Abstract
Research on emotional perception and learning indicates appetitive cues engage nucleus accumbens (NAc) and medial prefrontal cortex (mPFC), whereas amygdala activity is modulated by the emotional intensity of appetitive and aversive cues. This study sought to determine patterns of functional activation and connectivity among these regions during narrative emotional imagery. Using event‐related fMRI, we investigate activation of these structures when participants vividly imagine pleasant, neutral, and unpleasant scenes. Results indicate that pleasant imagery selectively activates NAc and mPFC, whereas amygdala activation was enhanced during both pleasant and unpleasant imagery. NAc and mPFC activity were each correlated with the rated pleasure of the imagined scenes, while amygdala activity was correlated with rated emotional arousal. Functional connectivity of NAc and mPFC was evident throughout imagery, regardless of hedonic content, while correlated activation of the amygdala with NAc and mPFC was specific to imagining pleasant scenes. These findings provide strong evidence that pleasurable text‐driven imagery engages a core appetitive circuit, including NAc, mPFC, and the amygdala. Hum Brain Mapp, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: liking, mental imagery, mesocorticolimbic, script‐driven imagery, psychophysiological interaction, ventral striatum, ventral medial prefrontal cortex
INTRODUCTION
Reading an exciting novel or listening to an engaging story can prompt vivid imagery of the described events and reported feelings of intense emotion. Research has repeatedly confirmed this phenomenon and demonstrated that text‐driven imagery prompts heightened autonomic and somatic reactions consistent with affective engagement [Cuthbert et al., 2003; Lang and McTeague, 2009; McTeague et al., 2009; Orr et al., 1993; Vrana et al., 1986; Vrana and Lang, 1990; Witvliet and Vrana, 1995]. Perhaps because the evocation of vivid fear imagery has been an important method in the psychological treatment of phobias and anxiety [Lang, 1977; Wolpe, 1958], clinical studies that utilize narrative imagery for symptom provocation [Cuthbert et al., 2003; Lang and McTeague, 2009; McTeague et al., 2009; Orr et al., 1993] have mainly assessed reactivity during aversive imagery. This emphasis also characterizes related neuroimaging research [Britton et al., 2005; Dougherty et al., 1999; Shin et al., 2000; Sinha et al., 2004] that consistently reports increased activation in the brain's aversive system [Lang and Davis, 2006], including the amygdala and related paralimbic regions.
While research on emotional perception and learning clearly indicates that the amygdala encodes the emotional intensity of both appetitive and aversive stimuli [Baxter and Murray, 2002; Davis and Whalen, 2001; McGaugh, 2004; Zald, 2003], there is debate as to whether NAc activation is driven by the pleasantness of a stimulus or its salience [Berridge et al., 2009]. Evidence from studies of pleasant visual perception [Aharon et al., 2001; Sabatinelli et al., 2007] and reward processing [Breiter et al., 2001; Cooper and Knutson, 2007; Knutson et al., 2005] indicates that the nucleus accumbens (NAc) and medial prefrontal cortex (mPFC) are selectively activated for pleasurable stimuli that are readily discerned. On the other hand, based on studies using a wide variety of populations, paradigms, and procedures, there is also evidence that NAc activity increases in response to both pain and pleasure [Leknes and Tracey, 2008]. This raises the question of whether mesolimbic activation during narrative emotional imagery will reflect the hedonic valence or the salience (emotional arousal) of the imagined scenes.
Consistent with the view that imagery and perception of emotional stimuli engage similar motivational circuitry [Lang, 1979, 1994], narrative imagery of drug craving heightened regional cerebral blood flow (rCBF) in NAc and amygdala in cocaine‐dependent men and women [Kilts et al., 2001, 2004], and imagery of pleasant sexual encounters/athletic success increased rCBF in ventral pallidum (an efferent target of NAc) in men [Rauch et al., 1999]. Yet because prior studies have not examined NAc activation during both pleasant and unpleasant imagery, it remains plausible that unpleasant imagery could also prompt increased activation of NAc, consistent with a salience account. The novel aim of this study is to determine, using a well‐founded imagery procedure [Cuthbert et al., 2003; Lang and McTeague, 2009; McTeague et al., 2009], if imagining prototypical pleasant scenes uniquely activates mesolimbic reward circuitry in healthy males and females, whether amygdala activation is found when imagining both pleasant and unpleasant scenes, and how functional connectivity among these three regions varies with hedonic content.
It is hypothesized that relative to neutral and unpleasant scenes, imagining pleasurable scenes will selectively activate NAc and mPFC, whereas compared to neutral imagery, processing arousing emotional content—pleasant or unpleasant—will heighten amygdala activation. We also examined the relationship between signal change in these regions and participants' ratings of pleasure and emotional arousal for the imagined scenes. Based on prior findings during emotional perception [Anderson et al., 2003; Cloutier et al., 2008; Knutson et al., 2005; Sabatinelli et al., 2005, 2007], pleasure ratings are expected to correlate positively with signal change in NAc and mPFC, while emotional arousal ratings should correlate positively with amygdala activity.
NAc and mPFC heavily innervate one another [Ongur and Price, 2000] and each region receives amygdala afferents [Friedman et al., 2002]. If these anatomical connections support a circuit underlying appetitive processing, functional connectivity among these structures is expected to vary with hedonic content. Predicated on the hypothesized sensitivity of NAc and mPFC to pleasure, temporal covariation of mesolimbic activation is anticipated during pleasant imagery and possibly overall. Because of the amygdala's sensitivity to emotional arousal, its functional connectivity with structures specifically sensitive to pleasure will reflect appetitive arousal. This suggests that functional connectivity of the amygdala with NAc or mPFC or both will occur only when imagining pleasant contents.
MATERIALS AND METHODS
Participants
Thirty‐two students (16 women, mean age = 19.1, SD = 1.5) from introductory psychology courses participated for course credit or for $20. Participants were right‐handed, had normal visual acuity, and reported no history of claustrophobia, psychopathology, or neurological insult in a telephone interview. Informed consent was obtained as stipulated by the institutional review board. Three participants were excluded due to excessive head motion or technical errors, resulting in a final sample of 29 participants.
