Abstract
The recently published comprehensive profiles of genomic alterations in glioma have led to a refinement in our understanding of the molecular events that underlie this cancer. Using state-of-the-art genomic tools, several laboratories have created and characterized accurate genetically engineered mouse models of glioma based on specific genetic alterations observed in human tumors. These in vivo brain tumor models faithfully recapitulate the histopathology, etiology, and biology of gliomas and provide an exceptional experimental system to discover novel therapeutic targets and test therapeutic agents. This review focuses on mouse models of glioma with a special emphasis on genetically engineered models developed around key genetic glioma signature mutations in the PDGFR, EGFR and NF1 genes and pathways. The resulting animal models have provided insight into many fundamental and mechanistic facets of tumor initiation, maintenance and resistance to therapeutic intervention and will continue to do so in the future.
Introduction
Histological and malignant profiling: glioma grades
Gliomas comprise a heterogeneous group of neoplasms that differ in location, morphological features, tendency for progression, and response to therapy. Histological grading according to Kernohan (Kernohan et al. 1949) and Ringertz (Ringertz 1950) dominated until 1993, when the World Health Organization (WHO) grading system was established classifying gliomas into grades I-IV (Huse and Holland 2010; Kleihues et al. 1995). WHO grade I gliomas generally behave in a benign fashion and can often be surgically resected without recurrence. Grade II-IV gliomas constitute the diffuse infiltrating gliomas and range in malignant character. Grade II tumors are categorized as astrocytomas, oligodendrogliomas, or mixed oligoastrocytomas, depending on the cells’ resemblance to astrocytes or oligodendrocytes. Grade III gliomas are more aggressive and include anaplastic astrocytomas and oligodendrogliomas. Grade IV gliomas, also known as glioblastoma multiforme (GBM), are the most aggressive subtype. GBMs either develop de novo (primary glioblastoma) or as lower grade gliomas that exhibit increasing malignancy over time (so-called secondary glioblastoma) (Hesselager and Holland 2003).
Transcriptional and proteomic profiling: glioma subgroups
DNA, RNA, and protein analyses of gliomas suggest that they are not a single tumor type, but fall into several distinct molecular subtypes. Studies published by multiple groups using a variety of technologies have come to similar conclusions regarding the number and nature of these molecular subtypes of glioma. One such study used correlative expression profiling to subdivide gliomas into groups based on similarities to known cell types. The resulting groups were termed Proneural, Proliferative and Mesenchymal (Phillips et al. 2006). A more recent analysis of The Cancer Genome Atlas (TCGA) data also identified several subclasses of glioma, termed Proneural, Neural, Classical, and Mesenchymal, that are strongly associated with genomic abnormalities in PDGFR, IDH1/IDH2, EGFR, or NF1 respectively (TCGA 2008; Verhaak et al. 2010). In a third report, a combination of proteomic and genomic analyses was used to subdivide tumors into three subclasses based on signal transduction pathway activation and genetic alterations. This study also resulted in the identification of the NF1, EGFR and PDGFR classes (Brennan et al. 2009). In summary, the data indicate that there are three distinct glioma subtypes that show either EGFR activation associated with amplification or mutation of receptor, PDGF-pathway activation, which is associated with PDGF ligand expression at the protein level and PDGFR amplification in half of the cases, or loss of NF1 expression. These signaling subtypes strongly correlate with distinct genomic and expression patterns.
The history of animal models of glioma
Although in vitro studies using human cell lines have been informative (Alley et al. 1988), cell culture experiments have inherent limitations including the inability to model invasion, angiogenesis, metastasis and the role of microenvironment in drug response. Therefore, many different animal modeling systems have been created to study tumor biology, and for use in preclinical trials to develop treatment modalities for this disease. These in vivo modeling systems fall into two basic categories: those that implant tumor cells into recipient mice and those that create tumors in mice de novo.
Implantation models can either be allografts where tumor cells derived from a given species are implanted back into the same species, or xenografts where tumor cells from a different species are implanted into immunosuppressed mice. Xenograft models can either be orthotopic (in the original site) or heterotypic (non-autochthonous site). The traditional allograft models uses cell lines that have been passaged and maintained in tissue culture for several decades. The tumors generated from these cell lines cultured in the presence of animal serum tend to not show the classical histologic appearance of human gliomas (Lee et al. 2006a), and they have not been predictive for response in preclinical trials. Studies of 31 cytotoxic cancer drugs in various preclinical cancer models demonstrated that murine allograft models show limited utility in predicting Phase II clinical trial performance (Voskoglou-Nomikos et al. 2003).
Recently, with the development of the cancer stem cell field, xenograft models of glioma have been modified and improved substantially. The use of orthotopic xenograft models has yielded valuable information on important aspects of GBM biology. For example, orthotopic GBM models have shed light on the sensitivities of different cell populations to the oncogenic activities of various genes (Bachoo et al. 2002). Tumor cells derived from freshly isolated human glioma tumors, when cultured in conditions optimized for cancer stem cell growth and injected orthotopically, more closely mirror the phenotype and genotype of the primary tumor than when cultured in serum-containing medium (Lee et al. 2006a). These tumors typically recapitulate the diffuse invasion of glioma cells into the normal brain structures, but do not exhibit the microvascular proliferation or pseudopalisading necrosis seen in human GBM. Moreover, serial passage of these cells in mice can lead to changes in tumor phenotype, suggesting that the progression from lower grade tumor to GBM may be modeled in such systems (Wang et al. 2009a). Several groups are currently using these modern xenograft models in preclinical testing. The advantage to such systems is that they are derived from human gliomas. These orthotopic models have also allowed for experiments aimed at studying the biology of GBM cancer stem cells (Dirks 2007; Dirks 2006; Singh et al. 2004a; Singh et al. 2003; Singh et al. 2004b) and have recently been utilized to recreate certain aspects of the microenvironment and cellular heterogeneity seen in human tumors (Inda et al. 2010).
Two major criticisms of xenograft models are: 1) the absence of a functional immune system in these animals and 2) the experimental methods by which these tumors are seeded. Most orthotopic xenograft glioma models are performed using human glioma cells injected intracranially in immunocompromised mice. It is becoming clear that cancer formation and progression involve immune surveillance-escaping paradigms, which, in an immunocompetent host, select for cancer developmental processes that are not represented in a xenograft system. In addition, DNA repair defects in these immunocompromised host mice can limit their capacity to tolerate novel treatments, including radiation therapy (Biedermann et al. 1991). Another potential drawback of xenograft models is the initiation of tumorigenesis by injection of a large number of cells into the recipient animals. This method of tumor initiation differs considerably from spontaneous tumor development, which is generally thought to involve the initial transformation of a single cell that acquires additional genetic hits as it evolves in situ. In light of these criticisms and with improved genetic tools at hand, many researchers have turned to the creation and utilization of model systems that may better represent key aspects of tumor biology.
A second approach to modeling cancer in animals is to use genetically engineered mouse models (GEMMs) with a close genetic resemblance to human disease. These GEMMs have played a key role in the study of cancer. First, they provide appropriate materials for comparative onco-genomic studies, which are aimed at identifying additional genes that are altered in the development of cancer. Second, these GEMMs have been used to validate the functionality of specific genes in tumorigenesis. GEMMs can also be used to analyze cooperative gene networks with specifically engineered mutations, thus allowing for the assignment of genetic lesions into defined pathways and the testing of drugs targeting these activities.
GEMMs of brain tumors
Although genetic modeling of gliomas in mice may superficially appear to be similar to modeling these tumors by implanting cell lines, there are important fundamental differences. Genetic models of glioma address the molecular causality for tumor initiation, progression, therapeutic response and histology. In this way, GEMMs have greatly contributed to our understanding of the molecular pathways responsible for tumor initiation, progression, and metastasis, and have expanded our knowledge of the role of the microenvironment in tumor biology. These models have shed light on the mechanistic role that oncogenes and tumor suppressor genes play in these processes. Studies with GEMMs have also improved our understanding of the role of individual genes and their mutated counterparts in tumorigenesis, as well as the cooperation between mutations in tumor development.
The first germline GEMMs for brain tumors were murine models that over-expressed viral oncogenes (Brinster et al. 1984). Subsequently, germline modification strategies for gain- and loss-of-function were developed for specific cellular genes. These lines of mice are maintained as populations, and the oncogenic alteration is maintained in a Mendelian fashion. In many ways, germline modification strategies mimic human heritable cancer predisposition syndromes. These models reduce the threshold for transformation and lead to the emergence of tumors from a population of genetically altered cells. As such, they feature genetic alterations that can contribute to cancer formation, rather than = alterations that are sufficient for cancer formation from normal cells. A germline alteration that is sufficient to convert normal cells to tumor in 100% of affected cells would generally be embryonically lethal and could not be maintained as a line of mice. In the majority of human cancers, most mutations occur somatically in a single cell or small group of specific cell types, and the identity of the mutation-bearing cell is one determinant of the characteristics of the resulting tumor cells (Kinzler and Vogelstein 1996).
Over time, genetic models evolved to more complex strategies where multiple genes could be gained or lost in specific cells and at specific developmental time points. Conditional strategies were developed that allow for the control of gene expression in both a tissue- and/or time-specific manner (Macleod and Jacks 1999; Talmadge et al. 2007). For example, tet-regulatable or cre-inducible alleles of genes can allow for the control of the timing, duration, and tissue compartment of gene expression or inactivation. In addition, several methods for somatic cell gene transfer have been developed using retroviral or adenoviral vectors delivering cre recombinase. The RCAS/tv-a system is based on use of a receptor for subgroup A avian leukosis virus (TVA) that allows multiple genes to be introduced somatically into a single mouse strain by using retroviral infection by subgroup A avian sarcoma leukosis viruses (ASLVs) (Federspiel et al. 1994). Replication-competent ALV splice acceptor (RCAS) viral vectors were derived from ASLVs; this vector is genetically modified to accept insertion of various oncogenes of interest (Greenhouse et al. 1988). All of the above-mentioned strategies have been used to generate brain tumor models.
Several brain tumor models have been developed by altering the signaling pathways that are disrupted in human gliomas such as those including Rb, RAS and AKT (Guha 1998; Henson et al. 1994; Holland et al. 2000; Ueki et al. 1996). Given the high frequency with which pRB function is impaired in human astrocytomas, a transgenic astrocytoma model was developed by tissue-specific inactivation of the Rb family proteins via the expression of SV40 T antigen (Danks et al. 1995), and later a fragment of the SV40 T antigen (T121), which effectively inactivates the Rb pathway, expressed under control of the GFAP promoter. When this gene is expressed later in development, nearly 100% of the mice develop lethal anaplastic astrocytomas within 10 months (Xiao et al. 2002). Furthermore, in this model, the combination of pten inactivation induces increased tumor cell survival and invasion along with angiogenesis (Xiao et al. 2005).
