Abstract
The chromosomal passenger complex (CPC) senses tension defects at the kinetochore to activate the spindle assembly checkpoint, and helps to position the cleavage furrow. The CPC, consisting of INCENP, Survivin, Borealin and Aurora B localizes to the inner centromere at metaphase and re-localizes to the spindle midzone at anaphase; several CPC functions are regulated by post-translational modification. Borealin is phosphorylated at multiple sites and phosphorylation at S219 causes Borealin to migrate more slowly upon electrophoresis. Here we find that Cdk1 can induce a mobility shift of Borealin, suggesting that S219 phosphorylation is under Cdk1 control. However, Cdk1 is inefficient at phosphorylating purified Borealin in vitro. A yeast orthologue of Borealin, Npl1, is dephosphorylated by the phosphatase Cdc14. We find no difference in the mobility shift of Borealin in human cells lacking either Cdc14A or Cdc14B. In contrast, the phosphatase inhibitor okadaic acid does delay the dephosphorylation of Borealin as cells exit mitosis. The proteasome inhibitor MG132 reduces Borealin phosphorylation in mitosis and increases the steady-state level of Borealin, especially in mutants lacking the C-terminus. However, a second, structurally unrelated proteasome inhibitor, lactacystin did not up-regulate Borealin. These results suggest that the effect of MG132 on Borealin is due to the inhibition of an intracellular protease other than the proteasome.
Keywords: centromere, checkpoint, chromosomal passenger complex, mitosis, PP2A
Post-translational modifications of the chromosomal passenger proteins are essential in regulation of the complex. For example, phosphorylation of INCENP by cyclin-dependent kinase (Cdk) during metaphase and dephosphorylation by Cdc14 during anaphase is essential for localization of the chromosomal passenger complex (CPC) to the central spindle in yeast (1). Borealin is expressed in a cell cycle-dependent manner and the levels of endogenous Borealin significantly increase during mitosis. Borealin is also phosphorylated during mitosis resulting in a decrease in its electrophoretic mobility (2). Phosphorylation at S219 of Borealin is sufficient to decrease its electrophoretic mobility, although other sites are likely phosphorylated in intact cells (3–5). Mutation of S219 to alanine in Borealin causes defects in mitotic progression indicating that phosphorylation is important in Borealin function. Mutation of additional sites creates a form of Borealin that is defective in localizing to the centromere, binding to Sgo1 and Sgo2, and in coordinating chromosome alignment (3, 6).
One of the primary candidate kinases that may phosphorylate Borealin S219 is Cdk1, which phosphorylates serines or threonines followed by proline (7). Other potential candidates include the mitogen-activated protein kinases, which are also proline-directed serine/threonine kinases (8). Here, we show that over-expression of Cdk1 induces Borealin to migrate more slowly suggesting that Cdk1 regulates Borealin phosphorylation. In contrast, purified Borealin is a relatively poor substrate for Cdk1 suggesting that additional factors may contribute to phosphorylation in cells. Borealin is dephosphorylated upon mitotic exit as indicated by a decrease in the slow migrating phosphorylated species (2). Potential candidates for Borealin dephosphorylation include Cdc14A or B, as well as PP2A, all of which have been implicated in dephosphorylating mitotic substrates (9–15). Clp1, a Cdc14 homologue of fission yeast is a bona fide interacting partner of the CPC, which co-localizes with the chromosomal passengers during mitosis (16). We now use somatic human knock-out cells to show that loss of either Cdc14A or Cdc14B does not affect Borealin dephosphorylation. However, okadaic acid delays the dephosphorylation of Borealin upon mitotic exit suggesting that Borealin dephosphorylation is regulated differently in yeast and animals.
Borealin expression is regulated in a cell cycle-dependent manner with increased levels of expression during G2 and mitosis. This increase in expression is partly due to higher levels of transcription but may also be the result of differences in protein degradation during mitosis. For example, higher levels of exogenous Borealin protein are found in mitotic cells even when the transfected Borealin is under the control of a CMV promoter that shows constant activity throughout the cell cycle (2). Here, we find that MG132 increases the steady-state level of Borealin during interphase but not mitosis. However, a second proteasome inhibitor, lactacystin did not affect Borealin levels suggesting that MG132 may inhibit Borealin proteolysis independently of its effects on the proteasome.
Experimental Procedures
Cell-culture conditions, transfections and drugs
Cells were grown in Dulbecco’s Modified Eagle Medium (Mediatech) with penicillin/streptomycin and 10% foetal bovine serum in a humidified atmosphere of 10% CO2 at 37°C. HeLaM cells, a subline of HeLa, were used for all experiments (17). HCT116 cells lacking Cdc14B and hTERT-RPE cells lacking Cdc14A were kindly provided by Dr Prasad Jallepalli (Memorial Sloan-Kettering Cancer Center). All chemicals were obtained from Fisher Scientific unless otherwise indicated. Nocodazole was used at a concentration of 200 ng/ml, hydroxyurea at 2 mM, MG132 at 5 µM, lactacystin at 20 µM, okadaic acid at 0.1 µM and puromycin was used at 1 μg/ml. Transient transfections were performed using either Fugene 6 (Roche) or Expressfect (Denville Scientific Inc) with similar results. In brief, the transfection reagent and DNA were used at a ratio of 3:1 and suspended in antibiotic and serum free DMEM to form DNA and polymer complexes. The mixture was incubated at room temperature for 20 min before being added to cells. The cells were incubated at 37°C, fresh DMEM containing serum and antibiotics was added 20 h post-transfection and cells incubated for another 24 h prior to harvesting. Truncated forms of Borealin were created using PCR-based mutagenesis and subsequently cloned into the pcDNA3.2 mammalian expression vector (5). All plasmids were confirmed by DNA sequencing.
