coronary heart disease (CHD) in a comparison of the top tertile of HCT (mean HCT = 46.3%) with the bottom tertile (mean HCT = 41.7%).14 In three studies of people with pre-existing CVD, the relative risk (RR) of mortality was higher for those with higher values of HCT (RR = 1.81; 95% CI 1.19–2.76). The associations of increased HCT with CHD and CVD have been confirmed in studies published since Danesh et al. meta-analysis.15,36,45 The association between elevated HCT and death may merely reflect the effects of smoking, which is known to raise HCT values.46 On the other hand, it is also plausible that a high HCT enhances acute thrombosis. In fact, experimental studies have shown that lower values of HCT are associated with a lower rate of arterial thrombosis.47 This is in agreement with the finding of an enhanced adhesion of platelets to the vascular endothelial wall with increasing HCT.48 The persistence of the association between both low and high values of HCT and mortality in our study among never-smokers and after adjustments for the smoking of cigarettes and use of other tobacco products supports the independence from smoking of the associations observed in our study.
Studies of specific groups with an increased risk of anaemia have shown associations between low values of HCT and mortality. Such groups include pregnant women (mainly in low- to middle-income countries)49; the elderly2,3,50; patients with chronic kidney disease,4 heart failure,6,7 or CHD51; and those susceptible to acute hemodynamic changes, including individuals with hip fracture52 or pulmonary embolism.53 Prolonged tissue hypoxia may have a role in the association between anaemia and increased mortality.54,55 Anaemia may have organ-specific effects. For example, it may contribute to CVD through an increased cardiac preload, reduced afterload, or increased cardiac output, with left-ventricular hypertrophy as a long-term consequence.54 Anaemia is generally associated with elevated levels of erythropoietin (EPO), which have effects on cardiovascular system that range from protective to adverse.56 Therefore, some of the health outcomes associated with anaemia may be related to effects of excess EPO. Furthermore, anaemia is sometimes to the result of nutritional deficiencies,57,58 which may be more prevalent in low- to middle-income regions,59 including Golestan Province, than in areas with higher incomes.60 The anaemia in these regions may also be accompanied by the risk of other consequences of nutritional deficiencies.7,49 The results of the present study are particularly relevant for low- and middle-income regions in which anaemia is common and in which there is a substantial population with suboptimal levels of HCT. According to the WHO Vitamin and Mineral Nutrition Information System for 1993–2005, approximately 42% and 25% of the populations in the low- and middle-income countries, respectively, were reported to be anemic, representing 367 million and 1.1 billion individuals, respectively.61
It might be argued that the association between low and high HCT and mortality is related to the association of HCT with risk factors for CVD and perhaps for other diseases. Our results confirm a positive association of HCT with such other risk factors for CVD as cigarette smoking, obesity, hypertension, and diabetes (among women). However, the association between HCT and mortality in our study persisted after adjustments for these other risk factors, suggesting an independent association of HCT with CVD. Nevertheless, we did not have data on cholesterol levels, and a history of diabetes was in our study based on self-reports. Further studies of the association between HCT and increased mortality, and the potential mechanistic factors for this association, are required.