Materials and Design
Forty‐two narratives were selected from the Affective Norms of English Text [ANET; Bradley and Lang, 2007; see Supporting Information Table S2 for a full listing] based on standardized ratings of pleasure and arousal and were categorized as pleasant (e.g., winning the lottery; n = 12), neutral (e.g., reading the newspaper; n = 6), or unpleasant (e.g., a car accident; n = 24).1 Prior to scanning, participants briefly read and imagined each scene and rated their experience of pleasure and arousal using graphic Self‐Assessment Manikin scales [Bradley and Lang, 1994; Lang, 1980]. Texts were written to be read in 12 s and their presentation counterbalanced so that no more than two trials of the same hedonic content occurred in succession. Multivariate comparison of sentence properties indicated that emotional and neutral sentences did not differ in word, syllable, or character counts [F(6,74) = 1.95, ns].
The imagery procedure consisted of 42 trials. Each trial began with visual presentation of a text for 12 s, backward projected onto a monitor (640 × 480 pixel resolution) situated behind the participant's head, and viewed using a head‐coil mounted mirror (IFIS‐SA, Invivo, Orlando, FL). The visual offset of the text signaled participants to continue imagining the scene they had just read. Twelve seconds later, a brief (400 ms) change in the color of the screen, perceptible as a flash, signaled that imagery should end and was followed by a fixed 12‐s intertrial interval. After the experiment, participants rated the overall difficulty of imagining the presented scenes (1 = very difficult to 9 = not difficult at all). On average participants reported little difficulty in completing the imagery task (mean difficulty rating = 6.78, SD = 1.13).
Procedure
After entering the scanner, participants were instructed to silently read each text as it appeared and to continue to imagine their active involvement in the described event, until a brief change in the color of the screen alerted the participant to stop imagining. Except during text presentations, participants were told to maintain their gaze on a fixation cross presented at the center of the monitor.
Data Acquisition and Analysis
A T1‐weighted anatomical volume was acquired using a Siemens 3T Allegra MR scanner. A total of 506 functional volumes (50 coronal slices, 2.5 mm thick, 0.5 mm gap) were collected using a T2*‐weighted echo planar imaging sequence (3 s TR, 35 ms TE, 160 mm FOV, 64 × 64 acquisition matrix).
Functional data were slice‐time adjusted, motion‐corrected, spatially smoothed (5‐mm FWHM Gaussian kernel), and converted to percent blood oxygen level‐dependent (BOLD) signal change using the Analysis of Functional Neuroimages software [Cox, 1996]. A multiple linear regression (MLM) model deconvolved hemodynamic responses for the three hedonic contents as a linear combination of eight uniform B‐spline basis functions. This generated a time series of beta coefficients at each voxel equaling the length of a single trial (24 s), beginning with text presentation and extending through the imagery period. Additional regressors modeled motion residuals and baseline drift. Resultant time series were spatially normalized [Talariach and Tournoux, 1988] and resampled to a 2.5 mm isotropic voxel size.
Using participants' average percent BOLD signal change during imagery following text presentation, three mixed effects ANOVAs identified voxels with greater activation during pleasant compared to neutral imagery, unpleasant compared to neutral imagery, and pleasant compared to unpleasant imagery. Nonparametric permutation tests were computed following recommended guidelines [Nichols and Holmes, 2002] to determine critical t‐statistic values for each contrast that corrected for multiple comparisons across the volume. In each permutation test, labels coding hedonic content were randomly reassigned within participants and checked for independence from previous permutation orders. A t‐statistic was then generated at each voxel and a Gaussian function fit to their distribution over the entire brain. The value of the t‐statistic at the 99.9 percentile of the fitted Gaussian distribution was selected to form a permutation distribution based on 10,000 randomizations. The resulting absolute thresholds that corrected for multiple comparisons at P < 0.05 were t = 3.71 (P < 0.0008 uncorrected) when contrasting pleasant to neutral imagery, t = 4.07 (P < 0.0003 uncorrected) when contrasting unpleasant to neutral imagery, and t = 4.2 (P < 0.0002 uncorrected) when contrasting pleasant to unpleasant imagery.
Functional regions of interest (ROI) for NAc and mPFC were based on cluster locations identified in the group contrast of pleasant compared to neutral imagery and for the amygdala based on the conjoined group contrasts of pleasant and unpleasant compared to neutral imagery. Average BOLD activity was extracted for each ROI from a 109 μl volume within the area of interest and surrounding a significant peak activation (P < 0.05 uncorrected) in each participants' spatially normalized contrast maps. Assuming that imagery begins as a text is read, we analyzed BOLD activity in an early window, during the 12‐s text presentation, and at a later 12‐s window when text was no longer on the screen. Mean BOLD signal change for each window was deviated from a baseline immediately prior to text presentation and then exported for ANOVA analyses. These included time period and hedonic content as within‐participant factors and gender as a between‐participant factor. Sphericity violations and all pairwise comparisons were controlled using the Greenhouse‐Geisser and Bonferroni corrections, respectively.
To examine correlations of NAc, mPFC, and amygdala activity with pleasure and arousal ratings, the imagined texts were ranked from low (1) to high (42) according to their rated pleasantness or arousal, for each participant. Mean group pleasure ratings were used to resolve ties when texts were rated as equally pleasant, and mean group arousal ratings used when texts were rated as equally arousing. Mean BOLD signal change in the postpresentation time window in NAc, mPFC, and amygdala at each rank was correlated with ranked pleasure or arousal ratings to test predictions regarding regional sensitivities to rated pleasure or emotional arousal. Correlations were similarly computed for each participant and categorized as either exceeding significance (r > 0.26, df = 42, one‐tailed P < 0.05) or not.
Functional connectivity analyses [Friston et al., 1997] assessed temporal correlations of NAc, mPFC, and amygdala BOLD activity when imagining emotional and neutral scenes. For each participant, BOLD time series extracted from each ROI were mean detrended and segmented to include activity in the post‐presentation time window, where effects of hedonic content were maximal. To represent the time‐varying interaction of BOLD activity and each imagined scenes' hedonic content, these time series were weighted by an equal length vector coding a contrast of pleasant versus unpleasant imagery. Iteratively specifying NAc, mPFC, or amygdala as the target ROI, the actual and weighted BOLD time series of the remaining two seed ROIs were entered as regressors in a MLM model predicting target ROI activity. This yielded two sets of correlation coefficients that described overall and valence‐dependent functional connectivity of the target and seed regions. Signed correlation coefficients for each participant were transformed to z statistics using Fisher's z‐transformation and analyzed at the group level using a one‐sample t‐test to determine if average correlation values differed from zero. Coactivation was further assessed, by averaging over participants' baseline‐deviated BOLD activity in NAc, mPFC, and amygdala for each text at each of the four time points following text presentations. Where indicated, correlation coefficients were then separately evaluated by hedonic content and compared to determine if they significantly differed from one another.