Western blotting
Western blotting was conducted as previously described (2). RIPA Buffer composed of 10 mM Tris (pH 7.4), 150 mM NaCl, 1% NP-40, 1% DOC, 0.1% SDS, 1 mM phenyl methane sulphonyl fluoride (PMSF), 1 mM DTT, protease inhibitors (1 µg/ml aprotinin, 2 µg/ml leupeptin 1 µg/ml pepstatin) and phosphatase inhibitors (1 mM sodium fluoride and 1 mM sodium vanadate) was used to lyse cells on ice. Cell lysates were resolved by SDS–PAGE (12.5% acrylamide at a ratio of 37.5:1 acrylamide: bisacrylamide). For better separation of phosphorylated and unphosphorylated Borealin, cell lysates in certain experiments were separated by extended electrophoresis using 12.5% acrylamide with a ratio of 29.2:0.8 acrylamide: bisacrylamide. The proteins were transferred to polyvinyldenefluoride (PVDF) membranes obtained from Millipore. Western blots were blocked in buffer composed of 0.05% (v/v) Tween and 5% (w/v) non-fat dry milk in PBS and antibodies for the western blots were diluted in the same buffer. Antibodies to Cdk1, Cyclin B1 and Ubiquitin were from Santa Cruz Biotechnology, antibodies to β-actin were from Neomarkers and antibodies to endogenous Borealin were raised against a Borealin–glutathione-S-transferase (GST) fusion protein (2). The Flag-tag was detected with anti-ECS antibodies directly conjugated to horse radish peroxidase (HRP; Bethyl Laboratories). Goat anti-mouse and goat anti-rabbit secondary antibodies conjugated to HRP (Biorad) were used and detected by enhanced chemiluminescence (Thermo Scientific).
Immunofluorescence
Immunofluoresence was carried out as we have described (2, 5). Briefly, HeLaM cells grown on coverslips were transiently transfected with green fluorescent protein (GFP)-tagged Cdc14B. Twenty-four hours post-transfection, cells were fixed with 2% formaldehyde and permeabilized with 1% SDS made in pre-warmed 1% PBS. The cells were blocked with PBS containing 0.1% BSA for 1 h at room temperature and then incubated with a rabbit Borealin antiserum for 2 h at room temperature to detect endogenous Borealin. Cells were incubated with Alexafluor-568 conjugated goat anti rabbit antibodies (Invitrogen) 1 h at room temperature to detect Borealin. Cdc14B was detected by GFP fluorescence. Nuclei were stained with Hoechst 33342 and coverslips mounted using Vectashield (Vector Laboratories). Images were analysed using an Axiophot fluorescence microscope.
Immunoprecipitation
Asynchronous WT8 cells treated with or without MG132 were lysed in buffer composed of 50 mM HEPES (pH 7.5), 5 mM EDTA, 150 mM NaCl, 1% Triton X-100, 10 mM N-ethylmalamide, protease inhibitors (1 µg/ml aprotinin, 2 µg/ml leupeptin 1 µg/ml pepstatin) and phosphatase inhibitors (1 mM sodium fluoride and 1 mM sodium vanadate). The lysates were immunoprecipitated with polyubiquitin affinity resin® or control resin (Calbiochem). Affinity matrix bound to the protein was resuspended in 2× gel loading buffer composed of 250 mM Tris HCl, pH 6.8, 4% SDS, 10% β-mercaptoethanol, 20% glycerol and bromophenol blue. The immunoprecipitates were separated by SDS–PAGE and transfers probed with an antibody to the Flag-tag conjugated to HRP to detect Borealin (anti-ECS-HRP; Bethyl Laboratories).
In vitro kinase assays
Wild-type Borealin and Borealin S219A were expressed and purified from Escherichia coli as GST fusion proteins using a pGEX-3X vector. Recombinant GST–Borealin and GST–BorealinS219A were phosphorylated in vitro with 25 ng of purified Cdk1/Cyclin-B1 (Cell Signaling Technology) in kinase buffer (20 mM HEPES pH 7.9, 5 mM MgCl2, 10% glycerol, 100 mM DTT, 10 mM ATP) in the presence 5 µCi of γ-(32P) ATP. The reaction was incubated at room temperature for 30 min and stopped by adding Laemmli buffer (2% SDS, 100 mM Tris, 0.05% BPB, 30% glycerol) and boiling for 5 min. The proteins were resolved by SDS–PAGE, stained with Coomassie blue, dried in a gel dryer (Bio-Rad Laboratories) and visualized by autoradiography (Typhoon Phosphor Imager).
Amino-terminal biotinylated peptides corresponding to Borealin S219 GNGSPLADAK, Borealin S219A GNGAPLADAK and Cdk1 optimal substrate HATPPKKKRK were obtained from Synthetic Biomolecules at a purity of 95%. Peptide substrates at a concentration of 1 µm each were phosphorylated in vitro using purified Cdk1/Cyclin B1 as per the protocol mentioned above. A PVDF membrane (Millipore) was saturated with 500 mg of avidin followed by blocking in 0.5% BSA for 1 h at room temperature each. The kinase reactions were spotted on pre-treated PVDF membrane and incubated for 1 h at room temperature. The PVDF membrane was washed and reactions visualized by autoradiography (Typhoon Phosphor Imager).
Amplification of recombinant adenoviruses and determination of titres
Recombinant adenoviruses were kindly provided by Dr David Morgan (UCSF) and included viruses encoding nuclear cyclin B1 (NB1) and mutant cyclin dependent kinase-1 (Cdk1-AF) all downstream of a tet operator and minimal CMV promoter (18). An additional virus expressing the tetracycline transactivator (TTA) downstream of a constitutive CMV promoter was included in all infections to drive expression of Cdk1/Cyclin B1. Adenoviruses were amplified in HEK 293 cells and viral supernatants re-suspended in 10% glycerol in PBS and stored at 4°C. To determine titres, virus supernatants were serially diluted and used to infect HEK 293 cells seeded in a 96-well plate that was incubated at 37°C for 5 days. Cells were stained with 50% ethanol saturated with methylene blue. The maximum dilution that still induced cytopathic effects was designated as the nominal titre. HeLaM cells were infected with the TTA virus plus the desired NB1, WTB1 or Cdk1-AF virus or viruses, each at a multiplicity of infection (MOI) of 50 plaque forming units per cell. The infected cells were incubated for 24 h at 37°C prior to harvesting.
Results
Borealin is phosphorylated in response to Cdk1 over expression
Borealin is phosphorylated at S219 during mitosis; mutation of this residue to alanine eliminated the slow migrating mitotic form of Borealin (5). The residues following S219 of Borealin conform to the partial Cdk1 consensus sequence [S/T] P (7). This prompted us to analyse the role of Cdk1 in the mitotic phosphorylation of Borealin. We used replication defective recombinant adenoviruses expressing a nuclear-targeted Cyclin B1 in which Cyclin B1 was fused to the nuclear localization signal of SV40 Large T antigen (NB1) and a constitutively active T14A Y15F mutant of Cdk1 (Cdk1AF). Interphase blocked HeLaM cells infected with adenoviruses expressing NB1 plus Cdk1AF showed a mobility shift characteristic of mitotic Borealin phosphorylation (Fig. 1A). Borealin was also shifted in cells expressing the Cdk1AF presumably due to the presence of endogenous Cyclin B1 (Fig. 1A). Control western blots showed the presence of the epitope-tagged exogenous Cdk1 and Cyclin B1 in appropriate samples (Fig. 1A). These results demonstrate that Borealin is phosphorylated in response to increased expression of Cdk1.
