Abstract
The current study examined emotional reflex reactions of participants threatened with respiratory distress caused by imposing a resistive load at inspiration. Cues signaling threat (breathing MAY be difficult) and safe periods were intermixed while startle reflexes, heart rate, skin conductance and facial EMG activity were measured. Compared to safe cues, threat cues elicited significant startle potentiation, enhanced skin conductance, heightened corrugator EMG changes and pronounced "fear bradycardia" consistent with defensive activation in the context of threatened respiratory dysfunction. These data indicate that anticipating respiratory resistance activates defensive responding which may mediate symptomatology in patients with panic and other anxiety disorders.
Breathing is fundamental to survival, and the occurrence of abrupt respiratory blockages that augur suffocation can be frightening events. Patients suffering from asthma or chronic obstructive pulmonary disease frequently call for medical assistance with persistent fears of respiratory dysfunction that adds significantly to disease morbidity (Davenport & Vovk, 2009). Suffocation fears are also predominant in anxiety disorders, most notably claustrophobia, agoraphobia, and panic (Klein, 1993; Roth, 2005; Timmons & Ley, 1994). Furthermore, comorbid anxiety disorders occur at higher rates in individuals with respiratory disease than in the general population (Brenes, 2003), emphasizing the survival threat implicit in respiratory distress and its exagerration in clinically anxious individuals.
In the current study, we measured emotional reflex reactions of unselected participants under threat of impeded breathing. Considering its diagnostic relevance for many anxiety disorders, a paradigm measuring reactivity in anticipation of respiratory resistance could be a useful tool for assessing anxiety disorder risk and resilience. Previous respiration studies examined defensive reflex reactions associated with CO2 inhalation (e.g., Fannes, et al., 2008) or when anticipating a paced hyperventilation task (Melzig, et al., 2008). The present study employs a more easily controlled challenge in which participants first experience one trial of specified inspiratory resistance, followed by an assessment of defensive reflex reactivity across multiple periods of anticipation and safety.
The present research methodology is modeled on recent studies of threat of receiving a painful electric shock (e.g, Bradley, Silakowski, & Lang, 2008; Bradley, Moulder, & Lang, 2005). Somatic and autonomic defense reactions were monitored in the context of visual cues signaling periods when it might be difficult to breathe ("threat") and periods when breathing would be unimpeded ("safe"). Throughout the experiment, the participant wore a nose clip and breathed through a mouthpiece equipped with a device that controlled inspiratory resistive load. When load was increased, breathing became more difficult. Participants initially experienced this sensation during a 60-second exposure trial. Then, blue or red screens signaled periods when the inspiratory load could occur again (i.e., “threat”) or periods where breathing would not be difficult (“safe”). The same inspiratory load was presented again halfway through the experiment.
Heart rate, skin conductance, and facial frowning (corrugator EMG) activity were continuously collected, and eyeblink reflexes elicited by acoustic startle probes were measured during threat and safe periods. Previous studies assessing defensive reactivity under threat of shock found potentiated startle reflexes, heightened skin conductance activity, "fear bradycardia", and increases in facial frowning (Bradley, et al., 2005, 2008; Grillon, et al., 1991; Grillon & Davis, 1995) during threat, compared to safe, periods. In the current study, considering that obstructed breathing represents a comparable threat, we expect that anticipating impeded respiration will prompt a similar profile of defensive activation.
Method
Participants
Fifteen (6 female) University of Florida psychology students received course credit for participation in this study, which was approved by the University of Florida Institutional Review Board.
Stimulus materials
Threat and safety cues
Cues were simple red or blue backgrounds projected on a screen in front of the participant. For half of the participants, a red background indicated that breathing might be difficult (“threat”) and a blue background indicated that breathing would be unimpeded (“safety”); for the other half of the participants, the conditions were reversed.
Inspiratory load
Throughout the experiment, participants breathed through a mouthpiece attached to a non-rebreathing valve and pneumotachograph. The inspiratory portion of the mouthpiece was connected to a tube equipped with a manually operated 3-way valve to which the experimenter added resistance (50 cm H2O/L/s) by applying a stopcock. Inspiratory flow was continuously recorded from the pneumotachograph (Validyne MP45) and the signal was integrated (Validyne FV156). The experimenter applied the stopcock at the start of an exhalation to instate the inspiratory load. The load was instated for 60 seconds in the presence of the threat cue on two occasions: once during an initial exposure trial prior to the start of the experiment, and once halfway through the experimental session.