RESULTS
Imagery Ratings
Table I lists mean pleasure and emotional arousal ratings when imagining each scene. Pleasure ratings indicated that compared to neutral scenes, pleasant scenes were rated as more pleasant, while unpleasant scenes were rated as more unpleasant [F(2,58) = 372, P < 0.0001, η2 = 0.93]. Both pleasant and unpleasant scenes were rated as more emotionally arousing than neutral scenes [F(2,58) = 106, P < 0.0001, η2 = 0.79] and did not differ in rated arousal.
Table I.
Mean pleasure and emotional arousal ratings for the imagined texts
| Hedonic content | Pleasure | Arousal |
|---|---|---|
| Pleasant | 8.25 (0.55) | 7.24 (1.20) |
| Neutral | 6.75 (1.03) | 3.23 (1.37) |
| Unpleasant | 2.78 (0.79) | 6.67 (1.07) |
Values in parentheses indicate standard deviations.
Volume Analyses
A set of volume analyses, corrected for multiple comparisons at P < 0.05, identified regions activated during either pleasant or unpleasant imagery compared to neutral imagery and during pleasant compared to unpleasant imagery (Table II; see Supporting Information Table S1 for a complete list of activated regions). As illustrated in Figure 1, greater activation was evident in NAc (Fig. 1A) and mPFC (Fig. 1B) during imagery of pleasant, compared to neutral texts. A contrast of pleasant and unpleasant imagery reinforced activation patterns found when contrasting pleasant and neutral imagery—specifically increased activation of ventral mPFC2 and NAc (Table II). There were no significant differences in activation of NAc or ventral mPFC when comparing unpleasant to neutral imagery.
Table II.
Mesocorticolimbic regions activated for contrasts of emotional imagery
| Region | μla | x | y | z | t b |
|---|---|---|---|---|---|
| Unpleasant > Neutral | |||||
| L Amygdala | 156 | −22 | −6 | −10 | 4.51 |
| R Amygdala | 63 | 30 | −4 | −12 | 4.27 |
| Pleasant > Neutral | |||||
| L Amygdala | 562 | −21 | −2 | −10 | 5.86 |
| R Amygdala | 63 | 18 | −6 | −10 | 4.30 |
| L Medial prefrontal cortex (BA 10) | 1,969 | −6 | 58 | 16 | 4.29 |
| L Nucleus accumbens | 189 | −10 | 8 | −6 | 3.72 |
| R Nucleus accumbens | 78 | 10 | 6 | −8 | 4.16 |
| Pleasant > Unpleasant | |||||
| L Medial prefrontal cortex (BA 10) | 2,047 | 0 | 55 | −2 | 6.26 |
| L Nucleus accumbens | 172 | −7 | 5 | −9 | 5.80 |
L, left; R, right.
Cluster size computed using 2.5‐mm3 voxel.
Significant at P < 0.05 corrected.
Figure 1.

Increased activation of NAc (A) and mPFC (B) was obtained during pleasant, compared to neutral, imagery, whereas amygdala activity (C) was increased during both pleasant and unpleasant, compared to neutral, imagery. Event‐related signal change is plotted for pleasant, neutral, and unpleasant scenes beginning with text presentation and continuing through subsequent imagery in NAc (D), mPFC (E), and the amygdala (F). Talairach coordinates are based on coronal slice selection. Error bars represent 95% confidence intervals [Loftus and Masson, 1994].
Examination of the event‐related time course of group‐averaged BOLD signal change for each region (Fig. 1D,E) indicated an increase in activity for pleasant scenes beginning during text presentation and that was selectively sustained during imagery, whereas during presentation of neutral and unpleasant texts, a signal decrease was obtained that reached a minimum during imagery.
Selective activation of NAc and mPFC for pleasant imagery contrasted with the heightened amygdala activation occurring during imagery of either pleasant or unpleasant scenes, compared to neutral scenes (Fig. 1C represents the conjunction of these contrasts). Increased BOLD activity that was initiated during text presentation was subsequently maintained during imagery of emotional texts, but decreased when imagining neutral scenes (Fig. 1F). There were no significant differences in activation of the amygdala for the contrasts of pleasant and unpleasant imagery.
ROI Analyses
NAc and mPFC
Modulation of NAc activity by hedonic content was significant in the individually sampled analysis of mean BOLD signal change [F(2,54) = 17.24, P < 0.0001, η2 = 0.13]. These effects were consistent across men and women. Pairwise analyses indicated larger signal increases in NAc both during text presentation [t(28) = 3.85, P < 0.005] and subsequent imagery [t(28) = 7.05, P < 0.0001] for pleasant, compared to unpleasant, scenes. When compared to neutral scenes, pleasant scenes elicited heightened NAc activity during text presentation [t(28) = 4.93, P < 0.0001], whereas activity prompted by unpleasant and neutral scenes did not differ at either time [Valence × Time, F(2,54) = 9.93, P < 0.0001, η2 = 0.02].
Mean signal change in mPFC also was significantly modulated by hedonic content [F(2,54) = 26.99, P < 0.0001, η2 = 0.35]. Pleasant scenes evoked greater signal increases in mPFC compared to unpleasant scenes, both during text presentation [t(28) = 5.65, P < 0.0001] and subsequent imagery [t(28) = 8.62, P < 0.0001]. Following text presentation, subsequent imagery of pleasant scenes evoked larger signal increases than neutral scenes [t(28) = 4.74, P < 0.0001], whereas unpleasant, compared to neutral, scenes prompted significantly larger decreases in mPFC both during text presentation [t(28) = 3.17, P < 0.05] and during subsequent imagery [t(28) = 3.18, P < 0.05; Valence × Time, F(2,54) = 11.09, P < 0.0001, η2 = 0.04].
Amygdala
Mean signal change in the amygdala was significantly modulated by emotional arousal [F(2,54) = 30.56, P < 0.0001, η2 = 0.25]. Imagining either pleasant [t(28) = 6.69, P < 0.0001] or unpleasant scenes [t(28) = 5.9, P < 0.0001] resulted in increased signal change, compared to neutral scenes. Heightened amygdala activation for emotional scenes was confined to imagery following text presentation [Valence × Time, F(2,54) = 51.47, P < 0.0001, η2 = 0.22]. There were no differences in amygdala activity between pleasant and unpleasant scenes and no gender effects.
