Abstract
Background At the APOE gene, encoding apolipoprotein E, genotypes of the ε2/ε3/ε4 alleles associated with higher LDL-cholesterol (LDL-C) levels are also associated with higher coronary risk. However, the association of APOE genotype with other cardiovascular biomarkers and risk of ischaemic stroke is less clear. We evaluated the association of APOE genotype with risk of ischaemic stroke and assessed whether the observed effect was consistent with the effects of APOE genotype on LDL-C or other lipids and biomarkers of cardiovascular risk.
Methods We conducted a systematic review of published and unpublished studies reporting on APOE genotype and ischaemic stroke. We pooled 41 studies (with a total of 9027 cases and 61 730 controls) using a Bayesian meta-analysis to calculate the odds ratios (ORs) for ischaemic stroke with APOE genotype. To better evaluate potential mechanisms for any observed effect, we also conducted a pooled analysis of primary data using 16 studies (up to 60 883 individuals) of European ancestry. We evaluated the association of APOE genotype with lipids, other circulating biomarkers of cardiovascular risk and carotid intima-media thickness (C-IMT).
Results The ORs for association of APOE genotypes with ischaemic stroke were: 1.09 (95% credible intervals (CrI): 0.84–1.43) for ε2/ε2; 0.85 (95% CrI: 0.78–0.92) for ε2/ε3; 1.05 (95% CrI: 0.89–1.24) for ε2/ε4; 1.05 (95% CrI: 0.99–1.12) for ε3/ε4; and 1.12 (95% CrI: 0.94–1.33) for ε4/ε4 using the ε3/ε3 genotype as the reference group. A regression analysis that investigated the effect of LDL-C (using APOE as the instrument) on ischaemic stroke showed a positive dose-response association with an OR of 1.33 (95% CrI: 1.17, 1.52) per 1 mmol/l increase in LDL-C. In the separate pooled analysis, APOE genotype was linearly and positively associated with levels of LDL-C (P-trend: 2 × 10−152), apolipoprotein B (P-trend: 8.7 × 10−06) and C-IMT (P-trend: 0.001), and negatively and linearly associated with apolipoprotein E (P-trend: 6 × 10−26) and HDL-C (P-trend: 1.6 × 10−12). Associations with lipoprotein(a), C-reactive protein and triglycerides were non-linear.
Conclusions In people of European ancestry, APOE genotype showed a positive dose-response association with LDL-C, C-IMT and ischaemic stroke. However, the association of APOE ε2/ε2 genotype with ischaemic stroke requires further investigation. This cross-domain concordance supports a causal role of LDL-C on ischaemic stroke.
Keywords: Stroke, lipids, apolipoprotein E, cardiovascular disease, systematic review, meta-analysis, biomarkers
Introduction
Worldwide, ischaemic stroke is the second leading cause of death after coronary heart disease (CHD) and is an important cause of disability, with a high burden of disease in low- and middle-income countries.1,2 Ischaemic stroke and CHD share several risk factors, including increasing age, high blood pressure, smoking and diabetes. However, although low-density lipoprotein cholesterol (LDL-C) is a known risk factor for CHD, its association with ischaemic stroke is uncertain,3 despite the fact that LDL-C-lowering statin drugs have been found to reduce both ischaemic stroke and CHD risk.4 This has prompted speculation that the beneficial effect of statins on ischaemic stroke risk might be mediated through alternative pathways.5
Numerous studies have examined associations of common genetic variants of the apolipoprotein E (APOE) gene and cardiovascular outcomes, including ischaemic stroke and CHD. The APOE gene has two common non-synonymous polymorphisms, rs429358 and rs7412, that together give rise to three distinct APOE ‘alleles’ (ε2, ε3, ε4); and these alleles in turn form six possible APOE ‘genotypes’ (ε2/ε2, ε2/ε3, ε2/ε4, ε3/ε3, ε3/ε4 and ε4/ε4).6
As confirmed by recent genome-wide association studies (GWAS), variants at the APOE locus are one of the strongest signals for LDL-C,7–10and follow a positive dose-response association (when ordered ε2/ε2, ε2/ε3, ε2/ε4, ε3/ε3, ε3/ε4 and ε4/ε4).
In addition to LDL-C, APOE genotype has been associated with high-density lipoprotein cholesterol (HDL-C) and triglycerides, though the magnitude and dose-response relationships appear to differ from those described for LDL-C.11 In addition, APOE genotype has been reported to be associated with some inflammatory biomarker such as C-reactive protein (CRP), mainly through the ε4 allele,12–16 but its association with other inflammatory and coagulation biomarkers is less clear.17,18 Evidence from a large meta-analysis showed that APOE genotype follows a positive dose-response association with CHD concordant with that observed for LDL-C, supporting a causal role of LDL-C in CHD.11 However, the association of APOE genotype with risk of ischaemic stroke is less clear and cannot be reliably extrapolated from the association with CHD. The largest published systematic review on APOE genotype and ischaemic stroke was inconclusive with only a tentative evidence for an association of the ε4 allele with high risk of ischaemic stroke.19 Furthermore, this analysis was based on ε2 and ε4 carriers, whereas an analysis based on all six APOE genotypes is needed to estimate the genotype dose-response association. A similar limitation relates to the APOE association with carotid intima-media thickness (C-IMT), a non-invasive measure of atherosclerosis linked to both CHD and ischaemic stroke risk, that is used as a surrogate outcome in randomised trials.20,21
Genome wide-association studies of ischaemic stroke and C-IMT have not identified any associations with genetic variation at the APOE locus.22,23 However, coverage of the single nucleotide polymorphisms (SNPs) contributing to or marking the APOE ε2/ε3/ε4 genotype is incomplete in arrays used in GWAS, leading to uncertainty regarding the association of APOE genotype with C-IMT and stroke risk. We therefore undertook a systematic review and meta-analysis to examine the association between APOE genotype and ischaemic stroke risk, including twice as many cases as in the previous largest meta-analysis.19 The large data set allowed the evaluation of the risk of ischaemic stroke conferred by each of the six APOE genotypes individually, as well as the trend in risk across the genotype categories, which has not been possible in prior studies. In addition, to evaluate the potential mechanisms for any observed effect, we undertook a separate pooled analysis of primary data on the relationship between APOE genotype and a wide range of lipids and inflammation and coagulation markers as well as C-IMT.
Methods
Two separate meta-analyses were performed to test (i) the association of APOE genotype with cardiovascular traits and C-IMT, and (ii) association of APOE with ischaemic stroke using both published and unpublished studies.
APOE genotype, cardiovascular traits and C-IMT
We developed a collaboration of 13 studies of European ancestry with information on the association of APOE genotypes with a wide range of blood lipids and apolipoproteins; inflammation, coagulation and metabolic markers; and C-IMT. The studies included were the English Longitudinal Study of Ageing (ELSA),24 the Northwick Park Heart Study II (NPHS II),25 the Whitehall II study (WHII),26 the Edinburgh Artery Study (EAS),27 the British Regional Heart Study (BRHS),28 the British Women’s Heart and Health Study (BWHHS),29 the Caerphilly and Speedwell studies (CaPS),30 the UCL Diabetes and Cardiovascular disease Study (UDACS) study,31 the Aspirin for Asymptomatic Atherosclerosis trial (AAAT),32 the European Atherosclerosis Research Study (EARS) I and II,33 the Czech sub-study from the World Health Organization (WHO) Multinational Monitoring of Trends and Determinants in Cardiovascular Disease Study (Czech-MONICA)34 and the Uppsala Longitudinal Study of Adult Men (ULSAM).35 These data were supplemented by published data for the European Prospective Investigation of Cancer Norfolk study (EPIC-N),36 Perth Carotid Ultrasound Disease Assessment Study (CUDAS)37 and the Rotterdam Study.38 Data request forms were based on a pre-specified analysis plan and did not include adjustment for lipid-lowering treatment. In a sub-sample of nine studies (11528 individuals), adjustment for age and gender for the APOE-LDL-C association was available. The complete data set comprised of 60 883 individuals (Table 1).
Table 1.
