Bovine CD1d is expressed on the cell surface but cannot present C26 α-galactosylceramide.
Keywords: animal models, CD1, immunogenetics, natural killer T cells (NKT cells)
Abstract
Although CD1d and NKT cells have been proposed to have highly conserved functions in mammals, data on functions of CD1d and NKT cells in species other than humans and rodents are lacking. Upon stimulation with the CD1d-presented synthetic antigen α-galactosylceramide, human and rodent type I invariant NKT cells release large amounts of cytokines. The two bovine CD1D (boCD1D) genes have structural features that suggest that they cannot be translated into functional proteins expressed on the cell surface. Here we provide evidence that despite an intron–exon structure and signal peptide that are different from all other known CD1 genes, boCD1D can be translated into a protein that is expressed on the cell surface. However, in vivo treatment of cattle (Bos taurus) with 0.1, 1, or 10 µg kg–1 of the most commonly used α-galactosylceramide, which has a C26 fatty acid, did not lead to an increase in body temperature and serum cytokine levels of the animals. This lack of reactivity is not due to a complete inability of boCD1d to present glycosphingolipids because α-galactosylceramide variants with shorter fatty acids could be presented by boCD1d to human NKT cells in vitro. This suggests that the natural ligands of boCD1d are smaller lipids.
Introduction
Because of their quick, strong responses and the availability of the potent synthetic antigen α-galactosylceramide (α-GalCer), type I NKT cells, also called invariant NKT cells, are currently targeted in anticancer treatment, vaccine development and immunotherapy for autoimmune diseases. CD1d can bind α-GalCer in the hydrophobic binding groove of CD1d, and recognition of this complex by the T cell receptor (TCR) of invariant NKT cells triggers the release of large amounts of cytokines, including IL-2, IL-4, IL-10, IL-8, IL-13, IL-21, GM-CSF, IFN-γ, TNF-α, MIP-1α and MIP-1 β (1–3). Because CD1d and the invariant TCR of NKT cells are highly conserved, mouse CD1d can present α-GalCer to human invariant NKT cells and vice versa (4–6).
With the availability of multiple mammalian genomes, it has become clear that CD1D genes are widespread. With the exception of marsupials, not a single mammalian genome has been reported to lack CD1D genes altogether (7). However, whether the presence of a CD1D gene in the genome always leads to the expression of functional CD1d protein on the cell surface and the development of a functional invariant NKT cell population in a species is unknown. Despite the absence of data directly addressing this question, it is thought that most mammals have a functional CD1d and invariant NKT cell system, with the notable exception of ruminants (8).
All MHC class I-like proteins, including CD1 proteins, consist of a heavy chain, which contains the three extracellular α domains, a transmembrane domain and a cytoplasmic tail. Upon translation and translocation into the endoplasmic reticulum, the signal peptide is cleaved off. The mature heavy chain forms a heterodimer with the β2 microglobulin molecule. MHC class I-like molecules also share a highly comparable intron–exon structure. The start codon and signal peptide lie on one exon and each of the three α domains, as well as the transmembrane domain and the cytoplasmic tail, are located on separate exons.
Even though CD1D genes are present in ruminant genomes and are transcribed, all of the studied ruminant CD1D genes have been shown to have mutations that eradicate the start codon and an intronic splice site, suggesting that functional protein might be absent (8, 9). CD1d proteins have not been detected in ruminants to date. Because the CD1d and invariant NKT cell system is such a prominent part of the immune system of humans and mice, two species belonging to different orders of mammalia, it is often assumed that the system has been broadly conserved during evolution and is also functional in the other CD1D gene-containing orders. Therefore, the previously described naturally occurring genetic distortion of the ruminant CD1D genes (8) and the ensuing suggestion that ruminants lack invariant NKT cells were unexpected and need further investigation.
Surprisingly, we found that the bovine CD1D (boCD1D) gene, which was already known to be transcribed, is also translated in vivo. This is possible because a sequence that is intronic in mice and humans functions as an exon in cattle and encodes an alternative start codon and peptide with signal peptide function. However, despite the expression of this CD1D gene product, we show that intravenously applied α-GalCer in cattle has no effect on serum cytokine levels and body temperature. In vitro activation of human NKT cells by shorter-chain α-GalCer presented by boCD1d suggests that the natural ligands of boCD1d are smaller lipids.
Methods
Animals
Three groups of three 4-month-old Holstein-Friesian calves, weighing ~120kg each, were treated by intravenous injections of 0.1, 1 and 10 µg kg–1 of α-GalCer in 5-ml sterile PBS in the jugular vein. α-GalCer was dried under a stream of N2 gas to remove organic solvents and sonicated at 50°C in PBS. Serum was collected once before and at 2, 4, 8, 16 and 30h after α-GalCer injection and stored at –20°C. The rectal temperature was measured at the same time points as serum collection and one day before treatment at the same hour as the post-α-GalCer time points. Experiments were approved by the Animal Ethical Committee of the University of Utrecht, the Netherlands.