Ratings of Emotional Experience and BOLD
For each participant, scenes were ranked based on pleasure ratings from least to most pleasurable. The proportion of a priori pleasant, neutral, and unpleasant texts comprising each rank is depicted by the pie charts in Figure 2.
Figure 2.
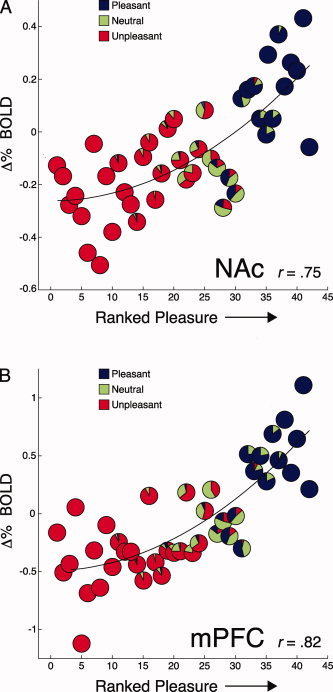
Pleasure ratings correlate with mesolimbic BOLD activity during imagery. Scenes were rank ordered by each participant's pleasure ratings and plotted against the mean signal change in NAc and mPFC at each rank. Pie charts depict the proportion of the a priori selected pleasant, neutral, and unpleasant texts at each rank [based on ANET standardized ratings; Bradley and Lang, 2007].
NAc and mPFC
A positive curvilinear trend strongly related mean BOLD signal change during imagery to ranked pleasure ratings for NAc [r = 0.75, F(2,39) = 25.3, P < 0.0001; Fig. 2A] and mPFC [r = 0.82, F(2,39) = 39.23, P < 0.0001; Fig. 2B].3 Positive curvilinear trends fit NAc activity in 79% of participants and were significant in 48% of the sample, and fit mPFC activity in 83% of participants, reaching significance in 55% of the sample. Pleasure rankings were also linearly related to mean signal change in each region [NAc: r = 0.72, F(1,40) = 43.9, P < 0.001; mPFC: r = 0.76, F(1,40) = 58, P < 0.0001], although a quadratic fit accounted for slightly, but significantly, more variance [NAc: F(1,39) = 3.36, P < 0.05; mPFC: F(1,39) = 9.03, P < 0.005]. When scenes were ranked by rated emotional arousal, there was no significant correlation with signal change in NAc (r = 0.16, ns) or mPFC (r = 0.26, ns).
Amygdala
BOLD signal change in the amygdala during imagery was unrelated to rankings based on pleasure ratings (r = 0.07, ns), but increased linearly when scenes were ranked according to participants' arousal ratings from least to most arousing [r = 0.44, F(1,40) = 8.9, P < 0.005]. A positive linear trend between arousal rankings and increased amygdala activity was evident in 68% of participants and significant in 25% of the sample.
Functional Connectivity
NAc and mPFC
The mean temporal correlation between activation in NAc and mPFC when imagining scenes following text presentation was significant [r = 0.27, t(28) = 8.10, P < 0.0001, η2 = 0.7], indicating their overall functional connectivity. Figure 3A presents the covariation of signal change in NAc and mPFC, averaged across participants for each time point in the imagery interval following text presentation for each scene. Signal change in each region was highly correlated overall (r = 0.71, P < 0.0001). Also shown is the distribution of signed correlation values when this same relationship was assessed in each participant (Fig. 3B). A positive linear correlation between NAc and mPFC activity during imagery was present in 86% of participants and was significant in 76% of the sample. When the covariation of mean BOLD signal change in NAc and mPFC was separately assessed by hedonic content, correlation coefficients when imagining pleasant (r = 0.57; Z = 2.92, P < 0.005) or unpleasant (r = 0.36; Z = 2.04, P < 0.05) scenes were larger than when imagining neutral scenes (r = −0.12), but did not differ from one another (Z = 1.54, ns).
Figure 3.
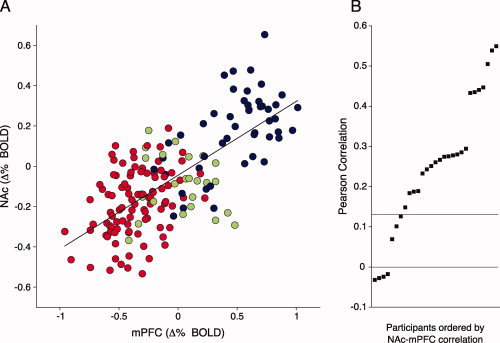
Functional connectivity of NAc and mPFC during imagery. The mean BOLD signal change during imagery in each region, averaged over participants for each of the four time points following text presentation for pleasant (blue), neutral (green), or unpleasant (red) scenes (A). The adjacent panel (B) shows the ordered distribution of the correlation between NAc and mPFC activity for each participant during imagery (r > 0.16. is significant; 0 < r < 0.16 is not significant, and r < 0 are in the unpredicted direction). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Amygdala
Functional connectivity of the amygdala with NAc and mPFC was heightened during pleasant, compared to neutral or unpleasant imagery. A significant temporal correlation across individuals was found in the BOLD time series between amygdala and NAc [t(28) = 2.65, P = 0.01, η2 = 0.19] and between the amygdala and mPFC [t(28) = 2.24, P < 0.05, η2 = 0.15] when the latter time series was linearly weighted by the a priori valence of the imagined scene. Figure 4 illustrates the covariation of the BOLD activity in amygdala with activity in NAc (A) and mPFC (B) during imagery of each scene—for the four time points following text presentation—separately assessed by hedonic content. Correlation of signal change in amygdala and NAc was larger during pleasant imagery (r = 0.51) compared to neutral (r = −0.04; Z = 2.24, P < 0.05) or unpleasant (r = 0.03; Z = 2.86, P < 0.005) imagery. Correlation of signal change in amygdala and mPFC was also larger during pleasant imagery (r = 0.59) compared to neutral (r = −0.01; Z = 2.62, P < 0.01) or unpleasant (r = 0.11; Z = 3.14, P < 0.005) imagery.
Figure 4.