The results of this study may have applications in in public health and disease prevention. First, individuals with low and high values of HCT, as an indicator of a higher risk of death, may be monitored regularly for common life-threatening conditions, including CVD. Regular monitoring of risk factors for CVD in persons with high values of HCT might also be considered because of the possible association of a high HCT with many other risk factors of CVD. Also, if a causal association is established between low values of HCT and overall mortality, correction of anaemia in the general population may be considered more seriously by the public, physicians, and health systems, particularly in low- and middle-income countries, where anaemia is more common. Results of our study support the need for further studies of any effects of reducing body-iron stores in reducing mortality in the general population. A beneficial effect of regular phlebotomy in decreasing overall mortality in patients with peripheral artery disease, especially in younger patients who had better compliance with phlebotomy and higher blood ferritin levels at entry, has been reported in a randomized multicenter trial.62,63 However, the results of observational studies of voluntary blood donation and health outcomes, including diabetes, cardiac events, and cancer, are mixed, with some such studies reporting a beneficial association64–66 and others finding no association.67–70
Our study has several strengths, including comprehensive and standardized follow-up of a large community-based cohort in a middle-income country, with the inclusion of both rural and urban participants; the inclusion of a wide range of HCT values; the availability of overall and subtype mortality data; and examination of the associations of HCT with disease and mortality in different models using continuous and categorical data. Limitations of the study include a self-reported history of diabetes, IHD, and cancer; a lack of data on blood lipids and other cellular blood components; and the unavailability of data on incident disease and other outcomes of interest (e.g. hospitalization and major cardiovascular events). Furthermore, we used a single measurement of HCT, whereas the normal range of HCT is subject to many day-to-day variables. However, we believe that the latter issue is not a major drawback for our study, because: (i) except for acute cases of hemodynamic changes, the day-to-day variation in HCT is likely to be small; and (ii) any measurement error in our prospective study is likely to be non-differential and to reduce the apparent association between high or low HCT and mortality. Hence, we conclude that this type of misclassification of exposure is unlikely to reverse the true trend of the association of low and high values of HCT with mortality.71 Furthermore, the association with mortality of low and high values of HCT obtained from single measurements can also be important per se, because these HCT levels are those usually measured in clinical practice. Additionally, the distribution of HCT values might differ in participants and non-participants in the study. We cannot examine this because we do not have HCT values for non-participants. However, with regard to the potential role of non-participation on the observed associations, these associations could have arisen spuriously if non-participants with low or high HCT had much greater longevities or non-participants with normal values of HCT had much shorter longevities than participants with similar HCT levels. Both scenarios seem unlikely.
In conclusion, we report a U-shaped relationship between HCT and mortality in which both low and high values of HCT are associated with increased overall mortality as well as with increased mortality from CVD and cancer. We have been able to demonstrate this relationship across a very wide range of HCT values for both men and women. These results are of particular importance for other low- and middle-income countries, where a substantial proportion of the population has suboptimal values of HCT.
Supplementary Data
Supplementary Data are available at IJE online.
Funding
