Abstract
Background Tubal ligation is a protective factor for ovarian cancer, but it is unknown whether this protection extends to all invasive histological subtypes or borderline tumors. We undertook an international collaborative study to examine the association between tubal ligation and ovarian cancer subtypes.
Methods We pooled primary data from 13 population-based case-control studies, including 10 157 patients with ovarian cancer (7942 invasive; 2215 borderline) and 13 904 control women. Invasive cases were analysed by histological type, grade and stage, and borderline cases were analysed by histological type. Pooled odds ratios were estimated using conditional logistic regression to match on site, race/ethnicity and age categories, and to adjust for age, oral contraceptive use duration and number of full-term births.
Results Tubal ligation was associated with significantly reduced risks of invasive serous (OR, 0.81; 95% CI, 0.74-0.89; P < 0.001), endometrioid (OR, 0.48; 95% CI, 0.40-0.59; P < 0.001), clear cell (OR, 0.52; 95% CI, 0.40-0.67; P < 0.001) and mucinous (OR, 0.68; 95% CI, 0.52-0.89; P = 0.005) cancers. The magnitude of risk reduction was significantly greater for invasive endometrioid (P < 0.0001) and clear cell (P = 0.0018) than for serous cancer. No significant associations were found with borderline serous or mucinous tumours.
Conclusions We found that the protective effects of tubal ligation on ovarian cancer risk were subtype-specific. These findings provide insights into distinct aetiologies of ovarian cancer subtypes and mechanisms underlying the protective effects of tubal ligation.
Keywords: Ovarian cancer, tubal ligation, tubal sterilization
Introduction
Worldwide, ovarian cancer is the seventh leading cause of cancer deaths in women and causes over 140 000 deaths each year.1 Ovarian cancer is a highly heterogeneous disease. Epithelial ovarian cancers may be invasive or borderline (low malignant potential) in behaviour, and may be serous, endometrioid, clear cell or mucinous in histology.2 Invasive serous cancers may be further subdivided into high-grade and low-grade subtypes3 and each disease subtype has distinct molecular, pathological and clinical features.4,5 The relative infrequency of most ovarian cancer subtypes, except for invasive serous high-grade cancer, has hindered efforts to elucidate their distinct aetiologies and risk factor profiles.
Tubal ligation, also referred to as tubal sterilization, is the most commonly used contraceptive method worldwide.6 Tubal ligation has been estimated to reduce ovarian cancer risk by about one-third overall,7 but its subtype-specific effects are unclear. Previous meta-analyses have reported that tubal ligation was associated with significantly reduced risks of invasive serous, endometrioid and clear cell cancers, but not invasive mucinous cancer.7,8 However, the published data on subtype-specific risks were limited, especially for the non-serous subtypes, and invasive serous high-grade and low-grade cancers and borderline subtypes were not separately examined. A better understanding of the effects of tubal ligation on ovarian cancer subtypes would help elucidate their distinct aetiologies and the underlying mechanisms by which this procedure prevents ovarian cancer.
We undertook an international collaborative study to examine the effects of tubal ligation on the risk of ovarian cancer subtypes using pooled data from 13 population-based case-control studies including 7942 invasive (4777 serous, 1273 endometrioid, 737 clear cell, 574 mucinous) and 2215 borderline (1309 serous, 906 mucinous) ovarian cancer patients, and 13 904 control women. We also examined whether younger age at tubal ligation or longer time since the procedure were associated with greater risk reductions. To our knowledge, this is the largest study of tubal ligation and ovarian cancer risk, enabling robust estimation of subtype-specific effects for the first time.
Methods
Study design and population
This study pooled primary data from 13 population-based case-control studies of ovarian cancer (Table 1). Twelve sites participate in the international Ovarian Cancer Association Consortium (OCAC),9 including nine conducted in the United States: CON,10 DOV,11 HAW,12,13 HOP,14 NCO,15,16 NEC,17,18 NJO,19,20 STA21 and USC;22 two in Europe, GER23 and MAL;24-26 and one in Australia, AUS.27 One additional population-based case-control study conducted in Canada (SON) was included.28 Cases were women newly diagnosed with invasive epithelial ovarian cancer (N = 7942) or borderline tumours (N = 2215). Eligible control women had at least one intact ovary and no history of ovarian cancer (N = 13 904). Controls were matched to cases on geographical region and age in all sites, and when warranted on race/ethnicity (Table 1). The present analysis included women aged 18–84 years at diagnosis (cases) or enrolment (controls) with information on tubal ligation status (missing for 74 women; 0.3%) and age at procedure (missing for 38 women who had a tubal ligation; 0.8%). Participants provided informed consent, and study protocols were approved by the respective institutional review board/human ethics committee for each site.
Table 1.
Description of 13 participating population-based ovarian cancer case-control studies
| Study name (abbreviation) | Country | Study years | Case ascertainment | Control ascertainmenta |
|---|---|---|---|---|
| Australian Cancer Study, Australian Ovarian Cancer Study (AUS)27 | Australia | 2002–2006 | Treatment centres, cancer registries | Electoral roll (compulsory enrolment) |
| Connecticut Ovarian Cancer Study (CON)10 | USA | 1998–2003 | Cancer registry, hospital records | Random digit dialling, Health Care Financing Administration records |
| Diseases of the Ovary and their Evaluation (DOV)11 | USA | 2002–2005 | Cancer registry | Random digit dialling |
| German Ovarian Cancer Study (GER)23 | Germany | 1993–1996 | Admissions to all hospitals serving the study regions | Population registries |
| Hawaii Ovarian Cancer Study (HAW)12,13 | USA | 1993–2008 | Cancer registry | Department of Health Annual Survey, Health Care Financing Administration records |
| Hormones and Ovarian Cancer Prediction (HOP)14 | USA | 2003–2009 | Cancer registries, pathology databases, physicians’ offices | Random digit dialling |
| Malignant Ovarian Cancer Study (MAL)24–26 | Denmark | 1995–1999 | Cancer registry, gynaecological departments | Random digit dialling |
| North Carolina Ovarian Cancer Study (NCO)15,16 | USA | 1999–2008 | Cancer registry | Random digit dialling |
| New England Case-Control Study of Ovarian Cancer (NEC)17,18 | USA | 1992–2003 | Cancer registries, hospital tumour boards | Random digit dialling, town books, drivers’ licence lists |
| New Jersey Ovarian Cancer Study (NJO)19,20 | USA | 2002–2008 | Cancer registry | Random digit dialling, Medicare and Medicaid Services, area sampling |
| Southern Ontario Ovarian Cancer Study (SON)28 | Canada | 1989–1992 | Cancer registry | Ministry of Revenue taxation roll |
| Genetic Epidemiology of Ovarian Cancer Study (STA)21 | USA | 1997–2002 | Cancer registry | Random digit dialling |
| Los Angeles County Case-Control Studies of Ovarian Cancer (USC)22 | USA | 1993–1999 | Cancer registry | Neighbourhood controls |
aControls were matched to cases on geographical region and age in all sites, and also on race/ethnicity in HAW, NCO, STA, and USC.