Studies contributing to the analysis of APOE genotypes and cardiovascular biomarkers
| Study (ref) | ELSA24 | NPHS-II25 | WHII26 | UDACS31 | EAS27 | AAAT32 | EARSI33 | EARSII33 | MONICA (CZECH)34 | ULSAM35 | BRHS28 | BWHHS54 | CaPS30b | EPIC Norfolk36 | CUDAS37b | Rotterdam38b |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study designa | P | P | P | CS | P | P | CC | CC | P | P | P | P | P | P | CS | P |
| Sampling frame | Respondents of HSE | General practices | Workplace | Diabetic patients | General practices | General practices | Universities | Universities | Administrative districts | Health survey | General practices | General practices | General practices | General Practices | Community Population | Administrative district |
| N with DNA | 5274 | 2775 | 5500 | 575 | 940 | 2833 | 1881 | 779 | 2562 | 879 | 3947 | 3500 | 1500 | ∼22 915 | 1111 | 5401 |
| % men | 48 | 100 | 77 | 59 | 50 | 28 | 51 | 100 | 46.5 | 100 | 100 | 0 | 100 | 46 | 50 | 59 |
| Year of DNA measurementc | 2004 | 1989–94 | 2002–04 | 2001–02 | 2004 | 1998 | 1990–91 | 1993–94 | 1997–98 & 2000–01 | 2004 | 1998–2000 | 1999–2001 | 1993–94 | 1997–2000 | 1995–96 | 1992 |
| Baseline year | 1998, 1999, 2001 | 1989–94 | 1985–88 | 2001 | 1987 | 1998 | 1990–91 | 1993–94 | 1997 | 1970–73 | 1978–80 | 1999–2001 | 1979–83 | 1993 | 1995–96 | 1990–93 |
| Physical measures | ||||||||||||||||
| BMI | 5274 | 2682 | 5591 | 544 | 904 | 1891 | 763 | 2562 | 874 | 3514 | 1317 | |||||
| Systolic BP | 5274 | 2683 | 5592 | 547 | 903 | 2397 | 1891 | 765 | 2562 | 878 | 3511 | 1331 | ||||
| Diastolic BP | 5274 | 2682 | 5592 | 547 | 901 | 2394 | 1891 | 765 | 2562 | 878 | 3511 | 1330 | ||||
| Lifestyle measures | ||||||||||||||||
| Alcohol | 2685 | 904 | 1888 | 764 | 3464 | 1332 | ||||||||||
| Blood measures | ||||||||||||||||
| Total-C | 5274 | 2662 | 5573 | 547 | 903 | 2385 | 1891 | 753 | 2562 | 879 | 3497 | 3240 | 1296 | 22 915 | ||
| LDL-C | 1677 | 5097 | 533 | 898 | 1887 | 742 | 874 | 3418 | 3161 | 1295 | 22 915 | |||||
| HDL-C | 5274 | 1774 | 898 | 1887 | 741 | 2562 | 877 | 3386 | 3236 | 1296 | 22 915 | |||||
| Triglycerides | 5274 | 2664 | 5196 | 547 | 903 | 1891 | 753 | 2562 | 879 | 2433 | 3239 | 1296 | ||||
| ApoAI | 2287 | 1855 | 765 | 357 | 1290 | |||||||||||
| ApoB | 2287 | 383 | 1853 | 765 | 357 | 1286 | ||||||||||
| ApoE | 5195 | 1881 | 765 | |||||||||||||
| Lp(a) | 2230 | 576 | 1860 | 357 | 1249 | |||||||||||
| Apo-AII | 1291 | |||||||||||||||
| Homocysteine | 1311 | 757 | 3456 | 1291 | ||||||||||||
| Glucose metabolism | ||||||||||||||||
| HbA1c | 5274 | 5547 | 544 | 3471 | 3154 | |||||||||||
| Glucose | 5274 | 4977 | 547 | 902 | 1890 | 738 | 2562 | 878 | 3495 | 3221 | 1297 | |||||
| Insulin | 4635 | 1766 | 707 | 867 | 2410 | 3303 | 672 | |||||||||
| Coagulation factors/markers | ||||||||||||||||
| Viscosity | 863 | 2383 | 3093 | 1286 | ||||||||||||
| Tp(a) | 166 | 767 | 3513 | 3250 | 1212 | |||||||||||
| vWF | 171 | 819 | 3513 | 3251 | 1178 | |||||||||||
| D-Dimer | 171 | 816 | 3509 | 3251 | 1178 | |||||||||||
| Factor VII | 2673 | 578 | 1624 | 3504 | 3234 | 934 | ||||||||||
| Prothrombin | 2658 | 572 | 901 | |||||||||||||
| Fib peptide A | 2658 | 899 | ||||||||||||||
| Inflammation markers | ||||||||||||||||
| Fibrinogen | 5274 | 2670 | 4879 | 885 | 2185 | 1697 | 334 | 3511 | 3242 | 1284 | ||||||
| I-CAM | 710 | |||||||||||||||
| V-CAM | 709 | |||||||||||||||
| E-selectin | 708 | |||||||||||||||
| IL-6 | 4893 | 537 | 635 | 2032 | 3253 | 767 | ||||||||||
| CRP | 5274 | 4927 | 536 | 611 | 2027 | 657 | 2562 | 3156 | 3506 | |||||||
| Ferritin | 5274 | |||||||||||||||
| Renal function | ||||||||||||||||
| Creatinine | 2664 | 543 | 861 | 3240 | 1284 | |||||||||||
| Macrovascular function/structure | ||||||||||||||||
| Carotid IMT | 3890 | 837 | 408 | 1105 | 5401 | |||||||||||
Empty cells denote data not applicable or not available.
aStudy design: P, prospective; CS, cross sectional; CC, case control.
bFrom published data.
cYear of measurement is the year when blood for DNA was obtained. The cardiovascular biomarker measurements are in general contemporaneous with this time.
Collectively the studies included information on several traits that were selected to interrogate the mechanisms by which variants at the APOE locus might exert their effects on ischaemic stroke. These were divided in two groups according to the level of evidence for their associations with APOE genotype. Traits with prior evidence for an association were lipids and apolipoproteins [total cholesterol, LDL-C, HDL-C, triglycerides (TG), apolipoprotein A1 (apoA1), apoB, apoE, and lipoprotein (a) (Lp(a)], inflammatory markers [CRP, fibrinogen, interleukin-6 (IL-6)] and C-IMT. Traits with unclear evidence for an association were coagulation factors [Factor VII, fibrinopeptide A, plasma viscosity, prothrombin fragment 1 + 2, tissue plasminogen activator (tPA), D-dimer, von Willebrand factor (vWF)), metabolic traits (homocysteine, insulin, glycated haemoglobin A1c (HbA1c), and creatinine], and some additional inflammatory markers (E-selectin, ICAM, VCAM and ferritin). In addition, we also included lifestyle factors (alcohol and BMI) and established risk factors for cardiovascular disease (blood pressure and glucose). For these traits, we did not anticipate an association with APOE genotypes, but their assessment served to confirm protection from confounding of the genetic associations as a result of randomized inheritance of parental alleles (Mendel’s second law).39 Further details of the studies and measures are in Table 1 and Supplementary Table S1 (available as Supplementary data at IJE online).
Statistical analysis: APOE, cardiovascular traits and C-IMT
ApoB, apoE, plasma viscosity, Lp(a), homocysteine, TG, IL-6 and CRP exhibited a skewed distribution and were natural log transformed. In each study, we calculated the mean and standard deviation for each APOE genotype group, and used fixed effects meta-analyses to obtain the standardized mean difference (SMD), weighted mean difference (WMD) and their 95% confidence interval (CI), respectively, for each trait by genotype using the common ε3/ε3 genotype as the reference. Random effect meta-analysis was also conducted as a subsidiary analysis and is reported in Supplementary Table S2, available as Supplementary data at IJE online.40 Data are presented in the order ε2/ε2, ε2/ε3, ε2/ε4, ε3/ε3, ε3/ε4 and ε4/ε4. I2 (and its 95% CI) were calculated for each comparison.41 Because of the multiple comparisons (5 genotype comparisons for 32 traits) we also estimated corrected P-values for each estimate using the Sidak method.42 In order to explore the potential effect that age and gender may have on the association of APOE genotypes with LDL-C, we used a sub-sample of nine studies (11 708 participants) and estimated the effect of the APOE genotypes with and without adjustment for age and gender. Given that studies included in the analysis span a long calendar period (1990 to 2004), we also stratified the studies in two equal time periods (before and after 1998) to explore the potential effect that year of collection of blood samples from which DNA was extracted may have on the APOE genotype-LDL-C association.
To evaluate a linear trend, meta-regression analysis was used on the summary estimated mean differences for each genotype using an additive model.43,44 The standard normal distribution was used to calculate P-values. This analysis was conducted using Stata software (version 11.2).
Meta-analysis of the of APOE genotype with ischaemic stroke
Search strategy
Following the HuGE Review Guidelines update,45 we searched EMBASE and MEDLINE via PubMed up to March 2011 for articles in any language restricted to ‘humans’ using free text and MeSH expansion of the following terms: apoe, apo e, apolipoprotein e, brain infarction, cerebrovascular accident, cerebrovascular disease, and stroke. Additional relevant studies were identified through reference lists of published meta-analyses, review articles and the studies included in the obtained papers.
Case-control and case-cohort studies of unrelated individuals were eligible if ischaemic stroke was verified by computerised tomography (CT) or magnetic resonance imaging (MRI), or if a physician confirmed the diagnosis of stroke, but not if self-reported. Studies reporting white-matter lesions and stroke in children were excluded. We used the largest data set for studies with serial publications and verified the decision with the study authors. We used the control group that most closely resembled the cases where more than one control group was studied. Two reviewers (T.A.K. and J.P.C.) reviewed the papers and consulted a third reviewer (A.D.H.) to resolve any disagreement.
Authors of identified studies were contacted to clarify information on genotype or stroke type where details were incomplete. We requested the following: (i) updated tabular data on six APOE genotypes by case-control status, (ii) blinding of lab staff, (iii) duplicate data in publications, (iv) type of stroke and (v) for studies with multiple ethnic groups, genotype counts were requested separately by ethnic group. Additionally we directly approached 88 studies that had reported on ischaemic stroke, to request information on APOE genotype, of which 38 replied (35 studies with ischaemic stroke and 3 with haemorrhagic stroke data).
Data were extracted into a pre-specified form and entered in Microsoft Excel. Details entered for each study were: study ID, author, year of publication, study location, study name, ethnicity, number of cases, source of cases and definition and exclusion criteria, control numbers, source of control and exclusion criteria, cases and controls matching variables (age and sex), genotype data on cases and controls, genotype frequencies, blinding of genotyping staff to identity of cases and controls, genotyping method, mean age of cases and controls and percentage of males in cases and controls. Any study showing results separately for more than one ethnicity has been counted as a different data set, so total studies represent total number of data sets.
A chi-square test on control group allele frequencies or whole study allele frequencies in cohort studies was used to assess departure from Hardy–Weinberg (HW) equilibrium.