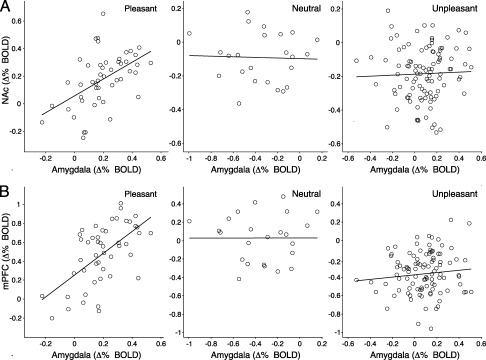
Pleasure‐specific connectivity of the amygdala with NAc and mPFC. Scatter plots depict the correlation of mean BOLD activity during imagery (averaged over participants for each of the four time points following text presentation) in the amygdala with NAc (A) and mPFC (B), separately evaluated for pleasant, neutral, and unpleasant scenes.
DISCUSSION
Narrative Emotional Imagery Engages Motivational Centers
Imagining pleasant events strongly activated NAc and mPFC, compared to imagining unpleasant or neutral scenes. Selective activation of these structures developed over time as participants continued to imagine the pleasant events they had just read. Activation of NAc and mPFC also showed a positive, graded response that correlated highly with pleasure ratings for the imagined texts. These results suggest a central role for NAc and mPFC in processing pleasurable events that is consistent across stimulus media, showing the same pattern of activation during pleasant imagery as previously observed in research assessing human reactions to tangible appetitive cues [Breiter et al., 1997, 2001; Knutson et al., 2005] or when viewing arousing pleasant pictures [Sabatinelli et al., 2007]. Peak activation coordinates reported here for pleasant imagery (NAc: ±10, 7, −7; mPFC: −6, 58, 16) are very close to those we reported previously [Sabatinelli et al., 2007] during pleasant picture perception (NAc: ±10, 9, −2; mPFC: −4, 39, 4), using the same MR hardware. The present results also clarify and expand on earlier findings [Rauch et al., 1999] of increased ventral pallidum activation in men when imagining pleasant sexual encounters and athletic success, as well as findings [Kilts et al., 2001, 2004] of increased NAc activation—although not mPFC activation—during imagery of drug use in substance abusers.
The finding of a pleasure‐specific increase in NAc during narrative imagery contrasts with some studies suggesting that increased NAc activity is driven by stimulus salience, rather than selectively by positive hedonic cues (see review by Leknes and Tracy [ 2008]). The database relating NAc activity to aversive stimulation is quite complex, and the modulation found appears to vary with species (human or animal), specific brain measure (e.g., single unit activity, dopamine release, PET, fMRI, etc.), type of aversive stimulation (pain, odor, taste, picture, image, etc.), and processing paradigm (perception, anticipation, classical conditioning, operant conditioning, etc.). When considering fMRI studies with human participants, however, the data are consistent in indicating that NAc activity does not increase when people view aversive pictures [Meseguer et al., 2007; Phan et al., 2004; Sabatinelli et al., 2007], are exposed to painful thermal stimulation [Becerra and Borsook, 2008; Becerra et al., 2001], or process cues signaling loss [Cooper and Knutson, 2007] or unpleasant odors [Gottfried et al., 2001]. The present study is consistent with these, indicating reward system activation during pleasant emotional imagery in human participants.
In fact, in the current study, BOLD activity during unpleasant imagery prompted marked signal decreases from baseline in both NAc and mPFC. Similar signal decreases to unpleasant content were found in these same structures in our previous study of picture perception [Sabatinelli et al., 2007]. BOLD signal decreases from baseline have also been found in NAc with the onset of painful stimulation [Aharon et al., 2006; Becerra and Borsook, 2008; Becerra et al., 2001]. Furthermore, using PET, decreased rCBF in mPFC was observed when contrasting traumatic and neutral imagery in patients with post‐traumatic stress disorder [Britton et al., 2005; Shin et al., 2004].
Research on the neurophysiology of the BOLD signal and rCBF [Goense and Logothetis, 2008; Logothetis et al., 2001; Mathiesen et al., 1998] indicates that hemodynamic measures generally reflect net excitation or inhibition of cortical microcircuits [Logothetis, 2008]. Deactivation of mesolimbic reward structures during unpleasant perception and imagery might represent inhibition of appetitive circuitry. Significantly greater deactivation of mPFC during unpleasant compared to neutral imagery, along with reports that mPFC deactivation during trauma imagery is correlated negatively with symptom severity in PTSD patients [Shin et al., 2004], further suggests that signal decreases in mPFC may code for aversion. However, whether the extent of NAc deactivation generally indexes aversive processing remains unclear as imagining neutral scenes and viewing neutral pictures [Sabatinelli et al., 2007] also prompt similar decreases.
As anticipated, heightened amygdala activation was found during both pleasant and unpleasant imagery and was positively correlated with emotional arousal ratings for the imagined scenes. This is consistent with similar patterns of amygdala activation found when people view pleasant and unpleasant emotionally arousing external stimuli [Cooper and Knutson, 2007; Sabatinelli et al., 2005, 2007; Zald, 2003], and complements extensive lesion and electrophysiological work in animals demonstrating amygdala involvement in both aversive [Davis and Whalen, 2001] and appetitive learning [Ambroggi et al., 2008; Baxter and Murray, 2002].
Functional Connectivity of an Appetitive Circuit
A strong, positive temporal correlation of BOLD activity in NAc and mPFC was evident during imagery regardless of content, whereas activity in the amygdala was specifically correlated with activity in NAc and mPFC during appetitive processing. Complete functional connectivity of NAc, mPFC, and the amygdala when imagining pleasant scenes is consistent with coactivation of these structures when viewing appetitive pictures [Sabatinelli et al., 2007] or processing monetary rewards [Cooper and Knutson, 2007]; as well as with evidence that intact afferents from both the mPFC [Ishikawa et al., 2008a] and amygdala [Ambroggi et al., 2008] are necessary for NAc neurons to display firing patterns predictive of subsequent appetitive behavior.
Functional coupling of NAc and mPFC signal change was expected given their common sensitivity to pleasure and known neuroanatomical connectivity [Ongur and Price, 2000]. Electrical stimulation of mPFC neurons is reported to evoke firing in NAc neurons [McGinty and Grace, 2009], and pharmacological inactivation of mPFC reduces reward‐seeking behaviors and related cue‐evoked firing of NAc neurons [Ishikawa et al., 2008a]. Another possibility is that linked mesolimbic function during pleasant imagery is attributable to coordinated dopamine release, as systemic injection of dopamine releasing agents increases BOLD activity in both NAc and mPFC [Breiter et al., 1997; Knutson and Gibbs, 2007].