The Golestan Cohort Study was supported by Tehran University of Medical Sciences (grant number: 81/15), Cancer Research UK (grant number: C20/A5860), the Intramural Research Program of the US National Cancer Institute, and various collaborative research agreements with the International Agency for Research on Cancer. R. Vedanthan is supported by Grant Number K01TW009218 from the Fogarty International Center, NIH.
Supplementary Material
Acknowledgements
Paolo Boffetta and Farhad Islami will act as guarantors for the paper.
Conflict of interest: None declared.
KEY MESSAGES.
Few studies have investigated the correlation between a wide range of low and high values of haematocrit (HCT) and mortality in the general population.
We found a U-shaped relationship between categories of HCT and mortality in both sexes, with both low and high values of HCT being associated with increased overall mortality.
These results may be of particular importance for low- and middle-income countries, where a substantial proportion of the population lives with suboptimal values of HCT.
References
- 1.Spivak JL. Anemia in the elderly: time for new blood in old vessels? Arch Intern Med. 2005;165:2187–89. doi: 10.1001/archinte.165.19.2187. [DOI] [PubMed] [Google Scholar]
- 2.Shavelle RM, Mackenzie R, Paculdo DR. Anemia and mortality in older persons: does the type of anemia affect survival? Int J Hematol. 2012;95:248–56. doi: 10.1007/s12185-012-1007-z. [DOI] [PubMed] [Google Scholar]
- 3.Culleton BF, Manns BJ, Zhang J, Tonelli M, Klarenbach S, Hemmelgarn BR. Impact of anemia on hospitalization and mortality in older adults. Blood. 2006;107:3841–46. doi: 10.1182/blood-2005-10-4308. [DOI] [PubMed] [Google Scholar]
- 4.Al Ahmad A, Rand WM, Manjunath G, et al. Reduced kidney function and anemia as risk factors for mortality in patients with left ventricular dysfunction. J Am Coll Cardiol. 2001;38:955–62. doi: 10.1016/s0735-1097(01)01470-x. [DOI] [PubMed] [Google Scholar]
- 5.Volkova N, Arab L. Evidence-based systematic literature review of hemoglobin/hematocrit and all-cause mortality in dialysis patients. Am J Kidney Dis. 2006;47:24–36. doi: 10.1053/j.ajkd.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Groenveld HF, Januzzi JL, Damman K, et al. Anemia and mortality in heart failure patients a systematic review and meta-analysis. J Am Coll Cardiol. 2008;52:818–27. doi: 10.1016/j.jacc.2008.04.061. [DOI] [PubMed] [Google Scholar]
- 7.van Veldhuisen DJ, Anker SD, Ponikowski P, Macdougall IC. Anemia and iron deficiency in heart failure: mechanisms and therapeutic approaches. Nat Rev Cardiol. 2011;8:485–93. doi: 10.1038/nrcardio.2011.77. [DOI] [PubMed] [Google Scholar]
- 8.Kato I, Nomura A, Stemmermann GN, Chyou PH. Prediagnostic hematocrit values and subsequent cancer risk. Cancer Epidemiol Biomarkers Prev. 1991;1:51–55. [PubMed] [Google Scholar]
- 9.Belperio PS, Rhew DC. Prevalence and outcomes of anemia in individuals with human immunodeficiency virus: a systematic review of the literature. Am J Med. 2004;116(Suppl 7A):S27–S43. doi: 10.1016/j.amjmed.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Zon LI, Groopman JE. Hematologic manifestations of the human immune deficiency virus (HIV) Semin Hematol. 1988;25:208–18. [PubMed] [Google Scholar]
- 11.Wilson A, Yu HT, Goodnough LT, Nissenson AR. Prevalence and outcomes of anemia in rheumatoid arthritis: a systematic review of the literature. Am J Med. 2004;116(Suppl 7A):S50–S57. doi: 10.1016/j.amjmed.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Furst DE, Chang H, Greenberg JD, et al. Prevalence of low hemoglobin levels and associations with other disease parameters in rheumatoid arthritis patients: evidence from the CORRONA registry. Clin Exp Rheumatol. 2009;27:560–66. [PubMed] [Google Scholar]
- 13.Wilson A, Reyes E, Ofman J. Prevalence and outcomes of anemia in inflammatory bowel disease: a systematic review of the literature. Am J Med. 2004;116(Suppl 7A):S44–S49. doi: 10.1016/j.amjmed.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 14.Danesh J, Collins R, Peto R, Lowe GD. Haematocrit, viscosity, erythrocyte sedimentation rate: meta-analyses of prospective studies of coronary heart disease. Eur Heart J. 2000;21:515–20. doi: 10.1053/euhj.1999.1699. [DOI] [PubMed] [Google Scholar]
