Therapeutic amino-acid copolymers stimulate innate myeloid-cell responses in EAE.
Keywords: BMDC, CCL22, Copaxone, EAE, macrophages, MHC II−/−, mice, regulatory T cells
Abstract
The effects of the amino acid copolymers used in the therapy of experimental autoimmune encephalomyelitis, poly(Y,E,A,K)n (Copaxone®) and poly(Y,F,A,K)n, on murine myeloid cells have been investigated. After administration of these copolymers to mice, increases in several splenic myeloid cell populations were observed, including CD11b+ CD11c+ dendritic cells. The latter were the major splenic cell type that secreted CCL22 (macrophage-derived chemokine) on stimulation with amino acid copolymers. CCL22 secretion was also stimulated from bone marrow-derived dendritic cells (BMDC) generated with GM-CSF in much larger amounts than from bone marrow-derived macrophages generated with M-CSF. Moreover, CCL22 secretion could also be obtained using BMDC generated from two different types of MHC II−/− mice, indicating that an innate immune receptor is involved. Finally, incubation of these BMDC or splenic dendritic cells with naive CD4+ CD25− T cells resulted in formation of CD4+ CD25HI Foxp3 T cells (~25% of which were Foxp3+). The number of these regulatory cells was doubled by pretreatment of BMDC with amino acid copolymers.
Introduction
The random amino acid copolymer [poly(Y,E,A,K)n] (copolymer 1, glatiramer acetate, Copaxone), called YEAK, was designed >40 years ago to be a mimic of myelin basic protein (MBP), an encephalitogen for experimental autoimmune encephalomyelitis (EAE). However, instead it was found to be an effective inhibitor of EAE induced in mice by MBP or peptides derived from it (1, 2). Later, it was introduced into therapy of multiple sclerosis (MS), for which it provides a modest amelioration in patients with relapsing remitting MS (3). Despite decades of work, knowledge of the mechanisms by which it ameliorates disease in mice or in humans remains incomplete. The focus in these studies was initially placed on T cells, which were shown to produce immunoregulatory and neuroprotective cytokines (4–9 and reviewed in ref. 10). The role of antigen-presenting myeloid cells has also been pointed out, particularly in recent work (7, 10–15), although the relationship between the T cells and antigen-presenting myeloid cells has remained enigmatic.
Recently, several additional random amino acid copolymers were synthesized and studied. Among these, poly(Y,F,A,K)n, called YFAK, has been the subject of additional study because it ameliorates EAE in mice much more effectively than YEAK (8, 9, 15–19). Two models were used principally: proteolipid protein (PLP)-139–151 induced EAE in SJL mice and MBP 85–99 induced EAE in ‘humanized’ mice in which the HLA-DR2 gene to which MS is linked replaced the mouse MHC gene I-As and a TCR from T cell clone Ob.1A12 derived from an MS patient was introduced as a transgene. The enhanced efficacy is thought to be due to the substitution of E in YEAK by F in YFAK because YEAK contains no amino acid that binds effectively to the P1 pocket of HLA-DR2 (the MHC class II protein with which MS is most closely associated) or to a corresponding pocket in the I-As protein of the SJL mouse. The mechanism by which this copolymer inhibits disease has been thought to be due to the generation of IL-10-secreting regulatory T cells that also secrete IL-4 and IL-13 (8, 9). Adoptive transfer of these regulatory T cells completely prevented subsequent induction of disease.
The most recent work sought to define the mechanism through which antigen-presenting cells participated in the action of this copolymer (15). The serum levels of the myeloid chemokines CCL22 [macrophage-derived chemokine (MDC)] and CXCL13 were found to be markedly elevated in treated mice. Moreover, bone marrow-derived macrophages (BMMϕ), as well a murine macrophage cell line, were found to secrete CCL22 on treatment with YFAK. These chemokines were also secreted into serum of MHC class II−/− mice, presumably through an innate immune receptor on these cells. Moreover, in successful Phase I clinical trials (20, 21), CCL22 was found elevated in the serum of patients with secondary progressive MS to whom YFAK had been administered. In addition, both YEAK and YFAK bind to human and mouse MHC II (22, 23) proteins and stimulate copolymer-specific T cells. Both the innate and the adaptive immune systems must therefore be involved in the mechanism.
In the present study, the role of myeloid cells has been further explored.
Methods
Mice and immunization
Pathogen-free SJL/J mice between 6 and 8 weeks of age were purchased from Jackson Laboratories (Bar Harbor, ME, USA). MHC II−/− mice [B6.SJL(129)-Ptprca/BoyAiTac H2-Ab1tm1Gru N7+N6] and age/sex-matched control mice (congenic B6.SJL mice) were purchased from Taconic Farms (Hudson, NY, USA). MHC II KO (B6.129S2-H2dlAb1-Ea/J) mice and age/sex-matched control mice (C57BL/6) were purchased from Jackson Laboratories. MyD88 KO [B6.129P2(SJL)-MyD88/tm1.1defr/J] mice (Jackson Laboratories) and control mice were a gift from Dr Koichi Kobayashi at Dana Farber Cancer Institute, Boston, MA, USA. All experiments with mice were performed with the approval of the animal care and use committees of Harvard University. The procedures for induction of EAE in SJL/J mice using PLP139–151 as the autoantigen have been described (17).
Copolymers and peptides
Copolymers and peptides were synthesized as described (16, 17). The sequence of PLP139–151 was HSLGKWLGHPDKF.
Flow cytometric analysis
Single-cell suspension from mouse spleen was used for surface and intracellular staining using fluorochrome conjugated anti-mouse CD3, CD4, CD8α, CD11b, CD11c, CD19, CD25, CD45R/B220, F4/80, Ly-6C, PDCA-1 and Gr-1 antibodies (BioLegend, San Diego, CA, USA). After surface CD4 and CD25 staining, intracellular Foxp3 staining was performed with a Foxp3 Fix/Perm Buffer set (BioLegend) following the manufacturer’s instructions. All samples were analyzed using FACSCalibur (BD Biosciences, Franklin Lakes, NJ, USA) or LSR II (BD Biosciences) and CellQuest Software (BD Biosciences) or FlowJo Software (Tree Star, Inc., Ashland, OR, USA). Cell sorting was performed using MoFlo (Beckman Coulter, Brea, CA, USA).
Isolation of splenic myeloid populations
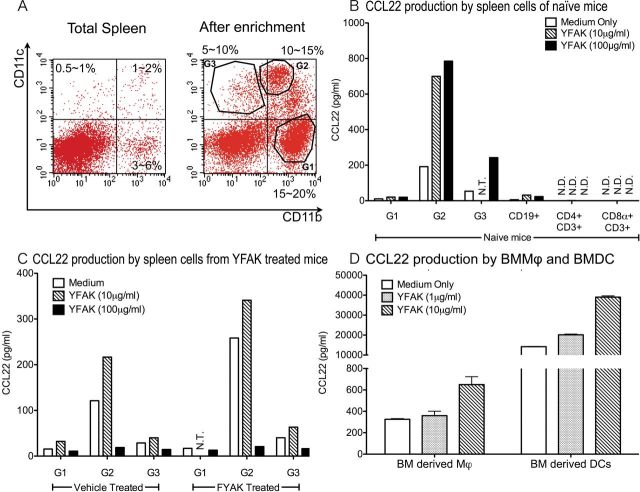
Single-cell suspensions of splenocytes were enriched using CD11b and CD11c magnetic beads (Miltenyi Biotech, Cologne, Germany). Three different subpopulations of splenic myeloid cells were further purified using the MoFlo Cell sorter (Beckman Coulter) after staining with PE-conjugated CD11b and allophycocyanin-conjugated CD11c, based on the scatter plot shown below in Fig. 3A.