Fig. 1.
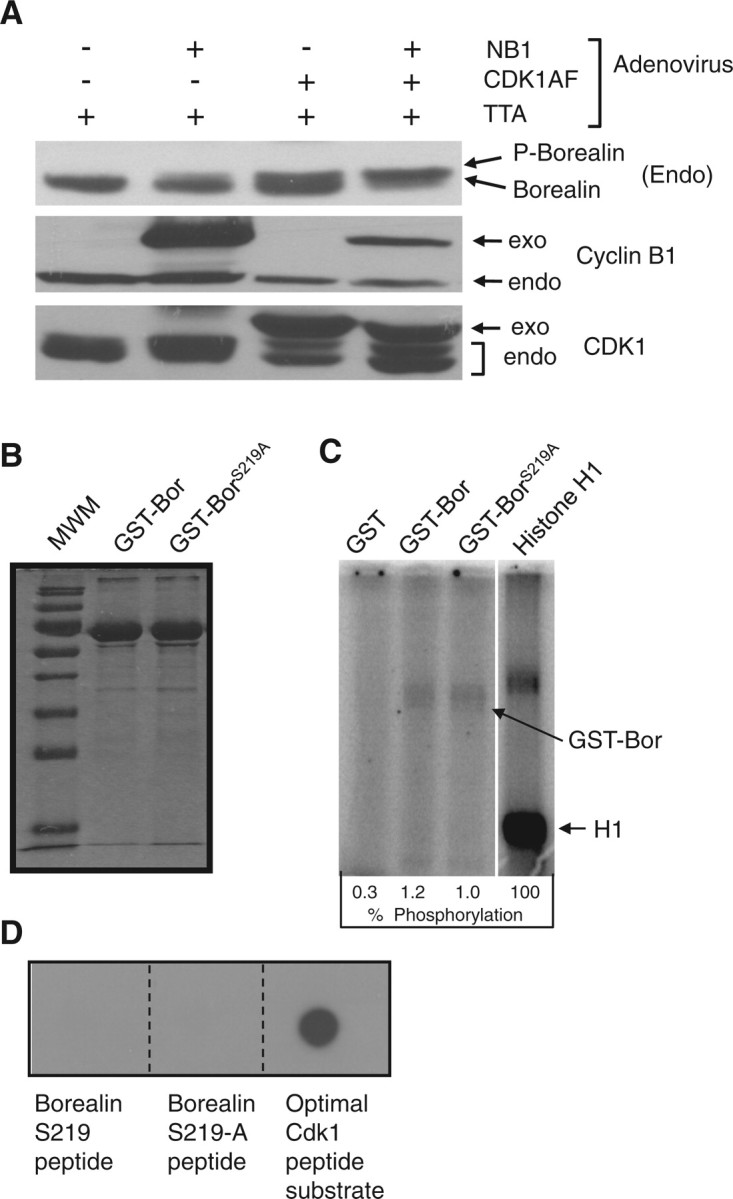
Effect of Cdk1 on Borealin phosphorylation. (A) Cdk1/Cyclin B1 induces Borealin mobility shift. HeLaM cells were blocked in S phase with hydroxyurea and then infected with recombinant adenoviruses as indicated at an MOI of 50. TTA virus encodes the tetracycline activator needed for expression from the recombinant adenoviruses. NB1, nuclear targeted Cyclin B1; Cdk1AF, Cdk1 T14A,Y15F. Lysates were analysed by western blotting with antibodies to endogenous Borealin, Cyclin B1, Cdk1 and β-actin to control for loading. Exo, exogenous; Endo, endogenous. (B) Purification of Borealin–GST. Wild-type Borealin (GST–Bor) and Borealin S219A (GST–BorS219A) were purified from E. coli as GST fusions. The proteins were separated by SDS–PAGE and stained with Coomassie blue. (C) Phosphorylation of GST–Borealin by Cdk1. GST–Bor, GST–BorS219A, histone H1 and GST were phosphorylated in vitro with purified Cdk1/Cyclin-B1 in the presence γ-(32P)ATP. The proteins were resolved by SDS–PAGE followed by autoradiography. Reactions included 1.0 µg of histone H1 which upon Coomassie blue staining was always lower in intensity compared to GST–Borealin (our unpublished data). Extent of phosphorylation is shown as a percent of H1 phosphorylation. (D) Cdk1 does not phosphorylate Borealin peptides. N-terminal biotinylated peptides containing Borealin S219, Borealin S219A and an optimal Cdk1 substrate were phosphorylated in vitro by purified Cdk1/Cyclin-B1 in the presence γ-(32P)ATP. The reactions were spotted on PVDF membrane saturated with avidin. The PVDF membrane was washed and visualized by autoradiography.
To determine whether Cdk1 directly phosphorylates Borealin, we used an in vitro kinase assay with purified Cdk1, and recombinant Borealin. Both full-length Borealin and the S219A mutant were phosphorylated by Cdk1 (Fig. 1B and C). However, Borealin phosphorylation was only ∼1% that of the optimal Cdk1 substrate Histone H1 (Fig. 1C). One possible explanation for the poor efficiency of phosphorylation is that our recombinant Borealin is denatured. However, GST–Bor was able to associate with Aurora B and Survivin from HelaM cell lysates suggesting the presence of at least some normal structure (Supplementary Fig. S1A). We also tested the ability of Cdk1 to phosphorylate a synthetic peptide encompassing S219 of Borealin. Cdk1 was unable to phosphorylate the S219 peptide but did phosphorylate a peptide containing a consensus Cdk1 substrate site (Fig. 1D). These results indicate that Borealin is not an optimal substrate for Cdk1-mediated phosphorylation in vitro.