To study the role of RAS signaling in gliomagenesis, transgenic lines were generated where constitutively active Ras (V12Ras) was expressed under the control of a human GFAP promoter. To investigate the importance of Ras gene dosage, a series of transgenic lines (GFAP-V12Ha-ras) with varying Ras expression levels were created. For instance, mice with extremely high V12Ha-ras expression and activity died of multifocal GBM within 2 weeks after birth. Moderately elevated levels of V12Ha-ras expression leads to astrocytomas of varying grades in 95% of animals, with 50% of these mice developing astrocytomas by 3 months of age (Ding et al. 2001; Shannon et al. 2005). GFAP-v-src transgenics have also been generated. These mice formed abnormal nests of proliferating astrocytes by 2 weeks after birth that evolved into overt malignant astrocytomas of the brain and spinal cord in 14% of mice by 65 weeks of age (Weissenberger et al. 1997). The RCAS/tv-a system has been used to transfer constitutively active mutant forms of K-Ras and Akt to nestin-expressing neural progenitor cells (Holland et al. 2000). Activation of Ras or Akt alone does not lead to tumor formation, and the combination of Ras and Akt is insufficient to induce tumors from GFAP-expressing astrocytes. This result demonstrated the importance of both activation of certain signaling cascades and the differentiation status of the cell of origin. The difference in tumorigenic potential of Ras in transgenics versus the somatic specific gene transfer method is most probably a result of secondary mutations that are occurring in transgenics. For example, when Ink4a-Arf null mice were crossed with Ntv-a or Gtv-a transgenic mice and infected with RCAS-KRas, they developed brain tumors with a sarcoma-like histology. This result implies that Ras is able to cooperate with either Akt activation (below) or Ink4a-Arf loss to produce gliomas with different histological features (Uhrbom et al. 2002; Uhrbom et al. 2005). The combination of RCAS/tv-a and Cre/Lox systems allows for the generation of Ras-driven gliomas that are deficient for PTEN (Hu et al. 2005). However, the gain-of-function component of the tumor models mentioned above is not driven by the specific genes or mutations that are found in gliomas in human, but rather by genes that are components of the signaling pathways activated in gliomas. Human gliomas most frequently achieve the activation of these signaling pathways via the activation of upstream receptor tyrosine kinases (RTKs). These models are relevant for studying the pathways that drive gliomas, despite the fact that activation of these signaling pathways is achieved by different means in human gliomas.
Postnatal, somatic cell mouse models of the three main subsets of human GBM
Ideally, animal models would mimic the human tumor in as many aspects as possible, including histology, tumor biology and the specific genetic alterations found in gliomas. In the next section we will discuss GEMMs that are driven by the genes known to be key drivers for each subtype of glioma described for human; the NF1, EGFR and PDGFR classes (Brennan et al. 2009; Phillips et al. 2006; Verhaak et al. 2010). These three classes are distinct and show either EGFR activation associated with amplification or mutation of receptor, PDGF-pathway activation, which is mainly ligand-driven, or loss of Nf-1 expression. There are mouse GEMMs matching each human group.
Mouse models of PDGF-driven gliomas
Platelet-derived growth factor (PDGF) was discovered in whole blood serum and was absent in cell free plasma; it was originally purified from human platelets (Heldin and Westermark 1990). Later, it was shown that PDGF is also produced by a number of other cell types, including macrophages, epithelial and endothelial cells. Reports followed showing that PDGF promotes cell proliferation, migration and survival of different cell types in vitro (Claesson-Welsh 1994). Subsequent studies showed there are five biologically active dimeric forms of the PDGF protein: AA, BB, AB, CC, DD (Gilbertson et al. 2001; Heldin et al. 1985; LaRochelle et al. 2001). The PDGF receptor has two types of subunits (PDGFRα and β), that are functionally and structurally related and belong to the receptor tyrosine kinase family (Claesson-Welsh 1994). Upon binding to PDGF, these receptors activate downstream signaling, including the PI3K/Akt, RAS/MAP kinase and PLC/PKC pathways. As for the role of PDGF in the brain, in vitro studies have shown that PDGF directly stimulates the proliferation and migration of glial progenitors (Frost et al. 2009; Noble et al. 1988). When these cells are grown in culture, a combination of PDGF and basic fibroblast growth factor (bFGF) promotes extended self-renewal and inhibits differentiation (Bogler et al. 1990). To further dissect the role of PDGF in vivo, PDGF knockout mice and transgenic mice over-expressing PDGF under the control of the neuron-specific enolase (NSE) promoter were generated. Data from these mice suggests that the number of progenitors in the CNS is regulated by the ambient levels of PDGF (Calver et al. 1998; van Heyningen et al. 2001).
The first indications of a role for PDGF in gliomagenesis came from studies showing that the intracerebral injection of simian sarcoma virus (SSV) into newborn marmosets induces glioma (Deinhardt and F New York: Raven Press, 1980). The v-sis oncogene of SSV was subsequently shown to be a retroviral homolog of the PDGF B-chain gene (Doolittle et al. 1983). Together with data suggesting that the v-sis gene product acts solely as a PDGF receptor agonist in transformation of human fibroblasts, these findings pointed to PDGF as a promising oncogenic candidate to study gliomagenesis. Since then, PDGF signaling has been widely implicated not only in brain tumors, but also in several other human malignances. Human glioma specimens and glioma cell lines frequently co-express PDGF ligands and receptors (Di Rocco et al. 1998; Goyal and Robinson 2008; Nister et al. 1988), and PDGFRα is amplified in some high-grade glioma cases (Shoshan et al. 1999), suggesting the existence of an autocrine loop comprised of PDGF and its receptors (Guha et al. 1995). Later, mouse models demonstrated that an autocrine loop is a crucial event in tumor development (Uhrbom et al. 2000) and verified that PDGF is causally related to the formation of gliomas (reviewed in (Calzolari and Malatesta 2010; Shih and Holland 2006)).
The two mouse models of PDGF-driven glioma that have been most extensively studied both use somatic cell type-specific gene transfer. The first of these models uses a recombinant Moloney murine leukemia virus (MoMuLV) containing the human PDGF B-chain gene (Uhrbom et al. 1998; Uhrbom et al. 2000). Injection of retrovirus into neonatal animals results in nonspecific gene transfer by infecting proliferating cells in the brain. Around 40% of mice developed tumors within 14–29 weeks with mixed histology, the most of which exhibit characteristics of GBM or primitive neuroectodermal tumor (PNET) (Uhrbom et al. 1998). Secondary genetic alterations induced by insertional mutagenesis have been used to identify genes that cooperate with PDGF expression in this glioma type (Johansson et al. 2004; Johansson et al. 2005).
The second strategy uses RCAS/tv-a (Fisher et al. 1999). Originally, two transgenic mouse lines were described that develop brain tumors when infected with RCAS vectors: Gtv-a, which expresses tv-a under the control of the glial fibrillary acidic protein (GFAP) promoter, and Ntv-a, which expresses tv-a under the control of the nestin promoter, which is active in neural stem/progenitor cells (Holland and Varmus 1998). Recently, a third tv-a transgenic line, Ctv-a, was created. In the Ctv-a mouse line, RCAS infection is restricted to myelinating oligodendrocyte progenitor cells (OPCs) expressing 2',3'-cyclic nucleotide 3'-phosphodiesterase (Lindberg et al. 2009). Newborn pups from these three tv-a transgenic mice have been used to overexpress PDGFB in order to create pediatric high- and low-grade gliomas in different locations such as cortex, brain stem, and cerebellum, thus allowing the investigation of the role of anatomical location in tumorigenesis and the effects of therapeutic modalities. Also, tumors have been generated where PDGF overexpression is combined with a loss of various tumor suppressors known to be involved in human gliomas, such as ink4a-arf, p53 and pten (Dai et al. 2001; Tchougounova et al. 2007).
Within the last few years, several lines of investigation have demonstrated that, although adult and pediatric gliomas are histologically indistinguishable, they differ in location, behavior, and molecular characteristics. These observations imply that the molecular pathways and pathophysiology of malignant gliomagenesis in these two populations are distinct. Such differences between adult and pediatric gliomas are likely to predict different therapeutic responses. For example, adult GBMs commonly demonstrate amplification of EGFR and inactivation by mutation or deletion of pten. In contrast, these alterations are uncommon events in pediatric malignant gliomas (Pollack et al. 2006). In order to design a model that reflects these differences, adult gliomas were recently modeled by overexpressing PDGFB in nestin- and GFAP-positive cells without or with a combination of various tumor suppressors (Hambardzumyan et al. 2009). These mice represent excellent tools for dissecting the role of location, genetic background, and cell of origin in response to radiation and chemotherapy in adult gliomas. Data obtained from adult preclinical studies can then be evaluated in parallel with data obtained from pediatric studies.
In response to the observation that human secondary gliomas commonly show mutation and/or loss of p53, often in combination with alterations in PDGF signaling, researchers engineered mouse models that contain these specific genetic alterations. In this transgenic model, human PDGFB (hPDGFB) was expressed under the control of the human GFAP (hGFAP) promoter, and then these mice were bred into p53 null mice. Tumors that displayed human GBM features developed between 2–6 months of age (Hede et al. 2009). Two independent genetically-engineered and conditionally-activated mouse models were used to generate PDGFB-driven primary intramedullary and astrocytic spinal gliomas that were histologically and pathologically indistinguishable from their human counterparts (Hitoshi et al. 2008). In the first model, a tetracycline responsive system was used to express hPDGFB (TRE/hPDGFB) from a GFAP promoter that regulated the tetracycline transcriptional activator (tet-off) tTA (GFAP/tTA/:TRE/hPDGFB) in a manner that could be repressed by doxycycline. In the second model, the hGFAP promoter drove hPDGF mRNA expression in selected mouse lines to study the development of spinal cord tumors (Hitoshi et al. 2008).
PDGF-driven mouse models generated by the RCAS/tv-a system were used to address several questions, including the cell of origin and the role of the microenvironment. Using either GFAP- or nestin-expressing cells to overexpress PDGF in combination with loss of Ink4a-ARF resulted in glioma formation with the same incidence and time frame. Stereotactic injection of RCAS-PDGF in nestin-expressing or GFAP-expressing cells shows similar incidence and latency when injected in either the SVZ or the cortex of adult mice (Hambardzumyan et al. 2009). Cerebellar and/or brain stem gliomas form when PDGF is overexpressed in GFAP- or nestin-expressing cells in cerebellum, although with longer latency relative to SVZ and cortex injections. These data suggest that in these experimental settings both nestin-positive stem/progenitor cells and GFAP-positive cells may be transformed into glioma cells. GFAP-positive cells represent a stem cell population in SVZ, but in the cortex and cerebellum they are astrocytes. Although it is know that most cortical astrocytes do not show GFAP-positivity by immunohistochemistry (Amankulor et al. 2009), stereotactic injection into the cortex causes astrocytes to activate and up-regulate GFAP, thereby becoming a target for infection by RCAS vectors. These results demonstrate that in some experimental conditions, differentiated astrocytes and stem/progenitor cells can serve as the cell of origin for gliomas. Recent studies have shown that myelinating oligodendrocyte progenitor cells (OPCs) can also serve as the glioma cell of origin. RCAS-PDGF injection into the cortex of Ctv-a (2',3'-cyclic nucleotide 3'-phosphodiesterase (Cnp) cnp-tv-a) mice resulted in efficient glioma formation (Lindberg et al. 2009). Overexpressing PDGF in nestin-expressing cells of adult and newborn mice, and in OPCs of newborns, results in formation of oligodendrogliomas. These tumors stain positive for Olig-2 and histologically resemble human oligodendrogliomas. When PDGF is overexpressed in GFAP-positive cells in newborn or adult mice, the formation of mixed gliomas is observed, with the resulting tumors resembling human mixed astro-oligo histology.