Six-month-old Holstein or Holstein cross-calves were infected via the intra-tracheal route with 2000 colony-forming units of Mycobacterium bovis (strain AF2122/97). Serum samples were collected 9 weeks post-infection. Disease was confirmed by post-mortem performed 9 weeks post-infection by the presence of visible pathology typical of bovine tuberculosis and the culture of M. bovis obtained from tissues.
Dairy cross-calves, 8–10 weeks old, were experimentally infected with 105 TCID50 [50% (median) tissue culture infectious dose] bovine viral diarrhea virus (BVDV; strain UK1362727) via intra-nasal inoculation. Serum samples were collected 8 days post-infection, at which point animals were pyrexic, leukopenic and viraemic. Work was carried out in accordance with UK legislation pertaining to care and use of animals under experimentation.
Bovine IFN-γ, IL-1β, IL-4, IL-10, IL-12 and MIP-1β detection
Simultaneous detection of IFN-γ, IL-1β, IL-4, IL-10, IL-12 and MIP-1β was performed in sera using a custom bovine multiplex cytokine–chemokine assay developed in collaboration with Meso Scale Discovery (MSD) (10). Multiplex 96-well plates were supplied with each of the commercially available target capture antibodies: bovine IFN-γ (Mabtech, Stockholm, Sweden), IL-4 (Endogen, Rockford, IL, USA), IL-10 and IL-12 (AbD-Serotec), IL-1β and human cross-reactive MIP-1β (MSD) pre-spotted on to spatially separated locations in each well. Incubations were performed at room temperature. Plates were blocked with MSD assay buffer prior to addition of 25 µl per well of test sera or standards. The standards were serially diluted in MSD dilution buffer of high concentrations (shown below in parentheses): IFN-γ (Endogen, 100ng ml-1); IL-1β [bovine IL-1β calibrator (MSD), 20ng ml-1]; IL-4 [bovine IL-4 calibrator (MSD), 2ng ml-1]; IL-10 (IAH, 30U ml-1); IL-12 (IAH, 1000U ml-1) and MIP-1β [human MIP-1β calibrator (MSD), 10ng ml-1]. Following a 2-h incubation, plates were washed and then incubated for a further 2h with a combined cocktail of biotinylated secondary detection antibodies [IFN-γ, IL-1β, IL-4, IL-10, IL-12, MIP-1β (all from MSD)] and Streptavidin–Sulfotag (MSD) in MSD dilution buffer. Minimum detectable levels were as follows: IFN-γ, 0.046ng ml-1; IL-1β, 0.043ng ml-1; IL-4, 3.19 pg ml-1, IL-10, 0.018U ml-1; IL-12, 1.83U ml-1 and MIP-1β, 1.32 pg ml-1. After a final wash, plates were coated with MSD Buffer-T and the luminescence signal was measured on an MSD-6000 instrument. Serum IFN-γ levels were also measured using the sandwich ELISA provided with the Bovigam® assay (Prionics AG, Zurich, Switzerland). Using this method, the minimum detectable level of IFN-γ was 0.016ng ml-1.
Molecular cloning of boCD1D transcript, transfections and flow cytometry
BoCD1D cDNA (accession number BT029852) was obtained from BACPAC Resources (Oakland, CA, USA) and cloned into pcDNA3.1 vector. Further, 293T and K562 cells were transfected with boCD1D cDNA in pcDNA3.1 or a chimeric construct of boCD1d in which the signal sequence was replaced by the boCD1b3 signal sequence (boCD1d/b) in pcDNA3.1 using FuGENE 6 reagent (Roche). 293T cells were harvested 48h after transfection and the cell surface was stained with antibodies raised against bovine thymocytes (CC43), bovine intestinal epithelial cells (CC118) (11), and anti-human CD1d clone CD1d42 (12), followed by goat anti-mouse PE. K562 cells were selected on 1mg ml-1 G418 for several weeks. K562 cells transfected with human CD1d have been described previously (13). C1R cells were electroporated as described previously, selected on 1mg ml-1 G418 and sorted for comparable levels of boCD1d or boCD1d/b at the cell surface.
Cellular assays and antigens
The human invariant NKT cell lines J3N.5 and J24L.17 and the murine NKT hybridoma DN3A4 were described previously (14, 15). C1R cells transfected with human CD1d, boCD1d or boCD1d with the signal sequence of boCD1b3 (5×104 cells per well) were mixed with a series of dilutions of α-GalCer. Before use, α-GalCer was dried and sonicated in tissue culture medium consisting of RPMI supplemented with 10% FCS and penicillin–streptomycin. Upon addition of invariant DN3A4 cells (5×104 cells per well), the cultures were incubated for 24h at 37°C and 5% CO2 before harvesting the culture supernatants for detecting the presence of murine IL-2 by ELISA (BD Pharmingen). Alternatively, 5×104 C1R cells were incubated with 1000 J3N.5 or J24L.17 NKT cells per well in a human IFN-γ ELISPOT assay (Mabtech). C12 and C26 α-GalCer were synthesized via a modification to a previously reported glycolipid synthesis (16). C16 α-GalCer was synthesized as described (17).
