Abstract
Metamorphosis, the transformation of one normal tissue or organ system into another, is a biological process rarely studied in higher vertebrates or mammals, but exemplified pathologically by the extremely disabling autosomal dominant disorder fibrodysplasia ossificans progressiva (FOP). The recurrent single nucleotide missense mutation in the gene encoding activin receptor IA/activin-like kinase-2 (ACVR1/ALK2), a bone morphogenetic protein type I receptor that causes skeletal metamorphosis in all classically affected individuals worldwide, is the first identified human metamorphogene. Physiological studies of this metamorphogene are beginning to provide deep insight into a highly conserved signaling pathway that regulates tissue stability following morphogenesis, and that when damaged at a highly specific locus (c.617G > A; R206H), and triggered by an inflammatory stimulus permits the renegade metamorphosis of normal functioning connective tissue into a highly ramified skeleton of heterotopic bone. A comprehensive understanding of the process of skeletal metamorphosis, as revealed by the rare condition FOP, will lead to the development of more effective treatments for FOP and, possibly, for more common disorders of skeletal metamorphosis.
Keywords: morphogen, metamorphogene, ACVR1, fibrodysplasia ossificans progressiva (FOP), bone morphogenetic protein (BMP), BMP receptor, heterotopic ossification
Introduction
Cancer, aging, degeneration, inflammation, and repair are well-known tissue processes that have received enormous attention in the scientific and medical literature. In contrast, the process of metamorphosis, the transformation of one normal tissue or organ system into another through a pathological process, is virtually unexplored and has been hidden for centuries behind the mask of an exceedingly rare and catastrophic human disease, fibrodysplasia ossificans progressiva (FOP). The genetic and molecular lessons of FOP are rapidly being revealed and its importance to medicine far exceeds its rarity [1].
Clinical features of FOP
Two clinical features define classic FOP: congenital malformation of the great toes and progressive heterotopic ossification in distinct anatomic patterns (Fig. 1) [2]. Individuals with FOP appear normal at birth except for malformations of the great toes, which are present in 100% of classically affected individuals [3]. During the first decade of life, children with FOP develop painful and highly inflammatory soft tissue swellings (or flare-ups) that progressively and permanently transform soft connective tissues, including aponeuroses, fascia, ligaments, tendons, and skeletal muscles, into an armament-like encasement of heterotopic bone [4,5]. Ribbons, sheets, and plates of heterotopic bone replace skeletal muscles and connective tissues through a process of endochondral ossification that leads to the formation of a highly ramified second skeleton with resultant permanent immobility [6–9]. Minor trauma such as intramuscular immunizations, mandibular blocks for dental work, muscle fatigue, blunt muscle trauma from bumps, bruises, falls, or influenza-like illnesses can trigger painful new flare-ups of FOP, leading to progressive heterotopic ossification (HO) [10–14].
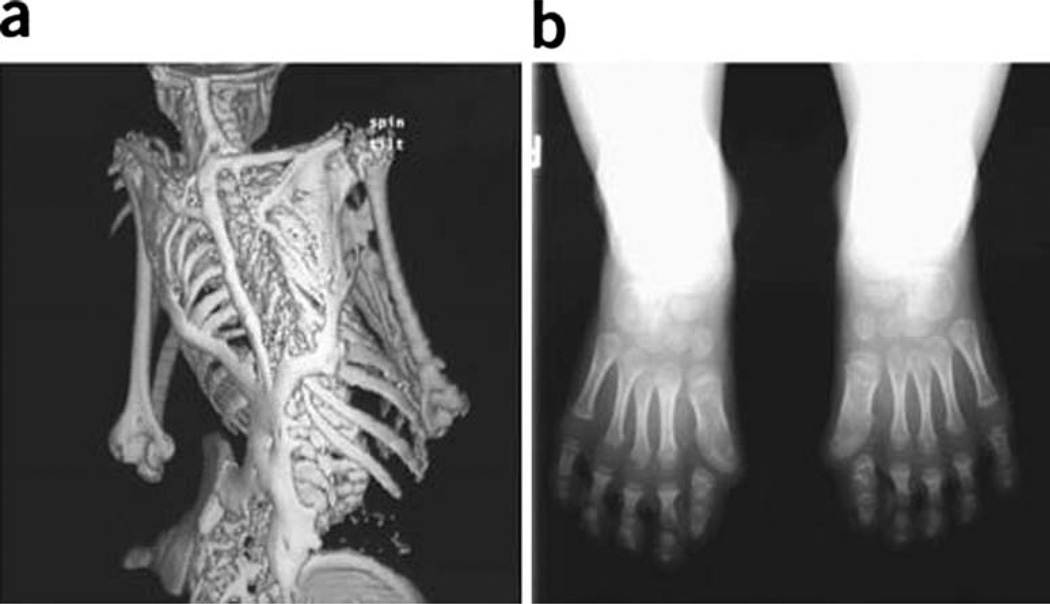
Fig. 1.
Characteristic clinical features of fibrodysplasia ossificans progressiva (FOP). a Extensive heterotopic bone formation typical of FOP is seen by three-dimensional reconstructed computed tomography (CT scan) of the back of a 12-year-old child. b Anteroposterior radiograph of the feet of a 3-year-old child shows symmetrical great toe malformations. [Originally published in Shore EM, et al. (2006) Nat Genet 38:525–527. Copyright held by the authors]
Surgical attempts to operatively remove heterotopic bone commonly lead to episodes of explosive and painful new bone growth [15–19]. HO in FOP progresses in characteristic anatomic and temporal patterns that mimic the patterns of normal embryonic skeletal formation. FOP involvement typically is seen first in the dorsal, axial, cranial, and proximal regions of the body and later in the ventral, appendicular, caudal, and distal regions [2,4,5,15,17]. Several skeletal muscles including the diaphragm, tongue, and extraocular muscles are enigmatically spared from FOP. Cardiac muscle and smooth muscle are not involved in the FOP process [2,15–17].
The clinical features of early lesional involvement in the axial regions are often different from those seen in the appendicular regions [20]. New lesions may appear rapidly. In the axial regions, swelling is often mistaken for tumors, as large bulbous lesional swellings may appear on the neck and back, whereas in the limbs, the swelling is often diffuse, and may be mistaken for acute thrombophlebitis, a complication that can occur in patients with FOP as a result of generalized immobility and associated venous stasis [20]. The qualitative differences in swelling in the axial versus the appendicular regions in patients with FOP may reflect regional differences in the anatomy of the subaponeurotic spaces as well as differences in the anatomy of the fascial compartments.
Bone formation in FOP is episodic, but disability is cumulative. Most patients with FOP are confined to a wheelchair by the third decade of life and require lifelong assistance in performing activities of daily living [2,15–19]. Severe weight loss may result from ankylosis of the jaw, and pneumonia or right-sided heart failure may complicate rigid fixation of the chest wall [21]. The severe disability of FOP results in low reproductive fitness, and fewer than ten multigenerational families are known worldwide [22]. The median age of survival is approximately 41 years, and death often results from complications of thoracic insufficiency syndrome [21].
Diagnosis and misdiagnosis of FOP
FOP is commonly misdiagnosed, as clinicians often fail to associate the rapidly developing soft tissue swellings that appear on the head, neck, and upper back with the malformed great toes [23]. FOP can be diagnosed clinically even before radiographic evidence of heterotopic ossification is seen if rapidly waxing and waning soft tissue lesions are associated with symmetrical malformations of the great toes [24]. When such associations are not made, FOP is commonly misdiagnosed as aggressive juvenile fibromatosis (extraabdominal desmoid tumors), lymphedema, or soft tissue sarcomas. Children often undergo unnecessary and harmful diagnostic biopsies that exacerbate the progression of the condition [23].
Additional skeletal anomalies in FOP
In addition to malformations of great toes and thumbs, early developmental anomalies are frequently observed in the cervical spine [25]. Stiffness of the neck is an early finding in most patients and can precede the appearance of HO. Characteristic anomalies of the cervical spine include large posterior elements, tall narrow vertebral bodies, and fusion of the facet joints between C2 and C7, findings that are strikingly similar to those seen in mice with homozygous deletions of the gene encoding Noggin, a secreted bone morphogenetic protein (BMP) antagonist [26], supporting that increased BMP pathway signaling underlies the disease [25]. Although mutations in the Noggin gene have been reported [27–29], these findings could not be reconfirmed and are thought to be in error [30–34].