PDGF-driven gliomas contain cancer stem-like cells as determined by the presence of a side population (SP) that forms neurospheres and exhibits higher tumorigenic ability compared to the main population (MP), mimicking the case for human gliomas (Bleau et al. 2009). Subsequent studies showed that cancer stem-like cells in PDGF-driven GBMs express the stem cell marker nestin and reside in specific niches in the perivascular regions of these tumors (Calabrese et al. 2007; Charles et al. 2010; Hambardzumyan et al. 2008). The existence of a perivascular stem cell niche was also shown for normal neuronal stem cell in the mouse brain (Shen et al. 2008; Tavazoie et al. 2008), as well as for human gliomas and medulloblastomas (Tavazoie et al. 2008). The microenvironment of the perivascular niche is provide important signaling stimuli to the cancer stem-like cell population, as exemplified by one study showing that nitric oxide released from tumor endothelial cells is able to activate notch signaling in the stem cells of this perivascular niche, a mechanism shown to be conserved in human PDGFR amplified human gliomas (Charles et al. 2010). These studies once again underline the utility of the PDGFB-driven models to study the biology of the PDGF/proneural subset of gliomas.
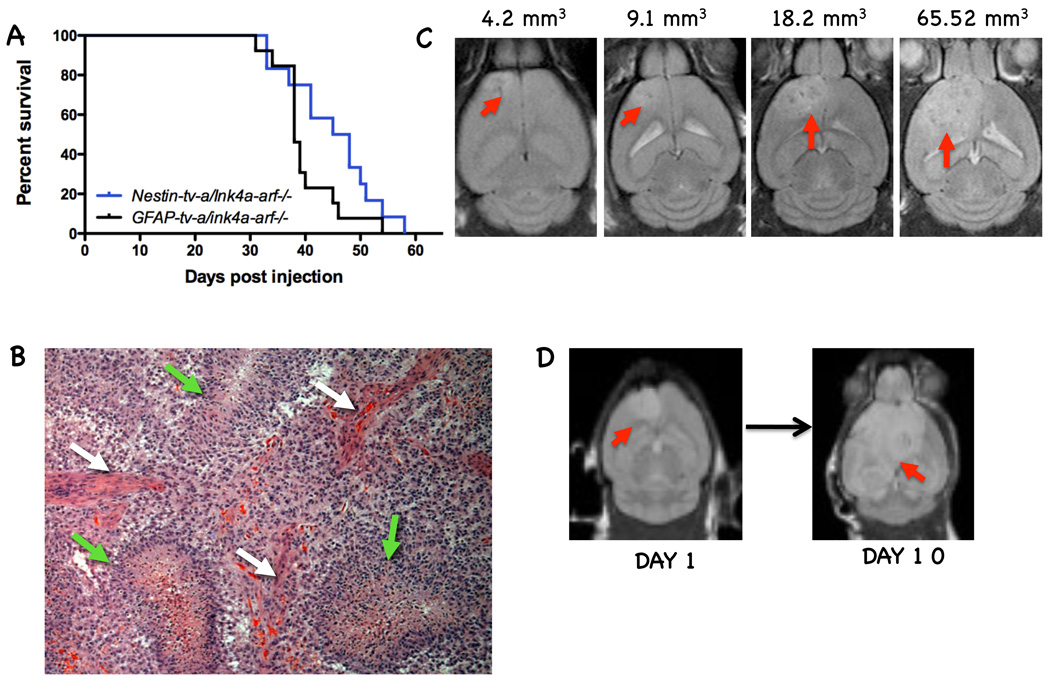
These PDGF-induced models have also been used in preclinical trials for the development of glioma therapies. PDGF overexpression in Nestin-tv-a/ink4a-arf-/- and GFAP-tv-a/ink4a-arf-/- backgrounds results in high-grade glioma formation with a 90–95% incidence (Fig. 1A,B). Enhancement on MRI scans was shown to correlate with regions of microvascular proliferation, and T2-weighted abnormalities correlate with invading tumor cells (Fig. 1C,D). Temozolomide increases survival for tumor-bearing mice (McConville et al. 2007). The mTOR inhibitor CCI779 and the PDGF/VEGF inhibitor PTK787 were shown to achieve similar growth arrests against this tumor type (Uhrbom et al. 2004). Perifosine, an inhibitor of Akt signaling, was also shown to have some efficacy in this model, prompting clinical trials for this agent in glioma patients (Momota et al. 2005). Furthermore, perifosine and radiation were tested alone and in combination in PDGF-driven brain stem gliomas and shown to have additive effects (Becher et al. 2010). Finally, the combination of CCI779 and perifosine has also been shown to be more effective than either drug alone (unpublished observations), prompting ongoing clinical trials of this drug combination in both adults and pediatric patients.
Figure 1.
Adult gliomas generated by overexpression of PDGF-B in nestin- and GFAP-positive cells represent tools for pre-clinical trials. A) Kaplan-Meier survival curves of PDGF-driven tumors in Nestin-tv-a/ink4a-arf-/- and GFAP-tv-a/ink4a-arf-/- backgrounds. B) Representative image of H&E staining of a PDGF-B-driven glioma demonstrate the existence of high-grade elements; white arrows point to microvascular proliferation and green arrows to pseudopalisading necrosis. C) T2-weighted images of mice 4 weeks post-injection of PDGF-B shows variable tumor size. Using the tumor volumes mice can be assigned to different treatment groups. D) T2-weighted images showing tumor growth at 10 days (day 10) after initial MRI (day 1).
Mouse models of the NF-1 group of gliomas
The NF1 tumor suppressor is responsible for the genetically heritable disease Neurofibromatosis type I. Neurofibromatosis type I arises as a germline mutation in about one in 3,500 births worldwide, and tumors of the nervous system are a cardinal feature of the disease (Trovo-Marqui and Tajara 2006). While peripheral nervous system tumors are predominant in Neurofibromatosis, as many as 50% of NF1 individuals develop benign pilocytic astrocytomas of the optic nerve (Singhal et al. 2002). In addition, the prevalence of malignant glioma in individuals harboring a germline NF1 mutation is considerably higher than the population at large (Gutmann et al. 2003; Gutmann et al. 2002). Thus, on this basis, the NF1 tumor suppressor can be considered a genetic predisposition factor for glioma. The recent publication of TCGA data for sporadic human glioma has provided compelling support for NF1 as a potentially causal agent in glioma, as NF1 was mutated at a similar frequency to other well-known tumor suppressors such as Pten, p53, Rb, and CDKN2A (TCGA 2008). Indeed, subsequent studies have begun to reveal NF1 mutations in a broader spectrum of solid tumors, albeit with a lesser proportion than seen in glioma (Ding et al. 2008; Sangha et al. 2008).
The NF1 gene encodes for a large protein, neurofibromin, whose only widely accepted function at this time is that of a GTPase-activating protein (RasGAP) for Ras family proteins. Therefore, NF1 tumor suppressor function in tumor development is attributed to inappropriate activation of cellular Ras proteins whose activity is normally tightly regulated and constrained by the presence of functional neurofibromin. When NF1 loss is considered as a Ras-activating mutation, the above-mentioned segregation of glioma subtypes based on mutation spectrum and gene expression profiles can be understood in a newly unifying light. The presence of EGFR amplification or activating mutation, PDGF expression and PDGFR amplification, or NF1 mutation can be considered as three alternative mechanisms of activation of the Ras pathway and its downstream effectors.
This line of thought raises many interesting questions. For example, are the alternative ways of activating the Ras pathway equally effective in generating glioma? Are these differential Ras activations occurring in the same susceptible cell of origin or instead do they represent distinct cellular origins and perhaps diseases? The more recently developed GEMMs described in this article represent refined tools that should help to provide answers to these and other critical questions, and also serve as substrates for therapeutic development.
The first NF1-based glioma mouse models were reported by Jacks and colleagues (Reilly et al. 2000). Mice harboring heterozygous loss of function mutations in cis for the NF1 and p53 tumor suppressor genes developed glioma spontaneously – the NF1 and p53 genes reside on the same human and mouse chromosomes (Cichowski et al. 1999; Vogel et al. 1999). One limitation of this mouse model was the incomplete and variable penetrance of tumor appearance. Incomplete penetrance makes pre-tumorigenic analysis virtually impossible, complicates preclinical trial interpretability, and adds considerable expense to mouse colony maintenance. However, a distinct strength of this mouse model was the discovery of differential tumor incidence depending on mouse strain genetic background. Some mouse strains harboring the cis NF1 and p53 mutations gave rise to tumors rarely while others had substantially increased tumor incidence (Reilly et al. 2000). These mice have been used in genetic mapping studies to identify modifier loci whose function impinges on the capacity of NF1 and p53 loss to generate gliomas (Reilly et al. 2004).