Statistical analyses
Statistical analysis was performed with R statistical software (version 2.10.1, www.R-project.org). Linear mixed-effects models were used to analyze the effect of α-GalCer treatment on serum cytokine levels, which included treatment group and time, and their interaction as fixed factors and animal as random factor.
Results
Transcription and translation of boCD1D genes previously considered pseudogenes
We have previously described two boCD1D genes and provided evidence for transcription of one of them, boCD1D1 (8). However, at that time, we considered the transcript to be non-functional because the sequence homologous to the murine and human signal peptide-encoding sequence starts with a mutated start codon in cattle (Fig. 1A). In addition, the part that is homologous to the intron that is present between the signal peptide-encoding exon and the α1 domain exon in mice and humans does not contain functional splice sites in cattle. Consistent with this, no transcripts with this intron properly spliced out could be cloned and were absent in the databases, whereas numerous transcripts were identified in which the intron was present. Re-evaluation of this transcript revealed an ATG at the beginning of this intron (Fig. 1A). We hypothesized that if translation starts at this ATG, the sequence that is intronic in humans and mice might be translated into an alternative peptide, possibly with signal peptide capacities, followed by a functional boCD1d protein.
Fig. 1.
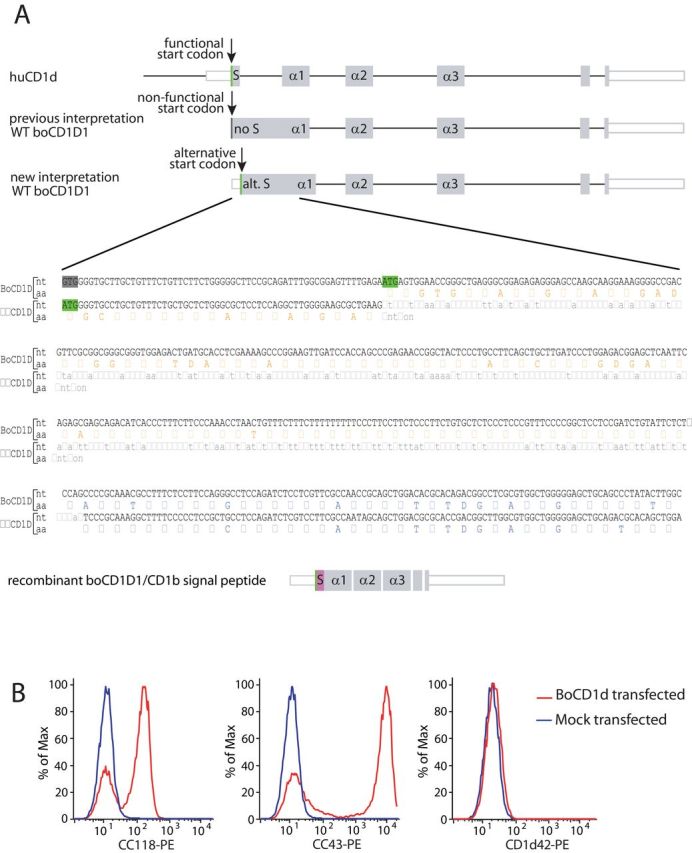
Structure of human and bovine CD1d (boCD1d) transcripts. (A) In the human CD1d transcript (huCD1d, accession number NM_001766), the intron (black horizontal lines)–exon (gray boxes) structure shows a pattern that is typical for all MHC class I-like molecules. The wild-type (WT) boCD1D1 transcript (accession numbers DQ192544 and BT029852) has previously been shown to contain a non-functional start codon (dark grey) at the same position where the human functional start codon lies (green). Our new interpretation of the boCD1D1 transcript shows a functional start codon in the part that is homologous to the human intron before the α1 domain. A recombinant construct has been created that contains the signal peptide of bovine CD1b3 (pink), followed by boCD1d from which the signal peptide has been removed. S: Signal peptide; alt. S: alternative signal peptide; intronic sequence: light grey; start codon: green; mutated start codon: dark grey; signal peptide amino acid (aa) sequence: orange; CD1 heavy-chain α1 domain aa sequence: blue. (B) 293T cells transfected with the boCD1D1 transcript or mock transfected were cell surface stained with the CC43, CC118 and CD1d42 antibodies.