Other skeletal anomalies often associated with FOP include short malformed thumbs, clinodactyly, short broad femoral necks, and proximal medial tibial osteochondromas. The latter two findings are also seen in patients who have multiple hereditary exostoses (MHE), although the genes associated with multiple hereditary exostoses are not mutated in patients who have FOP. Nevertheless, these shared clinical findings may illuminate common pathway anomalies [2,15–17,35].
Epidemiological, genetic, and environmental factors in FOP
FOP is extremely rare, with a worldwide prevalence of approximately 1 in 2 million. There is no ethnic, racial, gender, or geographic predisposition [17,19]. Most cases arise as a result of a spontaneous new mutation. When observed, genetic transmission is autosomal dominant and can be inherited from either mothers or fathers [22,36].
Both genetic and environmental factors affect the phenotype of FOP. A study of three pairs of monozygotic twins with FOP found that, within each pair, congenital great toe malformations were identical. However, postnatal heterotopic ossification varied greatly depending on life history and environmental exposure. This study indicated that genetic determinants strongly influence disease phenotype during prenatal development and that environmental factors strongly influence postnatal progression of heterotopic ossification [37].
The pathobiology of skeletal metamorphosis in FOP
The process of skeletal metamorphosis, as exemplified by FOP, does not involve the transdifferentiation of one mature cell into another, but rather a pathological process in which the normal structure and function of one tissue or organ are destroyed and replaced by those of another functioning tissue or organ [9,38].
The pathological stages of skeletal metamorphosis have been well described in FOP and correspond to the BMP-induced lesions described in several reports [6–8,39–44]. Skeletal metamorphosis in FOP begins with a soft tissue injury that triggers an inflammatory infiltrate and proceeds to muscle cell injury and death, replacement with a highly angiogenic fibroproliferative lesion, and maturation through a cartilage anlagen that culminates in the formation of a new skeletal element [6–9,38–44].
Despite detailed descriptions of FOP pathology in the medical literature, there has been a persistent misconception in the medical and scientific community about the process. For example, it is commonly believed that the process of skeletal metamorphosis involves a transdifferentiation of mature muscles cells into bone cells. That, in fact, does not appear to occur in vivo. Studies by Katagiri and colleagues have documented that C2C12 myoblasts, which are believed to be derived from satellite cells in murine thigh muscle, can express an osteogenic phenotype in the presence of high concentrations of recombinant BMP or constitutively active BMP type I receptors in vitro, and provide the template for understanding how dysregulation of the BMP signaling pathway can lead to altered progenitor cell fates [45,46]. Thus, mature muscles cells including myofibers do not transdifferentiate into mature bone cells. Rather, normal muscle tissue is replaced by normal bone tissue in a complex pathological process of skeletal metamorphosis that involves the epigenetic reprogramming of progenitor cell fates.
Morphogen receptor genes
The bone morphogenetic protein (BMP) signaling pathway is one of the most highly conserved signaling pathways in nature and regulates a myriad of developmental and post-developmental processes beginning in early embryogenesis and continuing through adult life [41,47–54]. A large body of evidence supports that BMPs act as morphogens in vertebrate development [41,51–55]. BMPs signal by binding to and activating heterotetrameric transmembrane complexes of type I and type II BMP receptors. Both type I and type II BMP receptors are serine/threonine kinases that have similar functional domains. Ligand binding occurs preferentially at the N-terminal extracellular domain of the BMP receptors, which is connected by a single transmembrane region to the C-terminal cytoplasmic kinase domain [51–54]. BMP signaling can be mediated by four known type I receptors: TSR (ALK1), ACVR1 (ALK2), BMPR1A (ALK3), and BMPR1B (ALK6). A unique feature of type I receptors is a cytoplasmic juxtamembrane region rich in glycine and serine residues (GS domain). Following ligand binding, serines and threonines in the GS domain are phosphorylated by the constitutively active BMP type II receptor. The BMP type I receptor is activated by these phosphorylation events and transmits downstream BMP signals through BMP pathway-specific Smads (Smads1, −5, −8) and p38 MAPK signaling pathways to regulate transcription of BMP-responsive target genes. Numerous comprehensive reviews are available on this seminal signaling pathway that will orient the reader to the concepts which follow here [51–54].
Discovery of the FOP metamorphogene
A large body of work has supported dysregulated BMP signaling in the pathogenesis of FOP [56–63]. FOP was recently mapped to chromosome 2q23-24 by genome-wide linkage analysis, a region that includes the ACVR1 gene. ACVR1/ALK2 is one of seven activin-like kinases (ALKs) in the human genome that all encode transforming growth factor-beta (TGF-β)/BMP type I serine/threonine transmembrane receptors involved in the specification of cell fate and differentiation in a wide variety of cells and tissues during embryonic development and postnatal life [64]. In all classically affected individuals worldwide, an identical heterozygous missense activating mutation (c.617G > A; R206H) was subsequently identified in the glycine-serine (GS) activation domain of ACVR1 [3].
This single nucleotide missense mutation transforms a morphogen receptor gene into a metamorphogene. The resultant mutant protein alters the basal set point and ligand-dependent sensitivity for BMP signaling in a cell-autonomous manner in connective tissue progenitor cells [63]. Identification of the mutant transmembrane receptor (and remarkably a single substituted amino acid residue in that receptor) provides a basis for elucidating the molecular pathophysiology of dysregulated BMP signaling and resultant skeletal metamorphosis in this illustrative and disabling condition [3,63].
Although ACVR1/ALK2 has been recognized as a BMP receptor, investigations of its functions in embryonic development and in regulating cell differentiation have been limited [3]. ACVR1/ALK2 is expressed in many tissues including skeletal muscle and chondrocytes. Constitutive activation of ACVR1/ALK2 induces alkaline phosphatase activity in C2C12 cells, upregulates BMP4, downregulates BMP antagonists, expands cartilage elements, induces ectopic chondrogenesis, and stimulates joint fusions, findings nearly identical to those seen in FOP [65]. Constitutive ACVR1/ALK2 expression (similar to that seen in FOP) in embryonic chick limbs induces expansion of chondrogenic anlage and induces joint fusions, suggesting that promiscuous ACVR1/ALK2 signaling alters cell fate and induces undifferentiated mesenchyme to form cartilage and bone [65]. Enhanced ACVR1/ALK2 activation in FOP is supported by recent data showing increased pathway-specific Smad phosphorylation and expression of BMP transcriptional targets in FOP cells as well as reversal of an ACVR1/ALK2-homologue loss of function phenotype by the ACVR1 (FOP) construct in mutant zebrafish embryos [66,67].
Protein homology mapping of mutant ACVR1
Protein homology modeling of the mutant ACVR1/ALK2 in FOP predicts changes in both ligand-independent BMP signaling and in ligand-stimulated BMP signaling in FOP cells [68]. We hypothesize that the canonical FOP mutation (ACVR1 c.617G>A; R206H), which replaces an arginine residue with a histidine at amino acid 206 in the GS domain of ACVR1/ALK2, affects the binding and downstream function of coregulatory proteins.
The GS domain of all TGF-β BMP type I receptors is a critical site for activation of pathway-specific Smad signaling proteins by constitutively active TFG-β/BMP type II receptors [69–72]. FKBP12 binds and stabilizes the inactive confirmation of all type I TGF-β/BMP receptors including ACVR1/ALK2. When bound to the GS domain, FKBP12 prevents leaky activation of type I receptors in the absence of ligand [69–72]. Importantly, FKBP12 also serves as a docking protein for the Smad–Smurf complexes that mediate ubiquitination, internalization, and degradation of ACVR1/ALK2, and is predicted to regulate the concentration of ACVR1/ALK2 and its BMP type I receptor oligomerization partners at the cell membrane [73,74].
The FOP mutation is predicted to impair FKBP12 binding and/or activity with resultant increased basal activity of BMP signaling in the absence of ligand as well as hyper-responsiveness of BMP signaling following ligand binding, two features of BMP signal dysregulation that have recently been demonstrated in FOP cells [63]. Thus, one scenario is that FKBP12 interaction with the GS domain may be altered in FOP, leading to promiscuous ACVR1/ALK2 activity (Fig. 2). Recent preliminary data strongly support this hypothesis, and it is the subject of intensive investigation.