Fig. 3.
CCL22 produced by dendritic cells and induced by YFAK stimulation. (A) Representative scatter plot of total spleen and enriched myeloid populations are shown. (B) Spleen cells were enriched with CD11b and CD11c magnetic beads followed by further isolation by MoFlo sorting. Naive splenic myeloid cell subpopulation G1:CD11b+ CD11c−, G2:CD11b+ CD11c+, G3:CD11b−CD11c+ secreted large amounts of CCL22, and splenic B cells secrete CCL22 in small amounts. Splenic T cells did not secrete any CCL22. Each cell population (1×106 cells ml−1) was stimulated with different doses of YFAK for 72h. CCL22 in supernatant was analyzed by ELISA. (C) Pretreatment of mice with YFAK enhanced CCL22 production from myeloid cell subpopulations. YFAK (150 µg day−1) or vehicle (PBS) was injected s.c. into SJL mice daily for 10 days. Spleen cell populations were isolated 1h after the final injections and analyzed as described in (B). (D) BMMϕ and BMDC were produced as described in Methods and assayed for CCL22 production as described in (B). NT, not tested; ND, not detected.
Bone marrow-derived macrophages and dendritic cells
BMMϕ were generated as described by Zhang et al. (24). Briefly, mouse bone marrow cells were isolated from tibia and femur and incubated in a CO2 incubator in complete BMEM:F12 (1:1) medium supplemented with 10ng ml−1 of M-CSF (ProSpec-Tany TechnoGene, Ltd, East Brunswick, NJ, USA) on day 1 and 3; macrophages were harvested at day 7. Bone marrow-derived dendritic cells (BMDC) were generated as described by Lutz et al. (25). Briefly, mouse bone marrow cells were isolated from tibia and femor and incubated in a CO2 incubator in complete DMEM medium supplemented with 10ng ml−1 of GM-CSF (Peprotech, Rocky Hill, NJ, USA) on day 1, 3, 6 and 8. Dendritic cells were harvested at day 10. To obtain YFAK-primed BMDC, 50 µg ml−1 of YFAK was included in the cell culture medium for the final 24h.
NF-κB reporter gene assay for activation of Toll-like receptor
NF-κB reporter gene assay for activation of Toll-like receptor (TLR) was performed with slight modification of the procedure of Ohnishi et al. (26). Briefly, HEK293T cells were transfected with an NF-κB reporter vector (Renilla luciferase reporter vector) and (A) mock, (B) hTLR1 (Invivogen, San Diego, CA, USA) and hTLR2 (Invivogen), (C) hTLR2 (Invivogen) and hTLR6 (pEFBOS-TLR6-myc; generously provided by Dr Shizuo Akira, Osaka University, Osaka, Japan), (D) hTLR2 and hTLR10 (pEFBOS-TLR10-myc; generously provided by Dr Shizuo Akira), (E) hTLR4 (Invivogen), MD2 (Invivogen) and CD14 (Invivogen) and (F) hTLR5 (Invivogen). After transfection, the cells were stimulated with copolymers, Pam2CSK4 (Invivogen), Pam3CSK4 (Invivogen), rec FLA-ST (Invivogen) or LPS (Invivogen) and incubated for 6h. Luciferase reporter gene activity was analyzed using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA).
Coculturing of BMDC/spleen dendritic cells with CD4+ CD25− cells
CD4+ CD25− cells were isolated from naive SJL/J spleen using the CD4+ CD25+ Regulatory T Cell Isolation Kit (Miltenyi Biotech). Purity of CD4+ CD25− cells in total enriched cells was >90%. CD11c+ cells were isolated from spleen using CD11c microbeads (Miltenyi Biotech). About >90% purity was achieved after passing through a second column. CD4+ CD25− cells (1×106) were incubated with or without BMDC/splenic DC (CD11c+ cells) for 7 days in a CO2 incubator in 2ml of complete DMEM medium with or without YFAK, YEAK or LPS (indicated dose). Cells were stained for CD4, CD25 and Foxp3 and analyzed by flow cytometry.
Cytokine analysis of cell culture supernatant with ELISA
Cytokine concentrations in cell culture supernatant were analyzed using ELISA kits according to the manufacturer’s instructions: CCL22 (R&D Systems, Minneapolis, MN, USA), transforming growth factor (TGF)-β1, IL-6, IL-13, IL-23, IL-27 (eBioscience, San Diego, CA, USA), IFN-γ, IL-1β and IL-10 (BioLegend).
Results
Effect of YFAK on the cellular composition of the spleen
Administration of YFAK to SJL mice was previously reported to result in a substantial enlargement of spleen size, amounting to 2–2.5 times the initial weight (8). The question of the cellular composition of the enlarged spleen has therefore been studied. Important differences between the earlier experiment and the present experiment should be noted. The immunization protocol used in the earlier study was a single injection of 75 µg of PLP139–151 together with 500 µg of YFAK in CFA followed by i.v. pertussis toxin (200ng) 1 day later and analysis at day 21. However, in the present study 150 µg of YFAK without CFA, PLP139–151 or pertussis toxin was administered daily for 10 days and spleens were removed for analysis at day 10, a protocol that more closely resembles the administration of copolymers in the treatment of MS. In the present study, only a small enlargement of spleen size was observed, amounting to 1.2 times control spleen weight. Analysis by flow cytometry indicated increases in the number of splenic CD11b+ CD11c+ myeloid cells and CD11b+ CD11c− myeloid cells; the former were presumably dendritic cells and the latter were monocytes/macrophages. A substantial decrease in the number of CD3+ CD4+ and CD3+ CD8+ T cells was also observed while no change was seen in the number of CD19+ CD45R+ B cells or CD11c+ CD11b− dendritic cells (Fig. 1).
Fig. 1.
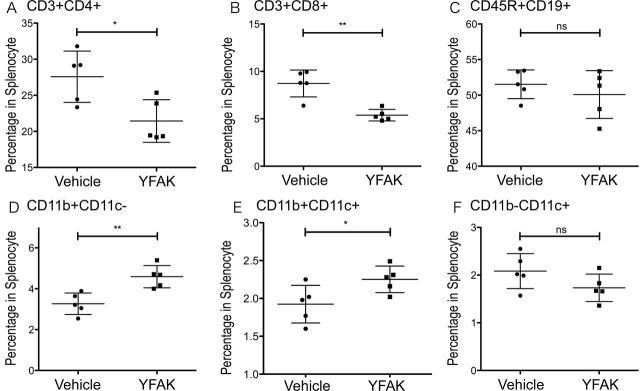
Effect of YFAK on cellular composition of the spleen of SJL mice. YFAK induces increased numbers of splenic CD11b+ CD11c− cells and CD11b+ CD11c+ cells, and also reduces CD4+ and CD8+ T cells. YFAK (150 µg day−1) or vehicle were injected daily for 10 days and spleen cells were obtained 1h after the final injections. Total spleen cells were analyzed using flow cytometry. Percentage of CD3+ CD4+ T cells (A), CD3+ CD8+ cells (B), CD19+ CD45R+ B cells (C), CD11b+ CD11c− monocytes/macrophages (D), CD11b+ CD11c+ dendritic cells (E) and CD11b− CD11c+ dendritic cells (F) in total spleen cells was analyzed.