Borealin and Cdc14B show an overlapping pattern of subcellular localization
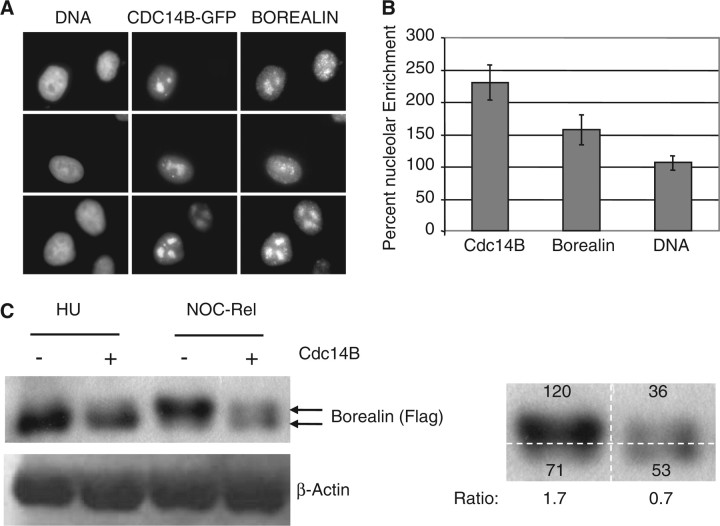
To determine whether Cdc14 phosphatases play a role in the dephosphorylation of Borealin, we tested the effect of over-expressing Cdc14B on Borealin. Interestingly, we observed that endogenous Borealin and GFP-tagged Cdc14B showed an overlapping pattern of localization (Fig. 2A and Supplementary Fig. S1B). Cdc14B has been reported to localize to the nucleolus suggesting that Borealin may also localize to this structure (19). We compared pixel intensities in nucleolar regions (as defined by high Cdc14B staining) with the rest of the nucleus. With this analysis, we found that Cdc14B was enriched 225% in the nucleolar area when compared with the rest of the nucleus. Borealin was enriched 156% in the nucleolus (Fig. 2B). These observations suggest that Cdc14B and Borealin co-localize to the nucleolus during interphase.
Fig. 2.
Regulation of Borealin by over-expressed Cdc14B. (A) Overlapping localization of Cdc14B and Borealin. HeLaM cells were transiently transfected with GFP-Cdc14B and 24 h later analysed by immunofluorescence with anti-sera to endogenous Borealin. Three separate fields are shown. (B) Nucleolar enrichment of Borealin and Cdc14B. Average pixel intensities within the nucleolar region was divided by the average pixel intensities in the rest of the nucleus. This ratio is expressed as a percent value. The nucleolar region was defined as the subnuclear region showing high Cdc14B staining. (C) Effect of Cdc14B over expression on Borealin. HeLaM were transiently transfected with Borealin-Flag with or without GFP-Cdc14B. The cells were blocked in S phase with hydroxyurea or in mitosis with nocodazole for 16 h. The ‘NOC-Rel’ sample corresponds to the t0 time point from a nocodazole release experiment. Specifically, cells for these samples were removed from the plate by mitotic shake-off and washed three times by re-suspending in PBS followed by centrifugation. Cell pellets were then frozen before being analysed by western blotting. During the collection and washing steps, Borealin had already begun to be dephosphorylated in cells transfected with Cdc14B. Results are representative of at least two experiments prepared in the same manner. β-Actin serves as a control for loading. The panel on the right indicates densitometric readings of two of the lanes from the gel to the left. Pixel intensities are shown in arbitrary units. Ratio: ratio of the slower band (phosphorylated) over the faster band (unphosphorylated).
Effect of Cdc14 over expression on Borealin
To further investigate a potential role of Cdc14B in the dephosphorylation of Borealin, we co-transfected HeLaM cells with GFP-Cdc14B and Borealin-Flag. Transfected cells were synchronized in mitosis by exposure to nocodazole. Nocodazole was either left on or removed. By 6 h after removal of nocodazole, Borealin was dephosphorylated equally in cells with or without over-expressed Cdc14B (our unpublished data). At the first time point of removal, in which cells were simply washed three times and frozen, we consistently observed partial dephosphorylation of Borealin in cells over-expressing Cdc14B (Fig. 2C). These observations suggest that high levels of Cdc14B may increase the rate of Borealin dephosphorylation.
Effects of proteasome inhibitors on Borealin levels and phosphorylation
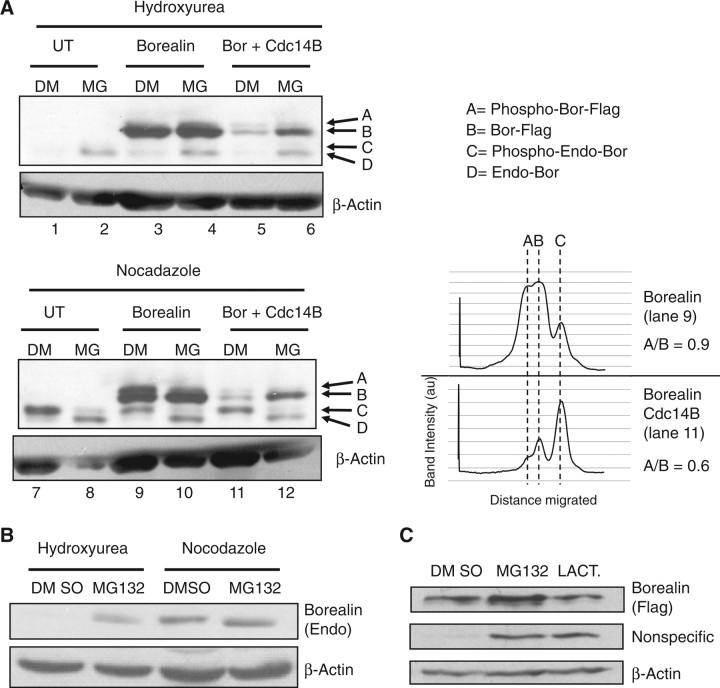
In some of our experiments, over-expressed Cdc14B was associated with a reduction in co-transfected Borealin. To test whether Cdc14B might enhance the degradation of Borealin, we tested the effect of MG132 in HeLaM cells co-transfected with Borealin-Flag and Cdc14B-GFP. Borealin was detected with an antibody that recognizes both endogenous and exogenous Borealin. With extensive electrophoresis, four species of Borealin were observed, two each derived from endogenous and exogenous Borealin. The two species represent phosphorylated and unphosphorylated Borealin. Furthermore, both species of exogenous Borealin migrate slower than endogenous protein due to the presence of the Flag-Tag (2). In cells first blocked in S phase with hydroxyurea, MG132 treatment increased the level of endogenous Borealin (Fig. 3A; bands C and D; lanes 1 versus 2; 3 versus 4; 5 versus 6). When cells were first arrested in mitosis with nocodazole, MG132 had no effect on the level of endogenous Borealin, but induced a shift from the slower (band C) to the faster migrating species (band D) (Fig. 3A; lanes 7 versus 8; 9 versus 10; 11 versus 12). MG132 had less of an effect on the level of exogenous Borealin (bands A and B) in S-phase blocked cells.