Epidemiological data were collected by in-person or telephone interviews and by self-administered questionnaires. Women were categorized as having had a tubal ligation if they reported having the procedure at least 2 years prior to the reference date, which was the date of diagnosis (cases) or enrolment (controls); 38 (0.8%) women who had the procedure within 2 years of the reference date were excluded. Twelve (0.2%) women who were older than 50 years at the time of tubal ligation were excluded because of possible error in the reported age or non-contraceptive reasons for having undergone the procedure. Sensitivity analyses showed that including women who had a tubal ligation within 2 years of enrolment or were >50 years of age at the time of the procedure made little difference (data not shown). Pathology data were obtained from cancer registries, pathology reports and medical records. Study pathologists reviewed the pathology reports for 47% of cases, and histological slides for 10% of cases.
Statistical analysis
We included age, race/ethnicity, oral contraceptive use and number of full-term pregnancies in all models. Age was modelled as both a continuous variable and as 5-year categories (<35, 35–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, 75+ years). Race/ethnicity was categorized as non-Hispanic White, Hispanic White, Black, Asian or other. Oral contraceptive use duration was categorized as never, <2, 2–4, 5–9 or 10+ years. Number of full-term pregnancies was categorized as 0, 1, 2, 3 or 4+. Potential confounding was also evaluated but not found for the following: age at last pregnancy, breastfeeding duration, age at menarche, menopausal status, use of hormone replacement therapy, history of endometriosis, hysterectomy, body mass index and family history of ovarian cancer in a first-degree relative. We modelled age at tubal ligation as a continuous variable and 5-year categories (<30, 30–34, 35–39, 40+ years), and years since tubal ligation as a continuous variable and 10-year categories (2–9, 10–19, 20–29, 30+ years). Calendar year of tubal ligation was categorized as <1970, 1970–74, 1975–79, 1980+, corresponding to approximate secular changes in tubal ligation methods. The most common procedures were Pomeroy ligation before 1970, laparoscopic unipolar electrocautery in the early 1970s and laparoscopic bipolar electrocautery since the late 1970s; use of less invasive laparoscopic methods such as rings and clips increased during the 1970s and stabilized during the 1980s.29,30
Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated using conditional logistic regression matched on sets determined by combinations of site, race/ethnicity and 5-year age categories, and adjusted for age as a continuous variable, oral contraception use and number of full-term pregnancies. There was little evidence of heterogeneity among the estimated effects across sites as assessed using likelihood ratio tests of the interactions with site. We conducted subtype-specific analyses of serous, endometrioid, clear cell and mucinous invasive ovarian cancer, and of serous and mucinous borderline tumours. Numbers of clear cell and endometrioid borderline tumours were too small for analysis. Invasive serous cancers were subdivided into low-grade (well differentiated) and high-grade (moderately or poorly differentiated) tumours because these subtypes may have distinct clinicopathological features and aetiology.3 Heterogeneity among subtype-specific effects was evaluated using Wald tests of differences among adjusted subtype-specific ORs estimated using polytomous logistic regression. Among women who had a tubal ligation, we tested for linear trends in the effects of age at the time of the procedure and years since the procedure, modelled as continuous variables. All statistical tests were two-sided and performed using Stata 11 (Stata Corporation, College Station, TX, USA).
Results
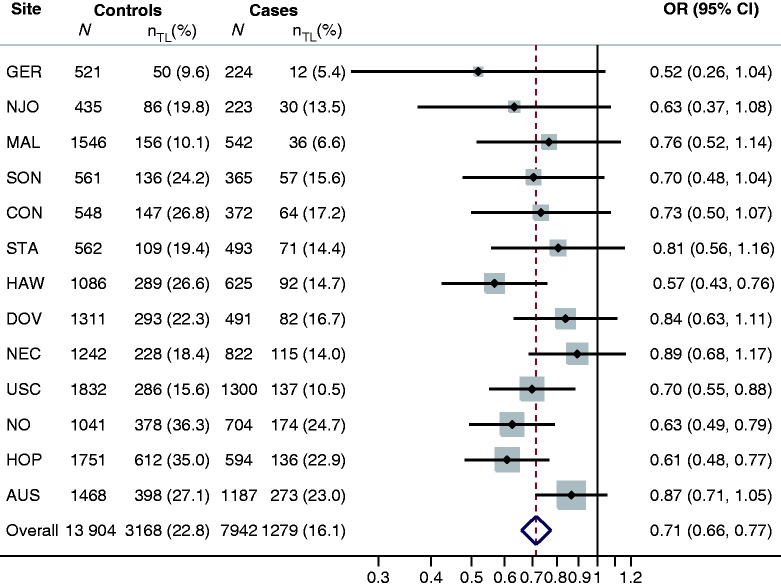
Characteristics of women with invasive epithelial ovarian cancer and borderline ovarian cancer and controls are shown in Table 2. Tubal ligation was less common among cases (invasive, 16.1% and borderline, 17.8%) than controls (22.8%), but the age at tubal ligation among women who had the procedure was similar in the three groups. The prevalence of tubal ligation among controls at each site ranged from 15.6% to 36.3% in the USA, 9.6% to 10.1% in Europe and was 27.1% and 24.2% in Australia and Canada, respectively (Figure 1).