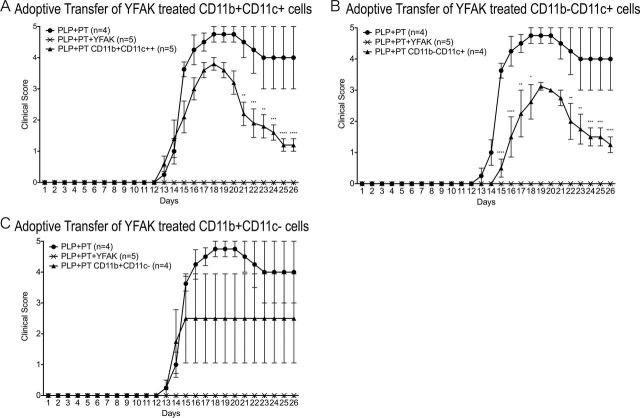
Adoptive transfer of 5×106 myeloid cells from Copaxone® (YEAK)-treated mice has been shown to modestly reduce the severity of EAE induced by MOG35-55 in C57Bl/6 mice (to which these mice are susceptible) (13). Adoptive transfer of myeloid cells separated from spleen after administration of YFAK to SJL mice was therefore also performed. Adoptive transfer into SJL mice of 2×106 of these CD11b+ CD11c+ or CD11b− CD11c+ cells, a similar number to that of unseparated CD11b+ monocytes used in the previous study (13), resulted in a modest amelioration of disease induced by PLP139–151 (Fig. 2), in parallel to that reported earlier. No significant amelioration was detected after adoptive transfer of CD11b+ CD11c− spleen cells at day 1 prior to administration of autoantigen. Notably, the extent of amelioration was much smaller than that observed on administration of YFAK to SJL mice (Fig. 2 or earlier) (16–18) or by adoptive transfer of 2×106 CD3+ splenocytes obtained from YFAK-treated mice that completely prevented appearance of disease (8).
Fig. 2.
Effect of adoptive transfer of splenic dendritic cells into SJL mice. YFAK-treated splenic dendritic cells have a modest suppressive effect on EAE. YFAK (150 µg day−1) was injected for 10 days daily and the spleens isolated 1h after the final injections. After enrichment with CD11b and CD11c magnetic beads, subtypes of myeloid cells were isolated by using a MoFlo cell sorter. Cells (1×106 per mouse) were transferred via tail vain injection followed by PLP139–151 (75 µg per mice) injection at day 1 and pertussis toxin injection on day 2. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.
CCL22 production by subsets of splenic myeloid cells after administration of YFAK
Recently, secretion of CCL22, a T cell chemoattractant, was shown to be an important marker of the effects of YFAK on splenic myeloid cells (15). Initially, CCL22 (also termed MDC) was found to be secreted in large amounts into plasma of mice treated with YFAK. Additionally, bone marrow cells were differentiated into macrophages by culture in IL-3 and tumour necrosis factor (TNF)α and were also found to secrete CCL22 in increased amounts when stimulated with YFAK, as did the RAW264.7 murine macrophage cell line. However, dendritic cells are also known to be a main source of CCL22 (27, 28). To determine which splenocytes from YFAK-treated mice secrete CCL22, first the various myeloid populations were isolated from naive SJL mouse spleen cells and separated into three prominent subsets of myeloid cells using FACS with allophycocyanin-CD11c and PE-CD11b, termed groups G1, G2 and G3 (Fig. 3A). Each of these populations is likely to be a mixture of many subsets of dendritic cells and/or monocytes/macrophages. However, the dominant phenotype of each of the populations indicated that G1 cells were predominantly monocytes, G2 cells myeloid dendritic cells and G3 cells plasmacytoid dendritic cells (Table 1). A small proportion of F4/80+ CD68+ splenic macrophages was found in the cells between G1 and G2 in Fig. 3(A), the number of which was too small for further examination in this study. The phenotype of BMDC obtained by treatment with GM-CSF was CD11c+ CD11b+ CD4− CD8− and would correspond most closely to population G2 in splenic myeloid cells.
Table 1. Phenotype of myeloid cells isolated from naive SJL mouse spleens.
| CD11b | CD11c | CD45R | MHC II | CD4 | CD8α | Ly-6C | PDCA-1 | F4/80 | CD68 | CD205 | 33D1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 | + | − | − | − | − | − | +/− | − | − | − | − | − |
| G2 | + | ++ | − | ++ | + | − | − | − | +/− | − | − (+)a | − (+)a |
| G3 | − | + | + | + | + | +/− | + | + | − | − | − | − |
aA majority of G2 cells was negative for CD205 or 33D1. A small portion was 33D1 positive and a smaller portion was CD205 positive.
Each population was analyzed for CCL22 secretion after stimulation with YFAK. Only the CD11b+ CD11c+ splenic myeloid cells secreted this cytokine. Little or no CCL22 secretion could be induced from the other myeloid populations or from B cells or T cells (Fig. 3B). Next, splenocytes were isolated from mice that had been pretreated with YFAK and, after separation, examined for CCL22 secretion with and without stimulation by YFAK (Fig. 3C). Again, the highest level of CCL22 secretion was observed using the CD11b+ CD11c+ myeloid population, both with and without additional in vitro stimulation by YFAK. These cells can be classified as classical dendritic cells rather than inflammatory dendritic cells (14) because they do not express Ly6C (29). Finally, bone marrow-derived myeloid cells were obtained after stimulation of bone marrow cells with either M-CSF to obtain BMMϕ or with GM-CSF to obtain BMDC. Although both populations secreted CCL22 spontaneously and could be induced to secrete larger amounts by YFAK, the amounts secreted by BMDC were in the range of 30–100 larger than that secreted by BMMϕ (Fig. 3D).
CCL22 production in myeloid cells from MHC II−/− mice
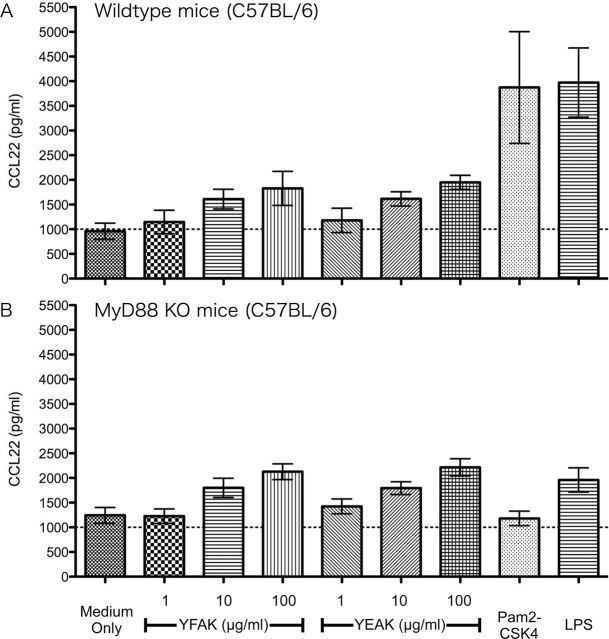
Previously, CCL22 was shown to be produced in vivo and secreted into the plasma of MHC II−/− mice that have been treated with YFAK and, to a much smaller extent, when treated with Copaxone (YEAK) (15). This observation has been confirmed and extended by using BMDC from two different MHC II−/− mouse strains. As in the previous study, initially BMDC were generated from C57Bl/6 mice that naturally lack expression of the I-Eα gene and in which the I-Aβ gene has been replaced by a mutant I-Aβ gene from SJL mice [mutant mice: B6.SJL(129)-Ptprca/BoyAiTac H2-Ab1tm1Gru N7+N6] and from wild-type congenic B6.SJL mice (Taconic Farms, Hudson, NY, USA). CCL22 production induced by YFAK or YEAK was the same from the BMDC of these MHC II−/− mice as from the BDMC in their wild-type counterparts (Fig. 4A). Next, MHC II knockout (KO) mice [B6.129S2-H2dlAb1-Ea/J (Jackson Laboratories)] in which all the MHC II genes had been deleted were utilized. The same result was obtained (Fig. 4B). The production of CCL22 by amino acid copolymers from BMDC does not depend on the presence of MHC II proteins.
