Abstract
BACKGROUND
Older men tend to have poorer semen quality and are generally at higher risks for infertility and abnormal reproductive outcomes.
METHODS
We employed proton-induced X-ray emission (PIXE, 3 MeV proton beam) to investigate the concentrations of zinc, copper, calcium, sulfur, chlorine, potassium, titanium, iron and nickel in washed sperm and seminal plasma from non-smoking groups of 10 older men (65–80 years old) and 10 younger men (22–28 years old) who were concurrently assayed for sperm function and genomicly defective sperm.
RESULTS
The older group showed elevated zinc, copper and calcium in sperm and elevated sulfur in seminal plasma compared with the younger men. The older group also showed reduced motility as well as increased sperm DNA fragmentation, achondroplasia mutations, DNA strand breaks and chromosomal aberrations. Sperm calcium and copper were positively associated with sperm DNA fragmentation (P < 0.03). Seminal sulfur was positively associated with sperm DNA fragmentation and chromosomal aberrations (P < 0.04), and negatively associated with sperm motility (P < 0.05). Sperm calcium was negatively associated with sperm motility, independent of male age (P = 0.01).
CONCLUSIONS
We identified major differences in elemental concentrations between sperm and seminal plasma and that higher sperm copper, sulfur and calcium are quantitatively associated with poorer semen quality and increased frequencies of genomic sperm defects.
Keywords: male aging, calcium, copper, semen, zinc
Introduction
There is an increasing trend for older men to father children (Rolf and Nieschlag, 2001; Sloter et al., 2004). Advancing male age has been associated with increased frequencies of sperm with chromosomal defects (Sloter et al., 2004, 2007), with mutations in genes associated with achondroplasia and Apert syndromes (Tiemann-Boege, et al., 2002; Glaser et al., 2003; Wyrobek et al., 2006) and with DNA damage measured by Sperm Comet and the sperm chromatin structure assay (SCSA) (Morris et al., 2002; Singh et al., 2003; Wyrobek et al., 2006; Schmid et al., 2007). Interestingly, male aging was not associated with an increase in the frequencies of aneuploid sperm (Wyrobek et al., 2006). Also, ∼60% of male infertility has been attributed to genetic causes (de la Rochebrochard and Thonneau, 2002), but the underlying mechanisms and roles of male aging are poorly understood (Ventura et al., 1997). The age-associated increases in sperm defects support the epidemiological findings that fathering children at older ages is associated with a greater risk of abnormal reproductive outcomes and children carrying specific gene mutations of paternal origin [see review in Wyrobek et al. (Wyrobek et al., 2006)]. Understanding the effects of male age on sperm quality is especially relevant for older men who are attending reproductive clinics because they are marginally fertile and seek assisted reproduction technologies in order to father children.
It is well known that certain elements are involved in normal spermatogenesis, sperm function and male fertility (Figa-Talamanca et al., 2001). Elements, such as zinc, magnesium, copper and calcium, are important for the maintenance of normal spermatogenesis, sperm maturation, DNA metabolism and repair and gene expression in germ cells (Schrag and Dixon, 1985; Benoff et al., 2000; Wong et al., 2001, 2000, 2002; Fenech, 2002; Yuyan et al., 2008). However, little is known about the relative concentrations of elements in sperm versus seminal plasma, whether concentrations of elements in either compartment are related to semen quality or genomic defects in sperm, and how these processes may be modified in the aging male.
Proton-induced X-ray emission (PIXE), which is based on the analyses of the energy spectra of X-rays emitted from samples bombarded with protons, is the preferred method for trace element detection in biological tissue (Forslind et al., 1991; Bench et al., 1996). The advantage of the PIXE technique over the more common methods of atomic absorption spectroscopy (Henkel et al., 1999) and inductively coupled argon plasma with mass spectrometry is its ability to detect trace elements with high sensitivity due to low background interference. PIXE is also 100 times more sensitive than electron micro-analysis systems for the identification and quantification of trace elements. PIXE is capable of detection sensitivity for trace elements generally down to a few parts per million (p.p.m.) (Forslind et al., 1991) but can be as sensitive to tenths or hundredths of p.p.m. with extended collection times and detectors with large collection efficiencies (Ryan, 2011). When ions pass through matter, they interact with the electrons in the atoms and, occasionally, a vacancy is produced by an excited electron. When this occurs in an inner shell, the vacancy is filled by an electron from an outer shell and an X-ray photon of characteristic energy is emitted. The PIXE method measures the energy of this photon in order to identify the atomic number and the amount of that element present. PIXE requires very small biological mass (<1 µg) compared with other methods, which is advantageous for semen analysis (Bench et al., 1998). In a previous application to semen samples, PIXE showed that the protein and DNA contents of sperm differed between fertile and infertile individuals and infertile men had elevated levels of sulfur within sperm heads compared with fertile individuals (Bench et al., 1996, 1998).
The current study uses a subset of samples from the California Age and Genetic Effects on Sperm (AGES) population. Analyses of genomic defects and gene mutations in sperm (aneuploidy, structural chromosome aberrations, DNA strand breakage, DNA fragmentation and achondroplasia mutations) and physiological end-points [sperm count, various motility parameters including computer-assisted semen analysis (CASA)] of this study population were previously described (Tiemann-Boege et al., 2002; Eskenazi et al., 2003; Sloter et al., 2006, 2007; Wyrobek et al., 2006; Schmid et al., 2007; Schmid et al., 2012). The AGES study has shown that male aging was associated with reduced semen quality (Eskenazi et al., 2003), increased frequencies of sperm-carrying structural aberrations (Sloter et al., 2007), DNA strand damage (Schmid et al., 2007; Schmid et al., 2012), DNA fragmentation and specific gene mutations (Wyrobek et al., 2006). In the AGES population, we also found that micronutrient intake was associated with improved motility (Eskenazi et al., 2005), lower frequencies of aneuploidy sperm (Young et al., 2008), and reduced sperm DNA damage in older men (Schmid et al., 2012).
The aims of the present study were to apply PIXE to semen samples collected in the well-characterized AGES study to: (i) measure the concentrations of specific elements and metals within isolated sperm and seminal plasma (zinc, copper, calcium, sulfur, chlorine, potassium, titanium, iron and nickel); (ii) determine whether older men had significantly different concentrations of these elements in their sperm or seminal plasma compared with younger men and (iii) determine whether changes in elemental concentrations could explain previously determined associations between male age and semen quality and genomic defects in sperm.
Materials and Methods
Participants
A group of 20 healthy male volunteers, 10 men aged 22–28 years and 10 men aged 65–80 years were selected from the AGES study (n = 97), which aimed to investigate whether there were differences in semen quality and genomic defects in sperm associated with male aging and nutritional factors (Eskenazi et al., 2003). These men represented the 10 youngest and the 10 oldest participants of the AGES study. Men who participated in the AGES study were recruited from a national laboratory in California. Men were eligible to participate if they currently worked or were retired from that workplace; had no current fertility or reproductive problems; had not smoked cigarettes in the last 6 months; had no vasectomy and no history of an undescended testicle, prostate cancer or azoospermia. The AGES study was approved by the Institutional Review Boards of all participating institutions, and written consent to participate was obtained from all participants.