Table 2.
Characteristics of women with invasive and borderline cancers and controls
| Controls | Invasive cases | Borderline cases | |
|---|---|---|---|
| Characteristic | N = 13 904 | N = 7942 | N = 2215 |
| Age (years), mean ± S.D. | 55.4 ± 12.3 | 56.9 ± 11.3 | 48.0 ± 12.9 |
| Race/ethnicity | |||
| Non-Hispanic White | 11 879 (85.4) | 6657 (83.8) | 1788 (80.7) |
| Hispanic White | 388 (2.8) | 277 (3.5) | 121 (5.5) |
| Black | 474 (3.4) | 228 (2.9) | 94 (4.2) |
| Asian | 612 (4.4) | 466 (5.9) | 100 (4.5) |
| Other | 551 (4.0) | 314 (4.0) | 112 (5.1) |
| Oral contraceptive use (years) | |||
| None | 4669 (33.6) | 3626 (45.7) | 699 (31.6) |
| <2 | 2361 (17.0) | 1471 (18.5) | 447 (20.2) |
| 2–4 | 2236 (16.1) | 1081 (13.6) | 355 (16.0) |
| 5–9 | 2254 (16.2) | 934 (11.8) | 355 (16.0) |
| 10+ | 2384 (17.1) | 830 (10.4) | 359 (16.2) |
| Full-term pregnancies | |||
| 0 | 2068 (14.9) | 1922 (24.2) | 689 (31.1) |
| 1 | 1936 (13.9) | 1163 (14.6) | 412 (18.6) |
| 2 | 4668 (33.6) | 2293 (28.9) | 578 (26.1) |
| 3 | 2982 (21.4) | 1445 (18.2) | 314 (14.2) |
| 4+ | 2250 (16.2) | 1119 (14.1) | 222 (10.0) |
| Prior tubal ligation | |||
| No | 10 736 (77.2) | 6663 (83.9) | 1821 (82.2) |
| Yesa | 3168 (22.8) | 1279 (16.1) | 394 (17.8) |
| Age at tubal ligation (years), | |||
| mean ± S.D. | 33.3 ± 5.5 | 33.2 ± 5.6 | 32.1 ± 5.6 |
aTubal ligation at least 2 years prior to the date of diagnosis for cases or interview for controls.
Figure 1.
Risk of invasive ovarian cancer associated with tubal ligation, by study site and overall. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated using conditional logistic regression, matched on sets determined by site, race/ethnicity (non-Hispanic White, Hispanic White, Black, Asian, other) and age (5-year categories), and adjusted for age (continuous), oral contraceptive use (none, <2, 2–4, 5–9, 10+ years), and number of full-term pregnancies (0, 1, 2, 3, 4+). The overall estimate was based upon analysis of the pooled data rather than meta-analysis of site-specific estimates. There was little heterogeneity among study sites (P = 0.38). The number (N) of cases and controls at each site who had a tubal ligation is denoted by nTL
Tubal ligation was associated with a 29% reduced risk of invasive ovarian cancer overall (OR, 0.71; 95% CI, 0.66-0.77; P < 0.001), after accounting for study site, age, race/ethnicity, oral contraceptive use and number of full-term births (Figure 1). Reduced risks were consistently found for every site, with little evidence of heterogeneity across sites (P = 0.38). In contrast, no significant associations were found between tubal ligation and risk of borderline ovarian tumors, either overall (OR, 0.98; 95% CI, 0.86-1.12; P = 0.80) or separately for serous borderline (OR, 0.98; 95% CI, 0.83-1.16; P = 0.80) or mucinous borderline (OR, 1.01; 95% CI, 0.83-1.23; P = 0.94) subtypes (Table 3).
Table 3.
Subtype-specific risk of invasive and borderline ovarian tumours associated with tubal ligation
| Cases | Prior tubal ligation | ORa (95% CI) | |
|---|---|---|---|
| Tumour behaviour, histology | N | N (%) | |
| Invasive | |||
| Serous | 4777 | 893 (18.7) | 0.81 (0.74-0.89) |
| High-gradeb | 3791 | 729 (19.2) | 0.80 (0.73-0.89) |
| Low-gradeb | 361 | 58 (16.1) | 0.89 (0.65-1.22) |
| Mucinous | 574 | 77 (13.4) | 0.68 (0.52-0.89) |
| Endometrioid | 1273 | 138 (10.8) | 0.48 (0.40-0.59) |
| Clear cell | 737 | 81 (11.0) | 0.52 (0.40-0.67) |
| Borderlinec | |||
| Serous | 1309 | 233 (17.8) | 0.98 (0.83-1.16) |
| Mucinous | 906 | 161 (17.8) | 1.01 (0.83-1.23) |
| Serous/mucinous | 2215 | 394 (17.8) | 0.98 (0.86-1.12) |
aConditional logistic regression stratified by site, race/ethnicity (non-Hispanic White, Hispanic White, Black, Asian, other) and age (5-year categories), and adjusted for age (continuous), oral contraceptive use (never, <2, 2–4, 5–9, 10+ years), and number of full-term pregnancies (0, 1, 2, 3, 4+); 13 904 control women were available for analysis.
bCases with invasive serous cancer but missing tumour grade (9.0%) were excluded; SON (561 controls, 212 serous cases) was excluded as grade information was not available.
cNJO (435 controls) was excluded as no borderline cases were available.