Functional connectivity of the amygdala with NAc and mPFC specific to pleasant imagery fits with the demonstrated sensitivity of these regions to ratings of emotional arousal or pleasure, respectively. Considering that signal change in both NAc and mPFC was unrelated to rated emotional arousal, mesolimbic connectivity with the amygdala during pleasant imagery suggests a pathway for specifically enhancing appetitive motivation. A lack of negative functional connectivity between the amygdala and reward structures during unpleasant imagery further emphasizes the specific involvement of this circuit in appetitive processing.
This view is supported by animal studies in which pharmacological inactivation of the basolateral amygdala following appetitive conditioning dampened cue‐evoked excitation of NAc neurons [Ambroggi et al., 2008] and reduced reward‐seeking behaviors in a dose‐dependent manner [Ishikawa et al., 2008b]. Chemical inactivation of the amygdala's central nucleus following food deprivation also reduced dopamine release in NAc and mPFC during food consumption [Ahn and Phillips, 2002], while opioid stimulation of the central nucleus increased appetitive and consummatory behaviors toward reward cues [Mahler and Berridge, 2009]. Interestingly, electrical stimulation of the amygdala is additionally found to increase neuronal firing in mPFC neurons projecting to NAc [McGinty and Grace, 2008] and coincident stimulation of amygdala and mPFC inputs to NAc increases neuronal firing above mPFC stimulation alone [McGinty and Grace, 2009]. Taken together, the functional connectivity patterns identified here imply that the NAc, mPFC, and the amygdala form an integrative circuit that is engaged when humans directly process pleasurable emotional content.
Limitations and New Directions
Because imagery is an inherently mental event, questions can arise regarding compliance with the task during experimentation. In this study, the palpable changes in BOLD signal in NAc, mPFC, and amygdala at the onset of the instructed imagery period (see Fig. 1) and their strong covariation with emotional ratings of experienced pleasure and arousal during imagery—both within and across participants (see Fig. 2A,B)—suggest compliance with the imagery task. Moreover, prior psychophysiological studies of both anxiety patients and healthy participants, using the same instructions and many of the same texts as in this study, have repeatedly confirmed that text‐driven imagery prompts heightened autonomic and somatic reaction consistent with emotional engagement [e.g., Cuthbert et al., 2003; Lang and McTeague, 2009; McTeague et al., 2009; Vrana et al., 1986]. Finally, participants rated the difficulty of imaging each scene, and most reported that it was an easy task.
Future neuroimaging work should, nevertheless, assess how connectivity of these structures during imagery relates to the appetitive motivational gradients defined by autonomic and somatic reflex indices [Bradley et al., 2001]. Another aim is to determine how depressive psychopathology alters appetitive circuitry. There is considerable evidence that altered connectivity of rostral anterior cingulate (rACC) with the amygdala and prefrontal cortices has a role in mediating symptoms in depressed patients [e.g., Yoshimura et al., in press] and that this circuit can be modified by cognitive therapy and pharmacological interventions [DeRubeis et al., 2008]. Increased activation of rACC during pleasant imagery was not found here with healthy participants, suggesting that the interaction between the cingulate and reward circuit may be specific to depression. Comparative studies with depressed patients during pleasant narrative imagery could clarify this issue and perhaps confirm a mechanism through which mood disorders compromise appetitive neural processing.
SUMMARY
Imagining pleasant, highly appetitive events selectively activated NAc and mPFC, and their activation correlated positively with rated pleasure of the imagined scenes. In contrast, imagery of either pleasant or unpleasant emotional scenes prompted increased amygdala activation, and its activation was positively correlated with rated emotional arousal. Functional connectivity analyses determined, furthermore, that coincident activation of all three regions—amygdala, NAc, and mPFC—occurred only when participants imaged pleasant scenes. Together, these findings provide strong evidence that pleasant and unpleasant imagery engage different motivational circuits—appetitive and aversive—paralleling previous findings from animal and human studies of emotional learning and perception. Moreover, these results suggest that the clinical use of narrative imagery might be usefully extended to mood disorders (e.g., depression and dysthymia) in which appetitive processing may be compromised.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Table 1.
Supporting Table 2.
Footnotes
1The additional 12 unpleasant scenes described specific fear‐relevant scenarios (dental and snake fear) and were included in the design to allow future comparisons with clinical populations. Analyses that excluded these categories yielded the same pattern of results.
2Activation of mPFC for the contrast of pleasant imagery versus unpleasant imagery was decidedly more ventral with respect to the frontal pole—overlapping BA 10/11—than that observed when contrasting pleasant and neutral imagery. It was also distinct from a smaller, dorsal cluster of increased activation in mPFC (BA 8) present when comparing both pleasant and unpleasant to neutral imagery (see Supporting Information Table S2).
3Correlations of similar size between NAc and mPFC activity and pleasure ratings were obtained when using mean pleasure ratings at each rank [NAc: r = 0.71, F(2,39) = 20.66, P < 0.0001; mPFc: r = 0.81, F(2,39) = 39.95, P < 0.0001], or when using mean pleasure ratings for each imagined scene [NAc: r = 0.72, F(2,39) = 21.15, P < 0.0001; mPFc: r = 0.85, F(2,39) = 21.15, P < 0.0001]. Likewise correlation between amygdala activation and arousal ratings were of similar magnitude when using either mean arousal ratings at each rank [r = 0.46, F(1,40) = 12.83, P < 0.001] or mean arousal ratings for each imagined scene [r = 0.46, F(1,40) = 12.21, P < 0.005].