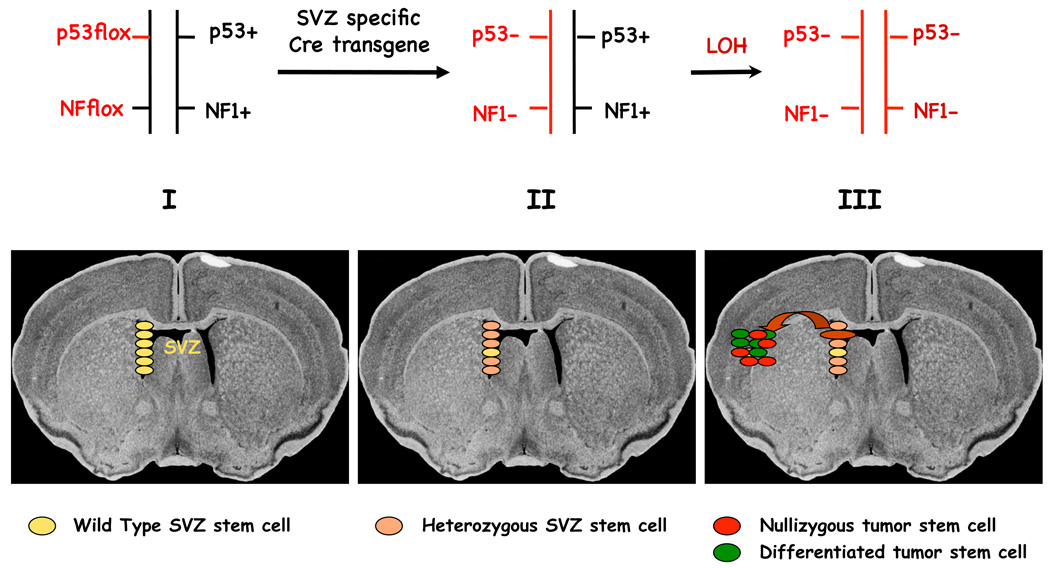
A second generation of NF1-based glioma models relies on the use of conditional (Cre/LoxP) tumor suppressor alleles coupled with Cre recombinase expressing transgenes that can specify the cell types in which tumor suppressor gene inactivation is induced. An added feature of these models was the inclusion of germline conditional or null mutations in only one allele for each tumor suppressor (NF1 and p53). Upon expression of the Cre recombinase, the resultant mice were rendered heterozygous for both tumor suppressor genes in the specified tissue types so that loss of the second allele and thus eradication of gene function (LOH) could only happen spontaneously. A Cre transgene that was demonstrated to be expressed in embryonic telencephalic progenitors as well as in adult neural stem cells (human GFAP promoter (Zhu et al. 2005; Zhuo et al. 2001)) was crossed to mice with combinations of null conditional and/or null NF1 and p53 alleles, resulting in a fully penetrant somatic model of glioma. Cells isolated from these tumors were shown to have undergone LOH for both the NF1 and p53 tumor suppressor genes, while non-tumor cells from brains of the same mice retained wild type NF1 and p53 alleles (Zhu et al. 2005). These mice developed secondary glioma with manifest pathologic evidence of Grade II, Grade III and Grade IV tumors, indicating that mutation in these two tumor suppressor genes was sufficient to generate malignant glioma (Fig. 2). In addition, genetic manipulations that are only possible in mouse models were added to this initial model, providing evidence that the sequence of tumor suppressor loss was critical. Tumors developed only when complete loss of p53 either preceded or coincided with that of NF1 loss (Wang et al. 2009b; Zhu et al. 2005). When NF1 loss preceded p53 loss, no tumors were observed. These results provided novel information about the importance of the sequence of mutational events in tumor formation.
Figure 2.
Neural Precursor Ablation of NF1 and p53 elicits gliomas in a somatic mouse model with 100% penetrance.
I. Lower panel: Subventricular zone (SVZ) stem/progenitor cells. Upper panel: Germ line genetic configuration of the NF1 and P53 tumor suppressor genes. The floxed alleles are equivalent to wild type but can be somatically mutated by Cre recombinase.
II. Lower panel: SVZ-specific Cre transgene induces recombination of floxed NF1 and p53 alleles, rendering most (beige), but not all (yellow) SVZ-derived cells heterozygous. Upper panel: Somatic genetic configuration of the two tumor suppressor genes following Cre recombination.
III. Lower panel: Stochastic loss of heterozygosity (LOH) renders rare cell(s) nullizygous for NF1 and p53 (red). Nullizygous cells migrate from the stem cell niche and seed tumors in the parenchyma. The cancer stem cells in the tumor are invasive but can also differentiate into non-tumorigenic cells (green). Upper panel: Genetic configuration of the tumor suppressor genes in the tumor (cancer stem cells and tumor-derived cells).
The complete penetrance of spontaneous tumor formation in these mouse models also permitted prospective histopathological analysis of pre-tumorigenic brains. These studies revealed that the first early abnormalities seen in the brains of the mice genetically destined to develop glioma were present in the subventricular zone, which is the major stem/progenitor niche of adult brains. Additional studies using pulse chase experiments with DNA synthesis-labeling agents such as bromodeoxyuridine (BrdU) supported this observation and permitted visualization of stem-like cells migrating away from the stem cell niche into the parenchyma (Kwon et al. 2008). Finally, these NF1-based GEMMs were used to test the contribution of Pten tumor suppressor loss. Pten mutations are prevalent in human high-grade gliomas, but have not been observed in preceding low-grade gliomas. It was found that inclusion of one heterozygous conditional Pten allele in the context of NF1 and p53 loss resulted in de novo high-grade tumors. These results provided experimental evidence for the idea that Pten mutation in human glioma may be a causal mutation in transition from lower-grade to high-grade tumors and in the appearance of primary glioma (Kwon et al. 2008).
GEMMs are proving to be an invaluable tool in investigating one of the important gaps in the knowledge in the glioma field - the natural history of the development and progression of gliomas. The availability of inducible Cre recombinase transgenes that allow both temporal and spatial induction of Cre recombinase affords a powerful new tool to ask such questions. For example, the use of a neural-specific nestin promoter/enhancer to drive a Cre recombinase-estrogen receptor fusion protein has been demonstrated to rely on the presence of the estrogen analog tamoxifen for activation specifically in the neural progenitor niches of the CNS (Chen et al. 2009). Activating Cre recombinase specifically in the stem/progenitor cells of the CNS has provided evidence that loss of NF1 and p53 in these cells is sufficient to initiate glioma development. Conversely, viral administration of Cre recombinase in adult brain to non-stem/progenitors induced local tumor suppressor loss and reactive astrogliosis with no emergence of tumors (Alcantara Llaguno et al. 2009). Such data are most consistent with the idea that, in these NF1-based models that use mutations frequently observed in human glioma, tumors arise from stem cells and not from more differentiated cells.
The experiments described above have centered on NF1 loss as the mutation responsible for activation of the Ras pathway in these tumors. Returning to earlier questions, it will be of interest to replace the NF1 mutation with PDGFR- or EGFR-activating mutations to experimentally examine whether these mutations have distinct clinical outcomes and whether the same or distinct cells in the brain are susceptible to form gliomas when sustaining each mutation. Thus, as described above, it is possible that only NF1-based gliomas need arise in the stem cell compartment, while activation of PDGFR or EGFR may support tumor development in more differentiated cell types.
The question of the cancer stem cell and its role in glioma can also be addressed with GEMMs of this type since tumorigenically inert reporter genes can be included in the genetic mix. The presence of reporter genes permits cell lineage tracing as well as the unbiased isolation of distinct tumor cell populations for analysis. Such studies should add considerable insight into the cancer stem cell hypothesis.
Mouse models of EGFR-driven gliomagenesis
The epidermal growth factor receptor (EGFR) is a proto-oncogene that is frequently amplified in a variety of cancers. It is one of the major oncogenic drivers of high-grade gliomas, where it has been shown to be overexpressed and mutated in over 50% of tumors (Frederick et al. 2000) (TCGA 2008; Verhaak et al. 2010). The EGF receptor is a member of the ErbB family of receptor tyrosine kinases that share structural and sequence homology (for a review see (Burgess 2008)). Evidence that members of this family play crucial roles in the development of neoplastic transformation come from their high percentage of mutations in various cancers. In addition to EGFR, ErbB2 (or Her2/neu) has been shown to be mutated in many neoplasms, with the most common being breast cancer.
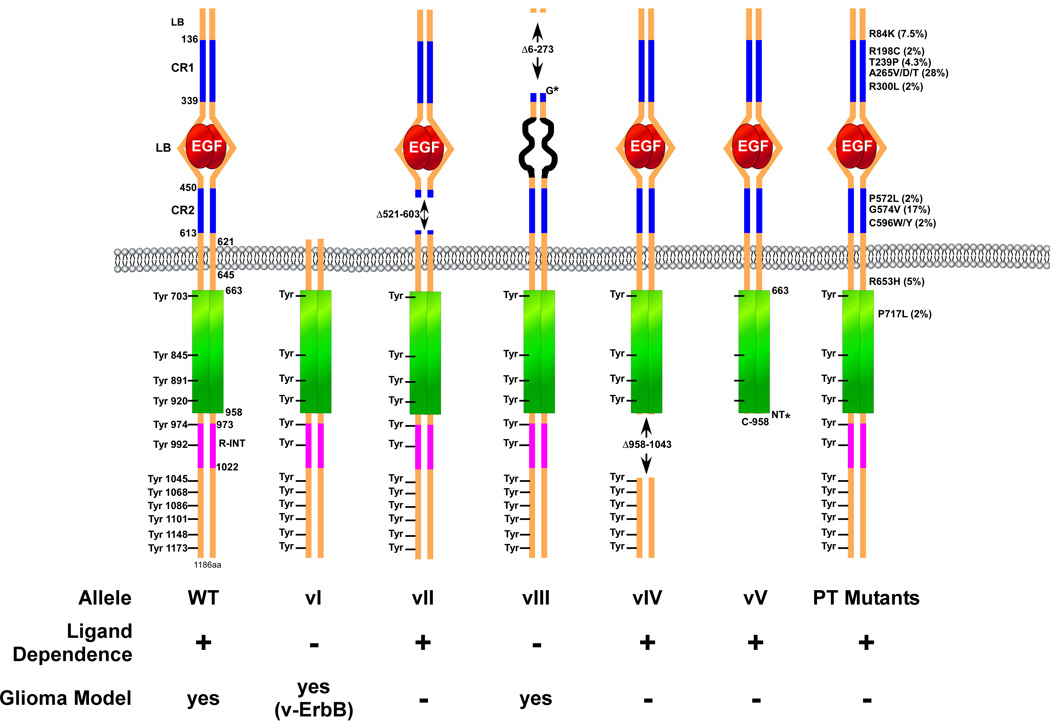
The mechanisms by which EGFR garners oncogenic characteristics are diverse. They include the establishment of autocrine and paracrine growth factor loops (reviewed in (Hatanpaa et al. 2010; Huang et al. 2009; Normanno et al. 2006; Salomon et al. 1995), overexpression and/or amplification of the EGFR gene, and deletions and mutations that cause the receptor to become independent of ligand and thus constitutively active (Frederick et al. 2000). Five different versions of deletion mutations in EGFR (vI to vV) have been reported (Fig. 3). EGFRvI is characterized by the deletion of the entire extracellular domain, which resembles the v-erbB oncoprotein and renders the protein constitutively active (Hayman and Enrietto 1991). The EGFRvII mutation consists of an extracellular domain deletion of 83 amino acids in the second cysteine rich region of the receptor. Interestingly, this mutation does not appear to confer oncogenic potential to the receptor (Mitsudomi and Yatabe 2009). In ~50% of malignant glioma tumors with an EGFR gene amplification event, an intragenic deletion encompassing exons 2 to 7 of the gene locus is observed. This event forms an in frame fusion of exons 1 and 8, which gives rise to a truncation deletion mutant of the receptor referred to as EGFRvIII. The EGFRvIII allele is the most common rearrangement of EGFR in malignant gliomas and has been demonstrated to encode a ligand-independent and constitutively active receptor, making it a potent oncogene. Recently, it was shown that EGFRvIII and wild type EGFR cooperate to promote glioma growth (Inda et al. 2010). A recent report demonstrates that EGFR vIV, which is a receptor that lacks internal segments distal to the intracellular tyrosine kinase domain, is also a potent oncogene (Pines et al. 2010). In addition, a carboxy-terminal end truncation mutant of EGFR (EGFRvV) has been observed in GBM. Interestingly, EGFRvV mutant receptors are found in tumors that carry the EGFRvIII allele (Frederick et al. 2000). Finally, a recent massive sequencing effort of the receptor from GBM tumors has uncovered several oncogenic missense point mutations in the extracellular domain of the receptor that in some cases have been shown to confer oncogenic potential (Lee et al. 2006b). With this many mutant alleles of EGFR, it remains to be determined if individual receptors contain more than one mutation. It is likely that these mutant EGFRs utilize signaling pathways that are sufficiently different from one another to affect responses to therapeutic receptor inhibition.