Tubal ligation was associated with significantly reduced risks of the four main histological types of invasive ovarian cancer (Table 3), and the magnitude of the risk reduction differed significantly among these subtypes (Wald P < 0.0001). Compared with women who did not have a tubal ligation, women who underwent the procedure had reduced risks of 19% (OR, 0.81; 95% CI, 0.74-0.89; P < 0.001) for invasive serous cancer, 32% (OR, 0.68; 95% CI, 0.52-0.89; P = 0.005) for invasive mucinous cancer, 48% (OR, 0.52; 95% CI, 0.40-0.67; P < 0.001) for clear cell cancer and 52% (OR, 0.48; 95% CI, 0.40-0.59; P < 0.001) for endometrioid cancer. Comparison of adjusted odds ratios estimated using polytomous logistic regression showed that tubal ligation was associated with a significantly smaller risk reduction for serous than for clear cell (Wald P = 0.0018) or endometrioid (Wald P < 0.0001) cancer. The risk reduction for mucinous cancer was intermediate in magnitude and did not differ significantly from that of the other histological types. For invasive serous cancers, the magnitude of risk reduction did not differ significantly between high-grade (OR, 0.80; 95% CI, 0.73-0.89; P < 0.001) and low-grade disease (OR, 0.89; 95% CI, 0.65-1.22; P = 0.46). There was no evidence of significant heterogeneity among study sites (P > 0.05) in any subtype-specific analysis. Exploratory analyses restricted to women with no history of hysterectomy yielded similar results suggesting that the reduced risk associated with tubal ligation was not explained by hysterectomy status (data not shown).
Table 4 shows the distribution of age at tubal ligation, years since the procedure and calendar year of the procedure among controls and women with invasive cancers overall and by subtype. Tubal ligation at any time was generally associated with reduced risks of all invasive subtypes except invasive serous low-grade cancer (Table 5). Furthermore, the risk reductions persisted for over 30 years following the procedure. Younger age at tubal ligation and longer time since the procedure were not associated with greater protection overall or for any subtype, despite tight control for reference age in all analyses and stratification by duration of oral contraceptive use (data not shown). For serous high-grade cancer, a slight trend towards greater protection with older age at tubal ligation and more recent procedures was found (P-trend = 0.01) which was of borderline significance after applying a Bonferroni correction for the number of disease subtypes examined. Tubal ligation procedures performed during any calendar period were generally associated with reduced risks of invasive ovarian cancer, with little evidence of heterogeneity (P > 0.05). Relatively few women had a tubal ligation prior to 1975 and the confidence intervals tended to be wider for estimates in these earlier time periods.
Table 4.
Timing of tubal ligation procedure among controls and cases with invasive ovarian cancer, by histological subtype
| Controls | Invasive cases | HGSCa | LGSCa | Mucinous | Endometrioid | Clear cell | |
|---|---|---|---|---|---|---|---|
| Tubal ligation status | N = 13 904 | N = 7942 | N = 3791 | N = 361 | N = 574 | N = 1273 | N = 737 |
| Never | 10 736 (77.2) | 6663 (83.9) | 3062 (80.8) | 303 (83.9) | 497 (86.6) | 1135 (89.2) | 656 (89.0) |
| Age at procedure (years) | |||||||
| <30 | 810 (5.8) | 329 (4.1) | 186 (4.9) | 15 (4.2) | 24 (4.2) | 41 (3.2) | 17 (2.3) |
| 30–34 | 1048 (7.5) | 428 (5.4) | 263 (6.9) | 20 (5.5) | 22 (3.8) | 43 (3.4) | 17 (2.3) |
| 35–39 | 868 (6.2) | 349 (4.4) | 186 (4.9) | 18 (5.0) | 23 (4.0) | 36 (2.8) | 32 (4.3) |
| 40+ | 442 (3.2) | 173 (2.2) | 94 (2.5) | 5 (1.4) | 8 (1.4) | 18 (1.4) | 15 (2.0) |
| Years since procedure | |||||||
| 2–9 | 364 (2.6) | 89 (1.1) | 38 (1.0) | 5 (1.4) | 15 (2.6) | 9 (0.7) | 6 (0.8) |
| 10–19 | 968 (7.0) | 364 (4.6) | 183 (4.8) | 23 (6.4) | 26 (4.5) | 44 (3.5) | 30 (4.1) |
| 20–29 | 1182 (8.5) | 555 (7.0) | 330 (8.7) | 18 (5.0) | 28 (4.9) | 60 (4.7) | 36 (4.9) |
| 30+ | 654 (4.7) | 271 (3.4) | 178 (4.7) | 12 (3.3) | 8 (1.4) | 25 (2.0) | 9 (1.2) |
| Calendar year of procedure | |||||||
| <1970 | 318 (2.3) | 147 (1.9) | 88 (2.3) | 5 (1.4) | 6 (1.1) | 16 (1.3) | 6 (0.8) |
| 1970–1974 | 496 (3.6) | 218 (2.7) | 133 (3.5) | 8 (2.2) | 12 (2.1) | 18 (1.4) | 11 (1.5) |
| 1975–1979 | 726 (5.2) | 329 (4.1) | 208 (5.5) | 9 (2.5) | 17 (3.0) | 29 (2.3) | 21 (2.9) |
| 1980+ | 1628 (11.7) | 585 (7.4) | 300 (7.9) | 36 (10.0) | 42 (7.3) | 75 (5.9) | 43 (5.8) |
aHigh-grade (HGSC) and low-grade serous cancer (LGSC); cases with invasive serous disease but missing tumour grade (9.0%) were excluded; grade information was not available for SON.
Table 5.