Trial structure
Throughout the session, a white fixation cross continuously appeared centrally on the projector screen. Each trial began with a 3 sec baseline in which a black background appeared. Threat and safety cues appeared for 12 seconds, followed by a variable (10–15 sec) intertrial interval (black background). Startle probes occurred on each trial at one of four counterbalanced latencies following cue onset (4, 5, 6, or 7 s). After the initial exposure to the load, the session consisted of 32 trials (16 threat, 16 safe), with the resistive load applied once again halfway through the session.
Procedure
The participant sat in a comfortable chair while the experimenter attached the sensors and mouthpiece. The experimenter then explained that the projector would periodically display a red or blue screen, and that anytime the screen was red (or blue, depending on group assignment), it was possible that it would become more difficult to breathe. During intertrial intervals, the participant was instructed to relax and wait for the next color cue. The initial exposure trial was then presented, in which the threat cue appeared while the inspiratory load was applied for 60 seconds. Instructions were then summarized and the experiment began. At the end of the session, the participant made separate ratings of the level of fear and suffocation experienced when breathing under the resistive load, using a 10-point scale (0=no fear/suffocation, 9=severe fear/suffocation).
Apparatus
VPM (version 11.7; Cook, 2001) controlled data acquisition and E-prime (Psychology Software Tools, Inc.) controlled events. Cues were displayed via an LCD projector on a screen 3m from the participant. Acoustic startle stimuli (50 ms, 95 dB) were presented over headphones.
In addition to inspiratory flow (described above), facial EMG was recorded at 20 Hz from 4mm electrodes placed over the left corrugator supercilii. Raw signals were amplified by 30,000, filtered (13–1000 Hz, Coulbourn V75-01) and integrated (V76-23A, 500 ms time constant). Skin conductance was recorded from 8 mm electrodes filled with NaCl paste on the hypothenar eminence of the left palm. Heart rate was recorded from 8mm electrodes on each forearm. A Schmitt trigger detected R-waves; inter-beat intervals were reduced to half-second bins.
For startle, 4mm Ag-AgCl electrodes were placed over the left orbicularis oculii (Fridlund & Cacioppo, 1986). Raw signals were amplified by 30,000 (Coulbourn V75-02), filtered from 8 to 1000 Hz (V75-48), sampled at 2000 Hz, and integrated (V-76-23A, 20 ms time constant). Offline, blinks were filtered between 28 Hz and 500 Hz, rectified, and integrated using a 20 ms time constant.
Data reduction and analysis
Blink data were reduced using a peak-scoring algorithm (Cook, 2001). Magnitude was transformed to microvolts, and trials on which there was no response were scored as zero. Blink data were excluded for 3 participants due to an excessive number of offscale responses, resulting in n=12. Heart rate, skin conductance, and corrugator EMG responses were calculated as change scores relative to the 1-second baseline prior to cue onset and averaged over the 12-second cue period. For respiratory data, a measure of inspiratory volume was obtained by computing the integral of the baseline-deviated inspiratory flow waveform for each 12-second cue period. SPSS (version 17.0) univariate repeated measures ANOVAs were performed for each measure, evaluating cue type (safe or threat) as a within-subjects factor.
Results
Exposure
Following exposure to the inspiratory resistive load, participants reported moderate fear (mean=4.0 out of 9, s.d.=2.3) and moderate sensation of suffocation (mean=4.5 out of 9, s.d.=2.3). Figure 1 illustrates that addition of the respiratory load during the initial exposure trial resulted in reliably reduced inspiratory flow (Figure 1 inlay), which was accompanied by a dramatic increase in skin conductance throughout the 60-second period.
Figure 1.