Figure 3.
Schematic representation of the most common EGFR mutant alleles observed in cancer and gliomas. The amino acid numbering represented is for the EGFR mature protein without the 24-residue signal peptide. The 12 known intracellular tyrosine phosphorylation sites are indicated. LB, ligand binding domain; CR1/2, cysteine-rich domain. Only those point mutants found at a frequency of >2% in gliomas are indicated and can be found on the COSMIC website (http://www.sanger.ac.uk/genetics/CGP/cosmic/).
The efforts of TCGA have resulted in a wealth of new information on GBMs. The Classical subtype of GBM tumors is overwhelmingly defined as overexpressing EGFR with concomitant loss of the Cdkn2a locus and loss of chromosome 10 (Verhaak et al. 2010). Although very informative, the TCGA data remains somewhat static and descriptive. For instance, the functionality of individual events cannot be determined solely from the genomic data. A major challenge now is to assign cancer relevance to the numerous GBM-associated genetic alterations in relevant and accurate model systems.
EGFR-Driven GEMMS of Malignant Glioma
The first mouse model of cancer using an oncogenic ErbB family member was the HER2/Neu oncogene expressed under the control of the MMTV promoter (Bouchard et al. 1989; Muller et al. 1988). This transgenic line developed mammary carcinoma and demonstrated full penetrance, relatively short latency and metastatic potential. The use of these transgenic animals and subsequently several more sophisticated derivatives thereof, has enlightened us on many critical aspects of mammary carcinogenesis biology.
The first reported glioma transgenic model using EGFR was created to explore the pathogenesis of oligodendroglioma (Weiss et al. 2003). Transgenic mice were designed to express the v-erbB oncogene under the control of the S100b promoter. The goal was to express an activated, transforming homologue of EGFR in oligodendroglia and astrocytes during early brain development. S100b is also expressed in CNS stem cells and the authors hypothesized that the S100b-v-erbB transgene would recapitulate the high levels of EGFR expression observed during the development of glioma.
In animals harboring the S100b-v-erbB transgene, 20% of the founder mice developed tumors. The resulting tumors had easily detectable levels of V-erbB protein, which was shown to be active by western blot analysis using an anti-phosphotyrosine antibody. The regional distribution of the transgene expression is consistent with that of the S100b gene. Histopathologically, the tumors shared numerous features that are characteristic of human low-grade oligodendroglioma, although in less than 10% of cases, the tumors resembled astrocytomas (Weiss et al. 2003). The authors concluded that the low proportion of astrocytomas in these mice might be due to the fact that overexpression of EGFR is an early event in oligodendroglioma formation, whereas it is a late event in astrocytoma genesis. Alternatively, this observation may also be due to the low transformability of the cells from which astrocytomas arise, as opposed to the oligodendroglia which can be more readily transformed by V-erbB. The tumor latency, penetrance and histopathological grade of this model were greatly enhanced by incorporating the loss of the tumor suppressor genes p53 or cdkn2a (a locus coding for p16Ink4a/p19ARF gene products) to the S100b-v-erbB transgene, suggesting that both EGFR oncogenicity and increasing tumor grade are facilitated by the loss of critical cell cycle-related TSG. Interestingly, loss of Rb1 had little effect on the penetrance, latency or grade of the tumors formed. This is probably due to the fact that in mice, compensatory mechanisms exist between the Rb family members (p107 and p130) in tumorigenesis. The incomplete penetrance and variable latency of this model suggest that additional genetic events contribute to the development of tumors. To address this issue, the authors performed comparative genome hybridization (CGH) on S100b-v-erbB transgene oligodendroglial tumors and demonstrated gain and loss of specific chromosomal regions that are orthologous with copy number alterations observed in human oligodendrogliomas, including deletions of chromosome 1p36. Given the preponderance of 1p/19q deletions in human oligodendrogliomas (Reifenberger and Louis 2003), this model is well suited for the discovery of novel genes related to oligodendroglial tumor development using genomic strategies.
Using an identical strategy, an S100b-v-erbB transgenic rat model of malignant glioma has recently been described (Ohgaki et al. 2006; Yokoo et al. 2008). Similar to its mouse counterpart, the rat model develops low grade and malignant anaplastic oligodendroglioma. Perhaps a striking difference between these two seemingly identical models is consistency in the area of tumor development in the rat model where over 80% of tumors were localized near or within the cerebellum (Ohgaki et al. 2006).
The S100b-v-erbB;p53 transgenic model was recently utilized to demonstrate the presence of so-called cancer stem cells in tumorspheres grown from these gliomas. The authors relied on the isolation and analysis of side population cells, a cellular phenotype common to stem cells and amenable to flow cytometry sorting, to demonstrate the presence of a population of glioma cells with higher tumorigenic potential (Harris et al. 2008).
The S100b-v-erbB transgenic model clearly demonstrated that expressing an activated allele of EGFR in an appropriate cell compartment drives tumorigenesis. Surprisingly, overexpression of either wild type EGFR or EGFRvIII variant under the glial fibrillary acidic protein (GFAP) promoter is incapable of driving tumorigenesis (Ding et al. 2003). This observation was also made using the RCAS/TVA retroviral system described earlier where EGFRvIII overexpression in either neuroglial precursors (Nestin positive cells) or astrocytic cells (GFAP positive cells) did not yield gliomas with high efficiency (Holland et al. 1998). This is a surprising result given the oncogenic power of EGFRvIII. In light of the S100b-v-erbB transgenic described above, it appears that the oncogenic power of EGFR is dependent upon the cell compartment of expression, and perhaps also on the context of additional genomic events
Co-expression of EGFRvIII with an activated allele of Ha-rasv12 resulted in a switch in histopathology of the resulting gliomas from a mixed astroglioma in GFAP-Ha-rasv12 mice to an oligodendroglioma in GFAP-EGFRvIII;GFAP-Ha-rasv12 compound transgenic mice (Ding et al. 2003). This result suggests that the aberrant expression of activated EGFR in progenitor cells provided a permissive context for oligodendroglioma formation by inappropriate EGFR signaling, thus facilitating the development of oligodendroglioma. Pulsed somatic expression of EGFRvIII by adenoviral delivery in the same activated Ha-rasv12 model led to an accelerated astrocytic glioma formation rather than a switch in histopathological phenotype (Wei et al. 2006). Based on these observations, the authors proposed that inappropriate mitogenic growth factor signaling conferred by EGFRvIII overexpression results in phenotypic changes in astroglial cell differentiation, which translates into a switch in histopathological tumor type. Using these models, it would be interesting to study the differences in signaling pathway utilization between the germline and somatic EGFRvIII expression, given the drastic change in phenotypic outcome.
The adenoviral delivery of EGFRvIII in the above-mentioned model served as a transient expression system. In human gliomas, EGFRvIII expression is sustained and represents an early event in tumorigenesis. To model this observation, a conditional transgenic model based on somatic induction of EGFRvIII expression in adult animals has recently been described (Zhu et al. 2009). A minigene that is composed of a strong ubiquitous promoter (known as CAG) followed by a floxed stop cassette and the cDNA coding for the human EGFRvIII receptor was knocked into the 3’UTR of the collagen 1a1 gene. Insertion of foreign sequences within this locus has been demonstrated to leave the collagen 1a1 expression unperturbed and allows for efficient single integration transgenesis (Beard et al. 2006). Thus, in this system, the expression of EGFRvIII is conditional to the activity of Cre recombinase. Expression of EGFRvIII in adult animals is triggered by stereotactic injection of an adenovirus expressing Cre recombinase. The authors have reported that expression of EGFRvIII alone is insufficient to promote cell transformation in vivo and requires a concomitant loss of the tumor suppressor locus cdkn2a and/or PTEN (Zhu et al. 2009). The gliomas arising from this model have characteristics of GBM, are fully penetrant and display a relatively short latency. Interestingly, the authors also noticed that overexpression of wild type EGFR to levels identical to those observed in human GBMs is inefficient at forming tumors under the same conditions. This result highlights the concept that physiologically relevant overexpression of wild type EGFR is not, in and of itself, an oncogenic event. There appears to be a threshold of EGFR expression above which receptor auto-activation occurs. This level of expression is rarely seen in tumor samples, which begs the question: by what other mechanism(s) do tumor cells utilize wild type EGFR? It appears that for non-self activating levels of EGFR expression, the receptor is dependent on the presence of EGFR ligand(s), often observed in tumor cells as autocrine or paracrine loops. Perhaps this is the reason why modeling malignant gliomas in the mouse using wild type EGFR as a driver of tumorigenesis remains an elusive endeavor.
The genetically tractable model system described by Zhu and colleagues offers opportunities to study signaling events downstream of EGFRvIII in an in vivo environment. This mouse model also provides a means by which resistance to therapeutic intervention can be systematically addressed in a whole animal system. Signaling proteomics profiling from tumors derived from this model will allow for the identification of key nodal signaling members that are mediators of tumor survival and resistance to therapeutic intervention in a context that closely represents the reality of the human condition.
Future directions
We have made considerable progress towards a better understanding of the molecular characteristics of human gliomas. Over the years, we created various culture systems and mouse models to study this disease. The knowledge gained from each step was successfully applied to the next step of improvement where we have now generated mouse models that closely resemble each subtype of human glioma. We also learned to appreciate the role that the microenvironment and tumor heterogeneity plays in several aspects of glioma biology. Despite this progress, there are several areas that will require further attention if we are to achieve clinically relevant advancements. For instance, these models must be used to design more efficient and effective clinical trials. The simultaneous use of these preclinical models capable of recapitulating the three main GBM subtypes (Proneural-PDGF, Classical-EGFR and Mesenchymal-NF1) for each drug tested would help predict responses in the different human subgroups.