Odds ratiosa (95% CI) for the risk of invasive ovarian cancer associated with tubal ligation status
| Invasive cases | HGSCb | LGSCb | Mucinous | Endometrioid | Clear cell | |
|---|---|---|---|---|---|---|
| Tubal ligation status | N = 7942 | N = 3791 | N = 361 | N = 574 | N = 1273 | N = 737 |
| Never | Referent | Referent | Referent | Referent | Referent | Referent |
| Age at procedure (years) | ||||||
| <30 | 0.73 (0.63-0.84) | 0.81 (0.68-0.97) | 0.85 (0.49-1.48) | 0.77 (0.50-1.20) | 0.53 (0.38-0.75) | 0.42 (0.25-0.69) |
| 30–34 | 0.76 (0.67-0.86) | 0.89 (0.77-1.04) | 0.98 (0.60-1.59) | 0.60 (0.38-0.94) | 0.48 (0.35-0.66) | 0.32 (0.19-0.54) |
| 35–39 | 0.71 (0.62-0.81) | 0.74 (0.62-0.88) | 1.04 (0.63-1.73) | 0.79 (0.51-1.22) | 0.46 (0.33-0.66) | 0.79 (0.54-1.15) |
| 40+ | 0.64 (0.53-0.78) | 0.72 (0.57-0.92) | 0.51 (0.20-1.29) | 0.51 (0.25-1.04) | 0.44 (0.27-0.72) | 0.66 (0.38-1.14) |
| P-trendc | 0.070 | 0.010 | 0.701 | 0.995 | 0.487 | 0.299 |
| Years since procedure | ||||||
| 2–9 years | 0.56 (0.44-0.72) | 0.68 (0.47-0.96) | 0.62 (0.25-1.56) | 0.78 (0.44-1.36) | 0.27 (0.14-0.54) | 0.42 (0.18-0.98) |
| 10–19 years | 0.70 (0.61-0.80) | 0.80 (0.67-0.96) | 1.09 (0.69-1.73) | 0.67 (0.44-1.02) | 0.44 (0.32-0.61) | 0.61 (0.41-0.90) |
| 20–29 years | 0.77 (0.69-0.87) | 0.82 (0.71-0.94) | 0.74 (0.44-1.25) | 0.77 (0.51-1.16) | 0.58 (0.43-0.77) | 0.56 (0.39-0.81) |
| 30+ years | 0.72 (0.61-0.84) | 0.82 (0.68-0.99) | 0.99 (0.52-1.88) | 0.45 (0.22-0.92) | 0.53 (0.34-0.81) | 0.33 (0.17-0.66) |
| P-trendc | 0.070 | 0.010 | 0.701 | 0.995 | 0.487 | 0.299 |
| Calendar year of procedure | ||||||
| <1970 | 0.76 (0.61-0.93) | 0.85 (0.66-1.10) | 0.64 (0.25-1.64) | 0.52 (0.23-1.20) | 0.64 (0.38-1.08) | 0.49 (0.21-1.14) |
| 1970–1974 | 0.72 (0.60-0.85) | 0.83 (0.67-1.03) | 0.93 (0.44-1.95) | 0.79 (0.43-1.45) | 0.43 (0.26-0.70) | 0.44 (0.23-0.83) |
| 1975–1979 | 0.73 (0.63-0.85) | 0.87 (0.73-1.03) | 0.61 (0.31-1.23) | 0.71 (0.43-1.19) | 0.43 (0.29-0.63) | 0.54 (0.34-0.86) |
| 1980+ | 0.70 (0.63-0.79) | 0.75 (0.65-0.86) | 1.05 (0.72-1.54) | 0.68 (0.48-0.95) | 0.49 (0.38-0.64) | 0.54 (0.39-0.75) |
| Phetd | 0.923 | 0.527 | 0.472 | 0.876 | 0.632 | 0.941 |
aConditional logistic regression stratified by site, race/ethnicity (non-Hispanic White, Hispanic White, Black, Asian, other) and age (5-year categories), and adjusted for age (continuous), oral contraceptive use (never, <2, 2–4, 5–9, 10+ years), and number of full-term pregnancies (0, 1, 2, 3, 4+).
bHigh-grade (HGSC) and low-grade serous cancer (LGSC); cases with invasive serous disease but missing tumour grade (9.0%) were excluded; grade information was not available for SON.
cTest for linear trend among women with tubal ligations modelling age at or years since the procedure as continuous variables. Note that these two trend tests yield nearly identical P-values when reference age is tightly controlled by both matching (5-year categories) and adjusting (continuous variable) for residual confounding using conditional logistic regression.
dTest for heterogeneity among the estimated coefficients for each of the four calendar periods.
We explored the association of tubal ligation with stage, grade and age at diagnosis of invasive ovarian cancer (data not shown). No significant associations were found between tubal ligation and the stage at diagnosis for any invasive subtype. Among women with invasive endometrioid cancer, tubal ligation was associated with higher tumour grade (P = 0.001, unadjusted). A polytomous logistic regression model showed that tubal ligation tended to be associated with greater reductions in the risk of well differentiated (OR, 0.26; 95% CI, 0.16-0.43) than moderately (OR, 0.55; 95% CI, 0.40-0.75) or poorly (OR, 0.68; 95% CI, 0.50-0.92) differentiated endometrioid cancers (Phet = 0.005), after adjusting for site, age, race, oral contraceptive use and parity. No significant associations were found between tubal ligation and tumour grade at diagnosis for any other invasive subtype. Among women with invasive clear cell cancer, tubal ligation was associated with fewer early-onset (before 50 years of age) cases (P < 0.001). However, after adjusting for site, age, race, oral contraceptive use and parity, tubal ligation was not associated with significantly greater reductions in the risk of early-onset (OR, 0.31; 95% CI, 0.16-0.59) versus late-onset (OR, 0.58; 95% CI, 0.44-0.77) clear cell cancer (Phet = 0.08). No significant associations were found between tubal ligation and age at onset of any other invasive subtype.
Discussion
In this pooled analysis of primary data from 13 population-based case-control studies, tubal ligation was associated with significantly reduced risks of invasive endometrioid, clear cell, mucinous and serous high-grade ovarian cancer. Furthermore, the magnitude of risk reduction was greater for invasive endometrioid and clear cell than for serous cancer. In contrast, tubal ligation was not associated with risks of borderline serous and mucinous tumours. To our knowledge, this is the largest study of tubal ligation and ovarian cancer risk, enabling robust estimation of effects on uncommon disease subtypes for the first time. These findings provide information about the subtype-specific aetiology of ovarian cancer, and inform current theories regarding the underlying mechanisms by which tubal ligation may prevent disease occurrence.
The major strengths of this international collaborative effort were the inclusion of studies with the same population-based incident case-control design, application of standard data harmonization and analysis methods, and large sample size. One limitation was the reliance on subtype classification by individual studies rather than by central pathological review of tumour slides. Histological classification by different pathologists has been shown to be reasonably reproducible for cell type, but less reproducible for grade.31 Misclassification of histological type would be expected to reduce the ability to detect subtype-specific differences. Misclassification of grade would most likely have resulted in the inclusion of some serous high-grade among the serous low-grade cancers which could have biased the latter results away from the null, but no significant association with serous low-grade cancer was found. Overall, there was no evidence of study heterogeneity of the effects of tubal ligation on any disease subtype, suggesting that the findings are likely to be robust and generalizable. A second limitation was the potential for recall bias. However, a woman’s self-reported tubal ligation history has been shown to be highly accurate compared with medical records,32 and recall bias likely had minimal impact on the study findings. Finally, we cannot exclude the possibility that unmeasured confounders that act differently among disease subtypes could contribute to the observed subtype-specific differences.