To test this hypothesis, we transfected 293T cells with a full-length wild-type (WT) boCD1D1 transcript. Sequencing confirmed that the insert contains 45bp 5′ of the location of the murine and human start codons, corresponding to the mutated start codon in cattle, and this 5′ stretch did not contain any additional ATG sequences. Of the available anti-human CD1d and anti-boCD1 antibodies, only CC43 and CC118 recognized the surface of transfected cells but not the mock-transfected cells (Fig. 1B). CC43, which was raised against bovine thymocytes, and CC118, which was raised against bovine intestinal epithelial cells, have previously been suggested to recognize boCD1 based on their staining pattern in vivo (11), but they do not recognize boCD1a and boCD1b3 (8). The identification of the boCD1D gene product as the target molecule for these antibodies is consistent with all published data. The fact that this molecule translocates to the cell surface implies that a signal peptide-like function is performed by the sequence that is homologous to the human and murine introns. Indeed, the 99-amino acid (aa)-long peptide that is predicted before the α1 domain contains a hydrophobic segment consistent with the signal peptide function of random sequences (18). The SignalP program (available at www.cbs.dtu.dk) does not predict a signal peptidase cleavage site, suggesting that the peptide results in a membrane anchor.
Serum cytokine response upon intravenous application of α-GalCer in cattle
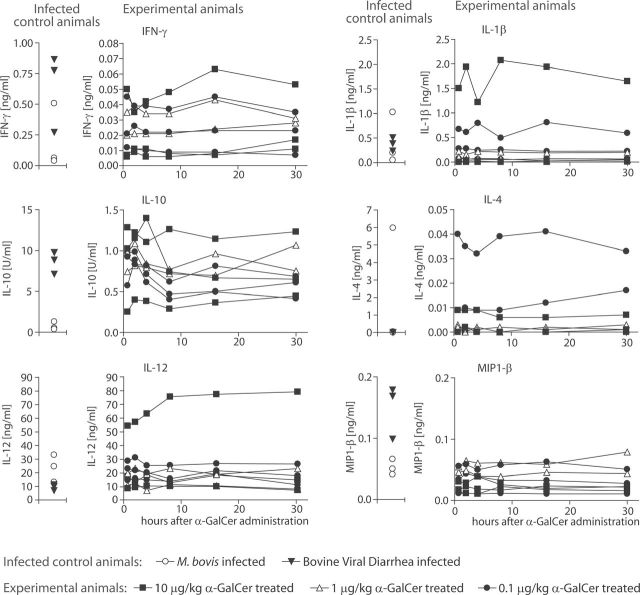
To investigate whether the boCD1D gene product performs the conserved function of presenting α-GalCer to T cells in vivo, we injected three groups of three calves (four months of age) intravenously with 0.1, 1 and 10 µg kg–1 of α-GalCer and determined serum IL-1β, IL-4, IL-6, IL-10, IL-12, IFN-γ and MIP-1β levels using the MSD technique. The α-GalCer that was injected was identical to KRN7000, containing a C18 phyotosphingosine and a C26 fatty acid, which is highly antigenic for human and murine NKT cells. From each animal, serum was collected just before (time point 0), and at 2, 4, 8, 16 and 30h after injection (Fig. 2). To show that the MSD technique that we used was adequate for the detection of cytokines in bovine sera and to provide a rough indication of physiological cytokine level ranges during the acute phase of infection, we included sera of animals with acute BVDV or M. bovis infections (left part of each panel), as well as a wide concentration range of recombinant standards. For IL-4, IL-10, IFN-γ and MIP-1β, the serum level of at least one infected control animal was an order of magnitude higher than that of the α-GalCer-treated animals (please note that the scale of the y-axes of the IL-4, IL-10 and IFN-γ graphs is different for the control animals and the α-GalCer-treated animals). These results, in addition to the standard curves obtained with recombinant cytokines, show that the detection method for serum cytokine levels was adequate.
Fig. 2.
Serum cytokine responses in cattle upon α-galactosylceramide (α-GalCer) treatment. Three groups of three calves were treated with 0.1 (n = 3), 1 (n = 2) and 10 µg kg–1 (n = 3) α-GalCer intravenously and serum cytokine levels were determined before (time point 0) and at the indicated time points after treatment. Similar symbols connected with a line represent data from one individual animal. As technical controls, animals suffering from M. bovis (n = 3) or bovine viral diarrhea virus (n = 3) were included.
There were no statistically significant increases in serum cytokine levels in any of the α-GalCer-treated groups. For each cytokine in each animal, the level before treatment (time point 0) never increased >1.5-fold upon treatment. Each animal, represented by similar symbols connected by a line, apparently has a certain level of each cytokine before injection of α-GalCer, and these levels remain fairly constant in time, but may be different from those of other animals. Comparable intravenous α-GalCer injections in mice typically show a 20-fold increase in cytokine levels, with the peak of the response, depending on the cytokine, ~4–16h after intravenous injection (19–21). Using the standard sandwich ELISA as provided with the Bovigam assay, the IFN-γ data were replicated with essentially identical results (data not shown).