Finally, these models will play a critical role in the study of glioma recurrence. Most patients in the clinic succumb from recurrent disease. Tumors that emerge from therapeutic treatment are known to be substantially different than the original tumor. The systems described above are beautiful models of the initiation and maintenance of gliomas, but do not address the changes that occur to tumor cells when confronted with therapeutic agents. Recurrence is currently not modeled well in mice. Recurrent disease could be achieved by using standard clinical therapies on existing models to study initial response and recurrent dissemination of glioma. Novel therapeutic agents and strategies targeted specifically to recurrent tumors can then be tested on accurate mouse models of recurrence. These studies will require considerable investments in time, funds and energy and can benefit greatly from collaborative efforts. The cancer mouse modeling community needs to establish standard operating procedures for treatment protocols somewhat similar to those for human trials, so that multi-center studies can become feasible. Only then will the full value of modeling cancer in genetically engineered mice be achieved.
Acknowledgements
LFP would like to thank Renee McKay for assistance in preparation of this manuscript. LFP is a recipient of funding from the NCI (R01 CA131313), Goldhirsh Foundation, McDonnell Foundation (JSMF-220020206), and CPRIT (RP 100782), and is an ACS Research Professor. AC is a recipient of funding from the NCI (U01 CA141556), ACS Research Scholar Grant (117409) and from the Goldhirsh Foundation. DH is supported by Grant #IRG-91-022-15 from the American Cancer Society and Seed Money from Cleveland Clinic Foundation.
References
- Alcantara Llaguno S, Chen J, Kwon CH, Jackson EL, Li Y, Burns DK, Alvarez-Buylla A, Parada LF. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell. 2009;15(1):45–56. doi: 10.1016/j.ccr.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, Abbott BJ, Mayo JG, Shoemaker RH, Boyd MR. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988;48(3):589–601. [PubMed] [Google Scholar]
- Amankulor NM, Hambardzumyan D, Pyonteck SM, Becher OJ, Joyce JA, Holland EC. Sonic hedgehog pathway activation is induced by acute brain injury and regulated by injury-related inflammation. J Neurosci. 2009;29(33):10299–10308. doi: 10.1523/JNEUROSCI.2500-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachoo RM, Maher EA, Ligon KL, Sharpless NE, Chan SS, You MJ, Tang Y, DeFrances J, Stover E, Weissleder R, et al. Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 2002;1(3):269–277. doi: 10.1016/s1535-6108(02)00046-6. [DOI] [PubMed] [Google Scholar]
- Beard C, Hochedlinger K, Plath K, Wutz A, Jaenisch R. Efficient method to generate single-copy transgenic mice by site-specific integration in embryonic stem cells. Genesis. 2006;44(1):23–28. doi: 10.1002/gene.20180. [DOI] [PubMed] [Google Scholar]
- Becher OJ, Hambardzumyan D, Walker TR, Helmy K, Nazarian J, Albrecht S, Hiner RL, Gall S, Huse JT, Jabado N, et al. Preclinical evaluation of radiation and perifosine in a genetically and histologically accurate model of brainstem glioma. Cancer Res. 2010;70(6):2548–2557. doi: 10.1158/0008-5472.CAN-09-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedermann KA, Sun JR, Giaccia AJ, Tosto LM, Brown JM. scid mutation in mice confers hypersensitivity to ionizing radiation and a deficiency in DNA double-strand break repair. Proc Natl Acad Sci U S A. 1991;88(4):1394–1397. doi: 10.1073/pnas.88.4.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleau AM, Hambardzumyan D, Ozawa T, Fomchenko EI, Huse JT, Brennan CW, Holland EC. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell. 2009;4(3):226–235. doi: 10.1016/j.stem.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogler O, Wren D, Barnett SC, Land H, Noble M. Cooperation between two growth factors promotes extended self-renewal and inhibits differentiation of oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells. Proc Natl Acad Sci U S A. 1990;87(16):6368–6372. doi: 10.1073/pnas.87.16.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard L, Lamarre L, Tremblay PJ, Jolicoeur P. Stochastic appearance of mammary tumors in transgenic mice carrying the MMTV/c-neu oncogene. Cell. 1989;57(6):931–936. doi: 10.1016/0092-8674(89)90331-0. [DOI] [PubMed] [Google Scholar]
- Brennan C, Momota H, Hambardzumyan D, Ozawa T, Tandon A, Pedraza A, Holland E. Glioblastoma subclasses can be defined by activity among signal transduction pathways and associated genomic alterations. PLoS One. 2009;4(11):e7752. doi: 10.1371/journal.pone.0007752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster RL, Chen HY, Messing A, van Dyke T, Levine AJ, Palmiter RD. Transgenic mice harboring SV40 T-antigen genes develop characteristic brain tumors. Cell. 1984;37(2):367–379. doi: 10.1016/0092-8674(84)90367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess AW. EGFR family: structure physiology signalling and therapeutic targets. Growth Factors. 2008;26(5):263–274. doi: 10.1080/08977190802312844. [DOI] [PubMed] [Google Scholar]
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11(1):69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Calver AR, Hall AC, Yu WP, Walsh FS, Heath JK, Betsholtz C, Richardson WD. Oligodendrocyte population dynamics and the role of PDGF in vivo. Neuron. 1998;20(5):869–882. doi: 10.1016/s0896-6273(00)80469-9. [DOI] [PubMed] [Google Scholar]
- Calzolari F, Malatesta P. Recent insights into PDGF-Induced gliomagenesis. Brain Pathol. 2010;20(3):527–538. doi: 10.1111/j.1750-3639.2009.00335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles N, Ozawa T, Squatrito M, Bleau AM, Brennan CW, Hambardzumyan D, Holland EC. Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell. 2010;6(2):141–152. doi: 10.1016/j.stem.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Kwon CH, Lin L, Li Y, Parada LF. Inducible site-specific recombination in neural stem/progenitor cells. Genesis. 2009;47(2):122–131. doi: 10.1002/dvg.20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichowski K, Shih TS, Schmitt E, Santiago S, Reilly K, McLaughlin ME, Bronson RT, Jacks T. Mouse models of tumor development in neurofibromatosis type 1. Science. 1999;286(5447):2172–2176. doi: 10.1126/science.286.5447.2172. [DOI] [PubMed] [Google Scholar]
- Claesson-Welsh L. Platelet-derived growth factor receptor signals. J Biol Chem. 1994;269(51):32023–32026. [PubMed] [Google Scholar]
- Dai C, Celestino JC, Okada Y, Louis DN, Fuller GN, Holland EC. PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev. 2001;15(15):1913–1925. doi: 10.1101/gad.903001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danks RA, Orian JM, Gonzales MF, Tan SS, Alexander B, Mikoshiba K, Kaye AH. Transformation of astrocytes in transgenic mice expressing SV40 T antigen under the transcriptional control of the glial fibrillary acidic protein promoter. Cancer Res. 1995;55(19):4302–4310. [PubMed] [Google Scholar]
- Deinhardt F. Biology of primate retroviruses. In: Klein G, editor. Viral Oncology. New York: Raven Press; 1980. pp. 357–398. [Google Scholar]
- Di Rocco F, Carroll RS, Zhang J, Black PM. Platelet-derived growth factor and its receptor expression in human oligodendrogliomas. Neurosurgery. 1998;42(2):341–346. doi: 10.1097/00006123-199802000-00080. [DOI] [PubMed] [Google Scholar]
- Ding H, Roncari L, Shannon P, Wu X, Lau N, Karaskova J, Gutmann DH, Squire JA, Nagy A, Guha A. Astrocyte-specific expression of activated p21-ras results in malignant astrocytoma formation in a transgenic mouse model of human gliomas. Cancer Res. 2001;61(9):3826–3836. [PubMed] [Google Scholar]
- Ding H, Shannon P, Lau N, Wu X, Roncari L, Baldwin RL, Takebayashi H, Nagy A, Gutmann DH, Guha A. Oligodendrogliomas result from the expression of an activated mutant epidermal growth factor receptor in a RAS transgenic mouse astrocytoma model. Cancer Res. 2003;63(5):1106–1113. [PubMed] [Google Scholar]
- Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, Sougnez C, Greulich H, Muzny DM, Morgan MB. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455(7216):1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirks P. Bmi1 and cell of origin determinants of brain tumor phenotype. Cancer Cell. 2007;12(4):295–297. doi: 10.1016/j.ccr.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Dirks PB. Cancer: stem cells and brain tumours. Nature. 2006;444(7120):687–688. doi: 10.1038/444687a. [DOI] [PubMed] [Google Scholar]
- Doolittle RF, Hunkapiller MW, Hood LE, Devare SG, Robbins KC, Aaronson SA, Antoniades HN. Simian sarcoma virus onc gene, v-sis, is derived from the gene (or genes) encoding a platelet-derived growth factor. Science. 1983;221(4607):275–277. doi: 10.1126/science.6304883. [DOI] [PubMed] [Google Scholar]
- Federspiel MJ, Bates P, Young JA, Varmus HE, Hughes SH. A system for tissue-specific gene targeting: transgenic mice susceptible to subgroup A avian leukosis virus-based retroviral vectors. Proc Natl Acad Sci U S A. 1994;91(23):11241–11245. doi: 10.1073/pnas.91.23.11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher GH, Orsulic S, Holland E, Hively WP, Li Y, Lewis BC, Williams BO, Varmus HE. Development of a flexible and specific gene delivery system for production of murine tumor models. Oncogene. 1999;18(38):5253–5260. doi: 10.1038/sj.onc.1203087. [DOI] [PubMed] [Google Scholar]
- Frederick L, Wang XY, Eley G, James CD. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res. 2000;60(5):1383–1387. [PubMed] [Google Scholar]
- Frost EE, Zhou Z, Krasnesky K, Armstrong RC. Initiation of oligodendrocyte progenitor cell migration by a PDGF-A activated extracellular regulated kinase (ERK) signaling pathway. Neurochem Res. 2009;34(1):169–181. doi: 10.1007/s11064-008-9748-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson DG, Duff ME, West JW, Kelly JD, Sheppard PO, Hofstrand PD, Gao Z, Shoemaker K, Bukowski TR, Moore M, et al. Platelet-derived growth factor C (PDGF-C), a novel growth factor that binds to PDGF alpha and beta receptor. J Biol Chem. 2001;276(29):27406–27414. doi: 10.1074/jbc.M101056200. [DOI] [PubMed] [Google Scholar]