Consistent with our findings, previous studies have reported a protective association of tubal ligation with invasive serous cancer,7,8,33 and no association with borderline tumours.7 However, previous studies of invasive endometrioid, clear cell and mucinous ovarian cancers have yielded inconsistent or inconclusive results largely because of limited numbers of patients and few published data for these rarer subtypes.7,8,33 Whereas a protective association with endometrioid cancer was reported by a meta-analysis of four case-control studies,7 no association was found in an earlier pooled analysis of 10 case-control studies, most conducted during the 1970s and 1980s, that included a total of 373 cases.33 Previous studies have reported suggestive inverse associations with invasive clear cell cancer and non-significant inverse associations with invasive mucinous cancer, but were limited by small numbers and study heterogeneity.7,8,33 In contrast, there was no evidence of study heterogeneity in the present study, which included substantially larger numbers of patients than previous studies.
Several mechanisms have been proposed to explain the protective effects of tubal ligation on ovarian cancer risk. Tubal ligation has been hypothesized to reduce blood flow to the ovary resulting in altered levels of hormones and growth factors, block the retrograde flow of carcinogenic or inflammatory agents from the vagina into the peritoneal cavity, and induce immunity to mucins which are overexpressed in ovarian cancer.34–38 Although these mechanisms may each contribute to overall risk reduction, none can explain the subtype-specific differences we found.
We hypothesize that differences in the subtype-specific effects of tubal ligation may be explained by their different cells of origin and the extent to which tubal ligation ablates or obstructs these cells from seeding the ovaries. There is growing evidence that most ovarian cancers arise from tissues embryologically derived from the Müllerian ducts.39 Endometrioid and clear cell ovarian cancers are believed to originate from exfoliated endometrial cells,40 and are associated with endometriosis41 and mutations in the ARID1A gene.42 In contrast, many serous high-grade cancers are believed to originate from the distal fimbriated end of the Fallopian tube.3 We hypothesize that tubal ligation is significantly more protective for endometrioid and clear cell cancers than for serous high-grade cancer because the location of the ligation near the utero-tubal junction prevents the retrograde transport and ovarian seeding by cells originating from the endometrium but not the distal tubes.
Invasive low-grade serous cancers are thought to arise from borderline tumours and have frequent KRAS and BRAF mutations, unlike serous high-grade cancers which are characterized by TP53 mutations.3 Thus, the absence of an association of tubal ligation with both invasive low-grade and borderline serous cancer would be biologically consistent, although the number of patients with invasive low-grade disease was small and the confidence interval for the estimated effect was broad. Mucinous and non-mucinous cancers have different risk factor profiles,26,43 and the origin of mucinous tumours is uncertain. They have been suggested to arise from paratubal and paraovarian embryological remnants of the Müllerian ducts39 and transitional-type epithelium located at the tubal-mesothelial junction where the fimbria contact the peritoneum.40 Due to their location, tubal ligation may not prevent ovarian seeding by these cells of origin and may reduce the risk of invasive mucinous cancer predominantly through other mechanisms.
The present finding that the protective effects of tubal ligation were not enhanced with younger age at tubal ligation or longer duration since the procedure is consistent with most previous studies.7,8 This finding suggests that having a tubal ligation at any age may reduce a woman’s risk of developing ovarian cancer, and that the maximum protective effects of tubal ligation may be achieved within a few years following the procedure. These data are consistent with a risk model in which tubal ligation confers an absolute decrease in the age-specific incidence rate of ovarian cancer, where the magnitude of the decrease is independent of age, and the age-specific incidence curves are parallel for women with and without the procedure.44 Such a decrease could plausibly result from abrupt and permanent reduction in the pool of potential precursor cells,44 as occurs when the tubal ligation procedure ablates or prevents ovarian seeding by pre-cancerous cells from the uterus and Fallopian tube.
In summary, in this large collaborative study, tubal ligation was associated with reduced risks of invasive ovarian cancer that were greatest for the endometrioid and clear cell subtypes, intermediate for mucinous cancer and smallest for serous high-grade cancer. These findings show that tubal ligation has subtype-specific effects, and suggest that the protective effects of tubal ligation are mediated by ablation of or prevention of ovarian seeding by distinct cells of origin for each subtype. The protective effects of tubal ligation did not appear to diminish with older age at the time of the procedure, and persisted for at least three decades. These findings highlight the potential of tubal ligation as a preventive intervention for ovarian cancer. To guide clinical practice, future studies are needed to determine whether tubal ligation procedures, such as bilateral salpingectomy, that ablate a greater portion of the Fallopian tube than standard procedures would result in greater reductions in the risk of ovarian cancer.
Funding
This work was supported by donations from the family and friends of Kathryn Sladek Smith to the Ovarian Cancer Research Fund. It was also supported by the U.S. National Institutes of Health: R01CA074850, R01CA080742 (CON), R01CA112523, R01CA87538 (DOV), R01CA58598, N01CN55424, N01PC67001 (HAW), R01CA95023 (HOP), R01CA61107 (MAL), R01CA76016 (NCO), R01CA54419, P50CA105009 (NEC), K07CA095666, R01CA83918, K22CA138563, R01CA120429 (NJO), U01CA71966, U01CA69417, R01CA16056, K07CA143047 (STA), R01CA136891, R01CA14089, R01CA17054, R01CA61132, R01CA63464, N01PC67010, R03CA113148 (USC); the U.S. Department of Defense: DAMD17-01-1-0729, W81XWH0610220 (AUS), DAMD17-02-1-0669 (HOP), DAMD17-02-1-0666 (NCO), W81XWH-10-1-02802 (NEC); the California Cancer Research Program 00-01389V-20170, 2II0200 (USC); National Health and Medical Research Council of Australia 199600 (AUS); Cancer Councils of New South Wales, Victoria, Queensland, South Australia and Tasmania (AUS); Cancer Foundation of Western Australia (AUS); German Federal Ministry of Education and Research, Programme of Clinical Biomedical Research 01 GB 9401 (GER); German Cancer Research Center (GER); Danish Cancer Society research grant 94 222 52 (MAL); the Mermaid I project (MAL); the Cancer Institute of New Jersey (NJO); Radboud University Nijmegen Medical Centre (NTH); National Health Research and Development Program of Health and Welfare Canada Grant 6613-1415-53 (SON).