REFERENCES
- Aharon I,Etcoff N,Ariely D,Chabris CF,O'Connor E,Breiter HC ( 2001): Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron 32: 537–551. [DOI] [PubMed] [Google Scholar]
- Aharon I,Becerra L,Chabris CF,Borsook D ( 2006): Noxious heat induces fMRI activation in two anatomically distinct clusters within the nucleus accumbens. Neurosci Lett 392: 159–164. [DOI] [PubMed] [Google Scholar]
- Ahn S,Phillips AG ( 2002): Modulation by central and basolateral amygdalar nuclei of dopaminergic correlates of feeding to satiety in the rat nucleus accumbens and medial prefrontal cortex. J Neurosci 22: 10958–10965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambroggi F,Ishikawa A,Fields HL,Nicola SM ( 2008): Basolateral amygdala neurons facilitate reward‐seeking behavior by exciting nucleus accumbens neurons. Neuron 59: 648–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AK,Christoff K,Stappen I,Panitz D,Ghahremani DG,Glover G,Gabrieli JD,Sobel N ( 2003): Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci 6: 196–202. [DOI] [PubMed] [Google Scholar]
- Baxter MG,Murray EA ( 2002): The amygdala and reward. Nat Rev Neurosci 3: 563–573. [DOI] [PubMed] [Google Scholar]
- Bradley MM,Lang PJ ( 1994): Measuring emotion: The self‐assessment manikin and the semantic differential. J Behav Ther Exp Psychiatry 25: 49–59. [DOI] [PubMed] [Google Scholar]
- Bradley MM,Lang PJ ( 2007): Affective Norms for English Text (ANET): Affective Ratings of Text and Instruction Manual. Technical Report No. D‐1. Gainesville, FL: University of Florida; 32 p. [Google Scholar]
- Bradley MM,Codispoti M,Cuthbert BN,Lang PJ ( 2001): Emotion and motivation I: Defensive and appetitive reactions in picture processing. Emotion 1: 276–298. [PubMed] [Google Scholar]
- Becerra L,Breiter HC,Wise R,Gonzalez RG,Borsook D ( 2001): Reward circuitry activation by noxious thermal stimuli. Neuron 32: 927–946. [DOI] [PubMed] [Google Scholar]
- Becerra L,Borsook D ( 2008): Signal valence in the nucleus accumbens to pain onset and offset. Eur J Pain 12: 866–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC,Robinson TE,Aldridge JW ( 2009): Dissecting components of reward: ‘Liking’, ‘wanting’, and learning. Curr Opin Pharmacol 9: 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC,Gollub RL,Weisskoff RM,Kennedy DN,Makris N,Berke JD,Goodman JM,Kantor HL,Gastfriend DR,Riorden JP,Mathew RT,Rosen BR,Hyman SE ( 1997): Acute effects of cocaine on human brain activity and emotion. Neuron 19: 591–611. [DOI] [PubMed] [Google Scholar]
- Breiter HC,Aharon I,Khaneman D,Dale A,Shizgal P ( 2001): Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron 30: 618–639. [DOI] [PubMed] [Google Scholar]
- Britton JC,Phan KL,Taylor SF,Fig LM,Liberzon I ( 2005): Corticolimbic blood flow in posttraumatic stress disorder during script‐driven imagery. Biol Psychiatry 57: 832–840. [DOI] [PubMed] [Google Scholar]
- Cloutier J,Heatherton TF,Whalen PJ,Kelley WM ( 2008): Are attractive people rewarding? Sex differences in the neural substrates of facial attractiveness. J Cogn Neurosci 20: 941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JC,Knutson B ( 2007): Valence and salience contribute to nucleus accumbens activation. Neuroimage 39: 538–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW ( 1996): AFNI: Software for analysis and visualization of functional magnetic resonance imaging. Comput Biomed Res 29: 162–173. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN,Lang PJ,Strauss C,Drobes D,Patrick CJ,Bradley MM ( 2003): The psychophysiology of anxiety disorder: Fear memory imagery. Psychophysiology 40: 407–422. [DOI] [PubMed] [Google Scholar]
- Davis M,Whalen PJ ( 2001): The amygdala: Vigilance and emotion. Mol Psychiatry 6: 13–34. [DOI] [PubMed] [Google Scholar]
- DeRubeis RJ,Siegle GJ,Hollon SD ( 2008): Cognitive therapy versus medication for depression: Treatment outcomes and neural mechanisms. Nat Rev Neurosci 9: 788–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DD,Shin LM,Alpert NM,Pitman RK,Orr SP,Lasko M,Macklin ML,Fischman AJ,Rauch SL ( 1999): Anger in healthy men: A PET study using script‐driven imagery. Biol Psychiatry 46: 466–472. [DOI] [PubMed] [Google Scholar]
- Friedman DP,Aggleton JP,Saunders RC ( 2002): Comparison of hippocampal, amygdala, and perirhinal projections to the nucleus accumbens: Combined anterograde and retrograde tracing study in the macaque brain. J Comp Neurol 450: 345–365. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Buechel C,Fink GR,Morris J,Rolls E,Dolan RJ ( 1997): Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6: 218–229. [DOI] [PubMed] [Google Scholar]
- Goense JB,Logothetis NK ( 2008): Neurophysiology of the BOLD fMRI signal in awake monkeys. Curr Biol 18: 631–640. [DOI] [PubMed] [Google Scholar]
- Gottfried JA,O'Doherty J,Dolan RD ( 2001): Appetitive and aversive olfactory learning in humans studied using event‐related functional magnetic resonance imaging. J Neurosci Sci 22: 10829–10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa A,Ambroggi F,Nicola SM,Fields HL ( 2008a): Dorsomedial prefrontal cortex contribution to behavioral and nucleus accumbens neuronal responses to incentive cues. J Neurosci 28: 5088–5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa A,Ambroggi F,Nicola SM,Fields HL ( 2008b): Contributions of the amygdala and medial prefrontal cortex to incentive cue responding. Neuroscience 155: 573–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilts CD,Schweitzer JB,Quinn CK,Gross RE,Faber TL,Muhammad F,Ely TD,Hoffman JM,Drexler KP ( 2001): Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry 58: 334–341. [DOI] [PubMed] [Google Scholar]
- Kilts CD,Gross RE,Schweitzer JB,Ely TD,Quinn CK,Drexler KPG ( 2004): The neural correlates of cue‐induced craving in cocaine‐dependent women. Am J Psychiatry 161: 233–241. [DOI] [PubMed] [Google Scholar]
- Knutson B,Taylor J,Kaufman M,Peterson R,Glover G ( 2005): Distributed neural representation of expected value. J Neurosci 25: 4806–4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B,Gibbs SE ( 2007): Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology 191: 813–822. [DOI] [PubMed] [Google Scholar]
- Lang PJ ( 1977): Imagery in therapy: An information processing analysis of fear. Behav Ther 8: 862–886. [DOI] [PubMed] [Google Scholar]