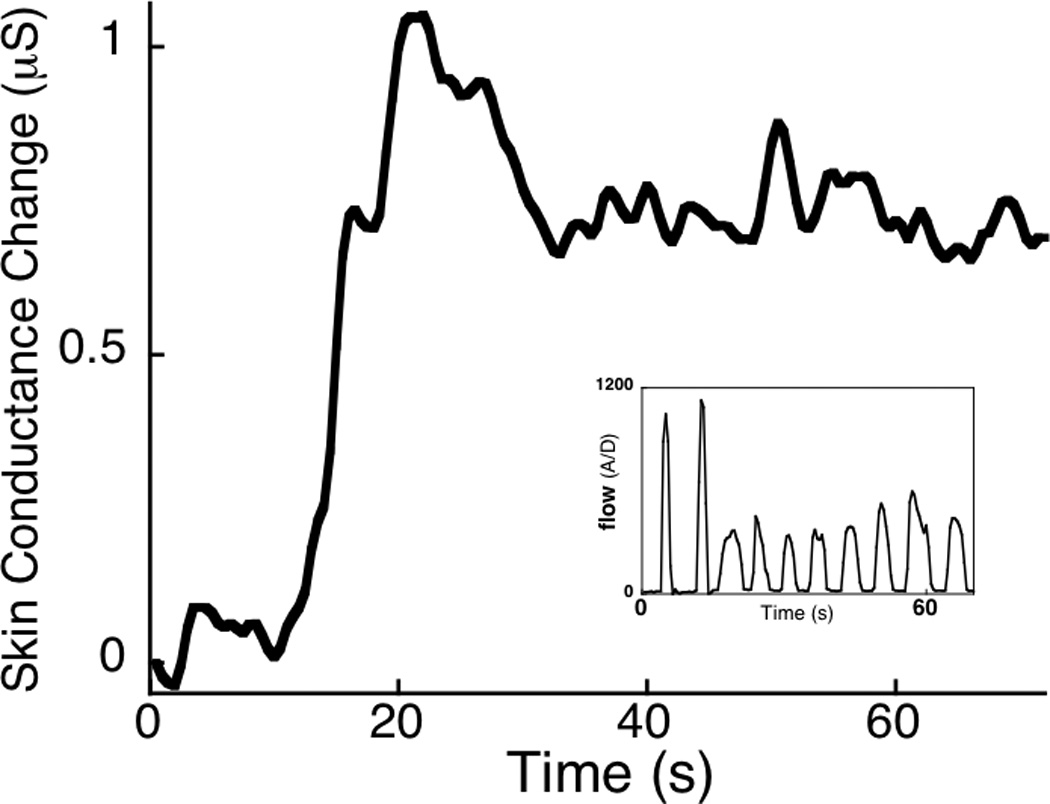
When the inspiratory resistive load was applied, skin conductance increased across the 60-second exposure trial. Inlay shows an example inspiratory flow waveform for one participant during exposure to the load, illustrating that inspiratory flow was reliably impeded across the 60-second period. µS=microsiemens.
Cued threat and safety
Figure 2 (top left panel) illustrates that startle probes presented in the context of cues threatening respiratory distress elicited potentiated startle blinks, compared to probes presented during safe periods, F(1,11)=11.1, p<0.01, η2p=0.50. Figure 2 (top right panel) illustrates that skin conductance change from the pre-cue baseline significantly increased during threat of respiratory distress, compared to safe periods, F(1,14)=6.8, p<0.05, η2p=0.33 (see Table 1). Consistent with the hypothesis that the threat of respiratory distress is aversive, corrugator muscle (i.e., frown) tension also significantly increased when viewing threat, compared to safety, cues, F(1,14)=7.1, p<0.05, η2p=0.34 (Figure 2, bottom left panel). Relatedly, significant cardiac deceleration was observed when participants viewed threat cues, compared to safe cues (Figure 2, bottom right panel), F(1,14)=6.5, p<0.05, η2p=0.32. Inspiratory volume was significantly greater when viewing cues signaling threat of respiratory distress, compared to cues that signaled unobstructed breathing, F(1,14)=7.15, p<0.05, η2p=0.34.
Figure 2.