Fig. 4.
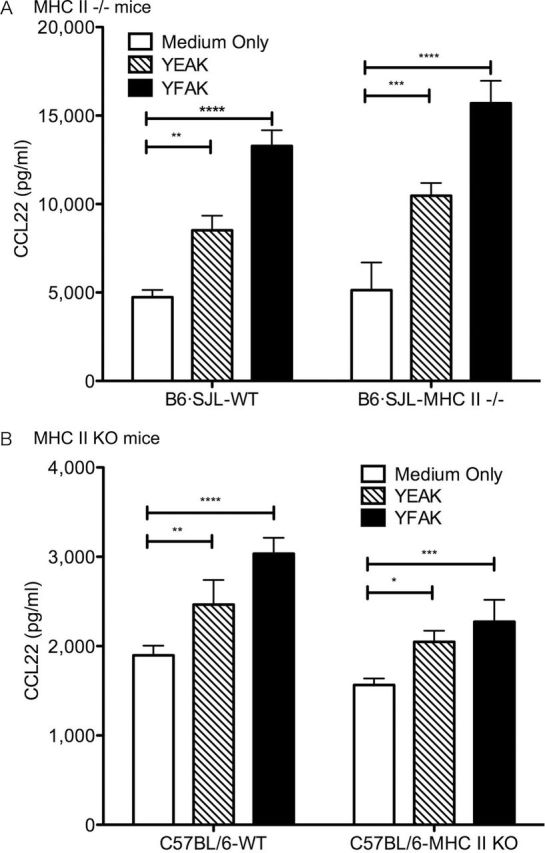
MHC class II is not required to induce CCL22. (A) BMDC were generated from B6.SJL MHC II−/− mice and congenic B6.SJL control mice, and stimulated with 100 µg ml−1 of YFAK or YEAK for 3 days. CCL22 production in supernatant was analyzed by ELISA. Each condition has four replicates, and similar result was obtained in two different experiments. (B) BMDC were generated from MHC class II KO mice and control C57Bl/6 mice, and stimulated with 50 µg ml−1 of YFAK or YEAK for 3 days. CCL22 production in supernatant was analyzed by ELISA. Each condition has four replicates, and similar result was obtained in two different experiments.
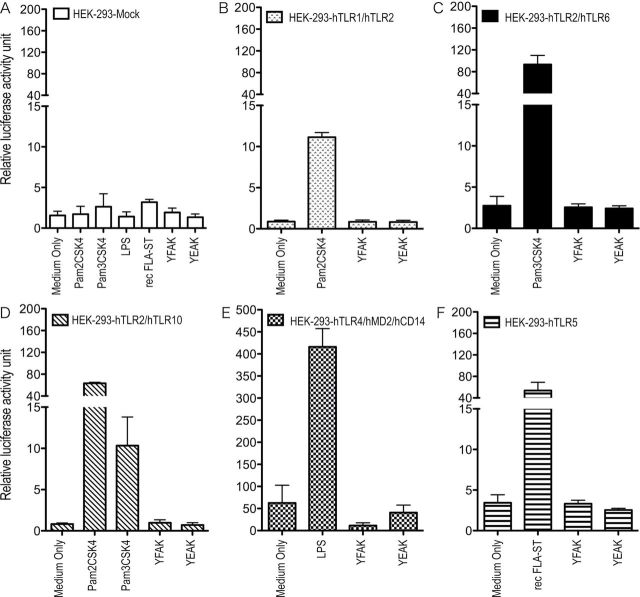
Efforts to identify the innate immune receptor for the amino acid copolymers that leads to CCL22 secretion are in progress. At the present stage, the possibility that this receptor is a MyD88-dependent TLR has been eliminated by the use of MyD88 KO mice that secreted CCL22 in the same amount as wild-type controls on stimulation by YFAK (Fig. 5) and by the use of HEK293 transfectants of TLR, none of which was activated by YFAK (Fig. 6). Moreover, TLR2−4− immortalized BMMϕ (generously provided by Dr Jonathon Kagan, Childrens Hospital, Boston, MA, USA) produced CCL22 at somewhat elevated levels, eliminating the possibility that the endosomal form of TLR4 might be the receptor. Additionally, most CLRs have been eliminated by siRNA knockdown of CARD9 and Sykb and by inhibition of Raf1 by BW5074, Sykb by piceatannol and SHP1/2 by NSC87877 (all components of the CLR signaling pathway) (30), as well as by overexpression of DC-SIGNR1 in Raw 264.7 cells (data not shown) generously provided by Dr Kayo Inaba (Kyoto University, Kyoto, Japan). The innate receptor for YFAK is likely to be an unusual molecule.
Fig. 5.
CCL22 production from BMDC utilizing normal B6 and B6-MyD88 KO mice. BMDC generated from C57Bl/6 mice (A) or MyD88 KO mice (B) were stimulated with different amount of copolymers, Pam2CSK4 (100ng ml−1) or LPS (1ng ml−1) for 72h. CCL22 concentration in the supernatant was measured by ELISA.
Fig. 6.
Luciferase assay of HEK293T cell transfectants. Mock- or hTLR1/2-, hTLR2/6-, hTLR2/10-, hTLR4/MD2/CD14- or hTLR5-transfected HEK293T cells were cotransfected with an NF-κB luciferase reporter vector were stimulated with YFAK (10 µg ml−1), YEAK (10 µg ml−1) or positive controls (Pam2CSK4 100ng ml−1, Pam3CSK4 100ng ml−1, LPS 100ng ml−1 or rec FLA-ST 100ng ml−1).