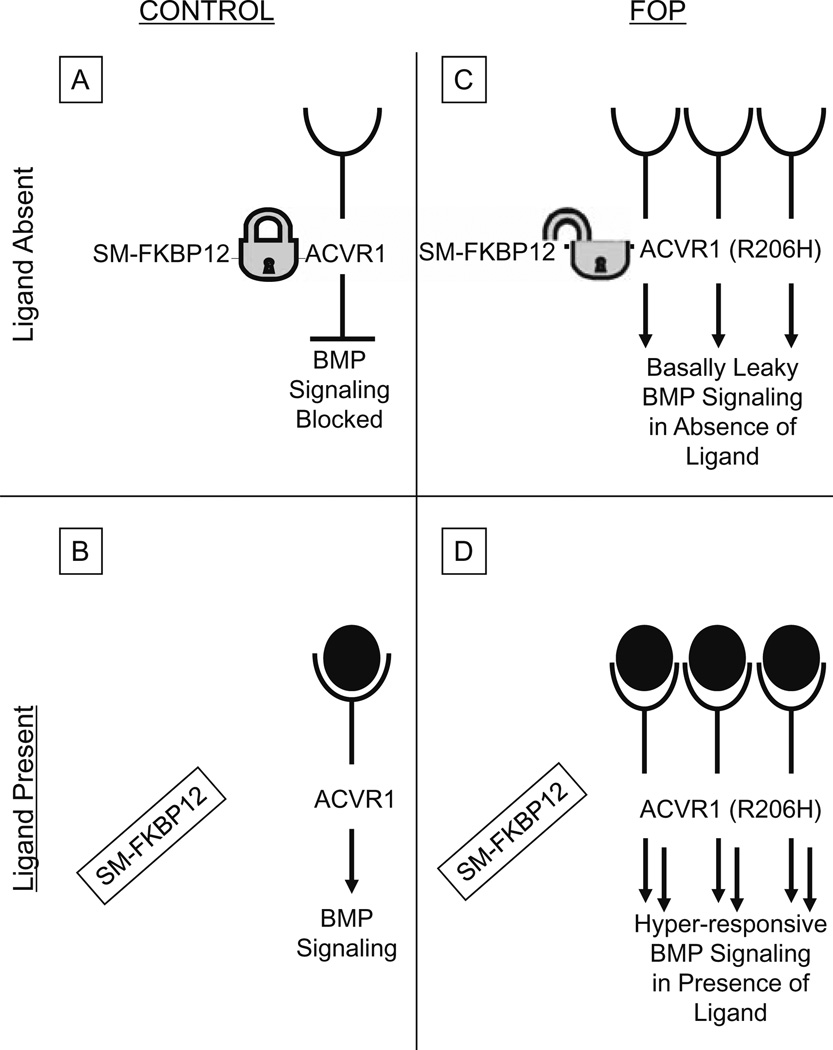
Fig. 2.
Hypothetical schema of bone morphogenetic protein (BMP) signaling in FOP cells. [Adapted from Kaplan FS, et al (2008) Fibrodysplasia ossificans progressiva. Best Pract Res Clin Rheumatol, in press.] In control cells (A), in the absence of ligand, the Smad/Smurf-FKBP12 (SM-FKBP12) complex binds ACVR1 (a BMP type I receptor) and prevents its promiscuous phosphorylation by the constitutively active type II BMP receptor (not shown). SM-FKBP12 also promotes ubiquitin-associated degradation of ACVR1 in the absence of ligand, thus maintaining low steady-state levels of ACVR1 at the cell membrane. Following ligand binding in control cells (B), SM-FKBP12 inhibition is released, thus allowing the constitutively active BMP type II receptor (not shown) to phosphorylate ACVR1 and promote SMAD1, −5, −8 phosphorylation and downstream BMP signaling. In FOP cells, SM-FKBP12 does not bind appropriately to the mutant receptor [ACVR1 (R206)]. Thus, inhibition of BMP signaling is impaired in the absence of ligand, and basal leakiness of BMP signaling occurs (C). Additionally, because the SM-FKBP12 complex cannot properly target the mutant ACVR1 (R206H) receptor for ubiquitin-associated degradation, ACVR1 might be expected to accumulate at the cell surface. Thus, in the presence of ligand (D), hyper-responsive BMP signaling might be predicted to occur. SM-FKBP12, Smad/Smurf-FKBP12 complex; arrows, signaling promoted; (by FOP mutation); open cups, extracellular ligand-binding domain of ACVR1; filled-in circles, BMP; filled-in circles inside of open cups, BMP ligand binding to ACVR1
The immune system and metamorphosis in FOP
Although dysregulation of the BMP signaling pathway can explain many features of skeletal metamorphosis in FOP, other features of the disease strongly implicate an underlying inflammatory trigger and/or a conducive inflammatory microenvironment [75] (Fig. 3).
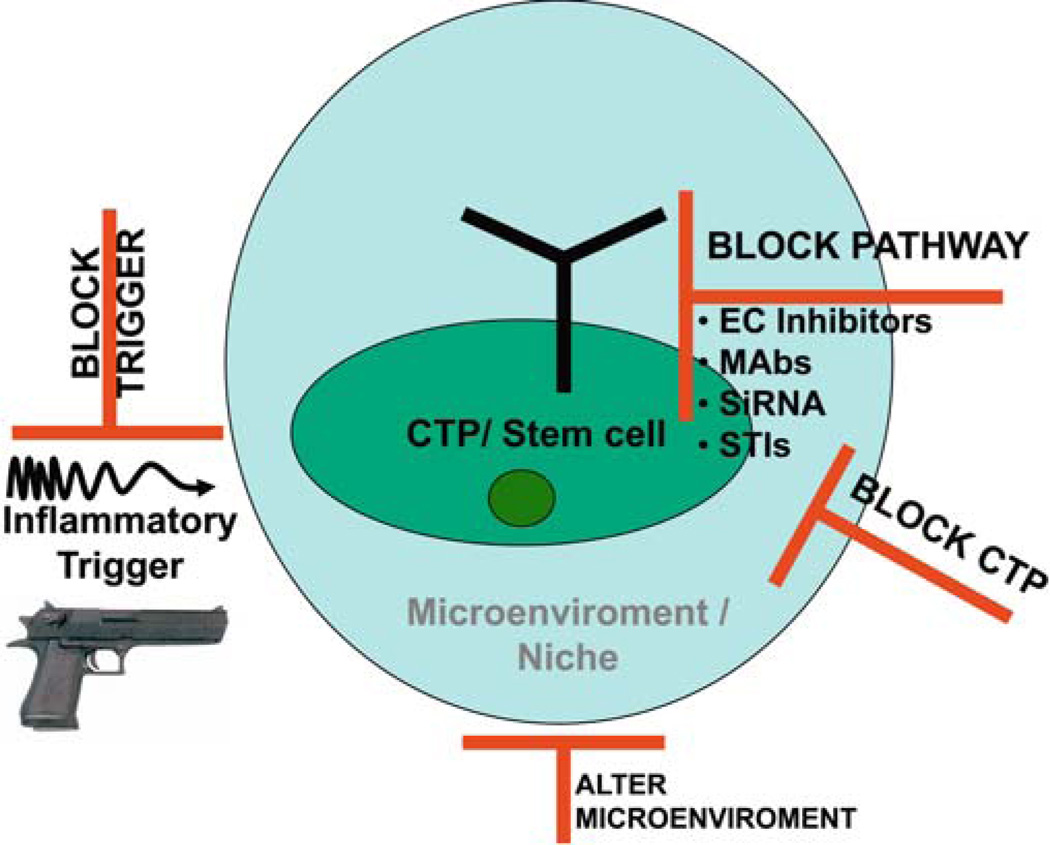
Fig. 3.