Body temperature upon intravenous application of α-GalCer
In human clinical trials and animal models where α-GalCer is administered, an increase in body temperature has sometimes been observed (22–24). To test whether cattle react to administration of α-GalCer with an increase in body temperature, the rectal temperature was measured just before and at 2, 4, 8, 16 and 30h after α-GalCer treatment (Fig. 3). Because body temperature follows a circadian rhythm, body temperature of the animals was also measured at the same hours a day before α-GalCer treatment (“8 calves untreated” in Fig. 3).
Fig. 3.
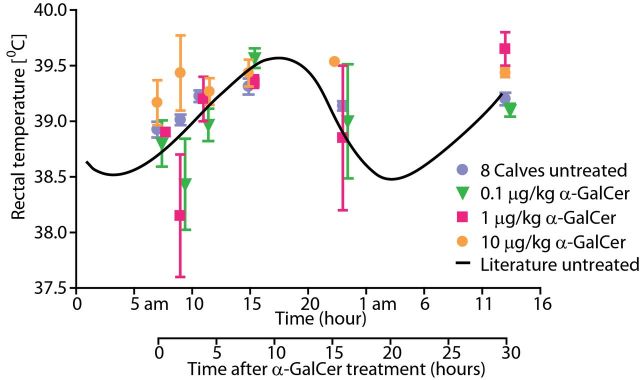
Body temperature of cattle treated intravenously with α-GalCer. The body temperature of calves treated with 0.1 (n = 3), 1 (n = 2) and 10 µg kg–1 (n = 3) α-GalCer was measured at the indicated time points after treatment. The circadian rhythm of the body temperature of healthy calves, as predicted from literature, is shown as a black line. One day before α-GalCer treatment, the body temperature of the calves was measured at the same hour as the post-α-GalCer treatment time points (in blue: “8 Calves untreated”).
Treatment with α-GalCer did not lead to a significant increase in body temperature. This was true when each of the three dose groups was compared with the untreated group and also when all treated animals were compared as one group with the untreated situation. To be able to visualize how the temperature data we collected fit in a normal circadian rhythm, the expected course of body temperature as based on published data is shown in Fig. 3 as a black line (25).
The boCD1D gene product presents shorter-chain α-GalCer to human NKT cells
The reason for the inability of α-GalCer to generate a response in cattle in vivo might reflect the lack of boCD1d to present α-GalCer or the absence of bovine NKT cells. If boCD1d is able to present α-GalCer to human or murine invariant NKT cells, these possibilities could be distinguished. Initial experiments showed that the lymphoblastoid cell line C1R transfected with boCD1d could not present α-GalCer to human and murine NKT cells (data not shown). Two testable explanations for this negative result are that (i) the highly unusual 99-aa-signal peptide is not cleaved off and has a negative effect on the capacity of boCD1d to present α-GalCer or (ii) the antigen-binding groove of boCD1d cannot bind α-GalCer with a C18 phyotosphingosine and a C26 fatty acid. For subsequent experiments, we also transfected the human lymphoblastoid cell line C1R with boCD1d in which the unusual 99-aa-signal peptide was replaced by the signal peptide of boCD1b3 (Fig. 1A), and we included α-GalCer with a C12 or a C16 fatty acid in our studies. Figure 4A shows that the human NKT clone J3N.5 is autoreactive to human CD1d, defined as reactivity without addition of α-GalCer, but not to boCD1d. However, it does respond to α-GalCer with a C12 or a C16 fatty acid presented by boCD1d, albeit sub-optimally, and not at all to α-GalCer with a C26 fatty acid. The human NKT clone J24L.17 and the murine NKT hybridoma DN3A4 are not autoreactive and respond to all three chain-length variants of α-GalCer when presented by human CD1d (Fig. 4B and C). Again, α-GalCer with a C12 or a C16 fatty acid are weakly recognized by the human NKT clone, but not the C26-fatty acid α-GalCer. The murine NKT hybridoma does not respond at all to boCD1d with any of the α-GalCer variants (Fig. 4C). The order of efficiency of α-GalCer presentation is as follows: human CD1d > boCD1d with the boCD1b signal sequence > WT boCD1d. The difference between the latter two may be influenced by the slightly higher surface expression of boCD1d in the presence of the boCD1b signal sequence compared with WT boCD1d (Fig. 4D), despite attempts to match expression levels by cell sorting. In all transfection experiments, we noticed that the cell surface expression level of boCD1d containing the boCD1b signal sequence was higher than that of boCD1d.
Fig. 4.