Men were mailed a semen collection container with instructions and a questionnaire covering medical and reproductive histories, sociodemographic characteristics (age, race, education), occupation, other possible exposures, as well as diet and lifestyle habits. Semen samples were analyzed for sperm count and motility upon collection (Eskenazi et al., 2003; Sloter et al., 2006) and immediately stored at −80°C until later analyses. The majority of sperm samples from both age groups were collected during fall and winter (80%).
Procedure for preparing sperm for PIXE
Semen aliquots were thawed and washed twice in 0.15 M ammonium acetate buffer (pH 7.4; Amereso, Solon, USA) to separate sperm from seminal plasma (Bench et al., 1996). Samples were centrifuged (4124g for 5 min) to sediment sperm and the pellets were resuspended in a small volume of 0.15 M ammonium acetate, pH 7.4. Seminal plasma and washed sperm (∼0.5 µl) were micro-pipetted onto separate ultraclean, 1-μm thick, transparent nylon foils stretched over a 15-mm diameter hole in a plastic support frame. Sperm or seminal plasma samples were allowed to settle onto the nylon surface for 5 min, and the excess buffer was removed with a Pasteur pipette and blotting paper.
Measurements of sperm motility
Computer-assisted semen analysis was performed using the HTM-Ceros semen analyzer (Hamilton Thorne Research, USA) according to the manufacturer's operation guidelines (Eskenazi et al., 2003; Sloter et al., 2006). Semen samples were maintained at room temperature until analyzed. Fifty microlitres of each sample were diluted 1:1 using Dulbecco's phosphate-buffered saline solution with 1.0 g/l of glucose and 0.3 g/l of bovine serum albumin (A9418, Sigma-Aldrich, St Louis, USA). All samples with >70 × 106/ml sperm were diluted to a standardized sperm concentration of ∼35 × 106/ml sperm. About 3–4 µl of diluted semen was pipetted into one side of a 2X-CEL 20-µm-depth chambered microscope slide (Hamilton Thorne Research) maintained at 37°C by a MiniTherm slide warmer (Hamilton Thorne Research). After 1 min, multiple microscope fields spanning the entire slide preparation were analyzed on an Olympus CH30 microscope (Olympus America, Center Valley, PA, USA) equipped with a ×10 negative phase objective for 0.5 s per field using a video frame rate of 60 Hz. When possible, at least 150 motile sperm were evaluated per drop of semen. Between two and four drops of diluted semen were evaluated per donor, and the median value was used for data analyses. Motile sperm includes rapid sperm plus progressive sperm. The percentage of rapid sperm equals the number of sperm with time-average velocity ≥25 µm/s divided by the total number of sperm analyzed × 100. The percentage of progressive sperm equals the number of sperm with time-average velocity ≥25 µm/s and the straightness of trajectory ≥80% divided by the total number of sperm analyzed× 100 (Eskenazi et al., 2003; Sloter et al., 2006).
Scanning transmission ion microscopy for the measurement of mass
Sperm and seminal plasma were examined in the nuclear microprobe facility located at the Lawrence Livermore National Laboratory. A beam of 3.0 MeV protons was used to quantify the mass of thin samples using a beam of not >2000 ions per second. These ions interact with the electron density of the sample causing a loss in energy of the traversing ions. The energy loss was accurately measured with a particle detector behind the sample specimen (Bench et al., 1996). An initial image was taken without any material in front of the detector to check the detector for defects and to provide for the zero energy loss data. Mylar foils of known areal density (mg/cm2) were used to check the calibration. The data were reduced by selecting a region of interest (ROI) that contains the sample deposit and an ROI of the mylar that was free from containing any sample. The area of the sample-free ROI allowed the calculation of the mass of mylar that was within the sample region, which was then subtracted from the sample mass.
Proton-induced X-ray emission for elemental analysis
The PIXE methods for measuring elemental concentrations in sperm were previously described in detail (Bench et al., 1996). Briefly, a beam of 3.0 MeV protons was used to eject inner-shell electrons from atoms within the specimen. As resulting electron vacancies are filled by outer-shell electrons, characteristic X-rays are emitted whose energies identify the particular element. X-rays were detected simultaneously for elements with atomic numbers >12 using solid-state energy dispersive detectors. The element limits of detection (LOD) in sperm and seminal plasma were determined by integrating the energy window corresponding to the background signal under the element peak.
The laboratory quality assurance procedure for using the nuclear microprobe at Lawrence Livermore National Laboratory has been previously described in detail (Bench et al., 1996). Background levels of the elements on the mylar foils were typically below minimum detectable limits of 5 ng/cm2 for each sample. Data were reduced off-line and analyzed with X-ray spectrum codes (Bench et al., 1996). A series of thin film standards containing the elements were used to calibrate the X-ray detection system, and elemental masses of the sperm were calculated with the thin film approximation. The measurement of elemental masses using this calibration procedure has an accuracy that is better than 95% (Bench et al., 1996).
Statistical analysis
Descriptive demographic statistics were calculated for this sample of 20 men, including duration of abstinence (≤5 days versus >5 days); season of sample collection (fall/winter versus spring/summer); body mass index (≤25 kg/m2 versus >25 kg/m2) and histories of previous tobacco use, regular alcohol use, vitamin supplement use in the last year, high blood pressure and kidney, bladder or urinary tract infection. The number and percentage of participants in each category by age group was compared using Fisher's exact test. Spearman correlations were performed to detect associations between levels of elements in washed sperm versus seminal plasma. We summarized each elemental concentration as the median and inter-quartile range by age group and tested their differences using the Mann–Whitney rank sum test. We used unadjusted and age-adjusted linear regression to determine whether these elements were associated with semen quality and genomic defects in sperm.
We constructed multivariable linear regression models to determine whether changes in elemental concentrations could explain previously determined associations between age and the various sperm/semen end-points. These models focused on elemental measures that differed by age (calcium, copper and zinc measured in sperm and sulfur measured in seminal plasma). The main independent variables were age group and the specific element, and the dependent variables were the various sperm/semen end-points. To approximate a normal distribution, we square-root transformed the outcome variables of sperm count, percentage progressively motile sperm, and total progressively motile sperm, and log-transformed DNA fragmentation, aneuploidy, comet, achrondroplasia and structural aberration. We also log transformed all elemental concentrations. Separate models were run for each element and each outcome. We compared the coefficients and P-values for age group in the crude linear regression models (without the element in the model) against the element-adjusted model to determine whether the age associations persisted. If the age variable was no longer significant or if the coefficients had changed >10% after adjusting by an element, we concluded that the element may play a role in the poorer genetic integrity of sperm or semen quality of older men. P-values of ≤0.05 were considered statistically significant.
Results
Characteristics of study population
Our study was performed on samples collected from the well-characterized AGES population, a non-clinical group of non-smoking men that included a younger group of 10 men with a median age of 23 years (age range = 22–28) and an older group of 10 men with a median age of 69 years (age range = 65–80). The older men tended to have slightly longer durations of sexual abstinence (P = 0.06) and were more likely to report prior tobacco use (P = 0.04) (Supplementary data, Table I).