Hypothetical approaches to prevention of skeletal metamorphosis in FOP. Four approaches to prevention of skeletal metamorphosis in FOP are diagrammed and include blocking the putative inflammatory trigger (gun), blocking the signal transduction pathway [using extracellular inhibitors, monoclonal antibodies (MAbs) against the renegade receptor, inhibitory RNA (siRNA) against the mutant receptor RNA, and signal transduction inhibitors (STIs)], blocking the repetitive connective tissue progenitor (CTP) cells that transduce the promiscuous signal, or blocking/altering the permissive microenvironmental niche. EC, extracellular
A recent study showed that aberrant expression of BMPs in soft tissue causes focal hypoxia and hypoxic stress within the target tissue, a prerequisite for the differentiation of stem cells to chondrocytes and subsequent formation of heterotopic bone [76]. Flare-ups of FOP are frequently associated with muscle fatigue and trauma-associated hypoxia [2,4,15,77]. Such physiological hypoxic stress is predicted to exacerbate tissue damage, to release inflammatory mediators and BMPs, and to mobilize free radicals and the subsequent stimulation of mutant ACVR1/ALK2 in as yet unidentified connective tissue progenitor cells that potentiate skeletal metamorphosis [76,78–84] (Fig. 3).
A major new area of FOP research is focused on the relationship of inflammatory triggers, mutant ACVR1/ALK2 receptors, and local environmental factors such as free radicals, pO2, and pH in the episodic flare-ups of the disease [68,75,76,78–84]. Recent protein modeling studies by Groppe et al. predict that the canonical FOP mutation creates a pH-sensitive switch within the cytoplasmic domain of the mutant ACVR1/ALK2 receptor that leads not only to ligand-independent activation but also to ligand-dependent hyper-responsiveness of mutant ACVR1/ALK2 in the microenvironment of a lowered intracellular pH [68].
Our working hypothesis on the pathophysiology of heterotopic skeletogenesis in FOP is that soft tissue injury creates an inflammatory and acidic tissue microenvironment in which prostaglandins, free radicals, and hypoxia in concert with inflammatory cells of hematopoietic origin stimulate resting connective tissue progenitor cells (CTPs) to become activated CTPs. These CTPs express the mutant ACVR1/ALK2 receptor that is putatively hyperactive in a mildly acidic intracellular environment and which results in misregulated BMP signaling through increased basal leakiness and conditional hyper-responsiveness to environmental BMPs [38] (Fig. 3). Once formed, the fibroproliferative cells in the FOP lesion produce robust amounts of BMP4, and overactivity of the BMP signaling is sufficient to drive the process of endochondral ossification to completeness in the absence of a continued inflammatory stimulus [38,58] (see Fig. 3).
The recent discovery of the FOP gene mutation in all familial and sporadic cases of classic FOP suggests a cell-autonomous basis for skeletal metamorphosis in FOP [61]. Protein modeling of the mutant receptor predicts destabilization of the GS activation domain consistent with basally leaky and conditional hyper-responsive BMP signaling as the cause of the ectopic chondrogenesis, osteogenesis, and joint fusion seen in FOP. Furthermore, these findings allow us to hypothesize that trauma and inflammation recruit osteogenic CTPs in which the activating mutation is expressed. These findings are consistent with in vivo observations in animal models showing that once the endochondral anlagen is induced, abrogation of the inflammatory response does not inhibit the formation of heterotopic bone [85].
Interactions between inflammatory cells and FOP mesenchymal cells in a microenvironment that is inductive and conducive to the initiation, progression, and sustenance of bone formation in FOP occurs at several steps (see Fig. 3). Recent studies have strongly suggested that migration of vascular progenitor cells to sites of inflammation is a key step in normal and disordered regenerative responses and likely plays a role as well in the pathology of skeletal metamorphosis [85,86].
Stem cells and skeletal metamorphosis in FOP
Stem cells, progenitor cells, and inflammation must therefore lie at the very heart of the process of metamorphosis (Fig. 3). Hematopoietic cells have been implicated in the skeletal metamorphosis of FOP, and their replacement has been postulated as a possible cure [85]. However, the definitive contribution of hematopoietic cells to the pathogenesis of skeletal metamorphosis has, until recently, remained obscure. A recent study in a patient with FOP who coincidentally developed intercurrent aplastic anemia demonstrated that bone marrow transplantation does not cure FOP. However, following transplantation of bone marrow from a normal donor, immunosuppression of the immune system appeared to ameliorate activation of skeletal metamorphosis in a genetically susceptible host. Thus, cells of hematopoietic origin may contribute to and perhaps even trigger the formation of an ectopic skeleton, although they are not sufficient to complete the process alone. Moreover, even a normal functioning immune system is apparently sufficient to trigger an FOP flare-up in a genetically susceptible host [85].
Which cells contribute to the fibroproliferative and chondrogenic mesenchymal anlagen in skeletal metamorphosis? The question is fascinating, important, and unresolved. Recent studies performed with two independent routes of investigation support the contention that such cells are not of hematopoietic origin, but arise from a different pool of connective tissue progenitor (CTP) cells residing in skeletal muscle and associated connective tissues, with possible lineage origins in endothelial cells, smooth muscle cells, neural cells, or other CTP cells [85]. Therefore, multiple sources of pluripotent stem cells or progenitors may contribute to the formation of an ectopic skeleton in the process of skeletal metamorphosis [85]. Detailed lineage-tracing experiments in transgenic mice with stable lineage markers will be necessary to definitively determine the origin of these cells. The development of a knock-in mouse that replicates the identical mutation of classic FOP is presently underway and will facilitate the identification of the autonomous connective tissue progenitor cell(s) that contribute to dysregulated BMP signaling in FOP.
Taken together, skeletal metamorphosis appears to be a stem cell or progenitor cell disorder, triggered by inflammation. One normal tissue is replaced with another, but first, nearly all vestiges of the original tissue, except its soft tissue scaffolding, neurovascular infrastructure, and progenitor cell repository, are destroyed and then replaced with a different tissue and a different epigenetic identity. Recent studies in FOP suggest that connective tissue progenitor cells resident in the local tissues are responsible for this metamorphosis, although the process is triggered by local inflammatory signals [75,85]. Essentially, skeletal metamorphosis is a disorder of dysregulated tissue repair. Metamorphosis thus unmasks the deep developmental restraints that must exist in normal tissue repair, and which are liberated, to the detriment of the host, by the specific recurrent mutation in the ACVR1/ALK2 (R206H) metamorphogene [3,85].
Prevention and treatment of skeletal metamorphosis in FOP
Presently, medical intervention for FOP is supportive. Guidelines for symptomatic management of disease flare-ups have been published and highlight the anecdotal utility of glucocorticoids in managing new flare-ups affecting the function of major joints in the appendicular skeleton [87]. Nonsteroidal antiinflammatory medications, COX-2 inhibitors, leukotriene inhibitors, and mast cell stabilizers are useful anecdotally in managing chronic discomfort and ongoing flare-ups, but presently there is no proven efficacy with any therapy in altering the natural history of the disease [87]. The rarity, variable severity, and episodic clinical course of FOP pose substantial uncertainties when evaluating experimental therapies [87]. A recent report documented the failure of bone marrow transplantation to cure the condition, but suggested that chronic immunosuppression may have some utility, although its general use is not recommended [85].
Surgical release of joint contractures is generally unsuccessful and risks new, trauma-induced heterotopic ossification. Osteotomy of heterotopic bone or surgical removal of heterotopic bone to mobilize joints is generally counterproductive because additional heterotopic ossification develops at the operative site. Rarely, a joint may be repositioned surgically to improve the patient’s overall functional status. Spinal bracing is ineffective, and surgical intervention is associated with numerous complications [88].
The ultimate goal of FOP research is the development of treatments that will prevent, halt, or even reverse the progression of the condition. The prevention and treatment of heterotopic ossification in FOP, as in any of the more common forms of heterotopic ossification, will ultimately be based on at least one of four principles: disrupting the relevant inductive signaling pathways, blocking the immunological and/or inflammatory triggers, suppressing the relevant osteoprogenitor cells in the target tissues, and modifying the tissue environment conducive to heterotopic osteogenesis (Fig. 4).