Fig. 3.
Effect of proteasome inhibitors on Borealin levels and phosphorylation. (A) HeLaM cells were transiently transfected with Borealin-Flag and GFP-Cdc14B Borealin-Flag alone. The cells were blocked in interphase with hydroxyurea (2 mM) or mitosis with nocodazole (100 ng/ml) with or without MG132 (5 µM) for 14 h. The lysates were separated by SDS–PAGE and analysed by western blotting with an antibody to Flag tagged Borealin. The transfers were stripped and re-probed with antibody to β-actin. To the right of the blots is a densitometric scan of selected lanes. (B) Up-regulation of endogenous Borealin after MG132 treatment. HeLaM cells were blocked in interphase with hydroxyurea (2 mM) or mitosis with nocodazole (200 ng/ml) with or without MG132 (5 µM) for 14 h. The lysates were analysed by western blotting with a rabbit antisera to endogenous Borealin. The transfers were stripped and re-probed with antibody to β-actin. UT, untransfected; DM, DMSO; MG, MG132. (C) Effect of lactacystin on Borealin expression. HeLaM cells were transfected with Flag-tagged Borealin, blocked in interphase with hydroxyurea and treated with lactacystin (20 µM) or MG132 (5 µM) for 14 h. Borealin was detected by western blotting with an antibody to the Flag tag followed by probing for actin as a loading control. A non-specific band detected with the Flag antibody was up-regulated in response to both MG132 and lactacystin.
Although co-transfection of Borealin with Cdc14B was associated with a reduction in the level of Borealin-Flag, the same was not observed with endogenous Borealin (Fig. 3A; compare lanes 3 to 5; lanes 4 to 6). This suggests that Cdc14B does not trigger proteolytic degradation of Borealin. Densitometric scans of lanes 9 and 11 indicated a slight reduction in the ratio of phosphorylated exogenous Borealin (band A versus B) in cells transfected with Cdc14B (lane 11) versus those just transfected with Borealin (lane 9) (Fig. 3A, right panel). This effect, although subtle, is similar to the dephosphorylation described in Figure 2C upon Cdc14B over-expression. More importantly, these experiments suggest that MG132 reduces the phosphorylation of Borealin during mitosis. Also, MG132 up-regulates Borealin levels during interphase but not mitosis (Fig. 3A and B). Interestingly, Borealin was not up-regulated in cells exposed to a second proteasome inhibitor lactacystin; however, a non-specific band was up-regulated suggesting that lactacystin was functional in our assay. Overall, these results suggest that a protease other than the proteasome may be responsible for the effects of MG132 on Borealin levels (Fig. 3C).
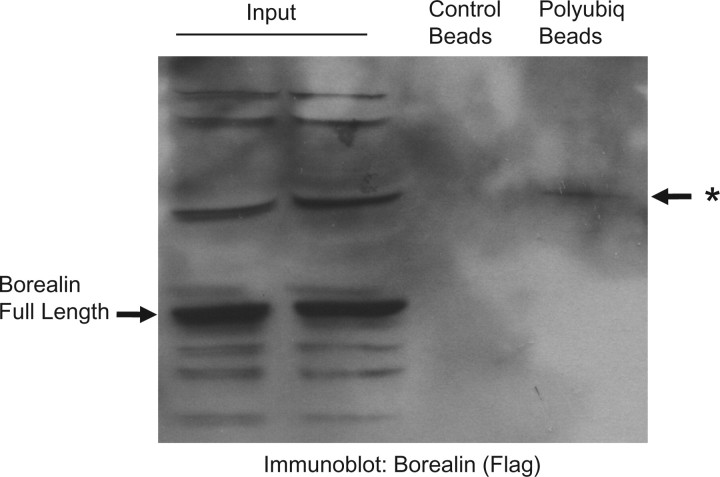
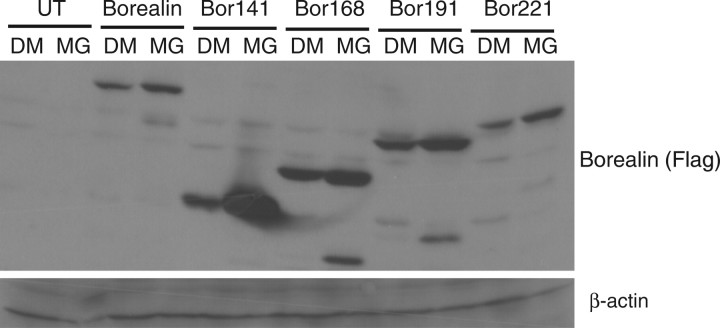
In experiments aimed at analysing Borealin ubiquitination, we detected a species of Borealin bound to an affinity column that recognizes poly-ubiquitin chains (Fig. 4). However, this band was relatively weak and we did not detect the ‘smear’ characteristic of multiple polyubiquitiniated species. To further dissect the regulation of Borealin steady-state levels, we analysed the effect of MG132 on C-terminal truncation mutants of Borealin. MG132 increased the level of all of the truncated forms; however, the mutant extending to amino acid 141 was more dramatically up-regulated compared with the other forms of the protein (Fig. 5). This observation suggests that the C-terminus of Borealin regulates its susceptibility to degradation.
Fig. 4.
Association of Borealin with ubiquitin chains. A clone of HeLaM cells stably expressing Borealin-Flag (‘WT8 cells’) were treated with MG132 and immunoprecipitated with polyubiquitin affinity resin or control resin. The immunoprecipitates were separated by SDS–PAGE and transfers probed with an antibody to the Flag-tag conjugated to HRP. The ubiquitinated species is indicated by an asterisks.
Fig. 5.
Effect of MG132 on the levels of truncated forms of Borealin. HeLaM cells were transfected with Flag-tagged full-length Borealin, Bor-141, Bor-168, Bor-191 or Bor-221. The cells were blocked in interphase with hydroxyurea and treated with MG132 for 14 h. The lysates were analysed by western blotting with an antibody that recognizes the Flag-tag. Blots were re-probed with an antibody to β-actin to serve as a loading control.