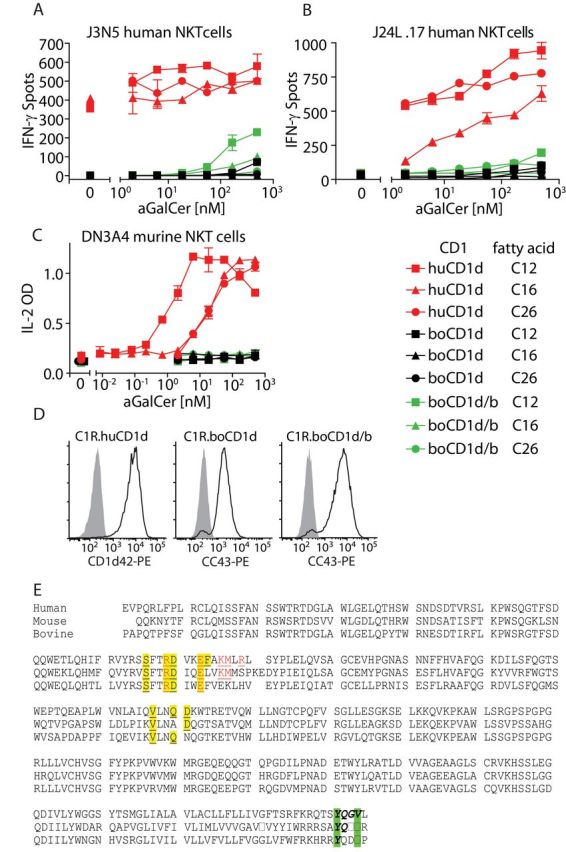
The boCD1D gene product presents α-GalCer to human NKT cells. C1R cells (5×104 cells per well) stably transfected with human CD1D, boCD1D1 or chimeric boCD1d protein in which the endogenous signal peptide was replaced by the signal peptide of boCD1b3 (boCD1d/b) were incubated with the indicated concentrations of α-GalCer and the human NKT cell clones J3N.5 (1000 cells per well) (A), or J24L.17 (B) in an IFN-γ ELISPOT assay. Alternatively, 5×104 C1R cells were incubated with the murine NKT hybridoma DN3A4 (5×104 cells per well); supernatants were harvested after 24h and tested for the presence of IL-2 by ELISA (C). The cell surface expression of human CD1d, boCD1D1 and boCD1d/b on the C1R cells at the day that the cellular assay was performed was confirmed by flow cytometry (D). This experiment has been replicated independently, with essentially the same results. (E) The human and murine CD1d protein sequences were aligned with the bovine CD1d sequence. Signal sequences were removed. Residues that are in the human CD1d sequence known to interact with (i) the human NKT TCR CDR3a are in yellow and are underlined and (ii) the human CDR2b are in red and are underlined (38). The YXXZ motif in the tail sequence is shown in green-bold-italics.
Thus, although α-GalCer with a C26 fatty acid fails to activate human NKT cells when presented by boCD1d, variants with chain lengths of C12 or C16 weakly stimulate human NKT cells, suggesting that boCD1d can bind and present α-GalCer, but only when it has a C12 or C16 fatty acid, and not when it has the common C26 fatty acid.
Discussion
Ruminants were thought to be the only group of mammals lacking functional CD1D genes (8). Here, we have demonstrated that, despite an intron–exon structure that is uncharacteristic for CD1 genes and a highly unusual signal peptide, the boCD1D gene can be expressed at the cell surface in vitro. In vivo expression on bovine thymocytes and B cells is implied by the published staining patterns of dendritic cells and B cells by the CC43 and CC118 antibodies (11), which we have shown here to recognize the boCD1D product.
Despite the expression of a boCD1D gene product, we have been unable to demonstrate bioactivity of α-GalCer with a C26 fatty acid in vivo in cattle, which is most likely caused by the inability of boCD1d to present this species of α-GalCer. However, variants of α-GalCer that contained a C12 or C16 fatty acid could be presented by boCD1d in vitro. Naturally occurring mammalian ceramides, which are thought to positively select and possibly expand and activate NKT cells in vivo, typically contain a C18 sphingosine, and the length of the fatty acid varies from C16 to C30, depending on the class of ceramide, tissue of origin, animal species and age of the animal (17, 26, 27). The mammalian ceramides that have been shown to stimulate NKT cells via presentation by CD1d include isoglobotrihexosylceramide, ganglioside GD3 and sulfatide (28–30). Even though the exact size limit for the fatty acid to enable binding of α-GalCer to boCD1d has not been determined in the functional experiments described here, it must probably be between C16 and C26. Together, these data suggest that boCD1d can only present a proportion of the mammalian ceramides, consisting of the ones with the shortest naturally occurring fatty acids. Mammalian phospholipids like phosphatidylinositol, phosphatidylcholine, phosphatidylglycerol and phosphatidylethanolamine, and bacterial lipids that are known to be presented by human and murine CD1d, are a little smaller and might fit well in the boCD1d groove (31–34). These differences in antigen-binding specificity between bovine versus murine and human CD1d may profoundly impact the selection and expansion of bovine NKT cells in vivo.
Our current data do not directly address the question whether cattle have an NKT cell population that is homologous to human and murine α-GalCer-reactive invariant NKT cells. In humans and mice, there are also populations of T cells (other than invariant NKT cells) that respond to CD1d. These cells recognize lipids without α-linked sugars, like phospholipids (31), sulfatides (35), or small aromatic molecules (36), and are called type II NKT cells (37). The boCD1d molecule we describe here might present comparable ligands.