Statistical analysis
We performed a Bayesian meta-analysis to estimate the unadjusted odds ratios (ORs) and 95% credible intervals (CrI) of ischaemic stroke for each genotype (ε2/ε2, ε2/ε3, ε2/ε4, ε3/ε4 and ε4/ε4) against the reference genotype ε3/ε3. This order was decided on the basis of association of APOE genotypes with LDL-C. A Bayesian model was used to estimate the five ORs simultaneously in one model using Markov Chain Monte Carlo techniques46 (for details of the model see Supplementary methods available at IJE online). The Bayesian approach has several advantages: It estimates simultaneously the five ORs mentioned above, taking into the fact that they are not independent from each other. It provides posterior credibility intervals that are more naturally interpreted than frequentist confidence intervals. It also allows for the use of the data coming from small studies without having to add any extra numbers to the situations when zero counts appear in an estimation of an OR.47
To explore the trend of these ORs (if any) we estimated a meta-logistic regression model with ischaemic stroke as the outcome and the APOE genotypes as the explanatory variable (for details of the model see Supplementary methods available at IJE online). APOE genotypes were coded numerically using the mean difference in LDL-C between each genotype and the ε3/3 group, as follows: ε2/2 = −0.756 mmol/l, ε2/3 = −0.481 mmol/l, ε2/4 = −0.241 mmol/l, ε3/3 = 0 mmol/l (reference), ε3/4 = 0.161 mmol/l, ε4/4 = 0.312 mmol/l. This mean difference for LDL-C was derived from our APOE-LDL-C analysis from studies in European ancestry populations. The summary OR derived from the trend analysis could be indirectly interpreted as the effect of LDL-C on ischaemic stroke through the use of APOE ε2/ε3/ε4 loci as the genetic instrument. To allow for a non-constant OR (equivalent to a non-linear trend in the log scale) we included the square of the explanatory variable (i.e. LDL-C2) in the regression model. If the posterior 95% CrI of the coefficient of the quadratic term included the null value, we repeated the analysis simplifying the model by removing the quadratic term and estimating only a linear trend.
We undertook several sensitivity analyses by re-estimating the logistic regression in different subsets of studies considered to be at lower risk of bias. These subsets were: studies with more than 100 cases, studies with control samples in HW equilibrium, studies with no zero cells for all APOE genotype categories, studies with genotyping staff blinded to case-control status, studies with imaging confirmed diagnosis of ischaemic stroke and studies that used new techniques for APOE genotyping (e.g. Taqman genotyping assay, One-tube LightTyper assay, Heteroduplex method, Pyrosequencing) that are less susceptible to genotyping miscalls.48,49 In order to evaluate the potential for publication bias (small-study bias) we, as a sensitivity analysis, re-estimated the effect of the APOE genotype on published versus unpublished studies, excluding small studies and re-quantifying the APOE-effect.
For the above-mentioned analyses we used fixed-effect models for the primary analysis; however, random effect models were also fitted and the results are reported as Supplementary material (available as Supplementary data at IJE online).
Priors
We used priors centred on the null that were non-informative in any specific direction but would cover a reasonable range of hypotheses by restricting their variance, e.g. an OR would have a prior distribution centred on 1, with a 95% probability of being between 0.03 and 30. For parameters in the log scale [log(OR) and log(odds)] we used normal distributions. For the standard deviation between studies in the random effects model we used a uniform prior between 0 and 1.5 times the observed standard deviation between the different studies. Bayesian analyses were performed using R (version 2.13) and JAGS software (version 2.2.0).
Results
APOE genotype, LDL-C and C-IMT
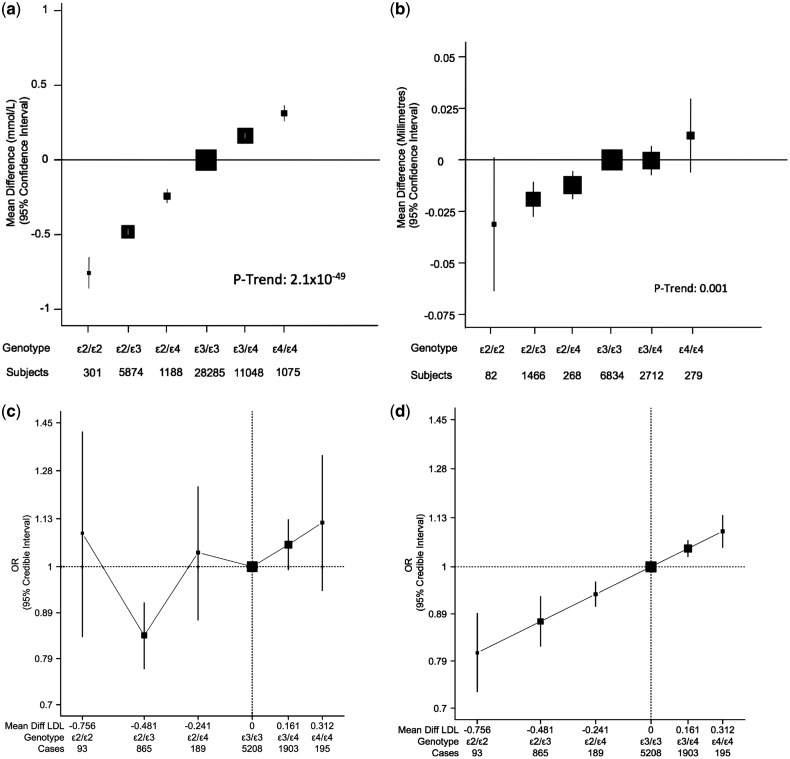
Twelve studies contributed data on APOE genotypes and LDL-C levels (Table 1). APOE genotypes exhibited a positive trend with LDL-C (47 771 subjects, P-trend: 2.1 × 10−49). With ε3/3 as reference, the mean differences (in mmol/l) were: −0.75 (95% CI: −0.86, −0.65) for ε2/2, −0.48 (95% CI −0.50, −0.46) for ε2/3, −0.24 (95% CI: −0.24, −0.01) for ε2/4, 0.16 (95% CI: 0.14, 0.18) for ε3/4 and 0.32 (95% CI: 0.26, 0.37) for ε4/4 (Figure 1a). There was a difference of 1.07 mmol/l between ε2/ε2 and ε4/ε4 individuals for LDL-C. These point estimates were used in the Bayesian meta-analysis to determine the coding of APOE genotypes and therefore to evaluate indirectly the effect of LDL-C on ischaemic stroke using a trend analysis. This positive dose-response between APOE genotypes and LDL-C remained unaltered in the sub-sample of studies that had provided data adjusted by age and gender (Supplementary Figure S1, available as Supplementary data at IJE online). Likewise, when the APOE-LDL-C analysis was stratified according to the time of DNA extraction, similar results were observed (Supplementary Figure S2, available as Supplementary data at IJE online).
Figure 1.
Association of APOE genotypes in studies of European ancestry individuals with (a) LDL-cholesterol (b) carotid intima-media thickness, (c) ischaemic stroke and (d) ischaemic stroke (trend analysis). Black boxes indicate summary estimates with their size proportional to weight. For (c) and (d), the x-axis is plotted in the log-scale with distance between APOE genotypes equal to mean difference of LDL-C in mmol/l. For (d), the effect estimate of trend analysis indicates an OR of 1.33 (95% CrI: 1.17, 1.52) per 1 mmol/l increase in LDL-C
Five studies contributed data on APOE genotypes and C-IMT (Table 1). We found a positive dose-response association between APOE genotypes and C-IMT (11 641 subjects, P-trend: 0.001) that strongly resembled the association between APOE genotype and LDL-C. There was a mean difference of 0.043 mm of C-IMT between ε2/ε2 and ε4/ε4 individuals (Figure 1b).
Association of APOE genotype with other lipids and apolipoproteins
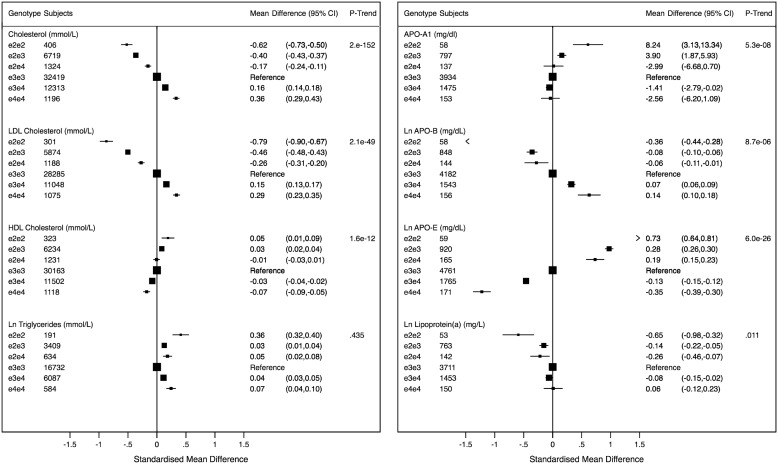
Sixteen studies contributed data on APOE genotypes and cardiovascular phenotypes. APOE genotype exhibited a positive trend with total cholesterol (54 377 subjects, P-trend: 2 × 10−152) and apoB (6931 individuals, P-trend: 8.7 × 10−06), similar to the association with LDL-C described above (Figure 2). We observed a negative trend of APOE genotype with levels of apoE (7841 individuals, P-trend: 6 × 10−26) and a weak negative trend association with HDL-C (50 571 individuals, P-trend: 1.6 × 10−49) and apoAI (6554 individuals, P-trend: 5.3 × 10−8). Associations of APOE genotype with other lipids and lipoproteins were more complex in shape. There was a non-linear relationship between APOE genotype and triglycerides (27 637 individuals), with both ε2/ε2 and ε4/ε4 individuals having higher concentrations of triglycerides, compared with the ε3/ε3 reference group. We also identified an association between APOE genotype and Lp(a) among 6272 individuals (Figure 2).
Figure 2.