Regulation of Borealin dephosphorylation
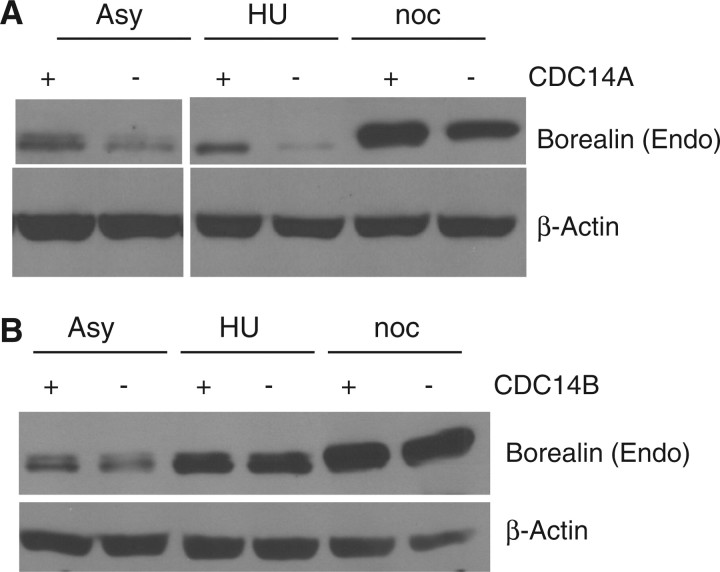
The increase in Borealin electrophoretic migration upon over-expression of Cdc14B suggested that this phosphatase might regulate Borealin (Figs 2C and 3A). Therefore, we measured the mobility of Borealin in human cells in which either Cdc14A or Cdc14B have been inactivated by homologous recombination (20). If one of these phosphatases normally targeted Borealin for dephosphorylation, we expected to observe a substantial increase in the amount of the slow migrating Borealin species in the knock-out cell line. In contrast, Borealin showed nearly identical migration patterns when comparing isogenic parental to Cdc14A knockout (Fig. 6A) or Cdc14B knock-out cell lines (Fig. 6B). These similarities in migration were observed in asynchronously growing cells or cells blocked in S phase with hydroxyurea or mitosis with nocodazole.
Fig. 6.
Borealin expression in cells lacking either Cdc14A or Cdc14B. Cells in which either Cdc14A or Cdc14B were knocked out by homologous recombination were analysed by western blotting to detect endogenous Borealin. Cells were either growing asynchronously (Asy) or arrested in S-phase with hydroxyurea (HU) or in mitosis with nocodazole (noc). (A) Borealin expression in hTERT-RPE cells either with or without Cdc14A. (B) Borealin expression in HCT116 cells either with or without Cdc14B.
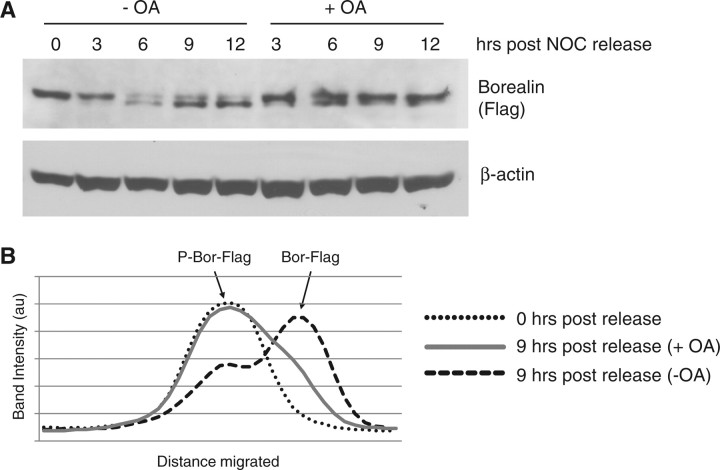
To further investigate the dephosphorylation of Borealin, we transfected HeLaM cells with Borealin-Flag and then synchronized the cells in mitosis with nocodazole. Cells were released from the block in the presence or absence of the phosphatase inhibitor okadaic acid. In the absence of okadaic acid, Borealin showed a shift to the dephosphoryated form by 6 h post nocodazole release (Fig. 7). Okadaic acid prevented this shift from occurring suggesting that Borealin remains phosphorylated in the presence of this inhibitor.
Fig. 7.
Effect of okadaic acid on Borealin dephosphorylation. HeLaM cells were transfected with full-length Borealin, and then synchronized with a single thymidine block. Cells were released from thymidine in the presence of nocodazole to block them in mitosis. Twenty-four hours later, mitotic cells were collected by shake-off, nocodazole was removed by washing and cells replated in the presence or absence of okadaic acid. (A) Western blot of Borealin after nocodazole release. Cell lysates were analysed by western blotting using an antibody that recognizes the Flag-tag. β-Actin served as a loading control. (B) Densitometric trace of Borealin levels 9 h after release. The slowly migrating band (‘P-Bor-Flag’) represents the phosphorylated Flag-tagged Borealin. Release from nocodazole in the absence of okadaic acid induced a shift to the faster migrating unphosphorylated species (‘Bor-Flag’). au, arbitrary units.
Discussion
Borealin plays an essential role as part of the CPC regulating chromosome segregation and cytokinesis. Phosphorylation of Borealin has been reported at several sites leading to enhancement of its mitotic functions (3, 5). For example, Borealin can be phosphorylated by Cdk1 leading to enhanced interaction with Sgo1 and Sgo2 and localization to the inner centromere (3). Phosphorylation at S219 is also needed for Borealin to suppress multinucleation that occurs after knocking down endogenous Borealin (5). S219 is a potential Cdk1 target site since it is directly followed by proline. We observed that over-expression of a constitutively active Cdk1 induced Borealin to migrate more slowly suggesting that Cdk1 is a physiologically relevant kinase regulating Borealin. As previously reported, Cdk1 was able to phosphorylate Borealin in vitro; however, our experiments suggest that this reaction is relatively inefficient. These findings suggest that additional intracellular factors present may enhance access of Cdk1 to Borealin. Cdk1 was completely unable to phosphorylate a peptide encompassing S219 of Borealin again suggesting that additional factors aid in Borealin phosphorylation. That S219 is phosphorylated in intact cells is supported by phospho-proteomic mapping experiments and the fact that the Borealin S219A mutant fails to show an increase in migration in mitotic cells (5, 21).