In vitro production of regulatory T cells by coincubation of BMDC and naive T cells in the presence of YFAK
An additional question is whether YFAK and BMDC could activate the formation in vitro of regulatory T cells from CD4+ CD25− naive T cells. First, naive CD4+ CD25− T cells were separated from the spleens of SJL mice and incubated with BMDC. After coculturing for 7 days, cell surface CD4 and CD25 and intracellular Foxp3 were analyzed by FACS (Fig. 7A and 7B). The experiments included incubation of CD4+ CD25− T cells with BMDC or with BMDC pretreated with 50 µg ml−1 YFAK, each with or without addition of YFAK to the incubation medium, for 24h at 37°C. Under these conditions, the number of CD4+ CD25+ cells increased from essentially 0% in the absence of any addition, to ~15% with BMDC alone and to ~30% with BMDC together with 50 µg ml−1 of YFAK (Fig. 7A). Notably, the enhanced conversion was largely due to the presence of an increased number of CD4+ CD25HI cells. A similar result was obtained using BMDC preincubated with 50 µg ml−1 YFAK and was further increased by addition of YFAK (5%). A smaller fraction of these brightest CD25HI cells (3–4%) also became Foxp3 positive when incubated with BMDC alone with a small but significant further increase in the presence of YFAK, while no Foxp3 was expressed in the CD25− or CD25LO cells.
Fig. 7.
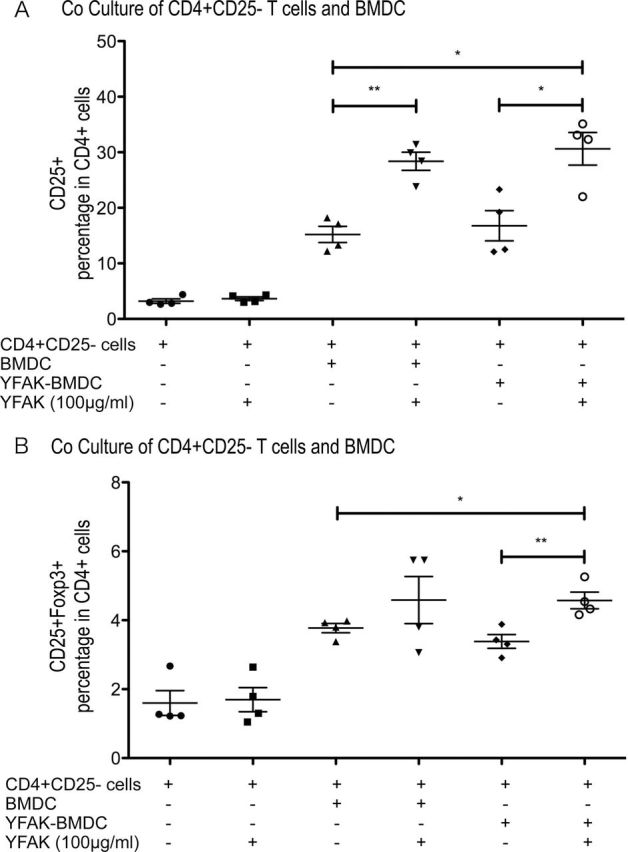
Coculture of CD4+ CD25− T cells with YFAK-treated BMDC. About 1×106 CD4+ CD25− cells isolated from spleens of naive SJL/J mice were cocultured with 1×106 BMDC supplemented with or without YFAK. After coculturing 7 days, cell surface CD4, CD25 and intracellular Foxp3 were analyzed by FACS.
Further, whether or not natural dendritic cells might induce regulatory T cells was investigated. Splenic CD11c+ cells were isolated from naive SJL/J mice and cocultured with or without splenic CD4+ CD25− cells for 7 days, in each case with or without YFAK or YEAK (Fig. 8A and 8B). The results were similar to those obtained with BMDC, although the percentage of CD4+ CD25− T cells converted in the presence of YFAK to CD4+ CD25+ cells (3%) or to CD4+ CD25HI Foxp3+ cells (1%) was much smaller with splenic dendritic cells than with BMDC. Furthermore, YEAK used in this experiment increased the number of CD4+ CD25+ cells but had no effect on the number of Foxp3+ cells, a result similar to that obtained with LPS as the activator.
Fig. 8.
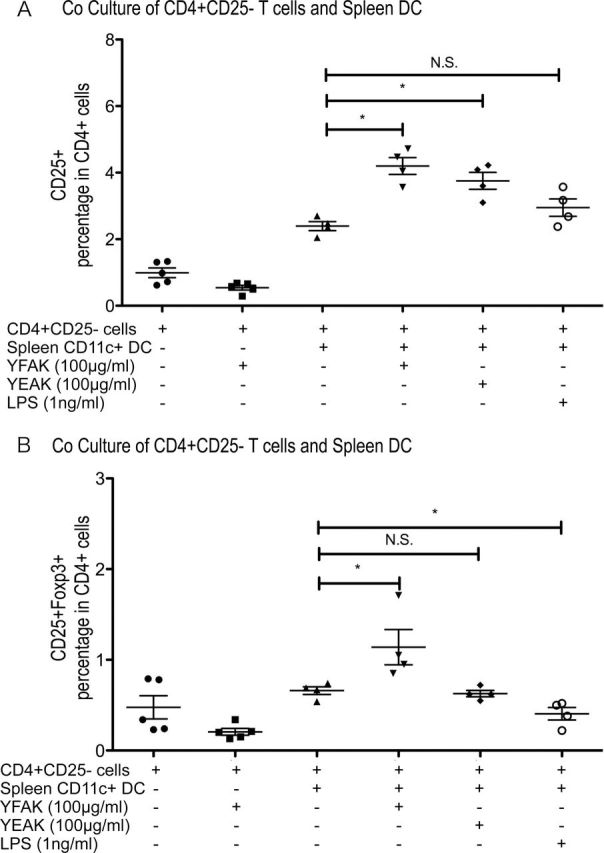
Coculture of CD25− CD4+ T cells with naive splenic dendritic cells. About 1×106 CD4+ CD25 cells isolated from spleens of naive SJL/J mice were cocultured in 2ml of complete DMEM with CD11c+ dendritic cells also isolated from spleen with or without YFAK, YEAK or LPS in the culture medium. After 7 days, cell surface CD4, CD25 and intracellular Foxp3 were analyzed by FACS.
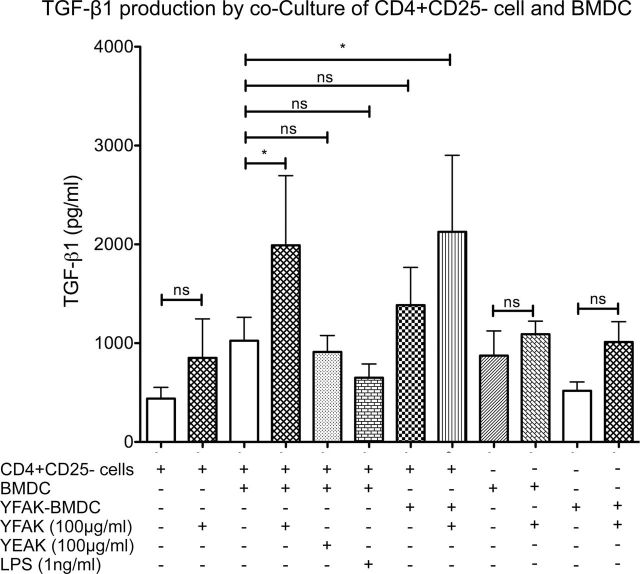
Cytokine production after induction of naive T cells by YFAK
Among the cytokines examined only an increase in the secretion of TGF-β induced by YFAK was observed. The maximum amount secreted into the medium was seen using BMDC with YFAK in the medium and reached ~2000ng ml−1 (Fig. 9). No effect of YEAK or TGF-β secretion was seen. In addition, neither secretion of IL-6, IL-10, IL-13, IL-17 or IFN-γ could be induced by YFAK in coculture incubation nor IL-1β, IL-6 or IL-27 could be detected on stimulation of BMDC by YFAK.