- Goyal M, Robinson S. Expanding the role of surgery in intractable extratemporal pediatric epilepsy. Neurosurg Focus. 2008;25(3):E15. doi: 10.3171/FOC/2008/25/9/E15. [DOI] [PubMed] [Google Scholar]
- Greenhouse JJ, Petropoulos CJ, Crittenden LB, Hughes SH. Helper-independent retrovirus vectors with Rous-associated virus type O long terminal repeats. J Virol. 1988;62(12):4809–4812. doi: 10.1128/jvi.62.12.4809-4812.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha A. Ras activation in astrocytomas and neurofibromas. Can J Neurol Sci. 1998;25(4):267–281. doi: 10.1017/s0317167100034272. [DOI] [PubMed] [Google Scholar]
- Guha A, Dashner K, Black PM, Wagner JA, Stiles CD. Expression of PDGF and PDGF receptors in human astrocytoma operation specimens supports the existence of an autocrine loop. Int J Cancer. 1995;60(2):168–173. doi: 10.1002/ijc.2910600206. [DOI] [PubMed] [Google Scholar]
- Gutmann DH, James CD, Poyhonen M, Louis DN, Ferner R, Guha A, Hariharan S, Viskochil D, Perry A. Molecular analysis of astrocytomas presenting after age 10 in individuals with NF1. Neurology. 2003;61(10):1397–1400. doi: 10.1212/wnl.61.10.1397. [DOI] [PubMed] [Google Scholar]
- Gutmann DH, Rasmussen SA, Wolkenstein P, MacCollin MM, Guha A, Inskip PD, North KN, Poyhonen M, Birch PH, Friedman JM. Gliomas presenting after age 10 in individuals with neurofibromatosis type 1 (NF1) Neurology. 2002;59(5):759–761. doi: 10.1212/wnl.59.5.759. [DOI] [PubMed] [Google Scholar]
- Hambardzumyan D, Amankulor NM, Helmy KY, Becher OJ, Holland EC. Modeling Adult Gliomas Using RCAS/t-va Technology. Transl Oncol. 2009;2(2):89–95. doi: 10.1593/tlo.09100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambardzumyan D, Squatrito M, Carbajal E, Holland EC. Glioma formation, cancer stem cells, and akt signaling. Stem Cell Rev. 2008;4(3):203–210. doi: 10.1007/s12015-008-9021-5. [DOI] [PubMed] [Google Scholar]
- Harris MA, Yang H, Low BE, Mukherjee J, Guha A, Bronson RT, Shultz LD, Israel MA, Yun K. Cancer stem cells are enriched in the side population cells in a mouse model of glioma. Cancer Res. 2008;68(24):10051–10059. doi: 10.1158/0008-5472.CAN-08-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanpaa KJ, Burma S, Zhao D, Habib AA. Epidermal growth factor receptor in glioma: signal transduction, neuropathology, imaging, and radioresistance. Neoplasia. 2010;12(9):675–684. doi: 10.1593/neo.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman MJ, Enrietto PJ. Cell transformation by the epidermal growth factor receptor and v-erbB. Cancer Cells. 1991;3(8):302–307. [PubMed] [Google Scholar]
- Hede SM, Hansson I, Afink GB, Eriksson A, Nazarenko I, Andrae J, Genove G, Westermark B, Nister M. GFAP promoter driven transgenic expression of PDGFB in the mouse brain leads to glioblastoma in a Trp53 null background. Glia. 2009;57(11):1143–1153. doi: 10.1002/glia.20837. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Wasteson A, Westermark B. Platelet-derived growth factor. Mol Cell Endocrinol. 1985;39(3):169–187. doi: 10.1016/0303-7207(85)90061-9. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Westermark B. Platelet-derived growth factor: mechanism of action and possible in vivo function. Cell Regul. 1990;1(8):555–566. doi: 10.1091/mbc.1.8.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson JW, Schnitker BL, Correa KM, von Deimling A, Fassbender F, Xu HJ, Benedict WF, Yandell DW, Louis DN. The retinoblastoma gene is involved in malignant progression of astrocytomas. Ann Neurol. 1994;36(5):714–721. doi: 10.1002/ana.410360505. [DOI] [PubMed] [Google Scholar]
- Hesselager G, Holland EC. Using mice to decipher the molecular genetics of brain tumors. Neurosurgery. 2003;53(3):685–694. doi: 10.1227/01.neu.0000081304.57547.b5. discussion 695. [DOI] [PubMed] [Google Scholar]
- Hitoshi Y, Harris BT, Liu H, Popko B, Israel MA. Spinal glioma: platelet-derived growth factor B-mediated oncogenesis in the spinal cord. Cancer Res. 2008;68(20):8507–8515. doi: 10.1158/0008-5472.CAN-08-1063. [DOI] [PubMed] [Google Scholar]
- Holland EC, Celestino J, Dai C, Schaefer L, Sawaya RE, Fuller GN. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat Genet. 2000;25(1):55–57. doi: 10.1038/75596. [DOI] [PubMed] [Google Scholar]
- Holland EC, Hively WP, DePinho RA, Varmus HE. A constitutively active epidermal growth factor receptor cooperates with disruption of G1 cell-cycle arrest pathways to induce glioma-like lesions in mice. Genes Dev. 1998;12(23):3675–3685. doi: 10.1101/gad.12.23.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland EC, Varmus HE. Basic fibroblast growth factor induces cell migration and proliferation after glia-specific gene transfer in mice. Proc Natl Acad Sci U S A. 1998;95(3):1218–1223. doi: 10.1073/pnas.95.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Pandolfi PP, Li Y, Koutcher JA, Rosenblum M, Holland EC. mTOR promotes survival and astrocytic characteristics induced by Pten/AKT signaling in glioblastoma. Neoplasia. 2005;7(4):356–368. doi: 10.1593/neo.04595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PH, Xu AM, White FM. Oncogenic EGFR signaling networks in glioma. Sci Signal. 2009;2(87):re6. doi: 10.1126/scisignal.287re6. [DOI] [PubMed] [Google Scholar]
- Huse JT, Holland EC. Targeting brain cancer: advances in the molecular pathology of malignant glioma and medulloblastoma. Nat Rev Cancer. 2010;10(5):319–331. doi: 10.1038/nrc2818. [DOI] [PubMed] [Google Scholar]
- Inda MM, Bonavia R, Mukasa A, Narita Y, Sah DW, Vandenberg S, Brennan C, Johns TG, Bachoo R, Hadwiger P, et al. Tumor heterogeneity is an active process maintained by a mutant EGFR-induced cytokine circuit in glioblastoma. Genes Dev. 2010;24(16):1731–1745. doi: 10.1101/gad.1890510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson FK, Brodd J, Eklof C, Ferletta M, Hesselager G, Tiger CF, Uhrbom L, Westermark B. Identification of candidate cancer-causing genes in mouse brain tumors by retroviral tagging. Proc Natl Acad Sci U S A. 2004;101(31):11334–11337. doi: 10.1073/pnas.0402716101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson FK, Goransson H, Westermark B. Expression analysis of genes involved in brain tumor progression driven by retroviral insertional mutagenesis in mice. Oncogene. 2005;24(24):3896–3905. doi: 10.1038/sj.onc.1208553. [DOI] [PubMed] [Google Scholar]
- Kernohan JW, Mabon RF, et al. A simplified classification of the gliomas. Mayo Clin Proc. 1949;24(3):71–75. [PubMed] [Google Scholar]
- Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87(2):159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- Kleihues P, Soylemezoglu F, Schauble B, Scheithauer BW, Burger PC. Histopathology, classification, and grading of gliomas. Glia. 1995;15(3):211–221. doi: 10.1002/glia.440150303. [DOI] [PubMed] [Google Scholar]
- Kwon CH, Zhao D, Chen J, Alcantara S, Li Y, Burns DK, Mason RP, Lee EY, Wu H, Parada LF. Pten haploinsufficiency accelerates formation of high-grade astrocytomas. Cancer Res. 2008;68(9):3286–3294. doi: 10.1158/0008-5472.CAN-07-6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRochelle WJ, Jeffers M, McDonald WF, Chillakuru RA, Giese NA, Lokker NA, Sullivan C, Boldog FL, Yang M, Vernet C, et al. PDGF-D, a new protease-activated growth factor. Nat Cell Biol. 2001;3(5):517–521. doi: 10.1038/35074593. [DOI] [PubMed] [Google Scholar]
- Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006a;9(5):391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Lee JC, Vivanco I, Beroukhim R, Huang JH, Feng WL, DeBiasi RM, Yoshimoto K, King JC, Nghiemphu P, Yuza Y, et al. Epidermal growth factor receptor activation in glioblastoma through novel missense mutations in the extracellular domain. PLoS Med. 2006b;3(12):e485. doi: 10.1371/journal.pmed.0030485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg N, Kastemar M, Olofsson T, Smits A, Uhrbom L. Oligodendrocyte progenitor cells can act as cell of origin for experimental glioma. Oncogene. 2009;28(23):2266–2275. doi: 10.1038/onc.2009.76. [DOI] [PubMed] [Google Scholar]
- Macleod KF, Jacks T. Insights into cancer from transgenic mouse models. J Pathol. 1999;187(1):43–60. doi: 10.1002/(SICI)1096-9896(199901)187:1<43::AID-PATH246>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- McConville P, Hambardzumyan D, Moody JB, Leopold WR, Kreger AR, Woolliscroft MJ, Rehemtulla A, Ross BD, Holland EC. Magnetic resonance imaging determination of tumor grade and early response to temozolomide in a genetically engineered mouse model of glioma. Clin Cancer Res. 2007;13(10):2897–2904. doi: 10.1158/1078-0432.CCR-06-3058. [DOI] [PubMed] [Google Scholar]
- Mitsudomi T, Yatabe Y. Epidermal growth factor receptor in relation to tumor development: EGFR gene and cancer. FEBS J. 2009;277(2):301–308. doi: 10.1111/j.1742-4658.2009.07448.x. [DOI] [PubMed] [Google Scholar]
- Momota H, Nerio E, Holland EC. Perifosine inhibits multiple signaling pathways in glial progenitors and cooperates with temozolomide to arrest cell proliferation in gliomas in vivo. Cancer Res. 2005;65(16):7429–7435. doi: 10.1158/0008-5472.CAN-05-1042. [DOI] [PubMed] [Google Scholar]
- Muller WJ, Sinn E, Pattengale PK, Wallace R, Leder P. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell. 1988;54(1):105–115. doi: 10.1016/0092-8674(88)90184-5. [DOI] [PubMed] [Google Scholar]
- Nister M, Libermann TA, Betsholtz C, Pettersson M, Claesson-Welsh L, Heldin CH, Schlessinger J, Westermark B. Expression of messenger RNAs for platelet-derived growth factor and transforming growth factor-alpha and their receptors in human malignant glioma cell lines. Cancer Res. 1988;48(14):3910–3918. [PubMed] [Google Scholar]
- Noble M, Murray K, Stroobant P, Waterfield MD, Riddle P. Platelet-derived growth factor promotes division and motility and inhibits premature differentiation of the oligodendrocyte/type-2 astrocyte progenitor cell. Nature. 1988;333(6173):560–562. doi: 10.1038/333560a0. [DOI] [PubMed] [Google Scholar]
- Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F, Salomon DS. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366(1):2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Ohgaki H, Kita D, Favereaux A, Huang H, Homma T, Dessen P, Weiss WA, Kleihues P, Heppner FL. Brain tumors in S100beta-v-erbB transgenic rats. J Neuropathol Exp Neurol. 2006;65(12):1111–1117. doi: 10.1097/01.jnen.0000248544.28423.48. [DOI] [PubMed] [Google Scholar]
- Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, Misra A, Nigro JM, Colman H, Soroceanu L, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Pines G, Huang PH, Zwang Y, White FM, Yarden Y. EGFRvIV: a previously uncharacterized oncogenic mutant reveals a kinase autoinhibitory mechanism. Oncogene. 2010;29(43):5850–5860. doi: 10.1038/onc.2010.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack IF, Hamilton RL, James CD, Finkelstein SD, Burnham J, Yates AJ, Holmes EJ, Zhou T, Finlay JL. Rarity of PTEN deletions and EGFR amplification in malignant gliomas of childhood: results from the Children's Cancer Group 945 cohort. J Neurosurg. 2006;105(Suppl)(5):418–424. doi: 10.3171/ped.2006.105.5.418. [DOI] [PubMed] [Google Scholar]
- Reifenberger G, Louis DN. Oligodendroglioma: toward molecular definitions in diagnostic neuro-oncology. J Neuropathol Exp Neurol. 2003;62(2):111–126. doi: 10.1093/jnen/62.2.111. [DOI] [PubMed] [Google Scholar]
- Reilly KM, Loisel DA, Bronson RT, McLaughlin ME, Jacks T. Nf1;Trp53 mutant mice develop glioblastoma with evidence of strain-specific effects. Nat Genet. 2000;26(1):109–113. doi: 10.1038/79075. [DOI] [PubMed] [Google Scholar]
- Reilly KM, Tuskan RG, Christy E, Loisel DA, Ledger J, Bronson RT, Smith CD, Tsang S, Munroe DJ, Jacks T. Susceptibility to astrocytoma in mice mutant for Nf1 and Trp53 is linked to chromosome 11 and subject to epigenetic effects. Proc Natl Acad Sci U S A. 2004;101(35):13008–13013. doi: 10.1073/pnas.0401236101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringertz N. Grading of gliomas. Acta Pathol Microbiol Scand. 1950;27(1):51–64. [PubMed] [Google Scholar]
- Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19(3):183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- Sangha N, Wu R, Kuick R, Powers S, Mu D, Fiander D, Yuen K, Katabuchi H, Tashiro H, Fearon ER, et al. Neurofibromin 1 (NF1) defects are common in human ovarian serous carcinomas and co-occur with TP53 mutations. Neoplasia. 2008;10(12):1362–1372. doi: 10.1593/neo.08784. following 1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon P, Sabha N, Lau N, Kamnasaran D, Gutmann DH, Guha A. Pathological and molecular progression of astrocytomas in a GFAP:12 V-Ha-Ras mouse astrocytoma model. Am J Pathol. 2005;167(3):859–867. doi: 10.1016/S0002-9440(10)62057-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3(3):289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih AH, Holland EC. Platelet-derived growth factor (PDGF) and glial tumorigenesis. Cancer Lett. 2006;232(2):139–147. doi: 10.1016/j.canlet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Shoshan Y, Nishiyama A, Chang A, Mork S, Barnett GH, Cowell JK, Trapp BD, Staugaitis SM. Expression of oligodendrocyte progenitor cell antigens by gliomas: implications for the histogenesis of brain tumors. Proc Natl Acad Sci U S A. 1999;96(18):10361–10366. doi: 10.1073/pnas.96.18.10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Clarke ID, Hide T, Dirks PB. Cancer stem cells in nervous system tumors. Oncogene. 2004a;23(43):7267–7273. doi: 10.1038/sj.onc.1207946. [DOI] [PubMed] [Google Scholar]
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821–5828. [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004b;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Singhal S, Birch JM, Kerr B, Lashford L, Evans DG. Neurofibromatosis type 1 and sporadic optic gliomas. Arch Dis Child. 2002;87(1):65–70. doi: 10.1136/adc.87.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge JE, Singh RK, Fidler IJ, Raz A. Murine models to evaluate novel and conventional therapeutic strategies for cancer. Am J Pathol. 2007;170(3):793–804. doi: 10.2353/ajpath.2007.060929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3(3):279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TCGA. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchougounova E, Kastemar M, Brasater D, Holland EC, Westermark B, Uhrbom L. Loss of Arf causes tumor progression of PDGFB-induced oligodendroglioma. Oncogene. 2007;26(43):6289–6296. doi: 10.1038/sj.onc.1210455. [DOI] [PubMed] [Google Scholar]
- Trovo-Marqui AB, Tajara EH. Neurofibromin: a general outlook. Clin Genet. 2006;70(1):1–13. doi: 10.1111/j.1399-0004.2006.00639.x. [DOI] [PubMed] [Google Scholar]
- Ueki K, Ono Y, Henson JW, Efird JT, von Deimling A, Louis DN. CDKN2/p16 or RB alterations occur in the majority of glioblastomas and are inversely correlated. Cancer Res. 1996;56(1):150–153. [PubMed] [Google Scholar]
- Uhrbom L, Dai C, Celestino JC, Rosenblum MK, Fuller GN, Holland EC. Ink4a-Arf loss cooperates with KRas activation in astrocytes and neural progenitors to generate glioblastomas of various morphologies depending on activated Akt. Cancer Res. 2002;62(19):5551–5558. [PubMed] [Google Scholar]
- Uhrbom L, Hesselager G, Nister M, Westermark B. Induction of brain tumors in mice using a recombinant platelet-derived growth factor B-chain retrovirus. Cancer Res. 1998;58(23):5275–5279. [PubMed] [Google Scholar]
- Uhrbom L, Hesselager G, Ostman A, Nister M, Westermark B. Dependence of autocrine growth factor stimulation in platelet-derived growth factor-B-induced mouse brain tumor cells. Int J Cancer. 2000;85(3):398–406. doi: 10.1002/(sici)1097-0215(20000201)85:3<398::aid-ijc17>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Uhrbom L, Kastemar M, Johansson FK, Westermark B, Holland EC. Cell type-specific tumor suppression by Ink4a and Arf in Kras-induced mouse gliomagenesis. Cancer Res. 2005;65(6):2065–2069. doi: 10.1158/0008-5472.CAN-04-3588. [DOI] [PubMed] [Google Scholar]
- Uhrbom L, Nerio E, Holland EC. Dissecting tumor maintenance requirements using bioluminescence imaging of cell proliferation in a mouse glioma model. Nat Med. 2004;10(11):1257–1260. doi: 10.1038/nm1120. [DOI] [PubMed] [Google Scholar]
- van Heyningen P, Calver AR, Richardson WD. Control of progenitor cell number by mitogen supply and demand. Curr Biol. 2001;11(4):232–241. doi: 10.1016/s0960-9822(01)00075-6. [DOI] [PubMed] [Google Scholar]
- Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel KS, Klesse LJ, Velasco-Miguel S, Meyers K, Rushing EJ, Parada LF. Mouse tumor model for neurofibromatosis type 1. Science. 1999;286(5447):2176–2179. doi: 10.1126/science.286.5447.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskoglou-Nomikos T, Pater JL, Seymour L. Clinical predictive value of the in vitro cell line, human xenograft, and mouse allograft preclinical cancer models. Clin Cancer Res. 2003;9(11):4227–4239. [PubMed] [Google Scholar]
- Wang J, Miletic H, Sakariassen PO, Huszthy PC, Jacobsen H, Brekka N, Li X, Zhao P, Mork S, Chekenya M, et al. A reproducible brain tumour model established from human glioblastoma biopsies. BMC Cancer. 2009a;9:465. doi: 10.1186/1471-2407-9-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Yang J, Zheng H, Tomasek GJ, Zhang P, McKeever PE, Lee EY, Zhu Y. Expression of mutant p53 proteins implicates a lineage relationship between neural stem cells and malignant astrocytic glioma in a murine model. Cancer Cell. 2009b;15(6):514–526. doi: 10.1016/j.ccr.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q, Clarke L, Scheidenhelm DK, Qian B, Tong A, Sabha N, Karim Z, Bock NA, Reti R, Swoboda R, et al. High-grade glioma formation results from postnatal pten loss or mutant epidermal growth factor receptor expression in a transgenic mouse glioma model. Cancer Res. 2006;66(15):7429–7437. doi: 10.1158/0008-5472.CAN-06-0712. [DOI] [PubMed] [Google Scholar]
- Weiss WA, Burns MJ, Hackett C, Aldape K, Hill JR, Kuriyama H, Kuriyama N, Milshteyn N, Roberts T, Wendland MF, et al. Genetic determinants of malignancy in a mouse model for oligodendroglioma. Cancer Res. 2003;63(7):1589–1595. [PubMed] [Google Scholar]
- Weissenberger J, Steinbach JP, Malin G, Spada S, Rulicke T, Aguzzi A. Development and malignant progression of astrocytomas in GFAP-v-src transgenic mice. Oncogene. 1997;14(17):2005–2013. doi: 10.1038/sj.onc.1201168. [DOI] [PubMed] [Google Scholar]
- Xiao A, Wu H, Pandolfi PP, Louis DN, Van Dyke T. Astrocyte inactivation of the pRb pathway predisposes mice to malignant astrocytoma development that is accelerated by PTEN mutation. Cancer Cell. 2002;1(2):157–168. doi: 10.1016/s1535-6108(02)00029-6. [DOI] [PubMed] [Google Scholar]
- Xiao A, Yin C, Yang C, Di Cristofano A, Pandolfi PP, Van Dyke T. Somatic induction of Pten loss in a preclinical astrocytoma model reveals major roles in disease progression and avenues for target discovery and validation. Cancer Res. 2005;65(12):5172–5180. doi: 10.1158/0008-5472.CAN-04-3902. [DOI] [PubMed] [Google Scholar]
- Yokoo H, Tanaka Y, Nobusawa S, Nakazato Y, Ohgaki H. Immunohistochemical and ultrastructural characterization of brain tumors in S100beta-v-erbB transgenic rats. Neuropathology. 2008;28(6):591–598. doi: 10.1111/j.1440-1789.2008.00923.x. [DOI] [PubMed] [Google Scholar]
- Zhu H, Acquaviva J, Ramachandran P, Boskovitz A, Woolfenden S, Pfannl R, Bronson RT, Chen JW, Weissleder R, Housman DE, et al. Oncogenic EGFR signaling cooperates with loss of tumor suppressor gene functions in gliomagenesis. Proc Natl Acad Sci U S A. 2009;106(8):2712–2716. doi: 10.1073/pnas.0813314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Guignard F, Zhao D, Liu L, Burns DK, Mason RP, Messing A, Parada LF. Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces malignant astrocytoma. Cancer Cell. 2005;8(2):119–130. doi: 10.1016/j.ccr.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo L, Theis M, Alvarez-Maya I, Brenner M, Willecke K, Messing A. hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis. 2001;31(2):85–94. doi: 10.1002/gene.10008. [DOI] [PubMed] [Google Scholar]