APOE genotypes and lipid and apolipoprotein traits. The graphs are displayed in standardized scale to allow comparability and show standardized mean differences of biomarker levels with APOE genotypes with ε3/ε3 as reference. The values (on the right) correspond to absolute weighted mean difference. Black boxes indicate estimates proportional to counts and horizontal lines represent 95% CI. Ln, natural log transformed
Association of APOE genotype with inflammation markers
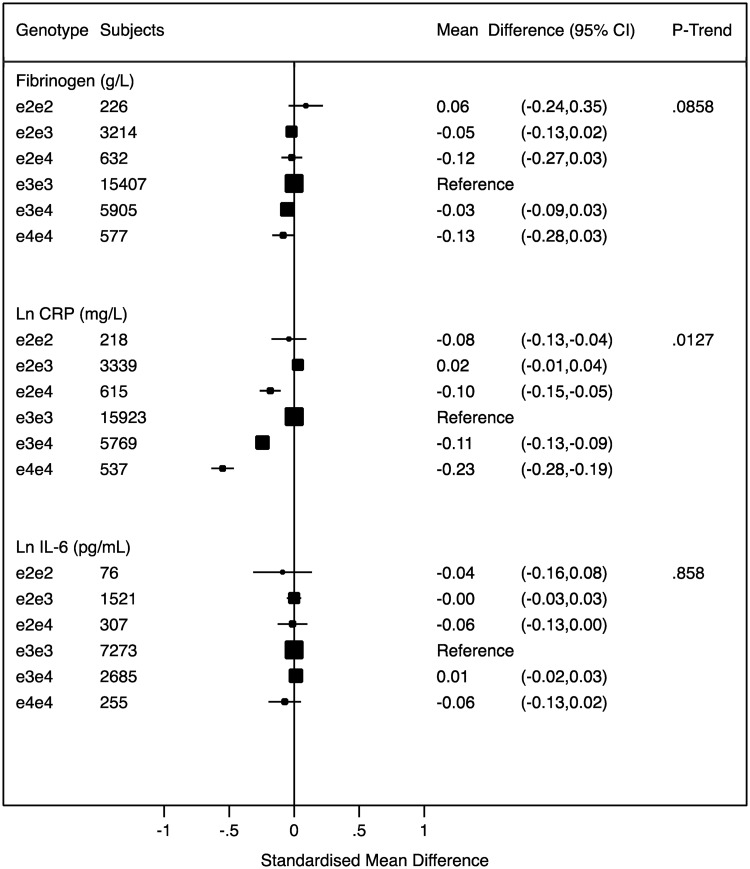
We found a marked association of APOE genotype with CRP concentration (26 401 individuals). With reference to the ε3/ε3 group, individuals with the ε4/ε4 genotype had the lowest concentration of CRP, whereas individuals with the ε2/ε4 genotype and the ε3/ε4 genotype had CRP levels approximately midway between the ε3/ε3 and ε4/ε4 groups. There was no difference in CRP levels among individuals with the ε2/ε2 or ε2/ε3 genotypes. We did not find any association of APOE genotype with another hepatocyte-derived inflammation marker, fibrinogen (25 961 individuals, P-trend: 0.09), with the macrophage/adipocyte-derived inflammation marker IL-6 (12 117 individuals, P-trend: 0.9) (Figure 3), or with a range of other inflammation markers (Supplementary Figure S3, available as Supplementary data at IJE online).
Figure 3.
APOE genotypes and mean differences for inflammatory traits. The graphs are displayed in standardized scale to allow comparability and show standardised mean differences of biomarker levels with APOE genotypes with ε3/ε3 as reference. The values (on the right) correspond to absolute weighted mean difference. Black boxes indicate estimates proportional to counts and horizontal lines represent 95% CI. Ln, natural log transformed
Association of APOE with coagulation markers, metabolic traits and other variables related to cardiovascular risk
We found no evidence for an association of APOE genotype with the coagulation markers factor VII (12 547 individuals), fibrinopeptide A (3 557 individuals), D-dimer (8925 individuals) or tPA (58 908 individuals), the endothelial activation markers vWF (8932 individuals) and E-selectin (708 individuals), systolic blood pressure (28 334 individuals), BMI (25 916 individuals), alcohol consumption (11 037 individuals) or indices of glycaemic control including HbA1c (17 990 individuals), fasting glucose (25 781 individuals) and insulin (14 360 individuals) (Figure S4).
Mean difference (95%CI), I2 (95% CI), unadjusted and Sidak-corrected P-values for each of the APOE genotype associations with cardiovascular traits are provided in Supplementary Table S2 (available as Supplementary data at IJE online).
APOE genotype and ischaemic stroke
Search results
We screened 1395 abstracts from the primary search and identified 249 potentially eligible articles. Of these, 2 studies included self-reported stroke, 7 contained duplicate data and a further 163 reported incomplete data or were not ischaemic stroke, and were excluded, leaving 77 studies reporting stroke that were included in this analysis (Supplementary Figure S5, available as Supplementary data at IJE online). Of these, 64 included information on ischaemic stroke and 19 gave information on haemorrhagic stroke (there was some overlap as 16 studies gave information on both). Thirteen studies reported strokes that were not classified as ischaemic or haemorrhagic. Of these, 10 were studies in European ancestry subjects28,35,36,50–54 (2265 stroke events) exclusively and these were counted as ischaemic stroke since 80% of stroke in European ancestry is ischaemic in nature.2 This took the total number of ischaemic stroke studies to 74 with total of 14 015 cases and 77 888 controls. This included data from 39 published studies 3635 cases and 42 024 controls from previously unpublished studies from 35 from complete unpublished data sets plus data sets updated from previous published studies.
A description of study design, source of controls, use of neuroimaging, genotyping method, blinding, and number of cases and controls, observed and expected genotype and allele frequencies (by ethnic groups) is given in Supplementary Tables S3, S4 and S5, available as Supplementary data at IJE online.
Association of APOE genotype with ischaemic stroke in European ancestry individuals
In 41 studies (14 prospective and 27 case-control studies), with a total of 9027 cases and 61 730 controls of European ancestry using the ε3/ε3 genotype as the reference group, the ORs for ischaemic stroke were 1.09 (95% CrI: 0.84, 1.43) for ε2/ε2, 0.85 (95% CrI: 0.78, 0.92) for ε2/ε3, 1.05 (95% CrI: 0.89, 1.24) for ε2/ε4, 1.05 (95% CrI: 0.99, 1.12) for ε3/ε4, and 1.12 (95% CrI: 0.94, 1.33) for ε4/ε4 (Figure 1c). We note the large uncertainty in OR estimates around the less common genotypes (ε2/ε2, ε2/ε4 and ε4/ε4), and in particular the ε2/ε2 genotype which only contributed 1% of the total cases.
We initially fitted a model that included a quadratic variable (LDL-C2) to allow for a non-linear trend, but the coefficient for this variable had a posterior credibility interval that included the null and we decided to remove this term and estimate a simpler model only with a linear trend. The results of the linear trend of the log-odds of APOE ε2/ε3/ε4 genotypes (coded in terms of the APOE effect on LDL-C) on ischaemic stroke are described in Figure 1d. In this regression model, the OR of ischaemic stroke per 1 mmol/l increase in LDL-C was 1.33 (95% CrI: 1.17, 1.52), equivalent to an OR of 0.75 (95% CrI: 0.66, 0.85) per 1 mmol/l reduction in LDL-C. An exploratory sensitivity analysis showed that this positive linear trend remained largely unaltered in all the subgroups (Supplementary Figure S6, available as Supplementary data at IJE online), with none of them showing strong evidence of a quadratic trend (the coefficient of the quadratic term had a posterior interval that included the null).
The random effects model showed very similar results to the fixed effects analysis despite the greater uncertainty in the effect estimates for less frequent APOE genotypes. The trend analysis was also concordant with the fixed effects model (Supplementary Figure S7, available as Supplementary data at IJE online).
Association of APOE genotype with ischaemic stroke in all ethnicities
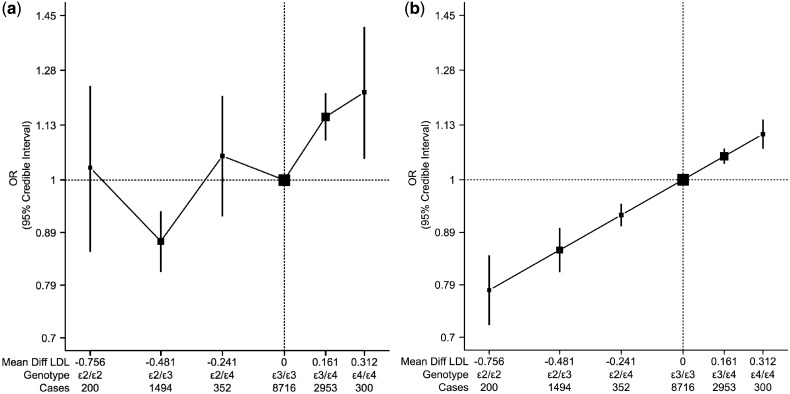
In 74 studies (16 prospective and 58 case-control studies), with a total of 14 015 cases and 77 888 controls in all populations using the ε3/ε3 genotype as the reference group, the ORs for ischaemic stroke were 1.05 (95% CrI: 0.87, 1.26) for ε2/ε2, 0.88 (95% CrI: 0.82, 0.94) for ε2/ε3, 1.06 (95% CrI: 0.93, 1.21) for ε2/ε4, 1.15 (95% CrI: 1.09, 1.21) for ε3/ε4, and 1.22 (95% CrI: 1.05, 1.41) for ε4/ε4 (Figure 4a).
Figure 4.