Fig. 9.
TGF-β1 secretion. Coculture of CD4+ CD25− cells and BMDC was performed as in Fig. 7. TGF-β1 concentration in the supernatant was measured by ELISA.
Discussion
These experiments indicate that myeloid cells play an important role in the mechanism by which YFAK protects mice from EAE, in agreement with many studies that have suggested a role for these cells in the mechanism of action of YEAK. This role is in addition to the role played by regulatory T cells. An important question is the relationship of these two types of cells. The present experiments suggest that myeloid cells are stimulated through innate immune receptors by these amino acid copolymers and that they have an important role in the generation of regulatory T cells.
A variety of cell types with immunoregulatory functions have been described. These include T cells of various types (31, 32) and myeloid cells. The regulatory myeloid populations include tolerogenic dendritic cells (33), regulatory or Type II macrophages (M2) of several types (34), monocyte-derived suppressor cells (35) and mast cells (36). In the present study, a small population of myeloid cells was found to expand in the spleen of mice treated with the random amino acid copolymer YFAK, the administration of which ameliorates EAE (Fig. 1). These cells were defined as CD11b+ CD11c+ dendritic cells. Adoptive transfer of these cells to mice undergoing induction of EAE by immunization with the autoantigen PLP139–151 ameliorated disease to a modest extent (Fig. 2). Notably, however, the extent of amelioration was much smaller than the complete prevention of disease provided by adoptive transfer of the same number of IL-10-secreting T cells (8).
In addition to their role in the amelioration of disease, a biochemical assay for the effects of YFAK and YEAK on cells has been described (15). Stimulation with YFAK of macrophages derived from murine bone marrow incubated with IL-3 and TNFα led to secretion of CCL22 (MDC), as did stimulation of the murine macrophage cell line RAW264.7. Moreover, treatment of mice with YFAK led to a rapid increase of the plasma level of CCL22 and CXCL13. To identify the myeloid cells that secrete CCL22 without treatment or after treatment of mice with YFAK, spleens were removed and splenocytes sorted by expression of CD11b and/or CD11c. In both cases, CD11b+ CD11c+ dendritic cells were the principal cells that could be induced by YFAK to secrete CCL22. Similarly, BMDC secreted much larger amounts of CCL22 than BMMϕ (Fig. 3). Although CCL22 was originally found secreted by macrophages and named ‘MDC’, it has also been known to be secreted by dendritic cells as a major source (28).
The secretion of CCL22 was induced through interaction with an innate immune receptor since MHC II−/− mice treated with YFAK or YEAK showed an increased plasma level of CCL22 to the same extent as their wild-type counterparts. In the present study, using BMDC generated from MHC II−/− mice and stimulated with YFAK, the same result was obtained. Moreover, two strains of MHC II−/− mice were used to further extend this result. (i) A strain in which the I-Aβ chain gene is mutated so that its product cannot pair with the I-Aα chain gene product. This mutant in C57Bl/6 mice is in a background in which the I-Eα chain gene is not expressed while the I-Eβ chain gene remains intact. The use of this strain raises the possibility that an I-Aα/I-Eβ chain cross-isotype heterodimer (37) could form under some circumstances. (ii) An MHC class II KO mouse strain in which the MHC class II region from I-Eα to I-Aβ1 has been deleted. Importantly, the possibility in this strain of formation of a mixed isotype MHC class II heterodimer is eliminated. BMDC produced from both of these strains showed increased production of CCL22 upon stimulation with YFAK or YEAK, with the effect of the former greater than that of the latter (Fig. 4). Thus an innate immune receptor must be involved in the secretion of CCL22. However, both the use of MyD88 KO mice and HEK293 transfectants with TLR1/2, TLR2/6, TLR2/10, TLR4 and TLR5 indicated that the innate immune receptor is not a TLR (Figs 5 and 6). These TLR represent all of the TLRs expressed at the surface.
The role of CCL22 in the amelioration of EAE induced by these random amino acid copolymers remains enigmatic. CCL22 is a chemoattractant for T cells expressing the receptor CCR4 (38). CCR4 has been reported to be expressed on activated Th2 cells, thymocytes and regulatory T cells (39–42). The hypothesis has therefore been proposed that its secretion by dendritic cells serves to bring Th2 cells in contact with the dendritic cells where they can be converted to regulatory T cells (Fig. 6 in ref. 15). However, the role of CCL22 in the generation of regulatory T cells has not been studied in detail. In the spleen, CCL22 might be required as a chemoattractant, although this might not necessarily be true when the cells are mixed together at high density in culture. In addition to this possible role, the CCL22/CCR4 system has an important function in attracting inflammatory macrophages in the induction of EAE (43, 44).
Finally, efforts were made to develop an in vitro system in which regulatory T cells could be generated (45, 46) and augmented by YFAK and/or YEA Naive CD4+ CD25– T cells isolated from splenocytes were incubated with BMDC that were prepared from naive mice, with or without YFAK in the incubation medium. The percentage of CD4+ DC25+ T cells formed under these circumstances was essentially doubled by the presence of YFAK in the incubation medium. Moreover, CD4+ CD25HI Foxp3+ cells were also formed in smaller amounts and their number was also essentially doubled by the presence of YFAK in the incubation medium. Similar effects were observed whether BMDC or natural dendritic cells obtained from spleen were used (Figs 7 and 8). The amounts of Foxp3+ cells that could be generated in these experiments were not sufficient to carry out direct in vitro or in vivo experiments demonstrating their immunosuppressive characteristics. Importantly, in both humans and in mice, nonregulatory CD4+ CD25+ Foxp3+ T cells have been described that are unstable and account for 1–2% of the total Foxp3+ population(47, 48). However, the T cells generated in this study can be distinguished from these nonregulatory Foxp3+ T cells because the latter are either CD25− or CD25LO. In the present study, the Foxp3+ cells generated in vitro occurred only in the brightest CD25HI population.
Analysis of cytokine secretion indicated that only TGF-β secretion could be detected (Fig. 9). Importantly, no IL-27 secretion from the dendritic cells was stimulated by YFAK. This cytokine is a critical inducer of IL-10 secretion on incubation with CD4+ CD25− T cells in the absence of myeloid cells (49, 50). Despite repeated attempts, no IL-10 or IL-13 secretion was found in the in vitro cultures in Figures 7 and 8, neither with BMDC nor with classical splenic dendritic cells, not unexpected in the absence of IL-27 secretion. The IL-27 that would be needed for induction of IL-10 secretion by T cells may be supplied by a different cell type than that used in Figs 7 and 8. Alternatively, it may be induced from the CD11b+ CD11c+ dendritic cells by a stimulus not supplied by the in vitro system, possibly a ligand of the aryl hydrocarbon receptor and/or an additional cytokine, e.g. TGF-β or IFN-γ (49–51).