- 15.Kunnas T, Solakivi T, Huuskonen K, Kalela A, Renko J, Nikkari ST. Hematocrit and the risk of coronary heart disease mortality in the TAMRISK study, a 28-year follow-up. Prev Med. 2009;49:45–47. doi: 10.1016/j.ypmed.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 16.Campbell MJ, Elwood PC, Mackean J, Waters WE. Mortality, haemoglobin level and haematocrit in women. J Chronic Dis. 1985;38:881–89. doi: 10.1016/0021-9681(85)90113-4. [DOI] [PubMed] [Google Scholar]
- 17.Islami F, Kamangar F, Nasrollahzadeh D, et al. Socio-economic status and oesophageal cancer: results from a population-based case-control study in a high-risk area. Int J Epidemiol. 2009;38:978–88. doi: 10.1093/ije/dyp195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Semnani S, Sadjadi A, Fahimi S, et al. Declining incidence of esophageal cancer in the Turkmen Plain, eastern part of the Caspian Littoral of Iran: a retrospective cancer surveillance. Cancer Detect Prev. 2006;30:14–19. doi: 10.1016/j.cdp.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Pourshams A, Khademi H, Malekshah AF, et al. Cohort Profile: The Golestan Cohort Study–a prospective study of oesophageal cancer in northern Iran. Int J Epidemiol. 2010;39:52–59. doi: 10.1093/ije/dyp161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bahrami H, Sadatsafavi M, Pourshams A, et al. Obesity and hypertension in an Iranian cohort study; Iranian women experience higher rates of obesity and hypertension than American women. BMC Public Health. 2006;6:158. doi: 10.1186/1471-2458-6-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Islami F, Malekshah AF, Kimiagar M, et al. Patterns of food and nutrient consumption in northern Iran, a high-risk area for esophageal cancer. Nutr Cancer. 2009;61:475–83. doi: 10.1080/01635580902803735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khademi H, Malekzadeh R, Pourshams A, et al. Opium use and mortality in Golestan Cohort Study: prospective cohort study of 50 000 adults in Iran. BMJ. 2012;344:e2502. doi: 10.1136/bmj.e2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khademi H, Etemadi A, Kamangar F, et al. Verbal autopsy: reliability and validity estimates for causes of death in the Golestan Cohort Study in Iran. PLoS One. 2010;5:e11183. doi: 10.1371/journal.pone.0011183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abnet CC, Saadatian-Elahi M, Pourshams A, et al. Reliability and validity of opiate use self-report in a population at high risk for esophageal cancer in Golestan, Iran. Cancer Epidemiol Biomarkers Prev. 2004;13:1068–70. [PubMed] [Google Scholar]
- 25.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 26.de Benoist B, McLean E, Egli I, Cogswell M, editors. Worldwide Prevalence of Anaemia 1993–2005: WHO Global Database on Anaemia. Geneva: World Health Organization; 2008. [Google Scholar]
- 27.Patnaik MM, Tefferi A. The complete evaluation of erythrocytosis: congenital and acquired. Leukemia. 2009;23:834–44. doi: 10.1038/leu.2009.54. [DOI] [PubMed] [Google Scholar]
- 28.Beutler E, Waalen J. The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood. 2006;107:1747–50. doi: 10.1182/blood-2005-07-3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hematocrit. MedlinePlus, a service of the US National Library of Medicine. Available at: http://www.nlm.nih.gov/medlineplus/ency/article/003646.htm (1 June 2012, date last accessed)
- 30.Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol. 1999;28:964–74. doi: 10.1093/ije/28.5.964. [DOI] [PubMed] [Google Scholar]
- 31.Sharma R, Francis DP, Pitt B, Poole-Wilson PA, Coats AJ, Anker SD. Haemoglobin predicts survival in patients with chronic heart failure: a substudy of the ELITE II trial. Eur Heart J. 2004;25:1021–28. doi: 10.1016/j.ehj.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 32.Mozaffarian D, Nye R, Levy WC. Anemia predicts mortality in severe heart failure: the prospective randomized amlodipine survival evaluation (PRAISE) J Am Coll Cardiol. 2003;41:1933–39. doi: 10.1016/s0735-1097(03)00425-x. [DOI] [PubMed] [Google Scholar]
- 33.Elwood PC, Waters WE, Benjamin IT, Sweetnam PM. Mortality and anaemia in women. Lancet. 1974;303:891–94. doi: 10.1016/s0140-6736(74)90346-8. [DOI] [PubMed] [Google Scholar]
- 34.Rossiter CE. Maternal mortality. BJOG. 1985;92(Suppl 5):100–15. [Google Scholar]