We have previously described the CD1D genes of other ruminants [African buffalo, sheep, bushbuck, bongo, N’Dama cattle and roe deer (9)], and because they all show equivalent genetic structure, it is likely that CD1D in these species is expressed and functions in a fashion similar to that in cattle, as we have described in the current study.
Funding
Nederlands Wetenschappelijk Onderzoek (Meervoud grant to I.V.R.); Ministry of Education and Training of Vietnam (Decision 322/QD-TTg for Overseas Training Projects to T.K.A.N.); the Burroughs Wellcome Fund Program in Translational Immunology (AIR048632 and AI 049313 to D.B.M.); and the National Insttitues of Health (AI074952 to D.M.Z.); A Personal Research Chair from Mr James Badrick, Royal Society Wolfson Research Merit Award, a Lister Institute-Jenner Research Fellowship, a Medical Council grant and grant from The Wellcome Trust (084923/B/08/7) to G.S.B.
Acknowledgements
CC118 antibody was kindly provided by Dr J. C. Hope, Compton, UK. The cell lines J3N.5 and J24L.17 were kindly provided by M. Brigl, Boston, USA, and J. Gumperz, Madison, USA.
References
- 1. Gumperz J. E., Miyake S., Yamamura T., Brenner M. B. 2002. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J. Exp. Med. 195: 625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee P. T., Benlagha K., Teyton L., Bendelac A. 2002. Distinct functional lineages of human V(alpha)24 natural killer T cells. J. Exp. Med. 195: 637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Snyder-Cappione J. E., Tincati C., Eccles-James I. G., et al. 2010. A comprehensive ex vivo functional analysis of human NKT cells reveals production of MIP1-α and MIP1-β, a lack of IL-17, and a Th1-bias in males. PLoS One 5: e15412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brossay L., Chioda M., Burdin N., et al. 1998. CD1d-mediated recognition of an alpha-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J. Exp. Med. 188: 1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Matsuda J. L., Naidenko O. V., Gapin L., et al. 2000. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J. Exp. Med. 192: 741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benlagha K., Weiss A., Beavis A., Teyton L., Bendelac A. 2000. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J. Exp. Med. 191: 1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baker M. L., Miller R. D. 2007. Evolution of mammalian CD1: marsupial CD1 is not orthologous to the eutherian isoforms and is a pseudogene in the opossum Monodelphis domestica. Immunology 121: 113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van Rhijn I., Koets A. P., Im J. S., et al. 2006. The bovine CD1 family contains group 1 CD1 proteins, but no functional CD1d. J. Immunol. 176: 4888 [DOI] [PubMed] [Google Scholar]
- 9. Looringh van Beeck F. A., Reinink P., Hermsen R., et al. 2009. Functional CD1d and/or NKT cell invariant chain transcript in horse, pig, African elephant and guinea pig, but not in ruminants. Mol. Immunol. 46: 1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coad M., Clifford D., Rhodes S. G., Hewinson R. G., Vordermeier H. M., Whelan A. O. 2010. Repeat tuberculin skin testing leads to desensitisation in naturally infected tuberculous cattle which is associated with elevated interleukin-10 and decreased interleukin-1 beta responses. Vet. Res. 41: 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Howard C. J., Sopp P., Parsons K. R., Bembridge G. P., Hall G. 1993. A new bovine leukocyte antigen cluster comprising two monoclonal antibodies, CC43 and CC118, possibly related to CD1. Vet. Immunol. Immunopathol. 39: 69 [DOI] [PubMed] [Google Scholar]
- 12. Exley M., Garcia J., Balk S. P., Porcelli S. 1997. Requirements for CD1d recognition by human invariant Valpha24+ CD4-CD8- T cells. J. Exp. Med. 186: 109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Jong A., Peña-Cruz V., Cheng T. Y., Clark R. A., Van Rhijn I., Moody D. B. 2010. CD1a-autoreactive T cells are a normal component of the human αβ T cell repertoire. Nat. Immunol. 11: 1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brigl M., van den Elzen P., Chen X., et al. 2006. Conserved and heterogeneous lipid antigen specificities of CD1d-restricted NKT cell receptors. J. Immunol. 176: 3625 [DOI] [PubMed] [Google Scholar]
- 15. Brossay L., Tangri S., Bix M., Cardell S., Locksley R., Kronenberg M. 1998. Mouse CD1-autoreactive T cells have diverse patterns of reactivity to CD1+ targets. J. Immunol. 160: 3681 [PubMed] [Google Scholar]