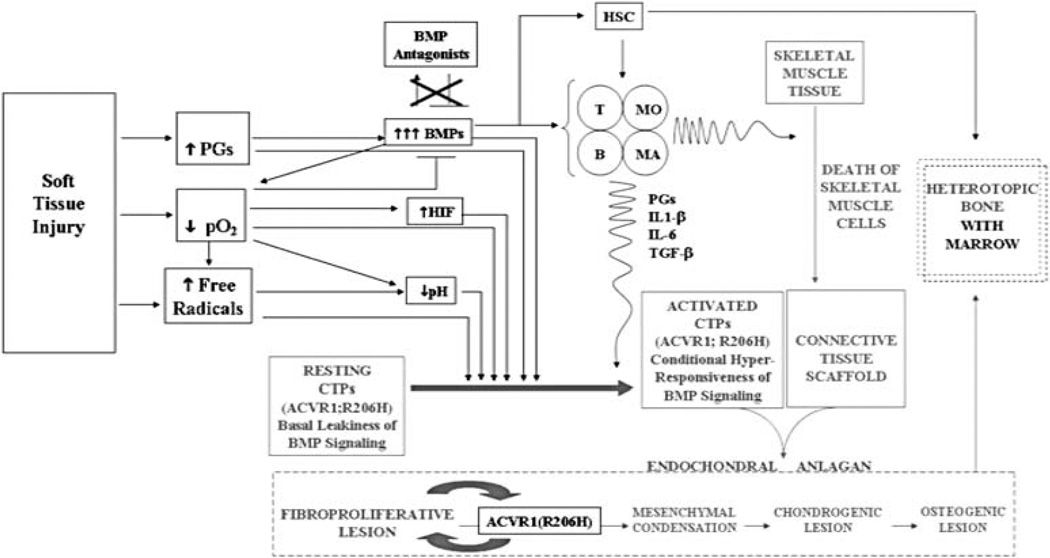
Fig. 4.
Hypothetical model of skeletal metamorphosis in fibrodysplasia ossificans progressiva. (Adapted from Kaplan FS, et al. (2007) Morphogen receptor genes and metamorphogens: skeleton keys to the metamorphosis. Ann N Y Acad Sci 1116:113–133 [38].) PGs, prostaglandins; pO2, tissue oxygen tension; HIF, hypoxia-inducing factor; CTPs, connective tissue progenitor cells; HSC, hematopoietic stem cells; T, T cells; B, B cells; Mo, monocytes; MA, mast cells; IL-1β, interleukin-1β; IL-6, interleukin 6; TGF-β, transforming growth factor-beta; straight arrows, progression; wavy arrows, influence
The identification of the recurrent heterozygous missense point mutation that causes FOP in all classically affected individuals provides a specific druggable target and a rational point of intervention in a critical signaling pathway [89]. Plausible therapeutic approaches to inhibiting BMP signaling in FOP include inhibitory RNA technology, monoclonal antibodies directed against ACVR1/ALK2, and orally available small molecule selective signal transduction inhibitors (STIs) of ACVR1/ALK2 [89], such as Dorsomorphin or its derivatives [90] which inhibit heterotopic ossification in inflammation-induced models of BMP pathway overactivity.
Metamorphosis and tissue engineering: harnessing the FOP metamorphogene
ACVR1/ALK2 (R206H) is the first identified human metamorphogene and provides a genetic basis for understanding the general biological principles that orchestrate the pathological transformation of one normal organ system into another. The immediate goal of FOP research is to understand the molecular and cellular pathophysiology of this process and to use that knowledge to develop pharmacological methods to block and prevent it. Conversely, it may be possible to harness the gene mutation that causes FOP to create new skeletal elements in a controlled way—for patients who have osteoporotic fractures, for those with severe bone loss from trauma or neoplasms, for those with fractures that fail to heal, for those with spinal fusions that are slow to heal, or for those with congenital malformations of the spine and limbs who need new bone. The discovery of the FOP gene, the first human metamorphogene, is a monumental milestone in an epic journey to understand the biological principles that regulate tissue stability and skeletal metamorphosis following embryogenesis.
Other examples of skeletal metamorphosis in humans
Classic FOP and its phenotypic and genotypic variants are the most dramatic, but not the only, examples of skeletal metamorphosis in humans. There are, for example, many acquired forms of skeletal metamorphosis that are spatially and temporally limited. Some are fairly common, such as the heterotopic ossification that occurs following closed head injury, spinal cord injury, total hip replacement, athletic injury, and blast injuries from war [18,91–94]. Additionally, approximately 13% of individuals with end-stage valvular heart disease form mature heterotopic bone in the aortic valve [91]. To date, there are few studies on the molecular pathogenesis in these more common disorders of skeletal metamorphosis. Where it has been examined, the histology is similar in all, and it would not be surprising if the ACVR1/ALK2 signaling pathway was involved in the more common as in the rarer forms of skeletal metamorphosis.
Nature does not use different genes, molecules, and pathways for common conditions or for rare ones. Rather, it is often the rare disease that reveals which gene, molecule, or pathway nature hijacks in its common infirmities [95]. William Harvey, the discoverer of the circulatory system, wrote in 1657:
“Nature is nowhere accustomed more openly to display her secret mysteries than in cases where she shows traces of her workings apart from the beaten path; nor is there any better way to advance the proper practice of medicine than to give our minds to the discovery of the usual law of nature by the careful investigation of cases of rarer forms of disease.” [96]
Acknowledgments
This work was supported in part by the Center for Research In FOP and Related Disorders, the International FOP Association, The Ian Cali Endowment, The Weldon Family Endowment, The Isaac & Rose Nassau Professorship of Orthopaedic Molecular Medicine, and by grants from The Academic Frontier Project of Saitama Medical University Research Center for Genomic Medicine, The Ministry of Education, Culture, Sports, Sciences, and Technology of Japan, and The United States National Institutes of Health (RO1-AR40196). Portions of this work have been modified and adapted from reference 38 [Kaplan FS, Groppe J, Pignolo RJ, Shore EM (2007) Morphogen receptor genes and metamorphogenes: skeleton keys to the metamorphosis. Ann N Y Acad Sci 1116:113–133] and reference 77 [Kaplan FS, Le Merrer M, Glaser DL, Pignolo RJ, Goldsby R, Kitterman JA, Groppe J, Shore EM (2008) Fibrodysplasia ossificans progressiva. Best Pract Res Clin Rheumatol 22:191–205].
Contributor Information
Frederick S. Kaplan, Email: frederick.kaplan@uphs.upenn.edu, Departments of Orthopaedic Surgery and Medicine, c/o Department of Orthopaedic Surgery, Hospital of the University of Pennsylvania, Silverstein 2, 3400 Spruce Street, Philadelphia, PA 19104, USA Tel. +1-215-349-8726; Fax +1-215-349-5928.
Qi Shen, Department of Orthopaedic Surgery, University of Pennsylvania School of Medicine, Philadelphia, PA, USA.
Vitali Lounev, Department of Orthopaedic Surgery, University of Pennsylvania School of Medicine, Philadelphia, PA, USA.
Petra Seemann, Max Planck Institute for Molecular Genetics, Development and Disease, Berlin, Germany.
Jay Groppe, Department of Biomedical Sciences, Baylor College of Dentistry, Texas A & M University Health Science Center, Dallas, TX, USA.
Takenobu Katagiri, Division of Pathophysiology, Research Center for Genomic Medicine, Saitama Medical University, Saitama, Japan.