- 35.Gagnon DR, Zhang TJ, Brand FN, Kannel WB. Hematocrit and the risk of cardiovascular disease–the Framingham study: a 34-year follow-up. Am Heart J. 1994;127:674–82. doi: 10.1016/0002-8703(94)90679-3. [DOI] [PubMed] [Google Scholar]
- 36.Brown DW, Giles WH, Croft JB. Hematocrit and the risk of coronary heart disease mortality. Am Heart J. 2001;142:657–63. doi: 10.1067/mhj.2001.118467. [DOI] [PubMed] [Google Scholar]
- 37.Kiyohara Y, Ueda K, Hasuo Y, et al. Hematocrit as a risk factor of cerebral infarction: long-term prospective population survey in a Japanese rural community. Stroke. 1986;17:687–92. doi: 10.1161/01.str.17.4.687. [DOI] [PubMed] [Google Scholar]
- 38.Artero A, Tarin JJ, Cano A. The adverse effects of estrogen and selective estrogen receptor modulators on hemostasis and thrombosis. Semin Thromb Hemost. 2012;38:797–807. doi: 10.1055/s-0032-1328883. [DOI] [PubMed] [Google Scholar]
- 39.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 40.Nelson HD, Walker M, Zakher B, Mitchell J. Rockville (MD): Menopausal hormone therapy for the primary prevention of chronic conditions: systematic reveiw to update the 2002 and 2005 U.S. Preventive Services Task Force recommendations. Agency for Healthcare Research and Quality (US) Report No.: 12-05168-EF-1; 2012. Available at: http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0046753/ [PubMed] [Google Scholar]
- 41.Pappa T, Alevizaki M. Endogenous sex steroids and cardio- and cerebro-vascular disease in the postmenopausal period. Eur J Endocrinol. 2012;167:145–56. doi: 10.1530/EJE-12-0215. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y, Zeleniuch-Jacquotte A, Arslan AA, et al. Endogenous hormones and coronary heart disease in postmenopausal women. Atherosclerosis. 2011;216:414–19. doi: 10.1016/j.atherosclerosis.2011.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scarabin-Carre V, Canonico M, Brailly-Tabard S, et al. High level of plasma estradiol as a new predictor of ischemic arterial disease in older postmenopausal women: the three-city cohort study. J Am Heart Assoc. 2012;1:e001388. doi: 10.1161/JAHA.112.001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rossouw JE, Prentice RL, Manson JE, et al. Relationships of coronary heart disease with 27-hydroxycholesterol, low-density lipoprotein cholesterol, and menopausal hormone therapy. Circulation. 2012;126:1577–86. doi: 10.1161/CIRCULATIONAHA.112.103218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skretteberg PT, Bodegard J, Kjeldsen SE, et al. Interaction between inflammation and blood viscosity predicts cardiovascular mortality. Scand Cardiovasc J. 2010;44:107–12. doi: 10.1080/14017430903171248. [DOI] [PubMed] [Google Scholar]
- 46.Wannamethee SG, Lowe GD, Shaper AG, Rumley A, Lennon L, Whincup PH. Associations between cigarette smoking, pipe/cigar smoking, and smoking cessation, and haemostatic and inflammatory markers for cardiovascular disease. Eur Heart J. 2005;26:1765–73. doi: 10.1093/eurheartj/ehi183. [DOI] [PubMed] [Google Scholar]
- 47.Quaknine-Orlando B, Samama CM, Riou B, et al. Role of the hematocrit in a rabbit model of arterial thrombosis and bleeding. Anesthesiology. 1999;90:1454–61. doi: 10.1097/00000542-199905000-00031. [DOI] [PubMed] [Google Scholar]
- 48.Karino T, Goldsmith HL. Role of blood cell-wall interactions in thrombogenesis and atherogenesis: a microrheological study. Biorheology. 1984;21:587–601. doi: 10.3233/bir-1984-21417. [DOI] [PubMed] [Google Scholar]
- 49.Brabin BJ, Premji Z, Verhoeff F. An analysis of anemia and child mortality. J Nutr. 2001;131:S636–S645. doi: 10.1093/jn/131.2.636S. [DOI] [PubMed] [Google Scholar]
- 50.Wu WC, Schifftner TL, Henderson WG, et al. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. JAMA. 2007;297:2481–88. doi: 10.1001/jama.297.22.2481. [DOI] [PubMed] [Google Scholar]
- 51.Shah AD, Nicholas O, Timmis AD, et al. Threshold haemoglobin levels and the prognosis of stable coronary disease: two new cohorts and a systematic review and meta-analysis. PLoS Med. 2011;8:e1000439. doi: 10.1371/journal.pmed.1000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laulund AS, Lauritzen JB, Duus BR, Mosfeldt M, Jorgensen HL. Routine blood tests as predictors of mortality in hip fracture patients. Injury. 2012;43:1014–20. doi: 10.1016/j.injury.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 53.Jimenez D, Escobar C, Marti D, et al. Association of anaemia and mortality in patients with acute pulmonary embolism. Thromb Haemost. 2009;102:153–58. doi: 10.1160/TH09-01-0003. [DOI] [PubMed] [Google Scholar]