Acknowledgements
The Australian group and all other groups gratefully acknowledge the contribution of all clinical and scientific collaborators. Some of the data used in the CON study were obtained from the Connecticut Tumor Registry in the Connecticut Department of Public Health. We assume full responsibility for the analyses and interpretation of these data.
Conflict of interest: None declared.
KEY MESSAGES.
Tubal ligation is associated with reduced risks of invasive ovarian cancer that were greatest for the endometrioid and clear cell subtypes, smallest for serous cancer and intermediate for mucinous cancer.
The subtype-specific effects of tubal ligation suggest that its protective effects are mediated in part by ablation of or prevention of ovarian seeding by distinct cells of origin for each subtype.
The protective effects of tubal ligation do not diminish with older age at the time of the procedure, and persist for at least three decades.
To guide clinical practice, future studies are needed to determine whether tubal ligation procedures, such as bilateral salpingectomy, that ablate a greater portion of the Fallopian tubes than standard procedures would result in greater reductions in the risk of ovarian cancer.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Seidman JD, Russell P, Kurman RJ. Surface epithelial tumors of the ovary. In: Kurman RJ, editor. Blaustein's Pathology of the Female Genital Tract. 5th edn. New York: Springer; 2002. [Google Scholar]
- 3.Vang R, Shih Ie M, Kurman RJ. Ovarian low-grade and high-grade serous carcinoma: pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems. Adv Anat Pathol. 2009;16:267–82. doi: 10.1097/PAP.0b013e3181b4fffa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaughan S, Coward JI, Bast RC, Jr, et al. Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer. 2011;11:719–25. doi: 10.1038/nrc3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Köbel M, Kalloger SE, Boyd N, et al. Ovarian carcinoma subtypes are different diseases: implications for biomarker studies. PLoS Med. 2008;5:e232. doi: 10.1371/journal.pmed.0050232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawrie TA, Nardin JM, Kulier R, Boulvain M. Techniques for the interruption of tubal patency for female sterilisation. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD003034.pub2. (2):CD003034. [DOI] [PubMed] [Google Scholar]
- 7.Cibula D, Widschwendter M, Majek O, Dusek L. Tubal ligation and the risk of ovarian cancer: review and meta-analysis. Hum Reprod Update. 2011;17:55–67. doi: 10.1093/humupd/dmq030. [DOI] [PubMed] [Google Scholar]
- 8.Rice MS, Murphy MA, Tworoger SS. Tubal ligation, hysterectomy and ovarian cancer: A meta-analysis. J Ovarian Res. 2012;5:13. doi: 10.1186/1757-2215-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berchuck A, Schildkraut JM, Pearce CL, Chenevix-Trench G, Pharoah PD. Role of genetic polymorphisms in ovarian cancer susceptibility: development of an international ovarian cancer association consortium. Adv Exp Med Biol. 2008;622:53–67. doi: 10.1007/978-0-387-68969-2_5. [DOI] [PubMed] [Google Scholar]
- 10.Risch HA, Bale AE, Beck PA, Zheng W. PGR +331 A/G and increased risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:1738–41. doi: 10.1158/1055-9965.EPI-06-0272. [DOI] [PubMed] [Google Scholar]
- 11.Rossing MA, Cushing-Haugen KL, Wicklund KG, Doherty JA, Weiss NS. Menopausal hormone therapy and risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:2548–56. doi: 10.1158/1055-9965.EPI-07-0550. [DOI] [PubMed] [Google Scholar]
- 12.Goodman MT, Lurie G, Thompson PJ, McDuffie KE, Carney ME. Association of two common single-nucleotide polymorphisms in the CYP19A1 locus and ovarian cancer risk. Endocr Relat Cancer. 2008;15:1055–60. doi: 10.1677/ERC-08-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lurie G, Wilkens LR, Thompson PJ, et al. Combined oral contraceptive use and epithelial ovarian cancer risk: time-related effects. Epidemiology. 2008;19:237–43. doi: 10.1097/EDE.0b013e31816334c5. [DOI] [PubMed] [Google Scholar]
- 14.Ness RB, Dodge RC, Edwards RP, Baker JA, Moysich KB. Contraception methods, beyond oral contraceptives and tubal ligation, and risk of ovarian cancer. Ann Epidemiol. 2011;21:188–96. doi: 10.1016/j.annepidem.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schildkraut JM, Iversen ES, Wilson MA, et al. Association between DNA damage response and repair genes and risk of invasive serous ovarian cancer. PLoS One. 2010;5:e10061. doi: 10.1371/journal.pone.0010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schildkraut JM, Moorman PG, Bland AE, et al. Cyclin E overexpression in epithelial ovarian cancer characterizes an etiologic subgroup. Cancer Epidemiol Biomarkers Prev. 2008;17:585–93. doi: 10.1158/1055-9965.EPI-07-0596. [DOI] [PubMed] [Google Scholar]
- 17.Terry KL, De Vivo I, Titus-Ernstoff L, Shih MC, Cramer DW. Androgen receptor cytosine, adenine, guanine repeats, and haplotypes in relation to ovarian cancer risk. Cancer Res. 2005;65:5974–81. doi: 10.1158/0008-5472.CAN-04-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terry KL, Tworoger SS, Goode EL, et al. MTHFR polymorphisms in relation to ovarian cancer risk. Gynecol Oncol. 2010;119:319–24. doi: 10.1016/j.ygyno.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bandera EV, King M, Chandran U, Paddock LE, Rodriguez-Rodriguez L, Olson SH. Phytoestrogen consumption from foods and supplements and epithelial ovarian cancer risk: a population-based case control study. BMC Womens Health. 2011;11:40. doi: 10.1186/1472-6874-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandran U, Bandera EV, Williams-King MG, et al. Healthy eating index and ovarian cancer risk. Cancer Causes Control. 2011;22:563–71. doi: 10.1007/s10552-011-9728-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGuire V, Felberg A, Mills M, et al. Relation of contraceptive and reproductive history to ovarian cancer risk in carriers and noncarriers of BRCA1 gene mutations. Am J Epidemiol. 2004;160:613–18. doi: 10.1093/aje/kwh284. [DOI] [PubMed] [Google Scholar]