- 16. Jervis P. J., Cox L. R., Besra G. S. 2011. Synthesis of a versatile building block for the preparation of 6-N-derivatized α-galactosyl ceramides: rapid access to biologically active glycolipids. J. Org. Chem. 76: 320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen J., Narayan S. B., Edinger A. L., Bennett M. J. 2012. Flow injection tandem mass spectrometric measurement of ceramides of multiple chain lengths in biological samples. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 883-884: 136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaiser C. A., Preuss D., Grisafi P., Botstein D. 1987. Many random sequences functionally replace the secretion signal sequence of yeast invertase. Science 235: 312 [DOI] [PubMed] [Google Scholar]
- 19. Smyth M. J., Crowe N. Y., Pellicci D. G., et al. 2002. Sequential production of interferon-gamma by NK1.1+ T cells and natural killer cells is essential for the antimetastatic effect of alpha-galactosylceramide. Blood 99: 1259 [DOI] [PubMed] [Google Scholar]
- 20. Fujii S., Shimizu K., Hemmi H., et al. 2006. Glycolipid alpha-C-galactosylceramide is a distinct inducer of dendritic cell function during innate and adaptive immune responses of mice. Proc. Natl. Acad. Sci. U.S.A. 103: 11252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Inui T., Nakagawa R., Ohkura S., et al. 2002. Age-associated augmentation of the synthetic ligand- mediated function of mouse NK1.1 ag+ T cells: their cytokine production and hepatotoxicity in vivo and in vitro. J. Immunol. 169: 6127 [DOI] [PubMed] [Google Scholar]
- 22. Woltman A. M., Ter Borg M. J., Binda R. S., et al. 2009. Alpha-galactosylceramide in chronic hepatitis B infection: results from a randomized placebo-controlled Phase I/II trial. Antivir. Ther. (Lond.) 14: 809 [DOI] [PubMed] [Google Scholar]
- 23. Motohashi S., Nagato K., Kunii N., et al. 2009. A phase I-II study of alpha-galactosylceramide-pulsed IL-2/GM-CSF-cultured peripheral blood mononuclear cells in patients with advanced and recurrent non-small cell lung cancer. J. Immunol. 182: 2492 [DOI] [PubMed] [Google Scholar]
- 24. Renukaradhya G. J., Manickam C., Khatri M., et al. 2011. Functional invariant NKT cells in pig lungs regulate the airway hyperreactivity: a potential animal model. J. Clin. Immunol. 31: 228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Piccione G., Caola G., Refinetti R. 2003. Daily and estrous rhythmicity of body temperature in domestic cattle. BMC Physiol. 3: 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kasumov T., Huang H., Chung Y. M., Zhang R., McCullough A. J., Kirwan J. P. 2010. Quantification of ceramide species in biological samples by liquid chromatography electrospray ionization tandem mass spectrometry. Anal. Biochem. 401: 154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pettus B. J., Kroesen B. J., Szulc Z. M., et al. 2004. Quantitative measurement of different ceramide species from crude cellular extracts by normal-phase high-performance liquid chromatography coupled to atmospheric pressure ionization mass spectrometry. Rapid Commun. Mass Spectrom. 18: 577 [DOI] [PubMed] [Google Scholar]
- 28. Zhou D., Mattner J., Cantu C., III, et al. 2004. Lysosomal glycosphingolipid recognition by NKT cells. Science 306: 1786 [DOI] [PubMed] [Google Scholar]
- 29. Wu D. Y., Segal N. H., Sidobre S., Kronenberg M., Chapman P. B. 2003. Cross-presentation of disialoganglioside GD3 to natural killer T cells. J. Exp. Med. 198: 173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jahng A., Maricic I., Aguilera C., Cardell S., Halder R. C., Kumar V. 2004. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J. Exp. Med. 199: 947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gumperz J. E., Roy C., Makowska A., et al. 2000. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity 12: 211 [DOI] [PubMed] [Google Scholar]
- 32. Brennan P. J., Tatituri R. V., Brigl M., et al. 2011. Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nat. Immunol. 12: 1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kinjo Y., Wu D., Kim G., et al. 2005. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature 434: 520 [DOI] [PubMed] [Google Scholar]
- 34. Kinjo Y., Tupin E., Wu D., et al. 2006. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat. Immunol. 7: 978 [DOI] [PubMed] [Google Scholar]
- 35. Arrenberg P., Halder R., Dai Y., Maricic I., Kumar V. 2010. Oligoclonality and innate-like features in the TCR repertoire of type II NKT cells reactive to a beta-linked self-glycolipid. Proc. Natl. Acad. Sci. U.S.A. 107: 10984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Van Rhijn I., Young D. C., Im J. S., et al. 2004. CD1d-restricted T cell activation by nonlipidic small molecules. Proc. Natl. Acad. Sci. U.S.A. 101: 13578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Godfrey D. I., MacDonald H. R., Kronenberg M., Smyth M. J., Van Kaer L. 2004. NKT cells: what’s in a name? Nat. Rev. Immunol. 4: 231 [DOI] [PubMed] [Google Scholar]
- 38. Borg N. A., Wun K. S., Kjer-Nielsen L., et al. 2007. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature 448: 44 [DOI] [PubMed] [Google Scholar]