Nbl1, the fission yeast Borealin orthologue, interacts with the Cdc14 phosphatase Clp1/Flp1 (16). Although over-expression of Cdc14B could accelerate the dephosphorylation of Borealin, human cells lacking either Cdc14A or Cdc14B did not show an increase in the amount of phosphorylated Borealin. The effects of over-expression may be the result of high levels of Cdc14B attacking substrates that it normally does not dephosphorylate. It is also possible that Cdc14A and Cdc14B act redundantly to dephosphorylate Borealin; knocking out one at a time may not be sufficient to cause persistent phosphorylation. Our results with okadaic acid instead suggest that other phosphatases dephosphorylate Borealin upon mitotic exit. Exposure of HeLaM cells to okadaic acid did delay the dephosphorylation of Borealin upon mitotic exit. Numerous phosphatases can be inhibited by okadaic acid, but one appealing candidate is PP2A since it dephosphorylates multiple mitotic substrates upon mitotic exit in animals. Furthermore, PP2A targets a number of substrates that are phosphorylated by Cdk1 during mitosis (9–13).
MG132 altered both the level of Borealin and its phosphorylation state. During interphase, but not mitosis, MG132 increased the steady-state level of Borealin. This suggests that Borealin is protected from degradation during mitosis. In mitotic cells, MG132 led to an apparent dephosphorylation of Borealin. One possible explanation is that the phosphatase that dephosphorylates Borealin is itself degraded by an MG132-sensitive protease during mitosis. Interestingly, the catalytic and regulatory ‘A’ subunits of PP2A have half-lives in excess of 10 h, whereas the regulatory ‘B’ subunit can be destabilized when the catalytic subunit is depleted from cells (22–25). Regardless of which okadaic acid-sensitive phosphatase targets Borealin, the simplest explanation for the effect of MG132 is that the phosphatase is inactivated by an MG132-sensitive protease during the nocodazole-induced mitotic arrest.
MG132 inhibits the proteasome but can also inhibit calpain and likely other proteases (26). Therefore, we tested the effect of lactacystin, a structurally unrelated compound that inhibits the proteasome much more efficiently than calpain (27). Lactacystin did not induce the accumulation of Borealin. One possibility is that calpain or some other MG123-sensitive protease degrades Borealin during interphase. It is also possible that MG132 is more effective at inhibiting the proteasome in our cells than lactacystin. Interestingly, we detected a species of Borealin bound to an affinity column that recognizes ubiquitin chains. This species of Borealin was not very abundant and we did not observe the characteristic ‘smear’ of polyubiquitinated forms of Borealin. These observations suggest that Borealin is potentially ubiquitinated; however, ubiquitination may be inefficient or rapidly reversed by deubiquitinating enzymes. The fact that lactacystin does not up-regulate Borealin suggests that ubiquitination may not be a major pathway for Borealin degradation.
In a survey of a number of truncated forms of Borealin, we observed that a version of Borealin extending to amino acid 141 was dramatically up-regulated by MG132. This observation suggests that regions in the C-terminus regulate Borealin stability. This effect may be mediated by interaction of the Borealin C-terminus with proteins that protect Borealin from degradation. It is also possible that the Borealin1–141 mutant protein is not properly folded and rapidly cleared by degradation. Evidence that argues against this interpretation is the fact that Borealin1–141 is still capable of localizing to the inner centromere and also retains some wild-type Borealin function in allowing chromosome segregation (Bekier, ME and Taylor, WR, manuscript in preparation). Structural studies have indicated that the region missing from the 1 to 141 mutant contains a dimerization domain (28). It is also possible that dimerization of Borealin affects its stability. Other than the putative dimerization domain, the C-terminal half of Borealin contains phosphorylation sites that can alter the interaction of the protein with the centromere (3, 5). It is also possible that these modifications play a role in Borealin stability.
Borealin plays an essential role as part of the CPC in regulating chromosome segregation and cytokinesis. These functions are regulated by phosphorylation, which is induced by Cdk1, and removed by an okadaic acid sensitive phosphatase. Furthermore, Borealin levels are reduced by an MG132-sensitive protease during interphase, but not mitosis. The C-terminus of Borealin appears to protect Borealin from degradation. These studies have uncovered several important layers of Borealin regulation.
Supplementary Data
Supplementary Data are available at JB Online.
Funding
National Institutes of Health (grant 1R15GM084410-01 to W.R.T.).
Conflict of interest
None declared.
Acknowledgements
The authors thank Prasad Jallepalli for cell lines lacking Cdc14A or Cdc14B, and David Morgan for recombinant adenoviruses.
Glossary
Abbreviations
- Cdk
cyclin-dependent kinase
- Cdk1AF
Cdk1 T14A Y15F
- CPC
chromosomal passenger complex
- GFP
green fluorescent protein
- GST
glutathione-S-transferase
- HRP
horse radish peroxidase
- MOI
multiplicity of infection
- NB1
nuclear targeted cyclin B1
- PMSF
phenylmethane sulphonylfluoride
- PVDF
polyvinyldenefluoride
- TTA
tetracycline transactivator
References
- 1.Pereira G, Schiebel E. Separase regulates INCENP-Aurora B anaphase spindle function through Cdc14. Science. 2003;302:2120–2124. doi: 10.1126/science.1091936. [DOI] [PubMed] [Google Scholar]
- 2.Kaur H, Stiff AC, Date DA, Taylor WR. Analysis of mitotic phosphorylation of borealin. BMC Cell Biol. 2007;8:5. doi: 10.1186/1471-2121-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsukahara T, Tanno Y, Watanabe Y. Phosphorylation of the CPC by Cdk1 promotes chromosome bi-orientation. Nature. 2010;467:719–723. doi: 10.1038/nature09390. [DOI] [PubMed] [Google Scholar]