Robert J. Pignolo, Department of Medicine, University of Pennsylvania School of Medicine, Philadelphia, PA, USA
Eileen M. Shore, Departments of Orthopaedic Surgery & Genetics, University of Pennsylvania School of Medicine, Philadelphia, PA, USA
References
- 1.Kaplan FS, Glaser DL, Pignolo RJ, Shore EM. Introduction. Clin Rev Bone Miner Metab. 2005;3(3–4):175–177. [Google Scholar]
- 2.Kaplan FS, Glaser DL, Shore EM, Deirmengian GK, Gupta R, Delai P, Morhart R, Smith R, Le Merrer M, Rogers JG, Connor JM, Kitterman JA. The phenotype of fibrodysplasia ossificans progressiva. Clin Rev Bone Miner Metab. 2005;3:183–188. [Google Scholar]
- 3.Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho T-J, Choi IH, Connor JM, Delai P, Glaser DL, Le Merrer M, Morhart R, Rogers JG, Smith R, Triffitt JT, Urtizberea JA, Zasloff M, Brown MA, Kaplan FS. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006;38:525–527. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 4.Cohen RB, Hahn GV, Tabas J, Peeper J, Levitz CL, Sando A, Sando N, Zasloff M, Kaplan FS. The natural history of heterotopic ossification in patients who have fibrodysplasia ossificans progressiva. J Bone Joint Surg Am. 1993;75:215–219. doi: 10.2106/00004623-199302000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Rocke DM, Zasloff M, Peeper J, Cohen RB, Kaplan FS. Age and joint-specific risk of initial heterotopic ossification in patients who have fibrodysplasia ossificans progressiva. Clin Orthop Relat Res. 1994;301:243–248. [PubMed] [Google Scholar]
- 6.Kaplan FS, Tabas J, Gannon FH, Finkel G, Hahn GV, Zasloff MA. The histopathology of fibrodysplasia ossificans progressiva: an endochondral process. J Bone Joint Surg Am. 1993;75:220–230. doi: 10.2106/00004623-199302000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Gannon FH, Valentine BA, Shore EM, Zasloff MA, Kaplan FS. Acute lymphocytic infiltration in an extremely early lesion of fibrodysplasia ossificans progressiva. Clin Orthop Relat Res. 1998;346:19–25. [PubMed] [Google Scholar]
- 8.Glaser DL, Economides AN, Wang L, Liu X, Kimble RD, Fandl JP, Wilson JM, Stahl N, Kaplan FS, Shore EM. In vivo somatic cell gene transfer of an engineered noggin mutein prevents BMP4-induced heterotopic ossification. J Bone Joint Surg Am. 2003;85:2332–2342. doi: 10.2106/00004623-200312000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Pignolo RJ, Suda RK, Kaplan FS. The fibrodysplasia ossificans progressiva lesion. Clin Rev Bone Miner Metab. 2005;3:195–200. [Google Scholar]
- 10.Lanchoney TF, Cohen RB, Rocke DM, Zasloff MA, Kaplan FS. Permanent heterotopic ossification at the injection site after diphtheria-tetanus-pertussis immunizations in children who have fibrodysplasia ossificans progressiva. J Pediatr. 1995;126:762–764. doi: 10.1016/s0022-3476(95)70408-6. [DOI] [PubMed] [Google Scholar]
- 11.Janoff HB, Zasloff MA, Kaplan FS. Submandibular swelling in patients with fibrodysplasia ossificans progressiva. Otolaryngol Head Neck Surg. 1996;114:599–604. doi: 10.1016/S0194-59989670253-X. [DOI] [PubMed] [Google Scholar]
- 12.Luchetti W, Cohen RB, Hahn GV, Rocke DM, Helpin M, Zasloff M, Kaplan FS. Severe restriction in jaw movement after routine injection of local anesthetic in patients who have fibrodysplasia ossificans progressiva. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:21–25. doi: 10.1016/s1079-2104(96)80141-7. [DOI] [PubMed] [Google Scholar]
- 13.Glaser DL, Rocke DM, Kaplan FS. Catastrophic falls in patients who have fibrodysplasia ossificans progressiva. Clin Orthop Relat Res. 1998;346:110–116. [PubMed] [Google Scholar]
- 14.Scarlett RF, Rocke DM, Kantanie S, Patel JB, Shore EM, Kaplan FS. Influenza-like viral illnesses and flare-ups of fibrodysplasia ossificans progressiva. Clin Orthop Relat Res. 2004;423:275–279. doi: 10.1097/01.blo.0000129557.38803.26. [DOI] [PubMed] [Google Scholar]
- 15.Connor JM, Evans DA. Fibrodysplasia ossificans progressiva. The clinical features and natural history of 34 patients. J Bone Joint Surg Br. 1982;64:76–83. doi: 10.1302/0301-620X.64B1.7068725. [DOI] [PubMed] [Google Scholar]
- 16.Smith R. Fibrodysplasia (myositis) ossificans progressiva: clinical lessons from a rare disease. Clin Orthop Relat Res. 1988;346:7–14. [PubMed] [Google Scholar]
- 17.Kaplan FS, Shore EM, Connor JM. Fibrodysplasia ossificans progressiva (FOP) In: Royce PM, Steinmann B, editors. Connective Tissue and Its Heritable Disorders: Molecular, Genetic, and Medical Aspects. 2nd edn. New York: Wiley-Liss; 2002. pp. 827–840. [Google Scholar]
- 18.Kaplan FS, Glaser DL, Hebela N, Shore EM. Heterotopic ossification. J Am Acad Orthop Surg. 2004;12:116–125. doi: 10.5435/00124635-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan FS, Glaser DL, Shore EM. Fibrodysplasia (myositis) ossificans progressiva. In: Favus MJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 6th edn. Washington, DC: The American Society for Bone and Mineral Research; 2006. pp. 450–453. [Google Scholar]
- 20.Moriatis JM, Gannon FH, Shore EM, Bilker W, Zasloff MA, Kaplan FS. Limb swelling in patients who have fibrodysplasia ossificans progressiva. Clin Orthop Relat Res. 1997;336:247–253. doi: 10.1097/00003086-199703000-00033. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan FS, Glaser DL. Thoracic insufficiency syndrome in patients with fibrodysplasia ossificans progressiva. Clin Rev Bone Miner Metab. 2005;3:213–216. [Google Scholar]
- 22.Shore EM, Feldman GJ, Xu M, Kaplan FS. The genetics of fibrodysplasia ossificans progressiva. Clin Rev Bone Miner Metab. 2005;3:201–204. [Google Scholar]
- 23.Kitterman JA, Kantanie S, Rocke DM, Kaplan FS. Iatrogenic harm caused by diagnostic errors in fibrodysplasia ossificans progressiva. Pediatrics. 2005;116:654–661. doi: 10.1542/peds.2005-0469. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan FS, Xu M, Glaser DL, Collins F, Connor M, Kitterman J, Sillence D, Zackai E, Ravitsky V, Zasloff M, Ganguly A, Shore E. Early diagnosis of fibrodysplasia ossificans progressiva (FOP) Pediatrics. 2008;121:e1295–e1300. doi: 10.1542/peds.2007-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaffer AA, Kaplan FS, Tracy MR, O’Brien ML, Dormans JP, Shore EM, Harland RM, Kusumi K. Developmental anomalies of the cervical spine in patients with fibrodysplasia ossificans progressiva are distinctly different from those in patients with Klippel–Feil syndrome. Spine. 2005;30:1379–1385. doi: 10.1097/01.brs.0000166619.22832.2c. [DOI] [PubMed] [Google Scholar]
- 26.Groppe J, Greenwald J, Wiater E, Rodriguez-Leon J, Economides A, Kwaitowski W, Affolter M, Vale WW, Izpisua-Belmonte JC, Choe S. Structural basis of BMP signalling inhibition by Noggin, a novel cystine knot protein. Nature (Lond) 2002;420:636–642. doi: 10.1038/nature01245. [DOI] [PubMed] [Google Scholar]
- 27.Lucotte G, Semonin O, Lutz P. A de novo heterozygous deletion of 42 base-pairs in the noggin gene of a fibrodysplasia ossificans progressiva. Clin Genet. 1999;56:469–470. doi: 10.1034/j.1399-0004.1999.560613.x. [DOI] [PubMed] [Google Scholar]
- 28.Semonin O, Fontaine K, Daviaud C, Ayuso C, Lucotte G. Identification of three novel mutations of the noggin gene in patients with fibrodysplasia ossificans progressiva. Am J Med Genet. 2001;102:314–317. doi: 10.1002/ajmg.1504. [DOI] [PubMed] [Google Scholar]