Association of APOE genotypes with ischaemic stroke in all ethnicities with (a) ischaemic stroke, (b) ischaemic stroke (trend analysis). Black boxes indicate summary estimates with their size proportional to weight. The x-axis is plotted in the log-scale with distance between APOE genotypes equal to mean difference of LDL-C in mmol/l. For (b), the effect estimate of trend analysis indicates an OR of 1.39 (95% CrI: 1.25, 1.54) per 1 mmol/l increase in LDL-C
A visual inspection of the trend analysis of the log-odds of APOE on ischaemic stroke indicated the presence of a positive dose-response over the greater part of the LDL-C range (indirectly measured by the APOE genotypes). Under the assumption of a common model across ethnic groups for the LDL-C–ischaemic stroke association, we fitted a linear model and estimate that the OR of ischaemic stroke for 1 mmol/l of increase in LDL-C was 1.39 (95% CrI: 1.25, 1.54) (Figure 4b). However, we also allow for the possibility of alternative models (i.e. quadratic model), and these results are included in our sensitivity analysis (Supplementary Figure S8, available as Supplementary data at IJE online). The random effects model produced very similar findings to the fixed effects model but with wider credible intervals (Supplementary Figure S9, available as Supplementary data at IJE online).
Assessment of the cumulative evidence, using the Venice criteria, suggest that the epidemiological credibility for the APOE-ischaemic stroke association is strong in European population studies and moderate or weak in all other ethnic groups (Supplementary Table S6, available as Supplementary data at IJE online).
The results of the sensitivity analysis that excluded small studies and the of one that re-estimated the effect for published and unpublished studies independently did not differ from the overall result, reducing the possibility that small-study bias influenced our results (Supplementary Figures S6 and S8, available as Supplementary data at IJE online).
In 11 studies with a total of 759 cases and 22 193 controls, using the ε3/ε3 genotype as the reference, no clear effect for any of the APOE genotypes with haemorrhagic stroke was observed (Figure S10, available as Supplementary data at IJE online).
Discussion
Using the largest collection of studies yet accumulated, our analysis of European populations confirmed the presence of a positive dose-response association between APOE genotype and LDL-C, and clarified the genetic effect on C-IMT, which followed the same dose-response trend observed for LDL-C. The pattern was concordant in the meta-analysis of European ancestry studies on ischaemic stroke that also showed a positive dose-response association when the APOE genotypes were ordered and coded to reflect their effects on LDL-C (Figure 1). The large degree of consistency across the different outcome domains evaluated, [LDL-C (intermediate phenotype), C-IMT (surrogate end-point) and ischaemic stroke (clinical event)] argues for a causal effect of LDL-C on ischaemic stroke, and provides an adequate explanation for the beneficial effects on ischaemic stroke observed with statins in randomized trials.
In this respect, it is important to note that the estimate derived from our regression model in European ancestry studies that used APOE genotypes as instrument for LDL-C showed an OR of ischaemic stroke of 0.75 (95% CrI: 0.66, 0.85) per 1 mmol/l LDL-C reduction, which is consistent with the rate ratio of 0.79 (95% CI: 0.74, 0.85) for ischaemic stroke for the same LDL-C reduction derived from randomized trials of statins.55
The large size of the data set synthesised in this report that included 2392 more cases of ischaemic stroke (including 6362 cases from unpublished and updated collections) than previous meta-analyses,19 permitted for the first time a reliable evaluation of the risk of ischaemic stroke in each of the six APOE genotype groups separately. Also, using APOE genotype as an instrument for LDL-C enabled the presence of a positive dose-response trend of LDL-C on ischaemic stroke to be determined. This is the largest study examining association of APOE genotypes with C-IMT to date, and shows that the genotypes are associated with atherosclerosis in a positive dose-response manner. This was similar in magnitude to the results of a previous meta-analysis examining this association.20 This current study also suggests that the APOE effect on ischaemic stroke is mediated via LDL-C, providing further evidence of a causal role for this lipid fraction, which has been a point of conflict over several decades of research.
The finding of a positive dose-response effect of LDL-C (indexed by APOE genotype) on ischaemic stroke in European ancestry individuals was supported by the results from our sensitivity analysis that was restricted to the subset of studies considered to be at low risk of bias according to study level characteristics, such as study size, outcome diagnosis, blinding of genotyping staff, or genotyping technique. However, it is important to note that despite the large sample size included in this analysis, we still observed considerable uncertainty on the effect estimates for ischaemic stroke for the less frequent APOE genotypes, in particular the ε2/ε2 genotype.
When we extended our analysis of the APOE loci on ischaemic stroke to genetic studies from all ethnic groups, a similar dose-response was observed for most of the association. However, a slight increase on ischaemic stroke for the section of the regression analysis associated with lower LDL-C levels, indexed by the genotypes ε2/ε3 and ε2/ε2, was observed. Our sensitivity analysis, however, indicated that a potential explanation for this apparent increase in risk could be due to genotyping errors as showed by the analysis restricted to the subset of studies in HW equilibrium, with blinding of the genotyping staff or those who used recent genotyping techniques. In this subset, the slight increase in risk of ischaemic stroke for ε2/ε2 genotypes was not seen and instead a positive dose-response relationship emerged (Supplementary Figure S6, available as Supplementary data at IJE online). A concern that some of the APOE genotypes may be prone to miscalling has been previously shown by some researchers,48 indicating that studies that used Hixson and Vernier’s PCR-RFLP method of genotyping or similar methods56,57 are more likely to have an excess of ε2/ε2 genotypes.48,58 This limitation is overcome by the newer APOE genotyping techniques (e.g. LightTyper assay and Taqman).49
A major limitation to this study is that we could not reliably assess the possibility of differential effects on the sub-types of ischaemic stroke. It has been postulated that different ischaemic stroke sub-types exhibit different associations with cholesterol and APOE genotype, suggesting differences in the underlying pathologies.19 Although information on haemorrhagic stroke was limited in our study, it was concordant with the lack of effect seen in observational studies (see Supplementary data at IJE online). However, our haemorrhagic stroke analysis had considerably less statistical power than our ischaemic stroke analysis. Larger studies are needed to answer this question with more confidence.59 The ε4 allele already has a validated association with Alzheimer’s disease;60 however, misclassification of Alzheimer’s with ischaemic stroke is very unlikely as the two diseases have very distinct clinical and imaging profiles, and thus we believe this misclassification did not play a role in our results.
Small-study bias can be a major limitation in any meta-analysis. The analysis of APOE genotypes and cardiovascular traits was entirely based on de novo data and included a sample size of 60 883 individuals. This avoids the impact of small-study bias which is mainly observed in meta-analyses based on published studies. With regard to the analysis of APOE genotypes with stroke, our two sensitivity analyses, i.e. excluding small studies and re-quantifying the APOE-effect and comparing published versus unpublished studies, did not show any evidence of small-study bias.
Mechanisms linking APOE genotype to ischaemic stroke risk
To evaluate the mechanism by which APOE genotype might alter ischaemic stroke risk, we undertook a detailed analysis of the association of APOE genotypes with a wide range of potential intermediate phenotypes including lipid, inflammation, coagulation, endothelial cell activation and metabolic markers measured in population-based and cross-sectional studies from European ancestry. Since genotype is determined at random at conception, intermediate phenotypes residing off the causal pathway from SNPs to disease, should be balanced evenly among the different genotypic groups, as they are in a randomized trial. In contrast, biomarkers that mediate the effect of genomic variation on disease risks should differ by genotype and the shape of the associations should be concordant.61,62 We identified robust associations of APOE genotype with total-, LDL- and HDL-C and triglycerides as reported in a recent systematic review.11 However, we extended the analysis to apolipoproteins E (the protein encoded by the APOE gene), apoA1, apoB, Lp(a), and 18 other biomarkers, including the well-studied inflammation marker CRP, among several thousand individuals. An exploratory, but biologically interesting finding was the fact that the shape of the APOE association with these traits varied considerably. Whereas a dose-response association was clearly observed with total-C, LDL-C, apoB, apoE and HDL-C, the effect on other traits appeared to be allele-specific, with lower Lp(a) and higher apoA1 being associated with the ε2 allele, and lower CRP with the ε4 allele. Although it is possible that these effects are due to a different biological mechanism of the APOE ε2 and ε4 variants on lipoprotein particle metabolism, it is also possible that these allele-specific associations could be due to different LD patterns between rarer, untyped, functional SNPs and the two genetic variants that comprise APOE ε2/ε3/ε4. It is often argued that except for rare variants, most of the observed phenotypic changes in lipoprotein biology can be explained by differences in the ε2/ε3/ε4 genotypes. We believe this view is simplistic and does not take into account the recent discoveries at this locus. Whole genome and dense gene-centric SNP arrays (e.g. the Illumina Cardiochip) have identified associations of SNPs in the APOE gene region with LDL-C8,10,63–65 and Alzheimer’s disease that encompass non-coding variants in the flanking genes and appear to be independent of the rs429358 and rs7412 SNPs (the two SNPS that encode ε2/ε3/ε4) in multivariate analysis.63 These include variants in PVRL2, APOC1 and TOMM40.66,67 This suggests possible functional roles for one or more of the flanking genes (and their encoded proteins), mechanisms relating to APOE expression (not just function), or both. However, the capture of both common and rare SNPs in the APOE gene cluster is not yet exhaustive, so it is uncertain which of the implicated variants is causal, which mark as yet unidentified causal variation or whether causal SNPs differ for the different associated traits and disease outcomes. Not only are low-frequency variants (<5% minor allele frequency) under-represented on whole genome arrays but surprisingly, there is also sparse coverage of the common alleles in the APOE region (including the omission of rs429358 and rs7412 SNPs) (Supplementary Figure S11 and Supplementary Table S7, available as Supplementary data at IJE online). The low degree of LD at this locus also makes it difficult to impute untyped variants, which means additional SNP-disease/trait association signals may have been overlooked by the prior GWAS. Early re-sequencing studies and isoelectric focusing analysis of APOE isoforms68–73 indicate the existence of many low-frequency, non-synonymous coding variants in this region, but these studies were narrowly focused on the APOE gene itself, rather than the flanking genes, and were neither exhaustive nor systematic.