Elements detected in sperm and seminal plasma
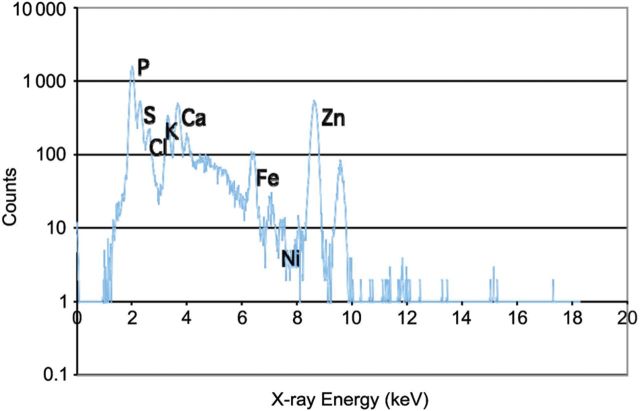
Nine elements of the PIXE spectrum were included in the analysis of isolated sperm: zinc, copper, calcium, sulfur, chlorine, potassium, titanium, iron and nickel (Fig. 1). An additional two elements (bromine and rubidium) were below the LOD in sperm and were measured only in seminal plasma. The concentrations of three elements were positively correlated between sperm and seminal plasma: sulfur (r = 0.54, P = 0.01), titanium (r = 0.50, P = 0.02) and zinc (r = 0.47, P = 0.04) (Supplementary data, Table II).
Figure 1.
The proton-induced X-ray emission spectrum of elemental concentrations in washed human sperm. P, phosphorus; S, sulfur; Cl, chlorine; K, potassium; Ca, calcium; Fe, iron; Ni, nickel; Zn, zinc.
Elemental concentrations in washed sperm and seminal plasma of young and old men
Table I summarizes the median and inter-quartile range of element concentrations measured by PIXE in sperm and seminal plasma of the young and old age groups. The sperm of the old group had significantly higher concentrations of calcium, copper and zinc compared with the young group; the median levels in the old group were 2.2 times higher for calcium (81 versus 38 fg per sperm; P = 0.02), 1.6 times higher for copper (0.08 versus 0.05 fg per sperm; P = 0.03) and 1.8 times higher for zinc (20 versus 11 fg per sperm; P = 0.05). No statistically significant differences between age groups were found for the five other elements: titanium, iron, nickel, chlorine and potassium, even though in all cases, concentrations were higher in the old group.
Table I.
Elemental concentrations in washed sperm (fg per sperm) and seminal plasma (p.p.m.) in the young and old groups of men.
| Element | Washed sperm (fg/sperm) |
Seminal plasma (p.p.m.) |
||||||
|---|---|---|---|---|---|---|---|---|
| Young group, 22–28 years (n = 10) |
Old group, 65–80 years (n = 10) |
Young group, 22–28 years (n = 10) |
Old group, 65–80 years (n = 10) |
|||||
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | |
| Sulfur | 66.78 | 47.96 | 144.53 | 128.03 | 3400.9 | 619.8 | 4624.9 | 1801.2* |
| Chlorine | 18.14 | 23.05 | 21.82 | 27.22 | 19219.6 | 15480.9 | 25715.3 | 5731.1 |
| Potassium | 29.01 | 30.19 | 31.69 | 100.08 | 49993.8 | 24773.9 | 52335.1 | 22459.0 |
| Calcium | 37.74 | 43.25 | 81.18 | 40.53* | 25086.0 | 10784.1 | 25489.7 | 13114.9 |
| Titanium | 1.03 | 0.51 | 1.67 | 2.81 | 63.7 | 82.4 | 69.4 | 109.0 |
| Iron | 1.21 | 1.25 | 1.74 | 1.79 | 28.7 | 26.2 | 29.0 | 24.0 |
| Nickel | 0.08 | 0.05 | 0.10 | 0.15 | 18.1 | 7.2 | 18.8 | 7.8 |
| Copper | 0.05 | 0.05 | 0.08 | 0.05* | 4.2 | 3.0 | 4.3 | 2.7 |
| Zinc | 10.98 | 7.96 | 19.66 | 21.44* | 3314.6 | 1448.6 | 4417.4 | 2175.2 |
| Bromine | N/A | N/A | N/A | N/A | 26.7 | 22.8 | 44.2 | 13.7 |
| Rubidium | N/A | N/A | N/A | N/A | 22.7 | 6.8 | 24.7 | 15.1 |
IQR, inter-quartile range; p.p.m., parts per million.
*P ≤ 0.05 by the Mann–Whitney test.
Sulfur was the only element out of 11, which showed an age-related difference in seminal plasma concentrations (median of 4600 p.p.m. in old group and 3400 p.p.m. in the young group, P = 0.01).
Age associations with elemental concentrations, semen quality and genomic defects in sperm of young and old men
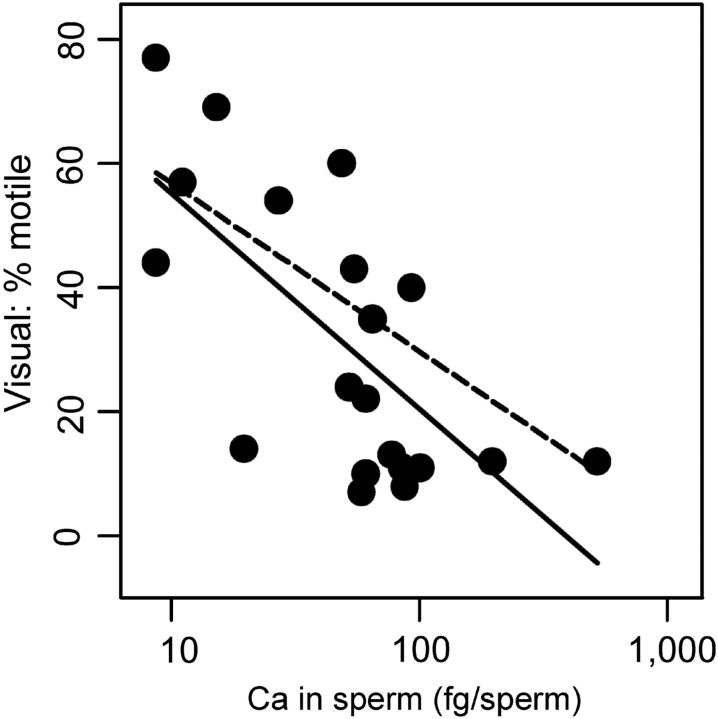
Table II lists the unadjusted associations between the elements found to differ by age and sperm outcomes. Higher sperm calcium was associated with lower sperm motility (by CASA and conventional analyses), and with an increased frequency of DNA fragmentation in sperm and sperm Comet damage (under alkaline but not neutral conditions). Higher sperm copper was also associated with lower sperm motility and higher DNA fragmentation. No statistically significant associations were found between sperm zinc and any of the semen quality and sperm end-points used in this study (Table II). The concentration of sulfur in seminal plasma was associated with lower sperm motility, increased sperm DNA fragmentation and increased frequencies of sperm with structural aberrations. When controlled for age (Supplementary data, Table III), the following associations were significant: sperm calcium and decreased sperm motility (P = 0.01) and seminal sulfur and increased sperm count (P = 0.04). Figure 2 shows the age-adjusted and unadjusted relationships between sperm calcium concentration and sperm motility (using visual assessment of sperm motility as the example of the motility effect).