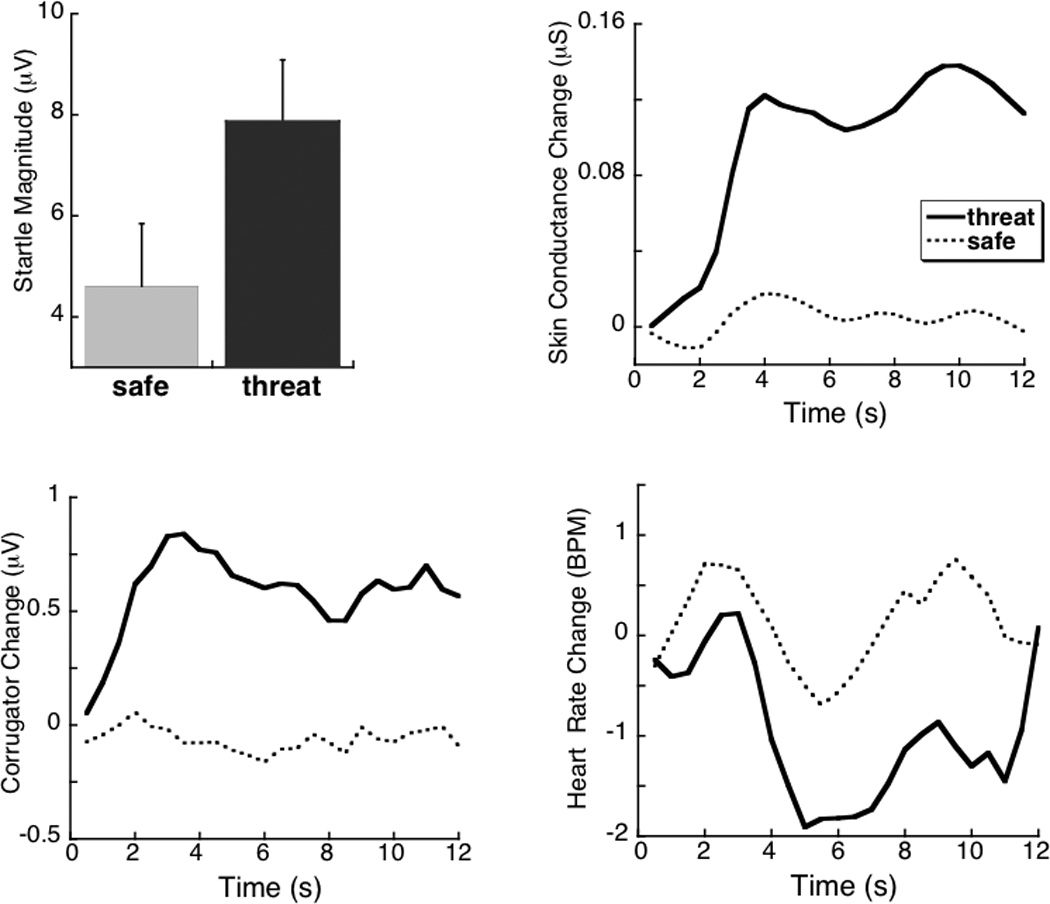
Top left: Reflexive startle blinks are larger in response to probes presented during cues signaling threat of respiratory distress, compared to cues signaling unobstructed breathing. Top right: Mean change in skin conductance level is heightened in the context of threat, compared to safe, cues. Bottom right: Mean heart rate shows deceleratory changes during threat, compared to safe, periods. Bottom left: Mean change in corrugator EMG (frown) activity is larger during threat, compared to safe, cues. µV=microvolts; µS=microsiemens; BPM=beats per minute. Error bars represent 95% confidence intervals (Loftus & Masson, 1994).
Table 1.
| Threat | Safe | |
|---|---|---|
| Skin conductance change (µSiemens) | 0.10 (0.03) | 0.00 (0.02) |
| Heart rate change (beats per min) | −0.95 (0.42) | 0.13 (0.38) |
| Corrugator EMG change (µV) | 0.58 (0.20) | −0.06 (0.14) |
| Inspiratory volume (ADU) | 74963 (2680) | 77739 (4189) |
Mean (SE) for each dependent measure as a function of cue type. Values reflect changes in skin conductance, heart rate, and corrugator electromyographic activity relative to a 1-second pre-cue baseline. µV=microvolts; ADU= analog-to-digital units.