Nevertheless, IL-10-secreting T cells have been generated from mouse spleen after treatment with YFAK or YEAK (8). The technique used was to induce proliferation of splenocytes in vitro with the copolymer from mice treated with YFAK and to repeat stimulation every 2 weeks for three or four rounds. The resultant T cell line secreted large amounts of IL-10 and IL-13 and was CD4+ CD25+ but Foxp3−, clearly related to Tr1 cells (52). Both the YFAK- and YEAK-specific oligoclonal lines were obtained. For example, ~60% of the cells in the YFAK line were Vβ14 or Vβ4 (9). Importantly, however, CD4+ CD25+ Foxp3+ cells do not proliferate in response to stimulation (31) and thus would not have been expected to expand under the conditions in which the CD4+ CD25+ Foxp3− IL-10-secreting regulatory T cells were obtained. Moreover, several studies have identified CD4+ CD25+ Foxp3+ cells in patients treated with YEAK (53, 54). Thus, the question of whether splenocytes from YFAK- and/or YEAK-treated mice have increased numbers of these two types of regulatory T cells and in what proportion requires further examination. Notably, under the conditions of in vitro generation of regulatory T cells studied here, only formation of CD4+ CD25+ Foxp3+ regulatory T cells that do not secrete IL-10 was found and could be increased by the presence of YFAK. A similar result has been reported for the in vitro induction of regulatory T cells induced by polysaccharide A obtained from the human commensal Bacteroides fragilis, both in vitro and in vivo, although in that system the CD4+ CD25+ Foxp3+ cells also secreted IL-10 (55–57). Whether a different myeloid cell or different culture conditions are required for the generation of Tr1-like copolymer-specific CD4+ CD25+ Foxp3− IL-10-secreting T cells remains a subject for further investigation.
Funding
National Institutes of Health (AI049524) and Health and Labor Science Research Grants for Research on Allergic Disease and Immunology from the Japanese Ministry of Health, Labor and Welfare.
References
- 1. Teitelbaum D., Meshorer A., Hirshfeld T., Arnon R., Sela M. 1971. Suppression of experimental allergic encephalomyelitis by a synthetic polypeptide. Eur. J. Immunol. 1: 242 [DOI] [PubMed] [Google Scholar]
- 2. Teitelbaum D., Fridkis-Hareli M., Arnon R., Sela M. 1996. Copolymer 1 inhibits chronic relapsing experimental allergic encephalomyelitis induced by proteolipid protein (PLP) peptides in mice and interferes with PLP-specific T cell responses. J. Neuroimmunol. 64: 209 [DOI] [PubMed] [Google Scholar]
- 3. Johnson K. P., Brooks B. R., Cohen J. A., et al. 1995. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. Neurology 45: 1268 [DOI] [PubMed] [Google Scholar]
- 4. Aharoni R., Teitelbaum D., Sela M., Arnon R. 1998. Bystander suppression of experimental autoimmune encephalomyelitis by T cell lines and clones of the Th2 type induced by copolymer 1. J. Neuroimmunol. 91: 135 [DOI] [PubMed] [Google Scholar]
- 5. Aharoni R., Eilam R., Domev H., Labunskay G., Sela M., Arnon R. 2005. The immunomodulator glatiramer acetate augments the expression of neurotrophic factors in brains of experimental autoimmune encephalomyelitis mice. Proc. Natl Acad. Sci. USA 102: 19045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Duda P. W., Schmied M. C., Cook S. L., Krieger J. I., Hafler D. A. 2000. Glatiramer acetate (Copaxone) induces degenerate, Th2-polarized immune responses in patients with multiple sclerosis. J. Clin. Invest. 105: 967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vieira P. L., Heystek H. C., Wormmeester J., Wierenga E. A., Kapsenberg M. L. 2003. Glatiramer acetate (copolymer-1, copaxone) promotes Th2 cell development and increased IL-10 production through modulation of dendritic cells. J. Immunol. 170: 4483 [DOI] [PubMed] [Google Scholar]
- 8. Stern J. N., Keskin D. B., Zhang H., Lv H., Kato Z., Strominger J. L. 2008. Amino acid copolymer-specific IL-10-secreting regulatory T cells that ameliorate autoimmune diseases in mice. Proc. Natl Acad. Sci. USA 105: 5172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang H., Stern J. N., Strominger J. L. 2009. T cell receptors in an IL-10-secreting amino acid copolymer-specific regulatory T cell line that mediates bystander immunosuppression. Proc. Natl Acad. Sci. USA 106: 3336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lalive P. H., Neuhaus O., Benkhoucha M., et al. 2011. Glatiramer acetate in the treatment of multiple sclerosis: emerging concepts regarding its mechanism of action. CNS Drugs 25: 401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hussien Y., Sanna A., Söderström M., Link H., Huang Y. M. 2001. Glatiramer acetate and IFN-beta act on dendritic cells in multiple sclerosis. J. Neuroimmunol. 121: 102 [DOI] [PubMed] [Google Scholar]
- 12. Kim H. J., Ifergan I., Antel J. P., et al. 2004. Type 2 monocyte and microglia differentiation mediated by glatiramer acetate therapy in patients with multiple sclerosis. J. Immunol. 172: 7144 [DOI] [PubMed] [Google Scholar]
- 13. Weber M. S., Prod’homme T., Youssef S., et al. 2007. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nat. Med. 13: 935 [DOI] [PubMed] [Google Scholar]
- 14. Toker A., Slaney C. Y., Bäckström B. T., Harper J. L. 2011. Glatiramer acetate treatment directly targets CD11b(+)Ly6G(-) monocytes and enhances the suppression of autoreactive T cells in experimental autoimmune encephalomyelitis. Scand. J. Immunol. 74: 235 [DOI] [PubMed] [Google Scholar]
- 15. Kovalchin J., Krieger J., Genova M., et al. 2011. Macrophage-specific chemokines induced via innate immunity by amino acid copolymers and their role in EAE. PLoS ONE 6: e26274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fridkis-Hareli M., Santambrogio L., Stern J. N., Fugger L., Brosnan C., Strominger J. L. 2002. Novel synthetic amino acid copolymers that inhibit autoantigen-specific T cell responses and suppress experimental autoimmune encephalomyelitis. J. Clin. Invest. 109: 1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stern J. N., Illes Z., Reddy J., et al. 2004. Amelioration of proteolipid protein 139-151-induced encephalomyelitis in SJL mice by modified amino acid copolymers and their mechanisms. Proc. Natl Acad. Sci. USA 101: 11743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Illes Z., Stern J. N., Reddy J., et al. 2004. Modified amino acid copolymers suppress myelin basic protein 85-99-induced encephalomyelitis in humanized mice through different effects on T cells. Proc. Natl Acad. Sci. USA 101: 11749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Illes Z., Stern J. N., Keskin D. B., et al. 2005. Copolymer effects on microglia and T cells in the central nervous system of humanized mice. Eur. J. Immunol. 35: 3683 [DOI] [PubMed] [Google Scholar]
- 20. Kovalchin J., Krieger J., Genova M., et al. 2010. Results of a phase I study in patients suffering from secondary-progressive multiple sclerosis demonstrating the safety of the amino acid copolymer PI-2301 and a possible induction of an anti-inflammatory cytokine response. J. Neuroimmunol. 225: 153 [DOI] [PubMed] [Google Scholar]
- 21. Kovalchin J., Krieger J., Collins K., et al. 2011. Safety, pharmacokinetic, and pharmacodynamic evaluations of PI-2301, a potent immunomodulator, in a first-in-human, single-ascending-dose study in healthy volunteers. J. Clin. Pharmacol. 51: 649 [DOI] [PubMed] [Google Scholar]