Table II.
Unadjusted associations between elemental compositions of sperm and semen (concentrations of sperm calcium, sperm copper and sperm zinc and seminal plasma sulfur) and semen quality and defects in the sperm genome.a
| Sperm/semen outcomes | Calcium in sperm |
Copper in sperm |
Zinc in sperm |
Sulfur in seminal plasma |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coef. | 95% CI | P-value | Coef. | 95% CI | P-value | Coef. | 95% CI | P-value | Coef. | 95% CI | P-value | |
| Sperm count (106) | −10.0 | (−20.8, 0.8) | 0.07 | −2.2 | (−13.6, 9.3) | 0.70 | −2.8 | (−16.5, 10.9) | 0.67 | 30.7 | (−16.7, 78.1) | 0.19 |
| Motility | ||||||||||||
| Visual: % motile | −34.6 | (−53.0, −16.3) | <0.005 | −16.8 | (−39.7, 6.1) | 0.14 | −17.9 | (−45.7, 9.9) | 0.19 | −102.9 | (−195.0, −10.8) | 0.03 |
| Visual: % progressively motile | −3.1 | (−4.9, −1.4) | <0.005 | −2.1 | (−4.1, 0.0) | 0.05 | −1.7 | (−4.3, 0.8) | 0.18 | −7.8 | (−16.7, 1.1) | 0.08 |
| Visual: total progressively motile sperm (106) | −9.1 | (−14.2, −4.0) | <0.005 | −3.0 | (−9.4, 3.4) | 0.34 | −3.8 | (−11.5, 3.8) | 0.31 | −8.9 | (−36.9, 19.2) | 0.52 |
| CASA: % motile | −38.6 | (−61.2, −15.9) | <0.005 | −22.2 | (−48.8, 4.4) | 0.10 | −22.9 | (−55.4, 9.6) | 0.16 | −126.3 | (−233.6, −18.9) | 0.02 |
| CASA: % progressively motile | −22.0 | (−33.7, −10.3) | <0.005 | −11.3 | (−25.8, 3.2) | 0.12 | −13.3 | (−30.7, 4.2) | 0.13 | −63.0 | (−122.5, −3.5) | 0.04 |
| CASA: % sperm with velocity >25 µm/s | −27.8 | (−42.2, −13.5) | <0.005 | −13.5 | (−31.6, 4.6) | 0.14 | −16.6 | (−38.2, 5.0) | 0.12 | −78.0 | (−152.0, −4.1) | 0.04 |
| Sperm DNA fragmentation | ||||||||||||
| SCSA: DNA fragmentation | 1.33 | (0.53, 2.12) | <0.005 | 0.94 | (0.14, 1.74) | 0.02 | 0.93 | (−0.19, 2.05) | 0.10 | 4.61 | (1.32, 7.90) | 0.01 |
| SCSA: mean DFI | 0.60 | (0.22, 0.99) | <0.005 | 0.39 | (−0.00, 0.78) | 0.05 | 0.38 | (−0.15, 0.92) | 0.15 | 2.14 | (0.59, 3.69) | 0.01 |
| SCSA: high DNA sustainability | −0.13 | (−0.75, 0.48) | 0.65 | 0.03 | (−0.52, 0.58) | 0.91 | −0.42 | (−1.10, 0.27) | 0.22 | −1.98 | (−4.15, 0.19) | 0.07 |
| Sperm aneuploidy | ||||||||||||
| X-Y-21 FISH: hyper-haploidy | −0.14 | (−0.29, 0.01) | 0.06 | −0.04 | (−0.20, 0.12) | 0.62 | −0.16 | (−0.33, 0.01) | 0.07 | −0.45 | (−1.10, 0.20) | 0.16 |
| X-Y-21 FISH: hypo-haploidy | −0.10 | (−0.27, 0.07) | 0.22 | 0.02 | (−0.15, 0.19) | 0.83 | 0.00 | (−0.21, 0.20) | 0.96 | −0.20 | (−0.93, 0.53) | 0.57 |
| X-Y-21 FISH: total hyper- and hypo-haploidy | −0.13 | (−0.27, 0.01) | 0.07 | −0.01 | (−0.16, 0.14) | 0.87 | −0.08 | (−0.26, 0.09) | 0.32 | −0.33 | (−0.95, 0.30) | 0.29 |
| X-Y-21 FISH: diploidy | −0.07 | (−0.44, 0.30) | 0.71 | 0.22 | (−0.12, 0.56) | 0.20 | −0.07 | (−0.50, 0.36) | 0.74 | 0.02 | (−1.54, 1.57) | 0.98 |
| Sperm comet | ||||||||||||
| Alkaline comet: % tail DNA | 0.13 | (−0.00, 0.26) | 0.05 | 0.08 | (−0.04, 0.20) | 0.18 | 0.06 | (−0.11, 0.22) | 0.48 | 0.22 | (−0.34, 0.77) | 0.41 |
| Neutral comet: % tail DNA | 0.01 | (−0.12, 0.13) | 0.91 | −0.02 | (−0.13, 0.09) | 0.74 | −0.07 | (−0.21, 0.06) | 0.26 | 0.05 | (−0.43, 0.53) | 0.82 |
| Sperm gene mutation | ||||||||||||
| Achrondroplasia | 0.11 | (−0.44, 0.67) | 0.67 | 0.40 | (−0.25, 1.05) | 0.21 | 0.25 | (−0.34, 0.83) | 0.37 | 1.50 | (−0.66, 3.65) | 0.15 |
| Sperm chromosomal aberrations | ||||||||||||
| ACM: breaks | 0.04 | (−0.15, 0.23) | 0.64 | 0.10 | (−0.08, 0.27) | 0.27 | 0.10 | (−0.11, 0.32) | 0.32 | 0.78 | (0.09, 1.47) | 0.03 |
| ACM: duplications and deletions | 0.04 | (−0.28, 0.36) | 0.80 | 0.26 | (−0.03, 0.55) | 0.07 | 0.17 | (−0.20, 0.54) | 0.34 | 1.17 | (−0.06, 2.40) | 0.06 |
| ACM: structural aberrations | 0.02 | (−0.21, 0.24) | 0.88 | 0.16 | (−0.04, 0.37) | 0.11 | 0.13 | (−0.12, 0.38) | 0.29 | 0.92 | (0.09, 1.75) | 0.03 |
aElemental concentrations were log transformed; sperm count, % progressively motile sperm and Total progressively motile sperm were square-root transformed; SCSA, Sperm aneuploidy, ACM, Sperm comet and a chrondroplasia mutation outcomes were log transformed; CASA, computer-assisted semen analysis; SCSA, sperm chromatin structure assay; DFI, DNA fragmentation index; X-Y-21 FISH, fluorescence in situ hybridization assay for detecting chromosomes X, Y and 21 in sperm; sperm ACM, fluorescence in situ hybridization assay used to detect structural and numerical aberrations of chromosome 1 in sperm.