The shape of the association of APOE genotype with an intermediate phenotype on the causal pathway should be consistent in the direction of the association with the disease end-point. Thus, the reported association of APOE genotype with CHD appears to be consistent with its effect on LDL-C. This is further supported by similar concordant associations of SNPs in the HMGCR, PCSK9 and SORT1 genes with both LDL-C and CHD risk.8,10,64,74 Taken together with data from observational studies and randomized trials, the findings endorse the role of LDL-cholesterol in the pathogenesis of CHD. The positive dose-response association of APOE genotype with ischaemic stroke risk in European population studies could be consistent with an almost complete mediating effect of LDL-C because both associations are positive and linear. None of the other biomarkers studied, including other lipids and apolipoproteins, as well as Lp(a), or inflammation markers (including CRP) exhibited associations that were consistent in shape or direction with that seen for ischaemic stroke. Indeed, it is important to note that the ε4 allele associated with high values of LDL-C and C-IMT as well as high stroke and CHD risk showed low CRP levels. Although these results appear to be in contradiction with observational studies, we considered that findings of APOE on CRP could be explained by differential pattern of LD, as described above. Moreover, recent large-scale Mendelian randomization studies have excluded CRP as causal factor in cardiovascular disease.75
The concordance in shape of the APOE ε2/ε3/ε4 with LDL-C and ischaemic stroke risk observed in European ancestry received further support from the APOE ε2/ε3/ε4 effects on C-IMT (derived from European ancestry studies) that also revealed a positive dose-response association (Figure 1). However, to confirm that the positive dose-response association of APOE loci on ischaemic stroke is mainly due to its effect on LDL-C would require access to large-scale cohorts with genotype information, LDL-C measures and ischaemic stroke outcomes in sufficiently large numbers to undertake appropriate Mendelian randomization/instrumental variables analyses.
C-IMT represents the structural change in arterial intima that strongly associates with both cardiovascular risk factors (LDL-C) and cardiovascular events, and it is also used as an intermediate marker of atherosclerosis.21 Overviews of statin trials have noted a linear relationship between the degree of LDL-C lowering and the change in C-IMT during follow-up, with modest LDL-C reductions being associated with reduced progression and more extreme LDL-C reductions being associated with regression of C-IMT.76 The linear dose-response association of APOE genotypes with C-IMT was concordant in shape with the association of APOE with LDL-C, and endorses C-IMT as a valid surrogate marker of LDL-C–mediated atherosclerosis.77,78 Similar to our study, a recent meta-analysis of APOE and C-IMT showed lower levels of C-IMT for ε2 carriers and higher values for ε4 carriers when compared with ε3/3.79 In contrast, the only GWAS of C-IMT23 reported a lack of association with APOE variant rs7412 that marks the ε4 allele (P-value: 0.7). Our study has the advantage of reporting the full range of APOE genotypes that might better represent this association.
Conclusions
In the most comprehensive study to date, we found a positive dose-response association of APOE genotype with ischaemic stroke in European populations. Concordance between the effects of the APOE genotype on LDL-C, C-IMT and ischaemic stroke risk was observed. Although this argues for a causal role of LDL-C in ischaemic stroke, and also helps to clarify the benefit of statins in ischaemic stroke, our finding must be interpreted with caution and it does not exclude the possibility that additional risk factors may be involved.
The use of the custom genotyping array Metabochip,80 which includes both APOE SNPs and a greater coverage around the APOE locus, will provide an opportunity to replicate our findings on C-IMT, but also to explore in further detail the allele-specific associations of APOE locus with certain cardiovascular traits.
Supplementary Data
Supplementary data are available at IJE online.
Funding
T.A.K. is funded by a Graduate Teaching Assistantship of London School of Hygiene & Tropical Medicine. A.D.H. was funded by a British Heart Foundation Senior Fellowship (FS/05/125). S.E.H, J.C. and P.J.T. are supported by the British Heart Foundation (PG2008/015). L.S. is funded by a Wellcome Trust Senior Fellowship in Clinical Science. T.R.G. was the recipient of a British Heart Foundation Intermediate Fellowship FS/05/065/19497 and T.R.G., I.D., S.E., D.A.L. and Y.B.S. are the recipients of a British Heart Foundation project grant PG/07/131/24254. D.A.L., G.D.S., T.R.G. and I.D. work in a UK Medical Research Council-supported centre. M.R.A. as funded by postdoctoral support from the University of Bristol which funded some set-up costs of Bristol Genetic Epidemiology Laboratory (M.R.A., T.R.G., I.D.). RCUK funding to I.D. and G.D.S. supported a Collaboration Development Workshop in Cardiovascular Genetics held in Beijing in March 2010. Tamuno Alfred is thanked for statistical support. J.S. is funded by the Swedish Research Council (2007-5942) and the Swedish Heart Lung Foundation (20041151). J.A.H. is funded by Centre for Cardiovascular Research and Institute for Clinical and Experimental Medicine project 00023001 (IKEM CR). A.J.S. is supported by the NESTOR stimulation programme for geriatric research in The Netherlands (Ministry of Health and Ministry of Education), the Netherlands Organization for Scientific Research (NWO), the Netherlands Prevention fund, the municipality of Rotterdam, the International Alzheimer Research Foundation (IARF), and the Fund for Scientific Research (FWO-F), Flanders, Belgium. T.K. (Tomohiro Katsuya) was supported by Grants-in-Aid for Scientific Research (18590265, 18590811, 19650188, 21390223) from the Ministry of Education, Science, Sports and Culture of Japan. R.H. is supported by the grants from the Ministry of Science and Technology of China (2011CB503900, 2009DFB30050 and 2006CB503805). M.A.N., A.B.S., J.H., B.B.W., S.R., T.G.B., R.D.B., J.F.M.: ISGS and SWISS received funding from grants from the National Institute of Neurological Disorders and Stroke (US), R01 NS39987 and R01 NS42733. This work was supported in part by the Intramural Research Program of the National Institute on Aging, National Institutes of Health, Department of Health and Human Services; project number Z01 AG000954-06. This work used samples and clinical data from the NINDS Human Genetics Resource Center DNA and Cell Line Repository (http://ccr.coriell.org/ninds), human subjects protocol numbers 2003-081 and 2004-147. The inclusion of BLSA samples was supported in part by the Intramural Research Program of the National Institute on Aging, National Institutes of Health, Department of Health and Human Services; project number Z01 AG000015-50, human subjects protocol number 2003-078. The ISGS study was funded by a grant from the National Institute for Neurological Disorders and Stroke (US; R01 NS42733) (J Meschia, P.I.). The SWISS study was funded by a grant from the National Institute for Neurological Disorders and Stroke (US; R01 NS39987 (J Meschia, P.I.). This study utilized the high-performance computational capabilities of the Biowulf Linux cluster at the National Institutes of Health, Bethesda, MD (http://biowulf.nih.gov).
Samples from the English Longitudinal Study of Ageing (ELSA) DNA Repository (EDNAR), received support under a grant (AG1764406S1) awarded by the National Institute on Aging (NIA). ELSA was developed by a team of researchers based at the National Centre for Social Research, University College London and the Institute of Fiscal Studies, UK. The data were collected by the National Centre for Social Research. Northwick Park Heart Study II (NPHS-II) was supported by the British Medical Research Council, the US National Institutes of Health (NHLBI 33014) and Du Pont Pharma, Wilmington, USA. The Whitehall II study (is supported by the US National Institutes of Health (NHLBl R01HL036310-20A2, NIA R01AG013196 and R01AG034454), the British Heart Foundation (RG/02/005) and the Medical Research Council, UK. Edinburgh Artery Study (EAS) was funded by the British Heart Foundation. The British Regional Heart Study (BRHS) is a British Heart Foundation Research Group and is supported by British Heart Foundation (RG/04/003). The views expressed in this publication are those of the authors and not necessarily those of the funding bodies. The British Women's Heart and Health Study (BWHHS) was funded by the Department of Health Policy Research Programme and the British Heart Foundation (BHF Project Grant No: PG/06/002 and DoH Project Grant No: 0090041). The Caerphilly Prospective Study (CaPS) was established by the former MRC Epidemiology Unit in Cardiff. Diabetes UK supported the creation of UDACS. The AAA Trial (AAAT) was funded by the British Heart Foundation, Chief Scientist Office in Scotland, Chest Heart and Stroke Scotland and Wellcome Trust. The EARSI study was supported by the European Community Concerted Action MRH4 COMAC Epidemiology, and the EARSII study was supported by the European Commission Contract BMH1-CT92-0206. Analyses in the Czech-MONICA study were supported by project grants No. 00023001 (I.K.E.M., C.R.) and 1M0510 (M.S.M.T., C.R.). The Uppsala Longitudinal Study of Adult Men (ULSAM) study was supported by the Swedish Research Council (2006–6555), Swedish Heart-Lung Foundation, Erik, Karin och Gösta Selanders Foundation, Thuréus Foundation, Lisa och Johan Grönbergs Foundation, Loo och Hans Ostermans Foundation and Uppsala University.
T.A.K., T.S., A.D.H. and J.P.C. designed the experiment and the analysis plan. T.A.K., T.S. and D.P. did the statistical analysis and drafted the report with A.D.H. and J.P.C. All authors provided critical revisions. All contributors had shared summary data and contributed to the interpretation of the results and to the redrafting of the report. All members of the coordinating centre contributed to the collection, standardization, analysis and interpretation of the data. T.A.K., T.S., D.P., J..PC. and A.D.H. had full access to all data in the study and had final responsibility to submit the report for publication. The study was conducted and analysed independently from its funders.
Conflict of interest: J.W. is 90% employed at GlaxoSmithKline while retaining a 10% appointment at London School of Hygiene & Tropical Medicine. A.D.H. is a member of the editorial board of Drug and Therapeutics Bulletin (a BMJ group publication) and has received honoraria for speaking at educational meetings most or all of which have been donated to charity. There are no other conflicts of interest.