- 22. Fridkis-Hareli M., Teitelbaum D., Gurevich E., et al. 1994. Direct binding of myelin basic protein and synthetic copolymer 1 to class II major histocompatibility complex molecules on living antigen-presenting cells—specificity and promiscuity. Proc. Natl Acad. Sci. USA 91: 4872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fridkis-Hareli M., Strominger J. L. 1998. Promiscuous binding of synthetic copolymer 1 to purified HLA-DR molecules. J. Immunol. 160: 4386 [PubMed] [Google Scholar]
- 24. Zhang X., Goncalves R., Mosser D. M. 2008. The isolation and characterization of murine macrophages. Curr. Protoc. Immunol. Chapter 14: Unit 14.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lutz M. B., Kukutsch N., Ogilvie A. L., et al. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Method 223: 77 [DOI] [PubMed] [Google Scholar]
- 26. Ohnishi H., Tochio H., Kato Z., et al. 2009. Structural basis for the multiple interactions of the MyD88 TIR domain in TLR4 signaling. Proc. Natl Acad. Sci. USA 106: 10260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Godiska R., Chantry D., Raport C. J., et al. 1997. Human macrophage-derived chemokine (MDC), a novel chemoattractant for monocytes, monocyte-derived dendritic cells, and natural killer cells. J. Exp. Med. 185: 1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vulcano M., Albanesi C., Stoppacciaro A., et al. 2001. Dendritic cells as a major source of macrophage-derived chemokine/CCL22 in vitro and in vivo . Eur. J. Immunol. 31: 812 [DOI] [PubMed] [Google Scholar]
- 29. Drutman S. B., Kendall J. C., Trombetta E. S. 2012. Inflammatory spleen monocytes can upregulate CD11c expression without converting into dendritic cells. J. Immunol. 188: 3603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Geijtenbeek T. B., Gringhuis S. I. 2009. Signalling through C-type lectin receptors: shaping immune responses. Nat. Rev. Immunol. 9: 465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sakaguchi S. 2011. Regulatory T cells: history and perspective. Methods Mol. Biol. 707: 3 [DOI] [PubMed] [Google Scholar]
- 32. Roncarolo M. G., Gregori S., Lucarelli B., Ciceri F., Bacchetta R. 2011. Clinical tolerance in allogeneic hematopoietic stem cell transplantation. Immunol. Rev. 241: 145 [DOI] [PubMed] [Google Scholar]
- 33. Steinman R. M., Hawiger D., Nussenzweig M. C. 2003. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21: 685 [DOI] [PubMed] [Google Scholar]
- 34. Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. 2004. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25: 677 [DOI] [PubMed] [Google Scholar]
- 35. Youn J. I., Gabrilovich D. I. 2010. The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur. J. Immunol. 40: 2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de Vries V. C., Noelle R. J. 2010. Mast cell mediators in tolerance. Curr. Opin. Immunol. 22: 643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Norcross M. A., Raghupathy R., Strominger J., Germain R. N. 1986. Transfected human B lymphoblastoid cells express the mouse Ad beta-chain in association with DR alpha. J. Immunol. 137: 1714 [PubMed] [Google Scholar]
- 38. Imai T., Chantry D., Raport C. J., et al. 1998. Macrophage-derived chemokine is a functional ligand for the CC chemokine receptor 4. J. Biol. Chem. 273: 1764 [DOI] [PubMed] [Google Scholar]
- 39. Chantry D., Romagnani P., Raport C. J., et al. 1999. Macrophage-derived chemokine is localized to thymic medullary epithelial cells and is a chemoattractant for CD3(+), CD4(+), CD8(low) thymocytes. Blood 94: 1890 [PubMed] [Google Scholar]
- 40. Columba-Cabezas S., Serafini B., Ambrosini E., et al. 2002. Induction of macrophage-derived chemokine/CCL22 expression in experimental autoimmune encephalomyelitis and cultured microglia: implications for disease regulation. J. Neuroimmunol. 130: 10 [DOI] [PubMed] [Google Scholar]
- 41. Tang H. L., Cyster J. G. 1999. Chemokine up-regulation and activated T cell attraction by maturing dendritic cells. Science 284: 819 [DOI] [PubMed] [Google Scholar]
- 42. Iellem A., Mariani M., Lang R., et al. 2001. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J. Exp. Med. 194: 847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Forde E. A., Dogan R. N., Karpus W. J. 2011. CCR4 contributes to the pathogenesis of experimental autoimmune encephalomyelitis by regulating inflammatory macrophage function. J. Neuroimmunol. 236: 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dogan R. N., Long N., Forde E., et al. 2011. CCL22 regulates experimental autoimmune encephalomyelitis by controlling inflammatory macrophage accumulation and effector function. J. Leukoc. Biol. 89: 93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yamazaki S., Dudziak D., Heidkamp G. F., et al. 2008. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J. Immunol. 181: 6923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bhattacharya P., Gopisetty A., Ganesh B. B., Sheng J. R., Prabhakar B. S. 2011. GM-CSF-induced, bone-marrow-derived dendritic cells can expand natural Tregs and induce adaptive Tregs by different mechanisms. J. Leukoc. Biol. 89: 235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Miyao T., Floess S., Setoguchi R., et al. 2012. Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity 36: 262 [DOI] [PubMed] [Google Scholar]
- 48. Tran D. Q., Ramsey H., Shevach E. M. 2007. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood 110: 2983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pot C., Jin H., Awasthi A., et al. 2009. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J. Immunol. 183: 797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pot C., Apetoh L., Awasthi A., Kuchroo V. K. 2010. Molecular pathways in the induction of interleukin-27-driven regulatory type 1 cells. J. Interferon Cytokine Res. 30: 381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wu H. Y., Quintana F. J., da Cunha A. P., et al. 2011. In vivo induction of Tr1 cells via mucosal dendritic cells and AHR signaling. PLoS ONE 6: e23618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Roncarolo M. G., Gregori S., Battaglia M., Bacchetta R., Fleischhauer K., Levings M. K. 2006. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol. Rev. 212: 28 [DOI] [PubMed] [Google Scholar]
- 53. Hong J., Li N., Zhang X., Zheng B., Zhang J. Z. 2005. Induction of CD4+CD25+ regulatory T cells by copolymer-I through activation of transcription factor Foxp3. Proc. Natl Acad. Sci. USA 102: 6449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Haas J., Korporal M., Balint B., Fritzsching B., Schwarz A., Wildemann B. 2009. Glatiramer acetate improves regulatory T-cell function by expansion of naive CD4(+)CD25(+)FOXP3(+)CD31(+) T-cells in patients with multiple sclerosis. J. Neuroimmunol. 216: 113 [DOI] [PubMed] [Google Scholar]
- 55. Ochoa-Repáraz J., Mielcarz D. W., Wang Y., et al. 2010. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 3: 487 [DOI] [PubMed] [Google Scholar]
- 56. Ochoa-Repáraz J., Mielcarz D. W., Ditrio L. E., et al. 2010. Central nervous system demyelinating disease protection by the human commensal Bacteroides fragilis depends on polysaccharide A expression. J. Immunol. 185: 4101 [DOI] [PubMed] [Google Scholar]
- 57. Round J. L., Mazmanian S. K. 2010. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl Acad. Sci. USA 107: 12204 [DOI] [PMC free article] [PubMed] [Google Scholar]