Figure 2.
Age-adjusted (dashed) and unadjusted (solid) associations of the concentration of calcium in sperm with sperm motility (% motile sperm).
Associations between male age and semen/sperm end-points adjusted for elemental measurements
As shown in Supplementary data, Table IV, we generally confirmed in our subgroup of 20 men the associations we previously reported between male age and various measures of semen quality and sperm damage assessed in the total AGES cohort (Eskenazi et al., 2003; Wyrobek et al., 2006; Schmid et al., 2007; Sloter et al., 2007). Using the younger men, as a reference, the group of older men had: (i) lower sperm motility (conventional medians: 12 versus 47%, P = 0.02); (ii) higher sperm DNA fragmentation measured by SCSA (4.1 versus 2.4, P < 0.01); (iii) a borderline significant increase in the number of single DNA strand breaks as measured by sperm Comet (1.7 versus 1.6%, P = 0.06) and (iv) higher frequencies of sperm with achrondroplasia mutations and with structural aberrations (1.7 versus 1.1, P = 0.01 and 1.5 versus 1.2, P = 0.01, respectively). In Table III, we control for the elements that were related to these end-points. After adjusting for calcium in sperm, we found that the associations between age and sperm motility and between age and alkaline Comet were markedly reduced. Similarly, when we controlled for seminal sulfur, the associations between age and motility decreased, whereas the associations between age and alkaline sperm Comet increased. The other age-related differences in sperm end-points changed by 10% or less after controlling for sperm calcium, sperm copper, sperm zinc or sulfur in seminal plasma.
Table III.
Associations between male aging and semen/sperm end-points adjusting for sperm calcium, sperm copper, sperm zinc and sulfur in seminal plasma.
| Sperm end-pointsa | n | Adjusted by calcium in sperm |
Adjusted by copper in sperm |
Adjusted by zinc in sperm |
Adjusted by sulfur in seminal plasma |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coef. | 95% CI | % Change in coefficient | Coef. | 95% CI | % Change in coefficient | Coef. | 95% CI | % Change in coefficient | Coef. | 95% CI | % Change in coefficient | ||
| % Motileb | 20 | −13.77 | (−31.26, 3.73) | −45 | −23.39 | (−44.93, −1.85) | −7 | −24.01 | (−45.08, −2.95) | −4 | −19.27 | (−43.26, 4.72) | −23 |
| % Progressively motilec | 20 | −10.14 | (−21.04, 0.76) | −40 | −15.92 | (−29.22, −2.61) | −7 | −15.80 | (−28.76, −2.83) | −7 | −14.64 | (−29.62, 0.34) | −14 |
| DNA fragmentationd | 19 | 1.43 | (0.99, 1.88) | −8 | 1.50 | (1.09, 1.92) | −3 | 1.54 | (1.16, 1.93) | 0 | 1.57 | (1.10, 2.03) | 1 |
| Alkaline COMETe | 17 | 0.08 | (−0.06, 0.21) | −32 | 0.10 | (−0.03, 0.23) | −10 | 0.12 | (−0.01, 0.25) | 5 | 0.25 | (0.06, 0.43) | 115 |
a% Progressively motile sperm was square-root transformed; SCSA and comet outcomes were log transformed to satisfy model assumptions.
bConventional semen quality analysis.
cComputer-assisted semen analysis (CASA).
dSperm chromatin structure assay (SCSA).
eAlkaline sperm comet analysis.
Discussion
Our study is the first to measure the concentrations of various elements in both sperm and seminal fluid using the sensitive PIXE method to investigate the effects of these elements on previously reported associations between male aging and the deterioration of certain semen quality and sperm genomic damage end-points. In this pilot study of 20 men from our larger AGES study, we found that older men (≥65 years) had significantly higher levels of zinc, copper and calcium in their sperm and higher levels of sulfur in their seminal fluid than younger men (≤28 years). We also found that higher sperm calcium was associated with lower sperm motility, increased frequency of DNA fragmentation in sperm and a marginal increase in sperm Comet damage; higher sperm copper was also associated with lower motility and higher DNA fragmentation. In addition, seminal plasma sulfur was negatively associated with sperm motility and structural aberrations and positively associated with DNA fragmentation. When we controlled for male age, only the negative association between sperm calcium and sperm motility remained, suggesting that sperm calcium concentrations are significantly associated with sperm motility, independent of male age. When we adjusted for calcium in sperm, the associations between overall aging and sperm motility and alkaline Comet damage were markedly reduced, implicating sperm calcium in motility as well as sperm single strand DNA breaks. However, when we controlled for sulfur in seminal plasma, although the associations between age and motility markedly decreased, the association between age and alkaline Comet increased.
Calcium has a broad role in male reproduction and sperm development. It is important for sperm physiology including motility, metabolism, acrosome reaction and fertilization, but only a small portion (2–4%) of the calcium in semen is present in ionized form (Meseguer et al., 2004). Calcium influxes through the calcium channels of the sperm plasma membrane for the acrosome reaction in the vicinity of the egg and plays a key role in mediating sperm fertility (Chung et al., 2011). Motility is also dependent on intracellular-free-calcium concentration in the principal piece of the flagellum (Marquez et al., 2007). The quantitative relationship between sperm intracellular calcium levels and sperm motility is still controversial (Marquez et al., 2007). It was shown that spermatozoa modulate their movement in response to an alteration in the intracellular calcium concentration which is dependent on the pH of the medium (Giroux-Widemann et al., 1991; Serres et al., 1991). Furthermore, calcium apparently switches on hyperactivation, a swimming pattern characterized by asymmetric flagellar beating and the development of high-amplitude flagellar waves, which is essential for fertilization (Bedu-Addo et al., 2008).Calcium plays a central role in both acrosome reaction and sperm chemotaxis, with spermatozoa failing to perform either of these crucial functions in the absence of extracellular calcium. The chemotaxis of sperm toward eggs is a widespread phenomenon that occurs in most forms of life from lower plants to mammals and plays important roles in ensuring fertilization (Yoshida and Yoshida, 2011). However, the relationship between intracellular calcium and the chemotactic response of a sperm flagellum is not well known (Bedu-Addo et al., 2008).
Our findings are consistent with those of Arver and Sjöberg who reported that low concentrations of ionized calcium in sperm were associated with better progressive motility (Arver and Sjoberg, 1982, 1983). In addition, average sperm path velocities (straight line velocity and linearity) measured by CASA showed significant inverse correlations to total calcium concentration in sperm (Meseguer et al., 2004). Straight line velocity expresses the forward linear progress that a sperm travels in a defined amount of time and is one of the most important clinical CASA parameters for motility. Marquez et al. showed that (i) adding calcium to demembranated human sperm suppressed motility and (ii) intracellular calcium concentrations following cryopreservation were negatively correlated with sperm motility and fertilizing ability (Marquez et al., 2007). These findings suggest that intracellular calcium concentrations regulate sperm motility and that the influx of extracellular calcium is required for successful fertilization.