Discussion
The present findings show that anticipation of only a brief, partial blockage of breathing prompts activation of the brain’s defense reflex system, reflected here in potentiated startle responses, activation of the sympathetic chain as indexed by increases in skin conductance, and facial displays of distress (i.e., marked corrugator muscle tension). Furthermore, the sustained cardiac deceleration seen during anticipated respiratory distress is consistent with a state of immobility and heightened vigilance (Bradley, 2009), as seen in the “fear bradycardia” found when animals confront a predator (Campbell, Wood, & McBride, 1997).
It has previously been shown (Davenport & Vovk, 2009; von Leupoldt, Sommer, Kegat, Eippert, Baumann, et al., 2008) that a respiratory load activates neural structures (e.g., amygdala, insula) within a motivational circuit typically implicated in affective experience. The current study shows that simply anticipating possible breathing difficulty prompts a range of reflex reactions mediated by this same defense circuit (Lang & Davis, 2006). Furthermore, the pattern of defensive engagement for participants under threat of disrupted breathing parallels that found for participants under the threat of receiving a painful electric shock (Bradley, et al., 2005, 2008; Grillon & Davis, 1995), which has also been related to defense circuit activation in brain imaging research (Costa, Bradley, Versace, & Lang, 2006; Dalton, Kalin, Grist, & Davidson, 2005). The similarity in defensive profiles when threatened with shock or disrupted breathing indicates that cues associated with threat to human life - whether painful or not -- activate a similar pattern of heightened vigilance and preparation for action (Bradley, 2009).
The strong defense reactions found here in healthy participants after exposure to a brief respiratory blockage—described as only moderately fear-provoking, on average—emphasize the broad evolutionary foundation for respiratory anxiety. Furthermore, a post-hoc analysis revealed that individuals reporting greater fear during exposure to inspiratory resistance subsequently showed greater sympathetic activation (i.e., skin conductance level) when viewing cues signaling threat of impeded breathing (r[13]=0.58, p<0.05). Although the small sample size encourages caution, the data support the view that greater fear of respiratory symptoms is associated with exaggerated sensitivity to threat cues, suggesting that the current paradigm could have meaningful clinical value.
These findings highlight the importance of refining treatments targeting suffocation fear in individuals with respiratory disease as well as anxiety disorders. In this regard, Coventry (2009) cites several pulmonary rehabilitation studies suggesting that incremental breathing exercises can be effective in reducing dyspnea-related anxiety. Similarly, a desensitization treatment using supervised anticipation of breathing under load, as in interoceptive exposure therapy for panic disorder (e.g., Craske, Barlow, & Meadows, 2000), could be beneficial in addressing anxiety associated with a dread of respiratory distress.
Fear of suffocation and dyspnea have been signaled as key features of panic and other anxiety diagnoses (Ley, 1989; Roth, 2005). The current data show that defensive activation and negative affect are reliable consequences in anticipation of even transient respiratory resistance. Thus, assessing the degree of defensive activation consequent on a controlled respiratory load could provide a safe method for measuring individual differences in resiliency, and potentially provide a tool for prognostic assessment in chronic fear and anxiety disorders.
Acknowledgments
This research was supported in part by NIMH grant P50 MH072850-04 to Peter J. Lang.