KEY MESSAGES.
The six APOE genotypes when ordered from ε2/ε2, ε2/ε3, ε2/ε4, ε3/ε3, ε3/ε4 and ε4/ε4 have a linear association with LDL-C and CIMT thickness.
The largest meta-analysis of APOE genotype with ischaemic stroke shows a positive linear association of increasing risk when ordered from ε2/ε2, ε2/ε3, ε2/ε4, ε3/ε3, ε3/ε4 and ε4/ε4 in European ancestry populations.
The concordance in the dose-response associations of APOE genotype with LDL-C, CIMT and ischaemic stroke provides strong support for a causal role of LDL-C in ischaemic stroke.
Supplementary Material
References
- 1.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–57. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 2.Feigin VL, Lawes CMM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet. 2003;2:43–53. doi: 10.1016/s1474-4422(03)00266-7. [DOI] [PubMed] [Google Scholar]
- 3.Lewington S, Whitlock G, Clarke R, et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55 000 vascular deaths. Lancet. 2007;370:1829–39. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 4.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–78. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 5.Amarenco P, Steg PGG. The paradox of cholesterol and stroke. Lancet. 2007;370:1803–04. doi: 10.1016/S0140-6736(07)61751-6. [DOI] [PubMed] [Google Scholar]
- 6.Mahley RW, Rall SC. Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–37. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- 7.Teslovich TM, Musunuru K, Smith AV, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–13. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aulchenko YS, Ripatti S, Lindqvist I, et al. Loci influencing lipid levels and coronary heart disease risk in 16 European population cohorts. Nat Genet. 2009;41:47–55. doi: 10.1038/ng.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallace C, Newhouse SJ, Braund P, et al. Genome-wide association study identifies genes for biomarkers of cardiovascular disease: serum urate and dyslipidemia. Am J Hum Genet. 2008;82:139–49. doi: 10.1016/j.ajhg.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willer CJ, Sanna S, Jackson AU, et al. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet. 2008;40:161–69. doi: 10.1038/ng.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennet AM, Di Angelantonio E, Ye Z, et al. Association of apolipoprotein E genotypes with lipid levels and coronary risk. JAMA. 2007;298:1300–11. doi: 10.1001/jama.298.11.1300. [DOI] [PubMed] [Google Scholar]
- 12.Grönroos P, Raitakari OT, Kähönen M, et al. Relation of Apolipoprotein E Polymorphism to Markers of Early Atherosclerotic Changes in Young Adults. Circulation. 2008;72:29–34. doi: 10.1253/circj.72.29. [DOI] [PubMed] [Google Scholar]
- 13.Rontu R, Ojala P, Hervonen A, et al. Apolipoprotein E genotype is related to plasma levels of C-reactive protein and lipids and to longevity in nonagenarians. Clin Endocrinol. 2006;64:265–70. doi: 10.1111/j.1365-2265.2006.02455.x. [DOI] [PubMed] [Google Scholar]
- 14.Berrahmoune H, Herbeth B, Siest G, Visvikis-Siest S. Heritability of serum hs-CRP concentration and 5-year changes in the Stanislas family study: association with apolipoprotein E alleles. Genes Immun. 2007;8:352–59. doi: 10.1038/sj.gene.6364395. [DOI] [PubMed] [Google Scholar]
- 15.Chasman DI, Kozlowski P, Zee RY, Kwiatkowski DJ, Ridker PM. Qualitative and quantitative effects of APOE genetic variation on plasma C-reactive protein, LDL-cholesterol, and apoE protein. Genes Immun. 2006;7:211–19. doi: 10.1038/sj.gene.6364289. [DOI] [PubMed] [Google Scholar]
- 16.Elliott P, Chambers JC, Zhang W, et al. Genetic Loci associated with C-reactive protein levels and risk of coronary heart disease. JAMA. 2009;302:37–48. doi: 10.1001/jama.2009.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marz W, Scharnagl H, Hoffmann MM, Boehm BO, Winkelmann BR, März W. The apolipoprotein E polymorphism is associated with circulating C-reactive protein (the Ludwigshafen risk and cardiovascular health study) Eur Heart J. 2004;25:2109–19. doi: 10.1016/j.ehj.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 18.Tsoi L-M, Wong K-Y, Liu Y-M, Ho Y-Y. Apoprotein E isoform-dependent expression and secretion of pro-inflammatory cytokines TNF-alpha and IL-6 in macrophages. ArchBiochem Biophys. 2007;460:33–40. doi: 10.1016/j.abb.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 19.Sudlow C, Martínez González NA, Kim J, Clark C. Does apolipoprotein E genotype influence the risk of ischaemic stroke, intracerebral hemorrhage, or subarachnoid hemorrhage? Systematic review and meta-analyses of 31 studies among 5961 cases and 17 965 controls. Stroke. 2006;37:364–70. doi: 10.1161/01.STR.0000199065.12908.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paternoster L, Martínez González NA, Lewis S, Sudlow C. Association between apolipoprotein E genotype and carotid intima-media thickness may suggest a specific effect on large artery atherothrombotic stroke. Stroke. 2008;39:48–54. doi: 10.1161/STROKEAHA.107.488866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polak JF, Pencina MJ, Pencina KM, et al. Carotid-wall intima-media thickness and cardiovascular events. N Engl J Med. 2011;365:213–21. doi: 10.1056/NEJMoa1012592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellenguez C, Bevan S, Gschwendtner A, et al. Genome-wide association study identifies a variant in HDAC9 associated with large vessel ischaemic stroke. Nat Genet. 2012;44:328–33. doi: 10.1038/ng.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bis JC, Kavousi M, Franceschini N, et al. Meta-analysis of genome-wide association studies from the CHARGE consortium identifies common variants associated with carotid intima media thickness and plaque. Nat Genet. 2011;43:940–47. doi: 10.1038/ng.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marmot M, Banks J, Blundell R, Lessof C. Health, wealth and lifestyles of the older population in England: The 2002 English Longitudinal Study of Ageing. Institute for Fiscal Studies. 2003 Jan:357–74. [Google Scholar]
- 25.Miller GJ, Bauer KA, Barzegar S, et al. The effects of quality and timing of venepuncture on markers of blood coagulation in healthy middle-aged men. Thromb Haemost. 1995;73:82–86. [PubMed] [Google Scholar]
- 26.Marmot M, Brunner E. Cohort Profile: the Whitehall II study. Int J Epidemiol. 2005;34:251–56. doi: 10.1093/ije/dyh372. [DOI] [PubMed] [Google Scholar]
- 27.Fowkes FG, Housley E, Cawood EH, Macintyre CC, Ruckley CV, Prescott RJ. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1991;20:384–92. doi: 10.1093/ije/20.2.384. [DOI] [PubMed] [Google Scholar]
- 28.Walker M, Whincup PH, Shaper AG. The British Regional Heart Study 1975-2004. Int J Epidemiol. 2004;33:1185–92. doi: 10.1093/ije/dyh295. [DOI] [PubMed] [Google Scholar]
- 29.Lawlor DA, Timpson N, Ebrahim S, Day INM, Davey Smith G. The association of oestrogen receptor alpha-haplotypes with cardiovascular risk factors in the British Women’s Heart and Health Study. Eur Heart J. 2006;27:1597–604. doi: 10.1093/eurheartj/ehi833. [DOI] [PubMed] [Google Scholar]
- 30.The Caerphilly and Speedwell Collaborative Group. Caerphilly and Speedwell collaborative heart disease studies. J Epidemiol Commun Health. 1984;38:259–62. doi: 10.1136/jech.38.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhamrait SS, Stephens JW, Cooper JA, et al. Cardiovascular risk in healthy men and markers of oxidative stress in diabetic men are associated with common variation in the gene for uncoupling protein 2. Eur Heart J. 2004;25:468–75. doi: 10.1016/j.ehj.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Price JF, Stewart MC, Deary IJ, et al. Low dose aspirin and cognitive function in middle aged to elderly adults: randomised controlled trial. BMJ. 2008;337:a1198. doi: 10.1136/bmj.a1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.The European Atherosclerosis Research Study (EARS): design and objectives. Int J Epidemiol. 1994;23:465–71. doi: 10.1093/ije/23.3.465. [DOI] [PubMed] [Google Scholar]
- 34.The World Health Organization MONICA Project (monitoring trends and determinants in cardiovascular disease): a major international collaboration. WHO MONICA Project Principal Investigators. J Clin Epidemiol. 1988;41:105–14. doi: 10.1016/0895-4356(88)90084-4. [DOI] [PubMed] [Google Scholar]
- 35.Wohlin M, Sundström J, Lannfelt L, et al. Apolipoprotein E epsilon4 genotype is independently associated with increased intima-media thickness in a recessive pattern. Lipids. 2007;42:451–56. doi: 10.1007/s11745-007-3045-5. [DOI] [PubMed] [Google Scholar]
- 36.Ward H, Mitrou PN, Bowman R, et al. APOE genotype, lipids, and coronary heart disease risk: a prospective population study. Arch Int Med. 2009;169:1424–29. doi: 10.1001/archinternmed.2009.234. [DOI] [PubMed] [Google Scholar]
- 37.Beilby JP, Hunt CCJ, Palmer LJ, et al. Apolipoprotein E gene polymorphisms are associated with carotid plaque formation but not with intima-media wall thickening: results from the Perth Carotid Ultrasound Disease Assessment Study (CUDAS) Stroke. 2003;34:869–74. doi: 10.1161/01.STR.0000062901.54157.12. [DOI] [PubMed] [Google Scholar]
- 38.Slooter AJ, Bots ML, Havekes LM, et al. Apolipoprotein E and carotid artery atherosclerosis: the Rotterdam study. Stroke. 2001;32:1947–52. doi: 10.1161/hs0901.095377. [DOI] [PubMed] [Google Scholar]