Copper plays an essential role in spermatogenesis and male infertility (Aydemir et al., 2006). The older men in our study had higher concentrations of copper in their sperm than the younger men, whereas seminal copper levels were similar between the two groups. Our analysis shows an association between higher concentrations of sperm copper and increased DNA fragmentation and poorer sperm motility. High levels of sperm copper are known to be detrimental to sperm maturation, motility and fertility (Skandhan, 1992; Massanyi et al., 2003, 2004) and blood serum copper was also negatively associated with motile and viable sperm (Telisman et al., 2000). Similarly, Aydemir et al. found higher copper levels in seminal plasma of subfertile males than in healthy males (Aydemir et al., 2006), yet we found no age-related differences in seminal copper in our study. These results suggest that sperm copper may be a more sensitive indicator than seminal copper for age-related effects on sperm motility.
Zinc is broadly involved in male reproduction and fertility (Evenson et al., 1993; Henkel et al., 1999, 2005; Telisman et al., 2000, 2007; Zhao and Xiong, 2005; Ebisch et al., 2007; Yuyan et al., 2008; Lee et al., 2009) and we observed that the older age group had increased sperm zinc. Our prior studies using PIXE analysis found zinc to be bound stoichiometrically with protamine 2 in sperm nuclei (Bench et al., 2000). In our current study, we found no significant associations between zinc concentrations in sperm or seminal plasma and any of the sperm or semen quality end-points used in our study.
Our group of older men showed higher concentrations of sulfur in seminal plasma, with significant associations between concentrations of seminal sulfur and poorer sperm motility, more DNA fragmentation and increased frequencies of sperm with chromosomal aberrations. After adjusting for age, these effects were no longer significant, but age-adjustment produced a minor association between sulfur in seminal plasma and sperm count. After adjusting for sulfur in sperm, there was an increased association between age and DNA breaks in the sperm. Prior data using PIXE (Bench et al., 1998) showed that sperm from infertile men were highly deficient in both sulfur and protamines. Our study did not find a change is sperm sulfur between the old and young men, which suggests that the semen quality of the older men was not like that of infertile men.
Our study had several methodological strengths and limitations. Its major strength is that we were able to perform elemental analyses on the same semen samples for which we obtained extensive measurements of semen quality and genetically defective sperm, and the study cohort's demographic characteristics, habits and medical and reproductive history were well documented. Another strength is that our sample was composed of relatively healthy non-smokers with no evidence of reproductive problems. However, these characteristics may also limit the generalization of study findings to sick persons, clinical groups and ethnically diverse populations (Jha et al., 1995). In the AGES study, only a single semen sample was provided by each man; thus, we cannot be sure that our single measurement represents the time-average element levels in the sperm and seminal plasma of these men. Our sample size of 20 men may be insufficient to detect small changes between the age groups, and did not allow us to consider multiple confounders of the association between elements and age or to determine whether the measured elements modified the relationships between age and the sperm end-points. In addition, multiple tests were performed between our various elements and various outcomes; therefore, it is possible that some findings may be due to chance alone.
In summary, we found that older men have significantly higher elemental concentrations of copper, calcium and zinc in their sperm and higher levels of sulfur in their seminal plasma. These findings demonstrate major differences in elemental concentrations of whole sperm and seminal plasma between younger and older men, and that these elements are quantitatively associated with increased risks for poorer semen quality and genomic defects in sperm of older men.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors' roles
T.E.S. and A.J.W. conceived of this study, organized its execution and drafted the manuscript. P.G.G. was responsible for the PIXE analyses. R.H.W. performed statistical analyses and manuscript preparation. B.E., F.M. and A.J.W. designed and conducted the original AGES study that provided the reference data for sperm quality and sperm genomic damage, and were vital to the interpretations and revisions of this manuscript.
Funding
The work was performed under the auspices of the U.S. Department of Energy by LLNL, contract W-7405-ENG-48, with funding support from Superfund P42 ES04705 from the National Institute of Environmental Health Sciences (Eskenazi and Wyrobek, study directors) and the Jennifer and Brian Maxwell Chair. This work was completed at LBNL contract DE-AC02-05CH11231.
Conflict of interest
None declared.
References
- Arver S, Sjoberg HE. Calcium fractions in seminal plasma and functional properties of human spermatozoa. Acta Physiol Scand. 1982;116:159–165. doi: 10.1111/j.1748-1716.1982.tb07125.x. doi:10.1111/j.1748-1716.1982.tb07125.x. [DOI] [PubMed] [Google Scholar]
- Arver S, Sjoberg HE. Ionized calcium in human seminal plasma. Scand J Clin Lab Invest Suppl. 1983;165:123–126. [PubMed] [Google Scholar]
- Aydemir B, Kiziler AR, Onaran I, Alici B, Ozkara H, Akyolcu MC. Impact of Cu and Fe concentrations on oxidative damage in male infertility. Biol Trace Elem Res. 2006;112:193–203. doi: 10.1385/BTER:112:3:193. doi:10.1385/BTER:112:3:193. [DOI] [PubMed] [Google Scholar]
- Bedu-Addo K, Costello S, Harper C, Machado-Oliveira G, Lefievre L, Ford C, Barratt C, Publicover S. Mobilisation of stored calcium in the neck region of human sperm—a mechanism for regulation of flagellar activity. Int J Dev Biol. 2008;52:615–626. doi: 10.1387/ijdb.072535kb. doi:10.1387/ijdb.072535kb. [DOI] [PubMed] [Google Scholar]