- 29.Lucotte G, La Garde JP and members of the FOP European Research Group. Mutations of the noggin and of the activin A type I receptor genes in fibrodysplasia ossificans progressiva (FOP) Genet Couns. 2007;18:349–352. [PubMed] [Google Scholar]
- 30.Xu M-Q, Feldman G, Le Merrer M, Shugart YY, Glaser DL, Urtizberea JA, Fardeau M, Connor JM, Triffitt J, Smith R, Shore EM, Kaplan FS. Linkage exclusion and mutational analysis of the noggin gene in patients with fibrodysplasia ossificans progressiva. Clin Genet. 2000;58:291–298. doi: 10.1034/j.1399-0004.2000.580407.x. [DOI] [PubMed] [Google Scholar]
- 31.Cohen MM., Jr Bone morphogenetic proteins with some comments on fibrodysplasia ossificans progressiva and noggin. Am J Med Genet. 2002;109:87–92. doi: 10.1002/ajmg.10289. [DOI] [PubMed] [Google Scholar]
- 32.Carey JC. Editor’s note. Am J Med Genet. 2002;109:160. [Google Scholar]
- 33.Xu M, Shore EM, Kaplan FS. Reported noggin mutations are PCR errors. Am J Med Genet. 2002;109:161. doi: 10.1002/ajmg.10288. [DOI] [PubMed] [Google Scholar]
- 34.Warman ML. Significant difference of opinion regarding the role of noggin in fibrodysplasia ossificans progressiva. Am J Med Genet. 2002;109:162. doi: 10.1002/ajmg.10290. [DOI] [PubMed] [Google Scholar]
- 35.Deirmengian GK, Hebela NM, O’Connell M, Glaser DL, Shore EM, Kaplan FS. Proximal tibial osteochondromas in patients with fibrodysplasia ossificans progressiva (FOP) J Bone Joint Surg Am. 2008;90:366–374. doi: 10.2106/JBJS.G.00774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaplan FS, McCluskey W, Hahn G, Tabas J, Muenke M, Zasloff MA. Genetic transmission of fibrodysplasia ossificans progressiva. J Bone Joint Surg Am. 1993;75:1214–1220. doi: 10.2106/00004623-199308000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Hebela N, Shore EM, Kaplan FS. Three pairs of monozygotic twins with fibrodysplasia ossificans progressiva: the role of environment in the progression of heterotopic ossification. Clin Rev Bone Miner Metab. 2005;3:205–208. [Google Scholar]
- 38.Kaplan FS, Groppe J, Pignolo RJ, Shore EM. Morphogen receptor genes and metamorphogenes: skeleton keys to the metamorphosis. Ann N Y Acad Sci. 2007;1116:113–133. doi: 10.1196/annals.1402.039. [DOI] [PubMed] [Google Scholar]
- 39.Urist MR. Bone formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 40.Urist MR, Nakagawa M, Nakata N, Nogami H. Experimental, myositis ossificans: cartilage and bone formation in muscle in response to a diffusable bone matrix-derived morphogen. Arch Pathol Lab Med. 1978;102:312–316. [PubMed] [Google Scholar]
- 41.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 42.Gannon FH, Glaser D, Caron R, Thompson LD, Shore EM, Kaplan FS. Mast cell involvement in fibrodysplasia ossificans progressiva. Hum Pathol. 2001;32:842–848. doi: 10.1053/hupa.2001.26464. [DOI] [PubMed] [Google Scholar]
- 43.Kan L, Hu M, Gomes WA, Kessler JA. Transgenic mice overexpressing BMP4 develop a fibrodysplasia ossificans progressiva (FOP)-like phenotype. Am J Pathol. 2004;165:1107–1115. doi: 10.1016/S0002-9440(10)63372-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kan L. The neuron-specific enolase-bone morphogenetic protein 4 transgenic mouse. Clin Rev Bone Miner Metab. 2005;3:235–237. [Google Scholar]
- 45.Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994;127:1755–1766. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akiyama S, Katagiri T, Namiki M, Yamaji N, Yamamoto N, Miyama K, Shibuya H, Ueno N, Wozney JM, Suda T. Constitutively active BMP type I receptors transduce BMP2 signals without the ligand in C2C12 myoblasts. Exp Cell Res. 1997;235:362–369. doi: 10.1006/excr.1997.3680. [DOI] [PubMed] [Google Scholar]
- 47.Massagué J. How cells read TGF-β signals. Nat Rev Mol Cell Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 48.Hoffman A, Gross G. BMP signaling pathways in cartilage and bone formation. Crit Rev Eukaryot Gene Expr. 2001;11:23–45. [PubMed] [Google Scholar]
- 49.ten Dijke P, Korchynskyi O, Valdimarsdottir G, Goumans MJ. Controlling cell fate by bone morphogenetic protein receptors. Mol Cell Endocrinol. 2003;211:105–113. doi: 10.1016/j.mce.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 50.Kloen P, Di Paola M, Borens O, Richmond J, Perino G, Helfet DL, Goumans MJ. BMP signaling components are expressed in human fracture callus. Bone (NY) 2003;33:362–371. doi: 10.1016/s8756-3282(03)00191-1. [DOI] [PubMed] [Google Scholar]
- 51.Gazzerro E, Canalis E. Bone morphogenetic proteins and their antagonists. Rev Endocr Metab Disord. 2006;7:51–65. doi: 10.1007/s11154-006-9000-6. [DOI] [PubMed] [Google Scholar]
- 52.Shi Y, Massagué J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 53.Massagué J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 54.Schmierer B, Hill CS. TGFβ-SMAD signal transduction: molecular specificity and functional flexibility. Mol Cell Biol. 2007;8:970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 55.Lander AD. Morpheus unbound: reimagining the morphogen gradient. Cell. 2007;128:245–256. doi: 10.1016/j.cell.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 56.Kaplan FS, Tabas JA, Zasloff MA. Fibrodysplasia ossificans progressiva (FOP): a clue from the fly? Calcif Tissue Int. 1990;47:117–125. doi: 10.1007/BF02555995. [DOI] [PubMed] [Google Scholar]
- 57.Shafritz AB, Shore EM, Gannon FH, Zasloff MA, Taub R, Muenke M, Kaplan FS. Over-expression of an osteogenic morphogen in fibrodysplasia ossificans progressiva. N Engl J Med. 1996;335:555–561. doi: 10.1056/NEJM199608223350804. [DOI] [PubMed] [Google Scholar]
- 58.Gannon FH, Kaplan FS, Olmsted E, Finkel G, Zasloff MA, Shore EM. Bone morphogenetic protein 2/4 in early fibromatous lesions of fibrodysplasia ossificans progressiva. Hum Pathol. 1997;28:339–343. doi: 10.1016/s0046-8177(97)90133-7. [DOI] [PubMed] [Google Scholar]
- 59.Ahn J, Serrano de la Peña L, Shore EM, Kaplan FS. Paresis of a bone morphogenetic protein-antagonist response in a genetic disorder of heterotopic skeletogenesis. J Bone Joint Surg Am. 2003;85:667–674. doi: 10.2106/00004623-200304000-00013. [DOI] [PubMed] [Google Scholar]
- 60.Serrano de la Peña L, Billings PC, Fiori JL, Ahn J, Shore EM, Kaplan FS. Fibrodysplasia ossificans progressiva, a disorder of ectopic osteogenesis, misregulates cell surface expression and trafficking of BMPRIA. J Bone Miner Res. 2005;20:1168–1176. doi: 10.1359/JBMR.050305. [DOI] [PubMed] [Google Scholar]
- 61.Fiori JL, Billings PC, Serrano de la Peña L, Kaplan FS, Shore EM. Dysregulation of the BMP-p38 MAPK signaling pathway in cells from patients with fibrodysplasia ossificans progressiva. J Bone Miner Res. 2006;21:902–909. doi: 10.1359/jbmr.060215. [DOI] [PubMed] [Google Scholar]
- 62.Kaplan FS, Fiori J, Serrano de la Peña L, Ahn J, Billings PC, Shore EM. Dysregulation of the BMP4 signaling pathway in fibrodysplasia ossificans progressiva. Ann N Y Acad Sci. 2006;1068:54–65. doi: 10.1196/annals.1346.008. [DOI] [PubMed] [Google Scholar]
- 63.Billings PC, Fiori JL, Bentwood JL, O’Connell MP, Jiao X, Nussbaum B, Caron RJ, Shore EM, Kaplan FS. Dysregulated BMP4 signaling and enhanced osteogenic differentiation of connective tissue progenitor cells from patients with fibrodysplasia ossificans progressiva. J Bone Miner Res. 2008;23:305–313. doi: 10.1359/JBMR.071030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harradine KA, Akhurst RJ. Mutations of TGF-β signaling molecules in human disease. Ann Med. 2006;38:403–414. doi: 10.1080/07853890600919911. [DOI] [PubMed] [Google Scholar]