- 22.Pike MC, Pearce CL, Peters R, Cozen W, Wan P, Wu AH. Hormonal factors and the risk of invasive ovarian cancer: a population-based case-control study. Fertil Steril. 2004;82:186–95. doi: 10.1016/j.fertnstert.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 23.Royar J, Becher H, Chang-Claude J. Low-dose oral contraceptives: protective effect on ovarian cancer risk. Int J Cancer. 2001;95:370–74. doi: 10.1002/1097-0215(20011120)95:6<370::aid-ijc1065>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 24.Glud E, Kjaer SK, Thomsen BL, et al. Hormone therapy and the impact of estrogen intake on the risk of ovarian cancer. Arch Intern Med. 2004;164:2253–59. doi: 10.1001/archinte.164.20.2253. [DOI] [PubMed] [Google Scholar]
- 25.Huusom LD, Frederiksen K, Hogdall EV, et al. Association of reproductive factors, oral contraceptive use and selected lifestyle factors with the risk of ovarian borderline tumors: a Danish case-control study. Cancer Causes Control. 2006;17:821–29. doi: 10.1007/s10552-006-0022-x. [DOI] [PubMed] [Google Scholar]
- 26.Soegaard M, Jensen A, Hogdall E, et al. Different risk factor profiles for mucinous and nonmucinous ovarian cancer: results from the Danish MALOVA study. Cancer Epidemiol Biomarkers Prev. 2007;16:1160–66. doi: 10.1158/1055-9965.EPI-07-0089. [DOI] [PubMed] [Google Scholar]
- 27.Merritt MA, Green AC, Nagle CM, Webb PM. Talcum powder, chronic pelvic inflammation and NSAIDs in relation to risk of epithelial ovarian cancer. Int J Cancer. 2008;122:170–76. doi: 10.1002/ijc.23017. [DOI] [PubMed] [Google Scholar]
- 28.Risch HA, Marrett LD, Howe GR. Parity, contraception, infertility, and the risk of epithelial ovarian cancer. Am J Epidemiol. 1994;140:585–97. doi: 10.1093/oxfordjournals.aje.a117296. [DOI] [PubMed] [Google Scholar]
- 29.Seiler JS. The evolution of tubal sterilization. Obstet Gynecol Surv. 1984;39:177–84. doi: 10.1097/00006254-198404000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Westhoff C, Davis A. Tubal sterilization: focus on the U.S. experience. Fertil Steril. 2000;73:913–22. doi: 10.1016/s0015-0282(00)00481-7. [DOI] [PubMed] [Google Scholar]
- 31.Gilks CB, Ionescu DN, Kalloger SE, et al. Tumor cell type can be reproducibly diagnosed and is of independent prognostic significance in patients with maximally debulked ovarian carcinoma. Hum Pathol. 2008;39:1239–51. doi: 10.1016/j.humpath.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Chilvers CE, Pike MC, Taylor CN, Hermon C, Crossley B, Smith SJ. General practitioner notes as a source of information for case-control studies in young women. UK National Case-Control Study Group. J Epidemiol Community Health. 1994;48:92–97. doi: 10.1136/jech.48.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurian AW, Balise RR, McGuire V, Whittemore AS. Histologic types of epithelial ovarian cancer: have they different risk factors? Gynecol Oncol. 2005;96:520–30. doi: 10.1016/j.ygyno.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 34.Woodruff JD. The pathogenesis of ovarian neoplasia. Johns Hopkins Med J. 1979;144:117–20. [PubMed] [Google Scholar]
- 35.Cramer DW, Welch WR. Determinants of ovarian cancer risk. II. Inferences regarding pathogenesis. J Natl Cancer Inst. 1983;71:717–21. [PubMed] [Google Scholar]
- 36.Whittemore AS, Harris R, Itnyre J. Characteristics relating to ovarian cancer risk: collaborative analysis of 12 US case-control studies. IV. The pathogenesis of epithelial ovarian cancer. Collaborative Ovarian Cancer Group. Am J Epidemiol. 1992;136:1212–20. doi: 10.1093/oxfordjournals.aje.a116429. [DOI] [PubMed] [Google Scholar]
- 37.Ness RB, Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. J Natl Cancer Inst. 1999;91:1459–67. doi: 10.1093/jnci/91.17.1459. [DOI] [PubMed] [Google Scholar]
- 38.Cramer DW, Titus-Ernstoff L, McKolanis JR, et al. Conditions associated with antibodies against the tumor-associated antigen MUC1 and their relationship to risk for ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1125–31. doi: 10.1158/1055-9965.EPI-05-0035. [DOI] [PubMed] [Google Scholar]
- 39.Dubeau L. The cell of origin of ovarian epithelial tumours. Lancet Oncol. 2008;9:1191–97. doi: 10.1016/S1470-2045(08)70308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurman RJ, Shih Ie M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–3. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pearce CL, Templeman C, Rossing MA, et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol. 2012;13:385–94. doi: 10.1016/S1470-2045(11)70404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiegand KC, Shah SP, Al-Agha OM, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363:1532–43. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Risch HA, Marrett LD, Jain M, Howe GR. Differences in risk factors for epithelial ovarian cancer by histologic type. Results of a case-control study. Am J Epidemiol. 1996;144:363–72. doi: 10.1093/oxfordjournals.aje.a008937. [DOI] [PubMed] [Google Scholar]
- 44.Whittemore AS. Quantitative theories of oncogenesis. In: Klein G, Weinhouse S, editors. Advances in Cancer Research. New York: Academic Press; 1978. [DOI] [PubMed] [Google Scholar]