- Bench GS, Friz AM, Corzett MH, Morse DH, Balhorn R. DNA and total protamine masses in individual sperm from fertile mammalian subjects. Cytometry. 1996;23:263–271. doi: 10.1002/(SICI)1097-0320(19960401)23:4<263::AID-CYTO1>3.0.CO;2-I. doi:10.1002/(SICI)1097-0320(19960401)23:4<263::AID-CYTO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Bench G, Corzett MH, De Yebra L, Oliva R, Balhorn R. Protein and DNA contents in sperm from an infertile human male possessing protamine defects that vary over time. Mol Reprod Dev. 1998;50:345–353. doi: 10.1002/(SICI)1098-2795(199807)50:3<345::AID-MRD11>3.0.CO;2-3. doi:10.1002/(SICI)1098-2795(199807)50:3<345::AID-MRD11>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Bench G, Corzett MH, Kramer CE, Grant PG, Balhorn R. Zinc is sufficiently abundant within mammalian sperm nuclei to bind stoichiometrically with protamine 2. Mol Reprod Dev. 2000;56:512–519. doi: 10.1002/1098-2795(200008)56:4<512::AID-MRD9>3.0.CO;2-M. doi:10.1002/1098-2795(200008)56:4<512::AID-MRD9>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Benoff S, Cooper GW, Centola GM, Jacob A, Hershlag A, Hurley IR. Metal ions and human sperm mannose receptors. Andrologia. 2000;32:317–329. doi: 10.1046/j.1439-0272.2000.00401.x. doi:10.1046/j.1439-0272.2000.00401.x. [DOI] [PubMed] [Google Scholar]
- Chung JJ, Navarro B, Krapivinsky G, Krapivinsky L, Clapham DE. A novel gene required for male fertility and functional CATSPER channel formation in spermatozoa. Nat Commun. 2011;2:153. doi: 10.1038/ncomms1153. doi:10.1038/ncomms1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Rochebrochard E, Thonneau P. Paternal age and maternal age are risk factors for miscarriage; results of a multicentre European study. Hum Reprod. 2002;17:1649–1656. doi: 10.1093/humrep/17.6.1649. doi:10.1093/humrep/17.6.1649. [DOI] [PubMed] [Google Scholar]
- Ebisch IM, Thomas CM, Peters WH, Braat DD, Steegers-Theunissen RP. The importance of folate, zinc and antioxidants in the pathogenesis and prevention of subfertility. Hum Reprod Update. 2007;13:163–174. doi: 10.1093/humupd/dml054. doi:10.1093/humupd/dml054. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Wyrobek AJ, Sloter E, Kidd SA, Moore L, Young S, Moore D. The association of age and semen quality in healthy men. Hum Reprod. 2003;18:447–454. doi: 10.1093/humrep/deg107. doi:10.1093/humrep/deg107. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Kidd SA, Marks AR, Sloter E, Block G, Wyrobek AJ. Antioxidant intake is associated with semen quality in healthy men. Hum Reprod. 2005;20:1006–1012. doi: 10.1093/humrep/deh725. doi:10.1093/humrep/deh725. [DOI] [PubMed] [Google Scholar]
- Evenson DP, Emerick RJ, Jost LK, Kayongo-Male H, Stewart SR. Zinc-silicon interactions influencing sperm chromatin integrity and testicular cell development in the rat as measured by flow cytometry. J Anim Sci. 1993;71:955–962. doi: 10.2527/1993.714955x. [DOI] [PubMed] [Google Scholar]
- Fenech M. Micronutrients and genomic stability: a new paradigm for recommended dietary allowances (RDAs) Food Chem Toxicol. 2002;40:1113–1117. doi: 10.1016/s0278-6915(02)00028-5. doi:10.1016/S0278-6915(02)00028-5. [DOI] [PubMed] [Google Scholar]
- Figa-Talamanca I, Traina ME, Urbani E. Occupational exposures to metals, solvents and pesticides: recent evidence on male reproductive effects and biological markers. Occup Med (Lond) 2001;51:174–188. doi: 10.1093/occmed/51.3.174. doi:10.1093/occmed/51.3.174. [DOI] [PubMed] [Google Scholar]
- Forslind B, Malmqvist KG, Pallon J. Proton induced X-ray emission analysis of biological specimens–past and future. Scanning Microsc. 1991;5:877–884. [PubMed] [Google Scholar]
- Giroux-Widemann V, Jouannet P, Pignot-Paintrand I, Feneux D. Effects of pH on the reactivation of human spermatozoa demembranated with Triton X-100. Mol Reprod Dev. 1991;29:157–162. doi: 10.1002/mrd.1080290211. doi:10.1002/mrd.1080290211. [DOI] [PubMed] [Google Scholar]
- Glaser RL, Broman KW, Schulman RL, Eskenazi B, Wyrobek AJ, Jabs EW. The paternal-age effect in Apert syndrome is due, in part, to the increased frequency of mutations in sperm. Am J Hum Genet. 2003;73:939–947. doi: 10.1086/378419. doi:10.1086/378419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel R, Bittner J, Weber R, Huther F, Miska W. Relevance of zinc in human sperm flagella and its relation to motility. Fertil Steril. 1999;71:1138–1143. doi: 10.1016/s0015-0282(99)00141-7. doi:10.1016/S0015-0282(99)00141-7. [DOI] [PubMed] [Google Scholar]
- Henkel R, Maass G, Schuppe HC, Jung A, Schubert J, Schill WB. Molecular aspects of declining sperm motility in older men. Fertil Steril. 2005;84:1430–1437. doi: 10.1016/j.fertnstert.2005.05.020. doi:10.1016/j.fertnstert.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Jha P, Flather M, Lonn E, Farkouh M, Yusuf S. The antioxidant vitamins and cardiovascular disease. A critical review of epidemiologic and clinical trial data. Ann Intern Med. 1995;123:860–872. doi: 10.7326/0003-4819-123-11-199512010-00009. [DOI] [PubMed] [Google Scholar]
- Lee SH, Song H, Park YS, Koong MK, Song IO, Jun JH. Poor sperm quality affects clinical outcomes of intracytoplasmic sperm injection in fresh and subsequent frozen-thawed cycles: potential paternal effects on pregnancy outcomes. Fertil Steril. 2009;91:798–804. doi: 10.1016/j.fertnstert.2007.12.061. doi:10.1016/j.fertnstert.2007.12.061. [DOI] [PubMed] [Google Scholar]
- Marquez B, Ignotz G, Suarez SS. Contributions of extracellular and intracellular Ca2+ to regulation of sperm motility: release of intracellular stores can hyperactivate CatSper1 and CatSper2 null sperm. Dev Biol. 2007;303:214–221. doi: 10.1016/j.ydbio.2006.11.007. doi:10.1016/j.ydbio.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massanyi P, Trandzik J, Nad P, Korenekova B, Skalicka M, Toman R, Lukac N, Strapak P, Halo M, Turcan J. Concentration of copper, iron, zinc, cadmium, lead, and nickel in boar semen and relation to the spermatozoa quality. J Environ Sci Health Part A Tox Hazard Subst Environ Eng. 2003;38:2643–2651. doi: 10.1081/ese-120024453. doi:10.1081/ESE-120024453. [DOI] [PubMed] [Google Scholar]
- Massanyi P, Trandzik J, Nad P, Korenekova B, Skalicka M, Toman R, Lukac N, Halo M, Strapak P. Concentration of copper, iron, zinc, cadmium, lead, and nickel in bull and ram semen and relation to the occurrence of pathological spermatozoa. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2004;39:3005–3014. doi:10.1081/ESE-200034832. [PubMed] [Google Scholar]