- 39.Davey Smith G, Ebrahim S. Mendelian randomization: prospects, potentials, and limitations. Int J Epidemiol. 2004;33:30–42. doi: 10.1093/ije/dyh132. [DOI] [PubMed] [Google Scholar]
- 40.Egger M, Davey Smith G, Altman D. Systematic Reviews in Health Care: Meta-analysis in Context. Oxford: Wiley-Blackwell; 2001. p. 512. [Google Scholar]
- 41.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abdi H. The Bonferonni and Šidák Corrections for Multiple Comparisons. In: Salkind NJ, editor. Encyclopedia of Measurement and Statistics. Thousand Oaks, CA: Sage: 2007. pp. 1–9. [Google Scholar]
- 43.Higgins JPT, Thompson SG. Controlling the risk of spurious findings from meta-regression. Stat Med. 2004;23:1663–82. doi: 10.1002/sim.1752. [DOI] [PubMed] [Google Scholar]
- 44.Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22:2693–710. doi: 10.1002/sim.1482. [DOI] [PubMed] [Google Scholar]
- 45.Sagoo GS, Little J, Higgins JPT. Systematic reviews of genetic association studies. Human Genome Epidemiology Network. PLoS Med. 2009;6:e28. doi: 10.1371/journal.pmed.1000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gilks W, Richardson S, Spiegelhalter D. Markov Chain Monte Carlo in Practice. Abingdon: Chapman and Hall/CRC/Taylor and Francis; 1995. [Google Scholar]
- 47.Warn DE, Thompson SG, Spiegelhalter DJ. Bayesian random effects meta-analysis of trials with binary outcomes: methods for the absolute risk difference and relative risk scales. Stat Med. 2002;21:1601–23. doi: 10.1002/sim.1189. [DOI] [PubMed] [Google Scholar]
- 48.Bolla MK, Wood N, Humphries SE. Rapid determination of apolipoprotein E genotype using a heteroduplex generator. J Lipid Res. 1999;40:2340–45. [PubMed] [Google Scholar]
- 49.Abdollahi MR, Guthrie PAI, Davey Smith G, Lawlor DA, Ebrahim S, Day INM. Integrated single-label liquid-phase assay of APOE codons 112 and 158 and a lipoprotein study in British women. Clin Chem. 2006;52:1420–23. doi: 10.1373/clinchem.2006.067082. [DOI] [PubMed] [Google Scholar]
- 50.van Vliet P, Mooijaart SP, de Craen AJM, Rensen PCN, van Heemst D, Westendorp RGJ. Plasma levels of apolipoprotein E and risk of stroke in old age. Ann NY Acad Sci. 2007;1100:140–47. doi: 10.1196/annals.1395.012. [DOI] [PubMed] [Google Scholar]
- 51.Zhu L, Fratiglioni L, Guo Z, et al. Incidence of dementia in relation to stroke and the apolipoprotein E epsilon4 allele in the very old. Findings from a population-based longitudinal study. Stroke. 2000;31:53–60. doi: 10.1161/01.str.31.1.53. [DOI] [PubMed] [Google Scholar]
- 52.Haan MN, Mungas DM, Gonzalez HM, Ortiz TA, Acharya A, Jagust WJ. Prevalence of dementia in older latinos: the influence of type 2 diabetes mellitus, stroke and genetic factors. J Am Ger Soc. 2003;51:169–77. doi: 10.1046/j.1532-5415.2003.51054.x. [DOI] [PubMed] [Google Scholar]
- 53.Fillenbaum GG, Blazer DG, Burchett BM, Saunders AM, Taylor DH. Apolipoprotein E epsilon4 and risk of mortality in African American and white older community residents. The Gerontologist. 2002;42:381–86. doi: 10.1093/geront/42.3.381. [DOI] [PubMed] [Google Scholar]
- 54.Lawlor D, Bedford C, Taylor M, Ebrahim S. Geographical variation in cardiovascular disease, risk factors, and their control in older women: British Women’s Heart and Health Study. J Epidemiol Community Health. 2003;57:134–40. doi: 10.1136/jech.57.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cholesterol Treatment Trialists’ Ctt Collaborators. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;6736:1–10. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–48. [PubMed] [Google Scholar]
- 57.Crook R, Hardy J, Duff K. Single-day apolipoprotein E genotyping. J Neurosci Methods. 1994;53:125–27. doi: 10.1016/0165-0270(94)90168-6. [DOI] [PubMed] [Google Scholar]
- 58.Appel E, Eisenberg S, Roitelman J. Improved PCR amplification/Hhal restriction for unambiguous determination of apolipoprotein E alleles. Clin Chem. 1995;41:187–90. [PubMed] [Google Scholar]
- 59.Ebrahim S, Sung J, Song Y-M, Ferrer RL, Lawlor DA, Davey Smith G. Serum cholesterol, haemorrhagic stroke, ischaemic stroke, and myocardial infarction: Korean national health system prospective cohort study. BMJ. 2006;333:22. doi: 10.1136/bmj.38855.610324.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc Natl Acad Sci U S A. 2006;103:5644–51. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Drenos F, Talmud PJ, Casas JP, et al. Integrated associations of genotypes with multiple blood biomarkers linked to coronary heart disease risk. Hum Mol Genet. 2009;18:2305–16. doi: 10.1093/hmg/ddp159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lawlor DA, Davey Smith G, Harbord R, Timpson N, Day I, Ebrahim S. Clustered environments and randomized genes: a fundamental distinction between conventional and genetic epidemiology. PLoS Med. 2007;4:e352. doi: 10.1371/journal.pmed.0040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Talmud P, Drenos F, Shah S, et al. Gene-centric association signals for lipids and apolipoproteins identified via the HumanCVD BeadChip. Am J Hum Genet. 2009;85:628–42. doi: 10.1016/j.ajhg.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kathiresan S, Melander O, Guiducci C, et al. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat Genet. 2008;40:189–97. doi: 10.1038/ng.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kathiresan S, Willer CJ, Peloso GM, et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat Genet. 2009;41:56–65. doi: 10.1038/ng.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roses AD, Lutz MW, Amrine-Madsen H, et al. A TOMM40 variable-length polymorphism predicts the age of late-onset Alzheimer’s disease. Pharmacogenomics J. 2010;10:375–84. doi: 10.1038/tpj.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abraham R, Moskvina V, Sims R, et al. A genome-wide association study for late-onset Alzheimer's disease using DNA pooling. BMC Med Genomics. 2008;1:44. doi: 10.1186/1755-8794-1-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klos K, Shimmin L, Ballantyne C, et al. APOE/C1/C4/C2 hepatic control region polymorphism influences plasma apoE and LDL cholesterol levels. Hum Mol Genet. 2008;17:2039–46. doi: 10.1093/hmg/ddn101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stengård JH, Frikke-Schmidt R, Tybjaerg-Hansen A, Nordestgaard BG, Sing CF. Variation in 5’ promoter region of the APOE gene contributes to predicting ischaemic heart disease (IHD) in the population at large: the Copenhagen City Heart Study. Ann Hum Genet. 2007;71:762–71. doi: 10.1111/j.1469-1809.2007.00370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stengård JH, Clark AG, Weiss KM, et al. Contributions of 18 additional DNA sequence variations in the gene encoding apolipoprotein E to explaining variation in quantitative measures of lipid metabolism. Am J Hum Genet. 2002;71(3):501–17.t. doi: 10.1086/342217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mahley R. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–30. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 72.van den Maagdenberg AM, Weng W, de Bruijn IH, et al. Characterization of five new mutants in the carboxyl-terminal domain of human apolipoprotein E: no cosegregation with severe hyperlipidemia. Am J Hum Genet. 1993;52:937–46. [PMC free article] [PubMed] [Google Scholar]
- 73.Mailly F, Xu CF, Xhignesse M, et al. Characterization of a new apolipoprotein E5 variant detected in two French-Canadian subjects. J Lipid Res. 1991;32:613–20. [PubMed] [Google Scholar]
- 74.Wellcome Trust Case Control Consortium. Genome-wide association study of 14 000 cases of seven common diseases and 3 000 shared controls. Nature. 2007;447:661–78. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.C Reactive Protein Coronary Heart Disease Genetics Collaboration. Association between C reactive protein and coronary heart disease: mendelian randomisation analysis based on individual participant data. BMJ. 2011;342:d548. doi: 10.1136/bmj.d548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Amarenco P, Labreuche J, Lavallée P, Touboul P-J. Statins in stroke prevention and carotid atherosclerosis: systematic review and up-to-date meta-analysis. Stroke. 2004;35:2902–09. doi: 10.1161/01.STR.0000147965.52712.fa. [DOI] [PubMed] [Google Scholar]
- 77.Amato M, Montorsi P, Ravani A, et al. Carotid intima-media thickness by B-mode ultrasound as surrogate of coronary atherosclerosis: correlation with quantitative coronary angiography and coronary intravascular ultrasound findings. Eur Heart J. 2007;28:2094–101. doi: 10.1093/eurheartj/ehm244. [DOI] [PubMed] [Google Scholar]
- 78.de Groot E, Hovingh GK, Wiegman A, et al. Measurement of arterial wall thickness as a surrogate marker for atherosclerosis. Circulation. 2004;109(Suppl. 1):III33–38. doi: 10.1161/01.CIR.0000131516.65699.ba. [DOI] [PubMed] [Google Scholar]
- 79.Paternoster L, Martinez-Gonzalez NA, Charleton R, Chung M, Lewis S, Sudlow CLM. Genetic effects on carotid intima-media thickness: systematic assessment and meta-analyses of candidate gene polymorphisms studied in more than 5000 subjects. Circulation. Cardiovascular genetics. 2010;3:15–21. doi: 10.1161/CIRCGENETICS.108.834366. [DOI] [PubMed] [Google Scholar]
- 80.Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380:572–80. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.