- 4.Gnad F, Ren S, Cox J, Olsen JV, Macek B, Oroshi M, Mann M. PHOSIDA (phosphorylation site database): management, structural and evolutionary investigation, and prediction of phosphosites. Genome Biol. 2007;8:R250. doi: 10.1186/gb-2007-8-11-r250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaur H, Bekier ME, Taylor WR. Regulation of Borealin by phosphorylation at serine 219. J. Cell. Biochem. 2010;111:1291–1298. doi: 10.1002/jcb.22853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamagishi Y, Honda T, Tanno Y, Watanabe Y. Two histone marks establish the inner centromere and chromosome bi-orientation. Science. 2010;330:239–243. doi: 10.1126/science.1194498. [DOI] [PubMed] [Google Scholar]
- 7.Ubersax JA, Woodbury EL, Quang PN, Paraz M, Blethrow JD, Shah K, Shokat KM, Morgan DO. Targets of the cyclin-dependent kinase Cdk1. Nature. 2003;425:859–864. doi: 10.1038/nature02062. [DOI] [PubMed] [Google Scholar]
- 8.Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voets E, Wolthuis RM. MASTL is the human orthologue of Greatwall kinase that facilitates mitotic entry, anaphase and cytokinesis. Cell Cycle. 2010;9:3591–3601. doi: 10.4161/cc.9.17.12832. [DOI] [PubMed] [Google Scholar]
- 10.Schmitz MH, Held M, Janssens V, Hutchins JR, Hudecz O, Ivanova E, Goris J, Trinkle-Mulcahy L, Lamond AI, Poser I, Hyman AA, Mechtler K, Peters JM, Gerlich DW. Live-cell imaging RNAi screen identifies PP2A-B55alpha and importin-beta1 as key mitotic exit regulators in human cells. Nat. Cell. Biol. 2010;12:886–893. doi: 10.1038/ncb2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mochida S, Maslen SL, Skehel M, Hunt T. Greatwall phosphorylates an inhibitor of protein phosphatase 2A that is essential for mitosis. Science. 2010;330:1670–1673. doi: 10.1126/science.1195689. [DOI] [PubMed] [Google Scholar]
- 12.Gharbi-Ayachi A, Labbe JC, Burgess A, Vigneron S, Strub JM, Brioudes E, Van-Dorsselaer A, Castro A, Lorca T. The substrate of Greatwall kinase, Arpp19, controls mitosis by inhibiting protein phosphatase 2A. Science. 2010;330:1673–1677. doi: 10.1126/science.1197048. [DOI] [PubMed] [Google Scholar]
- 13.Castilho PV, Williams BC, Mochida S, Zhao Y, Goldberg ML. The M phase kinase Greatwall (Gwl) promotes inactivation of PP2A/B55delta, a phosphatase directed against CDK phosphosites. Mol. Biol. Cell. 2009;20:4777–4789. doi: 10.1091/mbc.E09-07-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Visintin R, Craig K, Hwang ES, Prinz S, Tyers M, Amon A. The phosphatase Cdc14 triggers mitotic exit by reversal of Cdk-dependent phosphorylation. Mol. Cell. 1998;2:709–718. doi: 10.1016/s1097-2765(00)80286-5. [DOI] [PubMed] [Google Scholar]
- 15.Wan J, Xu H, Grunstein M. CDC14 of Saccharomyces cerevisiae. Cloning, sequence analysis, and transcription during the cell cycle. J. Biol. Chem. 1992;267:11274–11280. [PubMed] [Google Scholar]
- 16.Bohnert KA, Chen JS, Clifford DM, Vander Kooi CW, Gould KL. A link between aurora kinase and Clp1/Cdc14 regulation uncovered by the identification of a fission yeast borealin-like protein. Mol. Biol. Cell. 2009;20:3646–3659. doi: 10.1091/mbc.E09-04-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tiwari RK, Kusari J, Sen GC. Functional equivalents of interferon-mediated signals needed for induction of an mRNA can be generated by double-stranded RNA and growth factors. EMBO J. 1987;6:3373–3378. doi: 10.1002/j.1460-2075.1987.tb02659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin P, Hardy S, Morgan DO. Nuclear localization of cyclin B1 controls mitotic entry after DNA damage. J. Cell Biol. 1998;141:875–885. doi: 10.1083/jcb.141.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaiser BK, Zimmerman ZA, Charbonneau H, Jackson PK. Disruption of centrosome structure, chromosome segregation, and cytokinesis by misexpression of human Cdc14A phosphatase. Mol. Biol. Cell. 2002;13:2289–2300. doi: 10.1091/mbc.01-11-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mocciaro A, Berdougo E, Zeng K, Black E, Vagnarelli P, Earnshaw W, Gillespie D, Jallepalli P, Schiebel E. Vertebrate cells genetically deficient for Cdc14A or Cdc14B retain DNA damage checkpoint proficiency but are impaired in DNA repair. J. Cell Biol. 2010;189:631–639. doi: 10.1083/jcb.200910057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nousiainen M, Sillje HH, Sauer G, Nigg EA, Korner R. Phosphoproteome analysis of the human mitotic spindle. Proc. Natl Acad. Sci. USA. 2006;103:5391–5396. doi: 10.1073/pnas.0507066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silverstein AM, Barrow CA, Davis AJ, Mumby MC. Actions of PP2A on the MAP kinase pathway and apoptosis are mediated by distinct regulatory subunits. Proc. Natl Acad. Sci. USA. 2002;99:4221–4226. doi: 10.1073/pnas.072071699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glaser ND, Lukyanenko YO, Wang Y, Wilson GM, Rogers TB. JNK activation decreases PP2A regulatory subunit B56alpha expression and mRNA stability and increases AUF1 expression in cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H1183–H1192. doi: 10.1152/ajpheart.01162.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou J, Pham HT, Ruediger R, Walter G. Characterization of the Aalpha and Abeta subunit isoforms of protein phosphatase 2A: differences in expression, subunit interaction, and evolution. Biochem. J. 2003;369:387–398. doi: 10.1042/BJ20021244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baharians Z, Schonthal AH. Autoregulation of protein phosphatase type 2A expression. J. Biol. Chem. 1998;273:19019–19024. doi: 10.1074/jbc.273.30.19019. [DOI] [PubMed] [Google Scholar]
- 26.Tsubuki S, Saito Y, Tomioka M, Ito H, Kawashima S. Differential inhibition of calpain and proteasome activities by peptidyl aldehydes of di-leucine and tri-leucine. J. Biochem. 1996;119:572–576. doi: 10.1093/oxfordjournals.jbchem.a021280. [DOI] [PubMed] [Google Scholar]
- 27.Mellgren RL. Specificities of cell permeant peptidyl inhibitors for the proteinase activities of mu-calpain and the 20 S proteasome. J. Biol. Chem. 1997;272:29899–29903. doi: 10.1074/jbc.272.47.29899. [DOI] [PubMed] [Google Scholar]
- 28.Bourhis E, Lingel A, Phung Q, Fairbrother WJ, Cochran AG. Phosphorylation of a borealin dimerization domain is required for proper chromosome segregation. Biochemistry. 2009;48:6783–6793. doi: 10.1021/bi900530v. [DOI] [PubMed] [Google Scholar]