- Meseguer M, Garrido N, Martinez-Conejero JA, Simon C, Pellicer A, Remohi J. Relationship between standard semen parameters, calcium, cholesterol contents, and mitochondrial activity in ejaculated spermatozoa from fertile and infertile males. J Assist Reprod Genet. 2004;21:445–451. doi: 10.1007/s10815-004-8761-7. doi:10.1007/s10815-004-8761-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris ID, Ilott S, Dixon L, Brison DR. The spectrum of DNA damage in human sperm assessed by single cell gel electrophoresis (Comet assay) and its relationship to fertilization and embryo development. Hum Reprod. 2002;17:990–998. doi: 10.1093/humrep/17.4.990. doi:10.1093/humrep/17.4.990. [DOI] [PubMed] [Google Scholar]
- Rolf C, Nieschlag E. Reproductive functions, fertility and genetic risks of ageing men. Exp Clin Endocrinol Diabetes. 2001;109:68–74. doi: 10.1055/s-2001-14825. doi:10.1055/s-2001-14825. [DOI] [PubMed] [Google Scholar]
- Ryan CG. PIXE and the nuclear microprobe: tools for quantitative imaging of complex natural materials. Nucl Instrum Methods in Phys Res B. 2011;269:2151–2162. doi:10.1016/j.nimb.2011.02.046. [Google Scholar]
- Schmid TE, Eskenazi B, Baumgartner A, Marchetti F, Young S, Weldon R, Anderson D, Wyrobek AJ. The effects of male age on sperm DNA damage in healthy non-smokers. Hum Reprod. 2007;22:180–187. doi: 10.1093/humrep/del338. doi:10.1093/humrep/del338. [DOI] [PubMed] [Google Scholar]
- Schmid TE, Eskenazi B, Marchetti F, Young S, Weldon RH, Baumgartner A, Anderson D, Wyrobek AJ. Micronutrients intake is associated with improved sperm DNA quality in older men. Fertil and Steril. 2012 doi: 10.1016/j.fertnstert.2012.07.1126. (in press) doi:10.1093/humrep/del338. [DOI] [PubMed] [Google Scholar]
- Schrag SD, Dixon RL. Occupational exposures associated with male reproductive dysfunction. Annu Rev Pharmacol Toxicol. 1985;25:567–592. doi: 10.1146/annurev.pa.25.040185.003031. [DOI] [PubMed] [Google Scholar]
- Serres C, Feneux D, Berthon B. Decrease of internal free calcium and human sperm movement. Cell Motil Cytoskel. 1991;18:228–240. doi: 10.1002/cm.970180308. doi:10.1002/cm.970180308. [DOI] [PubMed] [Google Scholar]
- Singh NP, Muller CH, Berger RE. Effects of age on DNA double-strand breaks and apoptosis in human sperm. Fertil Steril. 2003;80:1420–1430. doi: 10.1016/j.fertnstert.2003.04.002. doi:10.1016/j.fertnstert.2003.04.002. [DOI] [PubMed] [Google Scholar]
- Skandhan KP. Review on copper in male reproduction and contraception. Rev Fr Gynecol Obstet. 1992;87:594–598. [PubMed] [Google Scholar]
- Sloter E, Nath J, Eskenazi B, Wyrobek AJ. Effects of male age on the frequencies of germinal and heritable chromosomal abnormalities in humans and rodents. Fertil Steril. 2004;81:925–943. doi: 10.1016/j.fertnstert.2003.07.043. doi:10.1016/j.fertnstert.2003.07.043. [DOI] [PubMed] [Google Scholar]
- Sloter E, Schmid TE, Marchetti F, Eskenazi B, Nath J, Wyrobek AJ. Quantitative effects of male age on sperm motion. Hum Reprod. 2006;21:2868–2875. doi: 10.1093/humrep/del250. doi:10.1093/humrep/del250. [DOI] [PubMed] [Google Scholar]
- Sloter ED, Marchetti F, Eskenazi B, Weldon RH, Nath J, Cabreros D, Wyrobek AJ. Frequency of human sperm carrying structural aberrations of chromosome 1 increases with advancing age. Fertil Steril. 2007;87:1077–1086. doi: 10.1016/j.fertnstert.2006.08.112. doi:10.1016/j.fertnstert.2006.08.112. [DOI] [PubMed] [Google Scholar]
- Telisman S, Cvitkovic P, Jurasovic J, Pizent A, Gavella M, Rocic B. Semen quality and reproductive endocrine function in relation to biomarkers of lead, cadmium, zinc, and copper in men. Environ Health Perspect. 2000;108:45–53. doi: 10.1289/ehp.0010845. doi:10.1289/ehp.0010845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telisman S, Colak B, Pizent A, Jurasovic J, Cvitkovic P. Reproductive toxicity of low-level lead exposure in men. Environ Res. 2007;105:256–266. doi: 10.1016/j.envres.2007.05.011. doi:10.1016/j.envres.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Tiemann-Boege I, Navidi W, Grewal R, Cohn D, Eskenazi B, Wyrobek AJ, Arnheim N. The observed human sperm mutation frequency cannot explain the achondroplasia paternal age effect. Proc Natl Acad Sci U S A. 2002;99:14952–14957. doi: 10.1073/pnas.232568699. doi:10.1073/pnas.232568699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura S, Martin J, Curtin S, Mathews T. Hyattsville, MD: National Center for Health Statistics; 1997. Report of Final Natality Statistics, 1995 Monthly Vital Statistics Report. [PubMed] [Google Scholar]
- Wong WY, Thomas CM, Merkus JM, Zielhuis GA, Steegers-Theunissen RP. Male factor subfertility: possible causes and the impact of nutritional factors. Fertil Steril. 2000;73:435–442. doi: 10.1016/s0015-0282(99)00551-8. doi:10.1016/S0015-0282(99)00551-8. [DOI] [PubMed] [Google Scholar]
- Wong WY, Flik G, Groenen PM, Swinkels DW, Thomas CM, Copius-Peereboom JH, Merkus HM, Steegers-Theunissen RP. The impact of calcium, magnesium, zinc, and copper in blood and seminal plasma on semen parameters in men. Reprod Toxicol. 2001;15:131–136. doi: 10.1016/s0890-6238(01)00113-7. doi:10.1016/S0890-6238(01)00113-7. [DOI] [PubMed] [Google Scholar]
- Wong WY, Merkus HM, Thomas CM, Menkveld R, Zielhuis GA, Steegers-Theunissen RP. Effects of folic acid and zinc sulfate on male factor subfertility: a double-blind, randomized, placebo-controlled trial. Fertil Steril. 2002;77:491–498. doi: 10.1016/s0015-0282(01)03229-0. doi:10.1016/S0015-0282(01)03229-0. [DOI] [PubMed] [Google Scholar]
- Wyrobek AJ, Eskenazi B, Young S, Arnheim N, Tiemann-Boege I, Jabs EW, Glaser RL, Pearson FS, Evenson D. Advancing age has differential effects on DNA damage, chromatin integrity, gene mutations, and aneuploidies in sperm. Proc Natl Acad Sci USA. 2006;103:9601–9606. doi: 10.1073/pnas.0506468103. doi:10.1073/pnas.0506468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Yoshida K. Sperm chemotaxis and regulation of flagellar movement by Ca2+ Mol Hum Reprod. 2011;17:457–465. doi: 10.1093/molehr/gar041. doi:10.1093/molehr/gar041. [DOI] [PubMed] [Google Scholar]
- Young SS, Eskenazi B, Marchetti FM, Block G, Wyrobek AJ. The association of folate, zinc and antioxidant intake with sperm aneuploidy in healthy non-smoking men. Hum Reprod. 2008;23:1014–1022. doi: 10.1093/humrep/den036. doi:10.1093/humrep/den036. [DOI] [PubMed] [Google Scholar]
- Yuyan L, Junqing W, Wei Y, Weijin Z, Ersheng G. Are serum zinc and copper levels related to semen quality. Fertil Steril. 2008;89:1008–1011. doi: 10.1016/j.fertnstert.2007.04.028. doi:10.1016/j.fertnstert.2007.04.028. [DOI] [PubMed] [Google Scholar]
- Zhao RP, Xiong CL. Zinc content analysis in serum, seminal plasma and spermatozoa of asthenozoospermic and oligoasthenozoospermic patients. Zhonghua Nan Ke Xue. 2005;11:680–682. [PubMed] [Google Scholar]