- 65.Zhang D, Schwarz EM, Rosier RN, Zuscik MJ, Puzas JE, O’Keefe RJ. Alk2 functions as a BMP type I receptor and induces Indian hedgehog in chondrocytes during skeletal development. J Bone Miner Res. 2003;18:1593–1604. doi: 10.1359/jbmr.2003.18.9.1593. [DOI] [PubMed] [Google Scholar]
- 66.Fukada T, Kohda M, Kanomata K, Nojima J, Kamizono J, Oda H, Nakayama K, Ohtake A, Miyazono K, Jimi E, Owan I, Okazaki Y, Katagiri T. A constitutively activated BMP receptor, ALK2, induces heterotopic bone formation in patients with fibrodysplasia ossificans progressiva. J Bone Miner Res. 2007;22(suppl 1):S10. [Google Scholar]
- 67.Shen Q, Xu M, Little SC, Kaplan FS, Mullins MC, Shore EM. Activation of BMP signaling by the FOP ACVR1 R206H mutation. J Bone Miner Res. 2007;22(suppl 1):S43. [Google Scholar]
- 68.Groppe JC, Shore EM, Kaplan FS. Functional modeling of the ACVR1 (R206H) mutation in FOP. Clin Orthop Relat Res. 2007;462:87–92. doi: 10.1097/BLO.0b013e318126c049. [DOI] [PubMed] [Google Scholar]
- 69.Wang T, Li B-Y, Danielson PD, Shah PC, Rockwell S, Lechleider RJ, Martin J, Manganaro T, Donahoe PK. The immunophilin FKBP12 functions as a common inhibitor of the TGF-β family type I receptors. Cell. 1996;86:435–444. doi: 10.1016/s0092-8674(00)80116-6. [DOI] [PubMed] [Google Scholar]
- 70.Chen Y-G, Liu F, Massagué J. Mechanism of TGF-β receptor inhibition by FKBP12. EMBO J. 1997;16:3866–3876. doi: 10.1093/emboj/16.13.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huse M, Chen YG, Massagué J, Kuriyan J. Crystal structure of the cytoplasmic domain of the type I TGF-β receptor complex with FKBP12. Cell. 1999;96:425–436. doi: 10.1016/s0092-8674(00)80555-3. [DOI] [PubMed] [Google Scholar]
- 72.Huse M, Muir TW, Xu L, Chen YG, Kuriyan J, Massagué J. The TGF-β receptor activation process: an inhibitor-to-substrate binding switch. Mol Cell. 2001;8:671–682. doi: 10.1016/s1097-2765(01)00332-x. [DOI] [PubMed] [Google Scholar]
- 73.Ebisawa T, Fukuchi M, Murakami G, Chiba T, Tanaka K, Imamura T, Miyazono K. Smurf1 interacts with transforming growth factor-β type 1 receptor through Smad 7 and induces receptor degradation. J Biol Chem. 2001;276:12477–12480. doi: 10.1074/jbc.C100008200. [DOI] [PubMed] [Google Scholar]
- 74.Yamaguchi T, Kurisaki A, Yamakawa N, Minakuchi K, Sugino H. FKBP12 functions as an adaptor of the Smad7-Smurf1 complex on activin type I receptor. J Mol Endocrinol. 2006;36:569–579. doi: 10.1677/jme.1.01966. [DOI] [PubMed] [Google Scholar]
- 75.Kaplan FS, Shore EM, Gupta R, Billings PC, Glaser DL, Pignolo RJ, Graf D, Kamoun M. Immunological features of fibrodysplasia ossificans progressiva and the dysregulated BMP4 pathway. Clin Rev Bone Miner Metab. 2005;3:189–193. [Google Scholar]
- 76.Olmsted-Davis E, Gannon FH, Ozen M, Ittmann MM, Gugala Z, Hipp JA, Moran KM, Fouletier-Dilling CM, Schumara-Martin S, Lindsey RW, Heggeness MH, Brenner MK, Davis AR. Hypoxic adipocytes pattern early heterotopic bone formation. Am J Pathol. 2007;170:620–632. doi: 10.2353/ajpath.2007.060692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaplan FS, Le Merrer M, Glaser DL, Pignolo RJ, Goldsby R, Kitterman JA, Groppe J, Shore EM. Fibrodysplasia ossificans progressiva. Best Pract Res Clin Rheumatol. 2008;22:191–205. doi: 10.1016/j.berh.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hakim M, Hage W, Lovering RM, Moorman CT, III, Curl LA, De Deyne PG. Dexamethasone and recovery of contractile tension after a muscle injury. Clin Orthop Relat Res. 2005;439:235–242. doi: 10.1097/01.blo.0000177716.70404.f9. [DOI] [PubMed] [Google Scholar]
- 79.Järvinen TA, Järvinen TL, Kääriäinen M, Kalimo H, Järvinen M. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33:745–764. doi: 10.1177/0363546505274714. [DOI] [PubMed] [Google Scholar]
- 80.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vanden Bossche LC, Van Maele G, Wojtowicz I, De Cock K, Vertriest S, De Muynck M, Rimbaut S, Vanderstraeten GG. Free radical scavengers are more effective than indomethacin in the prevention of experimentally induced heterotopic ossification. J Orthop Res. 2007;25:267–272. doi: 10.1002/jor.20296. [DOI] [PubMed] [Google Scholar]
- 82.Schipani E, Ryan HE, Didrickson S, Kobayashi T, Knight M, Johnson RS. Hypoxia in cartilage: HIF-1 alpha is essential for chondrocyte growth arrest and survival. Genes Dev. 2001;15:2865–2876. doi: 10.1101/gad.934301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pfander D, Cramer T, Schipani E, Johnson RS. HIF-1-alpha controls extracellular matrix synthesis by epiphyseal chondrocytes. J Cell Sci. 2003;116:1819–1826. doi: 10.1242/jcs.00385. [DOI] [PubMed] [Google Scholar]
- 84.Provot S, Ziny KD, Gunes Y, Kathri R, Le Q, Kronenberg HM, Johnson RS, Longaker MT, Giaccia AJ, Schipani E. Hif-1-alpha regulates differentiation of limb bud mesenchyme and joint development. J Cell Biol. 2007;177:451–464. doi: 10.1083/jcb.200612023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kaplan FS, Glaser DL, Shore EM, Pignolo RJ, Xu M, Zhang Y, Senitzer D, Forman SJ, Emerson SG. Hematopoietic stem-cell contribution to ectopic skeletogenesis. J Bone Joint Surg Am. 2007;89:347–357. doi: 10.2106/JBJS.F.00472. [DOI] [PubMed] [Google Scholar]
- 86.Stoick-Cooper CL. Advances in signaling in vertebrate regeneration as a prelude to regenerative medicine. Genes Dev. 2007;21:1292–1315. doi: 10.1101/gad.1540507. [DOI] [PubMed] [Google Scholar]
- 87.Glaser DL, Kaplan FS. Treatment considerations for the management of fibrodysplasia ossificans progressiva. Clin Rev Bone Miner Metab. 2005;3:243–250. [Google Scholar]
- 88.Shah PB, Zasloff MA, Drummond D, Kaplan FS. Spinal deformity in patients who have fibrodysplasia ossificans progressiva. J Bone Joint Surg Am. 1994;76:1442–1450. doi: 10.2106/00004623-199410000-00002. [DOI] [PubMed] [Google Scholar]
- 89.Kaplan FS, Glaser DL, Pignolo RJ, Shore EM. A new era for fibrodysplasia ossificans progressiva: a druggable target for the second skeleton. Expert Opin Biol Ther. 2007;7:705–712. doi: 10.1517/14712598.7.5.705. [DOI] [PubMed] [Google Scholar]
- 90.Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Bloch KD, Peterson RT. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mohler ER, III, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001;20:1522–1528. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 92.Pignolo RJ, Foley KL. Nonhereditary heterotopic ossification: implications for injury, arthropathy, and aging. Clin Rev Bone Miner Metab. 2005;3:261–266. [Google Scholar]
- 93.Wolf JM. The genetic key to a rare disease and its impact on orthopedics. Orthopedics. 2006;29:672. doi: 10.3928/01477447-20060801-16. [DOI] [PubMed] [Google Scholar]
- 94.Potter BK, Burns TC, Lacap AP, Granville RR, Gajkewski D. Heterotopic ossification in the residual limbs of traumatic and combat-related amputees. J Am Acad Orthop Surg. 2006;14:S191–S197. doi: 10.5435/00124635-200600001-00042. [DOI] [PubMed] [Google Scholar]
- 95.Kaplan FS. The key to the closet is the key to the kingdom: a common lesson of rare diseases. Orphan Disease Update. 2006;24:1–9. [Google Scholar]
- 96.Willis R. The Works of William Harvey. Philadelphia: University of Pennsylvania Press; 1989. pp. 616–617. [Google Scholar]