References
- Bradley MM, Silakowski T, Lang PJ. Fear of pain and defensive activation. Pain. 2008;137(1):156–163. doi: 10.1016/j.pain.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Moulder B, Lang PJ. When good things go bad: The reflex physiology of defense. Psychological Science. 2005;16:468–473. doi: 10.1111/j.0956-7976.2005.01558.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM. Natural selective attention: Orienting and emotion. Psychophysiology. 2009;46:1–11. doi: 10.1111/j.1469-8986.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenes GA. Anxiety and chronic obstructive pulmonary disease: Prevalence, impact, and treatment. Psychosomatic Medicine. 2003;65:963–970. doi: 10.1097/01.psy.0000097339.75789.81. [DOI] [PubMed] [Google Scholar]
- Campbell BA, Wood G, McBride T. Origins of orienting and defensive responses: An evolutionary perspective. In: Lang PJ, Simons RF, Balaban MT, editors. Attention and orienting: Sensory and motivational processes. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc.; 1997. pp. 41–67. [Google Scholar]
- Costa VD, Bradley MM, Versace F, Lang PJ. Fear relevance modulates frontal cortex activity during anticipation of pain. Program No.73.8. 2006 Neuroscience Meeting Planner. Atlanta, GA: Society for Neuroscience; 2006. Online. [Google Scholar]
- Coventry PA. Does pulmonary rehabilitation reduce anxiety and depression in chronic obstructive pulmonary disease? Current Opinion in Pulmonary Medicine. 2009;15:143–149. doi: 10.1097/MCP.0b013e3283218318. [DOI] [PubMed] [Google Scholar]
- Craske MG, Barlow DH, Meadows EA. Mastery of your anxiety and panic: Therapist guide for anxiety, panic, and agoraphobia (MAP-3) San Antonio, TX: Graywind/Psychological Corporation; 2000. [Google Scholar]
- Dalton KM, Kalin NH, Grist TM, Davdison RJ. Neural-cardiac coupling in threat-evoked anxiety. Journal of Cognitive Neuroscience. 2005;17(6):969–980. doi: 10.1162/0898929054021094. [DOI] [PubMed] [Google Scholar]
- Davenport PW, Vovk A. Cortical and subcortical central neural pathways in respiratory sensations. Respiratory Physiology & Neurobiology. 2009;167:72–86. doi: 10.1016/j.resp.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Fannes S, van Diest I, Meulders A, de Peuter S, Vansteenwegen D, van den Bergh O. To inhale or not to inhale: Conditioned avoidance in breathing behavior in an odor—20% CO2 paradigm. Biological Psychology. 2008;78(1):87–92. doi: 10.1016/j.biopsycho.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Woods SW, Merikangas K, Davis M. Fear-potentiated startle in humans: Effects of anticipatory anxiety on the acoustic blink reflex. Psychophysiology. 1991;28:588–595. doi: 10.1111/j.1469-8986.1991.tb01999.x. [DOI] [PubMed] [Google Scholar]
- Grillon C, Davis M. Acoustic startle and anticipatory anxiety in humans: Effects of monaural right and left ear stimulation. Biological Psychiatry. 1995;38:68–70. doi: 10.1111/j.1469-8986.1995.tb03307.x. [DOI] [PubMed] [Google Scholar]
- Klein DF. False suffocation alarms, spontaneous panics, and related conditions. Archives of General Psychiatry. 1993;50:306–317. doi: 10.1001/archpsyc.1993.01820160076009. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Davis M. Emotion, motivation, and the brain: Reflex foundations in animal and human research. Progress in Brain Research. 2006;156:3–29. doi: 10.1016/S0079-6123(06)56001-7. [DOI] [PubMed] [Google Scholar]
- Ley R. Dyspneic-fear and catastrophic cognitions in hyperventilatory panic attacks. Behavior Research and Therapy. 1989;5:549–554. doi: 10.1016/0005-7967(89)90089-2. [DOI] [PubMed] [Google Scholar]
- Loftus GR, Masson MEJ. Using confidence intervals in within-subject designs. Psychonomic Bulletin & Review. 1994;1:476–490. doi: 10.3758/BF03210951. [DOI] [PubMed] [Google Scholar]
- Melzig CA, Michalowski JM, Holtz H, Hamm AO. Anticipation of interoceptive threat in highly anxiety sensitive persons. Behaviour Research and Therapy. 2008;46:1126–1134. doi: 10.1016/j.brat.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Papp LA, Klein DF, Gorman JM. Carbon dioxide hypersensitivity, hyperventilation and panic disorder. American Journal of Psychiatry. 1993;150:1149–1157. doi: 10.1176/ajp.150.8.1149. [DOI] [PubMed] [Google Scholar]
- Roth WT. Physiological markers for anxiety: Panic disorder and phobias. International Journal of Psychophysiology. 2005;58:190–198. doi: 10.1016/j.ijpsycho.2005.01.015. [DOI] [PubMed] [Google Scholar]
- von Leupoldt A, Sommer T, Kegat S, Eippert F, Baumann HJ, Klose H, Dahme B, Buchel C. The unpleasantness of perceived dyspnea is processed in the anterior insula and amygdala. American Journal of Respiratory and Critical Care Medicine. 2008;177(9):1026–1032. doi: 10.1164/rccm.200712-1821OC. [DOI] [PubMed] [Google Scholar]