- 54.Metivier F, Marchais SJ, Guerin AP, Pannier B, London GM. Pathophysiology of anaemia: focus on the heart and blood vessels. Nephrol Dial Transplant. 2000;15(Suppl 3):14–18. doi: 10.1093/oxfordjournals.ndt.a027970. [DOI] [PubMed] [Google Scholar]
- 55.Hare GM. Anaemia and the brain. Curr Opin Anaesthesiol. 2004;17:363–9. doi: 10.1097/00001503-200410000-00003. [DOI] [PubMed] [Google Scholar]
- 56.Smith KJ, Bleyer AJ, Little WC, Sane DC. The cardiovascular effects of erythropoietin. Cardiovasc Res. 2003;59:538–48. doi: 10.1016/s0008-6363(03)00468-1. [DOI] [PubMed] [Google Scholar]
- 57.Balarajan Y, Ramakrishnan U, Ozaltin E, Shankar AH, Subramanian SV. Anaemia in low-income and middle-income countries. Lancet. 2011;378:2123–35. doi: 10.1016/S0140-6736(10)62304-5. [DOI] [PubMed] [Google Scholar]
- 58.Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet. 2007;370:511–20. doi: 10.1016/S0140-6736(07)61235-5. [DOI] [PubMed] [Google Scholar]
- 59.Lutter CK. Iron deficiency in young children in low-income countries and new approaches for its prevention. J Nutr. 2008;138:2523–28. doi: 10.3945/jn.108.095406. [DOI] [PubMed] [Google Scholar]
- 60.Gholamreza V. Anemia in north of Iran (south-east of Caspian Sea) Pak J Biol Sci. 2007;10:1703–07. doi: 10.3923/pjbs.2007.1703.1707. [DOI] [PubMed] [Google Scholar]
- 61.McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993-2005. Public Health Nutr. 2009;12:444–54. doi: 10.1017/S1368980008002401. [DOI] [PubMed] [Google Scholar]
- 62.Zacharski LR, Chow BK, Howes PS, et al. Reduction of iron stores and cardiovascular outcomes in patients with peripheral arterial disease: a randomized controlled trial. JAMA. 2007;297:603–10. doi: 10.1001/jama.297.6.603. [DOI] [PubMed] [Google Scholar]
- 63.Zacharski LR, Shamayeva G, Chow BK. Effect of controlled reduction of body iron stores on clinical outcomes in peripheral arterial disease. Am Heart J. 2011;162:949–57. doi: 10.1016/j.ahj.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 64.Salonen JT, Tuomainen TP, Salonen R, Lakka TA, Nyyssonen K. Donation of blood is associated with reduced risk of myocardial infarction. The Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Epidemiol. 1998;148:445–51. doi: 10.1093/oxfordjournals.aje.a009669. [DOI] [PubMed] [Google Scholar]
- 65.Fernandez-Real JM, Penarroja G, Castro A, Garcia-Bragado F, Hernandez-Aguado I, Ricart W. Blood letting in high-ferritin type 2 diabetes: effects on insulin sensitivity and beta-cell function. Diabetes. 2002;51:1000–04. doi: 10.2337/diabetes.51.4.1000. [DOI] [PubMed] [Google Scholar]
- 66.Meyers DG, Jensen KC, Menitove JE. A historical cohort study of the effect of lowering body iron through blood donation on incident cardiac events. Transfusion. 2002;42:1135–39. doi: 10.1046/j.1537-2995.2002.00186.x. [DOI] [PubMed] [Google Scholar]
- 67.Ascherio A, Rimm EB, Giovannucci E, Willett WC, Stampfer MJ. Blood donations and risk of coronary heart disease in men. Circulation. 2001;103:52–57. doi: 10.1161/01.cir.103.1.52. [DOI] [PubMed] [Google Scholar]
- 68.Jiang R, Ma J, Ascherio A, Stampfer MJ, Willett WC, Hu FB. Dietary iron intake and blood donations in relation to risk of type 2 diabetes in men: a prospective cohort study. Am J Clin Nutr. 2004;79:70–75. doi: 10.1093/ajcn/79.1.70. [DOI] [PubMed] [Google Scholar]
- 69.Edgren G, Reilly M, Hjalgrim H, et al. Donation frequency, iron loss, and risk of cancer among blood donors. J Natl Cancer Inst. 2008;100:572–79. doi: 10.1093/jnci/djn084. [DOI] [PubMed] [Google Scholar]
- 70.Menke A, Muntner P, Fernandez-Real JM, Guallar E. The association of biomarkers of iron status with mortality in US adults. Nutr Metab Cardiovasc Dis. 2012;22:734–40. doi: 10.1016/j.numecd.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weinberg CR, Umbach DM, Greenland S. When will nondifferential misclassification of an exposure preserve the direction of a trend? Am J Epidemiol. 1994;140:565–71. doi: 10.1093/oxfordjournals.aje.a117283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.