- Lang PJ ( 1979): A bio‐informational theory of emotional imagery. Psychophysiology 16: 495–512. [DOI] [PubMed] [Google Scholar]
- Lang PJ ( 1980): Behavioral treatment and bio‐behavioral assessment: Computer applications In: Sidowski JB, Johnson JH, Williams TA, editors. Techonology in Mental Heath Care Delivery Systems. Norwood, New Jersey: Ablex; p 119–137. [Google Scholar]
- Lang PJ ( 1994): The motivational organization of emotion: Affect‐reflex connections In: Van Goozen SHM, Van De Poll NE, Sergeant JA, editors. Emotions: Essays on Emotion Theory. Hillsdale, NJ: Erlbaum; pp 61–93. [Google Scholar]
- Lang PJ,Davis M ( 2006): Emotion, motivation, and the brain: Reflex foundations in animal and human research. Prog Brain Res 156: 3–34. [DOI] [PubMed] [Google Scholar]
- Lang PJ,McTeague LM ( 2009): The anxiety disorder spectrum: Fear imagery, physiological reactivity, and differential diagnosis. Anxiety Stress Coping 22: 5–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leknes S,Tracey I ( 2008): A common neurobiology for pain and pleasure. Nat Rev Neurosci 9: 314–320. [DOI] [PubMed] [Google Scholar]
- Loftus GR,Masson MEJ ( 1994): Using confidence intervals in within‐subject designs. Psychol Bull Rev 1: 476–490. [DOI] [PubMed] [Google Scholar]
- Logothetis NK,Pauls J,Augath M,Trinath T,Oeltermann A ( 2001): Neurophysiological investigation of the basis of the fMRI signal. Nature 412: 150–157. [DOI] [PubMed] [Google Scholar]
- Logothetis K ( 2008): What we can do and what we cannot do with fMRI. Nature 453: 869–878. [DOI] [PubMed] [Google Scholar]
- Mathiesen C,Caesar K,Akgören N,Lauritzen M ( 1998): Modification of activity‐dependent increases of cerebral blood flow by excitatory synaptic activity and spikes in rat cerebellar cortex. J Physiol 512: 555–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV,Berridge KC ( 2009): Which cue to “want?” Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue. J Neurosci 29: 6500–6513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL ( 2004): The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci 27: 1–28. [DOI] [PubMed] [Google Scholar]
- McGinty VB,Grace AA ( 2009): Timing‐dependent regulation of evoked spiking in nucleus accumbens neurons by integration of limbic and prefrontal cortical inputs. J Neurophysiol 101: 1823–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty VB,Grace AA ( 2008): Selective activation of medial prefrontal‐to‐accumbens projection neurons by amygdala stimulation and Pavlovian conditioned stimuli. Cereb Cortex 18: 1961–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague LM,Lang PJ,Laplante MC,Cuthbert BN,Strauss CC,Bradley MM ( 2009): Fearful imagery in social phobia: Generalization, comorbidity, and physiological reactivity. Biol Psychiatry 65: 374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meseguer V,Romero MJ,Barros‐Loscertales A,Belloch V,Bosch‐Morell F,Romero J,Avila C ( 2007): Mapping the appetitive and aversive systems with emotional pictures using a block‐design fMRI procedure. Piscotherma 19: 483–488. [PubMed] [Google Scholar]
- Nichols TE,Holmes AP ( 2002): Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp 15: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D,Price JL ( 2000): The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex 10: 206–219. [DOI] [PubMed] [Google Scholar]
- Orr SP,Pitman RK,Lasko NB,Herz LR ( 1993): Psychophysiologic assessment of posttraumatic stress disorder imagery in World War II and Korean combat veterans. J Abnorm Psychol 102: 152–159. [DOI] [PubMed] [Google Scholar]
- Phan KL,Taylor SF,Welsh RC,Ho S,Britton JC,Liberzon I ( 2004): Neural correlates of individual ratings of emotional salience: A trial related fMRI study. Neuroimage 21: 786–780. [DOI] [PubMed] [Google Scholar]
- Rauch SL,Shin LM,Dougherty DD,Alpert NM,Orr SP,Lasko M,Macklin ML,Fischman AJ,Pitman RK ( 1999): Neural activation during sexual and competitive arousal in healthy men. Psychiatry Res 91: 1–10. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D,Bradley MM,Fitzsimmons JR, Lang, PJ ( 2005): Parallel amygdala and inferotemporal activation reflect emotional intensity and fear relevance. Neuroimage 24: 1265–1270. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D,Bradley MM,Lang PJ,Costa VD,Versace F ( 2007): Pleasure rather than salience activates human nucleus accumbens and medial prefrontal cortex. J Neurophysiol 98: 1374–1379. [DOI] [PubMed] [Google Scholar]
- Shin LM,Dougherty DD,Orr SP,Pitman RK,Lasko M,Macklin ML,Alpert NM,Fischman AJ,Rauch SL ( 2000): Activation of anterior paralimbic structures during guilt‐related script‐driven imagery. Biol Psychiatry 48: 43–50. [DOI] [PubMed] [Google Scholar]
- Shin LM,Orr SP,Carson MA,Rauch SL,Macklin ML,Lasko NB,Peters PM,Metzger LJ,Dougherty DD,Cannistraro PA,Alpert NM,Fischman AJ,Pitman RK ( 2004): Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry 61: 168–176. [DOI] [PubMed] [Google Scholar]
- Sinha R,Lacadie C,Skudlarski P,Wexler BE ( 2004): Neural circuits underlying emotional distress in humans. Ann NY Acad Sci 1032: 254–257. [DOI] [PubMed] [Google Scholar]
- Talairach J,Tournoux P ( 1998): Co‐planar Stereotaxic Atlas of the Human Brain: An Approach to Medical Cerebral Imaging. Stuttgart: Thieme; 122 p. [Google Scholar]
- Vrana SR,Cuthbert BN,Lang PJ ( 1986): Fear imagery and text processing. Psychophysiology 23: 247–253. [DOI] [PubMed] [Google Scholar]
- Vrana SR,Lang PJ ( 1990): Fear imagery and the startle probe reflex. J Abnorm Psychol 99: 189–197. [DOI] [PubMed] [Google Scholar]
- Witvliet CV,Vrana SR ( 1995): Psychophysiological responses as indices of affective dimensions. Psychophysiology 32: 436–443. [DOI] [PubMed] [Google Scholar]
- Wolpe J ( 1958): Psychotherapy by Reciprocal Inhibition. Stanford: Stanford University Press; 239 p. [Google Scholar]
- Yoshimura S,Okamoto Y,Onoda K,Matsunaga M,Ueda K,Suzuki SI, Shigetoyamawaki: Rostral anterior cingulate cortex activity mediates the relationship between the depressive symptoms and the medial prefrontal cortex activity. J Affect Disord (in press). doi:10.1016/j.jad.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Zald D ( 2003): The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Brain Res Rev 41: 88–123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Table 1.
Supporting Table 2.


